Abstract
The purpose of this study was to determine the efficacy of a cognitive-behavioral based physical therapy (CBPT) program for improving outcomes in patients following lumbar spine surgery. A randomized controlled trial was conducted in 86 adults undergoing a laminectomy with or without arthrodesis for a lumbar degenerative condition. Patients were screened preoperatively for high fear of movement using the Tampa Scale for Kinesiophobia. Randomization to either CBPT or an Education program occurred at 6 weeks after surgery. Assessments were completed pre-treatment, post-treatment and at 3 month follow-up. The primary outcomes were pain and disability measured by the Brief Pain Inventory and Oswestry Disability Index. Secondary outcomes included general health (SF-12) and performance-based tests (5-Chair Stand, Timed Up and Go, 10 Meter Walk). Multivariable linear regression analyses found that CBPT participants had significantly greater decreases in pain and disability and increases in general health and physical performance compared to the Education group at 3 month follow-up. Results suggest a targeted CBPT program may result in significant and clinically meaningful improvement in postoperative outcomes. CBPT has the potential to be an evidence-based program that clinicians can recommend for patients at-risk for poor recovery following spine surgery.
Introduction
Degenerative lumbar conditions, such as spinal stenosis, lead to chronic pain, physical impairment, and reduced quality of life.2 The prevalence in the general population ranges from 20% to 25% and increases to above 45% in individuals greater than 60 years of age.31,34,35 Lumbar spinal stenosis is one of the most common diagnoses associated with spine surgery.2,18,72 The surgical technique for lumbar degenerative conditions is well established and studies have reported on the benefits of surgery compared to nonoperative management.24,39 The Spine Patient Outcomes Research Trial (SPORT), using as-treated analysis, found that surgery for lumbar stenosis had a significant advantage over nonoperative treatment at 2 and 4 years following surgery.84 However, as-treated SPORT findings demonstrated that the advantage of surgery was no longer significant after 5 years.45
The estimated percentage of people over 60 years is expected to increase steadily towards 2050.77 An increased number of people will experience age-associated degenerative conditions and chronic pain; spine surgery rates will continue to rise.4 Despite surgical advances, adults following lumbar spine surgery continue to have poorer physical and mental health outcomes compared to the general population.50,83 Studies have found persistent pain, functional disability and poor quality of life in up to 40% of individuals following spine surgery for lumbar degenerative conditions.9,32,48,84 The reoperation rate has been reported to range from 18% to 23% at 8 to 10 years after surgery.45
Archer et el.5,6,8 and others have found that fear of movement, avoidance coping, positive affect and depression are independently associated with persistent pain and disability and decreased physical function after lumbar spine surgery.17,29,47,69 Despite the literature recommending a biopsychosocial approach to postoperative care, 52,88 physical therapy programs after spine surgery continue to focus on trunk and lower extremity strengthening, flexibility, range of motion, and education on posture and proper body mechanics. Randomized trials to date have found no significant difference between traditional physical therapy and either no treatment, an educational booklet, or advice to keep active.1,46,51,52 These results suggest that an alternative approach to postoperative rehabilitation may be needed to address the psychosocial factors often associated with poor surgical spine outcomes.
The purpose of this study was to incorporate cognitive-behavioral strategies into physical therapy to improve outcomes in patients with chronic pain undergoing lumbar spine surgery. Individuals with high fear of movement were targeted in order to focus on adults at-risk for poor postoperative recovery.8,17,29,47 The program – Changing Behavior through Physical Therapy (CBPT) – was designed to decrease fear of movement and increase self-efficacy7 and be delivered by physical therapists. Since clinic-based rehabilitation can be impractical for many older adults, a telephone-delivery model was used to allow individuals with financial, geographic, and mobility constraints to participate in the study. We hypothesized that CBPT participants would have greater improvement in patient-reported pain, disability, and general health and performance-based tests compared to Education participants at 6 months after lumbar spine surgery for degenerative conditions.
Methods
Trial Design
This study was a randomized controlled trial. Participants were recruited from a single academic medical center and randomized to either CBPT or an Education program during a routine postoperative clinic visit at 6 weeks after surgery. At this visit, all participants also received standard care, which may include having lifting and/or driving restrictions removed and referral to traditional physical therapy. The Education program was chosen as a comparison to control for the time and attention of the therapist and for normal healing that occurs from 6 weeks to 3 months after surgery.
The investigators, participating surgeons, research personnel conducting the assessments, and patients were blinded to group assignment. Participants were informed that they would be randomly assigned to one of two different educational treatments and were asked not to discuss study procedures with their treating surgeon, medical staff, and research personnel. The study physical therapist was blinded to the aims and hypotheses of the study.
The overall study design included a clinic screening visit, preoperative assessment, pre-treatment assessment (6 weeks after surgery), treatment phase, post-treatment assessment (3 months after surgery), and 3-month follow-up assessment (6 months after surgery) (see ClinicalTrials.gov and NCT01131611). The Institutional Review Board at the participating site approved the study and all patients provided informed consent prior to study enrollment and data collection.
Sample Size and Power
The number of study participants was based on a sample size calculation for a comparison of treatment groups on change in the outcomes of pain intensity and interference, measured by the Brief Pain Inventory, disability measured by the Oswestry Disability Index, and general health measured by the 12-Item Short-Form Health Survey. Power was estimated by generating simulated data from available pilot data, then using the simulated data to estimate the original model parameters. A sample size of 80 was chosen to be able to detect minimum clinically important differences (MCID) in pain intensity of 1.2 to 2.0 points, pain interference of 1.6 to 2.2 points, disability of 10 to 12.8 points, and general health of 4.9 to 6.2 points during the postoperative period, with an 80% power while controlling type I error rate at 5%. These MCIDs were based on studies conducted in patients following lumbar spine surgery.
Participants
Participants for this study were recruited from 499 individuals, between March 2012 and April 2013, undergoing a laminectomy with or without arthrodesis for a lumbar degenerative condition (spinal stenosis, spondylosis with or without myelopathy, and degenerative spondylolisthesis). The following inclusion criteria were used for recruitment purposes: (1) 21 years of age or older; (2) English speaking; (3) back and/or lower extremity pain for greater than 6 months; (4) no history of neurological movement disorder; and (5) no presence of psychotic disease in the medical record. Participants also needed to report high fear of movement, based on a score of 39 or greater on the Tampa Scale for Kinesiophobia (TSK). A cut-off of 39 on the TSK has been found to identify individuals who have a high probability of dysfunctional pain beliefs and poor outcomes after spine surgery.5,6,57,79,80,85
Study exclusion criteria included: (1) spinal deformity as the primary indication for surgery; (2) surgery for pseudarthrosis, trauma, infection, or tumor; and (3) having microsurgical techniques as the primary procedure.
Study Procedures and Randomization
Eligible participants were approached for consent prior to surgery and completed a screening questionnaire to determine high fear of movement. Individuals who remained eligible completed an intake assessment, a battery of validated questionnaires that assessed pain, disability, general health, pain self-efficacy, depression and a series of performance-based tests. Participants returned to the clinic at 6 weeks after surgery for a standard postoperative visit. At this clinic visit, participants completed a pre-treatment assessment for the study and the first treatment session (CBPT or Education).
Randomization was administered through the Research Electronic Data Capture (REDCap) system28 and occurred immediately following the baseline assessment in order to initiate treatment. A computer-generated scheme randomized patients to either CBPT or Education in a 1:1 ratio in blocks of assignments. Since preliminary data demonstrated that surgery type and fear of movement influenced patient-reported outcomes, these assignments were frequency matched on type of surgery (fusion or no fusion) and screening score on the TSK (39–45, 46–49, 50–68), resulting in 6 strata.
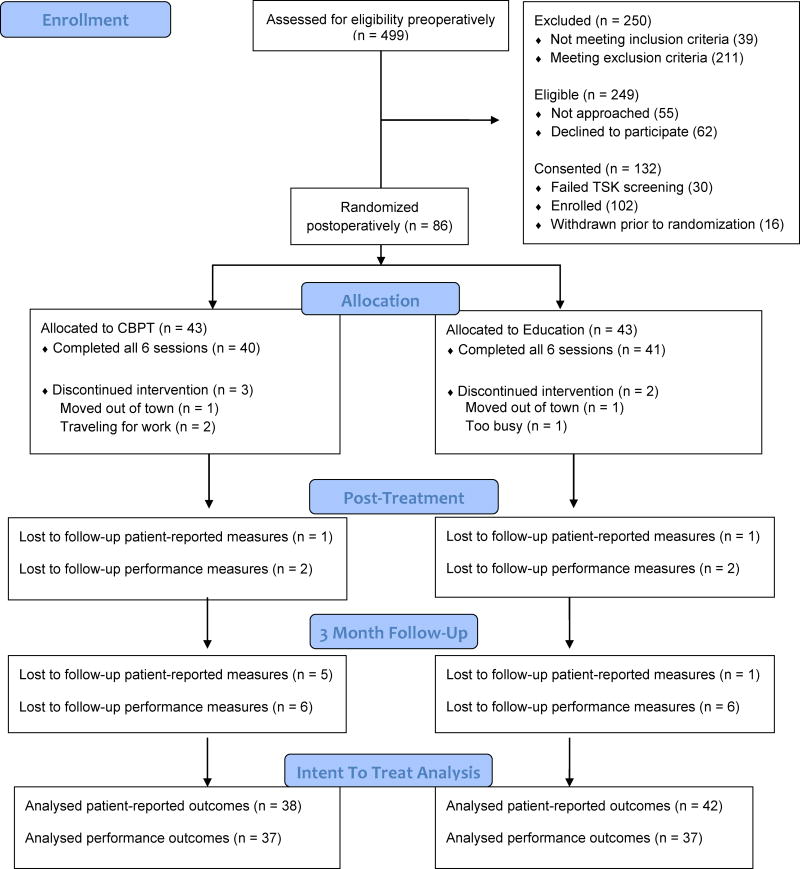
Participants returned for an in-person post-treatment assessment and a 3 month follow-up assessment at 3 and 6 months after surgery, respectively. All assessments included a self-report questionnaire that measured psychosocial characteristics (fear of movement and pain self-efficacy) and pain, disability, and general health outcomes as well as use of physical therapy and other health care services. Performance-based tests were completed to assess lower extremity strength, functional mobility, and gait speed. Participants that were unable to return to the clinic for follow-up visits were asked to complete questionnaires at home and return in self-addressed stamped envelopes. See Fig. 1 for a CONSORT flow diagram.
Figure 1.
Flow chart of recruitment and follow-up.
Participants were reimbursed $25 for their time in completing the baseline assessment and $100 for each of the in-person follow-up assessments.
Demographic and Depressive Symptoms
A preoperative intake assessment collected demographic and health information pertaining to age, sex, race, education, employment, smoking status, height and weight, co-morbid conditions, narcotic use, history of spinal surgery, and expectations of a successful surgery. Participants also provided information on depressive symptoms by completing the 9-item Patient Health Questionnaire (PHQ-9).40 A total score on the PHQ-9 can range from 0 to 27 with higher numbers indicating severe depressive symptoms. Participants rate each item on a 4-point Likert scale with scoring that ranges from ‘not at all’ to ‘nearly every day.’ In a psychometric study of the PHQ-9 compared to independent diagnoses made by mental health professionals, the instrument was both sensitive (0.75) and specific (0.90) for the diagnosis of major depression.40,41
Psychosocial Measures
Fear of movement was assessed with the 17-item TSK.38 A total score can range from 17 to 68. Participants rate items on a 4-point Likert scale with scoring alternatives ranging from ‘strongly disagree’ to ‘strongly agree.’ The MCID for the TSK has been reported to be 4 points in patients with back pain.89 The TSK has good internal consistency and test-retest reliability in surgical patients and patients with various musculoskeletal condition.22,67
The Pain Self-Efficacy Questionnaire (PSEQ) was used to measure the strength and generality of a person’s belief in his/her ability to accomplish a range of activities despite pain.58 Participants rate how confident they are on a 7-point scale from ‘not at all confident’ to ‘completely confident.’ Scores range from 0 to 60, with a score greater than 40 indicating high self-efficacy.55 The PSEQ has been found to have excellent internal consistency, good test-retest reliability, and construct validity through correlations with depression, anxiety, coping strategies, pain ratings, and work-related tasks in patients with chronic pain.58
Primary Outcome Measures
Pain Intensity and Pain Interference
The Brief Pain Inventory (BPI) was used to measure both pain intensity and pain interference with daily activity.12 The pain intensity scale includes 4 pain items assessing current, worst, least and average pain (0-no pain at all to 10-as bad as you can imagine). The pain interference scale is a 7-item scale measuring the degree to which pain interferes with areas of daily life: general activity, mood, walking, work, relations with others, sleep, and enjoyment of life (0-does not interfere to 10-completely interferes). The BPI has proven reliable (Cronbach’s alpha > 0.80) and valid (highly correlated with the SF-36 brief pain scale, the Roland Disability Questionnaire, the McGill Pain Questionnaire, and the Visual Analog Scale for pain) in both surgical patients and patients with chronic low back pain.36,53,92 The MCID for back and leg pain has been found to be 1.2 and 1.6, respectively, in patients following lumbar spine surgery.14,25
Disability
Low back disability was measured using the Oswestry Disability Index (ODI).21 The 10-item ODI assesses ten aspects of daily living: pain intensity, lifting, sitting, standing, walking, sleeping, hygiene, traveling, social and sex life. Ratings for each item are from 0 (high functioning) to 5 (low functioning). Total item scores are divided by the total possible score and multiplied by 100 to create a percentage of disability. Disability categories include: 0% to 20% (minimal disability), 21% to 40% (moderate disability), 41% to 60% (severe disability), 61% to 80% (crippled), and 81% to 100% (bed bound or exaggerated symptoms). The ODI has demonstrated excellent test-retest reliability (Pearson’s r > 0.80), adequate internal consistency (Cronbach’s alpha > 0.70), and validity with moderately high correlations with other disability measures.16,65 The MCID has been found to range from 10 to 12.8 points in patients following lumbar spine surgery.14,25,61,62
Secondary Outcome Measures
General Physical and Mental Health
General physical and mental health was measured with the physical and mental component scales of the 12-Item Short-Form Health Survey (SF-12).82 The physical component scale (PCS) assesses the four subdomains of physical functioning, role-physical, bodily pain, and general health and the mental component scale (MCS) assesses the 4 subdomains of vitality, social functioning, role-emotional, and mental health. Total subscale scores range from 0 to 100 and higher scores represent better health status. The PCS and MCS of the SF-12 have demonstrated responsiveness, good test–retest reliability, good internal consistency, and validity in both generalized and various patient populations.33,82 The MCID for the PCS and MCS has been estimated at 4.9 points in patients following lumbar arthrodesis.25
Performance-Based Function
The 5-Chair Stand test26 was used to assess lower extremity strength. Participants were asked to fold their arms across their chest and stand up from and sit down on a standard chair. If able to perform one time successfully, patients were asked to stand up and sit down 5 times as fast as possible starting in the sitting position and stopping after the fifth rise. Performance on the 5-Chair Stand test was measured in seconds. The 5-Chair Stand test has demonstrated good test-retest reliability and validity, with significant correlations with other measures of physical performance and self-reported disability.26 The MCID for the 5-Chair Stand has been estimated as a reduction of 2.3 seconds in patients with balance and vestibular disorders.54
The Timed Up and Go (TUG) test64 was used to assess functional mobility. Participants were asked to stand from a chair, walk 3 meters, turn around, walk back and sit down and the time to complete was recorded in seconds. The TUG has been shown to have excellent test-retest reliability and be a valid and responsive performance measure in older individuals.10,64 The major clinically important improvement for the TUG has been reported as a reduction in time ranging from 1.2 to 1.4 seconds in older adults with osteoarthritis.91
The 10-Meter Walk test27 was used to assess gait speed. Patients were given a 2-meter warm-up distance preceding the 10-meter distance and 2 meters beyond the 10 meters to continue walking. The time that it took to traverse the 10 meters at a comfortable pace was recorded. Two trials were conducted, with a brief rest as needed between trials. Measurements for both trials were averaged. Excellent interrater and intrarater reliability and good test-retest reliability for self-paced timed walking speed tests using a stopwatch have been reported [49]. Validity for walking speed tests has been determined by significant correlations with measures of function and mortality in older adults.27,49 The MCID for gait speed has been estimated to be 0.16 meters/second in patients with subacute stroke and substantial meaningful change has been found to be 0.10 meters/second in older adults.63,73
Treatments
Therapist Training
One physical therapist with no prior experience delivering cognitive-behavioral strategies participated in a training program for both the CBPT and Education programs. Formal training included eight hours of didactic and sixteen hours of experiential session-by-session training with a clinical psychologist (STW) and eight hours of training with a physical therapist who designed the programs (KRA). Knowledge and skills competence was determined through a written test after the first 2-day session and a skills test after the second 2-day session (i.e., scores needed to be > 85). After training, the CBPT and Education programs were implemented with research personnel and a pre-test occurred with 2 patients in each group. All sessions during the pre-test were audiotaped and reviewed with the physical therapist to evaluate adherence to the CBPT and Education treatment protocols and cognitive and behavioral competencies specific to the CBPT treatment.78
CBPT Program
The CBPT program is a cognitive-behavioral based approach to rehabilitation (see www.spine-surgery-recovery.com for more information). Brief cognitive-behavioral therapy (CBT) programs for pain developed by Woods and Asmundson,90 Williams and McCracken,87 and Turner et al.75 and a self-management program developed for older adults by Lorig44 provided the basis for the CBPT program. Specific cognitive-behavioral strategies were selected from these evidence-based CBT programs and adapted for use by physical therapists. The main goal of the CBPT program was to reduce pain and disability, through reductions in fear of movement and increases in self-efficacy. Patients received weekly sessions with a study physical therapist for 6 weeks. The first session was conducted in person and participants were given a manual to follow along with the study therapist. The remaining sessions were delivered over the telephone. All sessions were 30 minutes in length, except the first session, which was approximately 1 hour.
The CBPT program focused on empirically supported behavioral self-management, problem solving, cognitive restructuring, and relaxation training.74,87,90 The main components of the program include education on the relationship between the body, mind, and ones activity level, a graded activity plan (i.e., a comprehensive list of activities ordered from least to most difficult based on fear or pain) and weekly activity and walking goals. Goals were rated by patients on a scale from 0 to 10 (completely confident), and scores of 8 or greater indicated a realistic goal. A cognitive or behavioral strategy was introduced in each session, with the therapist helping patients identify enjoyable activities (i.e., distraction), replace negative thinking with positive thoughts, find a balance between rest and activity, and manage setbacks by recognizing high-risk situations and negative thoughts. Details of the CBPT intervention were previously published.7
Education Program
The Education program focused on postoperative recovery and consisted of topics commonly covered by physical therapists during outpatient treatment sessions. Sessions addressed benefits of physical therapy, proper biomechanics after surgery, importance of daily exercise, and ways to promote healing. Information on stress reduction, sleep hygiene, energy management, communication with health providers, and preventing future injury were also provided. Patients received weekly sessions with a study physical therapist for 6 weeks. The first session was conducted in person and participants were given a manual to follow along with the study therapist. The remaining sessions were delivered over the telephone. All sessions were 30 minutes in length, except the first session, which was approximately 1 hour.
Treatment Fidelity
The study physical therapist’s adherence to the CBPT and Education manuals were assessed by digitally recording all sessions and randomly selecting 30% of all sessions (balanced evenly across the sessions) to review. A clinical psychologist (STW) and a physical therapist (KRA) with expertise in the programs rated the CBPT and Education sessions for treatment integrity and potential contamination using a standardized checklist. The study therapist also completed a checklist of all the components delivered during each CBPT or Education session and made note of any protocol deviations. A therapist adherence score was determined for each session using a scale from 0 (completely nonadherent) to 100 (completely adherent).
Treatment Acceptability
Acceptability of the CBPT and Education programs was assessed post-treatment. Participants were asked to rate how helpful the program was to their recovery and how likely they were to recommend the program to a friend. These items were scored using an 11-point numeric rating scale with 0 being ‘not at all helpful or likely’ and 10 being ‘extremely helpful or likely’. Participants were also asked to rate the overall benefit of the program taking into account the effort put into it, the importance of changes in pain and activity due to the program, and the importance of the program compared to other services on a 5-point Likert scale. Finally, participants were asked through open-ended questions to comment on the strengths and weaknesses of the program.
Data Analysis
Descriptive statistics were used to summarize the mean scores and standard deviations (SD) or frequency of demographic, clinical, and psychosocial characteristics as well as outcomes measures. Group means and corresponding confidence intervals or frequency for preoperative variables and baseline measures were compared using student t-tests or chi-square tests to confirm balance between groups. The characteristics of the patients who were lost to follow-up were compared to those who completed the follow-up assessments. Missing items were less than 5% for the completed psychosocial and outcome scales and imputed based on a single mean imputation.
All analyses were intent-to-treat. The mean change from pre-treatment to post-treatment and 3 month follow-up was calculated for the primary and secondary outcome measures and psychosocial characteristics. Between-group differences of mean change from baseline to each follow-up time point were compared using repeated-measures analysis of variance. Standardized mean effect size differences of the programs were assessed with Cohen’s d and d = 0.20 indicated a small effect, d = 0.50 a medium effect, d = 0.80 a large effect, and d = 1.3 a very large effect.13,68 Separate multivariable linear regression models were then conducted for the outcomes at 3 month follow-up, controlling for a priori variables of the pre-treatment score of the outcome of interest, age, education, presence of comorbid conditions, and number of physical therapy visits since the baseline visit. Potential interactions between treatment and age and type of surgery were tested. Stata software (StataCorp, 2011, College Station, TX) was used to analyze the data. The level of significance was set at p < 0.05.
Results
Of the 194 eligible participants who were approached about the study, 132 (68%) were consented and 102 passed screening and were enrolled (Figure 1). Eight-six participants were randomized. Sixteen participants (16%) were not treated surgically for a lumbar degenerative condition and were withdrawn from the study prior to randomization. The dropout rate for the CBPT and Education programs was 7% and 5%, respectively. For the five individuals who did not complete all 6 sessions, reasons provided were moving out of town, traveling for work, and other time commitments. The follow-up rate for the patient-reported and performance-based outcomes post-treatment was 98% and 95% and at 3 month follow-up was 93% and 86%, respectively. There were no significant differences between patients with and without complete follow-up data on demographic, clinical, and psychosocial variables.
Participant demographic and clinical variables are presented in Table 1. No significant differences were noted across groups. Seventy-eight participants (91%) received clinic-based physical therapy during the treatment phase between 6 weeks and 3 months after surgery. The average number of physical therapy visits for the CBPT group was 8.6 (SD: 4.9) and 8.0 (SD: 4.2) for the Education group (p = 0.55). Forty patients (47%) continued with physical therapy between the post-treatment and 3 month follow-up time point, with the CBPT participants having an average of 6.5 visits (SD: 7.5) and the Education group 6.6 visits (SD: 10.3; p = 0.94).
Table 1.
Demographic and clinical characteristics of study participants (N=86).
| Characteristic | Total | CBPT N=43 | Education N=43 |
|---|---|---|---|
| Demographic | |||
| Age in years, Mean ± SD | 57.6 ± 12.2 | 56.9 ± 11.1 | 58.4 ± 13.3 |
| Female Sex, N (%) | 48 (55.8) | 25 (58.1) | 23 (53.5) |
| Self-Report White Race, N (%) | 69 (80.2) | 36 (83.7) | 33 (76.7) |
| More than High School Education, N (%) | 62 (72.1) | 30 (69.8) | 32 (74.4) |
| Married, N (%) | 61 (70.9) | 32 (74.4) | 29 (67.4) |
| Obese BMI Category, N (%) | 44 (51.1) | 23 (53.5) | 21 (48.8) |
| Employed Prior to Surgery, N (%) | |||
| Not Working | 29 (33.7) | 14 (32.6) | 15 (34.9) |
| Working | 39 (45.3) | 21 (48.8) | 18 (41.9) |
| Retired | 18 (21.0) | 8 (18.6) | 10 (23.2) |
| Current Smoker, N (%) | 17 (19.8) | 10 (23.3) | 7 (16.3) |
| Co-morbid conditions, N (%) | |||
| 0 | 6 (7.0) | 4 (9.3) | 2 (4.7) |
| 1–2 | 66 (76.7) | 32 (74.4) | 34 (79) |
| >2 | 14 (16.3) | 7 (16.3) | 7 (16.3) |
| Clinical | |||
| Fusion Surgery, N (%) | 60 (69.8) | 29 (67.4) | 31 (72.1) |
| Prior Spine Surgery, N (%) | 34 (39.5) | 17 (39.5) | 17 (39.5) |
| Duration of Preoperative Pain, Mean ± SD | 24.1 ± 27.4 | 25.1 ± 30.2 | 23.1 ± 24.5 |
| Taking Narcotics Prior to Surgery, N (%) | 47 (54.6) | 23 (53.5) | 24 (55.8) |
| Expectations of successful surgery, Mean ± SD | 8.9 (1.7) | 8.7 ± 2.1 | 9.2 ± 1.1 |
| Preoperative depression, PHQ-9 Mean ± SD | 10.3 (5.8) | 11 (5.6) | 9.6 (6) |
| Preoperative fear of movement, TSK Mean ± SD | 43.3 (5.3) | 43.5 (5) | 43.2 (5.6) |
| Preoperative pain self-efficacy, PSEQ Mean ± SD | 26.6 (11.3) | 25.5 (10.6) | 27.7 (12.1) |
| Preoperative back pain, BPI Mean ± SD | 6.7 (2.1) | 6.8 (1.9) | 6.5 (2.3) |
| Preoperative leg pain, BPI Mean ± SD | 7.1 (2.4) | 7.0 (2.6) | 7.1 (2.2) |
| Preoperative disability, ODI Mean ± SD | 49.1 (13.3) | 49.2 (13.7) | 49.0 (13.1) |
| Preoperative physical health, SF-12 Mean ± SD | 25.8 (5.8) | 25.4 (5.7) | 26.2 (6.1) |
| Preoperative mental health, SF-12 Mean ± SD | 46.8 (11.7) | 46 (11) | 47.7 (12.4) |
| 5-Chair Stand score, Mean seconds ± SD | 39.3 (21.5) | 38.0 (21.7) | 40.6 (21.5) |
| TUG score, Mean seconds ± SD | 20.0 (10.5) | 18.7 (9.8) | 21.3 (11.2) |
| 10-Meter Walk score, Mean m/s ± SD | 0.80 (0.32) | 0.79 (0.29) | 0.81 (0.35) |
SD = standard deviation; BMI = body mass index; PHQ-9 = 9-item Patient Health Questionnaire; TSK = Tampa Scale for Kinesiophobia (17–68); PSEQ = Pain Self-Efficacy Questionnaire (0–60); BPI = Brief Pain Inventory (0–10); ODI = Oswestry Disability Index (0–100); SF-12 = 12-item Short Form Health Survey (0–100); TUG = Timed Up and GO; m/s = meters/second
Treatment Fidelity and Acceptability
Adherence to the CBPT and Education programs was high, with no statistical differences between groups (97.7 vs. 98; p = 0.41). Both groups reported that the program was extremely helpful and it was extremely likely they would recommend the program to a friend (Table 2). The majority of CBPT participants (59.5%) reported that the benefits of the program far outweighed the effort compared to 45.2% of Education participants (p = 0.33). Significant differences were noted for the questions on the importance of changes in pain and activity, with 54.8% and 76.2% of CBPT participants and 21.4% and 33.3% of Education participants noting that their pain decreased and activity increased a meaningful amount, respectively (p < 0.01). No significant difference was found between groups on whether the program was more important than other services since leaving the hospital (CBPT: 45.2% vs. Education: 38.1%; p = 0.11).
Table 2.
Acceptability of CBPT and Education programs to study participants (N=84).
| Measure | CBPT (N=42) | Education (N=42) | |
|---|---|---|---|
| 1. Helpful (0–10), mean (SD) | 8.9 (1.7) | 8.1 (2.1) | |
| 2. Likely to recommend (0–10), mean (SD)* | 9.3 (1.6) | 8.3 (2.7) | |
| 3. Overall benefit, taking into account the effort put into it, N (%) | |||
| Benefits far outweighed the effort | 25 (59.5) | 19 (45.2) | |
| Benefits somewhat outweighed the effort | 5 (11.9) | 4 (9.5) | |
| Benefits equaled the effort | 11 (26.2) | 14 (33.3) | |
| Effort somewhat outweighed the benefits | 1 (2.4) | 2 (4.8) | |
| Effort far outweighed the benefits | 0 (0) | 3 (7.2) | |
| 4. Importance of changes in pain, N (%)* | |||
| Pain decreased a meaningful amount | 23 (54.8) | 9 (21.4) | |
| There | Some decrease in pain, but not enough to be meaningful | 6 (14.3) | 6 (14.3) |
| No change in pain | 13 (30.9) | 27 (64.3) | |
| Some increase in pain, but not enough to be meaningful | 0 (0) | 0 (0) | |
| Pain increased a meaningful amount | 0 (0) | 0 (0) | |
| 5. Importance of changes in activity, N (%)* | |||
| Activity increased a meaningful amount | 32 (76.2) | 14 (33.3) | |
| There | Some increase in activity, but not enough to be meaningful | 4 (9.5) | 7 (16.7) |
| No change in activity | 6 (14.3) | 20 (47.6) | |
| Some decrease in activity, but not enough to be meaningful | 0 (0) | 1 (2.4) | |
| Activity decreased a meaningful amount | 0 (0) | 0 (0) | |
| 6. Compared to other services, the importance of the program to recovery, N (%) | |||
| Much more important | 12 (28.5) | 6 (14.3) | |
| Somewhat more important | 7 (16.7) | 10 (23.8) | |
| As important | 21 (50) | 17 (40.5) | |
| Somewhat less important | 1 (2.4) | 5 (11.9) | |
| Much less important | 1 (2.4) | 4 (9.5) | |
p < 0.05 for significant differences across groups;
SD = standard deviation
The main strength of both programs from the patients’ perspective was the telephone-delivery format. Additional strengths noted were that the sessions provided encouragement/motivation/confidence to engage in the recovery process and that the sessions increased activity. Specific to the CBPT program, 40% reported that the program made them feel accountable to someone, 30% felt that the program made them more aware of what they could do about their condition, and 25% learned to discuss things more openly with their doctor or feel more connected to their medical staff. Weaknesses noted by participants included the following: 1) programs were not long enough; 2) more information was needed on the healing process and restrictions; 3) guidelines for recovery were needed; and 4) the programs needed to start closer to discharge from the hospital.
Primary Outcomes
Average primary outcome scores for the CBPT group demonstrated an improvement in back and leg pain, pain interference, and disability over time (Table 3). The Education group scores for leg pain and disability improved; however, average back pain and pain interference scores remain unchanged from post-treatment to 3-month follow-up.
Table 3.
Primary outcome scores and change from pre-treatment to post-treatment and 3 month follow-up by group
| Variable | CBPT Mean (SD) | Education Mean (SD) | Mean Change from Pre-Treatment | Between-Group Difference | ||
|---|---|---|---|---|---|---|
|
| ||||||
| CBPT | Education | CBPT vs. Education | P Value | |||
| BPI: Back Pain | ||||||
| Pre-Treatment | 3.0 (2.2) | 2.8 (2.0) | ||||
| Post-Treatment | 2.9 (2.6) | 2.5 (2.0) | -0.08 (-0.65 to 0.49) | -0.3 (-0.68 to 0.08) | 0.22 (-0.46 to 0.9) | 0.52 |
| 3 Month | 1.9 (2.0) | 2.5 (2.4) | -1.1 (-1.5 to -0.74) | -0.26 (-0.76 to 0.23) | -0.88 (-1.5 to -0.25) | 0.007 |
| BPI: Leg Pain | ||||||
| Pre-Treatment | 2.5 (2.6) | 2.2 (2.1) | ||||
| Post-Treatment | 2.1 (2.2) | 2.3 (2.2) | -0.48 (-0.91 to -0.06) | 0.05 (-0.34 to 0.44) | -0.53 (-1.1 to 0.04) | 0.07 |
| 3 Month | 1.3 (2.1) | 2.1 (2.6) | -1.3 (-1.9 to -0.72) | -0.1 (-0.75 to 0.55) | -1.2 (-2.1 to -0.34) | 0.007 |
| BPI: Interference | ||||||
| Pre-Treatment | 3.8 (3.0) | 3.1 (2.6) | ||||
| Post-Treatment | 3.2 (3.2) | 2.8 (2.9) | -0.65 (-1.16 to -0.14) | -0.3 (-0.84 to 0.24) | -0.35 (-1.1 to 0.38) | 0.34 |
| 3 Month | 2.1 (2.5) | 2.8 (2.8) | -1.7 (-2.4 to -1.1) | -0.26 (-0.89 to 0.38) | -1.5 (-2.4 to -0.57) | 0.002 |
| ODI Score | ||||||
| Pre-Treatment | 38.8 (17.3) | 34.0 (16.7) | ||||
| Post-Treatment | 28.6 (17.6) | 27.9 (19.4) | -9.8 (-12.1 to -7.5) | -6.1 (-10.5 to -1.7) | -3.7 (-8.6 to 1.2) | 0.14 |
| 3 Month | 21.1 (16.7) | 26.5 (20.5) | -17.3 (-20.3 to -14.4) | -7.5 (-12.1 to -2.9) | -9.8 (-15.3 to -4.4) | <0.001 |
SD = standard deviation; BPI = Brief Pain Inventory (0–10); ODI = Oswestry Disability Index (0–100)
Group differences in pain and disability were statistically significant at 3 month follow-up (p < 0.05), but not post-treatment (Table 3). The mean change from pre-treatment to 3-month follow-up for the CBPT group was above MCID for BPI pain interference score (-1.7 points [95%CI, -2.4 to -1.1]) and ODI score (-17.3 [95%CI, -20.3 to -14.4]). The effect size for back and leg pain was 0.62, pain interference was 0.72, and disability was 0.79.
Multivariable linear regression analyses controlling for the pre-treatment score of the outcome of interest, age, education, comorbid conditions, and number of physical therapy visits found that CBPT participants had BPI back pain scores that were -0.85 points lower (95%CI, -1.4 to -0.25; p = 0.006), BPI leg pain scores -1.1 points lower (95%CI, -1.9 to -0.27; p = 0.009), BPI pain interference scores -1.3 points lower (95%CI, -2.1 to -0.40; p = 0.005), and ODI scores -9.4 points lower (95%CI, -14.9 to -4.0; p = 0.001) than Education participants at 3 month follow-up. The regression models accounted for 64% and 44% of the variance for back and leg pain, 49% of the variance for the pain interference, and 59% of the variance for disability.
Secondary Outcomes: General Health
Average general health outcome scores for the CBPT group demonstrated an improvement in physical and mental health over time (Table 4). The Education group scores for physical health improved; however, average mental health scores remained relatively unchanged.
Table 4.
Secondary outcome scores and change from pre-treatment to post-treatment and 3 month follow-up by group
| Variable | CBPT Mean (SD) | Education Mean (SD) | Mean Change from Pre-Treatment | Between-Group Difference | ||
|---|---|---|---|---|---|---|
|
| ||||||
| CBPT | Education | CBPT vs. | P Value | |||
| SF-12: PCS | ||||||
| Pre-Treatment | 29.8 (0.5) | 32.0 (9.8) | ||||
| Post-Treatment | 36.5 (10.8) | 36.9 (11.6) | 6.6 (4.2 to 8.9) | 4.8 (2.1 to 7.6) | 1.7 (-1.9 to 5.3) | 0.34 |
| 3 Month | 43.0 (10.9) | 38.3 (11.4) | 13.4 (10.4 to 16.4) | 6.3 (3.3 to 9.3) | 7.1 (2.9 to 11.3) | 0.001 |
| SF-12: MCS | ||||||
| Pre-Treatment | 43.6 (11.9) | 53.9 (10.1) | ||||
| Post-Treatment | 50.9 (10.0) | 53.7 (12.2) | 7.4 (5.3 to 9.5) | -0.2 (-3.0 to 2.6) | 7.6 (4.2 to 11.1) | <0.001 |
| 3 Month | 56.6 (8.1) | 53.4 (12.0) | 12.5 (9.6 to 15.4) | -0.5 (-3.7 to 2.7) | 13.0 (8.7 to 17.2) | <0.001 |
| 5-Chair Stand, seconds | ||||||
| Pre-Treatment | 24.7 (18.9) | 20.3 (15.4) | ||||
| Post-Treatment | 22.6 (18.4) | 21.0 (16.2) | -2.7 (-5.4 to 0.01) | 0.4 (-3.2 to 4) | -3.1 (-7.5 to 1.4) | 0.17 |
| 3 Month | 13.6 (5.1) | 16.7 (11.7) | -11.6 (-17.3 to -5.9) | -4.6 (-8.2 to -0.91) | -7 (-13.7 to -0.37) | 0.04 |
| TUG, seconds | ||||||
| Pre-Treatment | 11.5 (5.0) | 11.6 (5.9) | ||||
| Post-Treatment | 10.0 (2.7) | 12.1 (6.4) | -1.7 (-2.8 to -0.55) | 0.33 (-1.2 to 1.9) | -2.0 (-3.9 to -0.11) | 0.04 |
| 3 Month | 9.6 (4.2) | 10.9 (6.2) | -2.1 (-3.3 to -0.9) | -0.54 (-1.9 to 0.78) | -1.6 (-3.3 to 0.19) | 0.08 |
| 10-Meter Walk, m/s | ||||||
| Pre-Treatment | 1.02 (0.26) | 1.07 (0.29) | ||||
| Post-Treatment | 1.19 (0.20) | 1.08 (0.30) | 0.10 (0.05 to 0.15) | 0.01 (-0.08 to 0.09) | 0.09 (-0.01 to 0.19) | 0.07 |
| 3 Month | 1.21 (0.22) | 1.17 (0.26) | 0.20 (0.13 to 0.26) | 0.10 (.004 to 0.19) | 0.10 (-0.14 to 0.21) | 0.08 |
SD = standard deviation; SF-12 = 12-item Short Form Health Survey (0–100); PCS = Physical Component Scale; MCS = Mental Health Component Scale; TUG = Timed Up and GO; m/s = meters/second
Group differences in general physical health was statistically significant at 3-month follow-up and in mental health at post-treatment and 3-month follow-up (p < 0.05; Table 4). The change scores for the SF-12 PCS of 6.6 and 13.4 and SF-12 MCS of 7.4 and 12.5 at 3 and 6 months after surgery in the CBPT group, respectively, were above the MCID value of 4.9-points. Physical and mental health effects sizes were 0.75 and 1.35, respectively.
Multivariable analyses found that SF-12 PCS and MCS scores were 6.4 points higher (95% CI, 2.3 to 10.6; p = 0.003) and 8.6 points higher (95% CI, 4.5 to 12.7; p < 0.001), respectively, in the CBPT group compared to the Education group indicating better overall health for CBPT participants at 3-month follow-up. The regression models accounted for 59% and 35% of the variance for physical and mental health.
Secondary Outcomes: Performance-Based Tests
Average physical performance outcome scores for the CBPT group demonstrated an improvement in performance-based function over time (Table 4). The Education group scores for 10-meter walk improved; however, the average time it took participants to complete the 5-chair stand and TUG tests increased from the pre-treatment to the post-treatment time-point.
The CBPT group had a greater mean improvement in the 5-Chair Stand and TUG tests test at 3-month follow-up and post-treatment, respectively (p < 0.05; Table 4). Change in seconds for the 5-Chair Stand and TUG tests were clinically significant with values greater than the MCID of 2.3 and 1.4, respectively. Effect sizes for the performance-based tests ranged from 0.41 to 0.49, with the largest effect found for the 5-Chair Stand test.
In multivariable analyses, CBPT participants had greater improvement in performance-based tests scores than the Education group at 3 month follow-up, with scores 4.3 seconds lower (95% CI: -7.7 to -0.82; p = 0.02) for the 5-Chair Stand, 1.8 seconds lower (95% CI: -3.2 to -0.16; p = 0.02) for the TUG, and 0.09 meters/second higher (95% CI: -0.008 to 0.18; p = 0.07) for the 10-Meter Walk test. Treatment effects were significant for the 5-Chair Stand and TUG tests. The regression models accounted for 52% of the variance for 5-Chair Stand, 62% of the variance for TUG, and 33% of the variance for 10-Meter Walk.
Psychosocial Characteristics
Average psychosocial scores for the CBPT and Education group demonstrated an improvement in fear of movement and pain self-efficacy over time (Table 5).
Table 5.
Psychosocial scores and change from pre-treatment to post-treatment and 3 month follow-up by group
| Variable | CBPT Mean (SD) | Education Mean (SD) | Mean Change from Pre-Treatment | Between-Group Difference | ||
|---|---|---|---|---|---|---|
|
| ||||||
| CBPT | Education | CBPT vs. Education | P Value | |||
| TSK Score | ||||||
| Pre-Treatment | 40.2 (7.3) | 38.7 (6.5) | ||||
| Post-Treatment | 37.5 (7.0) | 36.3 (7.2) | 2.6 (-4 to -1.2) | -2.2 (-3.9 to -0.48) | -0.4 (-2.6 to 1.8) | 0.70 |
| 3 Month | 33.9 (8.1) | 36.2 (8.4) | -5.9 (-7.7 to -4.2) | -2.3 (-4.4 to -0.16) | -3.6 (-6.3 to -0.93) | 0.009 |
| PSEQ Score | ||||||
| Pre-Treatment | 36.0 (16.8) | 41.5 (15.2) | ||||
| Post-Treatment | 42.7 (17.3) | 44.1 (16.5) | 6.5 (3.8 to 9.3) | 2.6 (-1.4 to 6.6) | 3.9 (-0.86 to 8.7) | 0.11 |
| 3 Month | 48.8 (14.3) | 43.5 (16.3) | 11.9 (8.7 to 15.1) | 1.9 (-2.2 to 6) | 10.0 (4.8 to 15.1) | <0.001 |
SD = standard deviation; TSK = Tampa Scale for Kinesiophobia (17–68); PSEQ = Pain Self-Efficacy Questionnaire (0–60)
Group differences in fear of movement and pain self-efficacy were statistically significant at 3-month follow-up (p < 0.05), but not post-treatment (Table 4). The 5.9-point decrease from pre-treatment to 3-month follow-up for the TSK was greater than the MCID of 4-points. The effect size for the TSK and PSEQ were 0.59 and 0.85, respectively.
Discussion
This trial was conducted to determine whether a CBPT program would lead to greater improvement in postoperative outcomes compared to an Education program in patients with chronic pain undergoing lumbar spine surgery for degenerative conditions. A targeted CBT-based rehabilitation approach decreased fear of movement and increased self-efficacy as well as improved patient-reported and performance-based outcomes at 6 months after surgery.
CBPT participants demonstrated greater improvement in back and leg pain and pain interference with activity. Change scores in the CBPT group between an early postoperative time point and 6 months after surgery are consistent with studies testing a psychologist-delivered CBT program (60-min sessions twice a week for 8 weeks) and a group behavioral physical therapy intervention (90 min sessions; 3 times over 8 weeks) in patients recovering from surgery for a degenerative condition.11,56 The minimal changes in back and leg pain for our Education program are similar to findings in trials examining traditional postoperative physical therapy and prospective cohort studies.1,8,39 Group differences at 3 month follow-up also support work by Abbott et al.3 that found a greater improvement in back pain after lumbar fusion with a 3 session psychomotor therapy program compared to exercise training.
Additional research is needed to determine whether larger and clinically meaningful improvements in pain can be obtained through a CBT-based approach. Improvement in back and leg intensity was statistically significant in our study but not clinically meaningful which may be due to the relatively low pain scores following surgery. The average back and leg pain scores pre-treatment (6 weeks after surgery) was 2.9 and 2.4, respectively. A more time-intensive or in-person CBPT program may be needed to achieve MCID and substantial clinical benefit thresholds ranging from 1.2 to 2.5-point net improvement.25
The hypothesis that CBPT participants would have greater improvement in disability and general health was supported. Disability and general health improvement in the CBPT group at 3 month follow-up was both statistically and clinically significant based on published MCID values. Our disability findings are consistent with trials by Abbott et al.3 and Monticone et al.56 comparing psychomotor therapy and CBT, respectively, to exercise training in patients after lumbar fusion. However, Abbott et al.3 did not find a significant difference in general health at 6-months after surgery, which may be accounted for by the short duration of the program (3 sessions) or early initiation (first 3 months after surgery). The large and clinically relevant changes noted in our study for disability and general physical and mental health may be due to the CBPT intervention’s focus on decreasing barriers to functional activity and walking rather than focusing solely on resolution of pain symptoms.
Significant differences between the CBPT and Education groups were found at 3-month follow-up, but not post-treatment for the pain, disability and physical health outcomes. The non-significant findings post-treatment may be due to the rapid improvement in pain and disability that occurs during the initial 3- month postoperative period, regardless of the postoperative management strategy.46,51,60 Another explanation may be that additional time is needed for patients to practice the cognitive and behavioral strategies presented in the CBPT program in order for improvements in pain and disability to occur.
Overall, these findings suggest that the CBPT program has the potential to be more effective for improving patients-reported outcomes than education and traditional clinic-based rehabilitation. Moderate to large effect sizes were found for the CBPT program during the postoperative recovery period (i.e., pre-treatment measure was 6 weeks after surgery). It is important to note that prior studies have used preoperative scores as the baseline measure; thus, may have overestimated treatment effects by capitalizing on the benefits of surgery. This study makes an important contribution by documenting that a CBT-based approach has the potential to make significant and clinically meaningful differences in outcomes beyond improvements that can be attributed to surgery.
The hypothesis regarding the performance-based tests was partially supported. Multivariable regression analyses found greater improvement in the 5-Chair Stand and TUG scores for CBPT participants at 3 month follow-up but not for the 10-Meter Walk. The high prevalence of comorbidities or fear of falling in this patient population may negatively affect gait speed to a greater extent than strength and mobility. Additional follow-up time may also be needed to detect meaningful change in 10-Meter Walk scores. The CBPT program produced a mean change in seconds for the 5-Chair Stand and TUG tests that can be considered clinically meaningful based on MCID values from previous studies.54.91 To date, this is the first study to assess the effects of a CBT-based intervention on physical performance in patients following spine surgery.
Clinical Implications
Research supports a comprehensive, biopsychosocial approach to postoperative spine management.59,70 Brief and telephone-administered CBT has been shown to improve pain and function in patients with chronic pain.42,43,75,81,86 However, rehabilitation in surgical populations has not traditionally focused on CBT. This study along with others supports the use of CBT-based interventions for patients having surgery for chronic musculoskeletal conditions.3,7,11,56,66
This study also has clinical implications with regard to targeted rehabilitation interventions. Findings are consistent with work in low back pain and whiplash populations that have found improvement in pain and disability following programs that specifically target the psychosocial variables contributing to poor outcomes.23,71 The literature recommends identifying the variables that mediate the effects of CBT in order to provide more effective and clinically relevant treatments.20
The CBPT program broadens the availability of effective CBT strategies by expanding the implementation from traditional providers (i.e., psychologists) to physical therapists. Several studies have reported on the benefits of CBT-based interventions when delivered by dental hygienists and nurses.15,19,76 Our work and that of others demonstrates that physical therapists can learn and successfully implement the cognitive-behavioral techniques needed to make meaningful differences in pain-related outcomes.7,23,30,37,71
Limitations
This study is limited by incomplete follow-up at 3 months after treatment, especially for the performance-based tests. However, this concern is minimized by our finding that there were no significant differences on baseline characteristics between patients with and without complete follow-up data. The sample size was underpowered to detect small to medium group differences in performance-based tests. It is important to consider clinical significance as well as statistical significance when interpreting these results. The Education group reported similar outcomes to those found in previous usual care arms and prospective cohort studies,2,8,39 but additional research is needed to determine the effectiveness of CBPT alone compared to usual care which typically consists of traditional clinic-based physical therapy. The treatments were delivered by a single physical therapist at a single center and to patients screened for high fear of movement, which limits the generalizability of our results. The long-term effectiveness of CBPT following spine surgery remains to be determined. Studies on CBT-based interventions following spine surgery have found inconsistent results. Monticone et al.56 reported positive findings at 1-year for a CBT program focusing on fear of movement and pain catastrophizing, while Abbott et al.3 and Christensen et al.11 found no group differences in back pain at 1 and 2 years following lumbar fusion. Finally, additional research is needed to determine the optimal time for delivery (i.e, addition of sessions preoperatively and/or immediately after surgery) and whether in-person administration would strengthen the effect of the CBPT program.
Conclusion
This randomized trial demonstrates that screening patients for fear of movement and using a targeted CBPT program results in significant and clinically meaningful improvement in pain, disability, general health, and physical performance after spine surgery for degenerative conditions. The CBPT program, delivered by physical therapists over the telephone, has the potential to be an evidence-based program that clinicians can recommend for patients at-risk for poor postoperative outcomes.
Perspective.
This study investigated a targeted cognitive-behavioral based physical therapy program for patients after lumbar spine surgery. Findings lend support to the hypothesis that incorporating cognitive-behavioral strategies into postoperative physical therapy may address psychosocial risk factors and improve pain, disability, general health, and physical performance outcomes.
Highlights.
Cognitive-behavioral based physical therapy (CBPT) compared to education after spine surgery.
CBPT participants had lower pain and disability at 6 months after surgery.
CBPT participants had higher physical and mental health at 6 months after surgery.
CBPT participants had better physical performance at 6 months after surgery.
An alternative approach to physical therapy may be warranted after spine surgery.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R21AR062880 and the Magistro Family Foundation grant through the Foundation for Physical Therapy. This study used REDCap as the secure database which was supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences.
Footnotes
The authors declare no conflict of interest in the preparation of this manuscript.
Disclosures: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R21AR062880 and the Magistro Family Foundation grant through the Foundation for Physical Therapy. This study used REDCap as the secure database which was supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kristin R. Archer, Email: kristin.archer@vanderbilt.edu, Department of Orthopaedic Surgery, Department of Physical Medicine and Rehabilitation, Vanderbilt University Medical Center, Nashville, TN.
Clinton J. Devin, Email: clinton.j.devin@vanderbilt.edu, Department of Orthopaedic Surgery, Vanderbilt University Medical Center, Nashville, TN.
Susan W. Vanston, Email: susan.w.vanston@vanderbilt.edu, Department of Orthopaedic Surgery, Vanderbilt University Medical Center, Nashville, TN.
Tatsuki Koyama, Email: tatsuki.koyama@vanderbilt.edu, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, TN.
Sharon Phillips, Email: Sharon.phillips@vanderbilt.edu, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, TN.
Steven Z. George, Email: szgeorge@phhp.ufl.edu, Department of Physical Therapy, University of Florida.
Matthew J. McGirt, Email: matt.mcgirt@cnsa.com, Carolina Neurosurgery and Spine Associates, Charlotte, NC.
Dan M. Spengler, Email: dan.m.spengler@vanderbilt.edu, Department of Orthopaedic Surgery, Vanderbilt University Medical Center, Nashville, TN.
Oran S. Aaronson, Email: oran.aaronson@vanderbilt.edu, Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN.
Joseph S. Cheng, Email: joseph.cheng@vanderbilt.edu, Department of Neurological Surgery, Vanderbilt University Medical Center, Nashville, TN.
Stephen T. Wegener, Email: swegener@jhmi.edu, Department of Physical Medicine and Rehabilitation, Johns Hopkins Medicine, Baltimore, MD.
References
- 1.Aalto TJ, Leinonen V, Herno A, Alen M, Kröger H, Turunen V, Savolainen S, Saari T, Airaksinen O. Postoperative rehabilitation does not improve functional outcome in lumbar spinal stenosis: a prospective study with 2-year postoperative follow-up. Eur Spine J. 2011;20:1331–1340. doi: 10.1007/s00586-011-1781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbas J, Hamoud K, May H, Peled N, Sarig R, Stein D, Alperovitch-Najemson D, Hershkovitz I. Socioeconomic and physical characteristics of individuals with degenerative lumbar spinal stenosis. Spine. 2013;38:E554–561. doi: 10.1097/BRS.0b013e31828a2846. [DOI] [PubMed] [Google Scholar]
- 3.Abbott AD, Tyni-Lenné R, Hedlund R. Early rehabilitation targeting cognition, behavior, and motor function after lumbar fusion: a randomized controlled trial. Spine. 2010;35:848–857. doi: 10.1097/BRS.0b013e3181d1049f. [DOI] [PubMed] [Google Scholar]
- 4.Anderson T, Bunger C, Sogaard R. Long-term health care utilization and costs of spinal fusion in elderly patients. Eur Spine J. 2013;22:977–984. doi: 10.1007/s00586-012-2479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer KR, Wegener ST, Seebach C, Song Y, Skolasky RL, Thornton C, Khanna AJ, Riley LH., 3rd The Effect of Fear of Movement Beliefs on Pain and Disability after Surgery for Lumbar and Cervical Degenerative Conditions. Spine. 2011;36:1554–1562. doi: 10.1097/BRS.0b013e3181f8c6f4. [DOI] [PubMed] [Google Scholar]
- 6.Archer KR, Phelps KD, Seebach CL, Song Y, Riley LH, 3rd, Wegener ST. Comparative Study of Short Forms of the Tampa Scale for Kinesiophobia: Fear of Movement in a Surgical Spine Population. Arch Phys Med Rehabil. 2012;93:1460–1462. doi: 10.1016/j.apmr.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Archer KR, Motzny N, Abraham CM, Yaffe D, Seebach CL, Devin CJ, Spengler DM, McGirt MJ, Aaronson OS, Cheng JS, Wegener ST. Cognitive-behavioral based physical therapy to improve surgical spine outcomes: a case series. Phys Ther. 2013;83:1140–1149. doi: 10.2522/ptj.20120426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archer KR, Seebach CL, Mathis S, Riley LH, Wegener ST. Early Postoperative Fear of Movement Predicts Pain, Disability, and Physical Health 6 Months after Spinal Surgery for Degenerative Conditions. Spine J. 2014;14:759–767. doi: 10.1016/j.spinee.2013.06.087. [DOI] [PubMed] [Google Scholar]
- 9.Atlas SJ, Keller RB, Wu YA, Deyo RA, Sinder DE. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the maine lumbar spine study. Spine. 2005;30:936–943. doi: 10.1097/01.brs.0000158953.57966.c0. [DOI] [PubMed] [Google Scholar]
- 10.Brooks D, Davis AM, Naglie G. Validity of 3 physical performance measures in inpatient geriatric rehabilitation. Arch Phys Med Rehabil. 2006;87:105–110. doi: 10.1016/j.apmr.2005.08.109. [DOI] [PubMed] [Google Scholar]
- 11.Christensen FB, Laurberg I, Bünger CE. Importance of the back-café concept to rehabilitation after lumbar spinal fusion: a randomized clinical study with a 2-year follow-up. Spine. 2003;28:2561–2569. doi: 10.1097/01.BRS.0000097890.96524.A1. [DOI] [PubMed] [Google Scholar]
- 12.Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 13.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale: Lawrence Erlbaum; 1988. [Google Scholar]
- 14.Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008;8:968–974. doi: 10.1016/j.spinee.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Dalton JA, Keefe FJ, Carlson J, Youngblood R. Tailoring cognitive-behavioral treatment for cancer pain. Pain Manag Nurs. 2004;5:3–18. doi: 10.1016/s1524-9042(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 16.Davidson M, Keating JL. A comparison of five low back disability questionnaires: reliability and responsiveness. Phys Ther. 2002;82:8–24. doi: 10.1093/ptj/82.1.8. [DOI] [PubMed] [Google Scholar]
- 17.den Boer JJ, Oostendorp R, Beems T, Munneke M, Evers A. Continued disability and pain after lumbar disc surgery. Pain. 2006;123:45–52. doi: 10.1016/j.pain.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. J Am Med Assoc. 2010;303:1259–1265. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dworkin SF, Huggins KH, Wilson L, Mancl L, Turner J, Massoth D, LeResche L, Truelove E. A randomized clinical trial using research diagnostic criteria for temporomandibular disorders-Axis II to target clinic cases for a tailored self-care TMD treatment program. J Orofac Pain. 2002;16:48–63. [PubMed] [Google Scholar]
- 20.Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol. 2014;69:152–166. doi: 10.1037/a0035747. [DOI] [PubMed] [Google Scholar]
- 21.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 22.French DJ, France CR, Vigneau F, French JA, Evans RT. Fear of movement/(re)injury in chronic pain: a psychometric assessment of the original English version of the Tampa scale for kinesiophobia (TSK) Pain. 2007;127:42–51. doi: 10.1016/j.pain.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 23.George SZ, Fritz JM, Bialosky JE, Donald DA. The effect of a fear-avoidance-based physical therapy intervention for patients with acute low back pain: results of a randomized clinical trial. Spine. 2003;28:2551–2560. doi: 10.1097/01.BRS.0000096677.84605.A2. [DOI] [PubMed] [Google Scholar]
- 24.Gibson JNA, Waddell G. Surgery for degenerative lumbar spondylosis. Cochrane Database of Systematic Reviews. 2005;(4):Art. No.: CD001352. doi: 10.1002/14651858.CD001352.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glassman SD, Copay AG, Berven SH, Polly DW, Subach BR, Carreon LY. Defining substantial clinical benefit following lumbar spine arthrodesis. J Bone Joint Surg Am. 2008;90:1839–1847. doi: 10.2106/JBJS.G.01095. [DOI] [PubMed] [Google Scholar]
- 26.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 27.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55:1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasenbring MI, Plaas H, Fischbein B, Willburger R. The relationship between activity and pain in patients 6 months after lumbar disc surgery: Do pain-related coping modes act as moderator variables? Eur J Pain. 2006;10:701–709. doi: 10.1016/j.ejpain.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Hay E, Lewis M, Vohara K, Main CJ, Watson P, Dziedzic KS, Sim J, Minns Lowe C, Croft PR. Comparison of physical treatments versus a brief pain- management programme for back pain in primary care: a randomized clinical trial in physiotherapy practice. Lancet. 2005;365:2024–2030. doi: 10.1016/S0140-6736(05)66696-2. [DOI] [PubMed] [Google Scholar]
- 31.Issak PS, Cunningham ME, Pumberger M, Hughes AP, Cammisa FP. Degenerative lumbar spinal stenosis: evaluation and management. J Am Acad Orthop Surg. 2012;20:527–535. doi: 10.5435/JAAOS-20-08-527. [DOI] [PubMed] [Google Scholar]
- 32.Jansson K, Németh G, Granath F, Jönsson B, Blomqvist P. Health-related quality of life (EQ-5D) before and one year after surgery for lumbar spinal stenosis. J Bone J Surg B. 2009;91:210–216. doi: 10.1302/0301-620X.91B2.21119. [DOI] [PubMed] [Google Scholar]
- 33.Jenkinson C, Layte R, Jenkinson D, Lawrence K, Petersen S, Paice C, Stradling J. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179–186. doi: 10.1093/oxfordjournals.pubmed.a024606. [DOI] [PubMed] [Google Scholar]
- 34.Kalichman L, Cole R, Kim Dh, Li L, Suri P, Guermazi A, Hunter DJ. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 1990;9:545–550. doi: 10.1016/j.spinee.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalichman L, Kim Dh, Li L, Guermazi A, Hunter DJ. Computed tomography-evaluated features of spinal degeneration: prevalence, intercorrelation, and association with self-reported low back pain. Spine J. 2010;10:200–208. doi: 10.1016/j.spinee.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Klaber Moffett JA, Jackson DA, Richmond S, Hahn S, Coulton S, Farrin A, Manca A, Torgerson DJ. Randomized trial of brief physiotherapy intervention compared with usual physiotherapy for neck pain patients: outcomes and patients’ preference. BMJ. 2005;330:75–80. doi: 10.1136/bmj.38286.493206.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kori SH, Miller RP, Todd DD. Kinesiophobia: a new region of chronic pain behavior. Pain Manag. 1990;3:35–43. [Google Scholar]
- 39.Kreiner DS, Shaffer WO, Baisden JL, Gilbert TJ, Summers JT, Toton JF, Hwang SW, Mendel RC, Reitman CA. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J. 2013;13:734–743. doi: 10.1016/j.spinee.2012.11.059. [DOI] [PubMed] [Google Scholar]
- 40.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroenke K, Spitzer RL, Williams JB, Lowe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Litt MD, Shafer DM, Kreutzer DL. Brief cognitive-behavioral treatment for TMD pain: long-term outcomes and moderators of treatment. Pain. 2010;151:110–116. doi: 10.1016/j.pain.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linton SJ, Andersson T. Can chronic disability be prevented? A randomized trial of a cognitive behavioral intervention and two forms of information for patients with spinal pain. Spine. 2000;25:2825–2831. doi: 10.1097/00007632-200011010-00017. [DOI] [PubMed] [Google Scholar]
- 44.Lorig KR, Ritter P, Stewart AL, Sobel DS, Brown BW, Jr, Bandura A, Gonzalez VM, Laurent DD, Holman HR. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39:1217–1223. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Lurie JD, Tosteson TD, Tosteson A, Abdu WA, Zhao W, Morgan TS, Weinstein JN. Long-term Outcomes of Lumbar Spinal Stenosis: Eight-Year Results of the Spine Patient Outcomes ResearchTrial (SPORT) Spine. 2015;40:63–76. doi: 10.1097/BRS.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mannion AF, Denzler R, Dvorak J, Muntener M, Grob D. A randomized controlled trial of post-operative rehabilitation after surgical compression of the lumbar spine. Eur Spine J. 2007;16:1101–1107. doi: 10.1007/s00586-007-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mannion AF, Elfering A, Staerkle R, Junge A, Grob D, Dvorak J, Jacobshagen N, Semmer NK, Boos N. Predictors of multidimensional outcome after spinal surgery. Eur Spine J. 2007;16:777–786. doi: 10.1007/s00586-006-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mannion AF, Denzler R, Dvorak J, Grob D. Five-year outcome of surgical decompression of the lumbar spine without fusion. Eur Spine J. 2010;19:1883–1891. doi: 10.1007/s00586-010-1535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marks R. Reliability and validity of selfpaced walking time measures for knee osteoarthritis. Arthritis Care Res. 1994;7:50–53. doi: 10.1002/art.1790070111. [DOI] [PubMed] [Google Scholar]
- 50.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, Sullivan SD. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 51.McGregor AH, Dore CJ, Morris TP, Morris S, Jamrozik K. Function after spinal treatment, exercise, and rehabilitation (FASTER) Spine. 2011;36:1711–1720. doi: 10.1097/BRS.0b013e318214e3e6. [DOI] [PubMed] [Google Scholar]
- 52.McGregor AH, Probyn K, Cro S, Doré CJ, Burton AK, Balagué F, Pincus T, Fairbank J. A Cochrane review. Rehabilitation following surgery for lumbar spinal stenosis. Spine. 2014;39:1044–1054. doi: 10.1097/BRS.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 53.Mendoza TR, Chen C, Brugger A, Hubbard R, Snabes M, Palmer SN, Zhang Q, Cleeland CS. The utility and validity of the modified brief pain inventory in a multiple-dose postoperative analgesic trial. Clin J Pain. 2004;20:357–362. doi: 10.1097/00002508-200409000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Meretta BM, Whitney SL, Marchetti GF, Sparto PJ, Muirhead RJ. The five times sit to stand test: responsiveness to change and concurrent validity in adults undergoing vestibular rehabilitation. J Vestib Res. 2006;16: 233–243. [PubMed] [Google Scholar]
- 55.Miles CL, Pincus T, Carnes D, Taylor SJ, Underwood M. Measuring pain self-efficacy. Clin J Pain. 2011;27:461–470. doi: 10.1097/AJP.0b013e318208c8a2. [DOI] [PubMed] [Google Scholar]
- 56.Monticone M, Ferrante S, Teli M, Rocca B, Foti C, Lovi A, Brayda Bruno M. Management of catastrophising and kinesiophobia improves rehabilitation after fusion for lumbar spondylolisthesis and stenosis. A randomised controlled trial. Eur Spine J. 2014;23:87–95. doi: 10.1007/s00586-013-2889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nederhand MJ, Ijzerman M, Hermens HJ, Turk DC, Zilvold G. Predictive value of fear avoidance in developing chronic neck pain disability: consequences in clinical decision making. Arch Phys Med Rehabil. 2004;85:496–501. doi: 10.1016/j.apmr.2003.06.019. [DOI] [PubMed] [Google Scholar]
- 58.Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain. 2007;11:153–163. doi: 10.1016/j.ejpain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 59.Ostelo RW, de Vet HC, Waddell G, Kerckhoffs MR, Leffers P, van Tulder M. Rehabilitation following first-time lumbar disc surgery: a systematic review within the framework of the cochrane collaboration. Spine. 2003;28:209–218. doi: 10.1097/01.BRS.0000042520.62951.28. [DOI] [PubMed] [Google Scholar]
- 60.Oestergaard LG, Nielsen CV, Bünger CE, Sogaard R, Fruensgaard S, Helmig P, Christensen FB. The effect of early initiation of rehabilitation after lumbar spinal fusion: a randomized clinical study. Spine. 2012;37:1803–1809. doi: 10.1097/BRS.0b013e31825a17ab. [DOI] [PubMed] [Google Scholar]
- 61.Parker SL, Adogwa O, Mendenhall SK, Shau DN, Anderson WN, Cheng JS, Devin CJ, McGirt MJ. Determination of minimum clinically important difference in pain, disability, and quality of life after extension of fusion for adjacent-segment disease. J Neurosurg Spine. 2012;16:61–67. doi: 10.3171/2011.8.SPINE1194. [DOI] [PubMed] [Google Scholar]
- 62.Parker SL, Adogwa O, Paul AR, Anderson WN, Aaronson O, Cheng JS, McGirt MJ. Utility of minimum clinically important difference in assessing pain, disability, and health state after transforaminal lumbar interbody fusion for degenerative lumbar spondylolisthesis. J Neurosurg Spine. 2011;14:598–604. doi: 10.3171/2010.12.SPINE10472. [DOI] [PubMed] [Google Scholar]
- 63.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 64.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 65.Pratt RK, Fairbank JC, Virr A. The reliability of the Shuttle Walking Test, the Swiss Spinal Stenosis Questionnaire, the Oxford Spinal Stenosis Score, and the Oswestry Disability Index in the assessment of patients with lumbar spinal stenosis. Spine. 2002;27:84–91. doi: 10.1097/00007632-200201010-00020. [DOI] [PubMed] [Google Scholar]
- 66.Riddle DL, Keefe FJ, Nay WT, McKee D, Attarian DE, Jensen MP. Pain coping skills training for patients with elevated pain catastrophizing who are scheduled for knee arthroplasty: a quasi-experimental study. Arch Phys Med Rehabil. 2011;92:859–865. doi: 10.1016/j.apmr.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roelofs J, Goubert L, Peters ML, Vlaeyen JW, Crombez G. The Tampa Scale for Kinesiophobia: further examination of psychometric properties in patients with chronic low back pain and fibromyalgia. Eur J Pain. 2004;8:495–502. doi: 10.1016/j.ejpain.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 68.Rosenthal JA. Qualitative descriptors of strength of association and effect size. J Soc Serv Res. 1996;21:37–59. [Google Scholar]
- 69.Seebach CL, Kirkhart M, Lating JM, Wegener ST, Song Y, Riley LH, 3rd, Archer KR. Examining the role of positive and negative affect in recovery from spine surgery. Pain. 2012;153:518–525. doi: 10.1016/j.pain.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 70.Soegaard R, Christensen FB, Lauerberg I, Bünger CE. Lumbar spinal fusion patients' demands to the primary health sector: evaluation of three rehabilitation protocols. A prospective randomized study. Eur Spine J. 2006;15:648–656. doi: 10.1007/s00586-005-0884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sullivan MJL, Adams H, Rhodenizer T, Stanish WD. A psychosocial risk factor-targeted intervention for the prevention of chronic pain and disability following whiplash injury. Phys Ther. 2006;86:8–18. doi: 10.1093/ptj/86.1.8. [DOI] [PubMed] [Google Scholar]
- 72.Szpalski M, Gunzburg R. Lumbar spinal stenosis in the elderly: an overview. Eur Spine J. 2013;12(Suppl 2):S170–175. doi: 10.1007/s00586-003-0612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP, Duncan PW. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. 2010;90:196–208. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turner JA, Mancl L, Aaron LA. Brief cognitive-behavioral therapy for temporomandibular disorder pain: effects on daily electronic outcome and process measures. Pain. 2005;117:377–387. doi: 10.1016/j.pain.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 75.Turner JA, Mancl L, Aaron LA. Short- and long-term efficacy of brief cognitive-behavioral therapy for patients with chronic temporomandibular disorder pain: a randomized controlled trial. Pain. 2006;121:181–194. doi: 10.1016/j.pain.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 76.Turner JA, Mancl L, Huggins KH, Sherman JJ, Lentz G, LeResche L. Targeting temporomandibular disorder pain treatment to hormonal fluctuations: A randomized clinical trial. Pain. 2011;152:2074–2084. doi: 10.1016/j.pain.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.United Nations DoEaSA. World Economics and Social Survey 2007 Development in an Aging World. New York: 2007. pp. 1–212. [Google Scholar]
- 78.van der Windt D, Hay E, Jellema P, Main C. Psychosocial interventions for low back pain in primary care: lessons learned from recent trials. Spine. 2008;33:81–89. doi: 10.1097/BRS.0b013e31815e39f9. [DOI] [PubMed] [Google Scholar]
- 79.Vlaeyen J, Kole-Snijders A, Boeren R, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- 80.Vlaeyen JW, de Jong J, Geilen M, Heits P, van Breukelen G. The treatment of fear of movement/ (re)injury in chronic low back pain: Further evidence on the effectiveness of exposure in vivo. Clin J Pain. 2002;18:251–261. doi: 10.1097/00002508-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 81.Von Korff M, Balderson BH, Saunders K, Miglioretti DL, Lin EH, Berry S, Moore JE, Turner JA. A trial of an activating intervention for chronic back pain in primary care and physical therapy settings. Pain. 2005;113:323–330. doi: 10.1016/j.pain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 82.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 83.Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T, Boden S, Hilibrand A, Goldberg H, Berven S, An H. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794–810. doi: 10.1056/NEJMoa0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weinstein JN, Tosteson TD, Lurie JD, Tosteson A, Blood E, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goodberg H, Berven S, An H. Surgical versus nonoperative treatment for lumbar spinal stenosis four year results of the spine patient outcomes research trial. Spine. 2010;35:1329–1338. doi: 10.1097/BRS.0b013e3181e0f04d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wideman TH, Adams H, Sullivan MJL. A prospective sequential analysis of the fear-avoidance model of pain. Pain. 2009;145:45–51. doi: 10.1016/j.pain.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 86.Williams DA, Cary M, Groner K, Chaplin W, Glazer L, Rodriguez A, Clauw D. Improving physical functional status in patients with fibromyalgia: A brief cognitive-behavioral intervention. J Rheumatol. 2002;29:1280–1286. [PubMed] [Google Scholar]
- 87.Williams AC, McCracken LM. Cognitive-behavioral therapy for chronic pain: an overview with specific references to fear and avoidance. In: Asmundson GG, Vlaeyen JW, Crombez G, editors. Understanding and Treating Fear of Pain. London: Oxford University Press; 2004. pp. 293–312. [Google Scholar]
- 88.Williams P. Decision making in surgical treatment of chronic low back pain: the performance of prognostic tests to select patients for lumbar spinal fusion. Acta Orthop Suppl. 2013;84:1–35. doi: 10.3109/17453674.2012.753565. [DOI] [PubMed] [Google Scholar]
- 89.Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117:137–144. doi: 10.1016/j.pain.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 90.Woods MP, Asmundson GJ. Evaluating the efficacy of graded in vivo exposure for the treatment of fear in patients with chronic back pain: a randomized controlled clinical trial. Pain. 2008;136:271–280. doi: 10.1016/j.pain.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 91.Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther. 2011;41:319–327. doi: 10.2519/jospt.2011.3515. [DOI] [PubMed] [Google Scholar]
- 92.Zalon ML. Comparison of pain measures in surgical patients. J Nurs Meas. 1999;7:135–152. [PubMed] [Google Scholar]