This review of the literature was conducted on the clinical experience with radiotherapy combined with either single-agent gemcitabine or gemcitabine/cisplatin-based polychemotherapy for the treatment of patients with locoregionally advanced squamous cell carcinoma of the head and neck. The review highlights the remarkable radiosensitizing potential of gemcitabine and suggests that even low dosages (less than 50 mg/m2 per week) provide a sufficient therapeutic ratio and therefore should be further investigated.
Keywords: Chemotherapy, Concomitant chemoradiotherapy, Gemcitabine, Head and neck cancer, Neoplasms, Oncology, Radiotherapy, Radiation, Toxicity
Abstract
Background.
Platinum-based concurrent chemoradiation (CCRT) improves locoregional control and overall survival of locoregionally advanced (LA) squamous cell carcinoma of the head and neck (SCCHN) when compared to radiotherapy alone, but this approach is hampered by significant toxicity. Therefore, alternative ways to enhance the radiation effects are worth investigating. Gemcitabine (2′,2′-difluorodeoxycytidine), in addition to its activity against a variety of solid tumors, including SCCHN, is one of the most potent radiosensitizers, and it has an overall favorable safety profile. In this paper, the clinical experience with gemcitabine-based chemoradiation in the treatment of patients with LA-SCCHN is reviewed.
Methods.
We conducted a review of the literature on the clinical experience with radiotherapy combined with either single-agent gemcitabine or gemcitabine/cisplatin-based polychemotherapy for the treatment of patients with LA-SCCHN. We also searched abstracts in databases of major international oncology meetings from the last 20 years. A meta-analysis was performed to calculate pooled proportions with 95% confidence intervals (CIs) for complete response rate and grade 3–4 acute mucositis rate.
Results.
A total of 13 papers were eligible for the literature review. For schedules using a gemcitabine dose intensity (DI) below 50 mg/m2 per week, the complete response rate was 86% (95% CI, 74%–93%) with grade 3–4 acute mucositis rate of 38% (95% CI, 27%–50%) and acceptable late toxicity. In one of the studies employing such low DIs, survival data were provided showing a 3-year overall survival of 50%. Compared with DI ≥50 mg/m2 per week, there was no difference in the complete response rate (71%; 95% CI, 55%–83%; p = .087) but a significantly higher (p < .001) grade 3–4 acute mucositis rate of 74% (95% CI, 62%–83%), often leading to treatment interruptions (survival data provided in 8 studies; 3-year overall survival, 27%–63%). Late toxicity comprising mainly dysphagia was generally underreported, whereas information about xerostomia and skin fibrosis was scarce.
Conclusion.
This review highlights the radiosensitizing potential of gemcitabine and suggests that even very low dosages (less than 50 mg/m2 per week) provide a sufficient therapeutic ratio and therefore should be further investigated. Refinements in radiation schemes, including intensity-modulated radiation therapy, in combination with low-dose gemcitabine and targeted agents, such as cetuximab, are currently being investigated.
Implications for Practice:
Cisplatin-based concurrent chemoradiation (CCRT) has become the standard treatment of locally advanced head and neck cancer (LAHNC). This approach is hampered by significant toxicity. This paper reviews the studies using gemcitabine as an alternative radio-sensitizer for CCRT in patients with LAHNC. In this capacity, despite its mild intrinsic toxicity, gemcitabine comes with high rates of severe mucositis when used in dosages exceeding 50 mg/m2 per week. CCRT with low-dose gemcitabine provides a sufficient therapeutic ratio, combining clinical activity, similar to the higher-dose regimens, with lower toxicity. Further investigation is warranted.
Introduction
Concurrent chemotherapy and radiation (CCRT) is the standard treatment for locoregionally advanced (LA) squamous cell carcinoma (SCCHN) of the head and neck (oral cavity, oropharynx, hypopharynx, and larynx) [1–4]. Platinum-based CCRT, in particular, is considered standard for patients with unresectable LA-SCCHN, patients with resectable yet nonsurgically treated cancer, and postoperative high-risk patients [5–9]. Platinum-based CCRT leads to better locoregional control and better larynx preservation and improves survival compared with radiotherapy alone [10–14]. However, the downside of this approach is the increased acute and late toxicity, which may lead to treatment-related deaths, or at a later time, lead to noncancer-related deaths [15–17].
Concerning the concurrent chemotherapy, single-agent cisplatin (100 mg/m2) on days 1, 22, and 43 is currently considered the optimal approach in carefully selected patients [12, 13]. The role of induction chemotherapy is still not fully elucidated. At this moment, there is no evidence that sequential administration of induction chemotherapy followed by CCRT is superior to CCRT alone, although some signals for a benefit in patients with oropharynx cancer and patients with bulky nodes are available [18–20].
Gemcitabine (2′,2′-difluorodeoxycytidine; dFdC; Gemzar, Eli Lilly and Company, Indianapolis, IN, http://www.lilly.com) is a fluorinated pyrimidine nucleoside analog with antitumor activity against a wide variety of solid tumors, including head and neck cancer, and a favorable toxicity profile when clinically used [21, 22]. In addition, it has synergistic activity with cisplatin and radiosensitizing properties [23–29]. Gemcitabine as a prodrug requires intracellular activation by phosphorylation to gemcitabine diphosphate (dFdCDP) and gemcitabine triphosphate (dFdCTP) for its antitumor activity [30]. dFdCDP affects DNA synthesis by preventing the de novo biosynthesis of deoxyribonucleotide 5′-triphosphates (in particular, deoxyadenosine triphosphate [dATP]) through inhibition of the enzyme ribonucleotide reductase. dFdCTP directly interferes with DNA synthesis in tumor cells through inhibition of DNA polymerization and incorporation of the fraudulent nucleotide into the growing DNA strand [31]. Finally, gemcitabine can be incorporated into RNA and can induce apoptosis [24, 32].
Gemcitabine is rapidly cleared from the plasma with a half-life of only a few minutes. However, due to the intracellular retention of the dFdCTP, the elimination is delayed up to 72 hours [33]. Moreover, the main metabolite of gemcitabine, difluorodeoxyuridine (dFdU), has a prolonged half-life and can be detected in the plasma for several days even after very low dosages of gemcitabine [33–35]. The radio-enhancement of gemcitabine is a complex phenomenon and seems to be dependent on drug exposure time and concentration [31, 36–38]. The key mechanism in the gemcitabine-induced radiosensitization has been stated to be the inhibition of ribonucleotide reductase, leading to depletion of dATP [31]. However, in addition, it has been hypothesized that the sustained presence of dFdU in the circulation after low-dose gemcitabine might contribute to the radio-enhancement effect of gemcitabine because of the radiosensitizing properties of dFdU [34, 35].
In this review, the efficacy and tolerance of gemcitabine used together with radiation as a single agent and as part of multiagent-based chemoradiotherapy, in combination with other cytotoxic agents in the treatment of LA-SCCHN, are summarized.
Methods
Search Strategy
We searched literature from the National Library of Medicine and the Cochrane Library to identify relevant available articles, with the last search updated on May 5, 2015. We also performed a bibliographic search of abstracts in the field of interest presented at top scientific meetings (American Association for Cancer Research, American Society of Clinical Oncology, American Society for Therapeutic Radiology and Oncology, European Society for Therapeutic Radiation and Oncology, European Society for Medical Oncology, and European Cancer Organisation) within the past 20 years. The key words and subject terms were searched (i.e., head and neck cancer, squamous cell carcinoma, locally or locoregionally advanced, radiotherapy or radiation, chemotherapy, gemcitabine). The language of the papers was restricted to English. References in the studies were reviewed to identify any additional studies that were not indexed by the electronic database. Literature data concerning treatment of nasopharynx cancer were not included in this review.
Statistical Methodology
Pooled proportions with 95% confidence intervals (CIs) for complete response (CR) rate and grade 3–4 acute mucositis rate were calculated using a meta-analysis with random effects model.
A generalized linear mixed model with logit link was used to study the relationship between dose of gemcitabine, dose of cisplatin and occurrence of CR, mucosal toxicity, and hematologic toxicity (Figs. 1–3). An interaction between dose of gemcitabine and cisplatin is added to account for a different effect of gemcitabine in the presence of cisplatin. Because data are clustered in trials, a random trial effect is entered in the model.
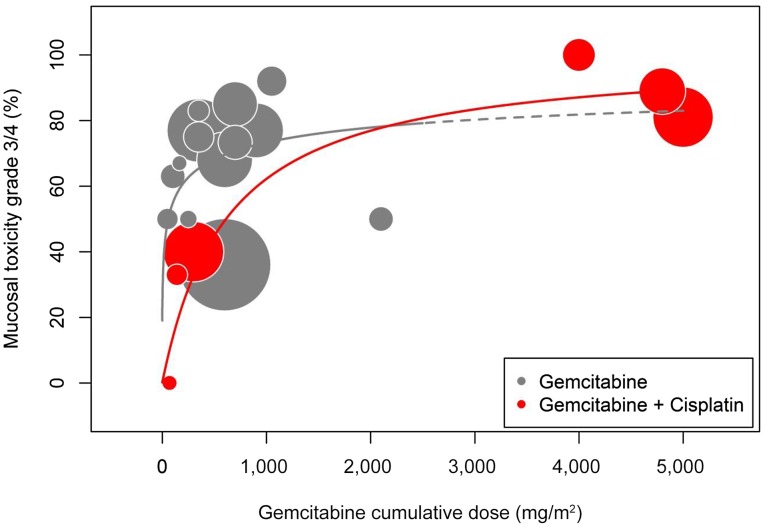
Figure 1.
Mucosal toxicity. Significant dose-related effect between cumulative dose of gemcitabine and incidence of severe acute mucositis was observed for chemoradiotherapy with gemcitabine and cisplatin (red bubbles) but not for chemoradiotherapy with gemcitabine alone (gray bubbles). However, taken together, a plateau phase above a cumulative dose of 300 mg/m2 (roughly corresponding to 50 mg/m2 per week) suggests that no clear dose-effect correlation for mucosal toxicity exists in that segment of the curve.
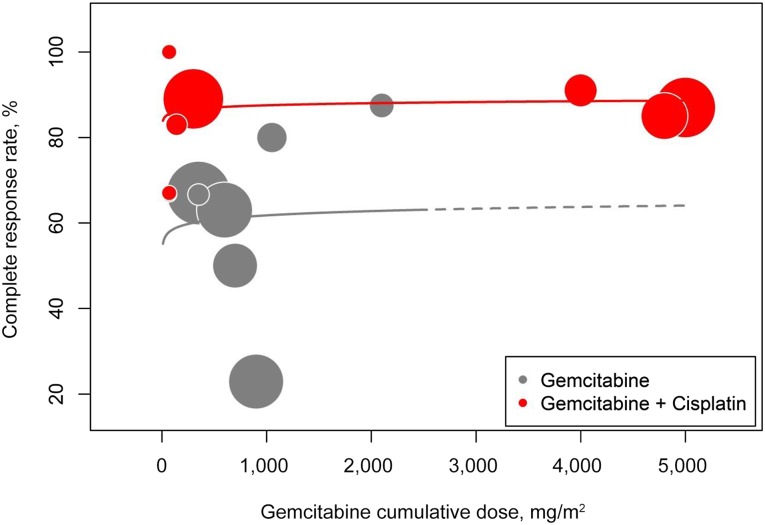
Figure 3.
Complete response rate. No correlation between cumulative dose of gemcitabine and complete response (CR) rate was observed. Importantly, very low-dose regimens integrating cumulative doses of gemcitabine <300 mg/m2 (roughly corresponding to 50 mg/m2 per week) still managed to yield a CR rate of 86% (95% CI, 74%–93%) [39, 52, 53]. Studies numbered 4 [41] and 13 [48] were not included in the analysis because only global response rates were given instead of a dose-specific response rate.
Data were visualized using bubble diagrams, where the size of the bubbles corresponds to the size of the trials. The statistical packages R version 3.1.0 (The R Foundation, Vienna, Austria, http://www.R-project.org/) and SAS version 9.4 (SAS Institute Inc, Cary, NC, USA, http://www.sas.com/en_us/software/sas9.html) were used for the analyses and graphs.
Results
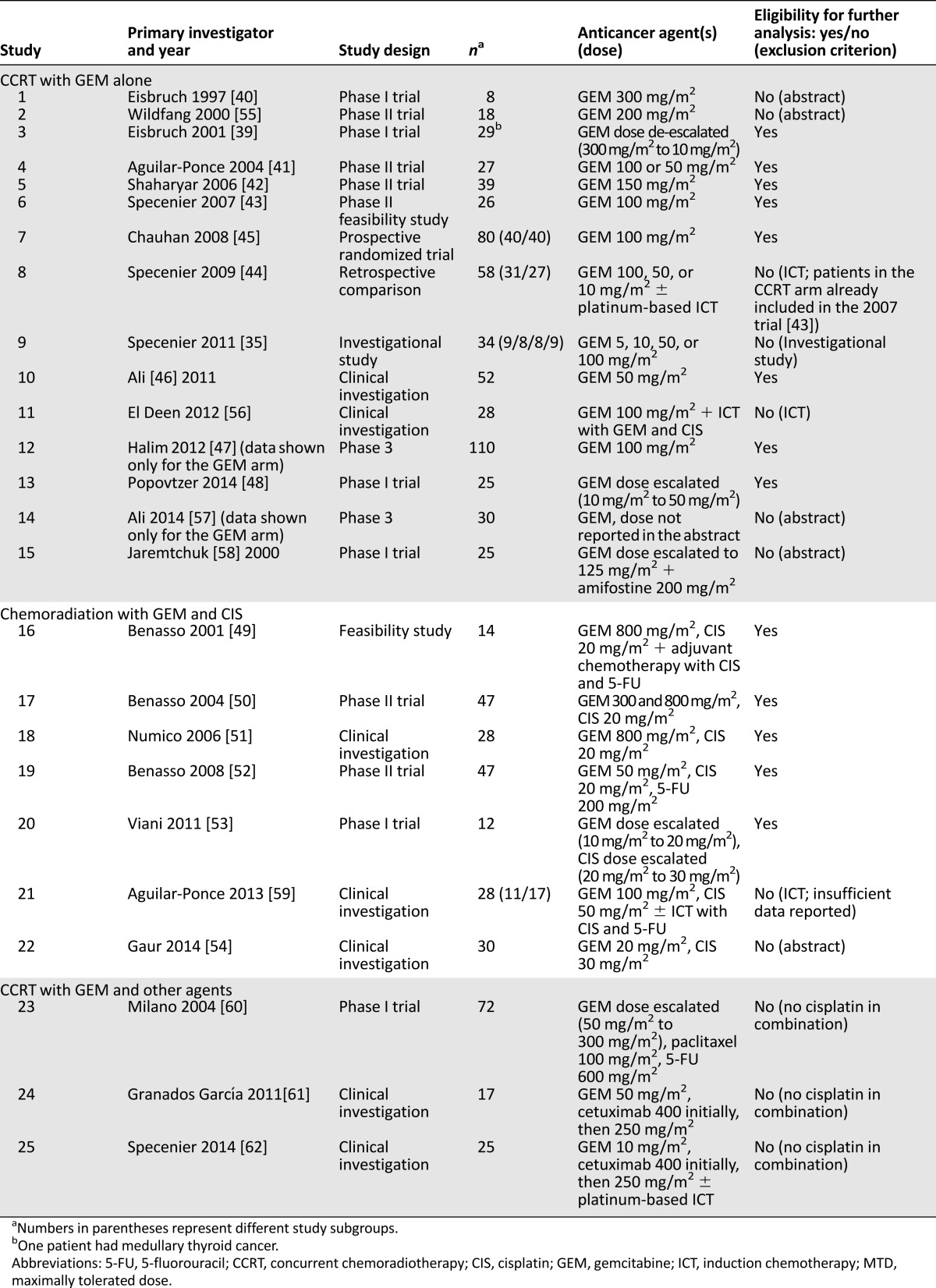
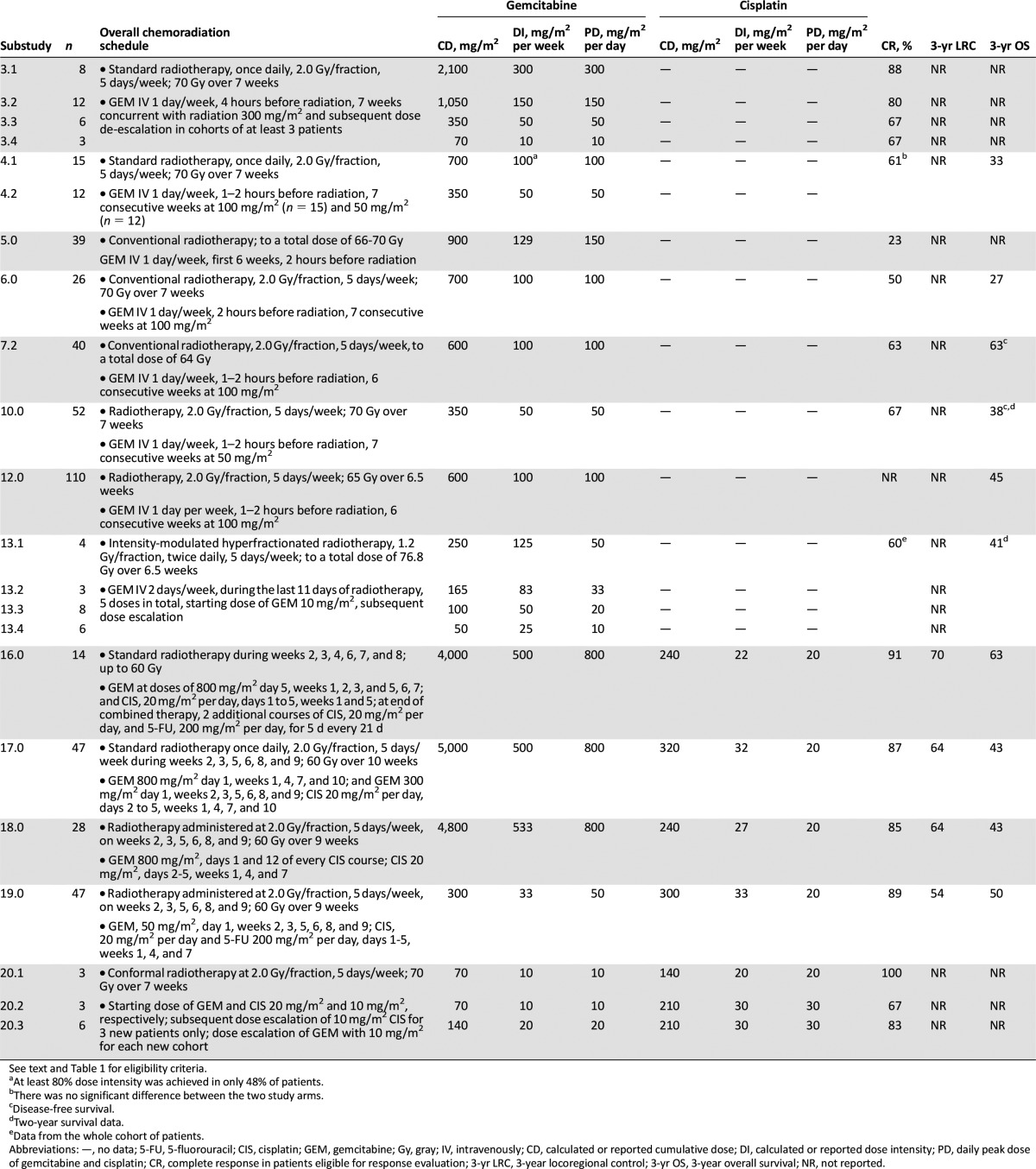
A summary of 25 clinical trials using chemoradiotherapy with gemcitabine for the treatment of LA-SCCHN is provided in Table 1 [35, 39–62]. Of the 25 trials, 13 studies published as full-text articles were eligible for further analysis. The exclusion criteria comprised (a) publication as an abstract only; (b) trials or substudies that were investigational; (c) trials or substudies that had induction chemotherapy (ICT) as part of the therapeutic regimen; and (d) when gemcitabine was part of multiagent-based chemoradiation in combination with other cytotoxic agents, cisplatin had to be part of the therapeutic regimen in order for the study to be eligible for further analysis. The details of the different chemoradiation schedules in the selected studies fully eligible for further analysis are given in Table 2, including types and schedules of radiation, details on gemcitabine and cisplatin cumulative doses, dose intensity (DI), and peak dose level and outcome. Table 3 provides an overview of the reported acute and late toxicity.
Table 1.
Summary of gemcitabine as single-agent or as part of multiagent chemotherapy combined with radiation for the treatment of locally advanced squamous cell carcinoma of the head and neck, with or without induction chemotherapy
Table 2.
Details and outcome of the chemoradiation schedules in the selected studies, including the respective substudies fully eligible for further analysis
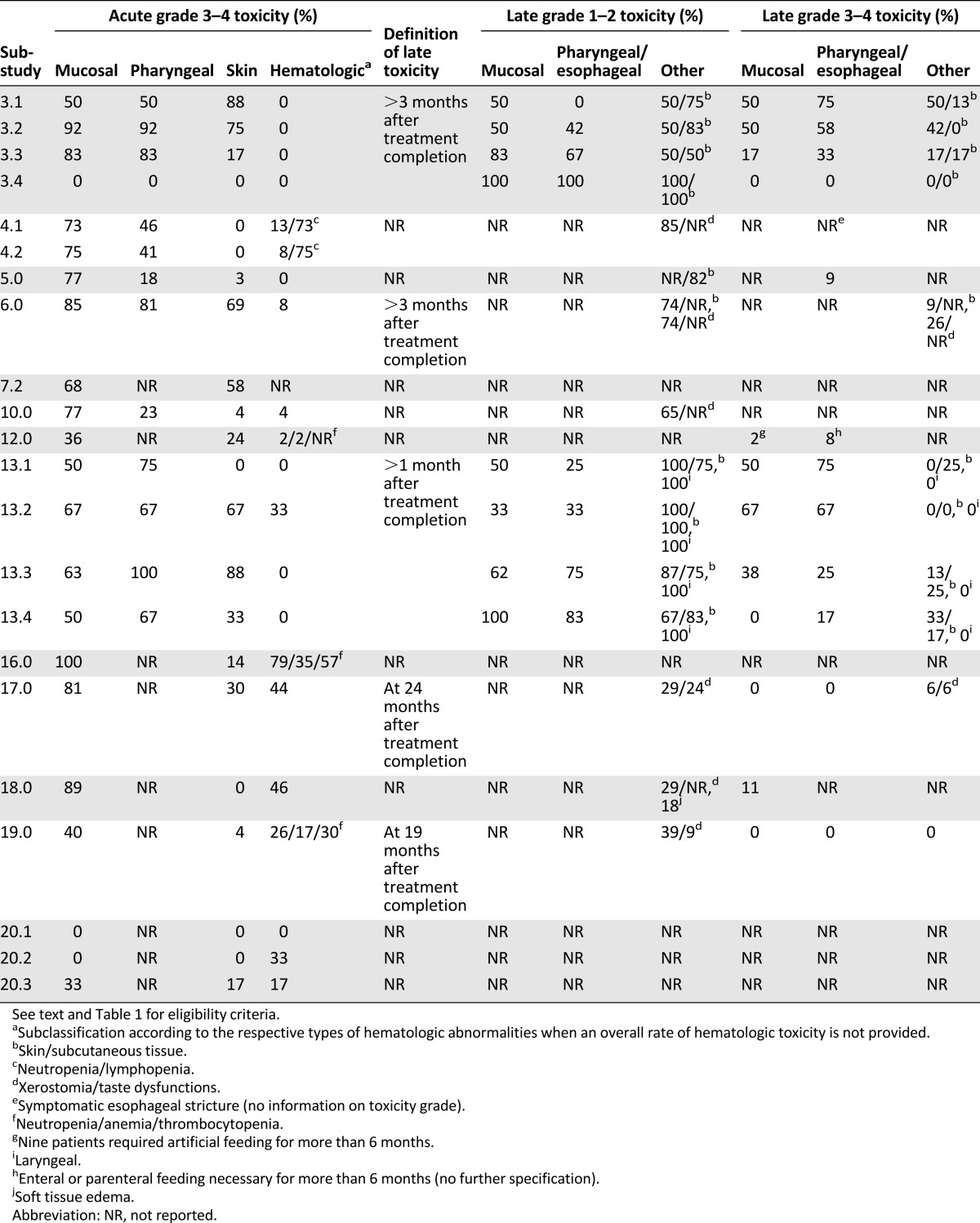
Table 3.
Overview of the reported acute and late toxicities in the eligible studies and substudies in patients evaluable for toxicity.
Studies Reporting on Chemoradiation With Gemcitabine as a Single Agent
Eisbruch et al. were the first to report on a phase I study examining the dose-limiting toxicity (DLT) of standard radiotherapy concurrent with gemcitabine in patients with LA-SCCHN, mostly of oropharyngeal origin [39]. The preliminary results with 300 mg/m2 weekly indicated that gemcitabine and radiotherapy with this regimen resulted in a high locoregional tumor control rate but at the cost of an excessive mucosal and pharyngeal toxicity [40]. The severe (grade 3–4) mucosal reactions were the reason for a gradual de-escalation of the gemcitabine dosage from 300 mg/m2 to 10 mg/m2 weekly. At the dosage levels of 50 to 300 mg/m2, a high rate of acute and, in particular, late toxicity was observed, and one grade 5 pharyngeal toxicity reported in the 300 mg/m2 per week group. In the cohorts receiving 300 and 150 mg/m2 of gemcitabine, confluent mucositis started during the third treatment week and lasted, on average, 7 weeks. In comparison, patients receiving 50 mg/m2 developed confluent mucositis later during the treatment schedule (weeks 4–6 during radiation) and it lasted for a shorter time (3 weeks on average). It is noteworthy that most patients receiving 50 mg/m2 up to 300 mg/m2 required gastric feeding tubes (100% receiving 300 mg/m2, 92% receiving 150 mg/m2, 83% receiving 50 mg/m2). Despite trials of esophageal dilatation, the need for gastric tube feeding persisted in 50%, 17%, and 33% of patients receiving gemcitabine 300, 150, and 50 mg/m2 per week, respectively. An important message of this study is that the researchers observed cases of late pharyngeal toxicity quite early after the treatment completion, which proved to be crucial for correct and timely dose de-escalation. In this particular phase I trial, no acute or late DLTs were observed at the 10 mg/m2 per week dosage level [39].
In a phase II study reported by Aguilar-Ponce, 2 groups of patients with primary tumors of the larynx and paranasal sinuses received gemcitabine 100 mg/m2 or 50 mg/m2 weekly during radiation. CR rates were similar, but excessive mucosal toxicity and one treatment-related death in the higher-dose group forced the researchers to lower the dose to 50 mg/m2. It is noteworthy that grade 3–4 lymphopenia occurred in 74% of patients in contrast with grade 3–4 neutropenia observed in 11% of patients [41].
Shaharyar et al. [42] evaluated the efficacy and toxicity of weekly administration of gemcitabine 150 mg/m2 during radiation in 39 patients with LA-SCCHN. Thirty-eight percent and 28% of the primary tumors were located in the oral cavity and hypopharynx, respectively. Although the overall response rate was high (94%), acute toxicities led to treatment interruption in 41% despite vigorous symptomatic and supportive care. One treatment-related death occurred [42].
In a phase II, single-institution feasibility study assessing the combination of weekly gemcitabine 100 mg/m2 concomitantly with radiotherapy in patients with LA-SCCHN (in particular, stage IV hypopharynx) by Specenier et al., an overall response rate of 100% was reported. In this study, grade 3–4 mucositis, pharyngitis, and dermatitis occurred in 85%, 81%, and 69% of patients, respectively [43]. In a later update of this study (a nonrandomized comparison of the same CCRT preceded by ICT), it was mentioned that none of the patients who were alive and without locoregional recurrence at 1 year were tube feeding-dependent. The use of ICT made this worse (33% dependent at 1 year) [44].
In a prospective, randomized trial by Chauhan et al. [45], 80 patients with LA-SCCHN (mostly of oropharyngeal origin) were randomly assigned to receive radiotherapy alone (n = 40) or radiation plus gemcitabine, given at a dosage of 100 mg/m2 per week. There was no benefit in terms of local control, yet there was a significant difference in disease-free survival in favor of the combined treatment at the cost of more severe acute toxicity (skin, mucosa, weight loss). Importantly, in the same publication, Chauhan et al. mentioned that when they used a higher dose intensity of 200 mg/m2 per week, they were confronted with an unacceptable toxicity profile (grade 3 mucositis in 100% of patients, 1 toxic death) [45].
A dose of 50 mg/m2 of gemcitabine during radiation was used by Ali et al. [46] in 52 patients with LA-SCCHN, mostly with laryngeal cancer. They reported an 88% overall response rate and a 67% CR rate, yet a 2-year disease-free survival of only 38% [46]. Halim et al. [47] randomly assigned LA-SCCHN patients to CCRT either with gemcitabine or paclitaxel. They found a modest but statistically significant advantage of paclitaxel, both in terms of safety and efficacy. The overall response rate in the gemcitabine-arm was 78%, with 80% of patients completing the treatment program [47].
Most recently, Popovtzer et al. [48] reported on a phase I trial attempting to raise the therapeutic ratio of gemcitabine-based CCRT (intensity-modulated radiotherapy) by using a twice-weekly drug regimen. They respectively explored 50, 33, 20, and 10 mg/m2 twice weekly during the last 2 weeks of radiotherapy. Despite favorable data from preclinical studies suggesting the maximal tolerated dose would be between 75 and 90 mg/m2 when given twice weekly, the observed increase was only marginal in comparison with the once-weekly schedule (2×20 mg/m2 vs. 1×10 mg/m2) [39, 48]. Importantly, the 2-year survival rate of 41% was similar between the lower (10 mg/m2 or 20 mg/m2) and higher-dose (33 mg/m2 or 50 mg/m2) groups, whereas none of the patients receiving 10 mg/m2 experienced a DLT or required a dose-modification [48].
Studies Reporting on Chemoradiation With Gemcitabine as Part of Multiagent Chemotherapy During Radiotherapy
In a feasibility study by Benasso et al., alternating chemoradiation with cisplatin and gemcitabine (800 mg/m2) was responsible for an unacceptably high incidence of toxicity (mostly hematologic and mucosal), leading to 3 treatment-related deaths and necessitating dose reductions of gemcitabine in 79% of patients [49].
In 2004, the same authors reported on a modification of the previous regimen in 47 patients with LA-SCCHN. The high acute toxicity rate was deemed manageable. Predominantly in terms of locoregional control, the inclusion of gemcitabine into the alternating regimen seemed to be better compared with the results from the authors’ own database on previously used alternating cisplatin-fluorouracil regimens [50].
Subsequently, in 2006, a third Italian trial further modified the alternating chemoradiation program, aiming to reduce the side effects while preserving efficacy [51]. Although this study and the 2004 trial from Benasso et al. reported an important decrease in hematologic toxicity (grade 3–4 neutropenia) compared with the first Benasso et al. study from 2001, there was no major change in nonhematologic (local) toxicity [49–51]. Moreover, in the 2004 and 2006 trials, 2 patients and 1 patient, respectively, died during the treatment as result of neutropenic fever, leading to toxic death rates of 2% and 7% [50, 51].
In 2008, Benasso et al. [52] reported on a phase II trial of low-dose gemcitabine (50 mg/m2) and radiation that alternated with 3 courses of cisplatin and 5-fluorouracil (Table 2). Compared with their 2004 study, the frequency of severe mucositis, dermatitis, and leukopenia decreased without negatively affecting the survival rates. However, the grade 3–4 mucosal toxicity tended to be higher than that of the authors’ previous results with the same regimen without gemcitabine (40% vs. 25%, p = .06), and 2 patients died during the therapy [52]. Notably, the same dose of gemcitabine in an uninterrupted CCRT schedule was found to induce acute grade 3–4 mucositis in 75% of evaluable patients [41] (Table 3).
In order to define the maximally tolerated dose and the DLT of weekly gemcitabine and cisplatin during CCRT in patients with LA-SCCHN, Viani et al. [53] performed a phase I study. The researchers concluded that the recommended phase II doses were 10 and 30 mg/m2 for gemcitabine and cisplatin, respectively, leading to an acceptable tolerability and compliance, with 83% of patients completing the therapy without interruptions. These encouraging results may be partly due to the uniformly very good performance status (PS 0) of the patients who participated in the study [53]. Most recently, the dosing schedule corresponding to dose level 3 (gemcitabine 20 mg/m2 plus cisplatin 30 mg/m2) that Viani et al. thought to be unsuitable for further exploration in a phase II protocol was prospectively studied by Gaur et al. [54] in a cohort of 30 patients. Apparently, a high overall response rate was achieved (100%), but indeed at the cost of grades 3 mucositis and dermatitis in 73% and 37% of patients, respectively [54].
Comparison Between Very Low-Dose (<50 mg/m2 per week) and Higher Dose (≥ 50 mg/m2 per Week) Gemcitabine-Based Chemoradiation
For schedules using very low-dose gemcitabine (<50 mg/m2 per week), a meta-analysis revealed a pooled proportion of CR rate of 86% (95% CI, 74%–93%), with a grade 3–4 acute mucositis rate of 38% (95% CI, 27%–50%) and acceptable late toxicity [39, 52, 53]. In one of the studies using such a low DI, survival data were provided showing a 3-year overall survival of 50% [52]. Studies 4 and 13 were not included in the meta-analysis because only global response rates were given instead of a dose-specific response rate [41, 48].
For higher DIs (≥50 mg/m2 per week), the CR rate was 71% (95% CI, 55%–83%), with a significantly greater (p<.001) severe acute mucositis rate of 74% (95% CI, 62%–83%), often leading to treatment interruptions [39, 41–43, 45–47, 49–51]. At those DIs, there was not any correlation found between the cumulative dose of gemcitabine and the severity of acute local toxicity. In terms of the CR rate, the difference between very low-dose and higher-dose gemcitabine did not reach statistical significance (p = .087). Correspondingly, based on survival data provided in 8 studies testing higher-dose gemcitabine, a 3-year overall survival ranged from 27% to 63% [41, 43, 45–47, 49–51].
Discussion
Concurrent chemoradiotherapy with single agent cisplatin is generally accepted as the most appropriate treatment for patients with LA-SCCHN. However, long-term outcomes in the majority of cases are still far from satisfactory. Moreover, the therapy-induced morbidity due to frequent acute and late adverse events has a negative impact on the quality of life of such patients. A recent meta-analysis of 3 large trials in patients with LA-SCCHN treated with cisplatin-based CCRT indicated that 43% of the patients suffered from severe late side effects, with long-term feeding tube dependency, pharyngeal dysfunction, and laryngeal dysfunction occurring in 20%, 27%, and 12% of patients, respectively [16]. Therefore, strategies in head and neck oncology focus on improvement of treatment efficacy and also on improving its safety profile. In this review, we explored the therapeutic potential of gemcitabine at both cytotoxic and radiosensitizing doses to illustrate the historical background and outline future perspectives. In total, 25 clinical studies on gemcitabine-based CCRT in LA-SCCHN were identified (14 using gemcitabine as a single agent, 11 in combination), 13 of which were eligible for further analysis.
When considering an appropriate gemcitabine-based CCRT regimen, several important factors have to be taken into account. These include the following: single-agent vs. multiagent chemotherapy with or without molecularly targeted agents; administration schedule; cumulative dose, dose intensity, and peak dose of each drug; characteristics of radiation delivery (dose, fractionation schedule, field size); acute and late side effects along with existing possibilities for their management; short and long-term outcomes; and quality of life of the cancer survivors. The rationale for using low-dose gemcitabine schedules to enhance the effect of radiation is supported by preclinical findings demonstrating the radiosensitizing properties of gemcitabine at noncytotoxic concentrations, and several clinical reports on the efficacy of gemcitabine in this setting at weekly doses well below those used alone or in combination with other cytotoxic agents in solid tumor treatment (10–50 mg/m2 vs. 1,000–1,250 mg/m2), whereas higher weekly dosages of gemcitabine (≥50 mg/m2) during chemoradiation in patients with SCCHN lead to unacceptable toxicity rates [31, 39–54].
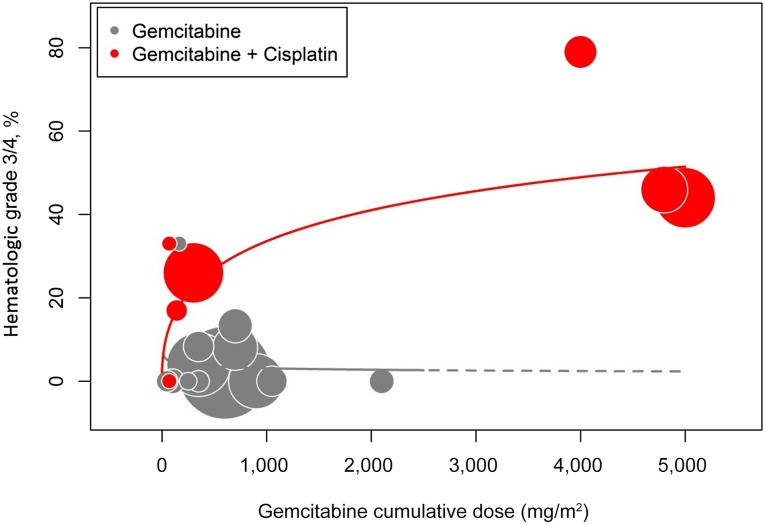
Unfortunately, gemcitabine does not elicit selective tumor radiosensitization. Despite the mild intrinsic toxicity of gemcitabine to mucous membranes, the presented literature data revealed a severe acute mucositis rate of 74% (95% CI, 62%–83%) with ≥50 mg/m2 per week of gemcitabine, often necessitating treatment interruptions [39, 41–43, 45–47, 49–51]. In contrast, in a large sample of 342 patients with laryngeal cancer assigned equally to radiotherapy with concurrent 3 weekly doses of cisplatin 100 mg/m2 and radiotherapy alone, the rates of grade 3–4 acute mucositis were 43% and 24% in both cohorts, respectively [8]. Moreover, there seems to be no clear correlation between the cumulative dose of gemcitabine above 300 mg/m2 (roughly corresponding to 50 mg/m2 per week) and the severity of acute mucosal toxicity (Fig. 1). It is noteworthy that our meta-analysis demonstrated a statistically significant difference in severe acute mucositis rate between very low-dose (<50 mg/m2 per week) and higher-dose (≥50 mg/m2 per week) gemcitabine-based chemoradiation schedules (38% vs. 74%, p<.001). In addition, although acute pharyngeal and skin reactions are quite prevalent in the analyzed series, hematologic toxicity is not of any importance when gemcitabine is used alone. It typically arises when gemcitabine is combined with cisplatin in this setting (Table 3; Fig. 2). In a phase I trial setting, the radio-enhancement of gemcitabine was also explored in combination with other cytotoxic agents (paclitaxel and 5-fluorouracil). However, the therapeutic potential was again hampered by severe acute local toxicity [60]. Therefore, separation of tumor and normal tissue radiosensitizations is one of the key elements in improving the therapeutic ratio of gemcitabine-based chemoradiation.
Figure 2.
Hematologic toxicity. The incidence of severe hematologic toxicity (typically neutropenia) remains low when gemcitabine is used alone (gray bubbles), but a significant dose-related effect arises when gemcitabine is combined with cisplatin (red bubbles). For studies in which an overall rate of hematologic toxicity was not provided, neutropenia was used as a surrogate marker.
Notwithstanding the fact that investigators in most trials of single-agent gemcitabine-based CCRT associated with high rates of severe acute mucositis deem this side effect manageable, we advocate for an increased alertness to serious late events and meticulous reporting thereof. In multivariable analysis, severe late side effects have been linked to the following independent risk factors: older age, advanced T stage, and tumor location in the larynx or hypopharynx [16]. As mentioned above, late pharyngeal toxicity developing within several months after the treatment completion led Eisbruch et al. to de-escalate the dose intensity of gemcitabine [39]. Although 10 out of the 13 eligible studies presented data on late toxicities, the extent and detail of information provided differed substantially, both in terms of definition of what was considered late toxicity as well as in terms of reporting which grade was considered severe [39, 41–43, 46–48, 50–52]. Late toxicity comprising mainly mucosal and pharyngeal/esophageal toxicity was generally underreported, whereas information about xerostomia and skin fibrosis was scarce. From a general point of view, late radiation effects are defined as those occurring in a follow-up period longer than 90 days (Common Terminology Criteria for Adverse Events, version 4.0). However, the ratios between patients evaluable for late toxicity and those evaluable for response, which are usually assessed within 1–3 months after treatment discontinuation, were 22/35, 17/39, and 23/38 in trials published by Shaharyar et al. in 2006, and Benasso et al. in 2004 and 2008, respectively [42, 50, 52]. Other investigators do not describe the duration of tube feeding or objective evaluation of swallowing function [41, 46, 51]. Taken together with data provided by Popovtzer et al. [48] and the current lack of large randomized trials focusing on long-term survival in gemcitabine-based chemoradiation trials, it seems that the safe level may in fact lie below 50 mg/m2 per week. Schedules integrating such dose levels, corresponding to cumulative doses of gemcitabine up to 300 mg/m2, still managed to yield a CR rate of 86% (95% CI, 74%–93%), which is not statistically different from the CR rate (71%; 95% CI, 55%–83%) achieved by higher dose gemcitabine-based chemoradiation schedules (p = .087) [39, 41–43, 45–47, 49–53] (Fig. 3). We believe that very low gemcitabine doses, even as low as 10 mg/m2, will have radiosensitizing potential because dFdU concentrations that showed radiosensitization in vitro could be measured for days in the plasma of patients receiving such low doses of gemcitabine [36]. Yet, within that context, it remains of interest that studies in cell lines have indicated that gemcitabine has a concentration-dependent radiosensitization effect [63].
Notwithstanding the fact that investigators in most trials of single-agent gemcitabine-based CCRT associated with high rates of severe acute mucositis deem this side effect manageable, we advocate for an increased alertness to serious late events and meticulous reporting thereof.
Principally, there are three methods to improve the therapeutic index of low-dose gemcitabine: (a) modifications in treatment schedules, (b) novel radiotherapy techniques, and (c) combination with other anticancer drugs. Favorable pharmacokinetics of gemcitabine permits dosing on a once- or twice-weekly basis [33]. In the study reported by Popovtzer et al. [48], 2×20 mg/m2 led to 2 DLTs in 8 patients with grade 4 mucositis and pharyngitis; however, this regimen was given in the last 2.5 weeks of hyperfractionated radiotherapy, and it was the only study in which intensity-modulated radiation therapy was used. For these reasons, a comparison with the other studies is rather difficult. With respect to novel radiotherapy techniques, new developments such as swallowing-sparing intensity-modulated radiotherapy or intensity-modulated proton radiotherapy may improve the tolerance of CCRT [64–67]. Finally, combining gemcitabine with other active agents has become a field of growing interest. Synergistic activity with cisplatin, cetuximab, or gefitinib has been described [30, 68, 69]. Using standard fractionated radiotherapy, researchers from Brazil selected weekly applications of very low-dose gemcitabine (10 mg/m2) and moderately low doses of cisplatin (30 mg/m2) as the recommended dosages for further study in phase II [53]. Disappointing experience with gemcitabine 800 mg/m2 incorporated into platinum-based alternating chemoradiotherapy regimens made Italian investigators ultimately reduce the dose of gemcitabine to 50 mg/m2 during standard fractionated radiotherapy, alternating this with 3 cycles of low-dose cisplatin plus 5-fluorouracil chemotherapy [49–52]. Interestingly, these investigators also reported that the addition of gemcitabine at these low doses seemed to improve outcome. They based this conclusion on the results obtained in patients treated by the same team in two consecutive controlled trials using the same methods of reporting. The 3-year local control rate, progression-free survival and overall survival in patients with stage IV disease treated with the very low-dose gemcitabine-containing alternating regimen were 54%, 45%, and 50%, respectively, vs. 40%, 28%, and 35%, respectively, when they used the same regimen without gemcitabine. This underlines the potential benefit of very low-dose gemcitabine in these circumstances [52]. The feasibility of these alternating regimens instead of CCRT represents an attractive approach in specialized institutions that needs to be further explored [70].
As the addition of cetuximab to platinum-based CCRT only adds toxicity to platinum-based CCRT without increasing efficacy, other cytotoxic agents with less systemic toxicity and with a different mode of action, such as gemcitabine, in combination with cetuximab and radiation, are an interesting field of research.
To overcome the higher toxicity rate associated with combined chemotherapy regimens, molecularly targeted agents have been sought to enhance the therapeutic ratio of gemcitabine-based CCRT. Two of the most promising strategies comprise EGFR inhibitors and checkpoint kinase 1 inhibitors. The rationale behind the latter approach is based on the knowledge that gemcitabine activates checkpoint kinases, which regulate the cell cycle progression. Preclinical studies using selective knockout of checkpoint kinase 1 in pancreas tumor cell lines confirmed the theoretical background [71]. With EGFR inhibitors, the research has advanced much further. Interesting early clinical data have already been reported (see below) following preclinical experiments, showing that EGFR inhibition, particularly with cetuximab, improves the effectiveness of gemcitabine-based chemoradiation [68, 69]. It is hypothesized that the synergistic effect of gemcitabine and anti-EGFR medication results from the fact that the latter is inhibiting EGFR phosphorylation and blocking the initial survival response (EGFR activation) induced by gemcitabine and thereby promoting apoptosis. Cetuximab/radiotherapy, because of its assumed better tolerance and compliance [72, 73], often serves as an alternative for cisplatin-based CCRT in patients who might have difficulties tolerating it, albeit that a recent literature-based meta-analysis suggested inferior efficacy [74]. As the addition of cetuximab to platinum-based CCRT only adds toxicity to platinum-based CCRT without increasing efficacy [75], other cytotoxic agents with less systemic toxicity and with a different mode of action, such as gemcitabine, in combination with cetuximab and radiation, are an interesting field of research.
A recent example of that is the so-called RAGE protocol, consisting of radiotherapy, gemcitabine, and cetuximab, given to patients with SCCHN who qualified for definitive treatment with CCRT. Preliminary data were presented in 2014 by Specenier et al. at the 24th American Head and Neck Society annual meeting in New York, NY [62]. They reported on 25 patients treated with weekly very low-dose gemcitabine (10 mg/m2) and cetuximab (250 mg/m2 after an initial loading dose of 400 mg/m2) and intensity-modulated radiotherapy (cumulative dose of 69.12 Gray with simultaneous integrated boost technique), 21 of whom received this combined approach after induction chemotherapy. The median treatment duration was 44 days. Twenty-four of the 25 patients had a treatment duration of 47 days or less, although 1 patient had a treatment duration of 50 days. Patients received 97% of the planned cetuximab and gemcitabine dose, and 100% of the planned radiotherapy dose. However, adequate supportive care was necessary because 17 of the 25 patients had grade 3–4 radiodermatitis, 24 had grade 3–4 mucositis, and 10 patients experienced severe weight loss (10%–20%). No conclusive data have been given yet on late toxicity and efficacy. Notably, among 17 assessable patients in a study by Granados García et al., a similarly high rate of grade 3–4 acute toxicities (88% lymphopenia, 71% mucositis, 24% rash, 6% xerostomia) was induced by 50 mg/m2 weekly dosages of gemcitabine combined with cetuximab (250 mg/m2 after an initial loading dose of 400 mg/m2) and concurrent standard fractionated radiotherapy [61]. Eight of 17 patients (47%) had treatment interruptions as the result of grade 3–4 toxicity (2 for hematologic toxicity, 4 for mucositis, 1 for skin rash, and 1 for nausea and vomiting). Toxicity was resolved within 1 week in most patients, but in 2 patients it took 3–5 weeks before the severe mucositis subsided. One patient required permanent study discontinuation due to acute abdominal pain. Despite the fact that toxicity was considered important, the high response rate (overall response rate = 100%, complete response rate = 82.4%) allowed the investigators to conclude that further studies with this triple combination were worth considering.
Conclusion
Despite the obvious limitations given by the heterogeneity of treatment regimens and patient populations in the trials included, the presented review indicates that low-dose gemcitabine given together with radiation can produce meaningful clinical activity either as a single agent or as part of a multiagent chemotherapy with cisplatin, with or without other anticancer drugs. Although there seems to be no difference in efficacy between very low-dose (<50 mg/m2 per week) and higher dose (≥50 mg/m2 per week) regimens, a significantly increased rate of severe acute mucositis can be observed (38% vs. 74%, p < .001) with the higher dose. The results therefore suggest that even very low doses provide a sufficient therapeutic ratio and should be further investigated.
Consequently, to further improve the outcome in terms of efficacy and toxicity, particularly with respect to the incidence of acute mucositis and late dysphagia, refinements in radiation schemes, the use of novel drug combinations, and a better selection of patients on the basis of validated biomarkers for a more personalized treatment approach are worth considering.
Footnotes
For Further Reading: David J. Iberri, A. Dimitrios Colevas. Balancing Safety and Efficacy of Epidermal Growth Factor Receptor Inhibitors in Patients With Squamous Cell Carcinoma of the Head and Neck. The Oncologist 2015;20:1393–1403.
Implications for Practice: Cetuximab and other inhibitors of the epidermal growth factor receptor (EGFR) have entered the medical oncologist’s arsenal against squamous cell carcinoma of the head and neck (SCCHN). They are modestly active as single agents and in combination with chemotherapy and radiotherapy. Despite their efficacy across multiple treatment settings, cetuximab and other EGFR inhibitors (EGFRIs) have not supplanted platinum-based therapies, which remain a standard of care for SCCHN. The modest benefits of EGFRI therapy must take into consideration patient, disease, and treatment characteristics and must be balanced against potential treatment toxicity.
Author Contributions
Conception/ Design: Olivier M. Vanderveken, Kristien Wouters, Jan Vermorken
Collection and/or assembly of data: Olivier M. Vanderveken, Petr Szturz, Pol Specenier, Jan Vermorken
Data analysis and interpretation: Olivier M. Vanderveken, Petr Szturz, Pol Specenier, Kristien Wouters, Jan Vermorken
Manuscript writing: Olivier M. Vanderveken, Petr Szturz, Dirk Van Gestel, Kristien Wouters, Jan Vermorken
Final approval of manuscript: Olivier M. Vanderveken, Petr Szturz, Pol Specenier, Marco C. Merlano, Marco Benasso, Dirk Van Gestel, Kristien Wouters, Carl Van Laer, Danielle Van den Weyngaert, Marc Peeters, Jan Vermorken
Disclosures
Marco C. Merlano: Merck Serono, Boehringer Ingelheim (C/A), Merck Serono (H); Marco Benasso: Merck Serono (C/A, H); Marc Peeters: Merck Serono, Lilly (C/A, H, RF); Jan Vermorken: Merck Serono (C/A, H, RF), Bristol-Myers Squibb (H, RF), Lilly (RF). The other authors indicated no financial relationships. The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Forastiere A, Koch W, Trotti A, et al. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 2.Seiwert TY, Cohen EE. State-of-the-art management of locally advanced head and neck cancer. Br J Cancer. 2005;92:1341–1348. doi: 10.1038/sj.bjc.6602510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermorken JB. Medical treatment in head and neck cancer. Ann Oncol. 2005;16(suppl 2):ii258–ii264. doi: 10.1093/annonc/mdi735. [DOI] [PubMed] [Google Scholar]
- 4.Seiwert TY, Salama JK, Vokes EE. The chemoradiation paradigm in head and neck cancer. Nat Clin Pract Oncol. 2007;4:156–171. doi: 10.1038/ncponc0750. [DOI] [PubMed] [Google Scholar]
- 5.Grégoire V, Lefebvre JL, Licitra L, et al. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v184–v186. doi: 10.1093/annonc/mdq185. [DOI] [PubMed] [Google Scholar]
- 6.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 7.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 8.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 9.Nwizu T, Ghi MG, Cohen EE, et al. The role of chemotherapy in locally advanced head and neck squamous cell carcinoma. Semin Radiat Oncol. 2012;22:198–206. doi: 10.1016/j.semradonc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Adelstein DJ, Lavertu P, Saxton JP, et al. Mature results of a phase III randomized trial comparing concurrent chemoradiotherapy with radiation therapy alone in patients with stage III and IV squamous cell carcinoma of the head and neck. Cancer. 2000;88:876–883. doi: 10.1002/(sici)1097-0142(20000215)88:4<876::aid-cncr19>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Merlano M, Benasso M, Corvò R, et al. Five-year update of a randomized trial of alternating radiotherapy and chemotherapy compared with radiotherapy alone in treatment of unresectable squamous cell carcinoma of the head and neck. J Natl Cancer Inst. 1996;88:583–589. doi: 10.1093/jnci/88.9.583. [DOI] [PubMed] [Google Scholar]
- 12.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. . Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 13.Pignon JP, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Wendt TG, Grabenbauer GG, Rödel CM, et al. Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: A randomized multicenter study. J Clin Oncol. 1998;16:1318–1324. doi: 10.1200/JCO.1998.16.4.1318. [DOI] [PubMed] [Google Scholar]
- 15.Givens DJ, Karnell LH, Gupta AK, et al. Adverse events associated with concurrent chemoradiation therapy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1209–1217. doi: 10.1001/archoto.2009.174. [DOI] [PubMed] [Google Scholar]
- 16.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31:845–852. doi: 10.1200/JCO.2012.43.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen EE, Karrison TG, Kocherginsky M, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32:2735–2743. doi: 10.1200/JCO.2013.54.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): A randomised phase 3 trial. Lancet Oncol. 2013;14:257–264. doi: 10.1016/S1470-2045(13)70011-1. [DOI] [PubMed] [Google Scholar]
- 20.Hitt R, Grau JJ, López-Pousa A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. 2014;25:216–225. doi: 10.1093/annonc/mdt461. [DOI] [PubMed] [Google Scholar]
- 21.Catimel G, Vermorken JB, Clavel M, et al. A phase II study of gemcitabine (LY 188011) in patients with advanced squamous cell carcinoma of the head and neck. Ann Oncol. 1994;5:543–547. doi: 10.1093/oxfordjournals.annonc.a058910. [DOI] [PubMed] [Google Scholar]
- 22.Raguse JD, Gath HJ, Bier J, et al. Gemcitabine in the treatment of advanced head and neck cancer. Clin Oncol (R Coll Radiol) 2005;17:425–429. doi: 10.1016/j.clon.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Pauwels B, Korst AE, Lardon F, et al. Combined modality therapy of gemcitabine and radiation. The Oncologist. 2005;10:34–51. doi: 10.1634/theoncologist.10-1-34. [DOI] [PubMed] [Google Scholar]
- 24.Pauwels B, Vermorken JB, Wouters A, et al. The role of apoptotic cell death in the radiosensitising effect of gemcitabine. Br J Cancer. 2009;101:628–636. doi: 10.1038/sj.bjc.6605145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braakhuis BJ, van Dongen GA, Vermorken JB, et al. Preclinical in vivo activity of 2′,2′-difluorodeoxycytidine (gemcitabine) against human head and neck cancer. Cancer Res. 1991;51:211–214. [PubMed] [Google Scholar]
- 26.Kroep JR, Peters GJ, van Moorsel CJ, et al. Gemcitabine-cisplatin: A schedule finding study. Ann Oncol. 1999;10:1503–1510. doi: 10.1023/a:1008339425708. [DOI] [PubMed] [Google Scholar]
- 27.Bergman AM, Ruiz van Haperen VW, Veerman G, et al. Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res. 1996;2:521–530. [PubMed] [Google Scholar]
- 28.Lawrence TS, Eisbruch A, McGinn CJ, et al. Radiosensitization by gemcitabine. Oncology (Williston Park) 1999;13(suppl 5):55–60. [PubMed] [Google Scholar]
- 29.Peters GJ, Bergman AM, Ruiz van Haperen VW, et al. Interaction between cisplatin and gemcitabine in vitro and in vivo. Semin Oncol. 1995;22(suppl 11):72–79. [PubMed] [Google Scholar]
- 30.Heinemann V, Hertel LW, Grindey GB, et al. Comparison of the cellular pharmacokinetics and toxicity of 2′,2′-difluorodeoxycytidine and 1-β-d-arabinofuranosylcytosine. Cancer Res. 1988;48:4024–4031. [PubMed] [Google Scholar]
- 31.Shewach DS, Hahn TM, Chang E, et al. Metabolism of 2′,2′-difluoro-2′-deoxycytidine and radiation sensitization of human colon carcinoma cells. Cancer Res. 1994;54:3218–3223. [PubMed] [Google Scholar]
- 32.Ruiz van Haperen VW, Veerman G, Vermorken JB, et al. 2′,2′-Difluoro-deoxycytidine (gemcitabine) incorporation into RNA and DNA of tumour cell lines. Biochem Pharmacol. 1993;46:762–766. doi: 10.1016/0006-2952(93)90566-f. [DOI] [PubMed] [Google Scholar]
- 33.Peters GJ, Clavel M, Noordhuis P, et al. Clinical phase I and pharmacology study of gemcitabine (2′, 2′-difluorodeoxycytidine) administered in a two-weekly schedule. J Chemother. 2007;19:212–221. doi: 10.1179/joc.2007.19.2.212. [DOI] [PubMed] [Google Scholar]
- 34.Pauwels B, Korst AE, Lambrechts HA, et al. The radiosensitising effect of difluorodeoxyuridine, a metabolite of gemcitabine, in vitro. Cancer Chemother Pharmacol. 2006;58:219–228. doi: 10.1007/s00280-005-0158-5. [DOI] [PubMed] [Google Scholar]
- 35.Specenier P, Guetens G, Dyck J, et al. Difluorodeoxyuridine plasma concentrations after low-dose gemcitabine during chemoradiation in head and neck cancer patients. Cancer Chemother Pharmacol. 2011;68:185–191. doi: 10.1007/s00280-010-1471-1. [DOI] [PubMed] [Google Scholar]
- 36.Rosier JF, Beauduin M, Bruniaux M, et al. The effect of 2′-2′ difluorodeoxycytidine (dFdC, gemcitabine) on radiation-induced cell lethality in two human head and neck squamous carcinoma cell lines differing in intrinsic radiosensitivity. Int J Radiat Biol. 1999;75:245–251. doi: 10.1080/095530099140708. [DOI] [PubMed] [Google Scholar]
- 37.Rosier JF, Bruniaux M, Husson B, et al. Role of 2′-2′ difluorodeoxycytidine (gemcitabine)-induced cell cycle dysregulation in radio-enhancement of human head and neck squamous cell carcinomas. Radiother Oncol. 2004;70:55–61. doi: 10.1016/j.radonc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Mose S, Karapetian M, Jüling-Pohlit L, et al. Radiation enhancement of gemcitabine in two human squamous cell carcinoma cell lines. Anticancer Res. 2000;20:401–405. [PubMed] [Google Scholar]
- 39.Eisbruch A, Shewach DS, Bradford CR, et al. Radiation concurrent with gemcitabine for locally advanced head and neck cancer: A phase I trial and intracellular drug incorporation study. J Clin Oncol. 2001;19:792–799. doi: 10.1200/JCO.2001.19.3.792. [DOI] [PubMed] [Google Scholar]
- 40.Eisbruch A, Shewach D, Urba S, et al. Phase I trial of radiation concurrent with low-dose gemcitabine for head and neck cancer: High mucosal and pharyngeal toxicity. Proc Am Soc Clin Oncol. 1997;16:386a. [Google Scholar]
- 41.Aguilar-Ponce J, Granados-García M, Villavicencio V, et al. Phase II trial of gemcitabine concurrent with radiation for locally advanced squamous cell carcinoma of the head and neck. Ann Oncol. 2004;15:301–306. doi: 10.1093/annonc/mdh071. [DOI] [PubMed] [Google Scholar]
- 42.Shaharyar A, Javed A, Shah I, et al. A phase II study of gemcitabine concurrent with radiation in locally advanced squamous cell carcinoma of head and neck: A trial of the Cancer Research Group Pakistan. Pak J Med Sci. 2006;22:258–264. [Google Scholar]
- 43.Specenier PM, Van den Weyngaert D, Van Laer C, et al. Phase II feasibility study of concurrent radiotherapy and gemcitabine in chemonaive patients with squamous cell carcinoma of the head and neck: Long-term follow-up data. Ann Oncol. 2007;18:1856–1860. doi: 10.1093/annonc/mdm346. [DOI] [PubMed] [Google Scholar]
- 44.Specenier PM, Weyler J, Van Laer C, et al. A non-randomized comparison of gemcitabine-based chemoradiation with or without induction chemotherapy for locally advanced squamous cell carcinoma of the head and neck. BMC Cancer. 2009;9:273. doi: 10.1186/1471-2407-9-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chauhan A, Singh H, Sharma T, et al. Gemcitabine concurrent with radiation therapy for locally advanced head and neck carcinomas. Afr Health Sci. 2008;8:149–155. [PMC free article] [PubMed] [Google Scholar]
- 46.Ali EM, Abdelraheem AG. Concurrent radiotherapy and chemotherapy for locally advanced squamous cell carcinoma of the head and neck. Head Neck Oncol. 2011;3:48. doi: 10.1186/1758-3284-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halim AA, Wahba HA, El-Hadaad HA, et al. Concomitant chemoradiotherapy using low-dose weekly gemcitabine versus low-dose weekly paclitaxel in locally advanced head and neck squamous cell carcinoma: A phase III study. Med Oncol. 2012;29:279–284. doi: 10.1007/s12032-010-9811-x. [DOI] [PubMed] [Google Scholar]
- 48.Popovtzer A, Normolle D, Worden FP, et al. Phase I trial of radiotherapy concurrent with twice-weekly gemcitabine for head and neck cancer: Translation from preclinical investigations aiming to improve the therapeutic ratio. Transl Oncol. 2014;7:479–483. doi: 10.1016/j.tranon.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benasso M, Merlano M, Sanguineti G, et al. Gemcitabine, cisplatin, and radiation in advanced, unresectable squamous cell carcinoma of the head and neck: A feasibility study. Am J Clin Oncol. 2001;24:618–622. doi: 10.1097/00000421-200112000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Benasso M, Corvò R, Ponzanelli A, et al. Alternating gemcitabine and cisplatin with gemcitabine and radiation in stage IV squamous cell carcinoma of the head and neck. Ann Oncol. 2004;15:646–652. doi: 10.1093/annonc/mdh138. [DOI] [PubMed] [Google Scholar]
- 51.Numico G, Russi EG, Vitiello R, et al. Gemcitabine and cisplatin in a concomitant alternating chemoradiotherapy program for locally advanced head-and-neck cancer: A pharmacology-guided schedule. Int J Radiat Oncol Biol Phys. 2006;66:731–737. doi: 10.1016/j.ijrobp.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 52.Benasso M, Vigo V, Bacigalupo A, et al. A phase II trial of low-dose gemcitabine and radiation alternated to cisplatin and 5-fluorouracil: An active and manageable regimen for stage IV squamous cell carcinoma of the head and neck. Radiother Oncol. 2008;89:44–50. doi: 10.1016/j.radonc.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Viani GA, Afonso SL, Tavares VC, et al. Weekly gemcitabine and cisplatin in combination with radiotherapy in patients with locally advanced head-and-neck cancer: Phase I study. Int J Radiat Oncol Biol Phys. 2011;81:e231–e235. doi: 10.1016/j.ijrobp.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Gaur S, Kumar S, Balasubramanian P. Concurrent chemoradiation with weekly gemcitabine and cisplatin in locally advanced carcinoma of the head and neck. Ann Oncol. 2014;25:iv347. [Google Scholar]
- 55.Wildfang I, Raub M, Guner SA. Low-dose gemcitabine with radiotherapy in advanced head and neck and thyroid cancer, a new radiosensitizing approach. J Cancer Res Clin Oncol. 2000;126:R40a. [Google Scholar]
- 56.El Deen DA, Toson EA, El Morsy SM. Gemcitabine-based induction chemotherapy and concurrent with radiation in advanced head and neck cancer. Med Oncol. 2012;29:3367–3373. doi: 10.1007/s12032-012-0269-x. [DOI] [PubMed] [Google Scholar]
- 57.Ali EM, Hassan EN, Elyamany A, et al. Gemcitabine versus cisplatin concomitant with radiotherapy for locally advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 2014;32:e17039a. [Google Scholar]
- 58.Jaremtchuk V, Zarba J, Keropian M, et al. Phase I study of gemcitabine combined with radiotherapy with or without amifostine in patients with locally advanced head and neck cancer. Proc Am Soc Clin Oncol. 2000;19:A1674. [Google Scholar]
- 59.Aguilar-Ponce JL, Granados-García M, Cruz López JC, et al. Alternating chemotherapy: Gemcitabine and cisplatin with concurrent radiotherapy for treatment of advanced head and neck cancer. Oral Oncol. 2013;49:249–254. doi: 10.1016/j.oraloncology.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Milano MT, Haraf DJ, Stenson KM, et al. Phase I study of concomitant chemoradiotherapy with paclitaxel, fluorouracil, gemcitabine, and twice-daily radiation in patients with poor-prognosis cancer of the head and neck. Clin Cancer Res. 2004;10:4922–4932. doi: 10.1158/1078-0432.CCR-03-0634. [DOI] [PubMed] [Google Scholar]
- 61.Granados García M, Chilaca Rosas MF, Lavín Lozano AJ, et al. Cetuximab concomitant with gemcitabine and radiotherapy in advanced squamous cell carcinomas of upper aerodigestive tract: A pilot study. Clin Transl Oncol. 2011;13:109–114. doi: 10.1007/s12094-011-0627-8. [DOI] [PubMed] [Google Scholar]
- 62.Specenier P, Van den Weyngaert D, Van Laer C et al. Radiotherapy, gemcitabine, and cetuximab (RAGE) in patients with locoregionally advanced squamous cell carcinoma of the head and neck (LA-SCCHN): A feasibility study. Abstract presented at: 5th World Congress of the International Federation of Head and Neck Oncologic Societies/Annual Meeting of the American Head and Neck Society; July 26–30, 2014; New York, NY. S339a. [Google Scholar]
- 63.Pauwels B, Korst AE, Andriessen V, et al. Unraveling the mechanism of radiosensitization by gemcitabine: The role of TP53. Radiat Res. 2005;164:642–650. doi: 10.1667/rr3445.1. [DOI] [PubMed] [Google Scholar]
- 64.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: Clinical and functional results. J Clin Oncol. 2010;28:2732–2738. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Laan HP, Christianen ME, Bijl HP, et al. The potential benefit of swallowing sparing intensity modulated radiotherapy to reduce swallowing dysfunction: An in silico planning comparative study. Radiother Oncol. 2012;103:76–81. doi: 10.1016/j.radonc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 66.van der Laan HP, van de Water TA, van Herpt HE, et al. The potential of intensity-modulated proton radiotherapy to reduce swallowing dysfunction in the treatment of head and neck cancer: A planning comparative study. Acta Oncol. 2013;52:561–569. doi: 10.3109/0284186X.2012.692885. [DOI] [PubMed] [Google Scholar]
- 67.Van Gestel D, Verellen D, Van De Voorde L, et al. The potential of helical tomotherapy in the treatment of head and neck cancer. The Oncologist. 2013;18:697–706. doi: 10.1634/theoncologist.2012-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chun PY, Feng FY, Scheurer AM, et al. Synergistic effects of gemcitabine and gefitinib in the treatment of head and neck carcinoma. Cancer Res. 2006;66:981–988. doi: 10.1158/0008-5472.CAN-05-2665. [DOI] [PubMed] [Google Scholar]
- 69.Feng FY, Lopez CA, Normolle DP, et al. Effect of epidermal growth factor receptor inhibitor class in the treatment of head and neck cancer with concurrent radiochemotherapy in vivo. Clin Cancer Res. 2007;13:2512–2518. doi: 10.1158/1078-0432.CCR-06-2582. [DOI] [PubMed] [Google Scholar]
- 70.Denaro N, Russi EG, Adamo V, et al. State-of-the-art and emerging treatment options in the management of head and neck cancer: News from 2013. Oncology. 2014;86:212–229. doi: 10.1159/000357712. [DOI] [PubMed] [Google Scholar]
- 71.Morgan MA, Parsels LA, Maybaum J, et al. Improving gemcitabine-mediated radiosensitization using molecularly targeted therapy: A review. Clin Cancer Res. 2008;14:6744–6750. doi: 10.1158/1078-0432.CCR-08-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 73.Curran D, Giralt J, Harari PM, et al. Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J Clin Oncol. 2007;25:2191–2197. doi: 10.1200/JCO.2006.08.8005. [DOI] [PubMed] [Google Scholar]
- 74.Petrelli F, Coinu A, Riboldi V, et al. Concomitant platinum-based chemotherapy or cetuximab with radiotherapy for locally advanced head and neck cancer: A systematic review and meta-analysis of published studies. Oral Oncol. 2014;50:1041–1048. doi: 10.1016/j.oraloncology.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 75.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]