This study aimed to evaluate the efficacy and safety of once-per-cycle balugrastim versus pegfilgrastim for neutrophil support in breast cancer patients receiving myelosuppressive chemotherapy. Once-per-cycle balugrastim is not inferior to pegfilgrastim in reducing cycle 1 duration of severe neutropenia in breast cancer patients receiving chemotherapy. Both drugs have comparable safety profiles.
Keywords: Balugrastim, Breast cancer, Granulocyte colony-stimulating factor, Myelosuppressive therapy, Pegfilgrastim
Abstract
Objectives.
This study aimed to evaluate the efficacy and safety of once-per-cycle balugrastim versus pegfilgrastim for neutrophil support in breast cancer patients receiving myelosuppressive chemotherapy.
Methods.
Breast cancer patients (n = 256) were randomized to 40 or 50 mg of subcutaneous balugrastim or 6 mg of pegfilgrastim ≈24 hours after chemotherapy (60 mg/m2 doxorubicin and 75 mg/m2 docetaxel, every 21 days for up to 4 cycles). The primary efficacy parameter was the duration of severe neutropenia (DSN) in cycle 1. Secondary parameters included DSN (cycles 2–4), absolute neutrophil count (ANC) nadir, febrile neutropenia rates, and time to ANC recovery (cycles 1–4). Safety, pharmacokinetics, and immunogenicity were assessed.
Results.
Mean cycle 1 DSN was 1.0 day with 40 mg of balugrastim, 1.3 with 50 mg of balugrastim, and 1.2 with pegfilgrastim (upper limit of 95% confidence intervals for between-group DSN differences was <1.0 day for both balugrastim doses versus pegfilgrastim). Between-group efficacy parameters were comparable except for time to ANC recovery in cycle 1 (40 mg of balugrastim, 2.0 days; 50 mg of balugrastim, 2.1; pegfilgrastim, 2.6). Median terminal elimination half-life was ≈37 hours for 40 mg of balugrastim, ≈36 for 50 mg of balugrastim, and ≈45 for pegfilgrastim. Antibody response to balugrastim was low and transient, with no neutralizing effect.
Conclusion.
Once-per-cycle balugrastim is not inferior to pegfilgrastim in reducing cycle 1 DSN in breast cancer patients receiving chemotherapy; both drugs have comparable safety profiles.
Implications for Practice:
This paper provides efficacy and safety data for a new, once-per-cycle granulocyte colony-stimulating factor, balugrastim, for the prevention of chemotherapy-induced neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. In this phase III trial, balugrastim was shown to be not inferior to pegfilgrastim in the duration of severe neutropenia in cycle 1 of doxorubicin/docetaxel chemotherapy, and the safety profiles of the two agents were similar. Once-per-cycle balugrastim is a safe and effective alternative to pegfilgrastim for hematopoietic support in patients with breast cancer receiving myelosuppressive chemotherapy associated with a greater than 20% risk of developing febrile neutropenia.
Introduction
Neutropenia is one of the most common toxicities in cancer patients receiving myelosuppressive chemotherapy [1]. Patients who develop neutropenia are at increased risk for infection with fever [1]. Febrile neutropenia (FN) often requires hospitalization and treatment with i.v. antibiotics, may necessitate chemotherapy dose reductions and/or delays, and may confer a mortality rate ranging from 8.0% to 14.3% depending on the type of cancer [2–5]. Severe neutropenia occurs most often during the initial cycles of chemotherapy [6], and increased duration is associated with an increased risk of infection [1, 7]. Because the incidence of FN across cancer types treated with commonly used chemotherapy regimens ranges from 5% to 44%, appropriate and effective prophylactic neutrophil support is an important clinical goal [3, 8].
Granulocyte colony-stimulating factor (G-CSF) products have demonstrated clinical benefit [9–12] in patients at risk for chemotherapy-induced neutropenia by stimulating neutrophil proliferation and function [13]. The prophylactic use of G-CSF products is therefore routinely recommended to maintain absolute neutrophil counts (ANC) in patients receiving chemotherapy regimens when the risk of FN is 20% or higher [6, 14, 15]. Filgrastim is an Escherichia coli-derived recombinant formulation of G-CSF that has a short elimination half-life (t1/2) and requires daily s.c. injections [16]. Attachment of a polyethylene glycol moiety to filgrastim (pegfilgrastim) extends its elimination half-life, facilitating once-per-chemotherapy-cycle dosing [16–18].
As an alternative to pegylation, a novel technology was used to develop balugrastim (Egranli [CG-10639]; Teva Pharmaceutical Industries, Netanya, Israel, http://www.tevapharm.com), a recombinant protein composed of human serum albumin and human G-CSF produced through recombinant DNA technology in Saccharomyces cereviseae [19]. The albumin domain prolongs the circulating half-life of balugrastim compared with G-CSFs, allowing once-per-cycle, fixed-dose, s.c. administration [20].
This phase III noninferiority study aimed to evaluate the efficacy and safety of 40- and 50-mg doses of balugrastim versus a 6-mg dose of pegfilgrastim (Neulasta; Amgen Inc., Thousand Oaks, CA, http://www.amgen.com) for the prophylaxis of chemotherapy-induced neutropenia. The doses in the current study were chosen based on the results of a dose-escalation phase II study that demonstrated similar safety and efficacy between the 40- and 50-mg doses of balugrastim and the active comparator, pegfilgrastim [21].
Materials and Methods
Study Design and Treatment
This multicenter, randomized, open-label, phase III noninferiority study compared the efficacy and safety of once-per-cycle 40 or 50 mg of balugrastim and 6 mg of pegfilgrastim in patients with breast cancer receiving chemotherapy with doxorubicin and docetaxel. The phase III portion of the study followed a phase II dose-escalation study of once-per-cycle 30, 40, and 50 mg of balugrastim and was designed to select 2 doses of balugrastim that demonstrated similar safety and efficacy to pegfilgrastim for further investigation in the phase III study.
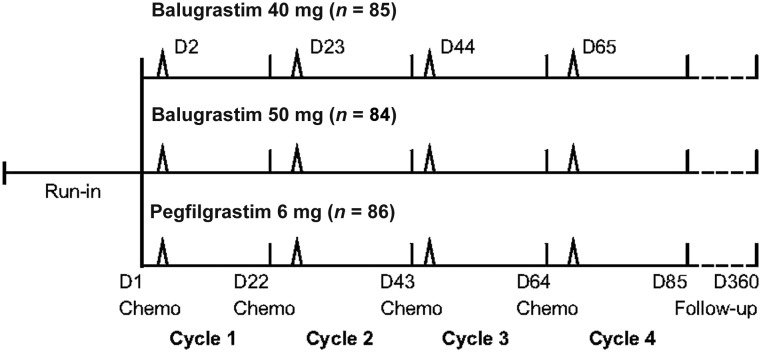
Patients were randomized in a 1:1:1 ratio to receive s.c. injections of 40 or 50 mg of balugrastim or 6 mg of pegfilgrastim once per cycle approximately 24 hours after chemotherapy administration for up to four 21-day chemotherapy cycles (Fig. 1). Randomization was stratified by body weight (<50, ≥50, <80, and ≥80 kg), prior chemotherapy exposure, and global location. Patients were allocated to treatment groups using a blocked randomization scheme with a block size of three. The randomization process was conducted by ClinPhone (Nottingham, U.K., http://www.clinphone.com) using computerized randomization and was delivered using an interactive voice response system. Chemotherapy consisted of doxorubicin 60 mg/m2 i.v. injection, followed by docetaxel 75 mg/m2 i.v. infusion. To reduce the incidence of fluid retention and hypersensitivity reactions, patients received oral corticosteroids for 3 days, starting 1 day before docetaxel administration. The use of antiemetics or other premedications was left to the physician’s discretion. Prior to receiving chemotherapy, patients were required to have an ANC of ≥1.0 × 109/L and a platelet count of ≥100 × 109/L. Patients were followed for 1 year after the final dose of study drug to assess overall survival.
Figure 1.
Phase III study design. Chemotherapy comprised doxorubicin 60 mg/m2 + docetaxel 75 mg/m2.
Abbreviations: Chemo, chemotherapy; D, day.
The local institutional review boards and ethics committees of the participating centers approved the study protocol, and the study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Written informed consent was obtained from all patients before the start of any study-related procedures.
Patients
Male and female patients aged 18 years or older with histologically or cytologically confirmed breast cancer who were scheduled to receive doxorubicin and docetaxel combination chemotherapy were eligible to participate in the study if they had an Eastern Cooperative Oncology Group (ECOG) performance status of ≤2, ANC of >1.5 × 109/L, platelet count of >100 × 109/L, and adequate hepatic and renal function.
Patients were excluded from the study if they had received more than 1 prior chemotherapy regimen, including adjuvant therapy within the last 12 months; a total lifetime cumulative anthracycline dose exceeding doxorubicin of >240 mg/m2 or equivalent dose of another anthracycline or anthracenedione; high-dose chemotherapy with hematopoietic stem cell transplant; chemotherapy, immunotherapy, or any investigational agent; or radiation (excluding spot irradiation for bone metastases). Other key exclusion criteria included previous exposure to G-CSF, granulocyte-macrophage CSF, or erythropoietin; systemic antibiotics within 72 hours of study chemotherapy; or concomitant trastuzumab. Pregnant or breastfeeding women were excluded, as were nonsterile men and women who did not agree to use an effective method of contraception throughout the study.
Outcomes
The primary efficacy parameter was the duration of severe neutropenia (DSN; number of days with ANC of <0.5 × 109/L) in cycle 1. Secondary parameters included DSN in cycles 2–4; incidence of FN (ANC of <0.5 × 109/L and oral temperature of ≥38.2°C occurring on the same day) and severe neutropenia in cycles 1–4; and time to ANC nadir, depth of ANC nadir, and time to ANC recovery (time from nadir ANC to ANC of ≥1.5 × 109/L, calculated for patients with ANC of <1.5 × 109/L after the start of chemotherapy) in cycles 1–4. Blood samples were collected to determine ANC 1 day before each chemotherapy cycle and until postnadir ANC was >2.0 × 109/L and then twice weekly. Serum samples for clinical chemistry panels were collected within 2 days before chemotherapy and then weekly during cycle 1 and 1 day before chemotherapy during cycles 2–4. Body temperature was monitored twice daily from days 5–15 of each chemotherapy cycle.
Pharmacokinetic parameters during cycle 1 for balugrastim and pegfilgrastim were derived using noncompartmental methods using Phoenix WinNonlin version 6.1. Balugrastim and pegfilgrastim concentrations were detected using a sandwich enzyme-linked immunosorbent assay (ELISA). Pharmacokinetic (PK) parameters included the area under the curve (AUC0-∞), apparent total clearance (CL/F), apparent volume of distribution (Vz/F), maximum serum concentration (Cmax), elimination half-life (t1/2, elim), and mean residence time. Blood samples for PK analysis were obtained during cycle 1 only, before study-drug administration, 24 hours after administration, and daily on days 5–8.
Immunogenicity samples were obtained before each chemotherapy cycle and at 6 and 12 months after the final dose of study drug. Screening, confirmation, and characterization assays were implemented for immunogenicity assessment. A direct-binding ELISA was used to evaluate development of antidrug antibodies against balugrastim and pegfilgrastim in balugrastim- or pegfilgrastim-treated patients, respectively. Confirmed antibalugrastim or antipegfilgrastim samples were tested for G-CSF-neutralizing activity. An additional assay was used to detect antidrug antibodies specifically against albumin domain in balugrastim-treated patients.
Adverse events (AEs) were continually monitored by the investigators throughout the course of the study, and an independent data safety monitoring committee periodically reviewed safety data after 60 subjects, 120 subjects, and 180 subjects completed cycle 1 of chemotherapy. AEs were reported from the start of study drug administration until 30 days after the last injection, summarized by type and frequency, and categorized using the Medical Dictionary for Regulatory Activities (version 11.1) system of organ classification. The investigator evaluated all AEs based on seriousness, causality (relationship to study drug), and grade using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Statistical Analysis
A sample size of at least 75 patients per treatment arm was chosen to provide 87% or greater power to establish noninferiority of balugrastim compared with pegfilgrastim with regard to the primary efficacy parameter, mean DSN in cycle 1. Sample size calculations included an estimate of 1.6 days as the within-treatment SD of cycle 1 DSN and a ≤20% dropout rate. All statistical analyses were performed using the SAS system.
Noninferiority tests for cycle 1 DSN were performed on the per-protocol population (randomized patients with no protocol violations that could affect the primary efficacy parameter; violations occurring after the measurement of DSN in cycle 1 were not considered relevant). Noninferiority tests were based on confidence intervals (CIs) for differences in mean DSN estimated by bootstrap resampling and stratified by previous chemotherapy and weight. Relative to pegfilgrastim, the noninferiority of balugrastim was established if either of the following conditions was met: the null hypothesis that the mean cycle 1 DSN for balugrastim exceeded the mean cycle 1 DSN for pegfilgrastim by at least 1 day was rejected if the 95% 2-sided CI for the difference in mean cycle 1 DSN was less than 1 day (1-sided α = 0.025) for both balugrastim treatment groups, or the null hypothesis that the mean cycle 1 DSN for balugrastim exceeded the mean cycle 1 DSN for pegfilgrastim by at least 1 day was rejected if the 97.5% 2-sided CI for the difference in mean cycle 1 DSN was less than 1 day (1-sided α = 0.0125) for either balugrastim treatment group.
All other efficacy analyses were performed on the per-protocol population and modified intent-to-treat (mITT) population (all randomized patients who received ≥1 dose of assigned study drug), and safety analyses were performed on the mITT population. Mean DSN (cycles 2–4), mean time to ANC recovery (ANC >1.5 × 109/L), and ANC nadir were assessed using conventional methods for calculating 95% CIs. As appropriate, the χ2 or Fisher’s exact test was used for testing differences in the incidence of FN and severe neutropenia (grade 4) and grade 3/4 neutropenia among treatment groups. All tests of treatment effects were conducted at a 2-sided α level of 0.05.
PK analyses were performed on the PK population (all randomized patients who had ≥1 PK sample collected; population eliminated those patients with insufficient data points and those with predose balugrastim serum concentrations >5% of Cmax). The complete PK population was a subset of the PK population and included only those with a definable t1/2, elim. Immunogenicity was assessed in the mITT population.
Results
Patient Disposition
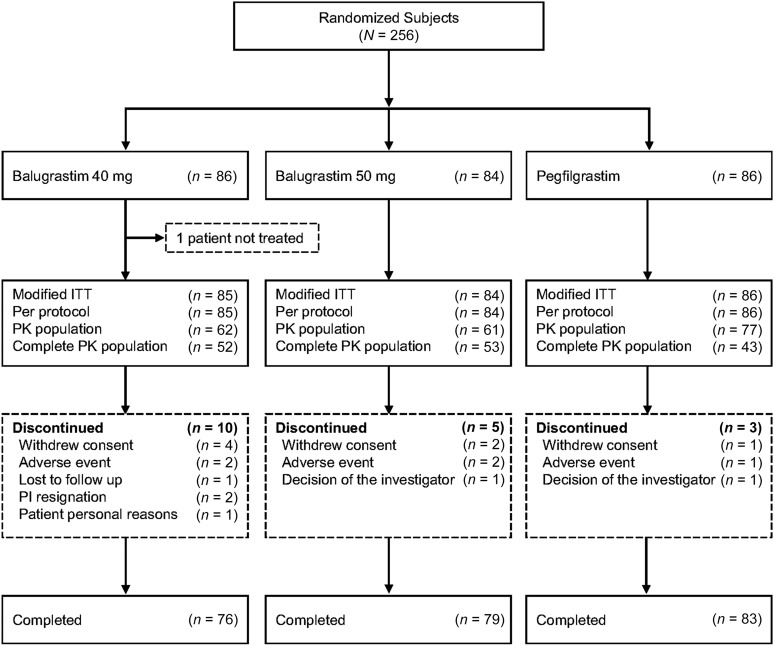
The study was conducted at 45 sites located in Russia (31 locations) and Ukraine (14 locations) between August 2008 and June 2009. Of the 256 patients enrolled (40 mg of balugrastim, n = 86; 50 mg of balugrastim, n = 84; pegfilgrastim, n = 86), 238 (93%) completed. One patient randomized to 40 mg of balugrastim was withdrawn before receiving study drug and was excluded from the mITT and per-protocol populations. The most common reasons for not completing the study were withdrawal of consent (n = 4, 40 mg of balugrastim; n = 2, 50 mg of balugrastim; n = 1, pegfilgrastim) and AEs (40 mg of balugrastim, death caused by malignant neoplasm progression [n = 1] and discontinuation because of cyclothymic disorder [n = 1]; 50 mg of balugrastim, discontinuation because of elevation of liver enzymes [n = 1]; and pegfilgrastim, death because of pulmonary edema [n = 1]) (Fig. 2). No AEs resulting in study withdrawal were considered by the investigators to be related to study drug. The per-protocol and mITT populations were identical.
Figure 2.
Patient disposition.
Abbreviations: ITT, intent to treat; PI, principal investigator; PK, pharmacokinetic.
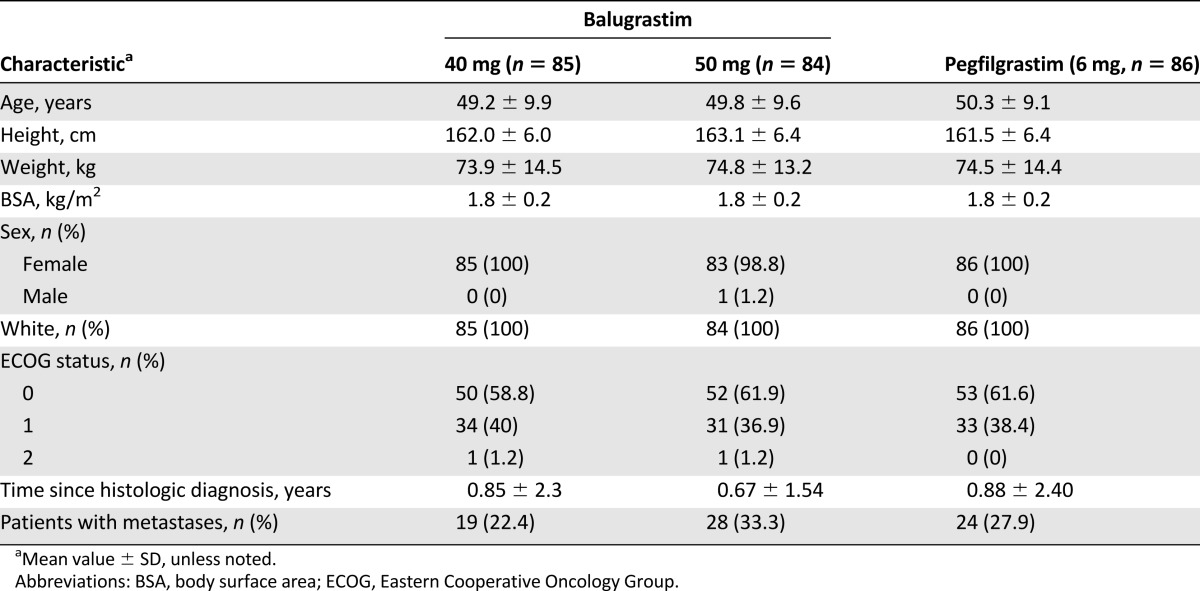
All 255 patients were white and the majority were female (n = 1, male; Table 1). Treatment groups were comparable at baseline for age, weight, body surface area, and ECOG performance status. Metastatic disease was present in 22%–33% of patients in the balugrastim treatment groups and in 28% of patients in the pegfilgrastim group.
Table 1.
Summary of baseline patient demographic and breast cancer characteristics (modified intent-to-treat population)
Efficacy
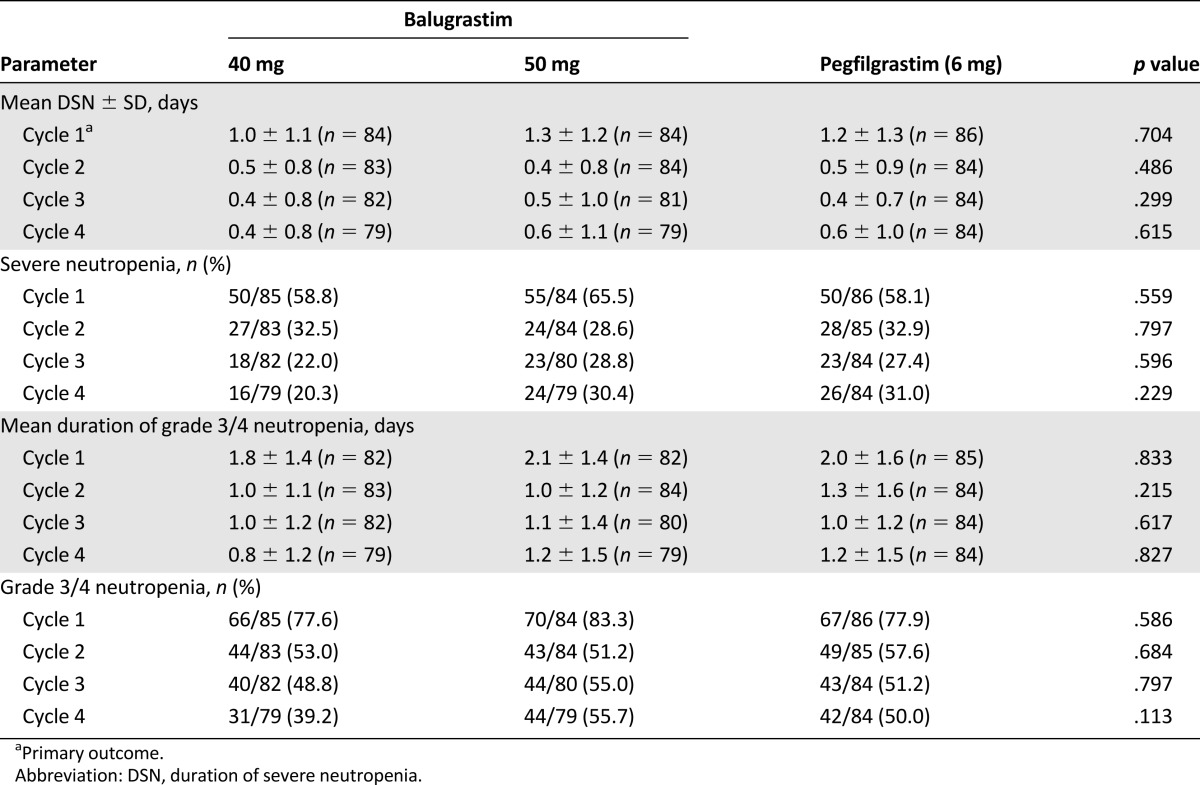
The primary efficacy parameter, DSN in cycle 1, was comparable in the 40-mg-balugrastim, 50-mg-balugrastim, and pegfilgrastim treatment groups (mean 1.0, 1.3, and 1.2 days, respectively [p = .704]; Table 2). The 95% and 97.5% 2-sided CIs for differences between balugrastim and pegfilgrastim for cycle 1 DSN were less than 1 day for both balugrastim doses, thereby establishing noninferiority of balugrastim to pegfilgrastim for the primary efficacy parameter. The upper limit of the 95% 2-sided CI for the difference between the 40-mg-balugrastim group and the pegfilgrastim group was 0.15 days, and the difference between the 50-mg-balugrastim group and the pegfilgrastim group was 0.41 days. The upper limit of the 97.5% CI versus pegfilgrastim was 0.21 days for 40 mg of balugrastim and 0.46 days for 50 mg of balugrastim.
Table 2.
Duration and incidence of severe neutropenia and grade 3/4 neutropenia for chemotherapy cycles 1 through 4 (modified intent-to-treat population)
The DSN in cycle 1 was similar across the ≥50-, <80-, and ≥80-kg patient subgroups in all treatment groups. Of the 3 patients in the <50 kg subgroup, 2 who were treated with balugrastim did not develop severe neutropenia, and 1 who was treated with pegfilgrastim had a DSN of 3 days during cycle 1.
Compared with cycle 1, the mean duration and incidence of severe neutropenia were reduced during cycles 2–4 (Table 2).The mean duration and incidence of grade 3/4 neutropenia also were shorter in cycles 2–4 than in cycle 1 (Table 2). There were no significant treatment effects related to the duration or incidence of severe neutropenia in any chemotherapy cycle for the doses evaluated.
No significant difference was observed in the proportion of patients experiencing FN during cycle 1 (3.5%, 40 mg of balugrastim; 6.0%, 50 mg of balugrastim; 2.3%, pegfilgrastim; p = .398). Three additional patients experienced FN during cycles 2 through 4: 2 in the 40-mg-balugrastim group and 1 in the pegfilgrastim group. The treatment effect was not significant in any chemotherapy cycle.
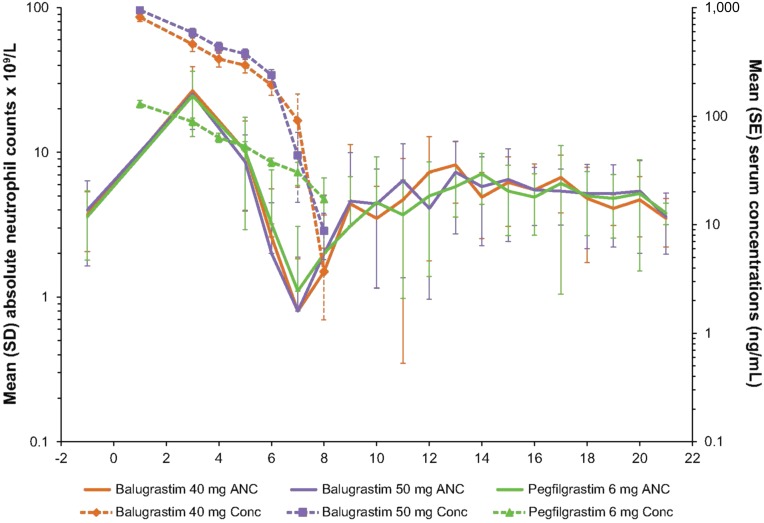
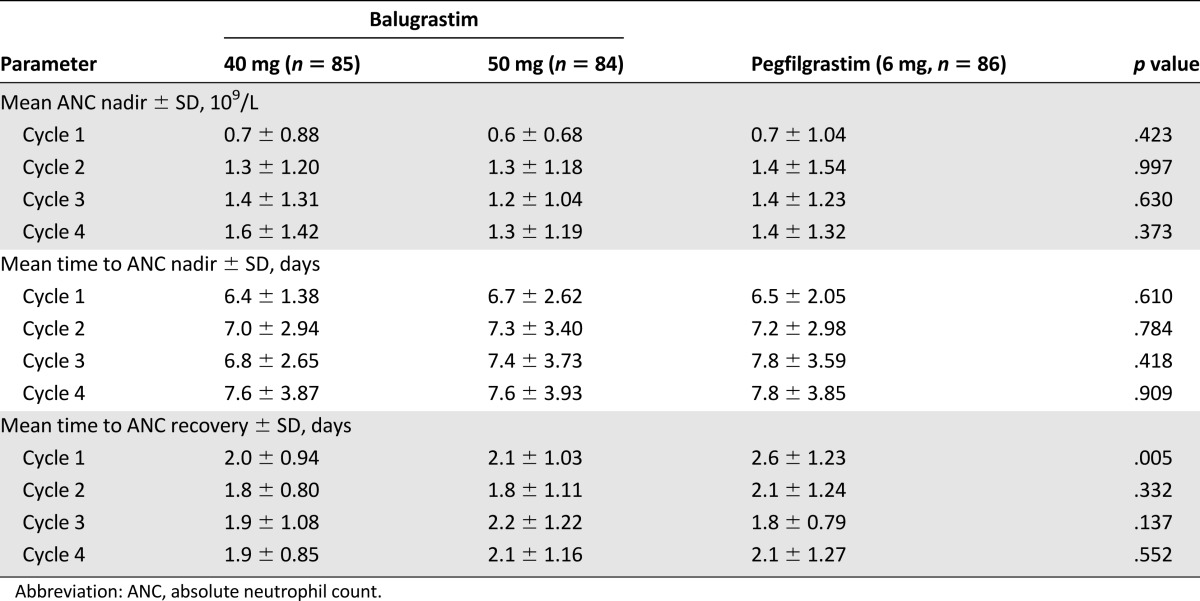
In cycle 1, no significant treatment effects occurred in time to ANC nadir and depth of nadir. The mean ANC nadir in cycle 1 ranged from 0.6 × 109/L to 0.7 × 109/L, which was reached at a mean of approximately 6.5 days across all treatment groups (Fig. 3; Table 3). In cycle 1, patients treated with 40 and 50 mg of balugrastim had a significantly shorter time to ANC recovery from nadir compared with patients treated with pegfilgrastim (2.0 and 2.1 days, respectively, vs. 2.6 days; p = .005). Within cycles 2–4, no significant differences were noted for any parameter (Table 3). Across treatment cycles, the mean values were consistent with the results in cycle 1.
Figure 3.
Mean ANC and study drug serum concentration-time profiles for balugrastim (40 and 50 mg) and pegfilgrastim (6 mg) during chemotherapy cycle 1.
Abbreviations: ANC, absolute neutrophil count; Conc, concentration.
Table 3.
ANC nadir, time to ANC nadir, and time to recovery for chemotherapy cycles 1 through 4 (modified intent-to-treat population)
Overall survival at the end of the 1-year follow-up period was 91.8%, 84.5%, and 90.7% for the 40-mg-balugrastim, 50-mg-balugrastim, and pegfilgrastim groups, respectively. No apparent differences were observed between treatment groups for new antitumor therapy, tumor response, or time to death.
Pharmacokinetics
The mean Cmax (coefficient of variation [CV%]) for 40 and 50 mg of balugrastim was 875 (76.3%) and 975 (74.1%) ng/mL, respectively. The mean Cmax (CV%) for pegfilgrastim was 164 (63.0%) ng/mL, and the median Tmax was approximately 24 hours for all treatment groups. The median terminal phase t1/2, elim was 37.4, 35.5, and 45.3 hours for the 40-mg-balugrastim, 50-mg-balugrastim, and pegfilgrastim groups, respectively. Mean estimates for CL/F were 1.34 and 1.18 L/hour for the 40-mg and 50-mg balugrastim groups, respectively, and 0.613 L/hour for the pegfilgrastim group. Mean estimates for Vz/F were 80.1, 69.2, and 39.7 L for the 40-mg-balugrastim, 50-mg-balugrastim, and pegfilgrastim groups, respectively.
Safety
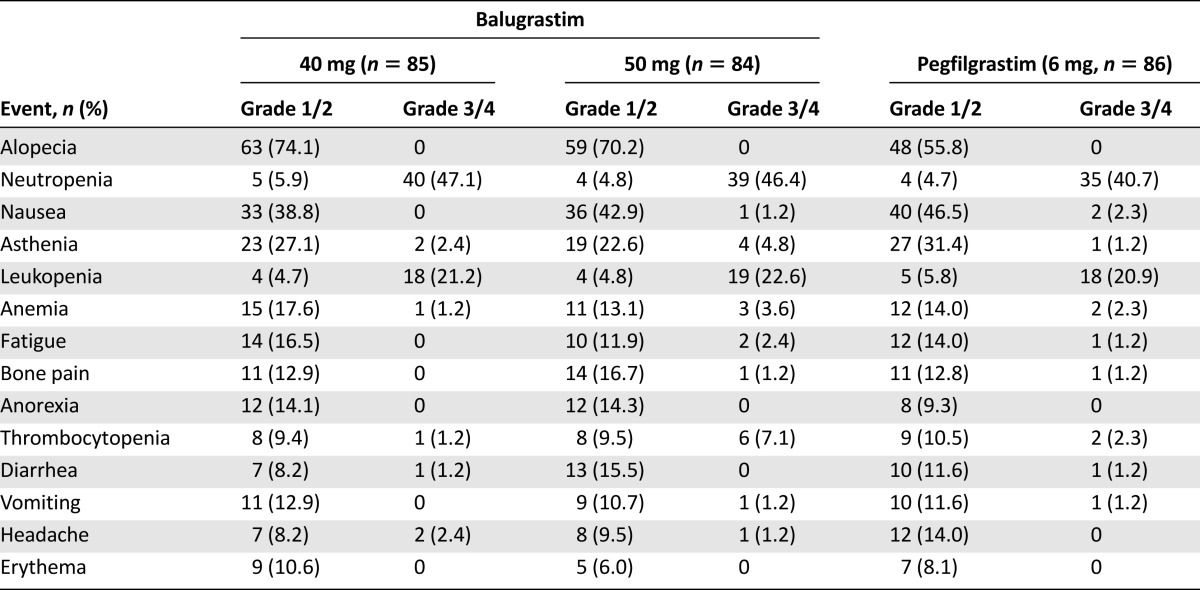
There was no consistent relationship between the incidence of AEs and balugrastim dose (Table 4), but the proportion of patients with grade 4 AEs was higher in the 50-mg-balugrastim group (46.4%) than in the other treatment groups (34.1%, 40 mg of balugrastim vs. 36.0%, pegfilgrastim). During the study, 1 patient randomized to the 40-mg-balugrastim group and 1 patient randomized to the pegfilgrastim group died because of malignant neoplasm progression and pulmonary edema, respectively. Serious AEs (SAEs) occurred in 6 and 8 patients in the 40- and 50-mg-balugrastim groups, respectively, and in 6 patients treated with pegfilgrastim. Reported SAEs included febrile neutropenia (n = 4), neutropenia (n = 1), malignant neoplasm progression (n = 1), cyclothymic disorder (n = 1), and uretic calculus (n = 1) in the 40-mg-balugrastim group; febrile neutropenia (n = 5) and bronchitis (n = 1) in the 50-mg-balugrastim group; and febrile neutropenia (n = 3), pyrexia (n = 1), abdominal wall abscess (n = 1), pneumonia (n = 1), and pulmonary edema (n = 1) in the pegfilgrastim group. Of the patients treated with balugrastim, 2 withdrew because of AEs (1 patient with cyclothymic disorder, 40 mg of balugrastim, and 1 patient with elevated liver enzymes, 50 mg of balugrastim). Two additional patients were withdrawn from study because of death, as described above.
Table 4.
Treatment drug-related adverse events reported in at least 10% of patients in any treatment group categorized by common terminology criteria grade (modified intent-to-treat population)
Complete blood count (CBC) and serum chemistry parameters were similar across treatment groups. Frequency distributions for the worst postbaseline grade were not significantly different for any CBC or serum chemistry parameter.
Immunogenicity
Immunogenicity was assessed in 169 patients treated with either balugrastim dose and in 86 patients treated with pegfilgrastim during the phase III portion of the 2-phase study. Antibodies to balugrastim were detected in two balugrastim-treated patients, and antibodies to pegfilgrastim were detected in one pegfilgrastim-treated patient. Antibody titers in both balugrastim- and pegfilgrastim-treated patients did not increase over time. None of the binding antibody-positive samples were neutralizing in the cell-based assay. In addition, low-titer, transient, drug-induced antibodies against the albumin domain of balugrastim were detected in six balugrastim-treated patients.
Discussion
Based on prospectively defined criteria, this study demonstrated that a single fixed-dose of 40 or 50 mg of balugrastim is not inferior to 6 mg of pegfilgrastim in reducing DSN during chemotherapy cycle 1 in breast cancer patients receiving doxorubicin and docetaxel. A 1-day difference in DSN would be anticipated to result in an approximate 10% difference in FN and was therefore selected in the present study and in previous pegfilgrastim versus filgrastim noninferiority studies as a meaningful and practical noninferiority margin [11, 22, 23]. The DSN ranged from 1.0 to 1.3 days across treatment groups during cycle 1, and the upper limits of the 95% and 97.5% 2-sided CIs for the differences among treatment groups were all less than 1 day; therefore, the null hypothesis that the difference between balugrastim and pegfilgrastim was at least 1 day was rejected.
The 40- and 50-mg-balugrastim doses were selected for evaluation based on a phase II, sequential dose-escalation pilot study of once-per-cycle 30, 40, and 50 mg of balugrastim versus 6 mg of pegfilgrastim in breast cancer patients receiving chemotherapy with doxorubicin and docetaxel [21]. The phase II study suggested that, compared with pegfilgrastim, 40 and 50 mg of balugrastim provided similarly effective prophylactic neutrophil support in breast cancer patients receiving myelosuppressive chemotherapy. These findings were confirmed in the current phase III study.
Balugrastim treatment also was associated with a significantly shorter time to ANC recovery in cycle 1 versus pegfilgrastim; however, recovery time was comparable between study drugs in cycles 2–4. No significant differences were noted between balugrastim and pegfilgrastim for any other secondary parameters in cycles 1–4.
Previous clinical studies have reported that primary prophylaxis with pegfilgrastim is as effective as filgrastim in reducing neutropenia and associated complications throughout a four-cycle course of doxorubicin and docetaxel chemotherapy [11]. Significant findings that favored pegfilgrastim over filgrastim, including DSN in cycles 2–4 and lower overall rates of FN, were reported, and trends toward higher nadir counts and a lower incidence of severe neutropenia also were noted. It was presumed that constant stimulation of neutrophils with longer-acting pegfilgrastim may be involved in such observations [11].
Previous studies have demonstrated a DSN of approximately 3.8 days in cycle 1 for breast cancer patients not treated with G-CSF during chemotherapy with doxorubicin and docetaxel [24]. Treatment with 6 mg of pegfilgrastim has demonstrated a decrease in DSN in cycle 1, with values ranging from 1.3 to 1.8 days, which is consistent with the values observed for pegfilgrastim during cycle 1 of the present study (1.2 days) [10, 11, 23].
The design of the current study was consistent with previous studies designed to demonstrate noninferiority of pegfilgrastim compared with filgrastim for the provision of neutrophil support in patients with breast cancer receiving chemotherapy with doxorubicin and docetaxel [11, 23]. The breast cancer patient population selected for this study had a greater than 20% risk of developing FN during chemotherapy with doxorubicin and docetaxel and represented the target population for G-CSF support [6, 14, 15].
Because of concerns that fixed G-CSF dosing may not be as clinically beneficial for heavier patients because of a decreased overall per-kg dose, a previous study stratified patients treated with pegfilgrastim by weight and demonstrated comparable DSNs in both heavier and lighter patients [23]. Similarly, in the current study, weight did not affect DSN in the balugrastim or pegfilgrastim treatment groups. However, because of relatively small sample sizes within weight strata, differences in mean DSN were not expected to be statistically significant.
Compared with filgrastim, which has an elimination t1/2 of approximately 3.5 hours [16], the present study indicated that the median extended elimination t1/2 of 40 mg of balugrastim (≈37 hours) was sufficient to support once-per-cycle administration in patients receiving chemotherapy with doxorubicin and docetaxel. Although the median elimination t1/2 of balugrastim was shorter than that of pegfilgrastim (45 hours), similar efficacy in terms of neutrophil support was clearly demonstrated in the balugrastim and pegfilgrastim treatment groups.
Balugrastim was well tolerated and, in general, the safety profiles of balugrastim and pegfilgrastim were comparable. The type and frequency of AEs were consistent with the underlying medical condition, and the safety profile was associated with that for chemotherapy with doxorubicin and docetaxel. The most common drug-related AEs, which included bone pain and asthenia, were expected with a G-CSF product [23, 24]. Although no apparent relationship existed between the incidence of AEs and the balugrastim dose, the proportion of patients with grade 4 AEs was higher in the 50-mg-balugrastim group. A dose of 40 mg of balugrastim also tended to perform better than the 50-mg dose for several efficacy parameters, including DSN and incidence of FN in cycle 1 and for severe neutropenia, suggesting that the 40-mg dose has a better benefit:risk profile.
Antibalugrastim binding antibodies were detected in two patients, and antipegfilgrastim binding antibodies were detected in one patient. Thus, the incidences of antibalugrastim or antipegfilgrastim binding antibodies were comparable between treatment groups. Antidrug antibody responses had low antibody titers and were negative for neutralizing activity.
Conclusion
At doses of 40 and 50 mg, balugrastim administered once per cycle is not inferior to pegfilgrastim in reducing DSN in cycle 1 in breast cancer patients receiving myelosuppressive chemotherapy. Once-per-cycle balugrastim is an effective alternative to pegfilgrastim for hematopoietic support in patients with breast cancer receiving myelosuppressive chemotherapy associated with a greater than 20% risk of FN, with a safety profile comparable to that of pegfilgrastim.
Acknowledgments
We thank the patients who participated in this study and the following study investigators: Anna V. Alyasova (Nizhny Novgorod, Russia), Viacheslav A. Askoldky (Cherkasy, Ukraine), Anna I. Bujnyakova (St. Petersburg, Russia), Olga N. Burdayeva (Arkhangelsk, Russia), Mikhail Yu Biakhov (Moscow, Russia), Nina A. Chekha (Magnitogorsk, Russia), Sergey V. Cheporov (Yaroslavl, Russia), Leonid P. Chybisov (Lugansk, Ukraine), Irina S. Davidenko (Krasnodar, Russia), Igor G. Drobner (Khmelnytsky, Ukraine), Oleksandr S. Dudnichenko (Kharkiv, Ukraine), Mikhail A. Gershanovich (St. Petersburg, Russia), Viktor E. Goldberg (Tomsk, Russia), Evegeny A. Gotovkin (Ivanovo, Russia), Mikhail V. Kopp (Samara, Russia), Volodymyr G. Komisarenki (Kryvyi Rih, Ukraine), Oleksiy O. Kovalyov (Zaporizhya, Ukraine), Dmetriy A. Krasnozhon (St. Petersburg, Russia), Olga V. Lazarevich (Republick of Karelia, Russia), Anatoly N. Makhson (Moscow Region, Russia), Alexey G. Manikhas (St. Petersburg, Russia), Marina P. Matrosova (Nizhny Novgorod, Russia), Elena I. Matyushina (Syktyvkar, Russia), Vladimir V. Milovanov (Tambov, Russia), Dmytro V. Myasoyedov (Kyiv, Ukraine), Sergei V. Orlov (St. Petersburg, Russia), Alexander P. Pecheniy (Orel, Russia), Sergiy E. Polenkov (Chernihiv, Ukraine), Genady G. Psaras (Marinupol, Ukraine), Sufia Z. Safina (Kazan, Russia), Igor E. Sedakov (Donetsk, Ukraine), Vladimir F. Semiglazov (St. Petersburg, Russia), Roman V. Senyutovich (Chernivtsi, Ukraine), Sergei A. Shinkarev (Lipetsk, Russia), Marina V. Shomova (Ryazan, Russia), Yaroslav V. Shparyk (Lviv, Ukraine), Eldar E. Topuzov (St. Petersburg, Russia), Sergei A. Tjulandin (Moscow, Russia), Dmitry P. Udovitsa (Krasnodar Krai, Russia), Vladimir I. Vladimirov (Stavropol Territory, Russia), and Igor V. Zhulkevych (Ternopil, Ukraine). The authors also thank Laurie Pukac for her expert review of the immunogenicity data. This study was sponsored by Teva Branded Pharmaceutical Products R&D, Inc. Medical writing assistance was provided by Lisa Grauer, of Chameleon Communications and Lisa Feder, of Peloton Advantage LLC and was funded by Teva Branded Pharmaceutical Products R&D, Inc. (Frazer, PA). Teva provided a full review of the article.
Author Contributions
Conception/Design: Steve Barash, Liat Adar
Provision of study material or patients: Oleg Gladkov, Vladimir Moiseyenko, Igor N. Bondarenko, Yaroslav Shparyk, Steve Barash, Liat Adar, Noa Avisar
Collection and/or assembly of data: Oleg Gladkov, Vladimir Moiseyenko, Igor N. Bondarenko, Yaroslav Shparyk, Steve Barash, Liat Adar, Noa Avisar
Data analysis and interpretation: Oleg Gladkov, Vladimir Moiseyenko, Igor N. Bondarenko, Yaroslav Shparyk, Steve Barash, Liat Adar, Noa Avisar
Manuscript writing: Oleg Gladkov, Vladimir Moiseyenko, Igor N. Bondarenko, Yaroslav Shparyk, Steve Barash, Liat Adar, Noa Avisar
Final approval of manuscript: Oleg Gladkov, Vladimir Moiseyenko, Igor N. Bondarenko, Yaroslav Shparyk, Steve Barash, Liat Adar, Noa Avisar
Disclosures
Steve Barash: Teva (E, OI); Liat Adar: Teva (E, OI); Noa Avisar: Teva (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Heuser M, Ganser A, Bokemeyer C, et al. Use of colony-stimulating factors for chemotherapy-associated neutropenia: Review of current guidelines. Semin Hematol. 2007;44:148–156. doi: 10.1053/j.seminhematol.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Altwairgi AK, Hopman WM, Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20:e171–e179. doi: 10.3747/co.20.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pettengell R, Schwenkglenks M, Leonard R, et al. Neutropenia occurrence and predictors of reduced chemotherapy delivery: Results from the INC-EU prospective observational European neutropenia study. Support Care Cancer. 2008;16:1299–1309. doi: 10.1007/s00520-008-0430-4. [DOI] [PubMed] [Google Scholar]
- 4.Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–2266. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 5.de Naurois J, Novitzky-Basso I, Gill MJ, et al. Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21(suppl 5):v252–v256. doi: 10.1093/annonc/mdq196. [DOI] [PubMed] [Google Scholar]
- 6.Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Bodey GP, Buckley M, Sathe YS, et al. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 8.Ozer H, Armitage JO, Bennett CL, et al. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: Evidence-based, clinical practice guidelines. J Clin Oncol. 2000;18:3558–3585. doi: 10.1200/JCO.2000.18.20.3558. [DOI] [PubMed] [Google Scholar]
- 9.Bohlius J, Herbst C, Reiser M, et al. Granulopoiesis-stimulating factors to prevent adverse effects in the treatment of malignant lymphoma. Cochrane Database Syst Rev. 2008:CD003189. doi: 10.1002/14651858.CD003189.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes FA, Jones SE, O’Shaughnessy J, et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: A multicenter dose-finding study in women with breast cancer. Ann Oncol. 2002;13:903–909. doi: 10.1093/annonc/mdf130. [DOI] [PubMed] [Google Scholar]
- 11.Holmes FA, O’Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002;20:727–731. doi: 10.1200/JCO.2002.20.3.727. [DOI] [PubMed] [Google Scholar]
- 12.Sung L, Nathan PC, Alibhai SM, et al. Meta-analysis: Effect of prophylactic hematopoietic colony-stimulating factors on mortality and outcomes of infection. Ann Intern Med. 2007;147:400–411. doi: 10.7326/0003-4819-147-6-200709180-00010. [DOI] [PubMed] [Google Scholar]
- 13.Page AV, Liles WC. Colony-stimulating factors in the prevention and management of infectious diseases. Infect Dis Clin North Am. 2011;25:803–817. doi: 10.1016/j.idc.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 14.NCCN Clinical Practice Guidelines in Oncology. Myeloid Growth Factors v.2.2014. National Comprehensive Cancer Network. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed July 29, 2015.
- 15.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 16.Neupogen [package insert]. Thousand Oaks, CA: Amgen Inc., 2013.
- 17.Neulasta [package insert]. Thousand Oaks, CA: Amgen Inc., 2014.
- 18.Yang BB, Kido A. Pharmacokinetics and pharmacodynamics of pegfilgrastim. Clin Pharmacokinet. 2011;50:295–306. doi: 10.2165/11586040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Halpern W, Riccobene TA, Agostini H, et al. Albugranin, a recombinant human granulocyte colony stimulating factor (G-CSF) genetically fused to recombinant human albumin induces prolonged myelopoietic effects in mice and monkeys. Pharm Res. 2002;19:1720–1729. doi: 10.1023/a:1020917732218. [DOI] [PubMed] [Google Scholar]
- 20.Pukac L, Barash S, Avisar N, et al. Balugrastim: A long-acting, once-per-cycle, fixed-dose filgrastim: Pharmacokinetics and pharmacodynamics in patients with breast cancer. Support Care Cancer. 2012;20(suppl 1):S231. [Abstract 970] [Google Scholar]
- 21.Gladkov O, Moiseyenko V, Bondarenko IN, et al. A randomized, non-inferiority study of balugrastim and pegfilgrastim in breast cancer patients receiving myelosuppressive therapy. Support Care Cancer. 2012;20(suppl 1):S234–S235. [Abstract 983] [Google Scholar]
- 22.Blackwell S, Crawford J. Filgrastim (r-metHuG-CSF) in the chemotherapy setting. In: Morstyn G, Dexter TM, editors. Filgrastim (r-metHuG-CSF) in Clinical Practice. New York, NY: Marcel Dekker; 1994. pp. 103–116. [Google Scholar]
- 23.Green MD, Koelbl H, Baselga J, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14:29–35. doi: 10.1093/annonc/mdg019. [DOI] [PubMed] [Google Scholar]
- 24.del Giglio A, Eniu A, Ganea-Motan D, et al. XM02 is superior to placebo and equivalent to Neupogen in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in cycle 1 in breast cancer patients receiving docetaxel/doxorubicin chemotherapy. BMC Cancer. 2008;8:332–338. doi: 10.1186/1471-2407-8-332. [DOI] [PMC free article] [PubMed] [Google Scholar]