The present study has demonstrated that a high human epidermal growth factor receptor 2-related fluorescence in situ hybridization ratio is a significant predictive factor for a pathologic complete response and recurrence-free survival, with a favorable tendency to predict overall survival in patients with locally advanced breast cancer receiving neoadjuvant systemic treatment with trastuzumab.
Keywords: HER2+ breast cancer, HER2/centromeric probe for chromosome 17 fluorescence in situ hybridization ratio, Trastuzumab, Predictive factor, Pathologic complete response
Abstract
Background.
The present study was performed to determine whether the human epidermal growth factor receptor-related 2 (HER2)/centromeric probe for chromosome 17 fluorescence in situ hybridization (FISH) ratio is a predictor of a pathologic complete response (pCR), recurrence-free survival (RFS), and/or overall survival (OS) in patients receiving neoadjuvant systemic treatment (NST) with trastuzumab (NST-T) for HER2+ locally advanced breast cancer (LABC).
Patients and Methods.
The present retrospective study included 555 patients with HER2+ LABC who had undergone NST and definitive surgery (1999–2012); 373 had concurrently received trastuzumab. HER2-positivity was considered present with an immunohistochemical score of 3+ and/or HER2 FISH ratio of ≥2.0. We used logistic regression analysis and Cox proportional hazard modeling to determine whether a high HER2 FISH ratio, either as a continuous variable or with a cutoff of ≥7.0, would predict for pCR (no invasive disease in the breast and no tumor in the ipsilateral axillary lymph nodes), RFS, and/or OS.
Results.
The pCR group’s median HER2 FISH ratio was significantly higher than that of the non-pCR group (6.4 vs. 5.2; p = .003). The logistic regression model demonstrated that the independent predictors of pCR included a high HER2 FISH ratio as a continuous variable (p = .04). The multicovariate Cox proportional hazard model showed that a high HER2 FISH ratio (with a cutoff of ≥7.0 or as a continuous variable) was a significant prognostic indicator of longer RFS time (p = .047 and p = .04, respectively). Similarly, a high HER2 FISH ratio of ≥7.0 was associated with longer OS (p = .06).
Conclusion.
A high HER2 FISH ratio is a predictor of pCR in patients with HER2+ LABC who receive NST-T.
Implications for Practice:
This study demonstrated the optimal predictive and prognostic value of a HER2/centromeric probe for chromosome 17 FISH ratio for primary HER2+ breast cancer treated with trastuzumab combined with neoadjuvant systemic treatment (NST-T). This suggests that a high HER2 FISH ratio is a potential indicator for a high pathologic complete response rate and a better prognosis when patients are treated with NST-T.
Abstract
摘要
背景. 本研究的目的是, 在接受新辅助系统治疗 (NST) 联合曲妥珠单抗 (NST-T) 治疗的人表皮生长因子受体 2 (HER2) 阳性的局部晚期乳腺癌 (LABC) 患者中, 确定 HER2/17 号染色体着丝粒探针荧光原位杂交(FISH) 比值是病理学完全缓解 (pCR)、无复发生存 (RFS) 和/或总生存 (OS) 的预测因素。
患者和方法. 本项回顾性研究在 1999 年∼2012 年间共纳入 555 例接受 NST 和根治术的 HER2+ LABC 患者, 373 例接受同期曲妥珠单抗治疗。免疫组化评分为 3+和/或 HER2 FISH 比值≥ 2.0 可判定为 HER2 阳性。我们使用 logistic 回归分析和 Cox 比例风险模型确定高 HER2 FISH 比值 (作为连续变量或者采用临界值≥7.0) 可预测 pCR (乳腺无浸润性疾病且同侧腋窝淋巴结无肿瘤)、RFS 和/或 OS。
结果. pCR组的中位HER2 FISH比值显著高于未达到pCR的患者 (6.4 vs 5.2, P = 0.003)。logistic 回归模型证实, 当高 HER2 FISH 比值作为连续变量时是 pCR 的独立预测因素 (P = 0.04)。多变量 Cox 比例风险模型显示, 高 HER2 FISH 比值 (采用临界值≥7.0 或作为连续变量) 是 RFS 持续时间较长的显著预后因素 (P值分别为 0.047 和 0.04)。与此相似, 当采用临界值≥7.0 时, 高 HER2 FISH 比值也与 OS 时间较长相关 (P = 0.06)。
结论. 高HER2 FISH 比值是接受NST-T治疗的HER2+ LABC患者pCR的预测因素。The Oncologist 2016;21:21–27
对临床实践的提示: 本研究证实, 对于接受曲妥珠单抗联合新辅助系统治疗 (NST-T) 的原发性 HER2+乳腺癌患者, HER2/17 号染色体着丝粒探针 FISH 比值具有理想的预测和预后价值。这提示对于接受 NST-T 治疗的患者, 高 HER2 FISH 比值是高病理学完全缓解率的潜在指标, 这类患者具有更好的预后。
Introduction
The use of neoadjuvant systemic treatment (NST) for breast cancer has been well established; and the patient outcomes with NST have been equivalent to those with adjuvant chemotherapy [1]. NST has several benefits, such as eradicating distant micrometastases [2], facilitating more conservative breast surgery [3], and enabling the operability of initially inoperable locally advanced disease [4]. Compared with patients with other breast cancer subtypes, patients with the human epidermal growth factor receptor 2 (HER2)-positive breast cancer subtype have had substantial benefit—specifically, a better pathologic complete response (pCR) and better patient outcomes—from trastuzumab (Herceptin; Genentech Inc., South San Francisco, CA, http://www.gene.com) combined with NST (NST-T) [5]. Randomized controlled trials have shown that in HER2+ breast cancer patients, chemotherapy-only NST produced pCR rates of 19%–28%; however, NST-T demonstrated significantly better pCR rates of 26%–65% and significantly better survival outcomes [5, 6]. Furthermore, an anti-HER2 doublet treatment strategy has become a standard option for patients with HER2+ breast cancer.
A high HER2/centromeric probe for chromosome 17 fluorescence in situ hybridization (HER2 FISH) ratio has been found to be a significant predictor of overall clinical response [7] and progression-free survival [8] in patients with metastatic breast cancer treated with NST-T. However, whether the HER2 FISH ratio predicts the trastuzumab response is unknown in the neoadjuvant setting. A predictor of trastuzumab response using pCR could be a surrogate marker of better survival outcomes, and a predictor of pCR could be useful for anti-HER2 dual therapy with trastuzumab. We hypothesized that a high HER2 FISH ratio would be a predictor of a pCR, extended recurrence-free survival (RFS), and overall survival (OS) in patients with locally advanced breast cancer (LABC) receiving NST-T.
Our primary objective was to determine whether a high HER2 FISH ratio is a surrogate marker of pCR to NST-T. Our secondary objective was to determine whether a high HER2 FISH ratio was a prognostic marker of RFS and OS.
Patients and Methods
Patient Selection
The study was a retrospective single-center investigation (protocol number PA12-1173). The patients were identified from the Breast Cancer Management System database (protocol no. 2004-0541) at The University of Texas MD Anderson Cancer Center. Patients with histologically confirmed stage III invasive breast carcinoma who had received an NST-based regimen with or without trastuzumab were included. All patients had also undergone definitive breast surgery. Invasive tumors had been histologically confirmed by core needle biopsy and/or punch biopsy, as clinically indicated, and clinically suspicious lymph nodes had been investigated by fine needle aspiration. Inflammatory breast cancer (IBC) was diagnosed by strictly adhering to the consensus diagnostic criteria. The pathologic evidence of dermal lymphatic invasion was noted but was not required for diagnosis. An IBC diagnosis must have involved multidisciplinary specialists (medical oncologists, surgical oncologists, and radiation oncologists). All tumors had been HER2-amplified according to a HER2 FISH ratio of ≥2.0 and/or 3+ overexpression on immunohistochemical (IHC) analysis.
The institutional review board at MD Anderson approved the study and waived informed consent, because the study was a retrospective medical record review involving no diagnostic or therapeutic intervention. The candidate patients included those treated from January 1, 1999, when NST-T was first used at MD Anderson Cancer Center, to December 31, 2012.
Follow-up information for patients in the Breast Cancer Management System database was obtained every 2 years by a direct review of the medical records and linkage to the MD Anderson Tumor Registry, which mails annual follow-up letters to patients registered at MD Anderson to confirm that they are alive and free of cancer if the registry had not obtained a patient update annually through another source, such as a patient examination at MD Anderson Cancer Center. The MD Anderson Tumor Registry checks the Social Security Death Index and the Texas Bureau of Vital Statistics for the life status of patients who did not respond to the letters.
Patient and Tumor Characteristics
The variables recorded from the patient medical records and the database included the demographic data (e.g., age, ethnicity, menopausal status, body mass index), date of diagnosis, date of recurrence, death, or last follow-up visit, estrogen receptor (ER)/progesterone receptor (PR) status, HER2 test results (IHC/FISH ratio), histologic findings (histologic subtype, nuclear grade, lymphovascular invasion), disease stage, neoadjuvant/adjuvant chemotherapy types, surgery type, and treatment response (pCR/non-pCR). We reviewed the patients’ staging evaluation findings, which included a complete medical history and physical examination; chest radiography, liver ultrasonography or computed tomography (CT), and positron emission tomography (PET) or a bone scan before NST; and mammography and additional ultrasound assessment of the breast and axilla. The cardiac evaluation included a baseline echocardiogram or cardiac scan. Patients with a history of uncompensated congestive heart failure or a cardiac ejection fraction of less than 45% were never treated with trastuzumab.
Treatment
The cytotoxic agents administered as a part of NST during the study period included an anthracycline and/or a taxane given in combination or sequential regimens with or without trastuzumab.
Local Therapy
The definitive surgery was mastectomy (82.0%) or conservative surgery, such as segmental mastectomy (12.8%) or lumpectomy (0.2%). Skin-sparing mastectomy (5.0%) was tested only in a clinical trial. A total of 500 patients (90.1%) received adjuvant radiation therapy.
Pathologic Evaluation
Diagnostic biopsy and resected specimens were evaluated at the MD Anderson Cancer Center. Diagnostic biopsy revealed the tumor receptor status. The ER and PR status was evaluated with an IHC staining assay. ER- and PR-positive status was defined as ≥10% positivity. HER2+ status was defined as an IHC score of 3+ and/or a FISH ratio of ≥2.0. A pCR was defined as no invasive disease in the breast and no tumor in the ipsilateral axillary lymph nodes.
Statistical Analysis
The primary clinical endpoint was the association between a high HER2 FISH ratio and pCR. The secondary endpoint was the association between this ratio and RFS and OS. The risk factors of interest included age, menopausal status, tumor histologic features, lymphatic invasion, vascular invasion, tumor grade, HER2 FISH ratio, ER status, PR status, hormonal therapy, neoadjuvant trastuzumab therapy, adjuvant trastuzumab therapy, adjuvant radiation therapy, and adjuvant chemotherapy. When we assessed the predictive and prognostic values of the HER2 FISH ratio, we included both continuous variables and dichotomized variables. We selected 7.0 as the HER2 FISH ratio cutoff according to the Martingale residuals from the null model. This graphic method has been widely used in survival analyses, especially in the data analysis of an exploratory nature. The Martingale residual plot showed that the cutoff point was approximately 7.0 (supplemental online Fig. 1). Therefore, we believe using 7.0 as the cutoff point was reasonable, and this will be investigated further in future studies.
The data were first summarized with standard descriptive statistics and frequency tabulation. The associations between categorical variables were assessed via cross-tabulation and the chi-square test. The Wilcoxon rank-sum test was used to compare continuous variables such as age and the HER2 FISH ratio. RFS and OS were estimated using the Kaplan-Meier method, and comparisons of the characteristics of the patient groups were assessed using the log-rank test. A logistic regression model was applied to assess the effect of covariates on pCR. Multicovariate Cox proportional hazard models were used to determine the effect of covariates on RFS and OS assessed with neoadjuvant and adjuvant trastuzumab administration. All computations were performed using SAS, version 9.3 (SAS Institute Inc., Cary, NC, http://www.sas.com) and R, version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org).
Results
Patient Demographics
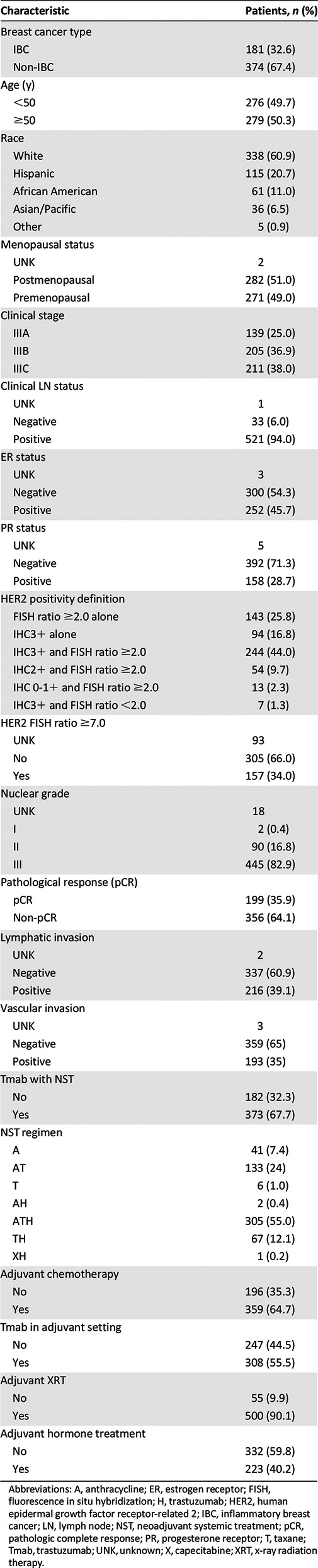
The median follow-up time for living patients was 3.4 years, and the median follow-up time, estimated using the reverse Kaplan-Meier method, was 4 years. We monitored 519 patients (93.5%) at our institution when we implemented the present study. The patient and tumor characteristics are listed in Table 1. All patients were candidates for NST-T; however, trastuzumab was not approved by the U.S. Food and Drug Administration for use in the neoadjuvant or adjuvant setting until November 2006. Trastuzumab was concurrently administered as neoadjuvant treatment to 373 patients (67.2%), and trastuzumab as a single agent or combined with systemic therapy was given as adjuvant treatment to 308 patients (55.5%). Most patients receiving NST received the anthracycline, taxane, and trastuzumab combination (305 patients [55.0%]).
Table 1.
Patient and tumor characteristics
Patient Characteristics
The median age at diagnosis was 51 years (range, 19–75) in the pCR group and 48.5 years (range, 21–83) in the non-pCR group (p = .22). The median body mass index at diagnosis was 28.8 kg/m2 (range, 13.5–77.1) in the pCR group and 28.3 kg/m2 (range, 18.4–59.5) in the non-pCR group (p = .60). The median HER2 FISH ratio was 6.4 (range, 1.39–16.44) in the pCR group and 5.19 (range, 1.17–24.3) in the non-pCR group (p = .003).
On univariate analyses, patient age ≥50 years (39.8% vs. 31.9%; p = .05), postmenopausal status (39.7% vs. 32.1%; p = .06), ER-negative status (41.3% vs. 29.0%; p = .003), PR-negative status (39.8% vs. 24.7%; p = .0008), and non-IBC (39.0% vs. 29.3%; p = .02) demonstrated borderline or significantly better pCR rates. The use of trastuzumab yielded significantly higher pCR rates when it was concomitantly administered with NST (40.5% vs. 26.4%; p = .001; supplemental online Table 1).
Association Between HER2 FISH Ratio and pCR
We acknowledge that pCR is a prognostic indicator, especially among those with hormone receptor (HR)-negative disease; however, we still do not know whether the HER2 FISH ratio can become a predictive or prognostic biomarker, regardless of HR status. Hence, we initially performed the present study, including both HR-positive and HR-negative cases. The HER2 FISH ratio as a continuous variable in the pCR group was significantly higher than that in the non-pCR group, and the pCR rates of the patients with a HER2 FISH ratio of ≥7.0 or <7.0 were 40.1% and 33.4%, respectively (p = .16).
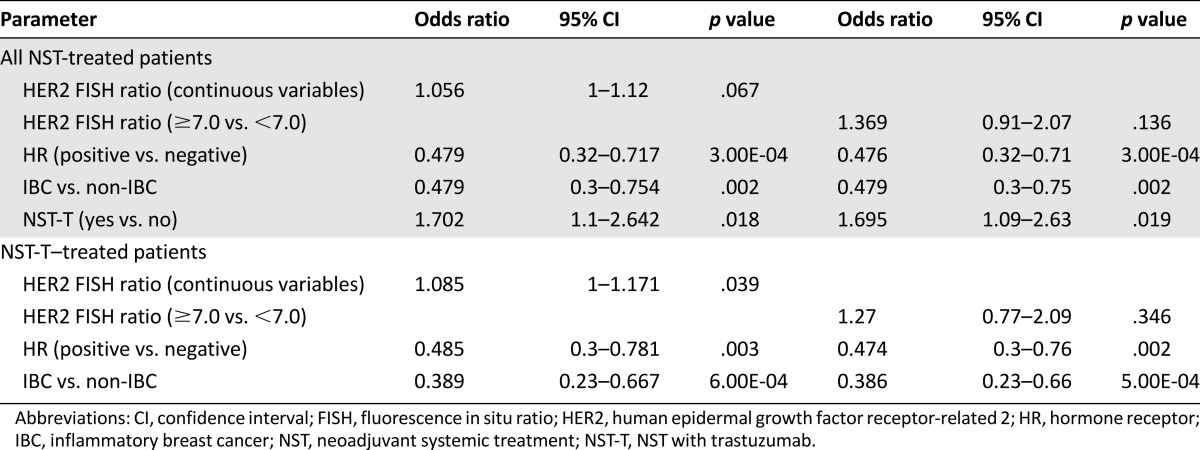
After adjustment for HR status and IBC status, a multicovariate logistic regression model revealed that the HER2 FISH ratio as a continuous variable was an independent predictor of pCR (p = .04) in patients who had received NST-T. However, after adjustment for HR, IBC status, and trastuzumab use, the model revealed only a trend for the HER2 FISH ratio as a continuous variable with which to predict pCR (p = .07) in all patient cohorts, including patients who had received only NST. Thus, a higher HER2 FISH ratio was an independent predictor of pCR in patients receiving NST-T rather than NST alone. Other factors such as HR-negative status and non-IBC remained independent predictors of pCR (Table 2). After adjustment for HR and IBC status, the model revealed that a HER2 FISH ratio of ≥7.0 was not an independent predictor of pCR (p = .35) in patients treated with NST-T. This dichotomized cutoff of the HER2 FISH ratio did not demonstrate significance, because this cutoff was determined for the extraction of better OS.
Table 2.
Logistic regression model for assessing independent predictors of pathologic complete response
Association Between HER2 FISH Ratio and Survival Outcome
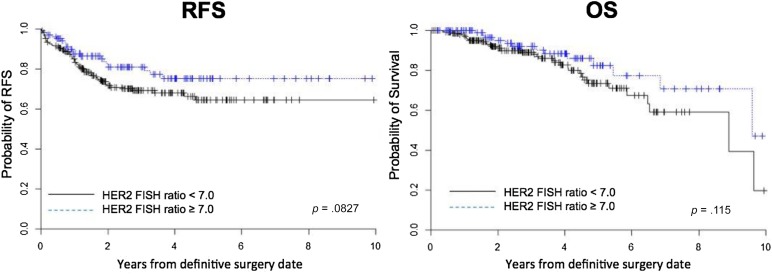
On univariate analysis, a log-rank test was used to compare the RFS and OS duration of patients grouped by certain characteristics. A dichotomized HER2 FISH ratio (≥7.0) was not associated with better RFS or OS. The 5-year RFS rate was 72.7% in the HER2 FISH ratio ≥7.0 group and 63.6% in the HER2 FISH ratio <7.0 group (supplemental online Table 2; p = .15). In addition, the 5-year OS rates were 80.2% in the HER2 FISH ratio ≥7.0 group and 73.7% in the HER2 FISH ratio <7.0 group (supplemental online Table 3; p = .12). Within the NST-T cohort, the HER2 FISH ratio ≥7.0 group had a marginally longer RFS (p = .08) but an even weaker benefit in OS (p = .12; Fig. 1).
Figure 1.
Kaplan-Meier curve for RFS and OS according to the HER2 FISH ratio (≥7.0 vs. <7.0) in the neoadjuvant systemic treatment with trastuzumab cohort.
Abbreviations: FISH, fluorescence in situ hybridization; OS, overall survival; RFS, recurrence-free survival.
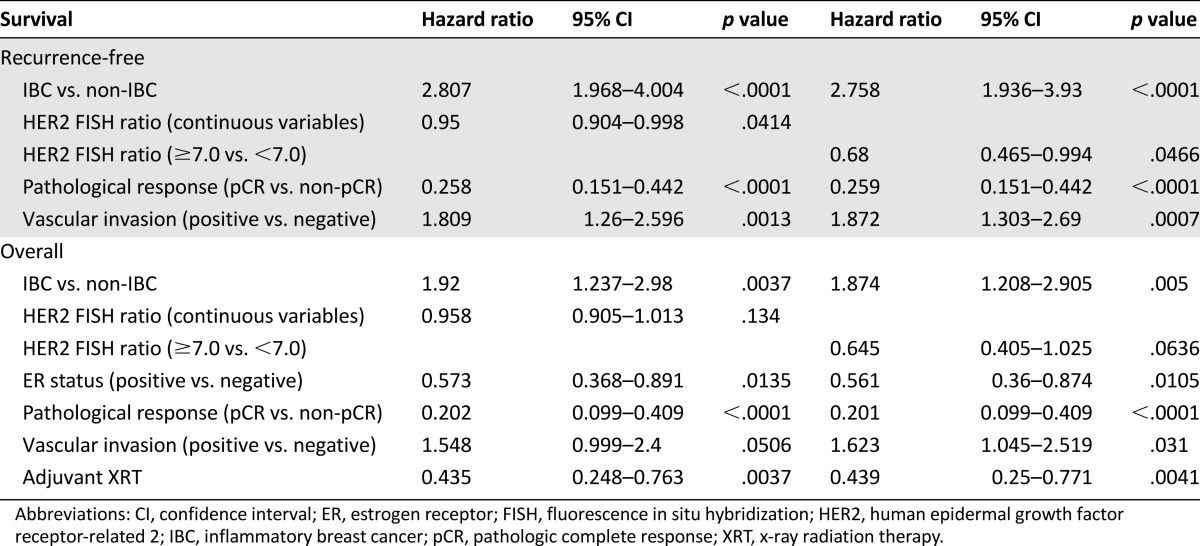
On multivariate analysis with a Cox regression model for RFS, the HER2 FISH ratio, as either a continuous variable or dichotomized variable (≥7.0), was significantly associated with better RFS (p = .04 and p = .047, respectively; Table 3) after adjustment for IBC, pCR status, vascular invasion, and NST-T.
Table 3.
Multivariate Cox regression model for recurrence-free survival and overall survival
A similar analysis showed a borderline association between a HER2 FISH ratio of ≥7.0 and longer OS (p = .06). However, the HER2 FISH ratio as a continuous variable was not a significant predictor of OS (p = .134; Table 3) after adjustment for other significant covariates such as IBC, ER status, pCR status, vascular invasion, adjuvant radiation therapy, and NST.
Discussion
We have demonstrated that a high HER2 FISH ratio as a continuous variable is a significant indicator of a higher pCR rate in LABC patients receiving NST-T. Furthermore, a high HER2 FISH ratio was an independent predictor of better RFS and showed a statistical trend as a prognostic indicator of OS when assessed with neoadjuvant and adjuvant trastuzumab administration. A subset analysis of patients receiving neoadjuvant chemotherapy revealed that a high HER2 FISH ratio was a significant predictive indicator of pCR in patients receiving NST-T. However, this was only a trend in all patients receiving NST with or without trastuzumab. Hence, these results suggest that a high HER2 FISH ratio might be a predictor of pCR in patients receiving a regimen that includes trastuzumab.
Our study has some similar aspects to that of the NOAH (neoadjuvant Herceptin) study [5, 9], which showed trastuzumab to be beneficial as a neoadjuvant treatment in patients with stage III HER2+ breast cancer, including IBC. The NOAH study also showed that trastuzumab improved the pCR rate in patients with LABC, including a substantial proportion (27.0%) with IBC. The results of our study were similar for the 32.6% of patients with IBC. Because we still do not know whether trastuzumab is similarly effective among IBC patients and non-IBC patients, this high proportion of IBC cases should be noted.
In previous studies that tested the predictors of trastuzumab response, HR-negative status was revealed to be a predictor of pCR but was also directly associated with poor survival outcomes [10–12]. Because we did not know whether the HER2 FISH ratio was a prognostic factor, we included HR-positive patients in the present study. Early prediction of pCR with the use of PET-CT [13] suggested that the imaging modality has predictive value in HER2+ breast cancer; however, repeated testing with PET-CT is burdensome to patients because of its high cost. Gene expression assays have also shown a possible predictive value in HER2+ breast cancer, although the test must be validated further and its practical usage determined [14].
The National Comprehensive Cancer Network guidelines have defined HER2+ breast cancer as an IHC score of 3+ and/or a FISH ratio of ≥2.0; however, in the present study, 20 patients had inconsistent results between the HER2 IHC scores and FISH ratios. A few retrospective studies have shown a high FISH ratio to be a predictor of the time to progression in patients with metastatic breast cancer [8]. However, the HERA (HERceptin Adjuvant) trial revealed that 1-year trastuzumab administration was a significantly better treatment option than was chemotherapy alone and that a high HER2 FISH ratio did not predict for better outcomes in the adjuvant setting [15]. Hence, we have speculated that a high HER2 FISH ratio is a predictor of the response when an ample tumor volume is present in the neoadjuvant setting. Furthermore, pCR is a strong surrogate marker of long-term survival, especially in patients with HR-negative disease [16], and we hypothesized that a high HER2 FISH ratio would be associated with a high pCR rate and better survival outcomes.
The novelty of the present study is that we adopted the HER2 FISH ratio as a possible predictive and prognostic biomarker of NST in patients with HER2+ LABC. We do not have a pragmatic predictive factor for HER2+ breast cancer that can be used in the clinical setting. However, we did develop a better treatment option: the anti-HER2 doublet treatment. The NeoSphere study (phase II) [17] and the TRYPHAENA study (phase II) [18] showed higher pCR rates with pertuzumab combined with trastuzumab-containing NST. Furthermore, the NEO-ALTTO study (phase III) [19] demonstrated lapatinib efficacy with higher pCR rates when combined with trastuzumab-containing NST. Anti-HER2 doublet treatment with trastuzumab and pertuzumab is currently allowed in the neoadjuvant setting, suggesting that a predictor of pCR with trastuzumab might be applicable for those receiving anti-HER2 doublet treatment.
The limitations of the present study included its retrospective nature and relatively short follow-up duration. Furthermore, pCR might not have been a prognostic indicator among the HR-positive cohort and our study might have demonstrated that a high HER2 FISH ratio is a predictor of pCR and is a prognostic indicator when assessed within an HR-negative cohort. However, we conducted exploratory testing to determine whether a high HER2 FISH ratio was possibly predictive and a prognostic factor of trastuzumab treatment, regardless of HR status. In addition, the present study included patients with more advanced disease and a higher proportion of IBC patients; hence, we need to demonstrate in further studies the association between higher HER2 FISH ratios and the clinical efficacy of a higher pCR rate and longer survival in IBC and non-IBC separately.
If a high HER2 FISH ratio is truly a predictor of better clinical outcomes in patients with HER2+ breast cancer, this biomarker provides justification for implementing a further neoadjuvant study with trastuzumab. Such future studies might identify pCR cases more precisely in advance and allow for the implementation of personalized medicine. In addition, our results could justify further development of a multiagent prospective study of various drugs combined with trastuzumab if the HER2 FISH ratio was moderately amplified.
Conclusion
Our findings have demonstrated that a high HER2 FISH ratio is a significant predictive factor for pCR and RFS and has a favorable tendency to predict OS in LABC patients receiving NST-T. Our results suggest that trastuzumab-containing regimens are effective for breast cancer with a high HER2 FISH ratio and thus that a biomarker-driven treatment strategy might be feasible among those with HER2+ breast cancer.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
Tamara K. Locke, Markeda L. Wade, and Joseph A. Munch from the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center provided scientific editing services. The present study was funded by a My Oncology Dream award from the Japan Cancer Society, the Morgan Welch Inflammatory Breast Cancer Research Program, and a grant from the State of Texas Rare and Aggressive Breast Cancer Research Program. Takahiro Kogawa is currently affiliated with the Department of Experimental Therapeutics/Breast Medical Oncology, National Cancer Center Hospital East, Chiba, Japan.
The abstract was presented at the 2013 European Cancer Congress Conference.
Author Contributions
Conception/design: Takahiro Kogawa, Tamer M. Fouad, Naoto T. Ueno
Provision of study material or patients: Naoto T. Ueno
Collection and/or assembly of data: Takahiro Kogawa, Tamer M. Fouad
Data analysis and interpretation: Takahiro Kogawa, Diane D. Liu, Jimin Wu, Yu Shen
Manuscript writing: Takahiro Kogawa, Tamer M. Fouad, Yu Shen, Hiroko Masuda, Takeo Fujii, Mariana Chavez-MacGregor, Ricardo H. Alvarez, Gabriel N. Hortobágyi, Vicente Valero, Naoto T. Ueno
Final approval of manuscript: Takahiro Kogawa, Tamer M. Fouad, Diane D. Liu, Jimin Wu, Yu Shen, Hiroko Masuda, Takeo Fujii, Mariana Chavez-MacGregor, Ricardo H. Alvarez, Gabriel N. Hortobágyi, Vicente Valero, Naoto T. Ueno
Disclosures
Mariana Chavez-MacGregor: Roche (C/A); Gabriel N. Hortobágyi: Antigen Express, Bayer, Metastat, Novartis Peregrine, Pfizer (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J Natl Cancer Inst. 2005;97:188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 2.Hortobagyi GN, Blumenschein GR, Spanos W, et al. Multimodal treatment of locoregionally advanced breast cancer. Cancer. 1983;51:763–768. doi: 10.1002/1097-0142(19830301)51:5<763::aid-cncr2820510502>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 4.Chia S, Swain SM, Byrd DR, et al. Locally advanced and inflammatory breast cancer. J Clin Oncol. 2008;26:786–790. doi: 10.1200/JCO.2008.15.0243. [DOI] [PubMed] [Google Scholar]
- 5.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): A randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 6.Pierga JY, Delaloge S, Espié M, et al. A multicenter randomized phase II study of sequential epirubicin/cyclophosphamide followed by docetaxel with or without celecoxib or trastuzumab according to HER2 status, as primary chemotherapy for localized invasive breast cancer patients. Breast Cancer Res Treat. 2010;122:429–437. doi: 10.1007/s10549-010-0939-3. [DOI] [PubMed] [Google Scholar]
- 7.Giuliani R, Durbecq V, Di Leo A, et al. Phosphorylated HER-2 tyrosine kinase and Her-2/neu gene amplification as predictive factors of response to trastuzumab in patients with HER-2 overexpressing metastatic breast cancer (MBC) Eur J Cancer. 2007;43:725–735. doi: 10.1016/j.ejca.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Han HS, Kim JS, Park JH, et al. Weekly paclitaxel and trastuzumab as a first-line therapy in patients with HER2-overexpressing metastatic breast cancer: Magnitude of HER2/neu amplification as a predictive factor for efficacy. J Korean Med Sci. 2009;24:910–917. doi: 10.3346/jkms.2009.24.5.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semiglazov V, Eiermann W, Zambetti M, et al. Surgery following neoadjuvant therapy in patients with HER2-positive locally advanced or inflammatory breast cancer participating in the NeOAdjuvant Herceptin (NOAH) study. Eur J Surg Oncol. 2011;37:856–863. doi: 10.1016/j.ejso.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Takada M, Ishiguro H, Nagai S, et al. Survival of HER2-positive primary breast cancer patients treated by neoadjuvant chemotherapy plus trastuzumab: A multicenter retrospective observational study (JBCRG-C03 study) Breast Cancer Res Treat. 2014;145:143–153. doi: 10.1007/s10549-014-2907-9. [DOI] [PubMed] [Google Scholar]
- 11.Alba E, Albanell J, de la Haba J, et al. Trastuzumab or lapatinib with standard chemotherapy for HER2-positive breast cancer: Results from the GEICAM/2006-14 trial. Br J Cancer. 2014;110:1139–1147. doi: 10.1038/bjc.2013.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: Results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humbert O, Cochet A, Riedinger JM, et al. HER2-positive breast cancer: 18F-FDG PET for early prediction of response to trastuzumab plus taxane-based neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41:1525–1533. doi: 10.1007/s00259-014-2739-1. [DOI] [PubMed] [Google Scholar]
- 14.Prat A, Bianchini G, Thomas M, et al. Research-based PAM50 subtype predictor identifies higher responses and improved survival outcomes in HER2-positive breast cancer in the NOAH study. Clin Cancer Res. 2014;20:511–521. doi: 10.1158/1078-0432.CCR-13-0239. [DOI] [PubMed] [Google Scholar]
- 15.Dowsett M, Procter M, McCaskill-Stevens W, et al. Disease-free survival according to degree of HER2 amplification for patients treated with adjuvant chemotherapy with or without 1 year of trastuzumab: The HERA trial. J Clin Oncol. 2009;27:2962–2969. doi: 10.1200/JCO.2008.19.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 17.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 18.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24:2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 19.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.