DEFINITION OF NEONATAL ABSTINENCE SYNDROME
Neonatal withdrawal symptoms have been noted following prenatal exposure to several drugs. Examples include opioids,1,2 benzodiazepines,3,4 mood-stabilizing medications,5 selective serotonin reuptake inhibitors,6 and nicotine.7 For all drug classes except opioids, these symptoms are usually self-limited and do not require pharmacologic treatment. Infants born to mothers with opioid abuse or receiving methadone maintenance often develop withdrawal symptoms, following the postpartum cessation of in utero exposure to opioids. This complex is known as the neonatal abstinence syndrome (NAS). The full mechanistic basis for the clinical presentation is unclear. Tolerance induced by long-term exposure to opioids is primarily mediated by receptor downregulation coupled with upregulation in the cyclic adenosine monophosphate (cAMP) pathway.8 Other putative mechanisms include neuroimmune activation, production of anti-opioid peptides, or activation of the spinal dynorphin system. Symptoms of withdrawal are hypothesized to be due to increased adenylyl cyclase activity and an abrupt increase in norepinephrine following removal of the mu opioid ligand. NAS is characterized by signs of central nervous system (CNS) hyperirritability, gastrointestinal dysfunction, respiratory distress, and vague autonomic symptoms. Common symptoms in order of frequency includes tremor, high-pitched cry, sneezing, increased muscle tone, regurgitation and vomiting, poor sleep, loose stools, sweating, excoriation, mottling, nasal stuffiness, low-grade fever, and tachypnea. Impaired weight gain and seizures are seen with untreated NAS. All infants with prolonged in utero opioid exposure will develop signs and symptoms of withdrawal of varying severity. However, the disorder encompasses a diverse spectrum, and those with milder symptoms respond well to supportive treatments. NAS symptoms severe enough to require pharmacologic treatment occur in 55% to 94% of infants born to opioid-dependent mothers.9
Current use of illicit drugs occurs in 4.4% of pregnant women.10 Heroin use during pregnancy is associated with fetal death and infant morbidity, including intrauterine growth retardation, placental insufficiency, postpartum hemorrhage, preeclampsia, and premature rupture of membranes.11,12 In an attempt to counteract these poor outcomes, methadone maintenance in opioid-dependent pregnant women has been used for the past 35 years, and is associated with improved birth weight and improvements in multiple domains.13–16 A more recent development is the expansion in the use and abuse of prescription opioids. Whereas the use of heroin decreased by 19% between 1998 and 2008, the abuse of prescription opioids during the same period increased by 41%.17 In 2010, 5.1 million individuals reported nonmedical abuse of prescription pain medications within the previous month, with 71% of abused pain relievers being obtained from friends or family, and either bought or taken without permission.10 The societal burden of NAS is difficult to assess, as is evident from the wide variations and implausible rates reported to regional authorities for hospitals in the defined geographic area with similar patient populations.18 Such variation is due to limited self-reporting of drug abuse, and underreporting of NAS using the International Classification of Diseases.19 In 1996, a survey by the National Institute of Drug Addiction estimated that 7000 cases occur each year, although the report conceded this is potentially an underestimation.20 More recently, the rate of NAS in the United States has increased from 1.2 to 3.9 per 1000 live births between 2000 and 2009.21 A similar incidence rate has been estimated in Australia.22
PREDICTORS OF NAS SEVERITY
The dose of maternal methadone dose as a covariate of the need for NAS treatment length has been examined extensively. Although a meta-analysis that evaluated studies by methodological quality did not identify a statistically significant difference in outcomes between high-dose and low-dose methadone, there is a suggestion of modest maternal dose dependency on outcomes.23 However, if such an effect does exist, it is small and not relevant in terms of choosing a maternal dose or differential treatment approaches in the treatment of infants. Lower maternal methadone doses have been associated with higher rates of illicit substitution, and a consensus view is that maternal doses of methadone should not be reduced solely to reduce NAS severity. High-quality randomized, controlled trial evidence from the MOTHER study has demonstrated that compared with methadone use, maternal buprenorphine is associated with a decreased need for morphine treatment in NAS and neonatal length of stay.24 The maternal study population in this study has been convincingly demonstrated to be similar to the patient population at large, strongly supporting the generalizability of results.25
Whereas Jansson and colleagues26 described worse NAS symptoms and pharmacotherapy in males, severity, need for therapy, or length of therapy were not influenced by gender in a cohort study by Holbrook and Kaltenbach.27 Similarly, there was no sex dependency in the large randomized MOTHER study, which compared use of methadone and buprenorphine in pregnant women.28 Intrapartum variability or decelerations in fetal heart rate do not predict the need for therapy in NAS.29 However, alternations in autonomic regulation, as measured by analysis of maternal26 or infant30 vagal tone, have been noted to be predictors of worse NAS symptomatology. It is postulated that infants who adapt to maternal methadone–induced autonomic changes are maladapted to more severe NAS following birth. Methadone exposure during pregnancy is associated with an approximately 2.5-fold increase in the rate of preterm birth.31,32 Preterm infants have a well-described natural history of NAS and a need for treatment that differs from term infants. The current NAS scoring instruments have not been examined in this population. Need for therapy33 and length of stay is shorter in the preterm population.34,35 The preterm population thus appears to be categorically different in terms of in utero opioid exposure.
Polydrug abuse during pregnancy is associated with impaired fetal markers (heart rate and variability) and greater need for postpartum pharmacologic therapy.36 A retrospective study by Seligman and colleagues34 demonstrated that the length of NAS treatment for all opioid only exposed infants between 2000 and 2006 was 31 days, compared with 38 days for polydrug-exposed term infants. Strikingly, a multivariate analysis of infants revealed a significant prolongation of treatment duration for NAS (31 vs 47 days, P<.01) of benzodiazepine versus non–benzodiazepine-exposed infants. Benzodiazepine withdrawal symptoms in adults include anxiety, tremors, anorexia, nausea, postural hypotension, and in severe cases seizures, delirium, and hyperpyrexia. The onset of symptoms is 12 to 24 hours for short-acting agents with a peak at 72 hours, whereas longer-acting agents such as diazepam are associated with an onset of 24 to 72 hours and a peak between 5 and 8 days following the last dose.37 Benzodiazepines cross the placenta,38,39 but maternal confounders have made it difficult to estimate adverse effects specific to in utero exposure of benzodiazepines.40 Whereas teratogenicity is unlikely in benzodiazepine-exposed infants,41–43 decreased birth weight and neonatal withdrawal have been noted,3 the latter manifested by hypotonia and hypoventilation or tremulousness.4 The half-life of diazepam in neonates is 31 days.44 Thus, in contrast to that of adults, initiation of neonatal withdrawal for many benzodiazepines can be delayed with an onset at a week and effects noticeable for weeks.45,46 There is no specific treatment for neonatal benzodiazepine withdrawal. Tobacco exposure is associated with worse NAS symptomatology.47,48 Analysis of meconium for tobacco, methadone or its metabolites, cocaine, or opioids other than methadone, however, are not predictive of NAS outcomes.49
LONG-TERM AND SHORT-TERM SEQUELAE
Environmental and social factors are more important influences upon childhood development than brief periods of prepartum and peripartum exposure to drugs of abuse.50,51 Infants exposed in utero to opioids show low birth weights, increased preterm birth, and reduced fetal growth parameters, but investigations have been hampered by the logistical difficulty of controlling for tobacco and other social factors associated with illicit drug use.52 Studies linking in utero opioids to impaired neurodevelopment53 have been criticized for not accounting for confounding of the child’s social and environmental milieu.54 The database for opioid exposure is less robust than that for cocaine.55 It is possible that there are subtle neurodevelopmental effects arising from in utero opioid exposure apart from effects caused by environmental and home settings.56 Even if this is real, however, these associations do not provide guidance about practical therapeutic decisions. For newborns, the benefits of maternal opioid therapy during pregnancy using methadone in a structured program clearly outweigh no therapy. There is evidence that untreated or undertreated women may seek street sources of opioids to treat withdrawal symptoms, which clearly has negative neonatal outcomes. Of importance, there is no evidence of long-term adverse outcomes in children treated with pharmacologic agents in comparison with infants who do not require treatment for NAS, or for treatment with different classes of agents.57,58 Although the database of information is smaller, in human and animal studies neonatal outcomes with in utero buprenorphine exposure are generally favorable compared with methadone.59
CURRENT THERAPIES
Variability of Current Practice Patterns
Few studies have examined NAS prevalence and treatment patterns. Nandakumar and Sankar60 published a survey of 17 neonatal units in the Northwest Region of the United Kingdom, revealing not only conflicting practices in scoring, identification, and management but also a deficit of reliable data to assist practitioners in determining the best regimen to treat NAS. In a survey conducted by Sarkar and Donn61 in United States neonatal intensive care units (NICU), the focus was primarily on determining the percentage of respondents using an abstinence scoring system, those with access to formal written policies or educational programs for NAS management, and practitioners using customary pharmacologic agents for withdrawal. The 13-question survey of 102 accredited fellowship programs (which had 75 respondents) did not include questions on NAS incidence or length of hospital stay. O’Grady and colleagues62 conducted a 15-question questionnaire of 235 neonatal units that sought to survey current NAS practices in the United Kingdom and Ireland. The survey assessed first-line and second-line agents, attitudes to breastfeeding by women on methadone, and the safety of infants discharged on medication. Crocetti and colleagues63 assessed the number of opiate-exposed neonates identified as having NAS, as well as policies and procedures for treatment in 27 hospitals throughout Maryland. In aggregate, these surveys demonstrate significant heterogeneity in diagnosis and treatment patterns. There are clear information gaps for identification, treatment, and length of hospital stay. Moreover, there is a lack of pharmacoeconomic analyses on costs and cost-effectiveness of treatment for NAS.
Framework for Treatment
The therapeutic framework of treatment begins with the identification of infants at risk for NAS. NAS is graded using a standard checklist that identifies and stratifies severity of disease based on signs and symptoms in multiple domains. Of several scoring systems used to gauge symptom severity and titrate drug dose,64–66 the Finnegan score (or modifications of it)67 is the most commonly used.61,62 A modification of the Finnegan score used in the multicenter MOTHER study of buprenorphine use for pregnant women68,69 is the standard instrument used in other randomized NAS research trials.70,71 The Finnegan instrument was created to assess severity of disease in those with known opioid exposure. On day 2 of life a score of 7 corresponds with the 95th percentile for nonexposed infants, meaning any score of 8 or greater is highly suggestive of in utero opioid exposure even in those denying opioid use during pregnancy.72 Nonpharmacologic therapies should be used for all infants with in utero opioid exposure. These treatments include swaddling,73 the use of small calorically dense formulas, rooming in, breastfeeding, and minimization of excessive external stimulation. Infants with mild symptoms should be observed in the hospital for at least 4 days. For infants with severe symptomatology manifested by seizures, poor weight gain, and elevated values in a NAS-specific scoring instrument, pharmacologic therapy is indicated. Ideal treatment uses a protocol-driven use of drug titration to control symptoms. Both symptom-driven (ie, weight-independent fixed dose titration based on severity of NAS scores) and weight-based dosing regimens have been used, with neither being identified as the standard approach.74 Regardless of the manner of dose titration, infants who do not have control of symptoms despite high doses of the initial therapy are treated with a secondary drug. After stabilization, symptom scores are used to gradually wean the controlling drug or drugs; this occurs typically in an inpatient setting, as it allows careful observation and dose titration of infants. Some institutions stabilize an infant in an inpatient setting, with terminal weaning done as an outpatient. The use of outpatient treatment in highly selected patients is associated with shorter inpatient stays, but extended total duration of therapy.75
The rationale to use pharmacologic therapy is to ensure proper feeding and development, and foster the maternal infant bond. The ideal specific drug used would safely achieve these therapeutic goals, while at the same time minimizing the total duration of therapy and length of hospitalization. The most commonly used initial therapy is an opioid, while phenobarbital or clonidine are the primary adjunctive agents. Although used more commonly, phenobarbital has not been demonstrated to have improved safety or efficacy in comparison with clonidine as an adjunctive therapy. A comparison of these agents as adjuncts is currently being investigated (NCT01175668). The role of initial dual therapy of phenobarbital with an opioid has been described, but has not been compared in a large number of patients.76 The value of this approach has not been established, but anecdotally may provide benefit in infants with polysubstance use.
Opioids
Cochrane reviews,77,78 the American Academy of Pediatrics,9,79 and expert reviews80,81 identify opioid replacement as the ideal treatment for the withdrawal symptoms associated with in utero exposure to opiates. Opioid replacement as a first-line agent (1) improves weight gain but lengthens hospitalizations compared with supportive care, (2) reduces seizure rates and possibly duration of therapy compared with phenobarbital, and (3) reduces treatment failure compared with diazepam.77 Although many of the studies cited in the Cochrane review had methodological flaws, the standard of care practice that has developed since the 1970s has generally supported this approach. There is limited evidence from which definitive recommendations can be made regarding differential safety and efficacy of specific opioids. Moreover, high-quality data on the optimal dose regimens or comparative effectiveness on use of adjunctive agents are lacking. Current practice patterns for these have been developed empirically, and remain an area that would benefit from investigations of higher quality.
Morphine
Morphine is the most commonly used opioid for replacement therapy. Paregoric, a previously commonly used morphine source, was never subject to any formal evaluation by the Food and Drug Administration, and is no longer available. Diluted, deodorized tincture of opium (DTO) has a morphine concentration of 0.4 mg/mL and an ethanol concentration of 0.19%. This agent has been largely replaced by an ethanol-free morphine solution of 0.4 mg/mL concentration. Preservative-free morphine hydrochloride solution for neonatal administration is stable at 4°C for at least 6 months.82 Because of the relatively short half-life of morphine, best outcomes have been demonstrated when morphine doses are given no longer than 4 hours apart.83 Accordingly, infants who are sleeping at the nominal dose time should be awakened for drug administration.
Morphine in humans is metabolized primarily to morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) via uridine 5′-diphosphate glucuronosyl transferase (UGT)-2B7. The ontogeny of UGT development is dynamic in the immediate postpartum period, demonstrated by reduced M6G/morphine ratio in neonates younger than 7 days as well as an associated reduced postsurgical morphine requirement in comparison with older neonates.84 Nonlinear mixed-effects models have been used to estimate active metabolite formation as well as elimination.85 Clearance generally correlates with glomerular filtration, with minimal fecal elimination or metabolism to normorphine. The large interpatient and intrapatient variability of intravenous morphine pharmacokinetics and pharmacodynamics in neonates is due in part to a dynamic acquisition of metabolic enzymes, renal function, and changes in fat and extracellular fluid balance.84–86 Of note, the pharmacokinetics profile of orally administered morphine in neonates is currently unknown. An area of therapeutic need would be the characterization of the concentration-response relationship. Such a relationship, created with modeling and simulation, would be of use in designing an optimized dose regimen.
The initial dose of morphine was 0.12 to 0.6 mg/kg/d in a survey of 17 pediatric units in the United Kingdom.60 These investigators had the opinion that a higher initial dose may be associated with better control of symptoms, but acknowledged that evidence to support this intuition was lacking. A dose of 0.24 mg/kg/d was recommended by the 1998 report of the American Academy of Pediatrics (AAP),9 although this protocol outlined drop-unit doses that would make fine titration difficult. Neither the 2012 AAP Committee on Drugs NAS report79 nor the Cochrane review of the topic identified a favored specific dose.77 There is no generally accepted maximum dose of morphine used for NAS. A survey of neonatal units in the United Kingdom revealed that typical maximum doses were up to 1.3 mg/kg/d, and that one-third determined dose according to symptom control rather than a maximum predefined level.62 Specific protocols for dose titration are based either on a weight-based increase in dose based on scores above a specific NAS score, or a weight-independent dose based on a graded severity of NAS score. Table 1 outlines these 2 commonly used approaches.
Table 1.
Morphine regimens, based on Finnegan scoring every 4 hours
| Weight Based | Symptom Based | |
|---|---|---|
Initial Dose:
|
Initial Dose:
|
|
| Single NAS score | Dose every 4 h (mg) | |
| 9–12 | 0.04 | |
| 13–16 | 0.08 | |
| 17–20 | 0.12 | |
| 21–24 | 0.16 | |
| ≥25 | 0.20 | |
| ||
| Single NAS score | Increase Dose (mg) | |
| 0–9 | None | |
| 9–12 | 0.02 | |
| 13–16 | 0.04 | |
| 17–20 | 0.06 | |
Weaning Dose:
| ||
| Two NAS scores | Increase Dose (mg) | |
| 9–12 | 0.01 | |
| 13–16 | 0.02 | |
| 17–20 | 0.04 | |
Cease therapy when dose is 0.02 mg Adjunctive Treatment:
| ||
Phenobarbital loading dose of 20 mg/kg followed by 5 mg/kg/d, or clonidine.
Data from Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after metha-done or buprenorphine exposure. N Engl J Med 2010;363(24):2320–31; and Jansson LM, Velez M, Harrow C. The opioid-exposed newborn: assessment and pharmacologic management. J Opioid Manag 2009;5(1):47–55.
Methadone
Methadone is a long-acting opioid commonly used for abstinence treatment. The longer half-life of methadone provides less of a flux between peak and trough levels, while also providing ease of administration at less frequent intervals. Oral bioavailability in adults is high, but variable.87 The pharmacokinetics of methadone in the pediatric and neonatal populations has been simulated using physiologic-based pharmacokinetic modeling that suggests significant interpatient and developmental variability, but decreased systemic exposure with age.88 This model has not been validated by rich patient-level data. There is scant published clinical trial evidence to guide its use in the neonatal population. In a single small study, outcomes with methadone were similar to those for phenobarbital or diazepam.89 Comparisons with oral morphine are limited to a single retrospective review of 46 patients, in which there was no significant difference in length of stay between treatments.90 A standard dose has not been established, but the protocol used by Lainwala and colleagues90 is provided in Box 1. Methadone use remains relatively uncommon, ranging from fewer than 2% of units in the United Kingdom62 to as many as 20% in the United States.61 The long dosing interval has led some sites to use methadone as extended outpatient dosing. Compared with full inpatient treatment, infants discharged home on methadone have shorter hospitalizations but longer duration of therapy, though at least in one study having a similar total of methadone administered.91 Because of the likely variability of pharmacokinetics, frequent outpatient follow-up is required to allow careful monitoring and dose titration based on symptoms.
Box 1. Methadone protocol for inpatient use.
Initial loading dose 0.1 mg/kg/dose
Additional 0.025 mg/kg/dose given every 4 h for continuing NAS scores greater than 8 until symptoms are controlled or a maximum dose of 0.5 mg/kg/d reached
Maintenance dose determined by calculating the total methadone dose given over previous 24 hours
Maintenance dose administered in 2 divided doses every 12 hours
Data from Lainwala S, Brown ER, Weinschenk NP, et al. A retrospective study of length of hospital stay in infants treated for neonatal abstinence syndrome with methadone versus oral morphine preparations. Adv Neonatal Care 2005;5(5):265–72.
Buprenorphine
Buprenorphine is a long-acting partial μ opioid receptor agonist that in adults is more effective for withdrawal symptoms than clonidine, and possibly methadone.92 Use of buprenorphine in this population has gained favor, in part because of its properties of improved safety, particularly with regard to respiratory depression. Buprenorphine has compared favorably with methadone for use in pregnant women.24 In NAS, the use of buprenorphine has been explored in 2 open-label, placebo-controlled trials.70,93 A total of 50 infants were randomized in a 1:1 ratio to oral morphine every 4 hours or sublingual buprenorphine administered every 8 hours. The optimized initial dose was 15.9 μg/kg/d, with a maximum of 60 μg/kg/d. Doses were increased 25% until control of symptoms was obtained, and decreased by 10% until cessation of therapy when the dose was 10% of the initial dose. Doses were not adjusted for actual weight, and were instead based on the weight at initiation of therapy. Although the initial goals of this phase 1 investigation was the feasibility and safety of buprenorphine to treat NAS, an efficacy advantage over morphine was demonstrated. When the results from both cohorts were combined, treatment with buprenorphine revealed a mean length of treatment of 23 days, compared with a mean length of 34 days using standard of care oral morphine (Fig. 1). Following log transformation to satisfy normality assumptions, the length of treatment was on average 36% shorter (95% confidence interval [CI]: 17%–51%; P = .001) in the buprenorphine arm than in those administered oral morphine, and the length of stay was on average 29% shorter (95% CI: 10%–44%; P = .006). Caveats to these findings are an open-label study design and that, while consistent with retrospective studies at the same institution,34 the duration of treatment and length of stay in both arms was somewhat longer than has been reported at other institutions.
Fig. 1.
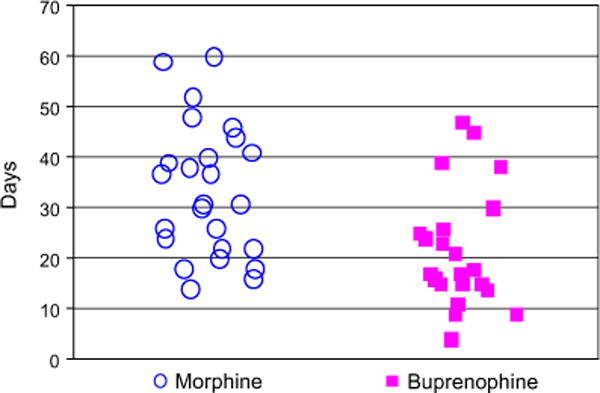
Length of treatment: open-label morphine versus buprenorphine by patient.
Adjunctive therapy with phenobarbital was required in 6 of 25 infants in the buprenorphine group compared with 2 of 25 randomized to morphine. It is unclear whether this finding is due to a ceiling effect of buprenorphine as a partial agonist in a subset of patients with more severe disease, or if the predefined maximum dose of buprenorphine was set too low. Pharmacokinetic sampling in this trial unexpectedly revealed amelioration of withdrawal symptoms at plasma concentrations of buprenorphine below the 0.7 ng/mL threshold, estimated for relief of symptoms in adults.94 This finding could be due to a different volume of distribution of the drug in the neonate, or a pharmacodynamic profile of withdrawal that fundamentally differs from that in adults.
Drugs for sublingual administration are formulated using buprenorphine for injection (Buprenex or equivalent generic) at a concentration of 0.075 mg/mL in a 30% ethanol solution. Buprenorphine is stable at room temperature for at least 30 days in glass vials and for at least 7 days in plastic syringes. Buprenorphine is absorbed by the sublingual route within 2 minutes in adults.95 There was no evidence of aspiration in neonates after more than 1600 individual doses were administered in the phase 1 investigation. There were 2 serious adverse events. One infant developed cytomegalovirus in the immediate postpartum period and another had idiopathic seizure. Both events were judged to be unrelated to study treatment by the investigator, institutional review board, and data safety monitoring board.
Adjuncts
Phenobarbital
The use of phenobarbital (identified as phenobarbitone in the British nomenclature) is often used as a rescue therapy when maximum dose of opioid replacement therapy is reached without adequate resolution of symptoms, although it has also been used as an initial adjunct in combination therapy with an opioid76 or as initial monotherapy.96 Phenobarbital use has been examined in a Cochrane review, which concluded that opioids had a comparative advantage concerning incidence of seizures, duration of treatment, and nursery admissions, but not necessarily in the rate of treatment failure.78 The half-life of phenobarbital in neonates decreases from 115 hours after 1 week to 67 hours after 4 weeks.97 This prolonged half-life explains the improved outcomes through the use of a loading dose compared with dosing without a load.98 The typical loading dose is 20 mg/kg, followed by 5 mg/kg. Phenobarbital anecdotally seems to have particular utility in those infants with polydrug exposure in utero. Phenobarbital causes increased metabolism of many drugs metabolized by the cytochrome P450 system for patients of all agents, a finding confirmed in NAS infants cotreated with phenobarbital and buprenorphine.99 Questions raised about the potential for deleterious neurodevelopmental effects will be addressed by the ongoing PROPHENO trial (NCT01089504), scheduled to be completed in late 2014.
Clonidine
Clonidine is a centrally acting a-agonist that reduces global sympathetic tone and has been used in adult withdrawal syndromes. Clonidine is less efficacious in adults in comparison with opioids in the management of withdrawal symptoms.92 Several small retrospective examinations had suggested clonidine as a useful adjunct therapy in NAS (Table 2). Agthe and colleagues71 described a high-quality, randomized controlled trial of clonidine, 1 μg/kg every 4 hours compared with placebo as a parallel adjunct to oral morphine therapy (in the form of DTO). Clonidine solution for epidural injection (100 μg/mL) was diluted to 5 μg/mL and administered orally. The dual morphine/clonidine arm had a statistically significant shorter length of stay (11 days [95% CI: 8–15] vs 15 days [95% CI: 13–17]). In addition, the total dose of morphine was 7.7 mg with dual therapy compared with 19.2 mg with monotherapy (P = .03). Clonidine was generally well tolerated, with no serious hypotension or bradycardia. An episode of supraventricular tachycardia occurred in one patient 3 days after cessation of clonidine. Based on the mechanism of action of clonidine and potential for post-cessation sympathetic surge, it is plausible that this was causally related to cessation of the study drug. Three infants in the clonidine-treated group died of autopsy-verified myocarditis, sudden infant death syndrome, and homicide (methadone overdose). Each occurred at least 22 days after the cessation of the study drug and were assessed to be unrelated to the study drug.71 Xie and colleagues100 performed nonlinear mixed-effects modeling of clonidine pharmacokinetics and noted a rapid increase in clearance in the first month of life. A dose adjustment of 1.5 μg/kg every 4 hours starting the second week of life, based on modeling and simulation, was proposed. This dose adjustment has not been tested in a clinical trial setting.
Table 2.
Clonidine use
| Authors,Ref. Year | n | Clonidine Dose (μg/kg) | Outcome in Length of Stay (LOS) or Length of Treatment (LOT) | |
|---|---|---|---|---|
| Hoder et al,124 1984 | Case Series | 7 | 0.5–1.0 by mouth every 6 h | 13 d LOS |
| Leikin et al,125 2009 | Case Series | 14 | 0.5–1.0 by mouth every 6 h | 7 d LOT In utero exposures = 3 Iatrogenic NAS = 11 |
| Esmaeili et al,126 2010 | Case Series | 29 | 0.5–3.0 h intravenous | 14 d LOT 32 d LOS Chloral hydrate rescue |
| Agthe et al,71 2009 | Randomized controlled trial | 40 | 1.0 by mouth every 4 h (+ morphine) | 11 d LOT vs 15 for placebo |
Breastfeeding
The number of women in methadone programs who choose to breastfeed their newborns has been traditionally low, with more than half of those who start stopping after 6 days.101 However, it is expected that this number will increase both locally and nationwide as a result of specific campaigns. In 2011, the United States Surgeon General released A Call to Action to Support Breastfeeding, which calls for expansion of breastfeeding for American infants. This standpoint is supported by the Department of Health and Human Services in Healthy People 2020, as well as major medical societies.102 Methadone is passed on to neonates through breast milk, although the absolute amount is small (<0.2 mg/d) and does not appreciably change neonatal serum methadone concentrations.103 However, a pharmacodynamic effect is suggested, as breastfed infants have decreased severity of NAS or need for treatment with pharmacologic agents.104,105 Based on the small doses of drug transferred to the infant, it is not clear if this effect reflects the calming effect of the act of breastfeeding or the effect of drug.106 For mothers maintained on usual abstinence doses, the amount of buprenorphine transferred through breast milk is 0.1 to 1.2 μg/kg/d, which represents approximately 0.02% of the maternal dose.107–110 The bioavailability of buprenorphine transferred in breast milk is not characterized, but appears low based on measurement in neonatal blood and urine,110 and by minimal effects in suppression of NAS symptomatology.111–113 There are no reported safety concerns associated with breastfeeding, therefore despite the product insert that advises against breastfeeding, current national guidelines advocate breastfeeding for mothers prescribed buprenorphine.114
Pharmacogenetics
The interpatient variability seen in severity of withdrawal symptoms or response to therapies cannot be reduced to a monogenic etiology in either newborns or adults. However, several single-nucleotide polymorphisms (SNPs) in candidate genes have been identified that appear to determine response to opioids for pain or replacement abstinence therapy in adults, for predilection to substance abuse disorder,115 and social hedonic capacity.116 The μ opioid receptor (OPRM1) gene A118G SNP has been associated with differential morphine sensitivity, with decreased pain and morphine requirements with the AA genotype.117 An exploratory examination by Wachman and colleagues101 in 28 term infants with in utero opioid exposure revealed a significantly lower need for pharmacotherapy, lower doses, and shorter lengths of stay in patients with the AA variant compared with those with the GG variant. Catechol-O-methyltransferase (COMT), an enzyme that degrades catecholamines, was also examined. In adults, the COMT SNP (Val158Met) is associated with a lower required morphine dosage in patients with cancer,118 although the association with addiction is much less clear.119 Wachman and colleagues101 reported that COMT (Val158Met) was associated with a decreased need for therapy, dose of medications, and length of stay. Variants of p-glycoprotein (MDR1) were not associated with differential NAS outcomes. These intriguing findings, if verified in a larger cohort, may have implications for identifying those most at risk for the need of therapy. Enthusiasm is tempered, however, by the example of pharmacogenetic approaches to warfarin therapy in adults, in which there is limited practitioner uptake despite evidence of efficacy and easy-to-use algorithms.
FUTURE DIRECTIONS
Future directions may include the examination of the existing scales, particularly those based on the Finnegan scale, to discern whether there it is possible to simplify the scales to include those elements most closely correlated with clinical outcomes in the management of infants with known opioid exposure. A 3-point scale consisting of hyperactive Moro reflex, mild tremors when undisturbed, and increased muscle tone has been described as discriminative between opioid-exposed and non-opioid-exposed infants, but this has not yet been validated in a large sample.24
Dexmedetomidine is chemically similar to clonidine, but with a greater α2-receptor specificity.120 Dexmedetomidine has been proposed as a potential alternative for the treatment of iatrogenic pediatric opioid withdrawal syndromes, but has not been evaluated in the treatment of NAS.121 Lofexidine and guanfacine are other α2-agonists that have been investigated for the treatment of adult withdrawal but not pediatric withdrawal, but the size and quality of studies have been limited.122 These agents have no theoretical advantage over clonidine.
It is not clear whether a short-acting agent such as morphine, compared with longer half-life drugs such as buprenorphine or methadone, will provide better outcomes for infants who require pharmacologic therapy. Extrapolation from adult abstinence and control of withdrawal symptoms would suggest that longer-acting agents, by reducing the flux in drug concentration, would provide more uniform control of symptoms and a smoother transition to the postcessation period of therapy. However, it is also possible that morphine would provide more flexibility in titrating to a dynamic symptom complex by allowing quicker dose titration and attainment of steady state after dose adjustment. A double-blinded, double-dummy trial currently under way (NCT01452789) may provide insight into this question. The lack of published pharmacokinetic and outcomes data make methadone dosing empiric and non-evidence based.
The majority of treatment for NAS takes place in an inpatient setting, but there are institutions in which home management with phenobarbital and methadone are used. A formal comparison between these approaches would be useful. The correct location for treatment also needs to take into consideration not only the pharmacology of the replacement agent but also the dynamics of mother-infant dyad and of the social situation. In this way, any investigation should take these considerations into account in structuring a study, as well as in defining end points for examination.
Pharmacogenetics may assist in identifying infants at risk for requiring pharmacologic therapy for NAS, but will likely be only one of many covariates that would feed into a predictive disease-state model. Such a model could effectively link demographics, in utero exposures, disease severity, genetic factors, pharmacodynamic responses, pharmacokinetics, and other variables. It is likely that such a model would be actuated optimally in an electronic system that had system inputs from an electronic medical record. Modeling also will play an increasing role in the realm of quantitative methods, allowing use of the sparse data sets available in neonates. In such a fashion, pharmacometric simulations can predict dose response and help to inform the formulation of new dosing regimens or combination therapy. Using a “learn and confirm” paradigm, these models can be refined and optimized.123
SUMMARY
There is clearly an unmet medical need to develop improved pharmacologic treatment for infants with NAS. The mean hospital cost for an NAS admission in 2009 was $53,400.21 Ideally such treatment would provide improved symptom control without compromising safety, and would shorten treatment duration and length of hospital stay. If widely adopted, treatment with these features would have the potential to decrease resource utilization and costs of treating NAS, as well as to improve psychosocial and developmental outcomes in infants exposed to opioids in utero. Lastly, treatment protocols should be standardized per institution, and re-evaluated as new outcomes data become available.
KEY POINTS.
All infants with in utero exposure to opioids demonstrate signs and symptoms of withdrawal. Two-thirds of infants require pharmacologic therapy to ensure proper feeding and development.
Opioid replacement is the optimal primary therapy. The current standard is morphine, although there is significant heterogeneity in treatment regimens, with many centers using methadone.
Of predictive factors, lack of polysubstance exposure, prematurity, and maternal use of buprenorphine are most strongly associated with less severe withdrawal symptoms and need for pharmacologic therapy.
Emerging therapies include the use of buprenorphine for primary therapy, and clonidine as an adjunct.
Pharmacogenetic profiling of infants and the use of modeling and simulation to optimize dosing are emerging, but not fully developed, technologies that may change the treatment of the neonatal abstinence syndrome.
Acknowledgments
John N van den Anker is in part supported by NIH grants (R01HD060543, K24DA027992, R01HD048689 and U54HD071601) and European Union FP7 grants TINN (223614), TINN2 (260908), NEUROSIS (223060), and GRIP (261060).
References
- 1.Schneck H. Narcotic withdrawal symptoms in the newborn infant resulting from maternal addiction. J Pediatr. 1958;52:584–7. doi: 10.1016/s0022-3476(58)80037-2. [DOI] [PubMed] [Google Scholar]
- 2.Dikshit SK. Narcotic withdrawal syndrome in newborns. Indian J Pediatr. 1961;28:11–5. doi: 10.1007/BF02746112. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy JE, Siney C, Shaw NJ, et al. Outcome predictors in pregnant opiate and polydrug users. Eur J Pediatr. 1999;158(9):748–9. doi: 10.1007/s004310051193. [DOI] [PubMed] [Google Scholar]
- 4.Swortfiguer D, Cissoko H, Giraudeau B, et al. Neonatal consequences of benzodiazepines used during the last month of pregnancy. Arch Pediatr. 2005;12(9):1327–31. doi: 10.1016/j.arcped.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 5.ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin: clinical management guidelines for obstetrician-gynecologists number 92, April 2008. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001–20. doi: 10.1097/AOG.0b013e31816fd910. [DOI] [PubMed] [Google Scholar]
- 6.Koren G, Matsui D, Einarson A, et al. Is maternal use of selective serotonin reuptake inhibitors in the third trimester of pregnancy harmful to neonates? CMAJ. 2005;172(11):1457–9. doi: 10.1503/cmaj.1041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Law KL, Stroud LR, LaGasse LL, et al. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111(6 Pt 1):1318–23. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- 8.Anand KJ, Willson DF, Berger J, et al. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010;125(5):e1208–25. doi: 10.1542/peds.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Academy of Pediatrics Committee on Drugs. Neonatal drug withdrawal. Pediatrics. 1998;101(6):1079–88. [PubMed] [Google Scholar]
- 10.SAMHSA (Substance Abuse, and Mental Health Services Administration) Results from the 2010 national survey on drug use and health: summary of national findings. Rockville, (MD): HHS; 2011. (NSDUH Series H-41). [Google Scholar]
- 11.Finnegan LP, Kandall SR. In: Maternal and neonatal effects of alcohol and drugs. Lowinson JH, Ruiz P, Millman RB, et al., editors. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 805–39. [Google Scholar]
- 12.Kennare R, Heard A, Chan A. Substance use during pregnancy: risk factors and obstetric and perinatal outcomes in South Australia. Aust N Z J Obstet Gynaecol. 2005;45(3):220–5. doi: 10.1111/j.1479-828X.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- 13.Kandall SR, Albin S, Lowinson J, et al. Differential effects of maternal heroin and methadone use on birthweight. Pediatrics. 1976;58(5):681–5. [PubMed] [Google Scholar]
- 14.Bell J, Harvey-Dodds L. Pregnancy and injecting drug use. BMJ. 2008;336(7656):1303–5. doi: 10.1136/bmj.39514.554375.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulse GK, Milne E, English DR, et al. The relationship between maternal use of heroin and methadone and infant birth weight. Addiction. 1997;92(11):1571–9. [PubMed] [Google Scholar]
- 16.Finnegan LP, Reeser DS, Connaughton JF., Jr The effects of maternal drug dependence on neonatal mortality. Drug Alcohol Depend. 1977;2(2):131–40. doi: 10.1016/0376-8716(77)90013-8. [DOI] [PubMed] [Google Scholar]
- 17.Manchikanti L, Fellows B, Ailinani H, et al. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13(5):401–35. [PubMed] [Google Scholar]
- 18.Pennsylvania Department of Health. Bureau of Health Statistics & Research Data From The Annual Hospital Questionnaire Reporting Period: July 1, 2008 Through June 30. 2009 Report 14. 2009;2010(10/11):1. [Google Scholar]
- 19.Burns L, Mattick RP. Using population data to examine the prevalence and correlates of neonatal abstinence syndrome. Drug Alcohol Rev. 2007;26(5):487–92. doi: 10.1080/09595230701494416. [DOI] [PubMed] [Google Scholar]
- 20.National Pregnancy and Health Survey. NIDA research monograph: Estimated percentage and numbers (in thousands) of infants exposed prenatally to selected substances: Table 4-2. 1996 No. 96-3819:37. [Google Scholar]
- 21.Patrick SW, Schumacher RE, Benneyworth BD, et al. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307(18):1934–40. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell M, Nassar N, Leonard H, et al. Increasing prevalence of neonatal withdrawal syndrome: population study of maternal factors and child protection involvement. Pediatrics. 2009;123(4):e614–21. doi: 10.1542/peds.2008-2888. [DOI] [PubMed] [Google Scholar]
- 23.Cleary BJ, Donnelly J, Strawbridge J, et al. Methadone dose and neonatal abstinence syndrome-systematic review and meta-analysis. Addiction. 2010;105(12):2071–84. doi: 10.1111/j.1360-0443.2010.03120.x. [DOI] [PubMed] [Google Scholar]
- 24.Jones HE, Harrow C, O’Grady KE, et al. Neonatal abstinence scores in opioid-exposed and nonexposed neonates: a blinded comparison. J Opioid Manag. 2010;6(6):409–13. doi: 10.5055/jom.2010.0038. [DOI] [PubMed] [Google Scholar]
- 25.Stine SM, Heil SH, Kaltenbach K, et al. Characteristics of opioid-using pregnant women who accept or refuse participation in a clinical trial: screening results from the MOTHER study. Am J Drug Alcohol Abuse. 2009;35(6):429–33. doi: 10.3109/00952990903374080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansson LM, Dipietro JA, Elko A, et al. Maternal vagal tone change in response to methadone is associated with neonatal abstinence syndrome severity in exposed neonates. J Matern Fetal Neonatal Med. 2007;20(9):677–85. doi: 10.1080/14767050701490327. [DOI] [PubMed] [Google Scholar]
- 27.Holbrook A, Kaltenbach K. Gender and NAS: does sex matter? Drug Alcohol Depend. 2010;112(1–2):156–9. doi: 10.1016/j.drugalcdep.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Unger A, Jagsch R, Bawert A, et al. Are male neonates more vulnerable to neonatal abstinence syndrome than female neonates? Gend Med. 2011;8(6):355–64. doi: 10.1016/j.genm.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leeman LM, Brown SA, Albright B, et al. Association between intrapartum fetal heart rate patterns and neonatal abstinence syndrome in methadone exposed neonates. J Matern Fetal Neonatal Med. 2011;24(7):955–9. doi: 10.3109/14767058.2010.536863. [DOI] [PubMed] [Google Scholar]
- 30.Jansson LM, Dipietro JA, Elko A, et al. Infant autonomic functioning and neonatal abstinence syndrome. Drug Alcohol Depend. 2010;109(1–3):198–204. doi: 10.1016/j.drugalcdep.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almario CV, Seligman NS, Dysart KC, et al. Risk factors for preterm birth among opiate-addicted gravid women in a methadone treatment program. Am J Obstet Gynecol. 2009;201(3):326.e1–6. doi: 10.1016/j.ajog.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 32.Cleary BJ, Donnelly JM, Strawbridge JD, et al. Methadone and perinatal outcomes: a retrospective cohort study. Am J Obstet Gynecol. 2011;204(2):139.e1–9. doi: 10.1016/j.ajog.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Liu AJ, Jones MP, Murray H, et al. Perinatal risk factors for the neonatal abstinence syndrome in infants born to women on methadone maintenance therapy. Aust N Z J Obstet Gynaecol. 2010;50(3):253–8. doi: 10.1111/j.1479-828X.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 34.Seligman NS, Salva N, Hayes EJ, et al. Predicting length of treatment for neonatal abstinence syndrome in methadone-exposed neonates. Am J Obstet Gynecol. 2008;199(4):396.e1–7. doi: 10.1016/j.ajog.2008.06.088. [DOI] [PubMed] [Google Scholar]
- 35.Dysart K, Hsieh HC, Kaltenbach K, et al. Sequela of preterm versus term infants born to mothers on a methadone maintenance program: differential course of neonatal abstinence syndrome. J Perinat Med. 2007;35(4):344–6. doi: 10.1515/JPM.2007.063. [DOI] [PubMed] [Google Scholar]
- 36.Jansson LM, Di Pietro JA, Elko A, et al. Pregnancies exposed to methadone, methadone and other illicit substances, and poly-drugs without methadone: a comparison of fetal neurobehaviors and infant outcomes. Drug Alcohol Depend. 2012;122(3):213–9. doi: 10.1016/j.drugalcdep.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wesson DR, Smith DE, Ling W, et al. Sedative-hypnotics. In: Lowinson JH, Ruiz P, Millman RB, et al., editors. Substance abuse: a comprehensive textbook. 4th. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 302–12. [Google Scholar]
- 38.Myllynen P, Vahakangas K. An examination of whether human placental perfusion allows accurate prediction of placental drug transport: studies with diazepam. J Pharmacol Toxicol Methods. 2002;48(3):131–8. doi: 10.1016/S1056-8719(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 39.McGrath C, Buist A, Norman TR. Treatment of anxiety during pregnancy: effects of psychotropic drug treatment on the developing fetus. Drug Saf. 1999;20(2):171–86. doi: 10.2165/00002018-199920020-00006. [DOI] [PubMed] [Google Scholar]
- 40.Wikner BN, Stiller CO, Kallen B, et al. Use of benzodiazepines and benzodiazepine receptor agonists during pregnancy: maternal characteristics. Pharmacoepidemiol Drug Saf. 2007;16(9):988–94. doi: 10.1002/pds.1391. [DOI] [PubMed] [Google Scholar]
- 41.Czeizel AE, Eros E, Rockenbauer M, et al. Short-term oral diazepam treatment during pregnancy: a population-based teratological case-control study. Clin Drug Investig. 2003;23(7):451–62. doi: 10.2165/00044011-200323070-00004. [DOI] [PubMed] [Google Scholar]
- 42.Eros E, Czeizel AE, Rockenbauer M, et al. A population-based case-control teratologic study of nitrazepam, medazepam, tofisopam, alprazolum and clonazepam treatment during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2002;101(2):147–54. doi: 10.1016/s0301-2115(01)00545-0. [DOI] [PubMed] [Google Scholar]
- 43.Dolovich LR, Addis A, Vaillancourt JM, et al. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ. 1998;317(7162):839–43. doi: 10.1136/bmj.317.7162.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandelli M, Morselli PL, Nordio S, et al. Placental transfer to diazepam and its disposition in the newborn. Clin Pharmacol Ther. 1975;17(5):564–72. doi: 10.1002/cpt1975175564. [DOI] [PubMed] [Google Scholar]
- 45.Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatr Serv. 2002;53(1):39–49. doi: 10.1176/appi.ps.53.1.39. [DOI] [PubMed] [Google Scholar]
- 46.Oei J, Lui K. Management of the newborn infant affected by maternal opiates and other drugs of dependency. J Paediatr Child Health. 2007;43(1–2):9–18. doi: 10.1111/j.1440-1754.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- 47.Choo RE, Huestis MA, Schroeder JR, et al. Neonatal abstinence syndrome in methadone-exposed infants is altered by level of prenatal tobacco exposure. Drug Alcohol Depend. 2004;75(3):253–60. doi: 10.1016/j.drugalcdep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Winklbaur B, Baewert A, Jagsch R, et al. Association between prenatal tobacco exposure and outcome of neonates born to opioid-maintained mothers. Implications for treatment. Eur Addict Res. 2009;15(3):150–6. doi: 10.1159/000216466. [DOI] [PubMed] [Google Scholar]
- 49.Gray TR, Choo RE, Concheiro M, et al. Prenatal methadone exposure, meconium biomarker concentrations and neonatal abstinence syndrome. Addiction. 2010;105(12):2151–9. doi: 10.1111/j.1360-0443.2010.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arendt RE, Short EJ, Singer LT, et al. Children prenatally exposed to cocaine: developmental outcomes and environmental risks at seven years of age. J Dev Behav Pediatr. 2004;25(2):83–90. doi: 10.1097/00004703-200404000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frank DA, Augustyn M, Knight WG, et al. Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. JAMA. 2001;285(12):1613–25. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schempf AH. Illicit drug use and neonatal outcomes: a critical review. Obstet Gynecol Surv. 2007;62(11):749–57. doi: 10.1097/01.ogx.0000286562.31774.76. [DOI] [PubMed] [Google Scholar]
- 53.Hunt RW, Tzioumi D, Collins E, et al. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum Dev. 2008;84(1):29–35. doi: 10.1016/j.earlhumdev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Jones HE, Kaltenbach K, O’Grady KE. The complexity of examining developmental outcomes of children prenatally exposed to opiates. A response to Hunt et al. Adverse neurodevelopmental outcome of infants exposed to opiates in-utero. Early Human Development (2008, 84, 29–35) Early Hum Dev. 2009;85(4):271–2. doi: 10.1016/j.earlhumdev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Bandstra ES, Morrow CE, Mansoor E, et al. Prenatal drug exposure: infant and toddler outcomes. J Addict Dis. 2010;29(2):245–58. doi: 10.1080/10550881003684871. [DOI] [PubMed] [Google Scholar]
- 56.Lester BM, Lagasse LL. Children of addicted women. J Addict Dis. 2010;29(2):259–76. doi: 10.1080/10550881003684921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaltenbach K, Finnegan LP. Neonatal abstinence syndrome, pharmacotherapy and developmental outcome. Neurobehav Toxicol Teratol. 1986;8(4):353–5. [PubMed] [Google Scholar]
- 58.Messinger DS, Bauer CR, Das A, et al. The maternal lifestyle study: cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics. 2004;113(6):1677–85. doi: 10.1542/peds.113.6.1677. [DOI] [PubMed] [Google Scholar]
- 59.Farid WO, Dunlop SA, Tait RJ, et al. The effects of maternally administered methadone, buprenorphine and naltrexone on offspring: review of human and animal data. Curr Neuropharmacol. 2008;6(2):125–50. doi: 10.2174/157015908784533842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nandakumar N, Sankar VS. What is the best evidence based management of neonatal abstinence syndrome? Arch Dis Child Fetal Neonatal Ed. 2006;91(6):F463. doi: 10.1136/adc.2006.095166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol. 2006;26(1):15–7. doi: 10.1038/sj.jp.7211427. [DOI] [PubMed] [Google Scholar]
- 62.O’Grady MJ, Hopewell J, White MJ. Management of neonatal abstinence syndrome: a national survey and review of practice. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F249–52. doi: 10.1136/adc.2008.152769. [DOI] [PubMed] [Google Scholar]
- 63.Crocetti MT, Amin DD, Jansson LM. Variability in the evaluation and management of opiate-exposed newborns in Maryland. Clin Pediatr (Phila) 2007;46(7):632–5. doi: 10.1177/0009922807300699. [DOI] [PubMed] [Google Scholar]
- 64.Lipsitz PJ. A proposed narcotic withdrawal score for use with newborn infants. A pragmatic evaluation of its efficacy Clin Pediatr (Phila) 1975;14(6):592–4. doi: 10.1177/000992287501400613. [DOI] [PubMed] [Google Scholar]
- 65.Green M, Suffet F. The Neonatal Narcotic Withdrawal Index: a device for the improvement of care in the abstinence syndrome. Am J Drug Alcohol Abuse. 1981;8(2):203–13. doi: 10.3109/00952998108999125. [DOI] [PubMed] [Google Scholar]
- 66.Zahorodny W, Rom C, Whitney W, et al. The neonatal withdrawal inventory: a simplified score of newborn withdrawal. J Dev Behav Pediatr. 1998;19(2):89–93. doi: 10.1097/00004703-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 67.Finnegan LP, Kaltenbach K. In: Neonatal abstinence syndrome. Hoekelman RA, Friedman SB, Nelson N, et al., editors. St Louis (MO): Mosby; 1992. pp. 1367–78. [Google Scholar]
- 68.Jones HE, Johnson RE, Jasinski DR, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79(1):1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 69.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–31. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kraft WK, Dysart K, Greenspan JS, et al. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. Addiction. 2011;106(3):574–80. doi: 10.1111/j.1360-0443.2010.03170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Agthe AG, Kim GR, Mathias KB, et al. Clonidine as an adjunct therapy to opioids for neonatal abstinence syndrome: a randomized, controlled trial. Pediatrics. 2009;123(5):e849–56. doi: 10.1542/peds.2008-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zimmermann-Baer U, Notzli U, Rentsch K, et al. Finnegan neonatal abstinence scoring system: normal values for first 3 days and weeks 5–6 in non-addicted infants. Addiction. 2010;105(3):524–8. doi: 10.1111/j.1360-0443.2009.02802.x. [DOI] [PubMed] [Google Scholar]
- 73.van Sleuwen BE, Engelberts AC, Boere-Boonekamp MM, et al. Swaddling: a systematic review. Pediatrics. 2007;120(4):e1097–106. doi: 10.1542/peds.2006-2083. [DOI] [PubMed] [Google Scholar]
- 74.Jansson LM. Neonatal abstinence syndrome. Acta Paediatr. 2008;97(10):1321–3. doi: 10.1111/j.1651-2227.2008.00968.x. [DOI] [PubMed] [Google Scholar]
- 75.Oei J, Feller JM, Lui K. Coordinated outpatient care of the narcotic-dependent infant. J Paediatr Child Health. 2001;37(3):266–70. doi: 10.1046/j.1440-1754.2001.00657.x. [DOI] [PubMed] [Google Scholar]
- 76.Coyle MG, Ferguson A, Lagasse L, et al. Diluted tincture of opium (DTO) and phenobarbital versus DTO alone for neonatal opiate withdrawal in term infants. J Pediatr. 2002;140(5):561–4. doi: 10.1067/mpd.2002.123099. [DOI] [PubMed] [Google Scholar]
- 77.Osborn DA, Jeffery HE, Cole MJ. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2010;(10):CD002059. doi: 10.1002/14651858.CD002059.pub3. [DOI] [PubMed] [Google Scholar]
- 78.Osborn DA, Jeffery HE, Cole MJ. Sedatives for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2010;(10):CD002053. doi: 10.1002/14651858.CD002053.pub3. [DOI] [PubMed] [Google Scholar]
- 79.Hudak ML, Tan RC. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540–60. doi: 10.1542/peds.2011-3212. [DOI] [PubMed] [Google Scholar]
- 80.Jansson LM, Velez M. Neonatal abstinence syndrome. Curr Opin Pediatr. 2012;24(2):252–8. doi: 10.1097/MOP.0b013e32834fdc3a. [DOI] [PubMed] [Google Scholar]
- 81.Johnson K, Gerada C, Greenough A. Treatment of neonatal abstinence syndrome. Arch Dis Child Fetal Neonatal Ed. 2003;88(1):F2–5. doi: 10.1136/fn.88.1.F2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Colombini N, Elias R, Busuttil M, et al. Hospital morphine preparation for abstinence syndrome in newborns exposed to buprenorphine or methadone. Pharm World Sci. 2008;30(3):227–34. doi: 10.1007/s11096-007-9176-1. [DOI] [PubMed] [Google Scholar]
- 83.Jones HC. Shorter dosing interval of opiate solution shortens hospital stay for methadone babies. Fam Med. 1999;31(5):327–30. [PubMed] [Google Scholar]
- 84.Bouwmeester NJ, Hop WC, van Dijk M, et al. Postoperative pain in the neonate: age-related differences in morphine requirements and metabolism. Intensive Care Med. 2003;29(11):2009–15. doi: 10.1007/s00134-003-1899-4. [DOI] [PubMed] [Google Scholar]
- 85.Bouwmeester NJ, Anderson BJ, Tibboel D, et al. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth. 2004;92(2):208–17. doi: 10.1093/bja/aeh042. [DOI] [PubMed] [Google Scholar]
- 86.Barrett DA, Barker DP, Rutter N, et al. Morphine, morphine-6-glucuronide and morphine-3-glucuronide pharmacokinetics in newborn infants receiving diamorphine infusions. Br J Clin Pharmacol. 1996;41(6):531–7. doi: 10.1046/j.1365-2125.1996.03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferrari A, Coccia CP, Bertolini A, et al. Methadone–metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50(6):551–9. doi: 10.1016/j.phrs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 88.Yang F, Tong X, McCarver DG, et al. Population-based analysis of methadone distribution and metabolism using an age-dependent physiologically based pharmacokinetic model. J Pharmacokinet Pharmacodyn. 2006;33(4):485–518. doi: 10.1007/s10928-006-9018-0. [DOI] [PubMed] [Google Scholar]
- 89.Madden JD, Chappel JN, Zuspan F, et al. Observation and treatment of neonatal narcotic withdrawal. Am J Obstet Gynecol. 1977;127(2):199–201. doi: 10.1016/s0002-9378(16)33250-1. [DOI] [PubMed] [Google Scholar]
- 90.Lainwala S, Brown ER, Weinschenk NP, et al. A retrospective study of length of hospital stay in infants treated for neonatal abstinence syndrome with methadone versus oral morphine preparations. Adv Neonatal Care. 2005;5(5):265–72. doi: 10.1016/j.adnc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 91.Backes CH, Backes CR, Gardner D, et al. Neonatal abstinence syndrome: transitioning methadone-treated infants from an inpatient to an outpatient setting. J Perinatol. 2011 doi: 10.1038/jp.2011.114. [Epub ahead of print]. http://dx.doi.org/10.1038/jp.2011.114. [DOI] [PMC free article] [PubMed]
- 92.Gowing L, Ali R, White JM. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009;(3):CD002025. doi: 10.1002/14651858.CD002025.pub4. [DOI] [PubMed] [Google Scholar]
- 93.Kraft WK, Gibson E, Dysart K, et al. Sublingual buprenorphine for treatment of neonatal abstinence syndrome: a randomized trial. Pediatrics. 2008;122(3):e601–7. doi: 10.1542/peds.2008-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuhlman JJ, Jr, Levine B, Johnson RE, et al. Relationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphine. Addiction. 1998;93(4):549–59. doi: 10.1046/j.1360-0443.1998.93454910.x. [DOI] [PubMed] [Google Scholar]
- 95.Anagnostis EA, Sadaka RE, Sailor LA, et al. Formulation of buprenorphine for sublingual use in neonates. Journal of Pediatric Pharmacology & Therapeutics. 2011;16(4):281–4. doi: 10.5863/1551-6776-16.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jackson L, Ting A, McKay S, et al. A randomised controlled trial of morphine versus phenobarbitone for neonatal abstinence syndrome. Arch Dis Child Fetal Neonatal Ed. 2004;89(4):F300–4. doi: 10.1136/adc.2003.033555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pitlick W, Painter M, Pippenger C. Phenobarbital pharmacokinetics in neonates. Clin Pharmacol Ther. 1978;23(3):346–50. doi: 10.1002/cpt1978233346. [DOI] [PubMed] [Google Scholar]
- 98.Finnegan LP, Michael H, Leifer B. The use of phenobarbital in treating abstinence in newborns exposed in utero to psychoactive agents. NIDA Res Monogr. 1984;49:329. [PubMed] [Google Scholar]
- 99.Wu D, Kraft WK, Ehrlich ME, et al. Sublingual bioavailability of buprenorphine in newborns with neonatal abstinence syndrome—a case study on physiological and developmental changes using SIMCYP. Presented at the American College of Clinical Pharmacology meeting; Philadelphia (PA). 2009. Available at: http://jdc.jefferson.edu/petfp/24/. Accessed August 10, 2012. [Google Scholar]
- 100.Xie HG, Cao YJ, Gauda EB, et al. Clonidine clearance matures rapidly during the early postnatal period: a population pharmacokinetic analysis in newborns with neonatal abstinence syndrome. J Clin Pharmacol. 2011;51(4):502–11. doi: 10.1177/0091270010370587. [DOI] [PubMed] [Google Scholar]
- 101.Wachman EM, Brown MS, Paul JA, et al. Single nucleotide polymorphisms and variability in severity of neonatal abstinence syndrome. Pediatric Academic Societies. 2012 [Google Scholar]
- 102.Mass SB. Supporting breastfeeding in the United States: the Surgeon General’s call to action. Curr Opin Obstet Gynecol. 2011;23(6):460–4. doi: 10.1097/GCO.0b013e32834cdcb3. [DOI] [PubMed] [Google Scholar]
- 103.Jansson LM, Choo R, Velez ML, et al. Methadone maintenance and breastfeeding in the neonatal period. Pediatrics. 2008;121(1):106–14. doi: 10.1542/peds.2007-1182. [DOI] [PubMed] [Google Scholar]
- 104.Abdel-Latif ME, Pinner J, Clews S, et al. Effects of breast milk on the severity and outcome of neonatal abstinence syndrome among infants of drug-dependent mothers. Pediatrics. 2006;117(6):e1163–9. doi: 10.1542/peds.2005-1561. [DOI] [PubMed] [Google Scholar]
- 105.McQueen KA, Murphy-Oikonen J, Gerlach K, et al. The impact of infant feeding method on neonatal abstinence scores of methadone-exposed infants. Adv Neonatal Care. 2011;11(4):282–90. doi: 10.1097/ANC.0b013e318225a30c. [DOI] [PubMed] [Google Scholar]
- 106.Liu AJ, Nanan R. Methadone maintenance and breastfeeding in the neonatal period. Pediatrics. 2008;121(4):869. doi: 10.1542/peds.2008-0217. [DOI] [PubMed] [Google Scholar]
- 107.Grimm D, Pauly E, Poschl J, et al. Buprenorphine and norbuprenorphine concentrations in human breast milk samples determined by liquid chromatography-tandem mass spectrometry. Ther Drug Monit. 2005;27(4):526–30. doi: 10.1097/01.ftd.0000164612.83932.be. [DOI] [PubMed] [Google Scholar]
- 108.Marquet P, Chevrel J, Lavignasse P, et al. Buprenorphine withdrawal syndrome in a newborn. Clin Pharmacol Ther. 1997;62(5):569–71. doi: 10.1016/S0009-9236(97)90053-9. [DOI] [PubMed] [Google Scholar]
- 109.Ilett KF, Hackett LP, Gower S, et al. Estimated dose exposure of the neonate to buprenorphine and its metabolite norbuprenorphine via breastmilk during maternal buprenorphine substitution treatment. Breastfeed Med. 2011 doi: 10.1089/bfm.2011.0096. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 110.Lindemalm S, Nydert P, Svensson JO, et al. Transfer of buprenorphine into breast milk and calculation of infant drug dose. J Hum Lact. 2009;25(2):199–205. doi: 10.1177/0890334408328295. [DOI] [PubMed] [Google Scholar]
- 111.Johnson RE, Jones HE, Fischer G. Use of buprenorphine in pregnancy: patient management and effects on the neonate. Drug Alcohol Depend. 2003;70(Suppl 2):S87–101. doi: 10.1016/s0376-8716(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 112.Lejeune C, Aubisson S, Simmat-Durand L, et al. Withdrawal syndromes of newborns of pregnant drug abusers maintained under methadone or high-dose buprenorphine: 246 cases. Ann Med Interne (Paris) 2001;152(Suppl 7):21–7. [in French] [PubMed] [Google Scholar]
- 113.Loustauneau A, Auriacombe M, Daulouede JP, et al. Is buprenorphine a potential alternative to methadone for treating pregnant drug users? Inventory of clinical data in the literature. Ann Med Interne (Paris) 2002;153(Suppl 7):2. [in French] [PubMed] [Google Scholar]
- 114.SAMHSA (Substance Abuse, and Mental Health Services Administration) SAMHSA clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: treatment improvement protocol series, No. 40. Rockville (MD): USDHHS; 2004. [PubMed] [Google Scholar]
- 115.Skorpen F, Laugsand EA, Klepstad P, et al. Variable response to opioid treatment: any genetic predictors within sight? Palliat Med. 2008;22(4):310–27. doi: 10.1177/0269216308089302. [DOI] [PubMed] [Google Scholar]
- 116.Troisi A, Frazzetto G, Carola V, et al. Social hedonic capacity is associated with the A118G polymorphism of the mu-opioid receptor gene (OPRM1) in adult healthy volunteers and psychiatric patients. Soc Neurosci. 2011;6(1):88–97. doi: 10.1080/17470919.2010.482786. [DOI] [PubMed] [Google Scholar]
- 117.Campa D, Gioia A, Tomei A, et al. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin Pharmacol Ther. 2008;83(4):559–66. doi: 10.1038/sj.clpt.6100385. [DOI] [PubMed] [Google Scholar]
- 118.Rakvag TT, Ross JR, Sato H, et al. Genetic variation in the catechol-O-methyltransferase (COMT) gene and morphine requirements in cancer patients with pain. Mol Pain. 2008;4:64. doi: 10.1186/1744-8069-4-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tammimaki AE, Mannisto PT. Are genetic variants of COMT associated with addiction? Pharmacogenet Genomics. 2010;20(12):717–41. doi: 10.1097/FPC.0b013e328340bdf2. [DOI] [PubMed] [Google Scholar]
- 120.Gertler R, Brown HC, Mitchell DH, et al. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14(1):13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oschman A, McCabe T, Kuhn RJ. Dexmedetomidine for opioid and benzodiazepine withdrawal in pediatric patients. Am J Health Syst Pharm. 2011;68(13):1233–8. doi: 10.2146/ajhp100257. [DOI] [PubMed] [Google Scholar]
- 122.Gowing L, Farrell M, Ali R, et al. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009;(2):CD002024. doi: 10.1002/14651858.CD002024. [DOI] [PubMed] [Google Scholar]
- 123.De Cock RF, Piana C, Krekels EH, et al. The role of population PK-PD modelling in paediatric clinical research. Eur J Clin Pharmacol. 2011;67(Suppl 1):5–16. doi: 10.1007/s00228-009-0782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoder EL, Leckman JF, Poulsen J, et al. Clonidine treatment of neonatal narcotic abstinence syndrome. Psychiatry Res. 1984;13(3):243–51. doi: 10.1016/0165-1781(84)90039-8. [DOI] [PubMed] [Google Scholar]
- 125.Leikin JB, Mackendrick WP, Maloney GE, et al. Use of clonidine in the prevention and management of neonatal abstinence syndrome. Clin Toxicol (Phila) 2009;47(6):551–5. doi: 10.1080/15563650902980019. [DOI] [PubMed] [Google Scholar]
- 126.Esmaeili A, Keinhorst AK, Schuster T, et al. Treatment of neonatal abstinence syndrome with clonidine and chloral hydrate. Acta Paediatr. 2010;99(2):209–14. doi: 10.1111/j.1651-2227.2009.01547.x. [DOI] [PubMed] [Google Scholar]


