Abstract
Advances in cancer therapy have resulted in significant improvement in long-term survival for many types of cancer, but have also resulted in untoward side effects associated with treatment. One such complication that has become increasingly recognized is the development of cardiomyopathy and heart failure. Whether a previously healthy person from a cardiovascular perspective develops cancer therapy related cardiac dysfunction or a high-risk cardiovascular patient requires cancer therapy, the team of oncologists and cardiologists must be better equipped with an evidence-based approach to care for these patients across the spectrum. Although the toxicities associated with various cancer therapies are well recognized, limitations to our understanding of the appropriate course of action remain. In this first of a 2-part review, we discuss the epidemiologic, pathophysiologic, risk factors, and imaging aspects of cancer therapy related cardiac dysfunction and heart failure. In a subsequent second part, we discuss the prevention and treatment aspects, concluding with a section on evidence gap and future directions. We focus on adult patients in all stages of cancer therapy from pre-treatment surveillance, to ongoing therapy, and long-term follow up.
Keywords: cardiomyopathy, heart failure, treatment, left ventricular ejection fraction, left ventricular dysfunction, chemotherapy, cardiotoxicity, cancer therapy, anthracycline, trastuzumab
Early detection and treatment has transformed cancer from a uniformly fatal disease to one that in many cases is a chronic condition. Improved survival however, is often accompanied by treatment related complications, including adverse effects of cancer therapies on the heart. In the long-term, the risk of death from cardiovascular causes exceeds that of tumor recurrence for many forms of cancer.1, 2 Patnaik et al found that cardiovascular disease (CVD) was the leading cause of death among older female breast cancer survivors without an initial diagnosis of CVD.3 Risk of toxicity was increased in patients with advanced age, multiple comorbid conditions, and in those requiring prolonged or intensive treatment. Cancer therapies including cytotoxic chemotherapy, molecular targeted therapies and mediastinal irradiation have been linked to myocyte damage, left ventricular dysfunction (LVD), heart failure (HF), thrombogenesis, pericardial pathology, hypertension, ischemia, conduction and rhythm disturbances, and vasospasm.4, 5 HF as a result of cancer therapy has been linked to a 3.5 fold increased mortality risk compared with idiopathic cardiomyopathy6 In part-1 of this 2-part review, we describe the definitions, pathophysiology, risk factors, and imaging aspects relevant to cancer therapy related cardiac dysfunction.
DEFINITIONS
Several definitions of cancer therapy related cardiac dysfunction have been proposed, making development of uniformly accepted recommendations for diagnosis, surveillance, and treatment challenging. The National Cancer Institute (NCI) broadly defines cardiotoxicity as “toxicity that affects the heart”,7 and proposes the Common Terminology Criteria for Adverse Events (CTCAE) that defines LVD and HF based on severity into grades 1–5. Grade 1 is defined as asymptomatic elevations in biomarkers or abnormalities on imaging. Grades 2 and 3 consist of symptoms with mild and moderate exertion. Grade 4 includes severe, life threatening symptoms requiring hemodynamic support and grade 5 involves death.8 The Food and Drug Administration (FDA) defines anthracycline cardiotoxicity as >20% decrease in left ventricular ejection fraction (LVEF) when baseline LVEF is normal, or >10% decrease when baseline LVEF is not normal.9
The Cardiac Review and Evaluation Committee (CREC) supervising trastuzumab trials defines cardiotoxicity as a decrease in LVEF that is either global or more severe in the septum and decline in LVEF of at least 5% to <55% with accompanying signs or symptoms of HF, or a decline of at least 10% to <55% without HF signs or symptoms.10 Several trials have specified toxicity with different parameters making estimation of the prevalence of cardiac toxicity difficult. In the Herceptin Adjuvant (HERA) trial, definitions of asymptomatic LVEF reductions were different and included decrease by ≥10% from baseline to an LVEF <50%, and HF as above accompanied by symptoms.11 The Breast Cancer International Research Group (BCIRG) used >10% reduction from baseline LVEF assessment to define asymptomatic LVD.12 An expert consensus published by the American Society of Echocardiography and European Association of Cardiovascular Imaging, comprised of several well-respected cardiologists and oncologists within the field of cardio-oncology, defines Cancer Therapeutics Related Cardiac Dysfunction CTRCD) as a decrease in the LVEF of >10%, to a value less than 53% confirmed by repeat imaging. Further characterization is based on the presence or absence of symptoms.13These definitions include arbitrary cutoffs without taking into account baseline risk and are not guided by clinical outcomes. In addition, LVEF based definition has limitations including variable reproducibility and the fact that many patients with HF have preserved LVEF. A more comprehensive definition for diagnosis of cancer therapy-related cardiotoxicity should take into account other imaging and biomarker based abnormalities as well.
CLASSIFICATION
Several attempts have been made to classify cardiotoxicity. Ewer and Lippman proposed cardiotoxicity based on the type and extent of structural abnormalities and degree of reversibility.14 Type I is irreversible and dose-related with myocyte injury, whereas type II includes reversibility with cessation of treatment, lack of dose-relationship, and absence of ultrastructural abnormalities. While intuitive, with anthracyclines as an example of type I and trastuzumab of type II cardiotoxicity, subsequent studies have raised concerns. This classification does not reflect the reality that anthracyclines and trastuzumab are rarely administered as sole agents, and are usually shortly preceded or followed with drugs belonging to other classes. Hence, it is likely that the final cardiotoxic effect results from a synergic/combined action. Although anthracyclines and trastuzumab are different in their mechanisms of cardiotoxicity, early recognition and initiation of neurohormonal antagonists may reverse LVD in both cases.15, 16 Trastuzumab cardiotoxicity may be associated with troponin elevation, and is not always reversible.15 Therefore it is more appropriate to understand the biological mechanisms of cardiotoxicity and the clinical features at different stages of presentation.
PATHOPHYSIOLOGY AND EPIDEMIOLOGY
Anthracyclines
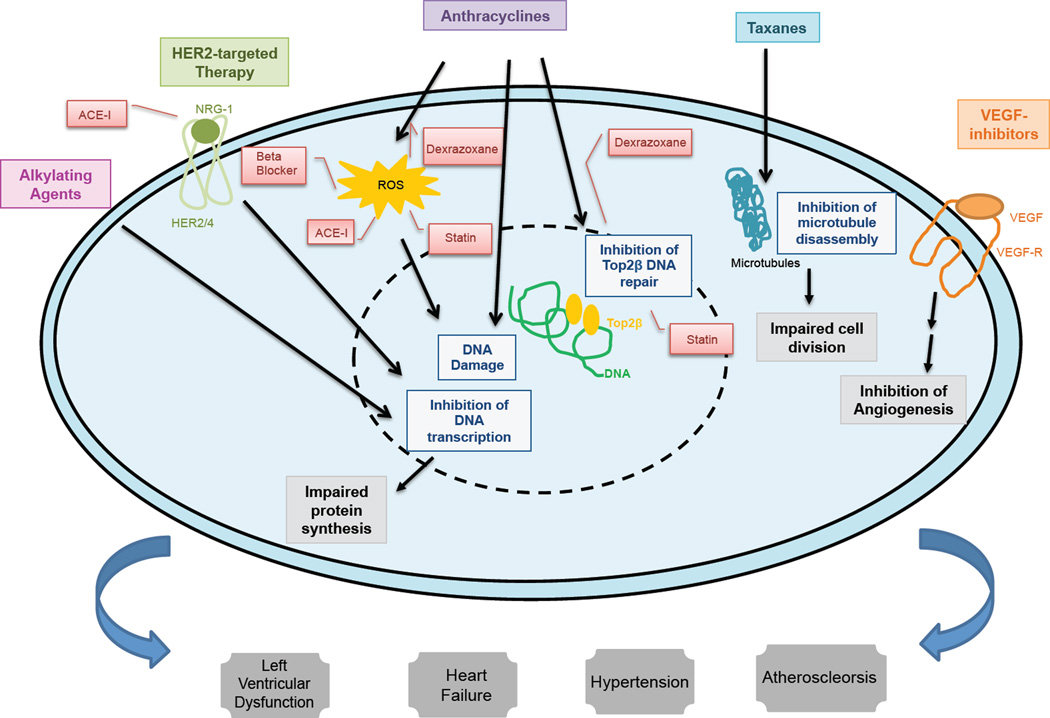
Anthracyclines (ex. doxorubicin, daunorubicin, epirubicin, idarubicin) are a class of highly effective chemotherapy agents used for the treatment of many solid and hematologic cancers.17 In breast cancer, doxorubicin and epirubicin are used in both the neoadjuvant [prior to definitive surgery] and adjuvant [following definitive surgery] setting, as well as in metastatic patients. The mechanisms of action of anthracyclines include intercalation into nuclear DNA to impair protein synthesis, production of reactive oxygen species, and inhibition of topoisomerase II to inhibit DNA repair. Topoisomerase, an enzyme involved in DNA transcription and replication, is a known target of anthracyclines.18 There are two isozymes of topoisomerase, Top 2-alpha, expressed in rapidly dividing cells, and Top 2-beta, expressed in quiescent cells such as cardiac myocytes.19 The cardiac toxicity of anthracyclines is thought to be mediated through the binding of these agents to DNA and Top2-beta in cardiac myocytes, resulting in a complex formation that ultimately culminates in cell death.20 (Figure 1) Older studies suggest an association between cumulative dosing and cardiotoxicity, with diastolic dysfunction reported at doses of 200 mg/m2 of doxorubicin and systolic dysfunction at 400–600 mg/m2.21 This has been challenged in recent literature suggests that LVD can occur at any dose as evidenced in 18.9% of patients receiving a doxorubicin dose of 240mg/m2 in combination with cyclophosphamide.22 Factors increasing the risk of anthracycline toxicity include the presence of other CVD risk factors, associated therapies like mediastinal irradiation, and concomitant therapy with agents such as cyclophosphamide, paclitaxel, and trastuzumab. 23 Anthracycline toxicity may be acute, sub-acute, or chronic. Acute toxicity is uncommon (~1%) and generally reversible, while early-onset chronic progressive toxicity (1.6–2.1%) developing during treatment, and late-onset chronic progressive types (1.6–5%) are more likely to be irreversible.24 This classification has been challenged as possible evolution of one phenomenon being clinically identified at various stages.16
Figure 1.
Pathophysiology of Cardiac Toxicity from Various Chemotherapeutics and Role of Preventative Therapies. Alkylating agents inhibit DNA transcription, impairing protein synthesis. Human epidermal receptor 2 (HER2/ErbB)-targeted therapy inhibits the activation of HER2 resulting in the inhibition of a signal transduction pathway that ultimately impairs DNA transcription. Anthracyclines intercalate into DNA, impairing protein synthesis, generate reactive oxygen species (ROS) resulting in DNA damage as well as inhibit topoisomerase II-beta (Top2B), impairing DNA repair. Taxanes impair microtubule function needed for cell division. Vascular endothelial growth factor (VEGF)-inhibitors blocks the activation of kinases resulting in the downstream inhibition of angiogenesis. Dexrazoxane, an iron-chelating agent may decrease the formation of ROS through prevention of anthracycline-iron complex formation as well as inhibit the formation of Top2B–DNA cleavage complexes which impair DNA repair. Beta-blockers, statins, and angiotensin converting enzyme inhibitors (ACE-I), through anti-oxidant properties, may inhibit the production of ROS. ACE-I’s may decrease angiotensin-induced blockade of the neuregulin-1 (NRG-1)/ErbB pathway. Statins have also been shown to inhibit Top2B–mediated DNA damage. The various cellular effects of these chemotherapy agents may ultimate result in cardiovascular manifestations in the form of left ventricular dysfunction, heart failure, hypertension, and atherosclerosis.
Alkylating agents
Alkylating agents (ex. Cyclophosphamide, ifosphamide, melphalan) inhibit DNA transcription, thereby affecting protein synthesis.25 Agents such as cyclophosphamide have been associated with development of LVD in 7–28% of patients and may be dose related (≥150 mg/kg and 1.5g/m2d), occurring shortly after initial administration.26 LVD has also been observed with ifosfamide at doses that exceed 12.5 g/m2.27
Microtubular polymerization inhibitors
Taxanes (ex. paclitaxel and docetaxel) bind to and inhibit disassembly of microtubules, interrupting cell division.28 Taxanes interfere with the metabolism and excretion of anthracyclines and may potentiate LVD risk, particularly with high dose anthracycline use. HF incidence with these agents is relatively low, with a reported 1.6% versus 0.7% incidence in patients with anthracycline-containing versus anthracycline-sparing regimens.12 Slower infusion or increasing intervals between paclitaxel and doxorubicin may attenuate toxicity.29 Newer adjuvant protocols and formulations might decrease the likelihood of toxicity.30
HER2-targeted cancer therapies
Overexpression of human epidermal growth factor receptor 2 (HER2/ERbB2) in breast cancer is a poor prognostic indicator, as these tumors tend to be more aggressive and associated with higher reoccurrence rates.31 Trastuzumab, a humanized anti-HER2 monoclonal antibody targeting the extracellular domain of this oncoprotein, has been shown in both the metastatic32 as well as the adjuvant33 setting to dramatically change the survival in HER2 positive breast cancer. HER2/ERbB2 is expressed on myocytes and plays a protective role against myocardial stress.21 The binding of cancer drugs to HER2 receptor may disrupt this cardioprotective pathway and result in cardiotoxicity. Trials with trastuzumabobserved increased incidence of LVD and its use with anthracyclines potentiates cardiotoxicty risk.10 Previous trials in the adjuvant setting reported HF in 1.7–4.1% and LVD in 7.1–18.6% of patients receiving trastuzumab, though in practice, incidence may be higher.34 Lapatanib, an oral tyrosine kinase inhibitor of HER2 and epidermal growth factor receptor (EGFR), is approved in the metastatic setting and pertuzumab, a monoclonal antibody that blocks the dimerization of HER2 with other HER2 receptors, is approved in both the neoadjuvant and metastatic setting in combination with trastuzumab and docetaxel.35 Combination treatment using two anti-HER2 agents improves rates of pathologic complete responses in the neoadjuvant setting and survival in the metastatic settings, with comparable cardiotoxicity.36 T-DM1 is a HER2-targeted antibody-drug conjugate combining trastuzumab with a cytotoxic agent DM1, reported a 2.7% incidence of LVD.37 As the available treatments expand, new questions arise with respect to the development of cardiotoxicity.
VEGF inhibitors (monoclonal antibodies and small molecules e.g. tyrosine kinase Inhibitors)
VEGF inhibitors exert their action by inhibiting VEGF-mediated angiogenesis through various mechanisms.38 Small molecule tyrosine kinase inhibitors (sunitinib and sorafenib) are nonselective inhibitors of VEGF receptors, inhibiting up to 50 different kinases in addition to their intended target, thus producing unwanted effects.39 These agents have been linked to hypertension, ischemia, LVD and HF.40 Initial reports of sunitinib showed a 10% incidence of LVD, but with recovery upon completion of therapy,41 and a 1.5–4.1% incident of HF.42 The real world experience is in fact worse, with ~14% of patients experiencing a >10% decline in EF.43 Bevacizumab is a monoclonal antibody that targets VEGF and is associated with a 5-fold increase in HF risk.44
Radiotherapy
Radiation induced heart disease is well recognized and may manifest years after exposure. Radiotherapy is associated with macrovascular, microvascular, and endothelial injury, valvular dysfunction, atherosclerosis, fibrosis and pericardial disease. LVD and HF can occur as acute radiation myocarditis,45 but more commonly develops as a long term consequence of fibrosis leading to ventricular dysfunction or restrictive cardiomyopathy.45The presence of other CVD risk factors, concomitant anthracycline use, and anterior or left chest irradiation all increase risk. Mediastinal irradiation increases CVD and HF risk even 40 years after initial exposure.46 In a recent study of patients receiving radiation, there was a linear increase in coronary events with radiation dose. Events occurred 5 years after initial exposure and continued through the third decade following exposure.47
RISK FACTORS
Identification of patients at risk is difficult though important. Patient related risk factors include those with preexisting cardiac risk factors such as hypertension, diabetes, smoking, previous LVD or HF, coronary disease, increasing age, female gender, and post-menopausal status.48 Genetic polymorphisms may predispose to cardiotoxicity at lower anthracycline dose,49 suggesting that genetic variation might modulate the risk of cardiovascular toxicity after cancer treatment. In an unselected group of cancer patients, even prior to chemotherapy, the presence of N-terminal pro brain natriuretic peptide (NT-proBNP), mid-regional pro atrial natriuretic peptide (MR-proANP), mid-regional pro adrenomedullin (MR-proADM), high sensitivity troponin T (hsTnT), and copeptin were associated with all-cause mortality.50 Therapy related risk factors include use of combination cancer therapy, particularly if administered simultaneously, or as bolus, addition of mediastinal irradiation, and higher doses. Certain agents such as anthracycline, trastuzumab, and cyclophosphamide carry higher risk while others such as bevacizumab, etoposide, and lapatinib carry lower risk.51 Prior treatment with anthracyclines increases the risk if a patient presents with recurrent disease or a new malignancy requiring further anthracycline therapy.52
RISK PREDICTION
Several risk scores have been published, though there is no consensus model. Herrmann et al. proposed a risk model including both cancer therapy and patient factors.53 Romond et al. used a model including age and baseline LVEF to estimate HF risk, also reporting LVEF recovery.54 Dranitsaris et al. developed and tested a cycle-based risk-prediction tool in metastatic breast cancer patients receiving doxorubicin or its pegylated liposomal form. This score included age, weight, anthracycline exposure, and performance status, adding points for each additional chemotherapy cycle.55 Ezaz et al developed a 7-point risk score for 3-year HF risk after trastuzumab therapy in older females. Risk factors such as coronary disease, atrial fibrillation, hypertension, diabetes, and renal failure all portended higher risk. HF incidence was higher than previously reported, with the oldest patients approaching 45% incidence.56 (Table 1)
Table 1.
Overview of Validated Risk Prediction Models
| Reference | Population | Risk Factors | n | Definition of Cardiotoxicity | Discrimination | Calibration | Validation |
|---|---|---|---|---|---|---|---|
| Ezaz et al. 201456 | Women with early-stage breast cancer who underwent surgery and adjuvant trastuzumab therapy |
Adjuvant therapy type, Age, CAD, AF, DM, HTN, renal failure |
1664 | ICD-9CM code for HF or CM that appears in at least one inpatient claim or 2 outpatient claims at least 30 days apart |
NR | NR | NR |
| Romond et al. 201254 | Women with HER2+ Breast Cancer |
Age, baseline LVEF | 1830 | LVEF drop >10% from baseline to <55% or drop >5% to level below lower limit of normal |
0.72 (0.70 after bootstrapping) |
NR | Bootstrapping |
| Dranitsaris et al. 200855 | Women with metastatic breast cancer receiving anthracycline-based chemotherapy |
Age, Weight, baseline anthracycline exposure, performance status, # cycles |
509 | (1) LVEF drop 20% but still normal range, (2) LVEF drop 10% if abnormal, (3) signs/symptoms HF |
0.84 | NR | Bootstrapping |
AF, atrial fibrillation; CAD, coronary artery disease; DM, diabetes mellitus, HF, heart failure; HTN, hypertension; LVEF, left ventricular ejection fraction NR, not reported
BIOMARKERS
A biomarker approach for early identification, risk stratification and monitoring of chemotherapy related cardiotoxicity holds promise, though challenges exist with respect to timing of measurement, optimal assays, and whether this strategy is best used alone or in conjunction with imaging.
Biomarker of Injury (ex. Troponin)
Troponin levels can be monitored before and after each therapy cycle and serve as a predictor of future cardiac dysfunction. A high negative predictive value with absence of troponin elevation has been reported in patients receiving high dose anthracyclines.57 Early increase in troponin I after anthracycline use predicted diastolic dysfunction in 34% of patients.58 Increased troponin I in patients receiving trastuzumab had a decreased likelihood of LVEF recovery and a higher incidence of cardiac events.15 Troponin I levels at completion of anthracycline treatment were predictive of subsequent reduction in LVEF and cardiac events.59 Smaller studies have looked at troponin use for identification of at-risk patients with newer agents such as anti-VEGF monoclonal antibodies, anti-VEGFR tyrosine kinase inhibitors, and a kinesin inhibitor.60
Biomarker of Load (ex. Natriuretic peptide)
Natriuretic peptides have been studied in chemotherapy-treated patients with variable results. In one study, BNP was predictive of LVD at 3-, 6-, and 12-month follow-up.61 In patients undergoing doxorubicin therapy, an increase in natriuretic peptides during the first 90 days was predictive of LVD at 4 years.62 Several studies however, have failed to show an association between these biomarkers and cardiac dysfunction,59, 63 and they may be more useful for their negative predictive value as part of a surveillance strategy. Further studies are needed to understand the role of natriuretic peptide use in this population, and to understand the differences in the various types of natriuretic peptides.
Other Biomarkers
High sensitivity C-reactive protein (CRP) predicts cardiotoxicity in patients treated with trastuzumab.64 Other studies of patients receiving anthracycline-containing regimen followed by taxanes and trastuzumab showed no association between CRP, galectin-3, ST2 or growth differentiation factor-15, and cardiotoxicity but one study reported that changes in myeloperoxidase levels were associated with cardiotoxicity.59, 65 In a recent study of multiple biomarkers, myeloperoxidase (MPO) levels rose early, persisted throughout the course of therapy, and were associated with cardiotoxicity.66 (Table 2)
Table 2.
The Role of Cardiac Biomarkers in Chemotherapy-induced Cardiotoxicity
| Reference | Patient Population | N | Chemotherapy | Biomarker | Value Cutoff | Timing of Measurement | Results |
|---|---|---|---|---|---|---|---|
| Ky et al. 201465 | HER2+ Breast Cancer | 78 | Doxorubicin, Cyclophosphamide, Paclitaxel, Trastuzumab |
TnI, CRP NT-proBNP GDF-15, MPO PIGF, sFlt-1 gal-3 |
-Biomarkers measured at baseline, 3, and 6 months -LVEF measured at baseline, 3,6,9,12,15 months |
-TnI and MPO rise at 3 months was associated with subsequent cardiotoxicity |
|
| Skovgaard et al. 201467 | Breast Cancer, Hematologic malignancies, Uterine/Ovarian Cancer |
333 | Anthracycline | BNP | 100pg/ml | No standard interval of measurements |
-BNP and LVEF independently predicted CHF -Only BNP was associated with overall mortality |
| Sawaya et al. 201259 | HER2+ Breast Cancer | 81 | Anthracycline, Paclitaxel, Trastuzumab |
Troponin NT-proBNP ST-2 |
≥30pg/mL >125pg/mL >35pg/mL |
Baseline 3,6,9,12,15 months | -Elevated TnI at 3 months was predictive of subsequent cardiotoxicity -No change in NT- proBNP and ST2 |
| Lipshultz et al. 201262 | Children with high- risk ALL |
156 | Doxorubicin | TnT NT-proBNP hsCRP |
Any detectable amount ≥150pm/mL <age 1; ≥100pm/mL >age 1; ≥1.9mg/L |
-Biomarkers measured at baseline, days 1–7 of doxorubicin induction, 7 days after a doxorubicin dose, and at end of doxorubicin therapy -LVEF measured at baseline, after therapy and every 2 years thereafter |
-Increase in TnT and NT-proBNP during first 90 days predicted cardiac dysfunction at 4 years |
| Onitilo et al. 201263 | HER2+ Breast Cancer | 54 | Trastuzumab adjuvant | BNP hs-CRP TnI |
≥200pg/mL ≥3mg/L ≥0.01ng/mL |
-Biomarkers measured at baseline, every 3 weeks up to 1 year -LVEF measured every 3–4 months |
-Only hs-CRP was associated with increased risk for clinically significant decline in LVEF |
| Morris et al. 201168 | HER2+ Breast Cancer | 95 | Doxorubicin, Cyclophosphamide, Paclitaxel, Trastuzumab, Lapatinib |
TnI CRP |
>0.04ng/mL(DF/HCC) >0.06ng/mL (MSKCC) ≥0.8mg/dL (MSKCC) ≥0.3mg/dL (DF/HCC) |
-Biomarker measured every 2 weeks during chemo, 6,9, 18 months -LVEF measured at months 0,6,9,18 |
-TnI rise (peaking at ~14 weeks) preceded maximal decline in LVEF but no relation to max LVEF decline -CRP did not correlate with LVEF decline |
| Romano et al. 201161 | Breast Cancer | 92 | Anthracycline, Taxane, 5- Fluorouracil, Cyclophosphamide |
NT-proBNP TnI |
>153pg/mL age ≤50; 222pg/mL age >50 5ng/ml age ≤50; 0.08ng/mL age >50 |
-Biomarkers measured at baseline, before and 24h after each drug administration -LVEF measured at baseline, every 2 cycles, end of chemo, 3, 6, 12 months follow up |
-NT-proBNP was predictive of LV impairment at 3,6, and 12 months follow-up |
| Cardinale et al. 201015 | Breast Cancer | 251 | Trastuzumab, Anthracycline, Cyclophosphamide, Paclitaxel |
TnI | >0.08ng/mL | -Biomarker measured before and after each cycle -LVEF measured at baseline, every 3 months during therapy and every 6 months after |
-TnI was an independent predictor of cardiotoxicity and LVEF recovery |
| Mavinkurve-Groothuis et al. 200969 | Various Pediatric Cancers |
122 | Anthracycline-containing regimen |
NT-proBNP TnT |
>10pmol/L M; >18pmol/L F >0.01ng/mL |
-Measured once | -Elevated NT-pro- BNP was associated with cumulative anthracycline dose |
| Dodos et al. 200870 | Solid or hematological malignancy |
100 | Anthracycline-containing regimen |
TnT NT-proBNP |
>0.010ng/mL <153pg/mL F <age 50; <334pg/mL F age 50–70; <88 pg/mL M <age 50; <227pg/mL M age 50–70 |
-Measured 24–72h, 1, 6, and 12 months after last course of chemo |
-TnT and proBNP did not predict cardiac dysfunction |
| Jingu et al. 200771 | Esophageal Cancer | 197 | Radiotherapy | BNP | -Measured before, <1 month, 1–2, 3–8, 9–24, >24hrs months after radiotherapy |
-BNP higher in patients who had high FDG accumulation |
|
| Sandri et al. 200372 | Various | 179 | Various regimens including Epirubicin, Cyclophosphamide, Taxotere, Carboplatin |
TnI | >0.08ng/mL | -Biomarkers measured at baseline, at end of infusion, 12, 24, 36, 72h after each cycle -LVEF measured at baseline, 1,2,3,4,7,12 months after end of treatment |
-Increase in TnI as early as first cycle predicted subsequent LVEF decline |
| Cardinale et al. 200073 | Breast Cancer, Ovarian cancer, Small-cell lung cancer, Hodgkin’s Lymphoma, non- Hodgkin’s Lymphoma |
204 | Various regimens including Epirubicin, Cyclophosphamide, Taxotere, Carboplatin, Etoposide |
TnI CK CK-MB |
>0.5ng/mL >190U/L >5ng/mL |
-Before, immediately after and then 12, 24, 36, 72h after every cycle of chemo |
-TnI elevation predicted future LVEF decline |
Studies ordered by Date with sample size >50 patients. ALL, acute lymphoblastic leukemia; CHF, congestive heart failure; CK, creatinine kinase; CK-MB, MB fraction; CRP, C-reactive protein; gal-3, galactin 3; GDF-15, growth differentiation factor 15; LVEF, left ventricular ejection fraction; MPO, myeloperoxidase; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PIGF, placental growth factor; sFlt-1, soluble fms-like tyrosine kinase receptor 1; ST-2, interleukin family member; TnI, troponin Ic
IMAGING
Echocardiography
Two Dimensional Echocardiography
Because of its widespread availability and safety, two-dimensional echocardiography (2DE) is increasingly utilized in monitoring cancer patients. 2DE allows for characterization of systolic and diastolic function, pulmonary pressures, valvular function, right ventricular function, and the pericardium. Assessment of LVEF is based on assumptions of cardiac geometry, depends on image quality, cannot detect small regional alterations in myocardial function, and may vary based on loading conditions.74 The American Society of Echocardiography (ASE) suggests the addition of contrast to 2DE for better definition of endocardial borders in patients with breast implants or mastectomy.75 One of the drawbacks of 2DE is in its inability to detect small (<10%) changes in LVEF, a limitation in cancer patients in whom subtle differences in cardiac function may have important implications on treatment dose adjustment or cessation.13,76
Three-dimensional echocardiography
Three-dimensional echocardiography (3DE) increases the accuracy of detecting more subtle changes in LVEF, with a higher reporoducibilty.77 Thavendiranathan et al. demonstrated that non-contrast 3DE had the highest inter- and intraobsever reproducibility for LVEF and LV volume detection in sequential 1 year follow up of patients receiving cancer therapy. 78 Unlike with 2DE, the study of use of contrast in 3DE has not been firmly established.78, 79
Diastolic Function
Diastolic dysfunction often precedes systolic dysfunction in patients receiving chemotherapy.80 Though findings have been inconsistent, changes in diastolic parameters such as isovolumetric relaxation and deceleration time have been shown in patients as early as 3 months following doxorubicin and were predictive of systolic dysfunction at 6 months, with a sensitivity similar to strain imaging.81 Early reductions in E and E/A ratio, or increase in E/e’, predict future decrement in systolic function years after chemotherapy.82 Still, the use of diastolic parameters to predict subsequent cancer related cardiotoxicity remains unclear, given the variability based on loading conditions in cancer patients.79
Strain and Speckle-Tracking
More recent techniques, including strain and speckle tracking may allow for earlier detection of more subtle changes in myocardial function. Strain imaging assesses myocardial function based on measurements of myocardial velocities in adjacent areas as they relate to distance between those areas during the cardiac cycle. Speckle tracking has largely replaced tissue Doppler imaging for analysis of myocardial deformation and holds promise in early prediction of chemotherapy cardiotoxicity. Global longitudinal strain (GLS) reduction precedes LVD in patients who later develop HF.59 Strain abnormalities can be seen early despite preserved LVEF. Persistent abnormalities were found in patients receiving high dose anthracyclines.83 In patients receiving trastuzumab alone or with anthracyclines, a change in GLS of >11% was the strongest predictor of cardiotoxicity.84 This technique is limited by availability, image quality, variability of quantification between vendors, and lack of universal definitions. In addition to measuring linear deformation, speckle tracking can assess torsion, a newer parameter requiring further study in patients receiving anthracyclines.85
Right Ventricular Function
Subclinical changes in right ventricular systolic and diastolic parameters have been described early after anthracycline therapy and correlated with elevations in NT-pro BNP levels.86 RV involvement after chemotherapy has been noted in earlier studies involving RV myocardial biopsies, but frequency is not known.87 In patients receiving isolated left ventricular mechanical support in the INTERMACS registry, markers of RV dysfunction were more common and more severe in chemotherapy-induced cardiotoxicity compared with ischemic or non-ischemic cardiomyopathy. These included higher levels of transaminases, lower systolic pulmonary pressures, moderate tricuspid regurgitation, and higher ratios of central venous to pulmonary capillary wedge pressures. Echocardiographic assessment of the RV should involve careful attention to chamber size, TAPSE, estimation of PASP, and RV diastolic parameters.88 Areas of future interest include utilization of speckle tracking for more in depth understanding of chemotherapy related RV involvement.
Cardiac Magnetic Resonance Imaging
Cardiac magnetic resonance imaging (CMR) is the gold standard for detection of ventricular volumes and function. CMR has a greater intra- and inter- observer reproducibility than echocardiography and may identify a higher prevalence of cardiomyopathy compared with echocardiography in cancer patients.89 CMR affords the opportunity for noninvasive tissue characterization including myocardial edema, inflammation, and fibrosis thus playing an important role in identification of early and late cardiotoxicity in cancer patients.90 Early increases in pre and post-contrast signal intensity may predict reductions in LVEF at 28 days and 6 months. Subepicardial delayed enhancement with gadolinium has been noted in the lateral wall of trastuzumab-treated patients who developed cardiomyopathy,91 however studies on delayed enhancement have demonstrated mixed results.90 CMR may also have prognostic value in detection of late cardiotoxicity. LV mass index has been shown to be an independent predictor of major adverse cardiac events in cancer patients with cardiomyopathy following treatment with anthracyclines.92 Larger patient cohorts require study in order to further define the role of CMR in the prediction of cardiotoxicity. As with use of CMR in the general cardiac population, higher cost, lack of universal availability and patient-related factors such as pacemakers and claustrophobia, limit its widespread use, though it plays an important role particularly in patients with technical limitations to echocardiography.
Radionuclide Imaging
Multiple-gated acquisition (MUGA) used to be the mainstay for cardiac functiontional assessment in cancer patients due to high reproducibility and availability,93 however these advantages are now limited due to changes in equipment and technique.79 Other limitations include the inability to obtain other structural and functional information, which can be obtained by echocardiogram. MUGA relies on EF being the most appropriate parameter to measure, at a higher cost compared to echocardiography. The largest disadvantage of MUGA is radiation exposure, which must be weighed against necessity on an individual basis when other options are available.
Positron Emission Tomography/Magnetic Resonance (PET/MR)
Positron emission tomography/magnetic resonance (PET/MR) is an emerging modality, though currently largely limited to research algorithms. In the assessment of cardiomyopathy, PET allows for the determination of myocardial perfusion and glucose metabolism. Furthermore, PET allows for the evaluation of myocardial viability.94,95 The combined use of PET/MR allows not only for the acquisition of complimentary data on cardiac structure and function, 96 but also limits exposure to radiation. 97 This is an important advantage when imaging cancer patients, who may have already been exposed to large amounts of radiation.
CONCLUSION
Cancer therapy related cardiotoxicity has become a topic of growing concern. Early toxicity can limit a patient’s ability to complete effective cancer therapy. Late onset toxicity impacts cardiac mortality among cancer survivors. This complex population of patients presents unique challenges to clinical care. Overlaps in clinical symptomatology can make the delineation between symptoms of cardiac dysfunction and expected side effects of chemotherapy difficult. Additional barriers include a lack of a universal definition of cardiotoxicity as well as the absence of established guidelines for monitoring and surveillance. Certain biomarkers and novel imaging techniques have been investigated, but further study is necessary to clarify and optimize their role in routine clinical practice. Enhanced recognition and awareness of this unique patient population, and more universally accepted definitions of cancer related cardiac toxicities, will allow advancement in the field of cardio-oncology.
Acknowledgments
BK is supported by NIH K23 HL095661 and R01 HL118018 and has an investigator-initiated research grant from Pfizer, Inc. AN is a consultant for Vertex Pharmaceuticals. MG reports consulting relationships with Abbott Laboratories, Astellas, AstraZeneca, Bayer Schering Pharma AG, Cardiorentis Ltd, CorThera, Cytokinetics, CytoPherx Inc, DebioPharm SA, Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Intersection Medical Inc, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Ono Parmaceuticals USA, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, sanofi-aventis, Sigma Tau, Solvay Pharmaceuticals, Sticares InterACT, Takeda Pharmaceuticals North America Inc, and Trevena Therapeutics. JB reports receiving research support from the National Institutes of Health, and European Union, and serve as a consultant to Amgen, Bayer, Cardiocell, Celladon, Novartis, Trevena, Relypsa, Z Pharma, and Zensun.
Footnotes
Disclosures: MWB is a consultant for Bristol Myers Squibb. CEH, LB, HS, and DJL, DC, ARL report no conflict of interest.
REFERENCES
- 1.Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, Hagerty KL, Somerfield MR, Vaughn DJ. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 2.Silber JH, Cnaan A, Clark BJ, Paridon SM, Chin AJ, Rychik J, Hogarty AN, Cohen MI, Barber G, Rutkowski M, Kimball TR, Delaat C, Steinherz LJ, Zhao H. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol. 2004;22:820–828. doi: 10.1200/JCO.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast cancer research : BCR. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowinsky EKMW, Guarnieri T, Fisherman JS, Christian MC, Donehower RC. Cardiac disturbances during the administration of taxol. J Clin Oncol. 1991;9:1704–1712. doi: 10.1200/JCO.1991.9.9.1704. [DOI] [PubMed] [Google Scholar]
- 5.Sorrentino MFKJ, Eoderaro AE, Truesdell AG. 5-Fluorouracil induced cardiotoxicity: review of the literature. Cardiology Journal. 2012;19:453–458. doi: 10.5603/cj.2012.0084. [DOI] [PubMed] [Google Scholar]
- 6.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 7.NCI Dictionary of Cancer Terms. Definition of cardiotoxicity. < http://www.cancer.gov/dictionary?CdrID=44004%3E.
- 8.NCI. Common Terminology Criteria for Adverse Events (CTCAE) 2009. [Google Scholar]
- 9.FDA Drug Label for DOXIL- doxorubicin hydrochloride injection, suspension, liposomal. < http://dailymed.nlm.nih.gov.ezproxy.hsclib.sunysb.edu/dailymed/drugInfo.cfm?setid=21d9c619-7e94-49e2-ac41-31e9ea96554a%3E.
- 10.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 11.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 12.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plana JCGM, Barac A, Ewer M, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23:2900–2902. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 15.Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, Lamantia G, Colombo N, Cortinovis S, Dessanai MA, Nole F, Veglia F, Cipolla CM. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–3916. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 16.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 17.Blum RH, Carter SK, Adriamycin A new anticancer drug with significant clinical activity. Ann Intern Med. 1974;80:249–259. doi: 10.7326/0003-4819-80-2-249. [DOI] [PubMed] [Google Scholar]
- 18.Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984;226:466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- 19.Vejpongsa P, Yeh ET. Topoisomerase 2beta: a promising molecular target for primary prevention of anthracycline-induced cardiotoxicity. Clinical pharmacology and therapeutics. 2014;95:45–52. doi: 10.1038/clpt.2013.201. [DOI] [PubMed] [Google Scholar]
- 20.Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, Liu LF. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007;67:8839–8846. doi: 10.1158/0008-5472.CAN-07-1649. [DOI] [PubMed] [Google Scholar]
- 21.Monsuez JJ, Charniot JC, Vignat N, Artigou JY. Cardiac side-effects of cancer chemotherapy. International journal of cardiology. 2010;144:3–15. doi: 10.1016/j.ijcard.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Pein F, Sakiroglu O, Dahan M, Lebidois J, Merlet P, Shamsaldin A, Villain E, de Vathaire F, Sidi D, Hartmann O. Cardiac abnormalities 15 years and more after adriamycin therapy in 229 childhood survivors of a solid tumour at the Institut Gustave Roussy. Br J Cancer. 2004;91:37–44. doi: 10.1038/sj.bjc.6601904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volkova M, Russell R., 3rd Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Current cardiology reviews. 2011;7:214–220. doi: 10.2174/157340311799960645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Von Hoff DD, Layard MW, Basa P, Davis HL, Jr, Von Hoff AL, Rozencweig M, Muggia FM. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 25.Gershwin ME, Goetzl EJ, Steinberg AD. Cyclophosphamide: use in practice. Ann Intern Med. 1974;80:531–540. doi: 10.7326/0003-4819-80-4-531. [DOI] [PubMed] [Google Scholar]
- 26.Braverman AC, Antin JH, Plappert MT, Cook EF, Lee RT. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol. 1991;9:1215–1223. doi: 10.1200/JCO.1991.9.7.1215. [DOI] [PubMed] [Google Scholar]
- 27.Quezado ZM, Wilson WH, Cunnion RE, Parker MM, Reda D, Bryant G, Ognibene FP. High-dose ifosfamide is associated with severe, reversible cardiac dysfunction. Ann Intern Med. 1993;118:31–36. doi: 10.7326/0003-4819-118-1-199301010-00006. [DOI] [PubMed] [Google Scholar]
- 28.Field JJ, Kanakkanthara A, Miller JH. Microtubule-targeting agents are clinically successful due to both mitotic and interphase impairment of microtubule function. Bioorganic & medicinal chemistry. 2014;22:5050–5059. doi: 10.1016/j.bmc.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 29.Giordano SH, Booser DJ, Murray JL, Ibrahim NK, Rahman ZU, Valero V, Theriault RL, Rosales MF, Rivera E, Frye D, Ewer M, Ordonez NG, Buzdar AU, Hortobagyi GN. A detailed evaluation of cardiac toxicity: a phase II study of doxorubicin and one- or three-hour-infusion paclitaxel in patients with metastatic breast cancer. Clin Cancer Res. 2002;8:3360–3368. [PubMed] [Google Scholar]
- 30.Gianni L, Baselga J, Eiermann W, Guillem Porta V, Semiglazov V, Lluch A, Zambetti M, Sabadell D, Raab G, Llombart Cussac A, Bozhok A, Martinez-Agullo A, Greco M, Byakhov M, Lopez Lopez JJ, Mansutti M, Valagussa P, Bonadonna G. Feasibility and tolerability of sequential doxorubicin/paclitaxel followed by cyclophosphamide, methotrexate, and fluorouracil and its effects on tumor response as preoperative therapy. Clin Cancer Res. 2005;11:8715–8721. doi: 10.1158/1078-0432.CCR-05-0539. [DOI] [PubMed] [Google Scholar]
- 31.Pritchard KI, Shepherd LE, O'Malley FP, Andrulis IL, Tu D, Bramwell VH, Levine MN. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354:2103–2111. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 32.Goldenberg MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clinical therapeutics. 1999;21:309–318. doi: 10.1016/S0149-2918(00)88288-0. [DOI] [PubMed] [Google Scholar]
- 33.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang C-S, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Sütő T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. New England Journal of Medicine. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 34.Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KA, Davis RL, Habel LA, Yood MU, McCarty C, Magid DJ, Wagner EH. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Network NCC. Breast Cancer Version 3.2015. 2015. [Google Scholar]
- 36.Valachis A, Nearchou A, Lind P, Mauri D. Lapatinib, trastuzumab or the combination added to preoperative chemotherapy for breast cancer: a meta-analysis of randomized evidence. Breast Cancer Res Treat. 2012;135:655–662. doi: 10.1007/s10549-012-2189-z. [DOI] [PubMed] [Google Scholar]
- 37.Krop IE, Suter TM, Dang CT, Dirix L, Romieu G, Zamagni C, Citron ML, Campone M, Xu N, Smitt M, Gianni L. Feasibility and cardiac safety of trastuzumab emtansine after anthracycline-based chemotherapy as (neo)adjuvant therapy for human epidermal growth factor receptor 2-positive early-stage breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1136–1142. doi: 10.1200/JCO.2014.58.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. American journal of physiology Cell physiology. 2001;280:C1358–C1366. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- 39.Yang B, Papoian T. Tyrosine kinase inhibitor (TKI)-induced cardiotoxicity: approaches to narrow the gaps between preclinical safety evaluation and clinical outcome. J Appl Toxicol. 2012;32:945–951. doi: 10.1002/jat.2813. [DOI] [PubMed] [Google Scholar]
- 40.Groarke JD, Choueiri TK, Slosky D, Cheng S, Moslehi J. Recognizing and managing left ventricular dysfunction associated with therapeutic inhibition of the vascular endothelial growth factor signaling pathway. Curr Treat Options Cardiovasc Med. 2014;16:335. doi: 10.1007/s11936-014-0335-0. [DOI] [PubMed] [Google Scholar]
- 41.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 42.Richards CJ, Je Y, Schutz FA, Heng DY, Dallabrida SM, Moslehi JJ, Choueiri TK. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29:3450–3456. doi: 10.1200/JCO.2010.34.4309. [DOI] [PubMed] [Google Scholar]
- 43.Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart failure. 2013;1:72–78. doi: 10.1016/j.jchf.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Choueiri TK, Mayer EL, Je Y, Rosenberg JE, Nguyen PL, Azzi GR, Bellmunt J, Burstein HJ, Schutz FA. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:632–638. doi: 10.1200/JCO.2010.31.9129. [DOI] [PubMed] [Google Scholar]
- 45.Filopei J, Frishman W. Radiation-induced heart disease. Cardiology in review. 2012;20:184–188. doi: 10.1097/CRD.0b013e3182431c23. [DOI] [PubMed] [Google Scholar]
- 46.van Nimwegen FA, Schaapveld M, Janus CP, Krol AD, Petersen EJ, Raemaekers JM, Kok WE, Aleman BM, van Leeuwen FE. Cardiovascular disease after hodgkin lymphoma treatment: 40-year disease risk. JAMA internal medicine. 2015;175:1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 47.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 48.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102:14–25. doi: 10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blanco JG, Sun CL, Landier W, Chen L, Esparza-Duran D, Leisenring W, Mays A, Friedman DL, Ginsberg JP, Hudson MM, Neglia JP, Oeffinger KC, Ritchey AK, Villaluna D, Relling MV, Bhatia S. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children's Oncology Group. J Clin Oncol. 2012;30:1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pavo N, Raderer M, Hulsmann M, Neuhold S, Adlbrecht C, Strunk G, Goliasch G, Gisslinger H, Steger GG, Hejna M, Kostler W, Zochbauer-Muller S, Marosi C, Kornek G, Auerbach L, Schneider S, Parschalk B, Scheithauer W, Pirker R, Drach J, Zielinski C, Pacher R. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101:1874–1880. doi: 10.1136/heartjnl-2015-307848. [DOI] [PubMed] [Google Scholar]
- 51.Curigliano G, Mayer EL, Burstein HJ, Winer EP, Goldhirsch A. Cardiac toxicity from systemic cancer therapy: a comprehensive review. Prog Cardiovasc Dis. 2010;53:94–104. doi: 10.1016/j.pcad.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Valachis A, Nilsson C. Cardiac risk in the treatment of breast cancer: assessment and management. Breast cancer (Dove Medical Press) 2015;7:21–35. doi: 10.2147/BCTT.S47227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc. 2014;89:1287–1306. doi: 10.1016/j.mayocp.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 55.Dranitsaris G, Rayson D, Vincent M, Chang J, Gelmon K, Sandor D, Reardon G. The development of a predictive model to estimate cardiotoxic risk for patients with metastatic breast cancer receiving anthracyclines. Breast Cancer Res Treat. 2008;107:443–450. doi: 10.1007/s10549-007-9803-5. [DOI] [PubMed] [Google Scholar]
- 56.Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. Journal of the American Heart Association. 2014;3:e000472. doi: 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G, Fiorentini C, Cipolla CM. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 58.Cardinale D, Bacchiani G, Beggiato M, Colombo A, Cipolla CM. Strategies to prevent and treat cardiovascular risk in cancer patients. Semin Oncol. 2013;40:186–198. doi: 10.1053/j.seminoncol.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 59.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ederhy S, Massard C, Dufaitre G, Balheda R, Meuleman C, Rocca CG, Izzedine H, Cohen A, Soria JC. Frequency and management of troponin I elevation in patients treated with molecular targeted therapies in phase I trials. Invest New Drugs. 2012;30:611–615. doi: 10.1007/s10637-010-9546-8. [DOI] [PubMed] [Google Scholar]
- 61.Romano S, Fratini S, Ricevuto E, Procaccini V, Stifano G, Mancini M, Di Mauro M, Ficorella C, Penco M. Serial measurements of NT-proBNP are predictive of not-high-dose anthracycline cardiotoxicity in breast cancer patients. Br J Cancer. 2011;105:1663–1668. doi: 10.1038/bjc.2011.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lipshultz SE, Miller TL, Scully RE, Lipsitz SR, Rifai N, Silverman LB, Colan SD, Neuberg DS, Dahlberg SE, Henkel JM, Asselin BL, Athale UH, Clavell LA, Laverdiere C, Michon B, Schorin MA, Sallan SE. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30:1042–1049. doi: 10.1200/JCO.2010.30.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Onitilo AA, Engel JM, Stankowski RV, Liang H, Berg RL, Doi SA. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: a pilot study. Breast Cancer Res Treat. 2012;134:291–298. doi: 10.1007/s10549-012-2039-z. [DOI] [PubMed] [Google Scholar]
- 64.Onitilo AAEJ, Stankowski RV, Liang H, Berg RL, Doi SAR. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: a pilot study. Breast Cancer Res Treat. 2012;134:291–298. doi: 10.1007/s10549-012-2039-z. [DOI] [PubMed] [Google Scholar]
- 65.Ky B, Putt M, Sawaya H, French B, Januzzi JL, Jr, Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Putt M, Hahn VS, Januzzi JL, Sawaya H, Sebag IA, Plana JC, Picard MH, Carver JR, Halpern EF, Kuter I, Passeri J, Cohen V, Banchs J, Martin RP, Gerszten RE, Scherrer-Crosbie M, Ky B. Longitudinal Changes in Multiple Biomarkers Are Associated with Cardiotoxicity in Breast Cancer Patients Treated with Doxorubicin, Taxanes, and Trastuzumab. Clin Chem. 2015;61:1164–1172. doi: 10.1373/clinchem.2015.241232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Skovgaard D, Hasbak P, Kjaer A. BNP predicts chemotherapy-related cardiotoxicity and death: comparison with gated equilibrium radionuclide ventriculography. PLoS One. 2014;9:e96736. doi: 10.1371/journal.pone.0096736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris PG, Chen C, Steingart R, Fleisher M, Lin N, Moy B, Come S, Sugarman S, Abbruzzi A, Lehman R, Patil S, Dickler M, McArthur HL, Winer E, Norton L, Hudis CA, Dang CT. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res. 2011;17:3490–3499. doi: 10.1158/1078-0432.CCR-10-1359. [DOI] [PubMed] [Google Scholar]
- 69.Mavinkurve-Groothuis AM, Groot-Loonen J, Bellersen L, Pourier MS, Feuth T, Bokkerink JP, Hoogerbrugge PM, Kapusta L. Abnormal NT-pro-BNP levels in asymptomatic long-term survivors of childhood cancer treated with anthracyclines. Pediatr Blood Cancer. 2009;52:631–636. doi: 10.1002/pbc.21913. [DOI] [PubMed] [Google Scholar]
- 70.Dodos F, Halbsguth T, Erdmann E, Hoppe UC. Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin Res Cardiol. 2008;97:318–326. doi: 10.1007/s00392-007-0633-6. [DOI] [PubMed] [Google Scholar]
- 71.Jingu K, Nemoto K, Kaneta T, Oikawa M, Ogawa Y, Ariga H, Takeda K, Sakayauchi T, Fujimoto K, Narazaki K, Takai Y, Nakata E, Fukuda H, Takahashi S, Yamada S. Temporal change in brain natriuretic Peptide after radiotherapy for thoracic esophageal cancer. Int J Radiat Oncol Biol Phys. 2007;69:1417–1423. doi: 10.1016/j.ijrobp.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 72.Sandri MT, Cardinale D, Zorzino L, Passerini R, Lentati P, Martinoni A, Martinelli G, Cipolla CM. Minor increases in plasma troponin I predict decreased left ventricular ejection fraction after high-dose chemotherapy. Clin Chem. 2003;49:248–252. doi: 10.1373/49.2.248. [DOI] [PubMed] [Google Scholar]
- 73.Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, Cinieri S, Martinelli G, Cipolla CM, Fiorentini C. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000;36:517–522. doi: 10.1016/s0735-1097(00)00748-8. [DOI] [PubMed] [Google Scholar]
- 74.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC, Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) Circulation. 2003;108:1146–1162. doi: 10.1161/01.CIR.0000073597.57414.A9. [DOI] [PubMed] [Google Scholar]
- 75.Mulvagh SL, Rakowski H, Vannan MA, Abdelmoneim SS, Becher H, Bierig SM, Burns PN, Castello R, Coon PD, Hagen ME, Jollis JG, Kimball TR, Kitzman DW, Kronzon I, Labovitz AJ, Lang RM, Mathew J, Moir WS, Nagueh SF, Pearlman AS, Perez JE, Porter TR, Rosenbloom J, Strachan GM, Thanigaraj S, Wei K, Woo A, Yu EH, Zoghbi WA. American Society of Echocardiography Consensus Statement on the Clinical Applications of Ultrasonic Contrast Agents in Echocardiography. J Am Soc Echocardiogr. 2008;21:1179–1201. doi: 10.1016/j.echo.2008.09.009. quiz 1281. [DOI] [PubMed] [Google Scholar]
- 76.Kongbundansuk S, Hundley WG. Noninvasive imaging of cardiovascular injury related to the treatment of cancer. JACC Cardiovascular imaging. 2014;7:824–838. doi: 10.1016/j.jcmg.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jenkins C, Moir S, Chan J, Rakhit D, Haluska B, Marwick TH. Left ventricular volume measurement with echocardiography: a comparison of left ventricular opacification, three-dimensional echocardiography, or both with magnetic resonance imaging. Eur Heart J. 2009;30:98–106. doi: 10.1093/eurheartj/ehn484. [DOI] [PubMed] [Google Scholar]
- 78.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 79.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European heart journal cardiovascular Imaging. 2014;15:1063–1093. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oreto L, Todaro MC, Umland MM, Kramer C, Qamar R, Carerj S, Khandheria BK, Paterick TE. Use of echocardiography to evaluate the cardiac effects of therapies used in cancer treatment: what do we know? J Am Soc Echocardiogr. 2012;25:1141–1152. doi: 10.1016/j.echo.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 81.Stoddard MF, Seeger J, Liddell NE, Hadley TJ, Sullivan DM, Kupersmith J. Prolongation of isovolumetric relaxation time as assessed by Doppler echocardiography predicts doxorubicin-induced systolic dysfunction in humans. J Am Coll Cardiol. 1992;20:62–69. doi: 10.1016/0735-1097(92)90138-d. [DOI] [PubMed] [Google Scholar]
- 82.Tassan-Mangina S, Codorean D, Metivier M, Costa B, Himberlin C, Jouannaud C, Blaise AM, Elaerts J, Nazeyrollas P. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: early and late alterations of left ventricular function during a prospective study. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2006;7:141–146. doi: 10.1016/j.euje.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 83.Stoodley PW, Richards DA, Boyd A, Hui R, Harnett PR, Meikle SR, Byth K, Stuart K, Clarke JL, Thomas L. Left ventricular systolic function in HER2/neu negative breast cancer patients treated with anthracycline chemotherapy: a comparative analysis of left ventricular ejection fraction and myocardial strain imaging over 12 months. Eur J Cancer. 2013;49:3396–3403. doi: 10.1016/j.ejca.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 84.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–498. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 85.Motoki H, Koyama J, Nakazawa H, Aizawa K, Kasai H, Izawa A, Tomita T, Miyashita Y, Kumazaki S, Takahashi M, Ikeda U. Torsion analysis in the early detection of anthracycline-mediated cardiomyopathy. European heart journal cardiovascular Imaging. 2012;13:95–103. doi: 10.1093/ejechocard/jer172. [DOI] [PubMed] [Google Scholar]
- 86.Tanindi A, Demirci U, Tacoy G, Buyukberber S, Alsancak Y, Coskun U, Yalcin R, Benekli M. Assessment of right ventricular functions during cancer chemotherapy. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2011;12:834–840. doi: 10.1093/ejechocard/jer142. [DOI] [PubMed] [Google Scholar]
- 87.Mason JW, Bristow MR, Billingham ME, Daniels JR. Invasive and noninvasive methods of assessing adriamycin cardiotoxic effects in man: superiority of histopathologic assessment using endomyocardial biopsy. Cancer Treat Rep. 1978;62:857–864. [PubMed] [Google Scholar]
- 88.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhaes A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 89.Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, Daniel Donovan F, Metzger ML, Arevalo A, Durand JB, Joshi V, Hudson MM, Robison LL, Flamm SD. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2876–2884. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thavendiranathan P, Wintersperger BJ, Flamm SD, Marwick TH. Cardiac MRI in the assessment of cardiac injury and toxicity from cancer chemotherapy: a systematic review. Circ Cardiovasc Imaging. 2013;6:1080–1091. doi: 10.1161/CIRCIMAGING.113.000899. [DOI] [PubMed] [Google Scholar]
- 91.Fallah-Rad N, Lytwyn M, Fang T, Kirkpatrick I, Jassal DS. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2008;10:5. doi: 10.1186/1532-429X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Neilan TG, Coelho-Filho OR, Pena-Herrera D, Shah RV, Jerosch-Herold M, Francis SA, Moslehi J, Kwong RY. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol. 2012;110:1679–1686. doi: 10.1016/j.amjcard.2012.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiji RS, Kramer CM, Salerno M. Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs. J Nucl Cardiol. 2012;19:377–388. doi: 10.1007/s12350-012-9512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rischpler C, Nekolla SG, Dregely I, Schwaiger M. Hybrid PET/MR imaging of the heart: potential, initial experiences, and future prospects. J Nucl Med. 2013;54:402–415. doi: 10.2967/jnumed.112.105353. [DOI] [PubMed] [Google Scholar]
- 95.Nappi CEFG. State of the art in cardiac hybrid technology: PET/MR. Curr Cardiovasc Imaging Rep. 2013;6:338–345. doi: 10.1007/s12410-013-9213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hahn VS, Lenihan DJ, Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. Journal of the American Heart Association. 2014;3:e000665. doi: 10.1161/JAHA.113.000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Catana C, Guimaraes AR, Rosen BR. PET and MR imaging: the odd couple or a match made in heaven? J Nucl Med. 2013;54:815–824. doi: 10.2967/jnumed.112.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]