In melanoma, analysis of circulating tumor products (CTPs) may have clinical utility in areas such as screening, diagnostics, and clinical decision-making, and may serve as surveillance biomarkers or sources of real-time genetic or molecular characterization. CTP analysis reveals new biomarkers, patterns of treatment resistance, and mechanisms of metastasis development. This report compares and contrasts CTPs, reviews the translational evidence to date, and discusses how CTP research can benefit patients with melanoma.
Keywords: Circulating tumor cells, Circulating tumor DNA, Circulating mRNA, Melanoma, Clinical utility
Abstract
Circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), and messenger RNA (mRNA), collectively termed circulating tumor products (CTPs), represent areas of immense interest from scientists’ and clinicians’ perspectives. In melanoma, CTP analysis may have clinical utility in many areas, from screening and diagnosis to clinical decision-making aids, as surveillance biomarkers or sources of real-time genetic or molecular characterization. In addition, CTP analysis can be useful in the discovery of new biomarkers, patterns of treatment resistance, and mechanisms of metastasis development. Here, we compare and contrast CTCs, ctDNA, and mRNA, review the extent of translational evidence to date, and discuss how future studies involving both scientists and clinicians can help to further develop this tool for the benefit of melanoma patients.
Implications for Practice:
Scientific advancement has enabled the rapid development of tools to analyze circulating tumor cells, tumor DNA, and messenger RNA, collectively termed circulating tumor products (CTPs). A variety of techniques have emerged to detect and characterize melanoma CTPs; however, only a fraction has been applied to human subjects. This review summarizes the available human data that investigate clinical utility of CTP in cancer screening, melanoma diagnosis, prognosis, prediction, and genetic or molecular characterization. It provides a rationale for how CTPs may be useful for future research and discusses how clinicians can be involved in developing this exciting new technology.
Abstract
摘要
科学家和临床医生对循环肿瘤细胞 (CTC) 、循环肿瘤DNA (ctDNA) 和信使RNA (mRNA) ——统称为循环肿瘤产物 (CTP) 具有浓厚兴趣。在黑色素瘤中, CTP 分析作为生物标记物监测或实时基因或分子特征来源, 可能在从筛查到诊断到协助临床决策制定等许多方面具有临床用途。此外, CTP分析可能有助于发现新的生物标记物、治疗耐药模式和转移发生机制。我们在本文对CTC、ctDNA和mRNA进行了比较和对比, 回顾了迄今为止的基因转译证据, 并讨论了未来的研究应如何使科学家与临床医生共同参与进来, 帮助这一工具进一步发展, 从而有益于黑色素瘤患者。The Oncologist 2016;21:89–94
对临床实践的提示: 科学的进展使分析循环肿瘤细胞、肿瘤 DNA 和信使 RNA [统称为循环肿瘤产物 (CTP) ]的工具得以迅速发展。研究者开发出许多工具用于检测和分析黑色素瘤 CTP 特征, 但其中仅有很小的一部分用于人类受试者。本综述总结了现有调查 CTP 用于肿瘤筛查、黑色素瘤诊断、预后、预测和基因或分子特征的人类数据。这为 CTP 如何有助于未来研究提供了理论依据, 我们还讨论了临床医生应如何参与到这一令人振奋的新技术的开发中来。
Introduction
Circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), and circulating messenger RNA (mRNA), collectively termed circulating tumor products (CTPs), have attracted great interest and investment. In 2014 alone, more than 600 publications searchable on PubMed described the diversity of CTP-related isolation techniques, proof-of-principle findings, and implications for human disease management and clinical study design. In melanoma, several reviews have been written to summarize the state of the art in CTP detection, isolation, and genetic characterization, yet its potential contributions to precision medicine remain uncertain [1–7].
Several important questions have yet to be answered. What is the relative potential clinical utility of CTCs, ctDNA, and mRNA? Can measuring these CTPs be used to detect new or recurrent disease, or monitor response to therapy? What clinical protocols need to be designed to demonstrate the utility of these assays? Even if these trials are positive, will these data lead to changes in current practice paradigms?
Melanoma serves as an important clinical and scientific model for considering these issues. Melanoma is one of the most frequently diagnosed cancers in men and women living in developed countries [8]. In contrast to the stable or declining trends for most malignancies, incidence of melanoma has significantly increased in the U.S. over the past decade [9]. Melanoma, therefore, represents a significantly increasing case load in clinical oncology. Additionally, the management of melanoma lies at the forefront of the precision medicine revolution [10–14]. Variation in prognosis and the availability of powerful targeted therapies demands tools to better define risk stratification, inform the optimal timing of therapy initiation, and detect drug resistance. Melanoma’s variability in surface marker expression poses a unique biological challenge to reliable detection, thus inspiring great variation in CTC detection methodologies [1–3, 5–7]. On the other hand, well-described, melanoma-specific DNA mutations, such as BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations, allow for ease in conducting proof-of-concept studies. Therefore, the diversity of CTP detection techniques and rapidly changing treatment paradigms make melanoma an ideal model disease for discussing the potential benefit and challenges of CTP assays in the clinic.
Here, we review existing data on CTP studies in patients with melanoma and outline the key laboratory, clinical trial, and commercialization considerations that may pave the way toward a practice-changing technology. Although we highlight the utility of CTCs, ctDNA, and mRNA in various clinical applications, we do not directly compare the three, as they are not mutually exclusive technologies and their emergence in clinical medicine may very well overlap.
Overview of CTCs, ctDNA, and mRNA
We briefly review the techniques involved in isolating each CTP (Fig. 1), focusing on their potential for clinical application (Table 1). Ideally, CTPs must demonstrate strong test characteristics of high sensitivity and specificity, high negative or positive predictive value, robustness and reliability, reproducibility, and cost-effectiveness. While detailed comparisons between methodologies are outside the scope of this review, this has been excellently analyzed by Rodic et al. in 2014 [2], Nezos et al. in 2011 [5], and Medic et al. in 2007 [4].
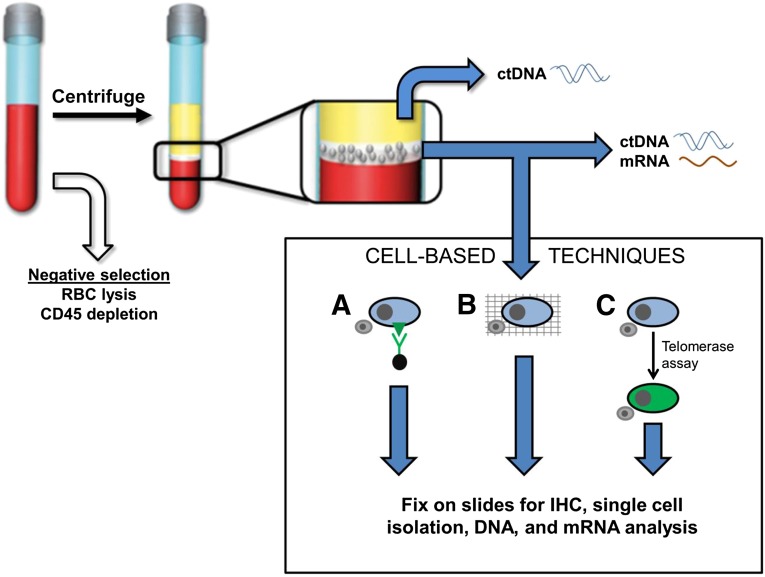
Figure 1.
Overview of circulating tumor cell (CTC), ctDNA, and mRNA isolation techniques. Negative selection applied to whole blood removes RBCs and CD-45-expressing leukocytes. After separation of blood components, ctDNA can be extracted from plasma (yellow layer). The mononuclear layer (white layer) can be used for ctDNA, mRNA, or CTC extraction (illustrated in the box labeled Cell-Based Techniques). CTC detection and isolation can result in downstream IHC analysis, single cell isolation, and DNA and mRNA analysis. Lane A: Surface-marker dependent detection methods use antibodies against melanoma-specific surface marker antigens. Antibodies may be linked to ferrous beads for magnetic pull-down or fluorescent proteins for visualization. White blood cells (WBCs) are represented in the illustration by a small gray cell. Lane B: Surface-marker independent detection methods whereby CTCs may be captured along with WBCs on a fine porous barrier. This is termed the ISET, or isolation by size of epithelial tumor cells, method. Lane C: A telomerase live-cell assay applied to CTCs and WBCs causes cells with elevated telomerase promoter activity to produce high levels of green fluorescent protein.
Abbreviations: ctDNA, circulating tumor DNA; IHC, immunohistochemistry; RBC, red blood cell.
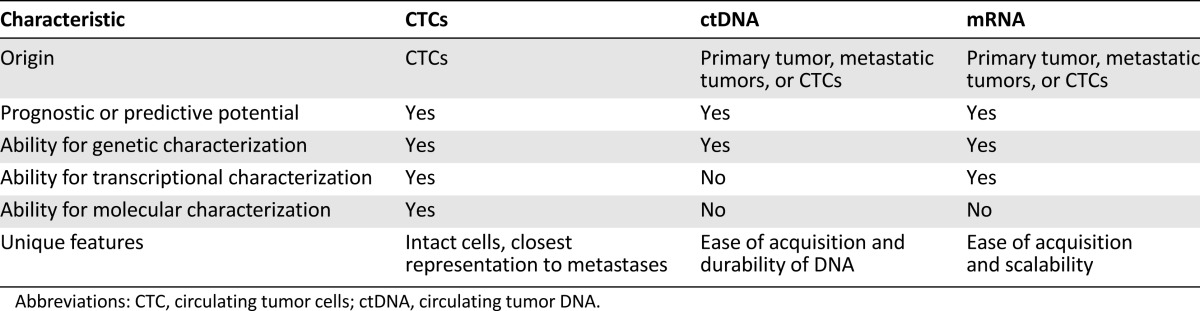
Table 1.
Comparison of CTCs, ctDNA, and mRNA
Circulating Tumor Cells
CTCs are intact cells shed from the primary tumor and detected within peripheral blood samples. In patients with melanoma, the number of detected CTCs range from 0 to more than 10,000 CTCs per 10 mL of blood [3, 15, 16]. In the same blood sample, there may be approximately 100 million leukocytes and 50 billion erythrocytes. Therefore, CTC assays face technical challenges of removing the overwhelming population of white and red blood cells while positively selecting for CTCs.
Rodic et al. recently published a systematic review of CTC detection in melanoma, categorizing isolation strategies into marker-dependent and marker-independent techniques (Fig. 1A, 1B) [2]. Marker-dependent strategies use melanoma-specific surface antigens and immunomagnetic beads to positively select for melanoma CTCs in blood. Surface markers such as high molecular weight melanoma-associated antigen (HMW-MAA), also known as melanoma-associated chondroitin sulfate proteoglycan (MCSP), CD146 or melanoma cell adhesion molecule (MCAM), and ATP-binding cassette subfamily B member 5 (ABCB5) are used either in isolation or combination to facilitate CTC capture [16–22]. In contrast, marker-independent strategies capitalize on CTCs’ physical properties of size and density. Size-based isolation techniques, such as isolation by size of epithelial tumor cells (ISET), use a porous filter to trap large cells (larger than 8 μm) regardless of surface marker expression. The cells can be evaluated for mRNA or DNA mutations or transferred onto a slide for immunohistochemistry (IHC) evaluation [23–25]. Density-based techniques use Ficoll-hypaque or Oncoquick centrifugation separation media to enrich for a layer of cells containing CTCs, suitable for further isolation [15, 26, 27].
What are the particular features of CTCs that may be most useful in the clinic (Table 1)? First, CTCs are intact cells. Among all CTPs, intact CTCs are the closest representation of “human tissue” compatible with IHC and traditional pathology protocols. Furthermore, CTCs may be pooled or analyzed as single cells to facilitate further understanding of CTC genomics, transcriptomics, and even proteomics. These characterizations of single CTCs, however, are prone to the pitfalls of tumor heterogeneity and sampling bias [28]. Clinical protocols such as tracking non-small cell lung cancer evolution through therapy (TRACERx) may help define expectations for heterogeneity in CTC analyses [29]. The study prospectively follows patients with lung cancer through multiregion and longitudinal tumor sampling, seeking to evaluate concordance between CTCs and the genetic composition of sampled metastases. Given the longitudinal nature of the study, the results may help inform the prevalence of CTC heterogeneity as a function of time, treatment, and treatment resistance.
CTCs are also thought to have the potential to seed metastases and, therefore, are valuable for metastasis research and identification of new therapeutic targets [3, 15]. Evidence demonstrating this causal relationship in melanoma and other human malignancies is limited, in part because of slowly maturing technologies in CTC identification and isolation [30]. In breast cancer, human CTCs have been found to give rise to bone, lung, and liver metastases in mice [31]. Small cell lung cancer CTCs have also been shown to produce CTC-derived explants in nude mice [32]. However, more research is needed to establish the putative causal relationship between CTCs and metastases. An important step toward this end is to differentiate live, dead, or dying cells, and identify subpopulations of CTCs relevant in metastasis research [3].
Circulating Tumor DNA
ctDNA refers to circulating DNA fragments containing cancer-specific mutations that are detectable in peripheral blood samples (Fig. 1) [33–35]. The major challenges in identifying ctDNA are in detecting low levels of ctDNA, accurate quantification, and differentiating tumor DNA from normal cell-free DNA circulating in the blood stream.
Diaz and Bardelli recently reviewed the state of the art in ctDNA detection [35]. They describe an evolution of DNA analysis technologies to accurately interrogate small fragments of DNA [35–38]. Approaches include massively parallel paired-end sequencing [39–44] and recently published techniques such as tagged-amplicon deep sequencing [45], cancer personalized profiling by deep sequencing [33], and droplet digital polymerase chain reactions (PCRs) [34, 46]. These strategies allow the extraction of a variety of personalized data about genomic alterations, including copy number variations, point mutations, rearrangements, and methylation patterns.
Compared with CTCs, ctDNA is easier to isolate using existing clinical protocols (Table 1). Many common blood tests, such as serum cholesterol or glucose levels, are already performed via commercially available serum separation tubes. Therefore, ctDNA analysis may integrate easily into the clinical laboratory workflow. Although ctDNA detection precludes single cell analysis, it may reveal important information about tumor heterogeneity. For example, serial sampling of ctDNA from patients with metastatic cancer may provide a means to survey the overall genetic composition of multiple metastases without requiring multiple biopsies [47, 48]. This may lead to downstream identification of candidate treatment resistance genes to facilitate research in drug resistance.
A major limitation of ctDNA analysis is its requirement for a priori knowledge of abnormal DNA sequences. The potential for discovery of new genetic abnormalities with therapeutic or prognostic implications is, therefore, restricted. While ctDNA is thought to be primarily derived from the solid tumor with a small fraction, if any, from CTCs, it is also possible that ctDNA may derive from noncancerous dysplastic tissues or any number of metastatic deposits. Therefore, conclusions made from ctDNA analysis hinge upon the reliability with which the primary tumor is the main source of the ctDNA and reflects changes in overall disease burden, drug sensitivity, or drug resistance.
mRNA
mRNA analysis is predicated on extracting RNA from the mononuclear layer of peripheral blood samples, composed of white blood cells and CTCs (Fig. 1) [49]. Sensitivity for melanoma, as opposed to circulating melanocytes, standardization of the choice of mRNA biomarkers, and agreement on clinically relevant thresholds remain consistent challenges for this approach [50, 51].
Since this technique was first described by Smith et al. in 1991, using tyrosinase mRNA PCR analysis, several additional candidate mRNA biomarkers have been proposed, including melanoma antigen recognized by T cells (MART-1), gp-100, melanoma-associated antigen 3 (MAGE-A3), paired box 3 transcription factor (PAX3), and β-1,4 N-acetylgalactosaminyltransferase (GalNAc-T) in various combinations [2, 4, 20, 21, 50, 52–57]. Even within the John Wayne Cancer Institute Group, who used this technique with two international multicenter trials published in the same year, the choice of biomarker cocktail has not been consistent [56, 57]. However, research groups tend to agree on the basic principles of the technique. Red blood cells in peripheral blood samples are lysed and RNA is extracted from the remaining intact cells, consisting presumably of white blood cells and CTCs. The RNA is converted to complementary DNA (cDNA) using reverse transcription. A cocktail of PCR primers for any of the abovementioned biomarkers is then added to cDNA and subsequently analyzed in quantitative PCR amplification.
The primary strength of mRNA analysis is its ease of acquisition and scalability (Table 1). reverse transcriptase PCR studies have already been successfully embedded in international multicenter clinical trials involving more than 1,000 patients in total [54, 56, 57]. However, mRNA analysis has not yet been used to identify key genetic abnormalities in melanoma, such as BRAF, c-Kit, and PTEN (phosphatase and tensin homolog). Unlike ctDNA isolation, RNA analysis has not provided clues as to differential protein amplification or expression profiles present among individuals with melanoma. A concerning study using tyrosinase mRNA as a biomarker found positive signal in a patient with a benign congenital nevus, bringing into question the specificity of tyrosinase and other melanocytic markers that serve as surrogates for CTCs [51]. Because of the long-term instability of RNA and cDNA when stored in freezers, the potential for using extracted products for downstream analysis and future applications is limited to approximately 1 year [49]. In addition, it is unclear to what extent these RNA products are derived from CTCs and not solid tumor. Even if they are CTC derivatives, whether these RNA products are derived from live or dying cells remains a further important question.
Applications Toward Melanoma Diagnosis
Screening
Cancer screening, especially through the use of a noninvasive blood test, is of great interest to translational researchers, public health workers, policy makers, clinicians, and patients alike. CTPs can have high sensitivity for early-stage disease, and studies have successfully described the ability to detect ctDNA or mRNA in early-stage melanoma and other cancers [44, 53]. The implications are enormous, as many cancers such as ovarian and pancreatic carcinomas tend to present clinically in later stages and may serve to benefit the most from early detection.
Considering current and past candidate cancer-screening tests, we learn that the barriers to implementation are quite high because of intense scrutiny over the cost-benefit ratio for these tests. The prostate-specific antigen test is the subject of unyielding controversy between the potential survival benefit of detecting prostate cancer and the harm of overdiagnosis and overtreatment [58]. Other important and well-known biomarkers, such as CA-125, CA19-9, and carcinoembryonic antigen are expressly not recommended for use in cancer screening because they fail to meet specificity requirements. To meet criteria for a screening biomarker, large-scale studies need to be conducted to demonstrate cost-effectiveness, improvement in disease outcomes as a result of early detection, reliable test performance, and technology scalability.
Melanoma, however, is already detected at an early stage because of increased public awareness and use of regular skin checks. Melanoma is less likely to benefit from an additional biochemical screening test compared with other malignancies that present more frequently at an advanced stage, such as ovarian and lung cancers. Therefore, the consideration for CTPs as screening biomarkers relies not only on the test performance and scalability, but also on the chosen disease sites for which cost-effectiveness analysis is performed.
Diagnostics
Another topic generating great enthusiasm is the potential for CTPs to aid in cancer diagnostics. Importantly, cancer diagnostics have been traditionally performed using tissue samples obtained after visual or radiographic observation of abnormal masses. Without the ability to obtain tissue and stain for tumor-specific markers, the diagnosis of cancer is typically limited to radiologic appearance, location of disease, and clinical experience alone. In current clinical practice among oncologists, pathologists, and radiologists, there remains a strong preference for tissue and the visualization of tumor-specific staining as necessary criteria for cancer diagnoses and subsequent treatment.
Among CTPs, only intact CTCs fixed to slides are able to satisfy the minimal requirements for cancer diagnosis—the presence of tissue that can be subjected to staining for tumor-specific antigens (Fig. 2). However, no studies have yet investigated CTCs in melanoma diagnosis. While circulating mRNA can be found in patients with early-stage disease [53], the potential of mRNA or ctDNA as a diagnostic tool seems limited because of the incompatibility of this technology with commonly used methods for melanoma cancer diagnosis.
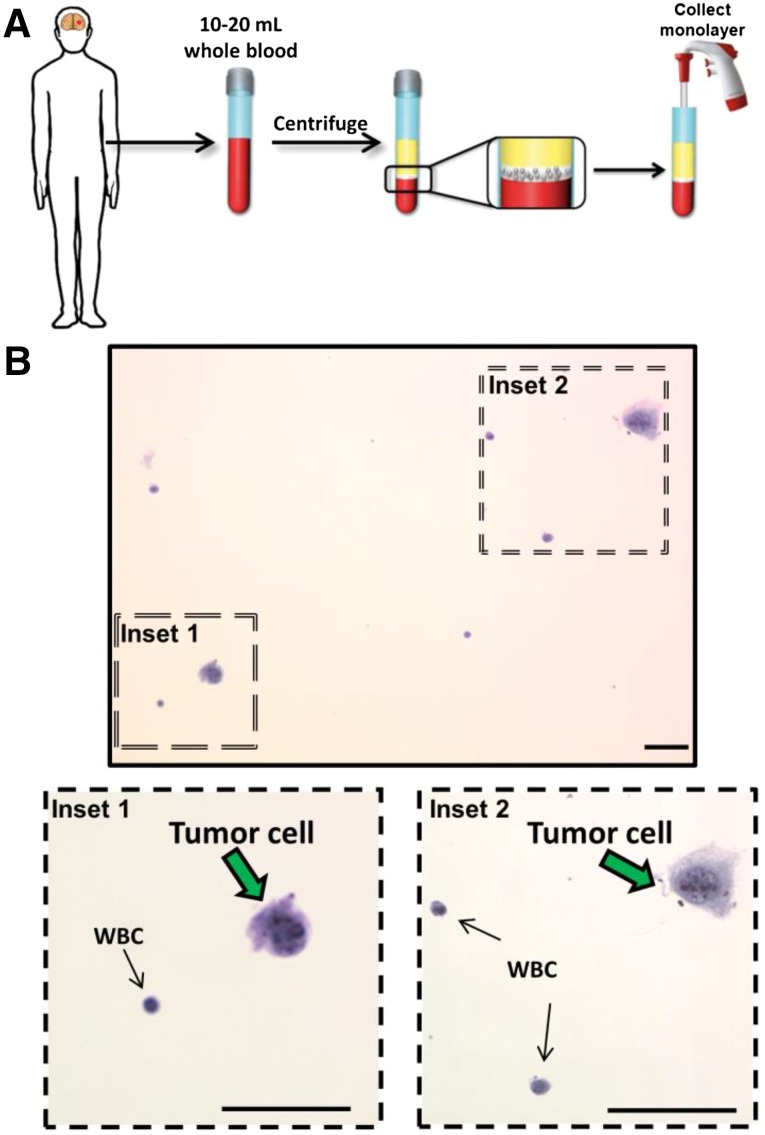
Figure 2.
Schematic of a potential cancer diagnosis strategy using circulating tumor cells (CTCs) as stainable tissue. (A): Patients’ blood samples are centrifuged and the monolayer of CTCs and WBCs are collected. (B): After ThinPrep transfer onto a microscope slide, H&E stain is applied. In this proof-of-concept example, cultured cancer cells are spiked into control blood samples and identified after H&E staining. Inset 1 demonstrates a tumor cell adjacent to a benign and small WBC. Inset 2 demonstrates a tumor cell adjacent to 2 WBCs. Scale bars = 50 μm.
Abbreviation: WBC, white blood cell.
An important and emerging alternative application of CTPs to diagnostics is the ability to corroborate radiologic findings in the subset of patients for whom tissue biopsy is unsafe.
An important and emerging alternative application of CTPs to diagnostics is the ability to corroborate radiologic findings in the subset of patients for whom tissue biopsy is unsafe. For instance, consider the frail patient with prior history of melanoma resection who presents with new development of multiple subcentimeter radiographic findings thought to represent lung or brain metastases. In this scenario, if an alternative method such as CTCs, ctDNA, or mRNA can reliably, sensitively, and specifically confirm the presence of cancer, there may be an important role for CTPs in diagnostics.
Directing Clinical Decision-Making
In light of the increased complexity of treatment options for patients with melanoma, particularly the advent of novel immune and checkpoint antibody treatments, the need for new biomarkers to direct clinical decision making in melanoma is great [59].
CTPs as Prognostic Biomarkers
CTCs are already U.S. Food and Drug Administration approved as overall survival (OS) and progression-free survival (PFS) prognostic biomarkers in prostate, breast, and colon cancer [60–62]. In melanoma, the majority of studies analyzing patient samples also seek to make conclusions about the prognostic value of CTCs, ctDNA, and mRNA (Table 2).
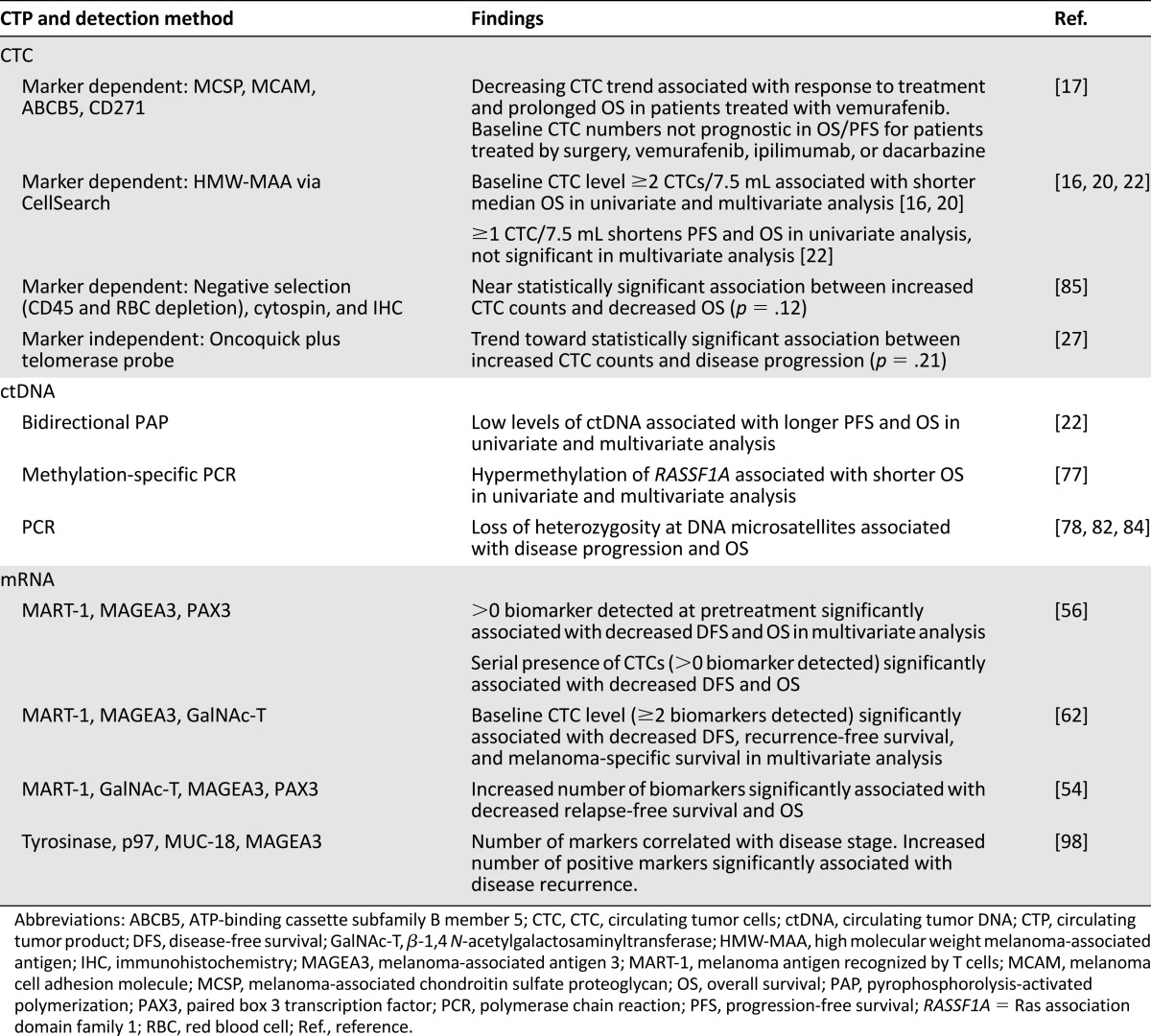
Table 2.
Evidence suggesting prognostic significance of CTCs, ctDNA, and mRNA in patients with melanoma
Many groups have already shown statistically significant associations between the detection of CTPs and poor clinical outcomes in OS and PFS (Table 2). Sample sizes for these studies tend to be small, so findings should be considered cautiously optimistic and still investigational. Even in studies with large sample sizes (n > 200), results should be interpreted with caution, as these studies pooled patients from both arms of multicenter therapeutic clinical trials. Thus, the OS and PFS rates may not be reflective of prognoses expected with standard treatment [55–57].
The added value of CTP prognostic information is an important consideration for application in clinical melanoma management. Melanoma already has a number of validated histological, clinical, and blood-based prognostic biomarkers. CTP prognostic claims, therefore, must compete with existing metrics for physician adoption and, importantly, insurance coverage. This is illustrated in the example of the CellSearch CTC platform (Janssen Diagnostics, Raritan, NJ, https://www.cellsearchctc.com) in metastatic breast cancer. Despite validation of prognostic utility, the added clinical value is limited [60, 63, 64]. This has been directly expressed in Medicare local coverage determinations not to cover CTC analyses and is indirectly evident in differential coverage decisions by private insurance companies across the U.S. [65, 66].
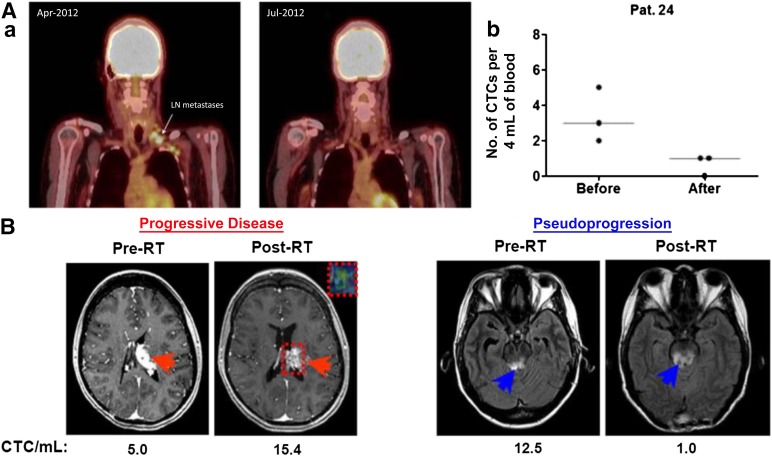
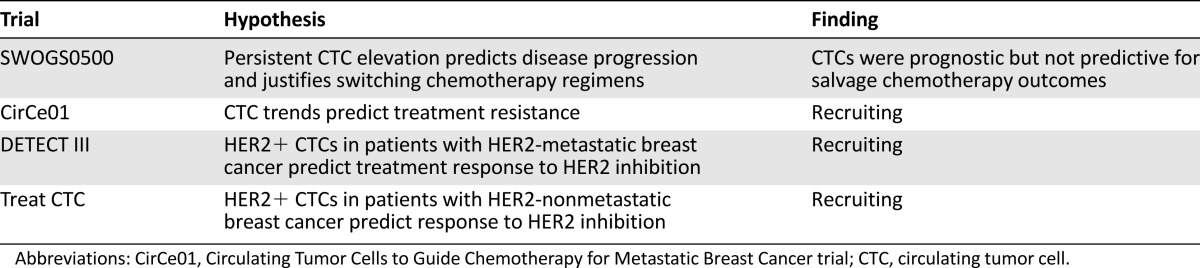
Another important application of CTPs as prognostic biomarkers lies in the correlation between CTPs and traditional prognosticators such as stage and disease volume [22, 53–55]. Of particular use to clinical melanoma management is the ability to further risk stratify patients with stage II (sentinel lymph node negative) disease into groups of low and high risk for recurrence. While studies have shown direct mRNA analysis of sentinel lymph nodes can lead to upstaging of disease and offer prognostic value, this finding has not been corroborated using CTP analysis in peripheral blood draws [67, 68]. CTP trends have also been used as markers of disease burden, response to therapy, and predictors of clinical outcomes (Fig. 3A) [17, 20]. These trends may be especially helpful in diseases for which radiographic imaging is difficult to interpret in the postoperative or postradiation setting (i.e., pseudoprogression vs. true progression after immunotherapy in patients with melanoma). Figure 3B illustrates the benefit of CTP analysis in evaluating pseudoprogression in glioblastoma.
Figure 3.
CTC levels may correspond to disease status. (Aa): Illustration of therapeutic response in a patient with metastatic melanoma who was treated with vemurafenib (reproduced from Klinac et al. [17], original vertical display edited to horizontal). Left panel: Representative images of the positron emission tomography scans before and during vemurafenib treatment. The arrow indicates lymph node metastasis detected prior to treatment and a complete metabolic response 2 months after treatment. Right panel: Reduction in the number of CTCs in 4 mL of whole blood in the same patient. A total of 12 mL of blood was collected at each time point (three 4-mL tubes). (Ab): The graph illustrates the number of CTCs found in each of the three blood samples and the median for each time point. (B): CTC trends differentiating between progressive disease and pseudoprogression in two patients with glioblastoma (reproduced with permission from MacArthur et al. [95]). Magnetic resonance imaging (MRI) was performed within 2 weeks prior to initiation of RT and approximately 1 month following completion of treatment. CTC results (given as number of CTCs per mL) are below the axial MR image at the respective time points. Red arrows indicate a left thalamic lesion prior to and following RT (left panels). The inset box delineated by the dotted red line in the post-RT image demonstrates the tumor area of interest and the associated advanced MRI relative cerebral blood volume map, which confirmed active tumor progression. Blue arrows indicate MR signal abnormality in the midbrain lesion and surrounding area on axial view prior to and following RT (right panels).
Abbreviations: CTC, circulating tumor cell; LN, lymph node; Pat. 24, patient 24; RT, radiation therapy.
CTPs as Predictive Biomarkers
CTP predictive potential for therapeutic outcome is perhaps its most compelling clinical application. This has been most recently modeled by the Oncotype DX assay for breast cancer (Genomic Health, Redwood City, CA, http://www.oncotypedx.com) [69–71]. Initially marketed as a prognostic biomarker [72], its transition to a predictive biomarker [73–75] for response to chemotherapy has contributed significantly to its increasing use among clinicians [76]. Thus, to appeal more strongly to clinicians, CTP studies should seek to progress from prognostic to prediction studies.
Few predictive studies have been reported in melanoma CTP-related publications. The presence of ctDNA in the form of methylated RASSF1A (Ras association domain family 1) or loss of heterozygosity at microsatellite regions has shown potential utility in predicting response to chemotherapy and/or immune modulating therapy [77, 78]. In another study of CTC trends in patients with melanoma who were treated with vemurafenib (n = 8), a decrease in CTC counts was associated good treatment response [17]. Such studies have been limited by small sample size and more patients are needed to elucidate the predictive value of CTP assays.
Among other disease sites, such as breast cancer, several international interventional clinical trials have been initiated to determine the predictive value of the CellSearch CTC enumeration platform (Table 3) [64]. The SWOG500 study, which opened for accrual in 2006, was the first clinical trial incorporating CTCs as a biomarker to inform treatment-arm stratification. This randomized phase III trial for metastatic breast cancer was designed to determine whether persistently high CTC levels (≥5 CTCs per 7.5 mL) after the first cycle of chemotherapy could indicate disease progression, and to determine whether an early switch to alternative chemotherapy would result in improved prognostic outcomes [63]. Early reported results redemonstrated the prognostic value of baseline CTCs but did not show improvement in overall survival from switching chemotherapies early. This study’s weaknesses include the heterogeneity of chemotherapy regimens allowed on the trial, poor outcomes of salvage chemotherapy regardless of switching treatment regimens, and lack of prior evidence that CTC values 3 weeks after treatment initiation can reliably assess treatment response. More studies are needed to determine how CTCs may offer the most predictive value. Three ongoing international clinical trials continue to investigate the association between CTC counts and surface marker expression profiles (i.e., human epidermal growth receptor 2 [HER2] expression) that may be associated with treatment response. Similarly designed predictive trials represent important next steps for melanoma CTP studies.
Table 3.
Clinical trials investigating the predictive value of CTCs in breast cancers
CTPs May Provide Genetic and Molecular Characterization
The ability to characterize a tumor’s changing genetic and molecular features offers valuable implications in precision medicine from clinical trial design to risk stratification and treatment response prediction [80]. In breast cancer, for example, CTC clinical trials are currently prescribing HER2 inhibition therapy to otherwise HER2-negative breast cancer patients on the basis of CTC analyses identifying HER2 positivity (Table 3) [79]. In melanoma management, the utility of genetic and molecular characterization is equally important, if not more so, because of the increased availability of targeted agents against BRAF and mitogen-activated protein kinase kinase (MEK), as well as other immunotherapies [10–14].
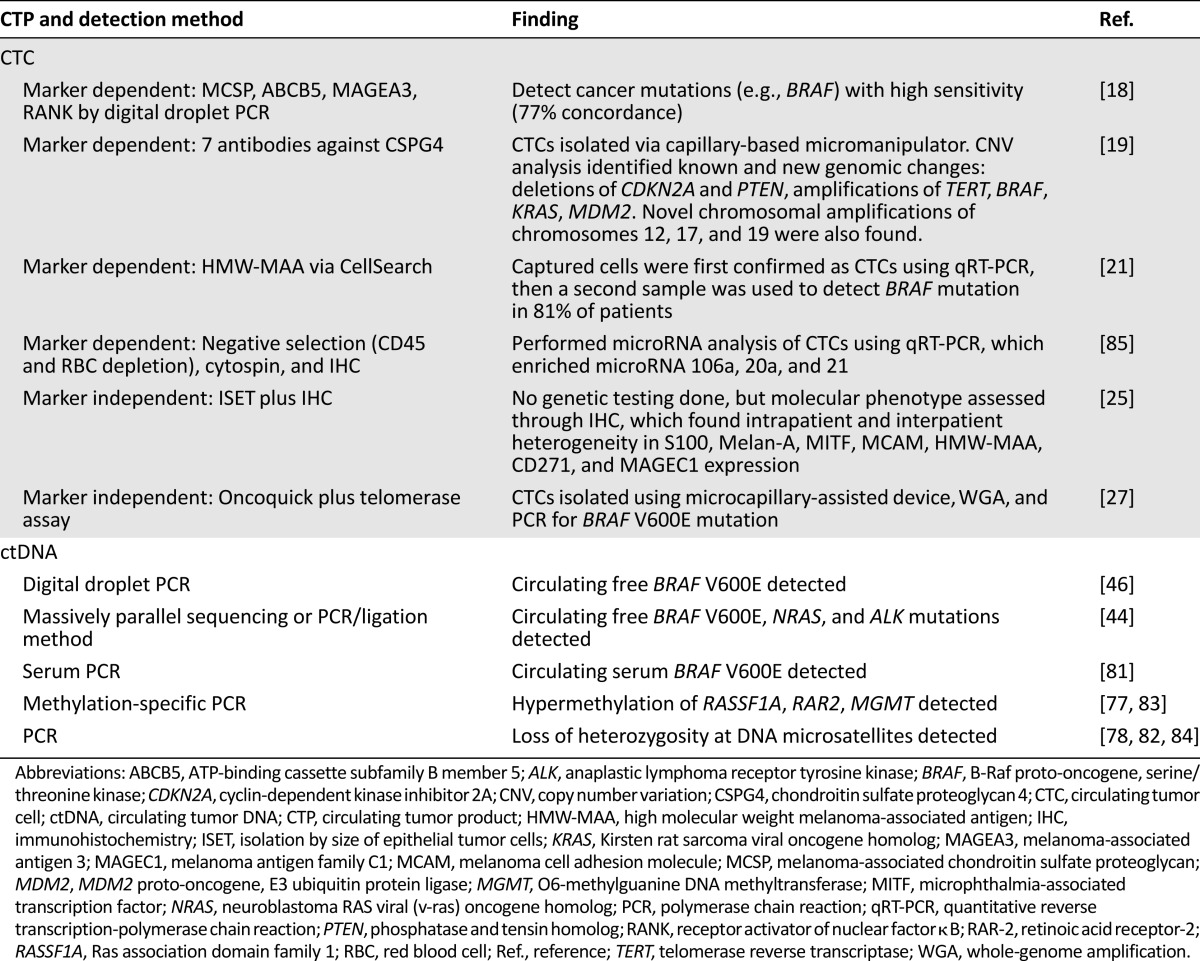
Genetic characterization has been widely achieved in melanoma CTP studies (Table 4). A subset of known melanoma-associated mutations with therapeutic relevance, such as BRAF V600E and Kirsten rat sarcoma viral oncogene homolog (KRAS), has been identified via CTC isolation and in ctDNA studies [18, 21, 27, 44, 46, 81]. Additional genetic studies have identified genomic changes with potential relevance as biomarkers, such as hypermethylated DNA and loss of heterozygosity at DNA microsatellite regions [77, 78, 82–84]. However, many CTC studies do not visually confirm the presence of CTCs prior to DNA extraction and analysis [18, 21, 85]. This may be motivated by ease of harvesting genetic material, but it also compromises the assurance that the detected mutations are derived from intact circulating cells. Currently, the only mechanism of isolating intact melanoma CTCs for DNA extraction and analysis is through microcapillary dissection [19, 27].
Table 4.
Genetic data obtained from CTCs and ctDNA in patients with melanoma
The identification of melanoma-specific genetic and molecular mutations has primarily served as proof-of-concept findings in many CTC and ctDNA studies, but these changes may also have potential prognostic and predictive significance [77, 78, 82–84]. For example, a CTP analysis that detects mutations (e.g., epidermal growth factor receptor [EGFR] T790M) or molecular changes (e.g., downregulation of programmed death-ligand 1 [PD-L1]) related to drug resistance may motivate earlier initiation of next-line therapy. Or, CTP analysis may identify patients who are eligible for targeted therapies when previous biopsies demonstrated they were ineligible (e.g., biopsy to CTP conversion from HER2− to HER2+ or PD-L1− to PD-L1+) [79]. Serving as a noninvasive biopsy of genetic and molecular changes, CTPs may, therefore, be helpful in illuminating new drug sensitivities or resistances.
Tumor Material Available for Future Discovery
CTPs may unlock new understanding of melanoma progression mechanisms that lead to the development of novel therapies and decision-making tools. For example, CTCs may be used to establish cell lines for long-term experimentation. Although no melanoma CTC cultures have been reported in the literature to date, CTCs have been successfully isolated and cultured in colon, gastric, and pancreatic cancers [86–88]. These are important recent developments resulting from maturing CTC isolation technologies. In the way that cultured human cancer cell lines revolutionized cancer research, cultured CTCs may have a profound impact on our understanding of cancer dynamics, metastasis, and drug resistance, and may enable identification of new drug targets.
In the absence of cell lines, emerging capabilities in single-cell and small-sample analysis enables genetic, transcriptional, and molecular discovery in CTPs. Melanoma ctDNA studies have already identified hypermethylation and loss of heterozygosity mutations with prognostic significance.
In the absence of cell lines, emerging capabilities in single-cell and small-sample analysis enables genetic, transcriptional, and molecular discovery in CTPs. Melanoma ctDNA studies have already identified hypermethylation and loss of heterozygosity mutations with prognostic significance [77, 78, 82, 84]. Array-based analyses incorporating the most common ctDNA mutations among all cancers may be used to identify new associations between known mutations and cancer sites [33]. A growing body of evidence is also evolving to define changes that initiate survival in circulation and metastasis development [30]. For example, several groups have detected increased expression of mRNA regulating epithelial-mesenchymal transition in CTCs from breast, colon, and head and neck cancers [89–92]. Other studies in lung cancer suggest survival in circulation and resistance to apoptotic stimuli may be mediated by CTC clustering [93, 94]. Distinguishing among live, dead, and dying cells may further facilitate ongoing research in CTCs and metastases [3]. Importantly, a methodology has been previously described to isolate live CTCs and has been effective for melanoma, glioma, bladder cancer, and non-small cell lung cancer [27, 95–97]. The continued development of this technique, along with other CTP analysis methods, enables better understanding of tumor changes that accumulate over the course of treatment.
The Role of the Clinical Researcher
Clinician input into the development of CTP technologies is critical. With such a diverse array of potential applications, insight into the types of CTP outcomes that will address the unmet needs in cancer management is extremely valuable. Furthermore, incorporating CTPs as secondary or exploratory outcomes in clinical trials may contribute significantly to the literature around predictive and prognostic value of these novel biomarkers. Clinicians are also likely able to foresee paradigm hurdles, such as how the importance of tissue staining in cancer diagnosis may pose barriers for the adoption of ctDNA technologies in diagnostics. Therefore, clinicians can offer valuable insight to the development of this technology and should feel empowered to further investigate how CTPs can benefit their patients in the future.
Conclusion
CTPs have strong potential to change the practice of melanoma management. Several studies have established their prognostic value, and future clinical protocols should be designed to further elucidate the predictive or diagnostic values of CTPs. As liquid biopsy specimens, CTPs offer the exciting potential to evaluate real-time changes in tumor genetics that confer new drug resistance or sensitivity. CTPs also enable the discovery of novel biomarkers, drug targets, and insight into the biology of melanoma metastasis. Given the variety of practice-changing CTP applications, involving clinical researchers to forecast and participate in the development of this technology is critical.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Melody J. Xu, Jay F. Dorsey, Ravi Amaravadi, Giorgos Karakousis, Charles B. Simone II, Xiaowei Xu, Lynn Schuchter, Gary D. Kao
Provision of study material or patients: Melody J. Xu, Jay F. Dorsey, Lynn Schuchter
Collection and/or assembly of data: Melody J. Xu, Jay F. Dorsey, Ravi Amaravadi, Charles B. Simone II, Xiaowei Xu, Wei Xu, Erica L. Carpenter, Lynn Schuchter, Gary D. Kao
Data analysis and interpretation: Melody J. Xu, Jay F. Dorsey, Ravi Amaravadi, Giorgos Karakousis, Charles B. Simone II, Xiaowei Xu, Wei Xu, Erica L. Carpenter, Lynn Schuchter, Gary D. Kao
Manuscript writing: Melody J. Xu, Ravi Amaravadi, Giorgos Karakousis, Charles B. Simone II, Xiaowei Xu, Erica L. Carpenter, Gary D. Kao
Final approval of manuscript: Melody J. Xu, Jay F. Dorsey, Ravi Amaravadi, Giorgos Karakousis, Charles B. Simone II, Xiaowei Xu, Wei Xu, Erica L. Carpenter, Lynn Schuchter, Gary D. Kao
Disclosures
Giorgos Karakousis: AMGEN, Castle Biosciences (H); Erica L. Carpenter: Janssen, Sysmex/Inostics (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Lianidou ES, Strati A, Markou A. Circulating tumor cells as promising novel biomarkers in solid cancers. Crit Rev Clin Lab Sci. 2014;51:160–171. doi: 10.3109/10408363.2014.896316. [DOI] [PubMed] [Google Scholar]
- 2.Rodic S, Mihalcioiu C, Saleh RR. Detection methods of circulating tumor cells in cutaneous melanoma: A systematic review. Crit Rev Oncol Hematol. 2014;91:74–92. doi: 10.1016/j.critrevonc.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Mumford BS, Robertson GP. Circulating melanoma cells in the diagnosis and monitoring of melanoma: An appraisal of clinical potential. Mol Diagn Ther. 2014;18:175–183. doi: 10.1007/s40291-013-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medic S, Pearce RL, Heenan PJ, et al. Molecular markers of circulating melanoma cells. Pigment Cell Res. 2007;20:80–91. doi: 10.1111/j.1600-0749.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 5.Nezos A, Msaouel P, Pissimissis N, et al. Methods of detection of circulating melanoma cells: a comparative overview. Cancer Treat Rev. 2011;37:284–290. doi: 10.1016/j.ctrv.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Kounalakis N, Goydos JS. Tumor cell and circulating markers in melanoma: Diagnosis, prognosis, and management. Curr Oncol Rep. 2005;7:377–382. doi: 10.1007/s11912-005-0065-2. [DOI] [PubMed] [Google Scholar]
- 7.Khoja L, Lorigan P, Dive C, et al. Circulating tumour cells as tumour biomarkers in melanoma: Detection methods and clinical relevance. Ann Oncol. 2015;26:33–39. doi: 10.1093/annonc/mdu207. [DOI] [PubMed] [Google Scholar]
- 8.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 10.Wolchok JD, Hodi FS, Weber JS, et al. Development of ipilimumab: A novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci. 2013;1291:1–13. doi: 10.1111/nyas.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: Clinical impact and future directions. Cancer Lett. 2007;253:180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Rao C, Bui T, Connelly M, et al. Circulating melanoma cells and survival in metastatic melanoma. Int J Oncol. 2011;38:755–760. doi: 10.3892/ijo.2011.896. [DOI] [PubMed] [Google Scholar]
- 17.Klinac D, Gray ES, Freeman JB, et al. Monitoring changes in circulating tumour cells as a prognostic indicator of overall survival and treatment response in patients with metastatic melanoma. BMC Cancer. 2014;14:423. doi: 10.1186/1471-2407-14-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid AL, Freeman JB, Millward M, et al. Detection of BRAF-V600E and V600K in melanoma circulating tumour cells by droplet digital PCR. Clin Biochem. 2015;48:999–1002. doi: 10.1016/j.clinbiochem.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz C, Li J, Luttgen MS, et al. Limited genomic heterogeneity of circulating melanoma cells in advanced stage patients. Phys Biol. 2015;12:016008. doi: 10.1088/1478-3975/12/1/016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoja L, Lorigan P, Zhou C, et al. Biomarker utility of circulating tumor cells in metastatic cutaneous melanoma. J Invest Dermatol. 2013;133:1582–1590. doi: 10.1038/jid.2012.468. [DOI] [PubMed] [Google Scholar]
- 21.Kitago M, Koyanagi K, Nakamura T, et al. mRNA expression and BRAF mutation in circulating melanoma cells isolated from peripheral blood with high molecular weight melanoma-associated antigen-specific monoclonal antibody beads. Clin Chem. 2009;55:757–764. doi: 10.1373/clinchem.2008.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bidard FC, Madic J, Mariani P, et al. Detection rate and prognostic value of circulating tumor cells and circulating tumor DNA in metastatic uveal melanoma. Int J Cancer. 2014;134:1207–1213. doi: 10.1002/ijc.28436. [DOI] [PubMed] [Google Scholar]
- 23.Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: A new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000;156:57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Giorgi V, Pinzani P, Salvianti F, et al. Application of a filtration- and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J Invest Dermatol. 2010;130:2440–2447. doi: 10.1038/jid.2010.141. [DOI] [PubMed] [Google Scholar]
- 25.Khoja L, Shenjere P, Hodgson C, et al. Prevalence and heterogeneity of circulating tumour cells in metastatic cutaneous melanoma. Melanoma Res. 2014;24:40–46. doi: 10.1097/CMR.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 26.Clawson GA, Kimchi E, Patrick SD, et al. Circulating tumor cells in melanoma patients. PLoS One. 2012;7:e41052. doi: 10.1371/journal.pone.0041052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu MJ, Cooke M, Steinmetz D, et al. A novel approach for the detection and genetic analysis of live melanoma circulating tumor cells. PLoS One. 2015;10:e0123376. doi: 10.1371/journal.pone.0123376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamal-Hanjani M, Quezada SA, Larkin J, et al. Translational implications of tumor heterogeneity. Clin Cancer Res. 2015;21:1258–1266. doi: 10.1158/1078-0432.CCR-14-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamal-Hanjani M, Hackshaw A, Ngai Y, et al. Tracking genomic cancer evolution for precision medicine: The lung TRACERx study. PLoS Biol. 2014;12:e1001906. doi: 10.1371/journal.pbio.1001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caixeiro NJ, Kienzle N, Lim SH, et al. Circulating tumour cells–a bona fide cause of metastatic cancer. Cancer Metastasis Rev. 2014;33:747–756. doi: 10.1007/s10555-014-9502-8. [DOI] [PubMed] [Google Scholar]
- 31.Baccelli I, Schneeweiss A, Riethdorf S, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 32.Hodgkinson CL, Morrow CJ, Li Y, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med. 2014;20:897–903. doi: 10.1038/nm.3600. [DOI] [PubMed] [Google Scholar]
- 33.Newman AM, Bratman SV, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipson EJ, Velculescu VE, Pritchard TS, et al. Circulating tumor DNA analysis as a real-time method for monitoring tumor burden in melanoma patients undergoing treatment with immune checkpoint blockade. J Immunother Cancer. 2014;2:42. doi: 10.1186/s40425-014-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz LA, Jr, Bardelli A. Liquid biopsies: Genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogelstein B, Kinzler KW. Digital PCR. Proc Natl Acad Sci USA. 1999;96:9236–9241. doi: 10.1073/pnas.96.16.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dressman D, Yan H, Traverso G, et al. Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proc Natl Acad Sci USA. 2003;100:8817–8822. doi: 10.1073/pnas.1133470100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q, Sommer SS. Pyrophosphorolysis-activated polymerization (PAP): Application to allele-specific amplification. Biotechniques. 2000;29:1072–1076, 1078, 1080 passim. doi: 10.2144/00295rr03. [DOI] [PubMed] [Google Scholar]
- 39.Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leary RJ, Kinde I, Diehl F, et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med. 2010;2:20ra14. doi: 10.1126/scitranslmed.3000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leary RJ, Sausen M, Kinde I, et al. Detection of chromosomal alterations in the circulation of cancer patients with whole-genome sequencing. Sci Transl Med. 2012;4:162ra154. doi: 10.1126/scitranslmed.3004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McBride DJ, Orpana AK, Sotiriou C, et al. Use of cancer-specific genomic rearrangements to quantify disease burden in plasma from patients with solid tumors. Genes Chromosomes Cancer. 2010;49:1062–1069. doi: 10.1002/gcc.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell PJ, Stephens PJ, Pleasance ED, et al. Identification of somatically acquired rearrangements in cancer using genome-wide massively parallel paired-end sequencing. Nat Genet. 2008;40:722–729. doi: 10.1038/ng.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forshew T, Murtaza M, Parkinson C, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012;4:136ra68. doi: 10.1126/scitranslmed.3003726. [DOI] [PubMed] [Google Scholar]
- 46.Sanmamed MF, Fernández-Landázuri S, Rodríguez C, et al. Quantitative cell-free circulating BRAFV600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin Chem. 2015;61:297–304. doi: 10.1373/clinchem.2014.230235. [DOI] [PubMed] [Google Scholar]
- 47.Murtaza M, Dawson SJ, Tsui DW, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108–112. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 48.Pantel K, Diaz LA, Jr, Polyak K. Tracking tumor resistance using ‘liquid biopsies’. Nat Med. 2013;19:676–677. doi: 10.1038/nm.3233. [DOI] [PubMed] [Google Scholar]
- 49.Kiyohara E, Hata K, Lam S, et al. Circulating tumor cells as prognostic biomarkers in cutaneous melanoma patients. Methods Mol Biol. 2014;1102:513–522. doi: 10.1007/978-1-62703-727-3_27. [DOI] [PubMed] [Google Scholar]
- 50.Mocellin S, Hoon D, Ambrosi A, et al. The prognostic value of circulating tumor cells in patients with melanoma: A systematic review and meta-analysis. Clin Cancer Res. 2006;12:4605–4613. doi: 10.1158/1078-0432.CCR-06-0823. [DOI] [PubMed] [Google Scholar]
- 51.De Giorgi V, Pinzani P, Salvianti F, et al. Circulating benign nevus cells detected by ISET technique: Warning for melanoma molecular diagnosis. Arch Dermatol. 2010;146:1120–1124. doi: 10.1001/archdermatol.2010.264. [DOI] [PubMed] [Google Scholar]
- 52.Smith B, Selby P, Southgate J, et al. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet. 1991;338:1227–1229. doi: 10.1016/0140-6736(91)92100-g. [DOI] [PubMed] [Google Scholar]
- 53.Koyanagi K, Kuo C, Nakagawa T, et al. Multimarker quantitative real-time PCR detection of circulating melanoma cells in peripheral blood: relation to disease stage in melanoma patients. Clin Chem. 2005;51:981–988. doi: 10.1373/clinchem.2004.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koyanagi K, O’Day SJ, Gonzalez R, et al. Serial monitoring of circulating melanoma cells during neoadjuvant biochemotherapy for stage III melanoma: Outcome prediction in a multicenter trial. J Clin Oncol. 2005;23:8057–8064. doi: 10.1200/JCO.2005.02.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koyanagi K, O’Day SJ, Boasberg P, et al. Serial monitoring of circulating tumor cells predicts outcome of induction biochemotherapy plus maintenance biotherapy for metastatic melanoma. Clin Cancer Res. 2010;16:2402–2408. doi: 10.1158/1078-0432.CCR-10-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoshimoto S, Faries MB, Morton DL, et al. Assessment of prognostic circulating tumor cells in a phase III trial of adjuvant immunotherapy after complete resection of stage IV melanoma. Ann Surg. 2012;255:357–362. doi: 10.1097/SLA.0b013e3182380f56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoshimoto S, Shingai T, Morton DL, et al. Association between circulating tumor cells and prognosis in patients with stage III melanoma with sentinel lymph node metastasis in a phase III international multicenter trial. J Clin Oncol. 2012;30:3819–3826. doi: 10.1200/JCO.2011.40.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barry MJ. Screening for prostate cancer–the controversy that refuses to die. N Engl J Med. 2009;360:1351–1354. doi: 10.1056/NEJMe0901166. [DOI] [PubMed] [Google Scholar]
- 59.Karagiannis P, Fittall M, Karagiannis SN. Evaluating biomarkers in melanoma. Front Oncol. 2014;4:383. doi: 10.3389/fonc.2014.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 61.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 62.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 63.Raimondi C, Gradilone A, Naso G, et al. Clinical utility of circulating tumor cell counting through CellSearch(®): The dilemma of a concept suspended in Limbo. Onco Targets Ther. 2014;7:619–625. doi: 10.2147/OTT.S46200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–414. doi: 10.1016/S1470-2045(14)70069-5. [DOI] [PubMed] [Google Scholar]
- 65.Final Comment and Response. Available at http://downloads.cms.gov/medicare-coverage-database/lcd_attachments/32218_1/DL32218_PATH033_FinalComments.pdf. Accessed May 19, 2015.
- 66.Janssen Diagnostics. Reimbursement. 2015. Available at https://www.cellsearchctc.com/support-resources/reimbursement. Accessed November 9, 2015.
- 67.Mocellin S, Hoon DS, Pilati P, et al. Sentinel lymph node molecular ultrastaging in patients with melanoma: A systematic review and meta-analysis of prognosis. J Clin Oncol. 2007;25:1588–1595. doi: 10.1200/JCO.2006.09.4573. [DOI] [PubMed] [Google Scholar]
- 68.Nicholl MB, Elashoff D, Takeuchi H, et al. Molecular upstaging based on paraffin-embedded sentinel lymph nodes: Ten-year follow-up confirms prognostic utility in melanoma patients. Ann Surg. 2011;253:116–122. doi: 10.1097/SLA.0b013e3181fca894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carlson JJ, Roth JA. The impact of the Oncotype Dx breast cancer assay in clinical practice: A systematic review and meta-analysis. Breast Cancer Res Treat. 2013;141:13–22. doi: 10.1007/s10549-013-2666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Partin JF, Mamounas EP. Impact of the 21-gene recurrence score assay compared with standard clinicopathologic guidelines in adjuvant therapy selection for node-negative, estrogen receptor-positive breast cancer. Ann Surg Oncol. 2011;18:3399–3406. doi: 10.1245/s10434-011-1698-z. [DOI] [PubMed] [Google Scholar]
- 71.Asad J, Jacobson AF, Estabrook A, et al. Does oncotype DX recurrence score affect the management of patients with early-stage breast cancer? Am J Surg. 2008;196:527–529. doi: 10.1016/j.amjsurg.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 72.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 73.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 74.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.National Cancer Institute. The TAILORx Breast Cancer Trial. Updated September 28, 2015. Available at http://www.cancer.gov/clinicaltrials/noteworthy-trials/tailorx. Accessed November 9, 2015.
- 76.Genomic Health. Genomic Health announces year-end 2014 financial results, provides 2015 financial outlook. February 10, 2015. Available at http://www.prnewswire.com/news-releases/genomic-health-announces-year-end-2014-financial-results-provides-2015-financial-outlook-300033988.html. Accessed February 13, 2015.
- 77.Mori T, O’Day SJ, Umetani N, et al. Predictive utility of circulating methylated DNA in serum of melanoma patients receiving biochemotherapy. J Clin Oncol. 2005;23:9351–9358. doi: 10.1200/JCO.2005.02.9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taback B, O’Day SJ, Boasberg PD, et al. Circulating DNA microsatellites: Molecular determinants of response to biochemotherapy in patients with metastatic melanoma. J Natl Cancer Inst. 2004;96:152–156. doi: 10.1093/jnci/djh011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bidard FC, Fehm T, Ignatiadis M, et al. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev. 2013;32:179–188. doi: 10.1007/s10555-012-9398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shinozaki M, O’Day SJ, Kitago M, et al. Utility of circulating B-RAF DNA mutation in serum for monitoring melanoma patients receiving biochemotherapy. Clin Cancer Res. 2007;13:2068–2074. doi: 10.1158/1078-0432.CCR-06-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fujimoto A, Takeuchi H, Taback B, et al. Allelic imbalance of 12q22-23 associated with APAF-1 locus correlates with poor disease outcome in cutaneous melanoma. Cancer Res. 2004;64:2245–2250. doi: 10.1158/0008-5472.can-03-2932. [DOI] [PubMed] [Google Scholar]
- 83.Hoon DS, Spugnardi M, Kuo C, et al. Profiling epigenetic inactivation of tumor suppressor genes in tumors and plasma from cutaneous melanoma patients. Oncogene. 2004;23:4014–4022. doi: 10.1038/sj.onc.1207505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fujiwara Y, Chi DD, Wang H, et al. Plasma DNA microsatellites as tumor-specific markers and indicators of tumor progression in melanoma patients. Cancer Res. 1999;59:1567–1571. [PubMed] [Google Scholar]
- 85.Joshi P, Jacobs B, Derakhshan A, et al. Enrichment of circulating melanoma cells (CMCs) using negative selection from patients with metastatic melanoma. Oncotarget. 2014;5:2450–2461. doi: 10.18632/oncotarget.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cayrefourcq L, Mazard T, Joosse S, et al. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 2015;75:892–901. doi: 10.1158/0008-5472.CAN-14-2613. [DOI] [PubMed] [Google Scholar]
- 87.Yuan D, Chen L, Li M, et al. Isolation and characterization of circulating tumor cells from human gastric cancer patients. J Cancer Res Clin Oncol. 2015;141:647–660. doi: 10.1007/s00432-014-1814-0. [DOI] [PubMed] [Google Scholar]
- 88.Bobek V, Gurlich R, Eliasova P, et al. Circulating tumor cells in pancreatic cancer patients: Enrichment and cultivation. World J Gastroenterol. 2014;20:17163–17170. doi: 10.3748/wjg.v20.i45.17163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Powell AA, Talasaz AH, Zhang H, et al. Single cell profiling of circulating tumor cells: Transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS One. 2012;7:e33788. doi: 10.1371/journal.pone.0033788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yokobori T, Iinuma H, Shimamura T, et al. Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial-mesenchymal transition and is associated with colorectal cancer prognosis. Cancer Res. 2013;73:2059–2069. doi: 10.1158/0008-5472.CAN-12-0326. [DOI] [PubMed] [Google Scholar]
- 91.Kallergi G, Papadaki MA, Politaki E, et al. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011;13:R59. doi: 10.1186/bcr2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Balasubramanian P, Lang JC, Jatana KR, et al. Multiparameter analysis, including EMT markers, on negatively enriched blood samples from patients with squamous cell carcinoma of the head and neck. PLoS One. 2012;7:e42048. doi: 10.1371/journal.pone.0042048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30:525–532. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- 94.Duda DG, Duyverman AM, Kohno M, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacArthur KM, Kao GD, Chandrasekaran S, et al. Detection of brain tumor cells in the peripheral blood by a telomerase promoter-based assay. Cancer Res. 2014;74:2152–2159. doi: 10.1158/0008-5472.CAN-13-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dorsey JF, Kao GD, MacArthur KM, et al. Tracking viable circulating tumor cells (CTCs) in the peripheral blood of non-small cell lung cancer (NSCLC) patients undergoing definitive radiation therapy: Pilot study results. Cancer. 2015;121:139–149. doi: 10.1002/cncr.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ju M, Kao GD, Steinmetz D, et al. Application of a telomerase-based circulating tumor cell (CTC) assay in bladder cancer patients receiving postoperative radiation therapy: a case study. Cancer Biol Ther. 2014;15:683–687. doi: 10.4161/cbt.28412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoon DS, Bostick P, Kuo C, et al. Molecular markers in blood as surrogate prognostic indicators of melanoma recurrence. Cancer Res. 2000;60:2253–2257. [PubMed] [Google Scholar]