Abstract
Effective and safe drug administration in young infants should be based on integrated knowledge concerning the evolving physiological characteristics of the infant who will receive the drug and the pharmacokinetic and pharmacodynamic characteristics of a given drug. Consequently, clinical pharmacology in neonates is as dynamic and diverse as the neonates we are entitled to take care of. Even more than median estimates, covariates of variability within the population are of clinical relevance. We aim to illustrate the complexity and the need for neonatal clinical pharmacology based on the gap between current and likely best clinical practice for two commonly administered compounds (aminoglycosides for infection and ibuprofen for patent ductus arteriosus) and one new compound (bevacizumab, to treat threshold retinopathy of prematurity). Progression has been made to render pharmacokinetic studies child size, e.g., low volume samples, optimal study design, and population pharmacokinetics. Challenges to further improve clinical pharmacology in neonates include, when appropriate, the validation of off-patent drug dosing regimens and of infant-tailored formulations. Knowledge integration, i.e., the use of available data to improve current drug use and to predict pharmacokinetics/pharmacodynamics for similar compounds is needed. Development of clinical research networks is helpful to achieve these goals.
Keywords: Pharmacodynamics, Pharmacokinetics, Newborn, Clinical pharmacology, Ontogeny, Maturation
Introduction: neonatal pathophysiology is reflected in neonatal clinical pharmacology
Drug dosing in young infants should be based on integrated knowledge concerning the specific diseases to be treated, the physiological characteristics of the infant receiving the drug, and the pharmacokinetic and pharmacodynamic parameters of this drug [1, 24, 43]. When we consider these physiological changes and the subsequent variability in physiological characteristics, we should be aware that maturational changes in physiology are most prominent in early infancy [15]. If we focus on weight changes to illustrate this, there is an initial decrease (6–12 %) in birth weight, with a subsequent increase of 50 % in the first 6 weeks of postnatal life. Moreover, weight doubles in the first 3–4 months to result in a threefold higher weight at the end of infancy. Consequently, total energy requirements change dramatically since these requirements are the sum of energy expenditure and energy deposition for growth [1, 24, 43]. These maturational physiological changes are further modulated by pathophysiological processes (e.g., perinatal asphyxia, cardiopathy, sepsis, renal failure, and patent ductus arteriosus) and treatment modalities (e.g., whole body cooling, extracorporeal membrane oxygenation (ECMO), or pharmacotherapy) applied. All these changes, both maturational (e.g., age and weight) and pathophysiological, are referred to as covariates in clinical pharmacology.
The aim of administering any drug is to reach an effective treatment of a given disease while avoiding disproportional side effects [16, 17]. Clinical pharmacology aims to predict drug-specific (side) effects based on pharmacokinetics and pharmacodynamics. Pharmacokinetics (PK) describe the relationship between a drug concentration at a specific site (e.g., plasma and cerebrospinal fluids) and time (“what the body does to the drug”). Pharmacodynamics (PD) describe the relationship between a drug concentration and (side) effects (“what the drug does to the body”) (Fig. 1). The above mentioned (patho)physiological changes in early neonatal life result in extensive inter- and intraindividual variability in PK and PD; in addition to median pharmacokinetic estimates or outcome variables, the range and its covariates are at least as crucial.
Fig. 1.
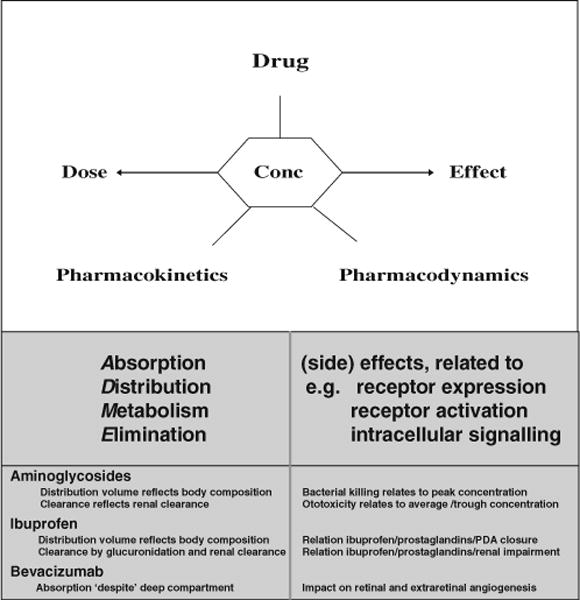
Relationship between a drug concentration and (side) effects
Pharmacokinetics in early infancy differ substantially from children or adults as a result of physiology-related maturation in absorption, distribution, and subsequent elimination, either through metabolic elimination or through primary renal elimination (ADME, pharmacokinetics). Body composition, protein binding, and compartment sizes change during infancy, all phases I (e.g., cytochromes) and II (e.g., glucuronidation) metabolic processes of drugs mature in an isoenzyme specific pattern, while renal function (glomerular filtration rate (GFR), tubular absorption/excretion) also displays age-dependent clearance [1, 15–17, 24, 43]. Age-dependent PD differences are much less explored, but relate to age-dependent effects (e.g., caffeine to prevent neonatal apnea and lidocaine to treat neonatal seizures) or side effects (e.g., cerebral palsy related to postnatal steroids, ototoxicity related to aminoglycosides, furosemide, and noise pollution) [15–17, 21, 29].
The perception that the effects of a given drug are different in neonates often arises from the fact that the PK has not yet been adequately studied in neonates [1, 24, 43]. The same dose (e.g., per kilogram) in a neonate will result in another concentration/time profile when compared to a child or adult. Consequently, we first have to get the dose right (PK, concentration/time profile) before we can search for population-specific PD (concentration/effect profile) differences. However, even after taking pharmacokinetic covariates (e.g., age and weight) into account, neonates often have altered PD. In general, the pharmacological response depends on the drug binding to a drug receptor. Age-dependent variations in receptor number, receptor affinity, or post receptor activation processes could influence the drug response. Yet, information about the effect of receptor ontogeny on interactions between drugs and receptors and the consequence of these interactions is very limited in neonates. Gamma-aminobutyric acid (GABAA) and motilin receptor ontogeny may serve as illustrations [5, 22].
Due to developmental shifts in chloride membrane transporters, immature neurons have higher intracellular chloride concentrations. In the immature neuron, this results in a chloride efflux when the GABAA receptor is activated instead of influx. This outward chloride flow results in cellular excitation instead of inhibition. Pharmacological manipulation of this chloride shift by bumetanide decreases the intracellular chloride concentration and potentiates the anticonvulsant action of phenobarbital through GABAA interaction. This chloride manipulation to switch from excitation to inhibition concept, i.e., the add-on effect of bumetanide in phenobarbital-resistant neonatal convulsions, is evaluated in the NEMO trial [5]. Likewise, the intestinal motilin receptor also displays age-dependent expression of intestinal motilin [22]. Since the motilin receptor only appears in older preterm infants, prokinetic agents interacting with these motilin receptors may not yet be effective in very preterm infants, only be partially useful in older preterm infants and more effective in full-term infants [22].
Despite the specific issues (PK/PD) of neonatal clinical pharmacology, many drugs in neonates are still prescribed off-label or remain unlicensed [8, 19, 21, 35]. This means that drugs are empirically dosed based on the extrapolation of observations in nonneonatal (i.e., children or adult) populations [2, 3, 11]. Off-label use, hereby, signifies the use of a drug in situations not covered by the product license. This may relate to age, dosage, frequency or route of administration, or due to extemporaneous formulation [8, 19, 21, 35]. Although off-label use is already common in ambulatory pediatrics (30 %), it is most prominent in neonatal (90 %) intensive care [8, 19, 21]. All stakeholders involved (e.g., pediatricians, policy makers, industry, and parents) need to be aware that such “daily practice” and invalidated off-label use of drugs may have important negative effects; newborns may receive ineffective doses of potentially effective medicines or may be harmed by medicines that might not be appropriate for their conditions. However, for some drugs, like aminoglycosides, these off-label dosing regimens have undergone extensive validation [1, 10, 12, 13, 20, 26, 28, 31, 32, 34, 37, 44, 45].
In this paper, we aim to illustrate the complexity and the need of neonatal clinical pharmacology (PK/PD) based on the gap between current and best clinical practice for two commonly administered compounds (aminoglycosides for neonatal infection and ibuprofen for patent ductus arteriosus) and one new emerging compound (bevacizumab for threshold retinopathy of prematurity).
Anti-infective drugs, aminoglycosides as illustration
The combination of in vitro and in vivo bactericidal characteristics resulted in a classification of antibiotics according to their specific PK/PD relationship [12, 26, 31, 37]. When we focus on bacterial growth inhibition or killing, a variable concentration response depending on this classification needs to be considered. Minimal inhibitory concentration (MIC) values and the most effective strategy to aim for relate to both the antibiotic and the pathogen (Fig. 1). For instance, maintaining the serum concentration of >4 times the MIC is the goal for beta-lactams, while reaching a peak/MIC ratio of >8 is applied for aminoglycosides [12, 26, 31, 37]. Since this strategy is focused on the pathogen, the challenge in neonates is to maximize these concepts to result in a more efficacious treatment in the context of their immature distribution and excretion pathways [12, 26, 31, 37]. Consequently, population-specific PK estimates and its covariates are of utmost importance to propose effective dosing regimens for antibiotics [2, 3, 10–12]. The translation of these concepts into effective prescription of aminoglycosides in neonates is further discussed as an illustration of the difficulties encountered to translate these concepts to effective and safe use of antibiotics in neonates [10–13, 23, 26, 31, 32, 34, 37, 44, 45, 47].
Because the bactericidal activity of aminoglycosides depends on the peak concentration with a persisted bactericidal effect after this peak decreased, extended interval dosing regimens have been developed [10, 26, 44, 45]. In essence, such extended interval regimens result in high peak levels and improved adherence to appropriate trough concentrations because of the extended time interval. Animal studies and trials in older children and adults suggest that such a “one pulse strategy” for aminoglycosides administration is superior (identical efficacy, but less toxicity) to a multiple doses per day regimen [26, 31, 34]. This is commonly achieved in children and adults with a once-daily administration, while in neonates, the time interval between consecutive doses may be longer. When developing “extended time” dosing guidelines for neonates, this means that both clearance and distribution volume (peak level) and its covariates need to be considered. Consequently, the available knowledge on aminoglycoside PK (both clearance and distribution volume) in neonates needs to be integrated in the design of such extended dosing guidelines.
Since aminoglycoside clearance reflects glomerular filtration rate (GFR), neonatal aminoglycoside clearance is between 1–5 % of adult clearance [10]. Within neonates, differences in aminoglycoside clearance can be expected to relate to ontogeny (e.g., gestational age, postnatal age, postmenstrual age, and weight) and disease characteristics (e.g., renal impairment due to ibuprofen or perinatal asphyxia) [10, 11, 26]. Moreover, the distribution volume for aminoglycosides is higher (liter per kilogram) in (pre)term neonates because of the higher extracellular water content [1, 10, 24]. While the lower elimination clearance necessitates a further extended dose interval to reach a safe trough level, the higher distribution volume necessitates higher doses [31].
At present, there are observations suggesting that pharmacokinetic properties (peak, trough) of an extended dose interval for aminoglycosides are superior to “multiple daily doses” since higher peak levels while avoiding toxic trough levels are observed [31, 34]. However, this superiority should be balanced with the feasibility to introduce more complex dosing guidelines in our daily clinical practice [22, 32, 34, 37]. As recently suggested for gentamicin in neonates, these more complex dosing guidelines result in a higher incidence of dosing errors [22, 32, 34, 37]. These dosing errors also relate to the multiple drug manipulations needed before the prescribed dose can be administered [36].
Pharmacodynamics and safety of antibiotics are in part unrelated to age (e.g., bacterial resistance), while other aspects (nephro- and ototoxicity, colonizing intestinal microbiota) display infancy-specific patterns that may be either protective or make this population more vulnerable [12, 13, 17, 20, 25, 28, 29, 31, 44, 45, 47]. The link between toxicity and high trough aminoglycosides levels is based on historical case series, when extended interval dosing regimens for aminoglycosides were not yet implemented, and relates to the median concentration, the total dose, and the length of treatment [13, 25, 27]. Megalin, a low-density lipoprotein receptor in the renal proximal tubule and in the labyrinth epithelium, is involved in aminoglycoside accumulation and toxicity [20, 28]. Since aminoglycoside binding to proximal renal tubular and cochlear cells is saturable, strategies including the one pulse strategy and the shortening in duration of aminoglycoside treatment when combined with other antibiotics likely increase the anti-infectious efficacy and decrease the risk of toxicities in neonates[20, 30, 33]. Because renal tubular uptake capacity is not yet mature, nephrotoxicity related to accumulation by aminoglycoside is less pronounced in neonates when compared to other populations [20, 28].
While the targets of antibiotics are pathogenic bacteria, other members of the microbiota are also affected by antibiotics [25, 47]. This is a specific safety (PD) concern in neonates since it is a unique time in human life where a myriad of bacteria should colonize a sterile gut [25, 37, 47]. The immune response at the submucosa intestinal level during infancy is a result of interactions between the infant and this progressive diversified intestinal microflora [47]. Interferences with this progressive diversification of the microflora may have impact on the immune interaction. Consequently, immune abnormalities in later life may be due to inadequate bacterial pressure on the intestinal mucosa in early infancy. Perinatal exposure to antibiotics, including aminoglycosides, may modulate this microbiota and is a population-specific, long-term outcome variable [25, 37, 47].
Patent ductus arteriosus
In fetal life, the patent ductus arteriosus (PDA) diverts placental oxygenated blood from the pulmonary artery into the fetal aorta. After birth, modifications in prostaglandins, its receptors, and the postnatal increase in arterial oxygen tension results in muscular constriction with subsequent anatomic closure of the ductus [6, 14, 18, 30, 41, 46, 48]. These processes (constriction and closure) may be delayed or even fail in preterm infants. Indomethacin and ibuprofen are both effective to induce PDA closure with minor differences between both compounds in the incidence of necrotizing enterocolitis or bronchopulmonary dysplasia [46]. Besides PDA closure, there are no other short-term outcome benefits. From a clinical pharmacology perspective (Fig. 1), we would like to make the point that integration of the available knowledge on PK and PD of ibuprofen has not yet been optimized [6, 14, 18, 30, 41, 46, 48].
First, there are data on ibuprofen PK and its covariates and one dose-finding, continual reassessment study of ibuprofen in preterm neonates with PDA. However, the PK information on covariates (age, weight) has not yet been introduced in prospective studies—“one dose still seems to fit all neonates” [14, 18, 30].
Second, also the PD part of this drug needs further considerations, including issues related to both diagnostic accuracy and long-term safety. In a recent analysis on the definitions of symptomatic PDA applied in prospective studies, a variety of clinical signs (e.g., murmur and hyperdynamic circulation) or echocardiographic markers (e.g., diameter ductus arteriosus and left atrium to aorta ratio) emerged without much data on sensitivity or specificity of the different indicators [48]. More recently, some groups introduced plasma biomarkers as a more valid indicator for symptomatic PDA. It is hereby striking that there was extensive variability in cutoff values between the different studies since the cutoff values of N-terminal fragment of pro-brain-type natriuretic peptide (NT-proBNP) varied with up to tenfold differences [6, 30, 48]. We claim that further evaluation of the impact of sample handling and validation of quantification methods are needed before such PDA biomarkers can be introduced in clinical care. For the issue on long-term outcome, a recent paper describes a link between ibuprofen exposure and reduction in renal nephrogenic zone width in a baboon model, which may suggest early cessation of nephrogenesis following ibuprofen exposure [40]. We are unaware of any long-term outcome studies following neonatal ibuprofen exposure.
Bevacizumab for treatment of retinopathy of prematurity
As recently discussed in this journal, retinopathy of prematurity (ROP) is a proliferative retinal vascular disease affecting the premature infant with an incompletely vascularized retina [7]. The spectrum of ophthalmological findings varies from minimal sequelae without visual impairment to bilateral retinal detachment and total blindness. Over the past two decades, major advances have been made in understanding the pathogenesis of ROP. Based on well-designed, collaborative multicenter studies, primary and secondary prevention (oxygen, nutrition) strategies, surgical indications, and surgical techniques (threshold ROP, laser surgery) have been validated [7].
The most recent advances relate to the intraocular injection of bevacizumab. The BEAT-ROP study provided the first evidence for intraocular injection of bevacizumab being less invasive and as effective for zones I–II retinopathy of prematurity, compared to primary laser surgery [4, 9, 38]. Obviously, there is a need for improvement since laser therapy is, in essence, an ablative surgery. From a clinical pharmacology perspective, there are, however, some PK and PD issues that need further considerations. First, although bevacizumab is injected in a deep compartment (the eye), this compound still appears in the general circulation [38]. This results in a decrease in serum vascular endothelial growth factor (VEGF), while we have to realize that VEGF is a relevant driver of microangiogenesis (e.g., the brain, lung, and kidney) [4, 9, 38]. Second, the issue of long-term visual outcome has not yet been thoroughly addressed since the need for subsequent laser surgery was the primary outcome variable, and not (long-term) visual outcome [4, 9, 38].
Discussion: on recent advances and future challenges
By describing some aspects of clinical pharmacology of aminoglycosides, ibuprofen, and bevacizumab, we tried to make the point that integration of the available knowledge on PK and PD is needed to further improve drug-related clinical care and outcome in neonates. The obvious aim of any prescription is effective treatment of a given disease while avoiding disproportional side effects. However, in the absence of PK and dose-finding studies, one can only speculate which dose to use. Similarly, in the absence of data on the relevant outcome variables (PD), one can still report on the association of a given compound and an effect. However, this treatment may result in unknown side effects because they are not yet reported. We would like to refer to some known neonatal drug “errors” to further illustrate this.
Inadvertent, i.e., extrapolated based on adult dosing, administration of chloramphenicol resulted in gray baby syndrome due to accumulation of chloramphenicol. This relates to the reduced glucuronidation capacity in early life. This is not an issue of population-specific toxicity, but relates to the reduced clearance capacity (PK) in neonates [1, 24]. Similarly, postnatal dexamethasone results in shorter ventilation time, but does not reduce the incidence of bronchopulmonary dysplasia and even results in an increased incidence of cerebral palsy and impaired neurodevelopmental outcome. This relates to a population-specific vulnerability (PD) [39]. Unfortunately, it took many more years before these negative long-term outcome data became apparent since the positive short-term outcome (i.e., extubation) delayed the evaluation of the impact of dexamethasone on neurodevelopmental outcome in former preterm neonates [39].
Since the implementation of the pediatric regulation in the USA and the subsequent initiatives in Europe and throughout the world, there is more clinical research in the field of neonatal product development for new compounds. This increase does not only result in more challenges such as how to perform these studies, but also creates opportunities for population-tailored approaches in both pediatric product development and clinical research [33]. Concerted efforts to improve research tools for pharmacokinetics and pharmacodynamics will likely result not only in improved drug therapy in infants, but will also be a potent driver to learn more about developmental physiology of infancy. The feasibility of performing pharmacokinetic studies in neonates and infants improved with the introduction of tailored sampling methods (e.g., saliva, dried spots blood, or urine) and more accurate quantification of metabolites in low-volume samples [27, 43]. More importantly, the analysis of sparse, unbalanced datasets and the burden for each individual infant can be minimized through modeling and simulation using nonlinear mixed effect methods or physiologically based pharmacokinetic (PBPK) modeling [2, 3, 11]. Population pharmacokinetic models can also be used in the design of studies and in the sampling strategy to obtain maximal information with a limited burden for every individual infant. It permits the exploration of the influence of different covariates such as body weight and age to explain the variability in drug concentration and/or response [2, 3, 11, 27, 43]. We refer the interested reader to some reviews on aspects of population PK in pediatrics [2, 3, 11].
However, we still fail to a certain extent to validate such models in the clinical setting, including aspects like feasibility. We, hereby, also refer to the aminoglycoside topic as an illustration that complex dosing guidelines itself result in new problems [22, 32, 34, 37]. Moreover, the ongoing research for new compounds and the availability of tailored research tools still leave us with challenges to further improve the clinical care in neonates, including prioritization and implementation [27, 42, 43].
Clinicians need to be aware of the fact that an important bulk of currently prescribed, off-patent drugs in neonates have not yet appropriately been evaluated in neonates despite the availability of specific incentives (e.g., paediatric use marketing authorization (PUMA)) [8, 16, 17, 19, 21, 33, 43]. The Neonatal Clinical Studies Group (CSG) of the Medicines for Children Research Network (MCRN) undertook a 2-week prospective survey to establish which medicines are used in neonatal units in the UK, how many babies are receiving them, and what clinicians considered important issues for future research [42]. Treatment of chronic lung disease and of PDA and vitamin supplements turned out to be high on their priority list. Such a research agenda for neonatal medicines can subsequently be considered in the development of priority lists of medicines by the competent authorities. The project called treat infections in neonates (TINN) is an illustration of such a research initiative based on a priority list [21, 43]. Besides compound- (e.g., fluconazole, micafungin, and azithromycin) specific results, such initiatives also result in network of units with experience in evaluating drugs in neonates. An additional important initiative called Global Research in Paediatrics (GRIP) will focus on international pediatric clinical pharmacology training and aims to facilitate the development and safe use of medicine in children through training and education [19, 21, 43].
In summary, clinical pharmacological research in neonates should be as dynamic and diverse as the neonates we are entitled to take care for. Even more important than the median estimates, we have to search for covariates of variability within this population. Progression has been made to render studies child size (e.g., low-volume samples, population PK, and optimal study design). Challenges to further improve clinical pharmacology in neonates include, when appropriated, the validation of off-patent drug dosing regimens and of infant-tailored formulations. Knowledge integration, i.e., the use of available data to improve current drug use and to predict pharmacokinetics/pharmacodynamics for similar compounds is needed. Development of clinical research networks should be helpful to achieve these goals. At least, we hope that we, hereby, were able to convince the readers that neonatal clinical pharmacology is a relevant discipline.
Acknowledgments
Karel Allegaert is supported by the Fund for Scientific Research, Flanders (Belgium) (F.W.O. Vlaanderen) by a Fundamental Clinical Investigatorship (1800209N) and a research grant (1506409N). Jean-Paul Langhendries is supported partly by the FP5 grants QLRT-2001-00389 and QKL1-CT-2002-30582, the FP6 grant EARNEST Food-CT-2005-007036, and FP7 grants TINN (223614) and TINN2 (260908). Johannes van den Anker is supported in part by NIH grants (R01HD060543, K24DA027992, R01HD048689, U54HD071601) and FP7 grants TINN (223614), TINN2 (260908), and NEUROSIS (223060).
Footnotes
Copyright of European Journal of Pediatrics is the property of Springer Science & Business Media B.V. and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder’s express written permission. However, users may print, download, or email articles for individual use.
Contributor Information
Karel Allegaert, Email: karel.allegaert@uz.kuleuven.ac.be, Neonatal Intensive Care Unit, Division of Woman and Child, University Hospitals Leuven, Herestraat 49, 3000 Leuven, Belgium.
Jean Paul Langhendries, CHC-Site St Vincent, NICU, Rue François Lefèbvre 207, Liege-Rocourt, Belgium.
John N. van den Anker, Division of Pediatric Clinical Pharmacology, Children’s National Medical Center, Washington, D.C., USA Departments of Pediatrics, Pharmacology, Physiology and Integrative Systems Biology, George Washington University School of Medicine and Health Sciences, Washington, D.C., USA; Intensive Care, Erasmus MC-Sophia Children’s Hospital, Rotterdam, the Netherlands.
References
- 1.Allegaert K, Verbesselt R, Naulaers G, van den Anker JN, Rayyan M, Debeer A, de Hoon J. Developmental pharmacology: neonates are not just small adults…. Acta Clin Belg. 2008;63:16–24. doi: 10.1179/acb.2008.003. [DOI] [PubMed] [Google Scholar]
- 2.Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr. 2006;165:819–829. doi: 10.1007/s00431-006-0189-x. [DOI] [PubMed] [Google Scholar]
- 3.Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: general principles. Eur J Pediatr. 2006;165:741–746. doi: 10.1007/s00431-006-0188-y. [DOI] [PubMed] [Google Scholar]
- 4.Avery RL. Bevacizumab (Avastin) for retinopathy of prematurity: wrong dose, wrong drug, or both? J AAPOS. 2012;16(1):2–4. doi: 10.1016/j.jaapos.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Ari Y. Blocking seizures with the diuretic bumetanide: promises and pitfalls. Epilepsia. 2012;53:394–396. doi: 10.1111/j.1528-1167.2011.03378.x. [DOI] [PubMed] [Google Scholar]
- 6.Benitz WE. Patent ductus arteriosus: to treat or not to treat? Arch Dis Child Fetal Neonatal Ed. 2012;97:F80–F82. doi: 10.1136/archdischild-2011-300381. [DOI] [PubMed] [Google Scholar]
- 7.Casteels I, Cassiman C, van Calster J, Allegaert K. Educational paper: retinopathy of prematurity. Eur J Pediatr. doi: 10.1007/s00431-011-1610-7. [DOI] [PubMed] [Google Scholar]
- 8.d’Aloja E, Paribello F, Demontis R, Müller M. Off-label drugs prescription in neonatology: a physician’s duty or a medical hazardous attitude? J Matern Fetal Neonatal Med. 2011;24(Suppl 1):99–100. doi: 10.3109/14767058.2011.607574. [DOI] [PubMed] [Google Scholar]
- 9.Darlow BA, Ells AL, Gilbert CE, Gole GA, Quinn GE. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed. 2011 doi: 10.1136/archdischild-2011-301148. [DOI] [PubMed] [Google Scholar]
- 10.De Cock RF, Allegaert K, Schreuder MF, Sherwin CM, de Hoog M, van den Anker JN, Danhof M, Knibbe CA. Maturation of the glomerular filtration rate in neonates, as reflected by amikacin clearance. Clin Pharmacokinet. 2012;51:105–117. doi: 10.2165/11595640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.De Cock RF, Piana C, Krekels EH, Danhof M, Allegaert K, Knibbe CA. The role of population PK-PD modelling in paediatric clinical research. Eur J Clin Pharmacol. 2011;67(Suppl 1):5–16. doi: 10.1007/s00228-009-0782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Hoog M, Mouton JW, van den Anker JN. New dosing strategies for antibacterial agents in the neonate. Semin Fetal Neonatal Med. 2005;10:185–194. doi: 10.1016/j.siny.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 13.De Hoog M, van Zanten BA, Hop WC, Overbosch E, Weisglas-Kuperus N, van den Anker JN. Newborn hearing screening: tobramycin and vancomycin are not risk factors for hearing loss. J Pediatr. 2003;142:41–46. doi: 10.1067/mpd.2003.mpd037. [DOI] [PubMed] [Google Scholar]
- 14.Desfrere L, Zohar S, Morville P, Brunhes A, Chevret S, Pons G, Moriette G, Rey E, Treluyer JM. Dose-finding study of ibuprofen in patent ductus arteriosus using the continual reassessment method. J Clin Pharm Ther. 2005;30:121–132. doi: 10.1111/j.1365-2710.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- 15.De Wildt SN. Profound changes in drug metabolism enzymes and possible effects on drug therapy in neonates and children. Expert Opin Drug Metab Toxicol. 2011;7:935–948. doi: 10.1517/17425255.2011.577739. [DOI] [PubMed] [Google Scholar]
- 16.Dotta A, Braguglia A, Salvatori G. Pharmacological research in neonatology. J Matern Fetal Neonatal Med. 2011;24(Suppl 1):44–46. doi: 10.3109/14767058.2011.607580. [DOI] [PubMed] [Google Scholar]
- 17.Du W, Tutag Lehr V, Lieh-Lai M, Koo W, Ward RM, Rieder MJ, Van Den Anker JN, Reeves JH, Mathew M, Lulic-Botica M, Aranda JV. An algorithm to detect adverse drug reactions in the neonatal intensive care unit: a new approach. J Clin Pharmacol. 2012 doi: 10.1177/0091270011433327. [DOI] [PubMed] [Google Scholar]
- 18.Hirt D, van Overmeire B, Treluyer JM, Langhendries JP, Marguglio A, Eisinger MJ, Schepens P, Urien S. An optimized ibuprofen dosing scheme for preterm neonates with patent ductus arteriosus, based on a population pharmacokinetic and pharmacodynamic study. Br J Clin Pharmacol. 2008;65:629–636. doi: 10.1111/j.1365-2125.2008.03118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoppu K, Anabwani G, Garcia-Bournissen F, Gazarian M, Kearns GL, Nakamura H, Peterson RG, Sri Ranganathan S, de Wildt SN. The status of paediatric medicines initiatives around the world—what has happened and what has not? Eur J Clin Pharmacol. 2012;68:1–10. doi: 10.1007/s00228-011-1089-1. [DOI] [PubMed] [Google Scholar]
- 20.Huth ME, Ricci AJ, Cheng AG. Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. Int J Otolaryngol. 2011;2011:937861. doi: 10.1155/2011/937861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacqz-Aigrain E. Drug policy in Europe Research and funding in neonates: current challenges, future perspectives, new opportunities. Early Hum Dev. 2011;87(Suppl 1):S27–S30. doi: 10.1016/j.earlhumdev.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Jadcherla SR, Klee G, Berseth CL. Regulation of migrating motor complexes by motilin and pancreatic polypeptide in human infants. Pediatr Res. 1997;42:365–369. doi: 10.1203/00006450-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Kadambari S, Heath PT, Sharland M, Lewis S, Nichols A, Turner MA. Variation in gentamicin and vancomcyin dosage and monitoring in UK neonatal units. J Antimicrob Chemother. 2011;66:2647–2650. doi: 10.1093/jac/dkr351. [DOI] [PubMed] [Google Scholar]
- 24.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 25.Langhendries JP, Maton P, François A, Marguglio A, Marion W, Smeets S, Philippet P. Implementation of the intestinal micro flora in the early stage and adequate immunity later on. Arch Pediatr. 2010;17(Suppl 3):S110–S118. doi: 10.1016/S0929-693X(10)70910-6. [DOI] [PubMed] [Google Scholar]
- 26.Langhendries JP, Battisti O, Bertrand JM, François A, Kalenga M, Darimont J, Scalais E, Wallemacq P. Adaptation in neonatology of the once-daily concept of aminoglycoside administration: evaluation of a dosing chart for amikacin in an intensive care unit. Biol Neonate. 1998;74:351–362. doi: 10.1159/000014053. [DOI] [PubMed] [Google Scholar]
- 27.Laughon MM, Benjamin DK, Jr, Capparelli EV, Kearns GL, Berezny K, Paul IM, Wade K, Barrett J, Smith PB, Cohen-Wolkowiez M. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4:643–652. doi: 10.1586/ecp.11.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79:33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- 29.McIntyre J, Choonara I. Drug toxicity in the neonate. Biol Neonate. 2004;86:218–221. doi: 10.1159/000079656. [DOI] [PubMed] [Google Scholar]
- 30.Meißner U, Chakrabarty R, Topf HG, Rascher W, Schroth M. Improved closure of patent ductus arteriosus with high doses of ibuprofen. Pediatr Cardiol. 2012 doi: 10.1007/s00246-012-0182-2. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed AF, Nielsen EI, Cars O, Friberg LE. Pharmacokinetic-pharmacodynamic model for gentamicin and its adaptive resistance with predictions of dosing schedules in newborn infants. Antimicrob Agents Chemother. 2012;56:179–188. doi: 10.1128/AAC.00694-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Patient Safety Agency, NHS. Neonatal services urged to follow new gentamicin safety guidance. 2010 www.npsa.nhs.uk/corporate/news. Accessed 15 Feb 2012.
- 33.Ramet J. What the paediatricians need—the launch of paediatric research in Europe. Eur J Pediatr. 2005;164:263–265. doi: 10.1007/s00431-005-1633-z. [DOI] [PubMed] [Google Scholar]
- 34.Rao SC, Srinivasjois R, Hagan R, Ahmed M. One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev. 2011;2011:CD005091. doi: 10.1002/14651858.CD005091.pub3. [DOI] [PubMed] [Google Scholar]
- 35.Reed MD. Reversing the myths obstructing the determination of optimal age- and disease-based drug dosing in pediatrics. J Pediatr Pharmacol Ther. 2011;16:4–13. [PMC free article] [PubMed] [Google Scholar]
- 36.Richey RH, Craig JV, Shah UU, Ford JL, Barker CE, Peak M, Nunn AJ, Turner MA. The manipulation of drugs to obtain the required dose: systematic review. J Adv Nurs. 2012 doi: 10.1111/j.1365-2648.2011.05916.x. [DOI] [PubMed] [Google Scholar]
- 37.Russell AB, Sharland M, Heath PT. Improving antibiotic prescribing in neonatal units: time to act. Arch Dis Child Fetal Neonatal Ed. 2012;97:F141–F146. doi: 10.1136/adc.2007.120709. [DOI] [PubMed] [Google Scholar]
- 38.Sato T, Wada K, Arahori H, Kuno N, Imoto K, Iwahashi-Shima C, Kusaka S. Serum concentrations of bevacizumab (Avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophtalmol. 2012;153:327–333. e1. doi: 10.1016/j.ajo.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Shinwell ES, Eventov-Friedman S. Impact of perinatal corticosteroids on neuromotor development and outcome: review of the literature and new meta-analysis. Semin Fetal Neonatal Med. 2009;14:164–170. doi: 10.1016/j.siny.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Sutherland MR, Yoder BA, McCurnin D, et al. Effects of ibuprofen treatment on the developing preterm baboon kidney. Am J Physiol Renal Physiol. 2012 doi: 10.1152/ajprenal.00216.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tosse V, Pillekamp F, Verde P, Hadzik B, Sabir H, Mayatepek E, Hoehn T. Urinary NT-proBNP, NGAL, and H-FABP may predict hemodynamic relevance of patent ductus arteriosus in very low birth weight infants. Neonatology. 2012;101:260–266. doi: 10.1159/000334826. [DOI] [PubMed] [Google Scholar]
- 42.Turner MA, Lewis S, Hawcutt DB, Field D. Prioritising neonatal medicines research: UK medicines for children research network scoping survey. BMC Pediatr. 2009;9:50. doi: 10.1186/1471-2431-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner MA. Neonatal drug development. Early Hum Dev. 2011;87:763–768. doi: 10.1016/j.earlhumdev.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 44.Van den Anker J. Use of aminoglycosides in preterm neonates: a simple task? J Pediatr. 2009;154:935. doi: 10.1016/j.jpeds.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Van den Anker JN, Allegaert K. Individualized dosing of aminoglycosides in neonates: mission accomplished or work in progress? Eur J Clin Pharmacol. 2009;65:1159–1160. doi: 10.1007/s00228-009-0688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Overmeire B, Smets K, Lecoutere D, Van de Broek H, Weyler J, Degroote K, Langhendries JP. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343:674–681. doi: 10.1056/NEJM200009073431001. [DOI] [PubMed] [Google Scholar]
- 47.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol. 2011;9:233–243. doi: 10.1038/nrmicro2536. [DOI] [PubMed] [Google Scholar]
- 48.Zonnenberg I, de Waal K. The definition of a haemodynamic significant duct in randomized controlled trials: a systematic literature review. Acta Paediatr. 2012;101:247–251. doi: 10.1111/j.1651-2227.2011.02468.x. [DOI] [PubMed] [Google Scholar]


