The frequency and risk factors for association between lymphomatoid papulosis (LyP) and another lymphoma were assessed in an 11-year retrospective study. Among 52 cases of lymphomas in 44 patients, lymphoma diagnosis occurred during the course of LyP in 21 cases (median delay: 5 years). Older age and presence of a T-cell clone in LyP lesions were the main prognostic factors for association between LyP and another lymphoma.
Keywords: Lymphomatoid papulosis, Lymphomas, Risk factors, Frequency
Abstract
Background.
Lymphomatoid papulosis (LyP) is classified as an indolent cutaneous lymphoma, but outcome dramatically worsens if LyP is associated with lymphoma. The frequency of this association remains unclear in the literature. Here, we assess the frequency and risk factors of association between LyP and another lymphoma in an 11-year retrospective study conducted in 8 dermatology departments belonging to the French Study Group on Cutaneous Lymphoma (FSGCL).
Patients and Methods.
Patients with LyP were identified and data extracted from the FSGCL registry between 1991 and 2006. Patients were followed up to January 2014. Age, sex, number of skin lesions, histologic subtype, and genotype were recorded at baseline. Risk factors were determined using univariate and multivariate analysis. Cumulative probability of association was calculated using the Kaplan-Meier method.
Results.
We observed 52 cases of lymphomas (cutaneous, n = 38; systemic, n = 14) in 44 of 106 patients (41%). Lymphoma diagnosis was concomitant with or prior to LyP diagnosis in 31 cases and occurred during the course of LyP in 21 cases (cutaneous, n = 14; systemic, n = 7; median delay: 5 years; interquartile range: 1.5–7 years). In multivariate analysis, main prognostic factors for association between LyP and another lymphoma were older age (odds ratio [OR]: 1.05 per year; 95% confidence interval [CI]: 1.01–1.08; p = .011) and presence of a T-cell clone in LyP lesions (OR: 7.55; 95% CI: 2.18–26.18; p = .001).
Conclusion.
Older age and presence of a T-cell clone in LyP lesions are risk factors for associated lymphomas in patients with LyP. These findings should help to identify patients who require close management in clinical practice.
Implications for Practice:
The management of lymphomatoid papulosis (LyP) is that of an indolent cutaneous lymphoma, based on its excellent prognosis. However, this good prognosis is altered if LyP is associated with lymphoma. Furthermore, risk factors for and frequency of this association remain unclear in the literature. The results presented here demonstrate a high rate of association between LyP and other lymphomas (41%) as well as a long median delay of occurrence (5 years), which emphasizes the need for prolonged follow-up of patients with LyP. Moreover, two main risk factors (i.e., older age and presence of a T-cell clone in LyP lesions) are highlighted, which should help clinical practitioners to identify patients who require close management.
Introduction
Lymphomatoid papulosis (LyP) is a chronic, recurrent, self-healing papulonecrotic or papulonodular skin disease that belongs to the spectrum of CD30+ lymphoproliferative disorders [1–4]. Besides the rare histological subtypes D and E recently described [5, 6], three main histopathologic subtypes have been identified. Type A, the most common, demonstrates scattered or small clusters of large CD30+ cells mixed with a polymorphic inflammatory infiltrate; type B, the least frequent, is characterized by a mycosis fungoides-like infiltrate of small to medium CD30− atypical cells with cerebriform nuclei; type C mimics a CD30+ anaplastic large T-cell lymphoma [3].
LyP is classified as an indolent cutaneous lymphoma because of its excellent prognosis, as demonstrated by a 5-year disease-specific survival rate of 100% [2–4]. Nevertheless, the prognosis of patients with LyP can be less favorable, namely in those who have an associated primary cutaneous or extracutaneous lymphoma [2–4]. The most frequently associated lymphomas are mycosis fungoides (MF), primary cutaneous anaplastic large cell lymphomas (pcALCL), or Hodgkin disease (HD). Diagnosis may precede or be concomitant with that of LyP, or occur during the course of LyP [7–21]
The exact frequency of the association between LyP and another lymphoma remains unclear in the literature. It varies from the commonly cited rates of 10%–20% up to 40% and even 60% in some recent studies [3, 7–20]. This discrepancy led Gruber et al. [19] to reassess the cumulative risk of developing an associated lymphoma in patients with LyP on the basis of the largest series [8, 12–14]. They observed an extremely high 80% rate of association after a 20- to 30-year follow-up period, leading them to recommend long-term follow-up in patients with LyP.
Risk factors for association between LyP and another cutaneous or extracutaneous lymphoma are still unknown because data from the literature are conflicting or derived from limited populations. Histological subtype of LyP has been reported to be associated with the presence of associated lymphomas. Type C has been suggested as a risk factor, whereas type B reportedly plays a protective role [7]. However, in a recent study, only a mixed histological subtype was shown to correlate with increased risk for associated lymphoma in patients with LyP [20]. Older age has also been pointed as a risk factor for association between LyP and another lymphoma in one study [7], but this finding was not confirmed in a more recent and larger one [18].
A retrospective study from the Mayo Clinic published in 2012 reported that detection of monoclonal T-cell receptor (TCR) gene rearrangement in the skin lesions of LyP was a potential predictor of an associated lymphoma [20]. Nevertheless, the predictive value of a monoclonal TCR gene rearrangement in the skin biopsy specimens of LyP remains debated. Indeed, monoclonal TCR gene rearrangement is detected in the majority of LyP lesions (i.e., 50%–100% [2, 3, 20–24]) but detection is not systematically associated with presence of lymphoma, because the commonly reported rate of association is only 10%–20% [3, 8, 9, 11–15, 20]. To disentangle these conflicting results, we reevaluated the frequency and risk factors for association between LyP and cutaneous or extracutaneous lymphomas in a large series of patients with LyP followed for a long duration.
Patients and Methods
This retrospective study was conducted from January 1991 to January 2014 in 8 dermatology departments belonging to the French Study Group on Cutaneous Lymphoma (FSGCL). Cases of LyP were identified from the registry of the group. This database was started in 1991 and includes all incident cases of primary cutaneous lymphomas after validation of diagnosis by both pathologist and dermatologist members of the group.
Inclusion criteria were the following (a) clinical and immunohistological diagnosis of LyP established on the basis of clinicopathologic criteria according to the World Health Organization-European Organization for Research and Treatment of Cancer (WHO-EORTC) classification [3, 4] and confirmed by the FSGCL, and (b) available data in the patient’s medical charts. Living patients without a minimum follow-up period of 6 months after diagnosis of LyP were excluded. We identified 118 patients with LyP from the FSGCL database between 1991 and 2006. Of these, 106 cases fulfilled the inclusion/exclusion criteria and were followed for a median duration of 11.5 years up to the endpoint of the study in January 2014. All medical records were reviewed.
The following baseline parameters present at the time of LyP diagnosis were recorded: age, sex, number of skin lesions (i.e., n < 5; 5 ≤ n ≤ 20; n > 20), histologic subtype of LyP, and presence of a monoclonal TCR gene rearrangement in the skin biopsy of LyP. Presence of another cutaneous or extracutaneous lymphoma at baseline or during the patient’s follow-up and its histologic type was also recorded.
Immunohistopathologic Analysis
Histological analysis of LyP skin biopsy specimens was performed on formalin-fixed, paraffin-embedded tissue sections using a hematoxylin-and-eosin stain. Immunophenotypical analysis was performed with a biotin-avidin-immunoperoxydase standard procedure and the following antileukocyte monoclonal antibodies: CD2, CD3, CD4, CD5, CD8, CD20, and CD30. Skin biopsy samples were examined by all FSGCL pathologists before registration in the database.
Molecular Analysis
Genotypic analysis of the T-cell receptor γ (TCR-γ) chain gene was performed independently of histopathologic examination in 73 of the 106 cases, using the validated polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE) technique [25, 26]. DNA from frozen skin samples was extracted using a phenol chloroform standard procedure and subsequently amplified with multiplex PCR-DGGE, as previously described [26]. At the end of the procedure, PCR products were electrophoresed on 6.5% polyacrylamide gel that contained a linearly increasing 10%–60% denaturating gradient. Gels were stained with ethidium bromide and photographed under UV illumination. The presence of a predominant T-cell clone was determined by a bright band on the gel as compared with negative and positive controls previously identified. This typical band was visible at 0.03 to 0.01 dilution, depending on the alleles. The polyclonal pattern was characterized by a smear and considered negative.
Statistical Analysis
Age, sex, number of skin lesions, histologic subtype of LyP, and positive TCR gene rearrangement in skin biopsy specimens were considered as potential risk factors for the association of LyP with another type of cutaneous or extracutaneous lymphoma. These variables were first compared using univariate analysis between patients with LyP with and those without an associated lymphoma. For number of skin lesions, the 3 subgroups previously described (i.e., n < 5; 5 ≤ n ≤ 20; n > 20) were matched in 2 subgroups with a cutoff at 20 to not decrease the power of the statistical test.
The Student’s t test was used to compare the mean of normal variables. For non-normal variables, the Mann-Whitney test was used. The chi-square test was used to compare categorical variables between the two groups of patients with LyP (with or without an associated lymphoma).
After assessing first-order interaction and confounding, binomial logistic regression was performed to identify factors independently associated with the presence of lymphomas in patients with LyP. The variables with a p value of less than .10 were considered for this multivariate analysis. Variables previously reported to be correlated with presence of an associated lymphoma in the literature (i.e., histological subtype of LyP, sex) [7, 18, 20] were also systematically incorporated into the model. The positive predictive value (PPV) and negative predictive value (NPV) of TCR clonality were also estimated.
For patients who had associated cutaneous or extracutaneous lymphomas during their follow-up, time to lymphoma was computed from diagnosis of LyP to that of the first associated lymphoma. A Kaplan-Meier curve was generated to estimate the cumulative probability of acquiring an associated lymphoma during the follow-up period. For all analyses, 2-sided p-values of less than .05 were considered statistically significant.
Data management and statistical analysis were performed using SPSS 17.0 software (IBM Corp., Chicago, IL, http://www-01.ibm.com). This retrospective study was approved by the ethics committee for noninterventional research of Rouen University Hospital (registered number: E2014-32).
Results
Population of Patients With LyP
The baseline clinical and histological characteristics of the 106 patients with LyP (65 men, 41 women) are shown in Table 1. The mean age (±SD) was 49 ± 18 years. The median delay of evolution of LyP before diagnosis was 12.0 months (interquartile range [IQR]: 1–48 months). Median follow-up time of living patients was 11.5 years (IQR: 8–15 years). At the end of the study (January 1, 2014), 25 patients had died, 12 patients were lost to follow-up, and 69 were still followed. Among deceased patients, 14 of 25 (56%) had a known associated cutaneous (n = 8) or systemic lymphoma (n = 6) that was identified as the cause of death in 2 of 8 and 5 of 6 cases, respectively (unknown cause, n = 5; breast cancer, n = 1; pulmonary embolism, n = 1).
Table 1.
Clinical and histological characteristics of 106 patients with LyP
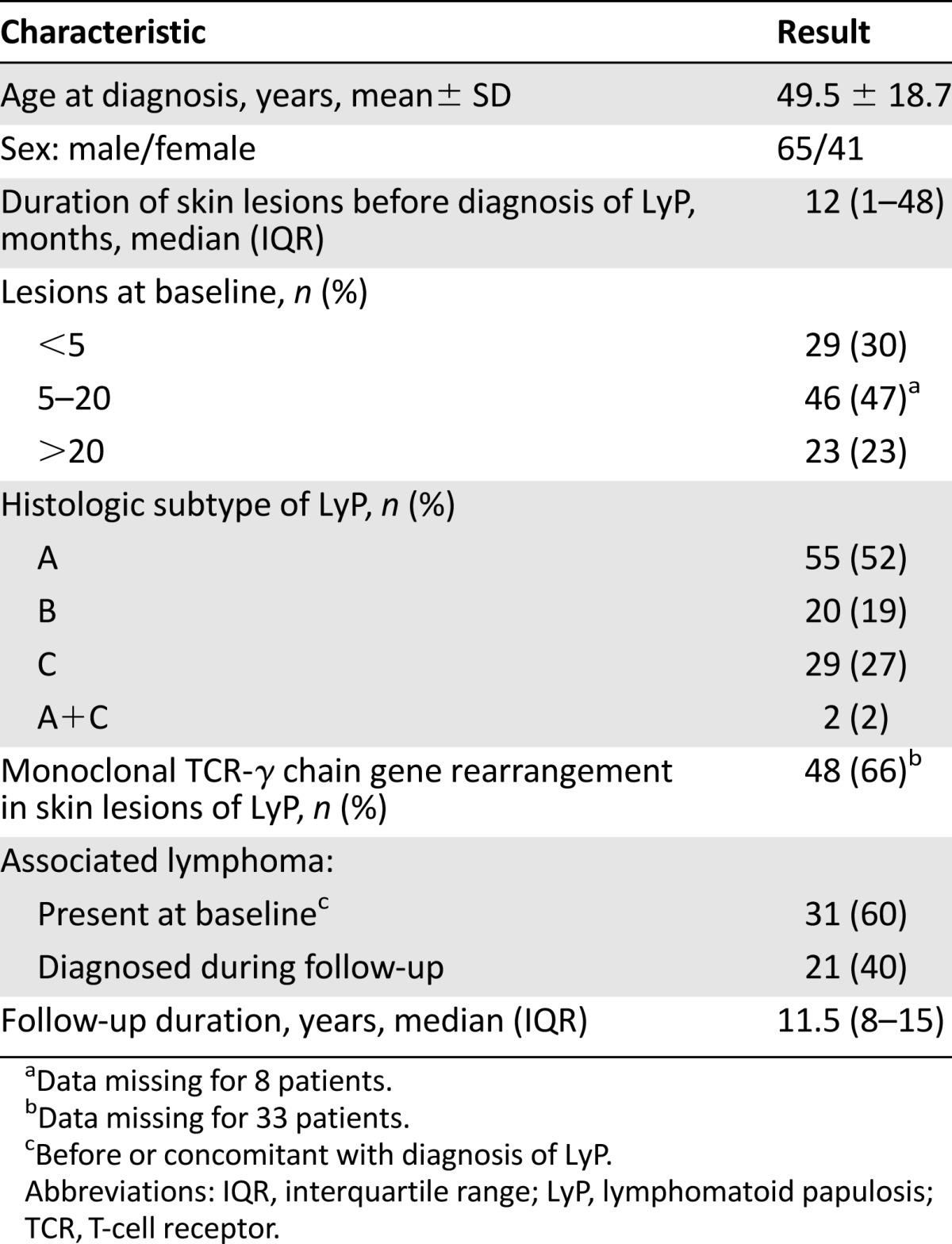
According to the WHO-EORTC classification, 55, 20, and 29 patients had type A, B, or C LyP, respectively. Two patients had unusual combined histologic features of type A and C. Molecular analysis was available in 73 cases. A monoclonal rearrangement of the TCR-γ gene chain was detected in 48 skin biopsy specimens (66%), with no difference between the 3 histologic subtypes of LyP: type A: 22 of 36 (61%); type B: 12 of 16 (75%); and type C: 14 of 21 (67%) (p = .619).
Prevalence and Main Characteristics of Associated Cutaneous or Extracutaneous Lymphoma
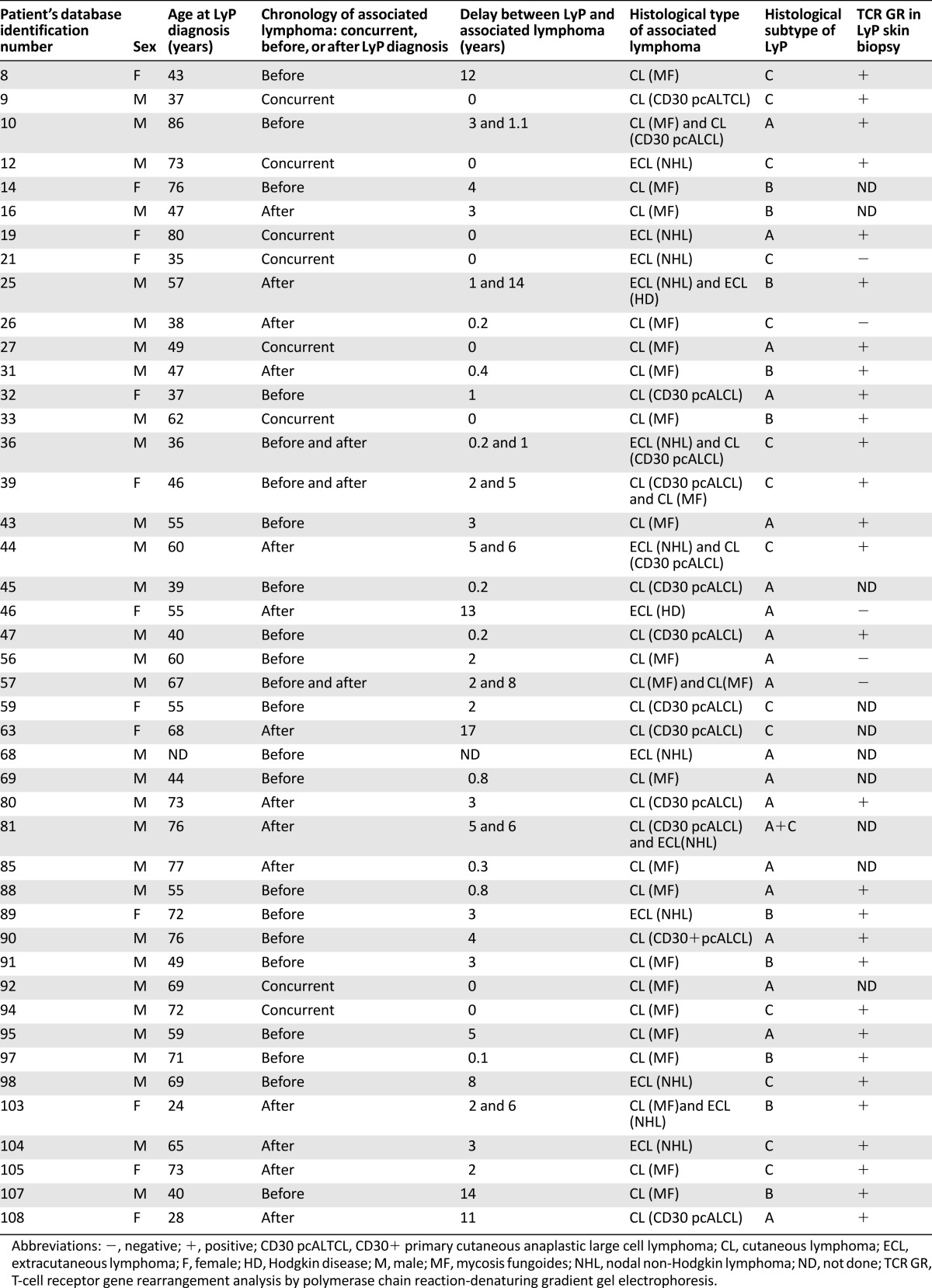
There were 52 cases of associated lymphomas observed in 44 patients with LyP (41%). Eight patients had 2 associated lymphomas (patient nos. 10, 25, 36, 39, 44, 57, 81, 103) (Table 2). Of the 44 patients with associated lymphoma, 19, 10, 14, and 1 had type A, B, C, and (A+C) LyP, respectively.
Table 2.
Characteristics of patients with LyP associated with another lymphoma
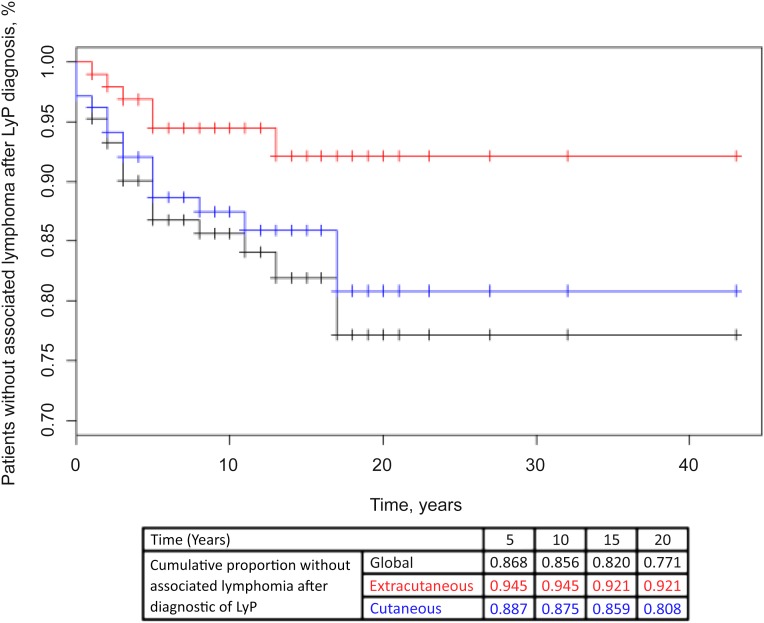
Associated lymphomas included primary cutaneous lymphoma in 38 cases, corresponding to MF and CD30+ pcALCL in 24 and 14 cases, respectively. Notably, the 14 cases of pcALCL were exclusively observed in patients with type A (n = 7), C (n = 6), or A+C (n = 1) LyP. Fourteen cases of extracutaneous lymphoma were noted (i.e., nodal non-Hodgkin lymphoma, n = 12; HD, n = 2). Nodal non-Hodgkin lymphomas corresponded to B-cell lymphomas in three cases (immunoblastic, Burkitt, and lymphoplasmacytic) and anaplastic large T-cell lymphomas in nine cases. Diagnosis of associated lymphomas was made before or concomitantly with that of LyP in 31 cases (60%), and during the follow-up period in 21 cases. These latter (40%) corresponded with 14 cases of primary cutaneous lymphoma and 7 cases of extracutaneous lymphoma, which occurred during the follow-up period in 17 patients after a median delay of 5 years after diagnosis of LyP (IQR: 1.5–7 years) (Fig. 1).
Figure 1.
Cumulative probability of acquiring an associated lymphoma during the follow-up period (Kaplan-Meier curve).
Abbreviation: LyP, lymphomatoid papulosis.
Of the 44 skin biopsy specimens from the 44 patients with LyP who had an associated lymphoma, 35 specimens were available for molecular assays. A monoclonal rearrangement of the TCR-γ gene chain was detected in 30 cases (86%). Among systemic (n = 7) or skin (n = 14) lymphomas developed during the follow-up period, the rate of detection of a monoclonal TCR gene rearrangement in LyP lesions at time of diagnosis was 83% and 80%, respectively. Conversely, only 2 of the 22 patients with a negative TCR gene rearrangement in LyP lesions and no associated lymphoma at time of LyP diagnosis further developed a lymphoma (systemic, n = 1; cutaneous, n = 1) during follow-up (NPV: 91%). Interestingly, the association between LyP and another skin or systemic lymphoma was not correlated with LyP status (i.e., partial or complete remission/stability of the lesions) at the last visit.
Risk Factors for Association Between Lymphomatoid Papulosis and Cutaneous or Extracutaneous Lymphoma
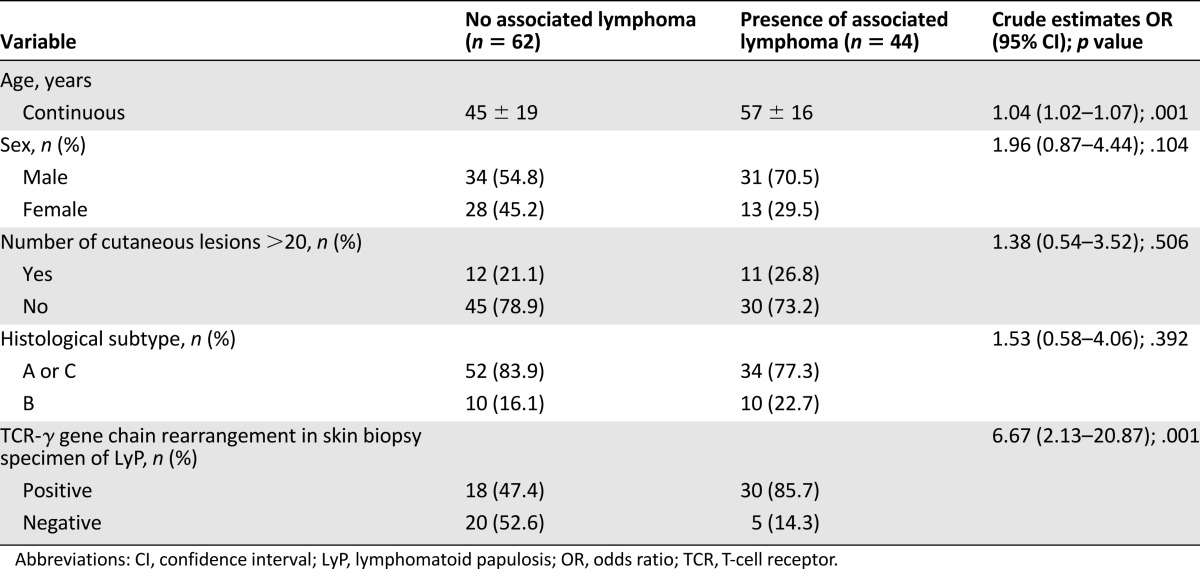
Results of univariate analysis assessing risk factors for association between LyP and another lymphoma are shown in Table 3. Because histological subtype B had been previously suggested to be a protector factor [7], we pooled types A and C versus type B in the analysis. Older age (odd ratio [OR]: 1.04 per year of age; 95% confidence interval [CI]: 1.02–1.07; p = .001), and detection of a monoclonal rearrangement of the TCR-γ gene chain in LyP lesions (OR: 6.67; 95% CI: 2.13–20.87; p = .001) were associated with presence of an associated cutaneous or extracutaneous lymphoma. As sex and histologic subtype of LyP had been previously reported to be associated with risk for lymphoma in patients with LyP [7, 18, 20], we included these variables in our model for multivariate analysis.
Table 3.
Univariate analysis of main risk factors for association of LyP and another type of lymphoma between patients with or without an associated lymphoma
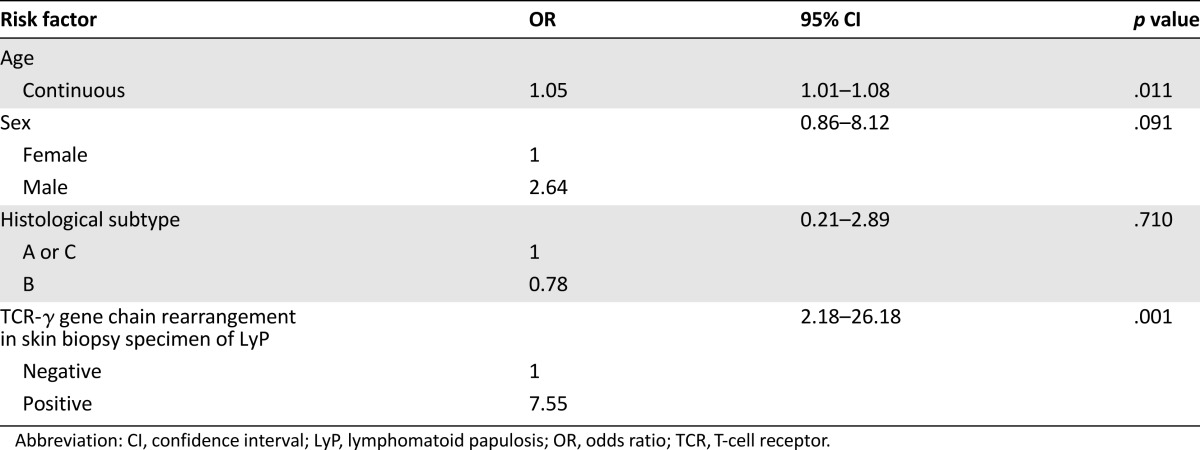
By multivariate analysis, detection of a T-cell clone in the skin lesions of LyP was even more strongly associated with risk for lymphoma (OR: 7.55; 95% CI: 2.18–26.18; p = .001). Older age (OR: 1.05 per year; 95% CI: 1.01–1.08; p = .011) but not LyP histological subtype (OR: 0.78; 95% CI: 0.21–2.89; p = .710) was also associated with risk for lymphoma (Table 4). The association between LyP-associated lymphoma and male sex was borderline statistically significant (OR: 2.64; 95% CI: 0.86–8.2; p = .091).
Table 4.
Factors independently associated with occurrence of another type of lymphoma in patients with LyP (binomial logistic regression model)
The PPV and NPV for detecting monoclonal TCR gene rearrangement in the skin lesions of LyP for association with another cutaneous or extracutaneous lymphoma were 62.5% and 80%, respectively.
Discussion
The main result of the present study is the demonstration that detection of a monoclonal rearrangement of the TCR gene in skin lesions from patients with LyP is a major risk factor for the occurrence of an associated lymphoma. Indeed, patients with a cutaneous T-cell clone had a 7.5-fold higher risk for associated lymphoma than patients with a polyclonal T-cell infiltrate in their skin lesions. The rather high 62% PPV and 80% NPV for the detection of a cutaneous T-cell clone in the skin biopsies of patients with LyP strongly suggest that this molecular assay might be a useful tool for clinicians. Interestingly, the detection of a TCR gene rearrangement in LyP lesions was also of major interest in the follow-up of patients with no associated lymphoma at the time of LyP diagnosis. Indeed, the rate of detection of a monoclonal TCR gene rearrangement in LyP lesions at the time of diagnosis was 83% and 80%, respectively, among cases that were further associated with a systemic (n = 7) or a skin (n = 14) lymphoma during follow-up. Moreover, only 2 of the 22 patients with a negative TCR gene rearrangement in LyP lesions and no associated lymphoma at time of LyP diagnosis developed a lymphoma during follow-up (NPV: 91%). These results suggest that molecular assay might help clinicians to determine which patients with LyP require a close follow-up with physical and possibly radiological evaluation.
Because associated lymphomas occurring after diagnosis of LyP arose after a rather long median delay of 5 years, our results emphasize the need for prolonged follow-up of patients with LyP who have a cutaneous T-cell clone, as previously highlighted by Gruber et al. [19]. Indeed, 25% of associated cutaneous or systemic lymphomas occurred after a very long delay of 6.5 years and 13 years, respectively, following diagnosis of LyP (Fig. 1). The need for prolonged follow-up of patients with LyP is also supported by the high 41% frequency of associated lymphomas in the present study. This rate is higher than the commonly reported 10%–20% rates [3, 8, 9, 11–15, 20], and is in accordance with the results from recent studies by Kunishige et al. and Liu et al., who reported much higher rates of associated lymphomas (40% and 61%, respectively) [16–18]. It is likely that the high proportion of associated lymphomas in these 2 series and the present study (41%) are due to the prolonged follow-up of patients (17.5, 12.3, and 11.3 years respectively), as compared with series that reported lower rates of associated lymphomas [16, 18]. As a strong case, the Kaplan-Meier curve shows the continuous occurrence of associated lymphomas during patients’ follow-up (Fig. 1).
The prognostic significance of detecting a T-cell clone in the skin lesions of LyP has been reported in a retrospective series from the Mayo Clinic, although the number of patients with LyP who had an associated lymphoma (17 cases) was rather limited [20]. Interestingly, the 7.5-fold higher risk for associated lymphoma in patients with a cutaneous T-cell clone in LyP lesions that we observed in the present study was of the same order of magnitude as that found in the Mayo Clinic series (OR: 5.7; 95% CI: 1.14–28.39; p = .02). These findings are in accordance with previous reports showing an identical T-cell clone in the lesions of LyP and associated lymphomas [22, 23, 27–29].
Older age was also associated with risk for lymphoma (OR: 1.05; 95% CI: 1.01–1.08; p = .011), because the mean age of patients with an associated lymphoma was 12 years older than that of patients without associated lymphoma (45 ± 19 years vs. 57 ± 16 years; p = .001). This finding is in accordance with the fact that MF, CD30+ pcALCL, and nodal non-Hodgkin lymphoma, which corresponded to 77% of associated lymphomas in the present series, usually affect people older than 50 years [3].
As in all retrospective studies, various biases are possible in this study. To avoid selection bias, we included exhaustively all consecutive cases of LyP registered in the FSGCL database followed in eight centers in France. The absence of major selection bias in our patients is also supported by similarities in clinical features, prevalence of histological subtypes, and frequency of molecular detection of T-cell clone in patients with LyP in our series compared with that found in previous studies [3, 8, 14, 20]. Indeed, the use of a common molecular assay allowed us to standardize T-cell clone detection in the eight different centers. The choice of TCR-γ detection method was guided by its high sensitivity, which is still superior to TCR-α or -β detection, as demonstrated by numerous previous studies [25, 26], including one published recently [21].
A diagnostic bias is unlikely because diagnoses of both LyP subtypes and type of lymphoma were validated by pathologists of the FSGCL. The absence of histological types D and E in the present series may be due to the time of LyP diagnosis between 1991 and 2006, whereas these 2 rare histological subtypes have been described more recently [4–6]. Attrition bias is a common problem in retrospective studies. However, we aimed at limiting the number of patients lost to follow-up before the end of the study. Indeed only 12 patients were unable to have their follow-up data recorded at the end of the study, leading to a long median follow-up duration of 11 years.
Not all patients in this series had an exhaustive diagnostic workup for a nodal lymphoma. This might have slightly underestimated the true rate of associated lymphomas. However, in the present study, there was little likelihood of misdiagnosis of associated lymphomas because the follow-up period for most patients was long.
Overall, the present study showed a 7.5-fold higher risk for associated lymphoma in patients with LyP who had a monoclonal rearrangement of the TCR-γ gene chain in their skin lesions. These findings should help dermatologists in clinical practice identify patients who require close management. Additionally, the long median delay of occurrence of associated lymphomas emphasizes the need for prolonged follow-up of patients with LyP with cutaneous T-cell clone.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
We thank Dr. Elisabeth Thomine for her critical review of the manuscript; Nikki Sabourin-Gibbs, Rouen University Hospital, for writing assistance and review of the manuscript in English; and Pierre Deschelotte, Delphine Anuset, Fatima Al Saif, and Annick Horville for their help in recording patients’ follow-up data.
Footnotes
For Further Reading:Brad Haverkos, Kelly Tyler, Alejandro A. Gru et al. Primary Cutaneous B-Cell Lymphoma: Management and Patterns of Recurrence at the Multimodality Cutaneous Lymphoma Clinic of The Ohio State University. The Oncologist 2015; 20:1161–1166.
Implications for Practice:Primary cutaneous B-cell lymphoma (PCBCL) is a rare malignancy with an increasing incidence. Clinicians must recognize the importance of a complete workup to accurately diagnose PCBCL, given the effect on prognosis and treatment. It was observed that nearly 20% of the patients who presented initially with cutaneous B-cell lymphoma were classified as having systemic B-cell lymphoma after whole body imaging. The findings from the present retrospective analysis of a single-institution cohort suggest that for early-stage indolent PCBCL, no front-line treatment strategy that decreases the risk of recurrence is obvious. No difference in the risk of recurrence between conservative skin-directed and other therapies was observed. These data support a continued need to compare front-line treatment therapies.
Author Contributions
Conception/Design: Nadège Cordel
Provision of study material or patients: Nadège Cordel, Michel D’Incan, Laurent Machet, Florent Grange, Eric Estève, Sophie Dalac, Saskia Ingen-Housz-Oro, Martine Bagot, Marie Beylot-Barry, Pascal Joly
Collection and/or assembly of data: Nadège Cordel, Benoît Tressières, Michel D’Incan, Pascal Joly
Data analysis and interpretation: Nadège Cordel, Benoît Tressières, Pascal Joly
Manuscript writing: Nadège Cordel, Pascal Joly
Final approval of manuscript: Nadège Cordel, Benoît Tressières, Michel D’Incan, Laurent Machet, Florent Grange, Eric Estève, Sophie Dalac, Saskia Ingen-Housz-Oro, Martine Bagot, Marie Beylot-Barry, Pascal Joly
Disclosures
The authors indicated no financial relationships.
References
- 1.Macaulay WL. Lymphomatoid papulosis. A continuing self-healing eruption, clinically benign–histologically malignant. Arch Dermatol. 1968;97:23–30. doi: 10.1001/archderm.97.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Willemze R, Beljaards RC. Spectrum of primary cutaneous CD30 (Ki-1)-positive lymphoproliferative disorders. A proposal for classification and guidelines for management and treatment. J Am Acad Dermatol. 1993;28:973–980. doi: 10.1016/0190-9622(93)70140-o. [DOI] [PubMed] [Google Scholar]
- 3.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 4.Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: Lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024–4035. doi: 10.1182/blood-2011-05-351346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardoso J, Duhra P, Thway Y, et al. Lymphomatoid papulosis type D: A newly described variant easily confused with cutaneous aggressive CD8-positive cytotoxic T-cell lymphoma. Am J Dermatopathol. 2012;34:762–765. doi: 10.1097/DAD.0b013e31825ba953. [DOI] [PubMed] [Google Scholar]
- 6.Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: A new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1–13. doi: 10.1097/PAS.0b013e3182648596. [DOI] [PubMed] [Google Scholar]
- 7.Beljaards RC, Willemze R. The prognosis of patients with lymphomatoid papulosis associated with malignant lymphomas. Br J Dermatol. 1992;126:596–602. doi: 10.1111/j.1365-2133.1992.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 8.Bekkenk MW, Geelen FAMJ, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: A report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653–3661. [PubMed] [Google Scholar]
- 9.Wang HH, Lach L, Kadin ME. Epidemiology of lymphomatoid papulosis. Cancer. 1992;70:2951–2957. doi: 10.1002/1097-0142(19921215)70:12<2951::aid-cncr2820701236>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Wang HH, Myers T, Lach LJ, et al. Increased risk of lymphoid and nonlymphoid malignancies in patients with lymphomatoid papulosis. Cancer. 1999;86:1240–1245. [PubMed] [Google Scholar]
- 11.Sina B, Burnett JW. Lymphomatoid papulosis. Case reports and literature review. Arch Dermatol. 1983;119:189–197. doi: 10.1001/archderm.119.3.189. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez NP, Pittelkow MR, Muller SA, et al. The clinicopathologic spectrum of lymphomatoid papulosis: Study of 31 cases. J Am Acad Dermatol. 1983;8:81–94. doi: 10.1016/s0190-9622(83)70011-3. [DOI] [PubMed] [Google Scholar]
- 13.Cabanillas F, Armitage J, Pugh WC, et al. Lymphomatoid papulosis: A T-cell dyscrasia with a propensity to transform into malignant lymphoma. Ann Intern Med. 1995;122:210–217. doi: 10.7326/0003-4819-122-3-199502010-00009. [DOI] [PubMed] [Google Scholar]
- 14.el-Azhary RA, Gibson LE, Kurtin PJ, et al. Lymphomatoid papulosis: A clinical and histopathologic review of 53 cases with leukocyte immunophenotyping, DNA flow cytometry, and T-cell receptor gene rearrangement studies. J Am Acad Dermatol. 1994;30:210–218. doi: 10.1016/s0190-9622(94)70019-2. [DOI] [PubMed] [Google Scholar]
- 15.El Shabrawi-Caelen L, Kerl H, Cerroni L. Lymphomatoid papulosis: Reappraisal of clinicopathologic presentation and classification into subtypes A, B, and C. Arch Dermatol. 2004;140:441–447. doi: 10.1001/archderm.140.4.441. [DOI] [PubMed] [Google Scholar]
- 16.Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: The Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049–1058. doi: 10.1016/s0190-9622(03)02484-8. [DOI] [PubMed] [Google Scholar]
- 17.Brown JR, Skarin AT. Clinical mimics of lymphoma. The Oncologist. 2004;9:406–416. doi: 10.1634/theoncologist.9-4-406. [DOI] [PubMed] [Google Scholar]
- 18.Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: A retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576–581. doi: 10.1111/j.1365-2230.2008.03024.x. [DOI] [PubMed] [Google Scholar]
- 19.Gruber R, Sepp NT, Fritsch PO, et al. Prognosis of lymphomatoid papulosis. The Oncologist. 2006;11:955–957; author reply 957. doi: 10.1634/theoncologist.11-8-955. [DOI] [PubMed] [Google Scholar]
- 20.de Souza A, el-Azhary RA, Camilleri MJ, et al. In search of prognostic indicators for lymphomatoid papulosis: A retrospective study of 123 patients. J Am Acad Dermatol. 2012;66:928–937. doi: 10.1016/j.jaad.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 21.de la Garza Bravo MM, Patel KP, Loghavi S, et al. Shared clonality in distinctive lesions of lymphomatoid papulosis and mycosis fungoides occurring in the same patients suggests a common origin. Hum Pathol. 2015;46:558–569. doi: 10.1016/j.humpath.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Chott A, Vonderheid EC, Olbricht S, et al. The dominant T cell clone is present in multiple regressing skin lesions and associated T cell lymphomas of patients with lymphomatoid papulosis. J Invest Dermatol. 1996;106:696–700. doi: 10.1111/1523-1747.ep12345532. [DOI] [PubMed] [Google Scholar]
- 23.Steinhoff M, Hummel M, Anagnostopoulos I, et al. Single-cell analysis of CD30+ cells in lymphomatoid papulosis demonstrates a common clonal T-cell origin. Blood. 2002;100:578–584. doi: 10.1182/blood-2001-12-0199. [DOI] [PubMed] [Google Scholar]
- 24.Gellrich S, Wernicke M, Wilks A, et al. The cell infiltrate in lymphomatoid papulosis comprises a mixture of polyclonal large atypical cells (CD30-positive) and smaller monoclonal T cells (CD30-negative) J Invest Dermatol. 2004;122:859–861. doi: 10.1111/j.0022-202X.2004.22304.x. [DOI] [PubMed] [Google Scholar]
- 25.Wood GS, Tung RM, Haeffner AC, et al. Detection of clonal T-cell receptor γ gene rearrangements in early mycosis fungoides/Sezary syndrome by polymerase chain reaction and denaturing gradient gel electrophoresis (PCR/DGGE) J Invest Dermatol. 1994;103:34–41. doi: 10.1111/1523-1747.ep12389114. [DOI] [PubMed] [Google Scholar]
- 26.Cordel N, Lenormand B, Courville P, et al. Usefulness of cutaneous T-cell clonality analysis for the diagnosis of cutaneous T-cell lymphoma in patients with erythroderma. Arch Pathol Lab Med. 2005;129:372–376. doi: 10.5858/2005-129-372-UOCTCA. [DOI] [PubMed] [Google Scholar]
- 27.Davis TH, Morton CC, Miller-Cassman R, et al. Hodgkin’s disease, lymphomatoid papulosis, and cutaneous T-cell lymphoma derived from a common T-cell clone. N Engl J Med. 1992;326:1115–1122. doi: 10.1056/NEJM199204233261704. [DOI] [PubMed] [Google Scholar]
- 28.Wood GS, Crooks CF, Uluer AZ. Lymphomatoid papulosis and associated cutaneous lymphoproliferative disorders exhibit a common clonal origin. J Invest Dermatol. 1995;105:51–55. doi: 10.1111/1523-1747.ep12312548. [DOI] [PubMed] [Google Scholar]
- 29.Lair G, Lenormand B, Bagot M, et al. Étude séquentielle du réarrangement des gènes du récepteur des lymphocytes T chez 5 patients ayant eu tout ou partie de la séquence: Maladie de Hodgkin, papulose lymphomatoide, mycosis fongoide, lymphome à grandes cellules CD30+ Ann Dermatol Venereol. 1996;123(suppl):S28. [Google Scholar]