Abstract
Objective
Physical activity (PA) adoption can improve quality of life (QOL) and related outcomes among breast cancer survivors. To disseminate a telephone-based PA intervention to cancer survivors, we partnered with the American Cancer Society’s Reach to Recovery program (RTR) whose volunteers (breast cancer survivors) provide information and emotional support to breast cancer survivors.
Method
This randomized controlled trial compared the effects of PA telephone counseling delivered by RTR volunteers (PA Plus RTR) vs. a contact control condition (RTR Control) in 6 New England states. RTR volunteers (n=18; mean age=54.9 years, mean years since breast cancer diagnosis=7.0) delivered a 12-week PA program to help participants adopt 30 minutes of moderate-intensity activity ≥5 days/week. Breast cancer survivors (n=76; mean age=55.62 years, mean years since diagnosis=1.11, Stage 0=6.58%, Stage 1=38.16%, Stage 2=44.74%, Stage 3=10.53%) were randomized to a study group.
Results
Using a series of generalized linear models, we assessed intervention effects on physical health, physical functioning, mental health, fatigue and QOL at 12 and 24 weeks, and examined whether these effects were moderated by age, marital status, chemotherapy use and baseline values of the outcomes. There were no significant intervention effects on the outcomes but there were significant moderator effects of age, chemotherapy use and baseline physical functioning, physical health, and breast cancer-specific symptoms (all p’s<.05).
Conclusions
Specific demographic and treatment variables and baseline psychosocial health moderate the impact of PA interventions on QOL.
Keywords: breast cancer, oncology, physical activity, quality of life, moderators
Background
Breast cancer is the most common cancer among women and its 5-year survival rates exceed 90% [1]. There has been increased interest in promoting moderate-to-vigorous physical activity (MVPA) through on-site, home-based (delivered by print, telephone calls, etc.) and combined programs [2] and there is evidence of improved psychosocial functioning (e.g., reduced depressive symptoms and improved quality of life)(QOL) among cancer survivors with increased MVPA [3–6]. The effects of physical activity (PA) on fatigue among cancer survivors is somewhat mixed [7, 8]. A majority of studies promoting PA have been conducted in research settings and trained research staff delivered the interventions. It is timely to consider extending the reach of these interventions to the community for wider application and to enhance dissemination.
One such approach is the use of peer-led interventions that have been effective with chronic disease management [9, 10] to scale up interventions and potentially to reduce intervention costs. Other advantages of peer-led interventions are that participants may be more receptive to advice and assistance provided by someone “like” them (e.g., age, disease condition, etc.). There is a small body of literature that demonstrates that peer-led interventions are effective in increasing PA in non-cancer populations [11, 12] and a recent review concluded that peer mentors are an under-utilized approach to PA promotion [13]. There are theoretical bases for the potential advantages of peer-delivered PA programs in that behaviors such as PA can be learned by observing and imitating other (modeling effects) and feedback and reinforcement from others can strengthen self-efficacy (social cognitive theory)[14]. Also, reinforcement, modeling, and mastery of skills by others can enhance the perception of competence which is central to several theories of motivation such as self-determination theory [15].
We completed a randomized controlled trial (RCT) in which volunteers with the American Cancer Society’s Reach to Recovery (RTR) program delivered a 12-week PA counseling intervention (PA Plus RTR) vs. contact control (RTR Control) to 76 breast cancer survivors in New England. Effects on self-reported and objective accelerometer data showed that the group that received the intervention significantly increased their MVPA at post-intervention (12 weeks) and at 24 weeks [16]. Although the primary goal of the trial was to examine the effects of PA counseling delivered by the ACS volunteers (coaches) on MVPA (results previously published, [16]), we were also interested in exploring potential QOL benefits of the peer-led intervention as secondary outcomes.
Consistent with the literature [3–8], our hypothesis was that the group receiving the PA counseling would report improved functioning in the psychosocial domains (specifically, mental and physical health, physical functioning, fatigue, and QOL) compared to the contact control group at 12 weeks and 24 weeks. In addition, we were interested in examining potential moderators of these intervention effects: age, marital status (partnered vs not partnered), use of chemotherapy and baseline values of the QOL outcomes. These variables were selected because prior research indicates that they influence PA intervention effects on QOL [7, 17].
Methods
Study Design
We compared the effects of a 12 week PA program (PA Plus RTR) vs. contact control (RTR Control) offered by RTR volunteers among breast cancer survivors in a RCT. Participants’ PA and other outcomes were assessed at baseline, 12 weeks and 24 weeks. The study received approval from the Institutional Review Boards at The Miriam Hospital (RI) and Women & Infants Hospital (RI).
Recruitment of ACS RTR Volunteers/Coaches
We recruited and trained 18 RTR coaches in the ACS New England Division. ACS staff recruited these coaches from among 335 RTR volunteers through email, print mailings, and personal contact. Thirty one volunteers expressed interest in the study, 28 were screened for eligibility, and 18 coaches completed training (64%)(mean age = 54.89 years, SD=7.76, mean 7.00 years post-diagnosis, mean 4.47 years volunteering with RTR, 95% White, 90.5% with some college education, 33.3% employed full time) [16]. All coaches completed informed consent procedures.
Eligibility criteria
To be eligible, coaches should have completed RTR training and been a RTR volunteer for at least a year. They had to be willing to: a) participate in group training, b) provide coaching to 4–5 participants, c) be supervised by telephone, and d) audiotape telephone contacts with study participants.
Training of Coaches
Training was conducted in small groups either in-person or using video-conferencing. The 4 session training program (approximately 2 hours per session) consisted of didactics on the PA program, intervention theory, monitoring patient safety, and issues relevant to human subjects’ certification and HIPAA requirements. The coaches received training in delivering the RTR Control condition as well as PA counseling and participated in role-plays to train skills such as showing empathy and reflective listening. The intervention was based on the transtheoretical approach and social cognitive theory [14,18]. To guide participants to become physically active, the coaches were trained to assess current MVPA and motivational readiness, identify barriers to MVPA, problem solve these barriers (social cognitive theory) [14] and negotiate PA goals for the following week (tailored to participant’s motivational readiness)[18]. The coaches were also trained to guide participants to exercise within the recommended range of intensity (55% to 65% estimated maximum heart rate). In the event of health symptoms related to exercise, coaches were trained to suspend the MVPA program for RTR Plus PA participants until medical clearance was obtained. If participants became distressed, coaches were trained to notify research staff so that appropriate referrals could be made.
Participants
Eligibility criteria for participants included: a) aged ≥21 years and diagnosed with Stage 0–3 breast cancer in the past 5 years, b) completed surgery (patients receiving on-going chemotherapy, radiation or hormone treatment were eligible), c) able to read and speak English; d) able to walk half-mile without stopping, e) sedentary: <30 minutes/week of vigorous PA or <90 minutes/week of moderate-intensity PA for the past six months, and f) access to a telephone and were willing to receive calls. Other medical or psychiatric problems (e.g., myocardial infarction and orthopedic problems) that might interfere with protocol adherence were exclusion criteria.
Participant Recruitment
Participants were chiefly recruited through print mailings sent by the Senior Operations Vice-President for Health Initiatives at the ACS to breast cancer constituents on mailing lists maintained by the ACS in 6 states (n=8111), electronic newsletters sent by the ACS, recruitment at ACS sponsored events in RI, and referrals from RTR coordinators. We also recruited via informational mailings by three hospitals and three private practices. Power analyses for the primary outcome (MVPA) showed that 108 participants were needed for the trial before attrition (99% power to detect differential change on MVPA at 12 weeks and 80% power at 24 weeks at a multiplicity-adjusted significance level alpha=0.025). The power estimates were based on the assumption that participant outcomes were independent within each ACS office.
Potential participants contacted the study staff using a toll-free number. Following a phone screen for eligibility, written informed consent was obtained and medical clearance for study participation was also obtained. In total, 595 potential participants were contacted, 304 were ineligible at initial contact or phone screen (51.1%), 123 were not interested (20.7%) and 168 were eligible at phone screen (28.2%). Reasons for ineligibility have been described previously [16]. Of the 168 potential participants, 31 were no longer interested, 61 became ineligible and the remaining 76 were eligible and randomized (76/168=45.2%).
Intervention Delivery
After baseline assessments were completed, the sample was stratified by age and whether or not they had received chemotherapy, and then randomized to a study group. The Intervention Coordinator assigned participants to coaches based on scheduling availability and similarity of cancer treatment(s). Each coach was asked to contact her participant in PA Plus RTR or RTR Control once a week over 12 weeks and audio-tape the calls.
PA Plus RTR group
The PA intervention that had been previously tested [19] consisted of telephone-delivered counseling tailored to participants’ motivational readiness [20]. Participants also received a pedometer (Digiwalker) and a heart rate monitor with instructions to use these during PA. Participants were instructed on maintaining PA logs (type of MVPA, duration, heart rate, rate of perceived exertion and pedometer steps) to facilitate self-monitoring (a technique from social cognitive theory and the transtheoretical approach)[14,18]. During the weekly calls, coaches were asked to build a supportive relationship with participants while assessing their motivational readiness, monitoring PA, identifying and problem solving barriers to PA (techniques based on constructs from social cognitive theory and the transtheoretical approach) [14,18,20] and identifying health concerns. The overall goal was to encourage participants to gradually increase the amount of MVPA (e.g., brisk walking) over 12 weeks to recommendations of ≥ 30 mins. of moderate-intensity PA on most days of the week [21]. As is typical of the RTR program, coaches responded to questions that participants asked about breast cancer and its treatment and provided informational and emotional support. At Week 2, 4, 8 and 12, each participant received a PA feedback report. Participants were also provided 12 exercise tipsheets that focused on PA topics and RTR informational booklets.
RTR Control Group
These participants were provided RTR informational booklets and coaches provided information and support for participants’ questions and concerns about breast cancer. During their weekly calls, the coach also administered the Weekly Symptom Questionnaire [22] that assesses problems such as headaches. The participants were asked not to join a structured program of MVPA during the 12-week intervention phase. After completing assessments at 24 weeks, they were provided the same PA tipsheets as those sent to the PA Plus RTR group.
Participant Measures
At baseline, we obtained demographic information from participants and disease and treatment variables from medical records. A Research Assistant (blind to the participant’s group assignment) was responsible for collecting all data by mail or by telephone. Participants received small incentives (e.g., $20 gift cards) for completing the assessments which included:
MOS 36-Item Short Form Health Survey (SF-36) [23,24] assesses eight health concepts (e.g., physical functioning, bodily pain). We obtained the Physical Functioning (PF) and Mental Health (MH) scores as well as overall physical and mental health as assessed by the Physical Component Summary (PCS) and Mental Component Summary (MCS) scores, respectively. The SF-36 yields continuous variables that ranges from a low score of 0 (e.g., poor functioning) to a high score of 100 (no limitations, high functioning) on each subscale. The PCS score was obtained by multiplying each subscale z-score by a physical factor score coefficient and summing the eight products. Similarly, the MCS score was obtained by multiplying each subscale z-score by its respective mental factor score coefficient and summing the eight products.
Functional Assessment of Cancer Therapy Scale-Fatigue (FACIT-F). This 13-item scale is a brief, reliable and valid measure of the physical and functional effects of fatigue [25]. The range of scores is 6 (high fatigue) to 52 (low fatigue).
Functional Assessment of Cancer Therapy Scale for Breast Cancer (FACT-B) is a 55-item scale that assesses QOL and is reliable and valid [26]. The range of scores is 0 to 144, with higher scores indicating a better QOL. We also examined the FACT Breast Symptom Index (FSBI) which contains 10 items such as “one or both of my arms are swollen or tender.” The range of scores is 0–36 with higher scores indicating fewer concerns specific to breast cancer.
Seven Day Physical Activity Recall (7 Day PAR) [27]. This interviewer-administered measure [28] assesses hours spent in sleep as well as moderate, hard, and very hard activity (leisure and occupational) over the past week. The effect on weekly minutes of MVPA was the primary outcome in the trial.
Analyses
We obtained descriptive statistics of baseline demographics, medical history and baseline QOL constructs summarized by group and aggregated across the sample. Although between-group differences for baseline demographic and medical history variables have been presented elsewhere [16], between-group differences in the baseline QOL constructs were tested using Analysis of Variance (ANOVA) for continuous measures.
Using a series of generalized linear models, we tested the association between intervention and outcomes: PF, MH, PCS, MCS, FACIT-F, FACT-B and the FSBI at 12 and 24 weeks, adjusting for baseline value of the outcome. As a subsequent step, we tested whether key baseline variables (age, chemotherapy use, partnered vs. not partnered and baseline values of the outcomes) were moderators of the intervention effects on QOL outcomes at 12 and 24 weeks, using a similar modeling approach, which also adjusted for baseline values of the outcomes. That is, a series of generalized linear models were run which included the main effect of the intervention, main effect of the potential moderator and the interaction between the two (intervention*moderator). A variable was considered a moderator of the intervention effect if the interaction with the assigned intervention was significantly different than zero.
Generalized linear models take a likelihood-based approach to estimation and thus make use of all available data without directly imputing missing values. Thus, results presented are on the Intent-to-Treat sample, such that all participants randomized were included in the analysis.
Finally, to better understand the results of the various moderator analyses, we explored the association between baseline values of the significant moderator variables and mean MVPA (as measured by 7-Day PAR) at follow-up, using Analysis of Variance and the correlation between weekly minutes of MPVA and QOL variables at follow-up (for significant results only).
All analysis was carried out using SAS 9.3 with significance level alpha=0.05.
Results
Seventy six breast cancer survivors were randomized to PA Plus RTR or RTR Control. Mean age of participants was 55.6 years at study entry and the majority of participants were diagnosed with Stage 1 or 2 cancer (See Table 1). There were no significant differences between groups at baseline with respect to baseline demographics, medical history or QOL constructs. Unadjusted mean values of the QOL constructs over time are presented in Table 2.
Table 1.
Description of Study Sample by Group
| PA Plus RTR (N=39) | RTR Control (N=37) | All (N=76) | |
|---|---|---|---|
| Age (years) | 55.64 (8.59) | 55.59(10.59) | 55.62(9.55) |
| Marital Status (% partnered) | 79.49%(31) | 86.49%(32) | 82.89%(64) |
| Race (%Caucasian) | 97.44%(38) | 100%(37) | 98.68%(75) |
| Ethnicity (%Hispanic/Latino) | 10.26%(4) | 2.70%(1) | 6.58%(5) |
| Education, %(n) At least Some College | 94.87%(37) | 83.78%(31) | 89.47%(68) |
| Employment, %(n) Full-time | 30.77%(12) | 48.65%(18) | 39.47%(30) |
| Stage | |||
| 0 | 7.69%(3) | 5.41%(2) | 6.58%(5) |
| 1 | 41.03%(16) | 35.14%(13) | 38.16%(29) |
| 2 | 41.03%(16) | 48.65%(18) | 44.74%(34) |
| 3 | 10.26%(4) | 10.81%(4) | 10.53%(8) |
| Mean Years Since Diagnosis | 1.05 (0.98) | 1.16 (1.14) | 1.11(1.05) |
| Treatment (% Chemotherapy) | 78.38%(29) | 64.86%(24) | 71.62%(53) |
| Baseline Activity Level (Min/Week) | 31.77(33.87) | 17.14(23.42) | 24.64(29.98) |
All values are means (standard deviations) unless otherwise noted.
Table 2.
Unadjusted Mean Values of the QOL Constructs over time
| PA Plus RTR | RTR Control | |
|---|---|---|
|
| ||
| SF-36 Physical Component Summary Score | ||
| Baseline | 49.19(8.76) | 46.40(7.36) |
| 12 Week | 52.00(6.71) | 50.93(6.94) |
| 24 Week | 51.49(7.07) | 50.63(6.81) |
|
| ||
| SF-36 Mental Component Summary Score | ||
| Baseline | 48.68(10.48) | 49.42(10.74) |
| 12 Week | 54.21(9.28) | 52.48(9.62) |
| 24 Week | 53.44(8.57) | 50.52(10.09) |
|
| ||
| SF-36 Physical Functioning | ||
| Baseline | 81.54(16.59) | 77.84(16.31) |
| 12 Week | 87.08(14.41) | 84.69(15.91) |
| 24 Week | 86.67(13.42) | 84.33(15.30) |
|
| ||
| SF-36 Mental Health | ||
| Baseline | 75.51(14.55) | 73.38(17.36) |
| 12 Week | 81.81(12.71) | 76.09(15.75) |
| 24 Week | 79.72(13.52) | 74.50(15.83) |
|
| ||
| Fatigue (FACIT-F) | ||
| Baseline | 37.33(8.93) | 34.70(12.53) |
| 12 Week | 43.83(7.28) | 41.22(8.49) |
| 24 Week | 42.20(7.77) | 40.40(9.32) |
|
| ||
| FACT-B | ||
| Baseline | 109.47(13.85) | 103.40(22.19) |
| 12 Week | 117.79(12.74) | 113.98(18.02) |
| 24 Week | 115.68(14.97) | 111.39(17.74) |
|
| ||
| FACT Breast Symptom Index (FSBI) | ||
| Baseline | 24.54(5.62) | 23.01(5.56) |
| 12 Week | 27.03(5.40) | 26.34(4.95) |
| 24 Week | 27.11(6.08) | 25.17(4.79) |
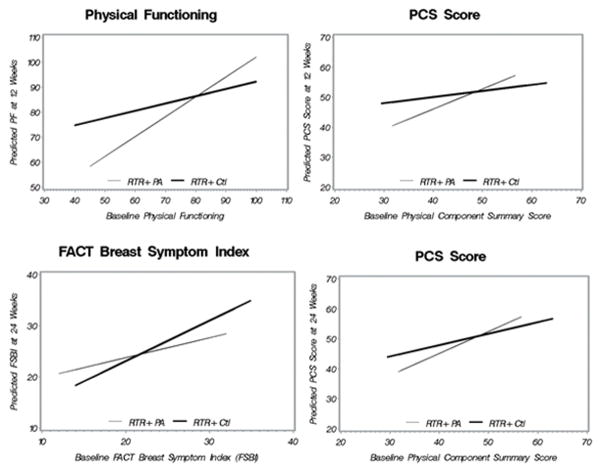
Results did not suggest intervention effects on changes in the QOL constructs from baseline to 12 and 24 weeks (p’s>.05). However, moderator relationships were found, suggesting that differences between groups in mean QOL scores at follow-up (controlling for baseline), depended on the value of the moderator. There was a significant intervention effect on PF at 12 weeks among those with poorer physical functioning at baseline (btreatment=40.65, SE=15.46, p<.01, bbase x treatment=−0.50, SE=0.19, p<.01), a significant effect on the PCS score at 12 weeks among those with lower baseline scores (btreatment=22.75, SE=9.39, p=.02, bbase x treatment=−0.47, SE=0.19, p=0.02), a significant effect on the PCS score at 24 weeks among those with lower baseline scores (btreatment=16.82, SE=8.88, p=0.05, bbase x treatment=−0.35, SE=0.18, p<.01 and a significant effect on the BSFI at 24 weeks for those with better QOL at baseline (btreatment=−8.71, SE=4.75, p=0.06, bbase x treatment=0.40, SE=0.19, p=0.03) (See Figure 1). Figure 1 presents the baseline value (x-axis) by predicted outcome value (y-axis) separately for each study group. In each case, the lines cross thus showing that the effect of the intervention on the outcome depended on the baseline value of the moderator and the relationship changed over the range of the moderator variable. There were no significant moderator effects of baseline value on the MCS score, MH, FACIT-F or FACT-B.
Figure 1.
Baseline Values as Moderators of 12 Week and 24 Outcomes
Further exploration of these effects suggested higher minutes/week of MVPA at 12 weeks among those with lower PF at baseline (p<.01), as well as higher mean minutes/week of MVPA at 24 weeks among those with higher scores on the FSBI at baseline (p<.01). In addition, there was a trend towards a significant correlation between minutes/week of MVPA and both PF and FSBI at each follow-up (rho’s ranging from 0.2–0.3 and p’s from 0.08–0.10).
Finally, age was a significant moderator of the intervention effects on PF at 24 weeks (btreatment=−35.48, SE=16.37, p=0.03, bage x treatment=0.62, SE=0.29, p=0.03) such that for those over 57 years, mean physical functioning was higher for those randomized to PA Plus RTR vs. RTR Control (see Figure 2) (57 years was the value of age for which the lines cross). Also, chemotherapy use was a significant moderator of PF at 24 weeks (btreatment=−10.25, SE=4.80, p=0.03, bchemo x treatment=12.98, SE=5.69, p=0.02) such that among those who had received chemotherapy, mean physical functioning was higher at 24 weeks for those randomized to PA Plus RTR vs RTR Control (see Figure 2).
Figure 2.
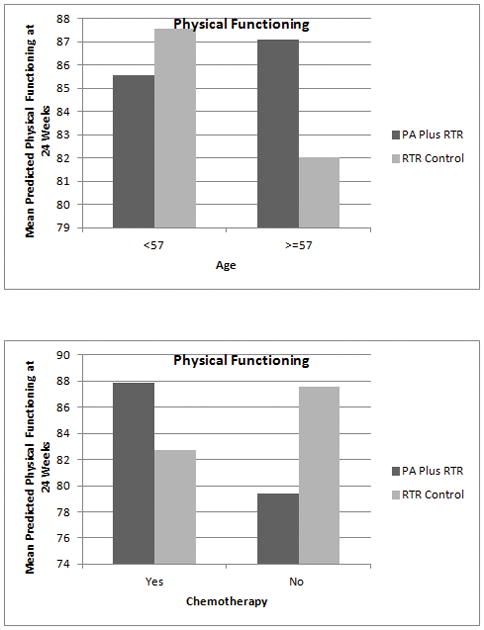
Age and Chemotherapy as Moderators of 24 Week Outcomes
Conclusions
Prior research concluded that PA interventions with breast cancer survivors show small-to-moderate effects on fatigue and overall QOL [29]. While these findings are promising, the authors called for the use of standardized measures and the investigation of potential moderators of the effects of PA interventions in future research. In addition, more research is needed to evaluate the impact of peer-led PA interventions. To address these issues, we explored the impact of a peer-led PA intervention on QOL outcomes and potential moderators of intervention effects.
The primary finding of this study was that, contrary to our hypotheses, the peer-led PA intervention did not lead to significantly greater improvement in QOL outcomes for the PA Plus RTR arm compared to the RTR Control group. These findings are somewhat inconsistent with several meta-analyses and reviews [5, 29,30] which generally conclude that PA interventions do have a positive, albeit small-to-moderate impact on QOL. It may be that our intervention was simply not strong enough to impact these outcomes. Indeed, we set a target level of 150 minutes/week of MVPA for the PA Plus RTR group. However, at 12 weeks, weekly MVPA participation in this group averaged only 120 minutes [16]. So, despite exercising more than those in the control arm, it is possible that the PA Plus RTR group did not meet a minimum threshold where QOL outcomes may be impacted. Such a result is a potential drawback of home-based PA interventions where there are fewer opportunities to help participants reach a specific level of exercise. It is also consistent with the results of several meta-analyses that concluded that on-site supervised exercise programs produced the largest effects on PA interventions on psychosocial outcomes [3, 4]. Secondly, our intervention was also not specifically designed to impact QOL outcomes. The RTR coaches were trained to be supportive and empathic to all participants regardless of group assignment. Indeed the standard RTR program that was administered to both study groups includes an emotional support component. Hence, it is possible that the MVPA intervention did not significantly improve QOL outcomes over and beyond the emotional support and assistance that both groups received.
A third potential explanation for the lack of statistically significant main effects of the PA intervention on QOL outcomes is that we did not purposefully recruit participants with poor QOL. Although we did assess baseline levels of PA and included only those who indicated they were sedentary, we did not target those with poorer QOL. For example, the participants’ mean baseline scores for PF (81.54), MH (75.51), PCS (49.19), and MCS (48.68) are comparable to the available population norms for these measures [31]. In essence, there may have been a “ceiling effect” for potential improvement in these outcomes because the study participants had relatively good baseline scores and thus had little room to improve. This is consistent with previous literature that suggest that PA interventions intended to impact psychosocial outcomes need to be targeted to populations with the greatest need to realize the greatest impact [17]. Finally, it may be also be that because we powered for the primary MVPA outcome, our study was simply under powered to detect statistically significant effects of the intervention on QOL outcomes.
Despite these null main effects, consistent with the recommendations of Speck and colleagues [29], we did examine potential moderators of the effects of the PA intervention on QOL outcomes. Previous research has shown that variables such as marital status, pre-intervention weight and physical health, baseline fatigue and preference for exercise modality may moderate the effects of a PA intervention on global QOL outcomes [32–34]. Although we explored marital status in our analyses, we did not find it to have a significant moderator effect. As proposed by Kalter and colleagues [34], it may be that the need for social support is greatest during active treatment: a majority of our participants had completed surgery, radiation and/or chemotherapy. The results of our study did indicate that the intervention was most effective in improving physical functioning among those with poorer physical health and physical functioning at baseline.
At the same time, we also found that the intervention was more effective in improving managing breast cancer specific symptoms among those who reported significantly less symptom burden at baseline. This finding was noted only for the 13-item FSBI, as opposed to the total FACT-B score. These seemingly contradictory findings could potentially be a function of the constructs assessed by the respective measures. Specifically, overall physical health as measured by the SF-36 PCS score and PF subscale, assess an individuals’ physical health status and conditioning (e.g., ability to climb a flight of stairs). In contrast, the FSBI assesses concerns about breast cancer-specific symptoms (e.g. pain, stiffness, numbness). Thus, it may be that the intervention is effective for those who are de-conditioned and asymptomatic but not for those with persistent breast cancer symptoms. The clinical implication is that while PA interventions may be appropriately prescribed to increase MVPA in breast cancer survivors, additional targeting of deconditioned but asymptomatic survivors may be recommended if impact on QOL outcomes is a primary aim.
Finally, we also found age and chemotherapy to have significant moderator effects, with beneficial effects of the intervention for participants who, at baseline, were older (over 57 years old) and had received chemotherapy. As with the potential explanation for the lack of significant main effects in our study, it may be that those who are younger and who have not had chemotherapy had better QOL at baseline. As a result, there was less scope for the intervention to have a significant impact on their QOL. Our results are somewhat similar to a recent study on the effects of group-based aerobic and resistance training plus cognitive-behavioral therapy (vs. wait-list controls) which found no moderating effects of age but did find that the intervention had a greater impact on global QOL among those survivors who received a combination of chemotherapy and radiotherapy, compared to those who received only one treatment modality [34].
We recognize that others have examined the psychosocial effects of peers providing emotional support to cancer survivors [35,36]. However, our study focused on the addition of an evidence-based PA program to an existent peer support program and hence, was not designed to assess the specific effects of peer support (since both groups received emotional support from the RTR coaches) on QOL.
To conclude, this study adds to the growing literature examining the impact of PA interventions on psychosocial outcomes. It raises interesting issues regarding the appropriate selection of study participants, sample size considerations, and the use of peer coaches to deliver such interventions. The majority of studies support the adoption of healthy lifestyles to improve cancer survivors’ QOL. However, in order for clinicians to prescribe a peer-led PA intervention for QOL benefits and to encourage community-based organizations to implement them, additional evidence is needed. Specifically, future research examining the role of PA interventions in QOL outcomes should consider the small-to-moderate effect sizes typically found in these studies and power on QOL outcomes during the study design phase. Designing studies that purposefully enroll survivors with poorer QOL may also allow for a closer examination of the potential for PA to positively impact these outcomes. Finally, additional research is needed to identify the mechanisms by which MVPA may impact QOL of breast cancer survivors.
Acknowledgments
We thank the American Cancer Society in New England (Vice-Presidents, Community Executives and staff) who assisted with coach recruitment and training and participant recruitment. We also thank the study team for study implementation.
This study was funded by a grant from the National Cancer Institute (R01 CA132854) to the first author.
Contributor Information
Bernardine Pinto, University of South Carolina
Kevin Stein, American Cancer Society
Shira Dunsiger, Miriam Hospital and Alpert Medical School of Brown University
References
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta, GA: 2013. [Google Scholar]
- 2.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c11200005768-201007000-00023. pii. [DOI] [PubMed] [Google Scholar]
- 3.Brown JC, Huedo-Medina TB, Pescatello LS, et al. The efficacy of exercise in reducing depressive symptoms among cancer survivors: a meta-analysis. PLoS One. 2012;7(1):e30955. doi: 10.1371/journal.pone.0030955PONE-D-11-17152. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craft LL, Vaniterson EH, Helenowski IB, Rademaker AW, Courneya KS. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(1):3–19. doi: 10.1158/1055-9965.EPI-11-06341055-9965.EPI-11-0634. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duijts SF, Faber MM, Oldenburg HS, van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors--a meta-analysis. Psychooncology. 2011;20(2):115–126. doi: 10.1002/pon.1728. [DOI] [PubMed] [Google Scholar]
- 6.Ferrer RA, Huedo-Medina TB, Johnson BT, Ryan S, Pescatello LS. Exercise interventions for cancer survivors: a meta-analysis of quality of life outcomes. Ann Behav Med. 2011;41(1):32–47. doi: 10.1007/s12160-010-9225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(1):123–133. doi: 10.1158/1055-9965.EPI-10-09881055-9965.EPI-10-0988. pii. [DOI] [PubMed] [Google Scholar]
- 8.Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, Wittink HM. The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of randomised controlled trials. Clin Oncol (R Coll Radiol) 2010;22(3):208–221. doi: 10.1016/j.clon.2009.12.005S0936-6555(09)00411-7. pii. [DOI] [PubMed] [Google Scholar]
- 9.Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 2010;153(8):507–515. doi: 10.7326/0003-4819-153-8-201010190-00007153/8/507. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ljungberg I, Kroll T, Libin A, Gordon S. Using peer mentoring for people with spinal cord injury to enhance self-efficacy beliefs and prevent medical complications. J Clin Nurs. 2011;20(3–4):351–358. doi: 10.1111/j.1365-2702.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 11.Castro CM, Pruitt LA, Buman MP, King AC. Physical activity program delivery by professionals versus volunteers: the TEAM randomized trial. Health Psychol. 2011;30(3):285–294. doi: 10.1037/a00219802011-09497-006. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buman MP, Giacobbi PR, Jr, Dzierzewski JM, et al. Peer volunteers improve long-term maintenance of physical activity with older adults: a randomized controlled trial. J Phys Act Health. 2011;8(Suppl 2):S257–266. [PMC free article] [PubMed] [Google Scholar]
- 13.Ginis KA, Nigg CR, Smith AL. Peer-delivered physical activity interventions: an overlooked opportunity for physical activity promotion. Transl Behav Med. 2013;3(4):434–443. doi: 10.1007/s13142-013-0215-2215. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Prentice-Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- 15.Deci EL, Ryan RM. Intrinsic Motivationand self-determination in human behavior. New York: Plenum; 1985. [Google Scholar]
- 16.Pinto BM, Stein K, Dunsiger S. Peers promoting physical activity among breast cancer survivors: A randomized controlled trial. Health Psychol. 2014 doi: 10.1037/hea0000120. 2014-32951-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buffart LM, Galvao DA, Brug J, Chinapaw MJ, Newton RU. Evidence-based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer Treat Rev. 2014;40(2):327–340. doi: 10.1016/j.ctrv.2013.06.007S0305-7372(13)00130-8. pii. [DOI] [PubMed] [Google Scholar]
- 18.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 19.Pinto BM, Frierson G, Rabin C, Trunzo J, Marcus B. A home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23:3577–3587. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 20.Marcus BH, Simkin LR. The stages of exercise behavior. J Sports Med Phys Fitness. 1993;33(1):83–88. [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion. U.S. Government Printing Office; Atlanta, GA: 1996. Physical Activity and Health: A Report of the Surgeon General. [Google Scholar]
- 22.Winningham M. Developing the Symptom Activity 27: An instrument to evaluate perception of symptom effects on activity. Oncol Nurs Forum. 1993;20:330. [Google Scholar]
- 23.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 25.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. S0885392496002746 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 27.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122(5):794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 28.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121(1):91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 29.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. 14/7/1588 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Ware JE, Kosinski M, Dewey JF. How to Score Version Two of the SF-36 Health Survey. QualityMetric, Inc; Lincoln, RI: 2000. [Google Scholar]
- 32.Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JK, Lane K, Yasui Y, Segal RJ. Moderators of the effects of exercise training in breast cancer patients receiving chemotherapy: a randomized controlled trial. Cancer. 2008;112:1845–1853. doi: 10.1002/cncr.23379. [DOI] [PubMed] [Google Scholar]
- 33.Courneya KS, Sellar CM, Stevinson C, McNeely ML, Friedenreich CM, Peddle CJ, Basi S, Chua N, Tankel K, Mazurek A, Reiman T. Moderator effects in a randomized controlled trial of exercise training in lymphoma patients. Cancer Epidemiol Biomarkers Prev. 2009;18:2600–2607. doi: 10.1158/1055-9965.EPI-09-0504. [DOI] [PubMed] [Google Scholar]
- 34.Kalter J, Buffart LM, Korstjens I, et al. Moderators of the effects of group-based physical exercise on cancer survivors’ quality of life. Support Care Cancer. 2015 Feb 14; doi: 10.1007/s00520-015-2622-z. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn J, Steginga SK, Rosoman N, Millicap D. A review of peer support in the context of cancer. J Psychosoc Oncol. 2003;21(2):55–67. doi: 10.1300/J077v21n02_04. [DOI] [Google Scholar]
- 36.Pistrang N, Jay Z, Gessler S, Barker C. Telehone peer support for women with gynaecological cancer: benefits and challenges for supporters. Psycho-Oncol. 2013;22:886–894. doi: 10.1002/pn.3080. [DOI] [PubMed] [Google Scholar]