Summary
Introduction
Cystic fibrosis (CF) is characterized by low circulating levels of insulin-like growth factor-1 (IGF-1), a hormone produced by the liver that governs anabolism and influences immune cell function. Because treatment of CF pulmonary exacerbation (CFPE) often improves body weight and lung function, we questioned whether serum IGF-1 trends were emblematic of these responses. Initially, we compared serum levels between healthy adults with CF and controls of similar age. We then measured serum IGF-1 throughout the CFPE cycle. We also investigated correlations among IGF-1 and other serum biomarkers during CFPE.
Methods
Anthopometric, spirometric, and demographic data were collected. Serum IGF-1 concentrations were measured by ELISA.
Results
CF subjects in their usual state of health had lower serum IGF-1 levels than controls. Serum IGF-1 concentrations fell significantly from baseline at the beginning of CFPE. Treatment with intravenous antibiotics was associated with significant improvement in serum IGF-1 levels, body mass index (BMI), and percent-predicted forced expiratory volume in 1 sec (FEV1%). At early and late CFPE, serum IGF-1 was directly correlated with FEV1%, serum iron, hemoglobin concentration, and transferrin saturation (TSAT) and indirectly correlated with alpha-1-antitrypsin.
Conclusions
This study not only supports the paradigm that CF is characterized by IGF-1 deficiency but also that trends in lung function, nutritional status, and serum IGF-1 are related. Improvements in all three parameters after antibiotics for CFPE likely highlight the connection between lung function and nutritional status in CF Close correlations among IGF-1 and iron-related hematologic parameters suggest that IGF-1 may participate in CF iron homeostasis, another process that is known to be influenced by CFPE.
Keywords: cystic fibrosis, IGF-1, exacerbation, iron, FEV1
Introduction
Insulin-like growth factor-1 (IGF-1) is an anabolic hormone that reduces protein catabolism, restricts gluconeogenesis and ketogenesis, and stimulates tissue glucose utilization.1 Several clinical studies, mostly in children, have shown that serum IGF-1 concentrations are abnormally low in cystic fibrosis (CF) 2–4 and that low levels are associated with impaired growth.5 IGF-1 deficiency appears to be an intrinsic attribute of this condition because circulating concentrations are reduced at birth in a porcine model system of CF and in human newborns.2 Nonetheless, subcutaneous injection of IGF-1 in prepubertal children with CF over a 6-month period does not increase linear growth.6
The impact of IGF-1 deficiency on host immune responses in CF is largely unexplained. In a murine model of Pseudomonas sepsis, IGF-1 treatment improved hepatic bacterial clearance and survival,7 demonstrating that this molecule plays a prominent role in controlling infection. In pigs with CF, bloodstream levels of IGF-1 and its major binding protein, IGFBP-3, increase significantly after intravenous antibiotics and enteral nutritional support,2 raising the possibility that serum IGF-1 improves after antibiotics in humans. The manner in which serum IGF-1 is related to nutritional status and lung function has not been reported during transitions from baseline health to early CF pulmonary exacerbation (CFPE) and from early CFPE to late CFPE (i.e., after antibiotics).
Treatment-related changes in several cytokines and proteins have been investigated during CFPE, including interleukin-6 (IL-6),8,9 interleukin-8 (IL-8),10 vascular endothelial growth factor (VEGF),10 tumor necrosis factor-alpha (TNF-α),11 and alpha-1-antitrypsin (A1AT).12 However, relationships between serum IGF-1 and each of these proposed biomarkers of CFPE have not been presented. Therefore, we determined correlation coefficients between serum IGF-1 levels and serum concentrations of these substances at early and late CFPE.
Materials and Methods
Study Enrollment and Definitions
Data for this study were obtained under the auspices of two protocols (#22506 and #23314) approved by the Committee for the Protection of Human Subjects at Dartmouth College, and all participants gave written informed consent. Control subjects were non-smokers of similar age to CF subjects with no evidence of a systemic disease. They did not take prescription medications, except for oral contraceptive pills in the case of women. Control subjects were required to be in their baseline state of health (i.e., free of symptoms suggesting acute infection) at the time of phlebotomy. Control subjects were informed of the study by advertisements posted in our clinic.
Eight CF subjects were evaluated during a period of baseline health, defined by an attending physician with long-term understanding of the subject's medical history as a state in which treatment escalation was unnecessary. The time between their last CFPE and baseline health encounter was not determined. These individuals and an additional four CF subjects performed spirometry and provided blood samples within 24 hr of hospital admission for CFPE (early CFPE) and within 24 hr of hospital discharge following therapy (late CFPE). Demographic and clinical data for the 12 subjects at early CFPE are provided in Table 1. All of these subjects participated in a study of iron homeostasis during CFPE,8 and serum IGF-1 concentrations were measured in samples collected during that investigation. The median interval between early and late CFPE was 12 days (range: 5–19 days). For all CF subjects, body mass index (BMI) was determined on the same day of spirometry and phlebotomy. CFPE was defined by an attending pulmonologist as new or acutely worsened sinopulmonary and constitutional signs and symptoms that included the following: increased cough frequency, increased daily sputum expectoration, wheezing, dyspnea, fevers, chills, sweating, anorexia, and weight loss, consistent with prior investigations at our institution.8,13 Exacerbation scores were not utilized.
Table 1. Characteristics of CF Subjects Evaluated During CFPE.
| Subject | Age (years) | Gender | Early BMI (kg/m2) | Early FEV1 (%) | CFTR genotype | Early IGF-1 (ng/ml) | Late IGF-1 (ng/ml) |
|---|---|---|---|---|---|---|---|
| 1 | 18 | M | 15.6 | 19 | dF508/dF508 | 1.0 | 196.4 |
| 2 | 23 | M | 18.5 | 57 | dF508/dF508 | 204.9 | 402.6 |
| 3 | 24 | M | 18.2 | 23 | dF508/EX6b_10dup | 114.2 | 106.2 |
| 4 | 22 | M | 18.1 | 46 | dF508/dF508 | 143.7 | 418.2 |
| 5 | 53 | M | 21.4 | 26 | dF508/dF508 | 182.8 | 320.3 |
| 6 | 40 | M | 18.4 | 21 | dF508/A455E | 1.0 | 1.0 |
| 7 | 29 | M | 17.2 | 22 | dF508/G542X | 239.3 | 208.8 |
| 8 | 41 | M | 27.1 | 51 | dF508/dF508 | 206.5 | 289.7 |
| 9 | 35 | F | 20.7 | 32 | dF508/dF508 | 106.9 | 284.9 |
| 10 | 41 | M | 23.3 | 49 | dF508/621 + 1G → T | 297.4 | 424.6 |
| 11 | 19 | F | 16.6 | 20 | dF508/c.3254A → C | 1.0 | 89.5 |
| 12 | 39 | F | 19.1 | 39 | dF508/711 + 1G → T | 64.9 | 197.4 |
Laboratory Testing
ELISA kits for human IGF-1 were obtained from R&D Systems (Minneapolis, MN) and were used according to the manufacturer's instructions. Serum hepcidin-25 was quantified by a competitive ELISA assay at Intrinsic LifeSciences (La Jolla, CA).14 Serum A1AT was measured by immunoturbidimetric method (Foundation for Blood Research, Scarborough, ME). Serum iron, hemoglobin, and transferrin saturation (TSAT) were quantified by autoanalyzer (COBAS c311, Roche Diagnostics USA, Indianapolis, IN). Serum IL-6, IL-8, VEGF, and TNF-α were assayed with multiplex ELISA kits (Bio-Rad, Inc., Hercules, CA) according to manufacturer instructions.
Statistical Analyses
Data are expressed as mean ± standard deviation unless otherwise noted. We have used unpaired Student's t-tests to compare IGF-1 levels in control and CF subjects and paired Student's t-tests to compare levels among CF subjects during different contexts. Correlations are presented as Spearman rank correlation coefficients (ρ). Chi-square analysis was used to explore relationships between short and long treatment duration and serum IGF-1 response. GraphPad Prism 5.04 for Windows (GraphPad Software, San Diego CA) was used for all calculations. A two-tailed P-value <0.05 was considered statistically significant.
Results
Characteristics of CF Subjects and Controls
Controls and CF adults were of comparable age. The median BMI of the 8 controls (24.9 kg/m) was 5.9 kg/m2 higher than that of the 8 CF subjects evaluated during baseline health (P = 0.003) and 5.6 kg/m2 higher than that of the 12 CF subjects who were evaluated at early CFPE (P = 0.002). In general, CF subjects enrolled in this study were mostly adult men with an advanced illness phenotype defined by severely impaired pulmonary function, chronic malnutrition, a preponderance of CF-related diabetes, hypoferremic anemia, and ineffective erythropoiesis that we have previously characterized.13 Table 1 describes demographic and clinical features of each subject at early CFPE.
Serum IGF-1 Levels Are Lower in CF Subjects Than Healthy Controls
Mean serum IGF-1 concentration in eight control subjects was 320.8 ng/ml higher than that of eight CF subjects in their baseline state of health (95% confidence interval for mean difference: 212.0–429.7 ng/ml) (Fig. 1). This observation is consistent with prior studies.15,16 In healthy controls, serum IGF-1 was not correlated with BMI (Spearman ρ = −0.10, P = 0.8). For the eight CF subjects in their baseline state of health, serum IGF-1 and BMI were also not significantly correlated (Spearman ρ = 0.38, P = 0.4).
Figure 1.
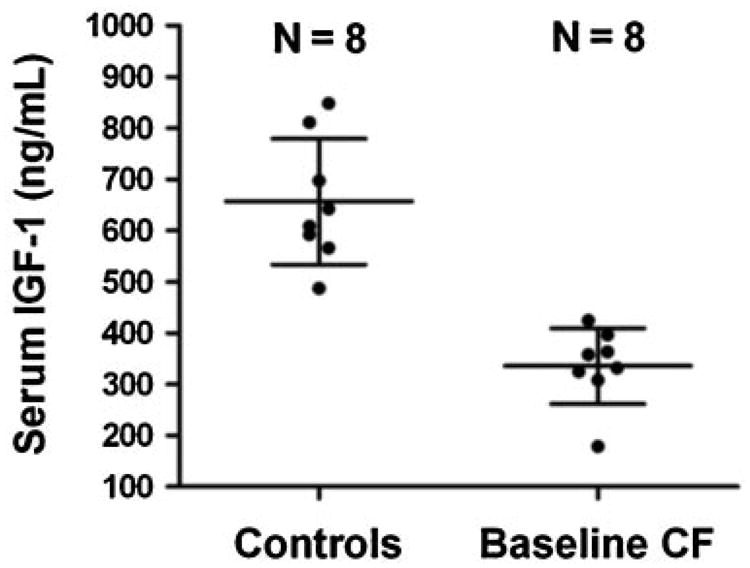
Comparison of serum IGF-1 concentrations between healthy controls and CF patients who subsequently experienced CFPE. The mean difference in (IGF-1) was 320.8 ± 50.7 ng/ml (95% CI of mean difference = 212.0–429.7). P-value for comparison by unpaired t-test <0.0001. Lines denote mean and SD.
Serum IGF-1 Levels Fall Significantly at the Onset of CFPE
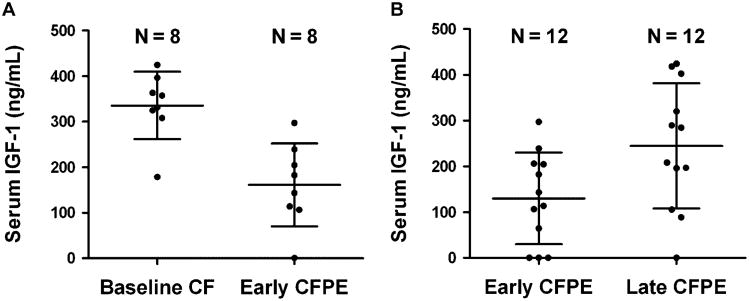
We first sought to identify whether serum IGF-1 levels fall in CF subjects as they progress from a compensated state of health (i.e., baseline) to early CFPE. Measurements were available at both time points from the eight subjects who were compared to healthy controls. Figure 2A shows that mean serum IGF-1 concentration was 174.4 ng/ml lower at early CFPE than baseline (95% confidence interval for mean difference: 102.4–246.5 ng/ml, P < 0.001). This represented a 48% drop from baseline (P < 0.05).
Figure 2.
Comparison of serum IGF-1 concentrations throughout the CFPE cycle. In eight subjects (panel A), mean serum IGF-1 fell by 174.4 ng/ml (95% CI of mean difference = 102.4–246.5, P < 0.001). In 12 subjects (panel B), including those compared in panel A, mean serum IGF-1 increased by 114.7 ng/ml (95% CI of mean difference = 55.6–173.7, P = 0.001). Lines denote mean and SD.
Serum IGF-1 Levels, BMI, and FEV1% Significantly Respond to CFPE Therapy
We measured serum IGF-1 levels in 12 CF subjects (including those compared in Fig. 2A) at early and late CFPE (Fig. 2B). In this cohort, mean serum IGF-1 concentration improved by 114.7 ng/ml after treatment (95% confidence interval for mean difference: 55.6–173.7 ng/ml, P = 0.001). These subjects also experienced a 0.7 kg/m2 increase in mean BMI (95% confidence interval for mean difference: 0.3–1.1 kg/m2, P = 0.004) and a 6.8% improvement in mean FEV1% (95% confidence interval for mean difference: 3.3–10.2%, P = 0.001).8 Mean serum IGF-1 concentrations at early and late CFPE in the four CF subjects without baseline measurements were similar to those of the eight CF subjects with baseline measurements (P = 0.2 and 0.1 for each subgroup comparison at the two time points). Serum IGF-1 levels returned to baseline in the eight subjects with late CFPE measurements for comparison (mean difference between baseline and late CFPE: 40.5 ng/ml, 95% confidence interval for mean difference: −67.7–148.7 ng/ml, P = 0.4). Serum IGF-1 responses for individual subjects are given in Table 1.
The Spearman correlation coefficient between treatment duration (days) and post-treatment difference in serum IGF-1 was 0.24 (P = 0.45) in the 12 subjects. We could not dichotomize treatment duration into any short and long intervals and appreciate corresponding significant differences in the magnitude of the serum IGF-1 response using Chi-square test.
Serum IGF-1 Is Correlated With Biomarkers of Iron Homeostasis
Because we observed discrete trends in serum IGF-1 during the CFPE treatment cycle, and CFPE therapy modulates iron homeostasis,8 we questioned whether the two phenomena were associated. Therefore, we calculated Spearman correlation coefficients between serum IGF-1 values and parameters relevant to iron homeostasis, specifically, serum iron, hepcidin-25, TSAT, and hemoglobin concentration. Table 2 shows that these all of these metrics except hepcidin-25 are significantly correlated with serum IGF-1 during early and late CFPE, suggesting that these relationships more generally exist regardless of exacerbation status.
Table 2. Spearman Correlation Coefficients for Relationships Among Serum IGF-1, Iron-Related Hematologic Parameters, FEV1%, and BMI During CFPE.
| Early CFPE | P-value | Late CFPE | P-value | |
|---|---|---|---|---|
| Serum IGF-1 and serum iron | 0.82 | 0.001 | 0.84 | 0.006 |
| Serum IGF-1 and hepcidin-25 | −0.28 | 0.38 | −0.14 | 0.66 |
| Serum IGF-1 and TSAT | 0.76 | 0.004 | 0.91 | <0.001 |
| Serum IGF-1 and hemoglobin | 0.75 | 0.005 | 0.69 | 0.01 |
| Serum IGF-1 and FEV1% | 0.67 | 0.02 | 0.78 | 0.003 |
| Serum IGF-1 and BMI | 0.75 | 0.02 | −0.15 | 0.60 |
IGF-1 Is Related to Alpha-1-Antitrypsin in Serum During CFPE Therapy
Because serum IGF-1 varied in response to antibiotic therapy for CFPE (Fig. 2B), and blood levels of several inflammatory mediators, including IL-6,8,9 IL-8,10VEGF,10 TNF- α,11 and A1AT12 have been quantified during CFPE, we investigated correlations between each of these parameters and IGF-1. Table 3 shows Spearman correlation coefficients for these relationships. Serum IGF-1 was indirectly correlated with serum A1AT, a circulating serine protease inhibitor that is distinctly elevated in CF,17 at early and late CFPE. There was a significant indirect correlation between IGF-1 and IL-6 in serum at late CFPE.
Table 3. Spearman Correlation Coefficients for Relationships Among Serum IGF-1 and Inflammatory Mediators During CFPE.
| Early CFPE | P-value | Late CFPE | P-value | |
|---|---|---|---|---|
| Serum IGF-1 and serum IL-6 | −0.48 | 0.11 | −0.85 | <0.001 |
| Serum IGF-1 and serum IL-8 | −0.16 | 0.61 | 0.08 | 0.83 |
| Serum IGF-1 and serum VEGF | −0.35 | 0.26 | 0.13 | 0.70 |
| Serum IGF-1 and serum TNF-α | −0.04 | 0.90 | 0.15 | 0.65 |
| Serum IGF-1 and serum A1AT | −0.66 | 0.02 | −0.71 | 0.009 |
Serum IGF-1 Is Related to FEV1% During CFPE Therapy and to BMI at Early CFPE
Pulmonary function (FEV1%) was significantly associated with serum IGF-1 at early and late CFPE (Table 2). In the 12 subjects followed through the CFPE cycle, serum IGF-1 correlated with BMI at early CFPE (Spearman ρ = 0.75, P = 0.02) but not at late CFPE (Spearman ρ = −0.15, P = 0.6).
Discussion
This study revealed several observations about IGF-1 in the context of CFPE. First, we demonstrated that CF is characterized by abnormally low serum IGF-1 levels. The onset of physician-diagnosed CFPE that warranted antibiotic treatment was distinguished by reduced circulating IGF-1 concentrations which improved within subjects after treatment. Serum IGF-1 was related to metrics of iron homeostasis and A1AT but not to other inflammatory mediators. Serum IGF-1 was correlated with FEV1% throughout the CFPE cycle and with BMI at early exacerbation. We conclude that serum IGF-1 likely reflects a link between nutritional sufficiency and lung function in CF. It may also mediate other physiologic processes, including iron utilization and protease inhibition.
Our finding of lower serum IGF-1 levels in CF subjects compared to age-matched controls supports the contention that CF is a state of IGF-1 insufficiency. By analyzing dried blood spots, Rogan et al.2 discovered that mean IGF-1 level (∼95 ng/ml) in 23 human newborns with CF was significantly lower than that (∼110 ng/ml) of 41 healthy human newborns. In older CF patients and controls (mean age: 26.6 and 28.0 years, respectively), Street et al.16 found that mean serum IGF-1 concentration was lower in the former population (231.7 ± 16.1 ng/ml vs. 302.2 ± 26.7 ng/ml). Data for stable CF subjects in Figure 1 are quite similar to those presented by Street et al.,16 although our controls had higher IGF-1 measurements. In distinction to our data from adults and those cited from newborns and adults,2,16 Ozen et al.3 did not detect any difference in serum IGF-1 levels between 37 CF and 23 healthy pre-pubertal children.
Our study is the second to demonstrate that serum IGF-1 increases in response to treatment of CFPE. In 33 pediatric and adult CF patients, Lebl et al.4 calculated standard deviation scores (SDS) for serum IGF-1 based on local population standards. These authors4 showed that the SDS for serum IGF-1 normalized after antibiotics and hyperalimentation. Indeed, higher serum IGF-1 levels after treatment for CFPE may largely reflect greater nutritional reserve, which is supported by a mean BMI improvement of 0.7 kg/m2 after CFPE treatment. Data in Figure 2A extends the perspective of Lebl et al.4 by showing that CFPE is heralded by a decline in serum IGF-1 from stable health. In a cross-sectional study of 92 pediatric and adolescent CF patients, Lebl et al.4 reported a direct correlation (r = 0.23, P = 0.05) between serum IGF-1 SDS and FEV1%, but they did not evaluate this relationship at early and late CFPE (Table 2). Our study was not designed to ask whether reductions in serum IGF-1 are more sensitive than a decline in lung function for the onset of CFPE, although this question warrants further consideration.
Our investigation is the first to identify correlations among serum IGF-1 and iron-related hematologic parameters during CFPE (Table 2). Iron is critically important for erythropoiesis, the production of hemoglobin-containing red blood cells in the bone marrow.18 Using human fetal bone marrow, Hanley et al.19 found that IGF-1 has an anti-apoptotic effect on myeloid progenitor cells, implicating IGF-1 in the prevention of anemia. Indeed, serum levels of IGF-1 and iron independently predict hemoglobin concentration in human adults.20,21 Moreover, in iron-deficient adolescent females, serum levels of IGF-1 and iron are directly correlated,22 congruent with data in Table 2. We have now demonstrated that systemic antibiotics and routine supportive care for CFPE23 improve serum iron8 and IGF-1 in adult CF patients, although these interventions do not alter hemoglobin concentration.8 The latter observation may reflect sources of type II error in our work, namely a short interval between biochemical tests and/or a small study population. Regardless, our data suggest that IGF-1 influences iron homeostasis in CF, and further mechanistic investigation is warranted.
Having discovered that serum IL-6 and hepcidin-25, two mediators of hypoferremia,24 fall after CFPE treatment,8 we questioned whether acute phase reactants correlated with IGF-1. In 17 children with CF who were studied during stable health, Street et al.25 identified a modest but statistically significant correlation between TNF-α and IGF-1 in serum. We found no such relationship during CFPE but did note that serum A1AT and IGF-1 were indirectly related at the onset and resolution of CFPE (Table 3). Recently, antibiotics have been shown to reduce blood levels of neutrophil-derived CD16b:A1AT complexes in adults with CF,26 suggesting that A1AT and IGF-1 are reflections of the inflammatory milieu in this disease.
Our finding of an indirect correlation between serum IGF-1 and IL-6 after CFPE treatment (Table 3) is unprecedented and congruent with clinical and in vitro observations. In an unselected cohort of 629 adults aged 50–89 years, Andreassen et al.27 identified a modest correlation between these parameters (r = −0.19, P < 0.001) and between serum IGF-1 and high sensitivity C-reactive protein (r = − 0.17, P < 0.001), suggesting that inflammation suppresses IGF-1 production. Indeed, IL-6 inhibits the induction of IGF-1 mRNA by growth hormone (GH) in hepatocytes, implicating this cytokine as a mediator of hepatic GH resistance.28 Therefore, the inflammatory nadir at the end of CFPE therapy8 coincides with improved anabolism.
Improvement in FEV1% is an expected outcome of antibiotic treatment for CFPE.29,30 Herein, we show that serum IGF-1 is significantly correlated with this important physiological variable during acute illness and the earliest phase of convalescence (Table 2). In a case– control study of CF children, Taylor et al.31 detected a modest correlation (r = 0.17) between serum IGF-1 SDS and FEV1%. Our finding that serum IGF-1 remains closely associated with FEV1% throughout the exacerbation cycle generalizes this perspective. Our study was underpowered to identify correlations between serum IGF-1 and BMI at late CFPE, as denoted by an insignificant P-value. Heterogeneity in weight gain might also explain why serum IGF-1 and BMI are only correlated at CFPE onset.
In summary, we provide further evidence that higher serum IGF-1 levels are associated with better health in CF. It is not yet clear whether serum IGF-1 is a biomarker of nutritional status or whether it reflects lung function, although this warrants further investigation. Given that CFPE onset is heralded by reductions in serum IGF-1 and that CFPE recovery is signified by the opposite trend justifies evaluation of this molecule in larger CF populations as a biomarker for treatment adequacy and as a potential surrogate endpoint in interventional studies. Our data establish a compelling basis for probing the manner in which IGF-1 influences iron homeostasis and erythropoiesis in CF and other inflammatory conditions.
Acknowledgments
The authors would like to thank Ms. Kathy Smith, BA, MAT-C, of the Dartmouth Immune Monitoring and Flow Cytometry Shared Resource for performing the multiplex cytokine assays. We also thank Ms. Gordana Olbina, PhD of Intrinsic LifeSciences, LLC, for performing the hepcidin-25 competitive ELISA. Dr. Gifford is supported by National Institutes of Health grant 8P20GM103413-10 awarded to Bruce A. Stanton, PhD. This work was also supported by a pilot grant from the SYNERGY Institute for Clinical and Translational Science at Dartmouth College (Dr. Ashare).
Funding source: National Institutes of Health, Number: 8P20GM103413-10
Footnotes
Conflict of interest: None.
References
- 1.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 2.Rogan MP, Reznikov LR, Pezzulo AA, Gansemer ND, Samuel M, Prather RS, Zabner J, Fredericks DC, McCray PB, Jr, Welsh MJ, Stoltz DA. Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proc Natl Acad Sci USA. 2010;107:20571–20575. doi: 10.1073/pnas.1015281107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozen M, Cokugras H, Ozen N, Camcioglu Y, Akcakaya N. Relation between serum Insulin-like growth factor-I and insulinlike growth factor-binding protein-3 levels, clinical status and growth parameters in prepubertal cystic fibrosis patients. Pediatr Int. 2004;46:429–435. doi: 10.1111/j.1442-200x.2004.01925.x. [DOI] [PubMed] [Google Scholar]
- 4.Lebl J, Zahradnikova M, Bartosova J, Zemkova D, Pechova M, Vavrova V. Insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in cystic fibrosis: a positive effect of antibiotic therapy and hyperalimentation. Acta Paediatr. 2001;90:868–872. [PubMed] [Google Scholar]
- 5.Laursen EM, Juul A, Lanng S, Hoiby N, Koch C, Muller J, Skakkebaek NE. Diminished concentrations of insulin-like growth factor I in cystic fibrosis. Arch Dis Child. 1995;72:494–497. doi: 10.1136/adc.72.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucuvalas JC, Chernausek SD, Alfaro MP, Krug SK, Ritschel W, Wilmott RW. Effect of insulinlike growth factor-1 treatment in children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2001;33:576–581. doi: 10.1097/00005176-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Ashare A, Nymon AB, Doerschug KC, Morrison JM, Monick MM, Hunninghake GW. Insulin-like growth factor-1 improves survival in sepsis via enhanced hepatic bacterial clearance. Am J Respir Crit Care Med. 2008;178:149–157. doi: 10.1164/rccm.200709-1400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gifford AH, Moulton LA, Dorman DB, Olbina G, Westerman M, Parker HW, Stanton BA, O'Toole GA. Iron homeostasis during cystic fibrosis pulmonary exacerbation. Clin Transl Sci. 2012;5:368–373. doi: 10.1111/j.1752-8062.2012.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shead EF, Haworth CS, Barker H, Bilton D, Compston JE. Osteoclast function, bone turnover and inflammatory cytokines during infective exacerbations of cystic fibrosis. J Cyst Fibros. 2010;9:93–98. doi: 10.1016/j.jcf.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Gray RD, Imrie M, Boyd AC, Porteous D, Innes JA, Greening AP. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J Cyst Fibros. 2010;9:193–198. doi: 10.1016/j.jcf.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Nixon LS, Yung B, Bell SC, Elborn JS, Shale DJ. Circulating immunoreactive interleukin-6 in cystic fibrosis. Am J Respir Crit Care Med. 1998;157:1764–1769. doi: 10.1164/ajrccm.157.6.9704086. [DOI] [PubMed] [Google Scholar]
- 12.Wolter JM, Rodwell RL, Bowler SD, McCormack JG. Cytokines and inflammatory mediators do not indicate acute infection in cystic fibrosis. Clin Diagn Lab Immunol. 1999;6:260–265. doi: 10.1128/cdli.6.2.260-265.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gifford AH, Miller SD, Jackson BP, Hampton TH, O'Toole GA, Stanton BA, Parker HW. Iron and CF-related anemia: expanding clinical and biochemical relationships. Pediatr Pulmonol. 2011;46:160–165. doi: 10.1002/ppul.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 15.Boguszewski MC, Kamoi TO, Bento Radominski R, Boguszewski CL, Rosberg S, Filho NA, Sandrini Neto R, Albertsson-Wikland K. Insulin-like growth factor-1, leptin, body composition, and clinical status interactions in children with cystic fibrosis. Horm Res. 2007;67:250–256. doi: 10.1159/000098480. [DOI] [PubMed] [Google Scholar]
- 16.Street ME, Ziveri MA, Spaggiari C, Viani I, Volta C, Grzincich GL, Virdis R, Bernasconi S. Inflammation is a modulator of the insulin-like growth factor (IGF)/IGF-binding protein system inducing reduced bioactivity of IGFs in cystic fibrosis. Eur J Endocrinol. 2006;154:47–52. doi: 10.1530/eje.1.02064. [DOI] [PubMed] [Google Scholar]
- 17.Brown MA, Morgan WJ, Finley PR, Scuderi P. Circulating levels of tumor necrosis factor and interleukin-1 in cystic fibrosis. Pediatr Pulmonol. 1991;10:86–91. doi: 10.1002/ppul.1950100209. [DOI] [PubMed] [Google Scholar]
- 18.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116:4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 19.Hanley MB, Napolitano LA, McCune JM. Growth hormone-induced stimulation of multilineage human hematopoiesis. Stem Cells. 2005;23:1170–1179. doi: 10.1634/stemcells.2004-0322. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson-Ehle H, Bengtsson BA, Lindstedt G, Mellstrom D. Insulin-like growth factor-1 is a predictor of blood haemoglobin concentration in 70-yr-old subjects. Eur J Haematol. 2005;74:111–116. doi: 10.1111/j.1600-0609.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- 21.Succurro E, Arturi F, Caruso V, Rudi S, Sciacqua A, Andreozzi F, Hribal ML, Perticone F, Sesti G. Low insulin-like growth factor-1 levels are associated with anaemia in adult non-diabetic subjects. Thromb Haemost. 2011;105:365–370. doi: 10.1160/TH10-06-0379. [DOI] [PubMed] [Google Scholar]
- 22.Choi JW, Kim SK. Association of serum insulin-like growth factor-I and erythropoiesis in relation to body iron status. Ann Clin Lab Sci. 2004;34:324–328. [PubMed] [Google Scholar]
- 23.Flume PA, Mogayzel PJ, Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, Marshall BC. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 24.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347–360. doi: 10.1146/annurev-med-050109-142444. [DOI] [PubMed] [Google Scholar]
- 25.Street ME, Spaggiari C, Volta C, Ziveri MA, Viani I, Rossi M, Pisi G, Grzincich G, Bernasconi S. The IGF system and cytokine interactions and relationships with longitudinal growth in prepubertal patients with cystic fibrosis. Clin Endocrinol (Oxf) 2009;70:593–598. doi: 10.1111/j.1365-2265.2008.03387.x. [DOI] [PubMed] [Google Scholar]
- 26.Reeves EP, Bergin DA, Fitzgerald S, Hayes E, Keenan J, Henry M, Meleady P, Vega-Carrascal I, Murray MA, Low TB, McCarthy C, O'Brien E, Clynes M, Gunaratnam C, McElvaney NG. A novel neutrophil derived inflammatory biomarker of pulmonary exacerbation in cystic fibrosis. J Cyst Fibros. 2012;11:100–107. doi: 10.1016/j.jcf.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Andreassen M, Raymond I, Hildebrandt P, Kistorp C, Rathcke C, Vestergaard H, Faber J, Kristensen LO. Associations between plasma insulin-like growth factor-I and the markers of inflammation interleukin 6, C-reactive protein and YKL-40 in an elderly background population. Inflamm Res. 2010;59:503–510. doi: 10.1007/s00011-009-0154-z. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed TA, Buzzelli MD, Lang CH, Capen JB, Shumate ML, Navaratnarajah M, Nagarajan M, Cooney RN. Interleukin-6 inhibits growth hormone-mediated gene expression in hepato-cytes. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1793–G1803. doi: 10.1152/ajpgi.00547.2006. [DOI] [PubMed] [Google Scholar]
- 29.VanDevanter DR, O'Riordan MA, Blumer JL, Konstan MW. Assessing time to pulmonary function benefit following antibiotic treatment of acute cystic fibrosis exacerbations. Respir Res. 2010;11:137. doi: 10.1186/1465-9921-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nazer D, Abdulhamid I, Thomas R, Pendleton S. Home versus hospital intravenous antibiotic therapy for acute pulmonary exacerbations in children with cystic fibrosis. Pediatr Pulmonol. 2006;41:744–749. doi: 10.1002/ppul.20433. [DOI] [PubMed] [Google Scholar]
- 31.Taylor AM, Bush A, Thomson A, Oades PJ, Marchant JL, Bruce-Morgan C, Holly J, Ahmed L, Dunger DB. Relation between insulin-like growth factor-I, body mass index, and clinical status in cystic fibrosis. Arch Dis Child. 1997;76:304–309. doi: 10.1136/adc.76.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]