Abstract
Knowledge about the safe and effective use of medicines in neonates has increased substantially but has resulted in few label changes. Drugs developed for use in adults are reshaped and tailored to specific neonatal indications. However, the use of drugs in neonates should not only mirror adult pharmacotherapy, but should be driven by their own specific needs. Therefore, building collaborative networks may assist to develop a newborn-driven research agenda addressing their clinical needs and diseases.
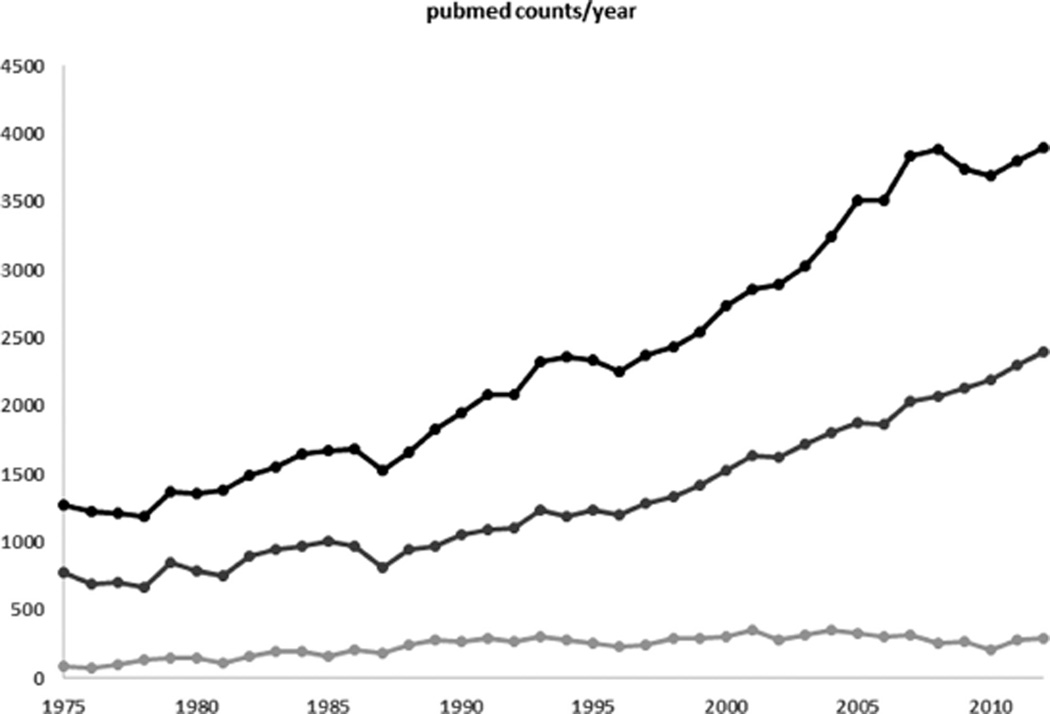
Drug therapy is a very powerful tool to improve both short- and long-term outcome in individual patients. This obviously also holds true in neonates and young infants. The ultimate goals of neonatal clinical pharmacology are to both predict proportional therapeutic effects (e.g., pain reduction, prevention, or treatment of an infection), as well as to avoid disproportional side effects (e.g., adverse drug events), thereby chasing the Holy Grail of “personalized, individualized, tailored” pharmacotherapy: prescribing the right compound integrated in the most appropriate, neonate-friendly formulation, and using the right dose in the right patient. We affirm that tremendous efforts have been made to improve the knowledge on neonatal drug therapy. This is also reflected in the increased scientific output as retrieved using a search for the number of hits for the words “newborn” and “pharmacotherapy, pharmacokinetics, or drug” from 1975 and 2013 (Figure 1).
Figure 1.
Number of annual/yearly (1975–2013) results (“hits”) in a PubMed search using the word “newborn” in combination with “pharmacotherapy,” “pharmacokinetics,” and “drug.” Compared to the 1975–1980 observations, there is a 2–4-fold increase in hits (black line “newborn and drug,” dark gray line “newborn and pharmacotherapy,” light gray line “newborn and pharmacokinetics”).
However, despite this increase in efforts and scientific output, we are not even close to finding this Holy Grail for neonatal drug therapy. At present, neonates and young infants are still regularly prescribed drugs in an off-label manner whereby dosing regimens of these drugs are simply derived from adult doses by adjusting for bodyweight and/or size. As developmental and maturational changes are complex processes, such simplified methods may result in subtherapeutic doses and lack of effect, or adverse toxic events. In addition to issues related to off-label use, suboptimal formulations (e.g., concentration, excipients), (poly)-pharmacy, immature organ functions, specific characteristics (e.g., preterm neonates at the threshold of viability) or treatment modalities (e.g., whole-body cooling), and difficulties to disentangle morbidity from adverse drug reactions (ADRs) add to the limited predictability of pharmacotherapy in early infancy.1
To improve the knowledge on ADR prevention and management, pharmacovigilance also needs to be adapted to neonates and infants. This includes, but is not limited to, strategies of error prevention (e.g., prescription, formulation, bedside manipulation, access), ADR signal detection through laboratory (e.g., population-specific reference laboratory values), or clinical outlier data signaling (overall high morbidity, how to detect a signal in the setting of extensive background noise?), or assessment through algorithm scoring (population specific compared to more common tools, e.g., Naranjo).2
To illustrate the relevance of such a more structured approach for ADR signal detection, we like to refer to the issue of postnatal dexamethasone exposure in neonates.3 Use of this potent drug resulted in shorter ventilation time, but had no effect on the incidence of bronchopulmonary dysplasia and actually resulted in an increase in neurodevelopmental impairment. This is due to a population-specific vulnerability. Unfortunately, it took many years before these negative long-term effects were recognized because the positive short-term outcome (i.e., extubation) delayed the evaluation of the impact of dexamethasone on neurodevelopmental outcome in former preterm neonates.3 Therefore, a more tailored pharmacovigilance strategy will likely result in improved knowledge on developmental toxicology, the most effective approach for secondary prevention, and improvement in neonatal pharmacotherapy.2
Obviously, if the mechanisms involved in compound-specific maturational toxicity (e.g., dexamethasone, oxygen) are understood, one can subsequently minimize drug toxicity in the future for the same, but likely also for similar compounds (e.g., high protein bound drugs and free bilirubin). Even for “obvious” compounds like oxygen, we are still in this cycle of pharmacovigilance, ADR signal detection, and a search for the mechanisms of underlying maturational toxicity.2 To illustrate this, we refer to the meta-analysis on optimal oxygenation in extreme low birth-weight infants (i.e., birthweight <1 kg) conducted by Saugstad and Aune.4 The relative risks (RRs) for mortality (1.14, 95% confidence interval [CI] 1.14–1.74) and necrotizing enterocolitis (1.25, 95% CI 1.05–1.49) were significantly higher, but for severe retinopathy of prematurity significantly lower (0.74, 95% CI 0.59–0.92) in preterm infants with a low (85–89%) vs. a high oxygen saturation target (91–95%). In contrast, there were no significant differences in bronchopulmonary dysplasia, brain injury, or patent ductus arteriosus between the low or high oxygen saturation target groups. Based on these results, the authors suggest that oxygen saturation should be targeted for the higher level (90–95%) in extreme low birthweight infants to reduce mortality at the price of a higher incidence of retinopathy of prematurity. However, it is still unknown whether this oxygen saturation should be constant throughout the entire postnatal period, or should be tailored to different stages of maturation (postnatal or postmenstrual age).4
In essence, neonatal drug therapy is as diverse and evolving as the individual neonate we are caring for: large intra- and interindividual variability is one of the main issues in neonatal medicine. In addition to, and even more relevant than mean or median estimates of clearance or effect, covariates of variability within the neonatal population are of utmost importance. As a consequence, improvements in neonatal pharmacotherapy should be driven by the integration of knowledge about the fast-evolving physiological characteristics of early infancy into pharmacotherapy. In addition to this knowledge integration, initiatives are needed to build research capacity through collaborative networks that will facilitate clinical research by covering aspects related to, e.g., safety (ADRs), efficacy, dosing, and formulations.1,5,6
WHY THE NEONATE IS THE LAST THERAPEUTIC ORPHAN
Despite the positive trends on scientific output illustrated in Figure 1, there are also reports that clearly indicate that effective knowledge (label changes, publication) about neonatal drug therapy is lagging behind when compared to other pediatric subpopulations.7 US and European initiatives such as the US Food and Drug Administration Safety and Innovation Act (FDASIA) and the Pediatric Regulation have resulted in significantly more pediatric studies, followed by an increase in labeling changes. Unfortunately, it seems that neonatal indications are not yet covered in this positive trend. Stiers and Ward7 recently reported that only a limited number of label changes (24/406, 6%) included drug labeling changes for neonates (1997–2010, FDA), claiming that “newborns were one of the last therapeutic orphans to be adopted.” Besides this limited number of changes, those authors also noticed that other labels were not changed despite the fact that some drugs such as intravenous acetaminophen, valganciclovir, caspofungin, or lansoprazole may be potentially very relevant compounds for use in young infants. These recently reported data (1997–2010) also illustrate the very limited progress made in label changes for neonates in recent years.7 Evaluating the studies conducted for pediatric exclusivity (US) between 1998–2004, Benjamin et al. already reported that only 31/253 (12%) included newborns. Although 115 therapeutic agents were evaluated in this time interval, therapeutic areas in neonates were limited to, e.g., pain/sedation (four studies), infectious diseases (10 studies), or gastrointestinal/reflux (eight studies) pathology.8
This disproportional underrepresentation and evaluation of neonates in pediatric studies has recently also been highlighted in the European setting of Pediatric Investigation Plans (PIPs).9 In the European Union (EU), the development of a new medicine should follow an agreed PIP. Dose finding thereby is a crucial issue due to the variability in drug disposition throughout pediatric life. Despite the fact that this variability is most pronounced in early infancy, Hampson et al. concluded that many (41%) of the studies (time interval = 2010–2012) were in fact dose-evaluating in nature rather than dose-finding. Moreover, only a minority of these programs included neonates. Even more relevant for this review, the median number of patients included in these neonatal pharmacokinetic (PK) studies was three for orphan and six for nonorphan drugs, with a very limited median number of samples collected in an individual neonate (orphan = 2–3 samples/case; nonorphan = 3–5 samples/case).9 The median number of patients in other pediatric cohorts (2–11 and 12–17 years) was 12 and 10 for orphan, 14 and 18 for nonorphan drugs.9 This confirms a similar pattern as reported by Pansieri et al.10 Among all trials (n = 138,948) registered within the Clinicaltrials.gov dataset, 22% (n = 5 30,912) were pediatric trials, but only 0.2% (n = 288) involved neonates. There was a 4-fold increase in pediatric studies from 4,328 to 16,275 between 1999 and 2012. In contrast, there was only a disappointing catch-up in the absolute number of studies in neonates from 32 to 190 trials. All therapeutic classes were, however, represented in the neonatal studies including the cardiovascular system, the central nervous system, and anti-infective drugs. We noticed the limited presence of “industry” as a sponsor (23%) when compared to pediatric (41.4%) or adult studies (65%).9 The majority are single-center studies (n = 164, 58%), and there is a modest catch-up of EU studies compared to those located in the US during the last 2 years in the analysis (2011 = 37 US to 22 EU studies; 2012 = 27 US to 24 EU studies), mainly driven by a decrease in US studies.10
WHAT MAKES NEONATAL CLINICAL PHARMACOLOGY DIFFERENT FROM PEDIATRIC CLINICAL PHARMACOLOGY
Aiming at a moving target
Tailored drug choice and dosing regimens should reflect the (patho)physiologic characteristics of the population considered and be adapted to the specific characteristics of the individual patient. Neonates are the subgroup of children from birth up to 28 days of postnatal age, or the equivalent maturational age (44 weeks postmenstrual age), and cover both preterm (<37 weeks gestational age at birth) and term neonates. They represent a particularly vulnerable subgroup within the pediatric population. This is because the fast developmental changes in early infancy result in extensive variability in both PK and pharmacodynamics (PD), including safety and adverse drug events. This extensive variability is not specific to neonatal drug therapy, but is the essence of early infancy.1,5,6 To further illustrate this, there is at least one log size difference in weight (<0.5 kg and up to 5 kg) in neonates at admission. Despite the initial weight loss in the first 8 to 10 days of postnatal life, bodyweight will increase by about 50% in the first 6 weeks of life, doubles by 3 to 4 months of age, to be 3 times higher at the end of the first year of life. Similarly, the growth rate in the first trimester of postnatal life is significantly higher when compared to the growth spurt during puberty (10 to 20 cm/year as compared to 5 to 10 cm/year). All the above-mentioned indicators (birthweight, weight gain, growth rate, body proportions, e.g., liver size) are reflections of a very dynamic, evolving biological system driven by maturation or ontogeny and results in extensive variability in drug disposition (PK) and effects (PD).1,5,6
Ontogeny hereby refers to the maturation or development of the expression of all genes involved in drug absorption, drug disposition, drug metabolism or drug elimination (ADME) with time (postnatal, gestational, or postmenstrual age), or weight as main covariates. All these genes, and related enzymes, have their specific maturation pattern of phenotypic activity.1,5,6 This ontogeny-driven, time-varying physiology is further aggravated by additional covariates like genetic variation or environmental (drug–drug, drug–nutrition, drug–treatment modalities, disease) characteristics. Although the topic of modeling and simulation in pediatric drug therapy is not covered in this review, but discussed elsewhere in this issue on pediatric pharmacology, we still would like to draw attention to a recent attempt to integrate this time-varying physiology of early infancy as compared to later pediatric life into physiologically based (PBPK) model-building approaches. In general, growth and maturation are considered in such models based on size and maturation of pathways of metabolic or elimination clearance. These covariates are generally fixed throughout a study. However, in the setting of fast maturation in early infancy, redefinition of these covariates during the study is warranted. Based on a dataset on sildenafil disposition, Abduljalil et al. illustrated that the resampling time in the first day of life is hourly, to increase to every 6 hours in the second part of the first week of postnatal life to every 48 hours after the first month of life11.
Maturation: the need for integration
Newborns differ from other populations, and also in their drug response. Maturational changes in early infancy can significantly affect PK processes, but also PD. The PK processes considered are absorption, distribution, metabolism, and excretion (ADME), while PD comprises the physiological and biological response to a given compound, and represents both efficacy and safety. 1,12 In essence, drug absorption is affected by age, formulation, dose, route of administration, as well as, e.g., food or other drugs that interact. Specific issues are gastric acid production, skin permeability, and gut permeability, including first-pass effects and the ontogeny of intestinal transporters. Differences in the distribution of the drug relate to body composition, blood flow, protein binding, and membrane permeability. The body composition in early infancy evolves, resulting in an age-dependent proportion of body water and fat. Drug metabolism is generally low in neonates and age-driven ontogeny of drug-metabolizing enzymes is the main contributor to maturational PK.14 Finally, renal elimination capacity (glomerular filtration rate, GFR) increases in the first 2 weeks of life to reach adult values at the end of infancy. Premature infants show similar trends, with a slower postnatal rise. Active tubular secretion and tubular reabsorption are also immature at birth (20–30% of adult values), reaching adult values after 6 months.15,16 The aspects of maturational PK (absorption, distribution, metabolism, excretion) have been reviewed regularly,1,4–6,12,13 but we would like to stress the need to integrate these different maturational processes in order to predict the concentration time profiles in individual patients. The maturational pattern of the individual renal or hepatic elimination processes may differ and this necessitates to integrate the ontogeny related knowledge of different elimination routes (metabolic vs. elimination clearance) to predict compound-specific, concentration–time profiles in neonates: there is no isolated neonatal liver or neonatal kidney, but newborns are in need of improved predictability.6
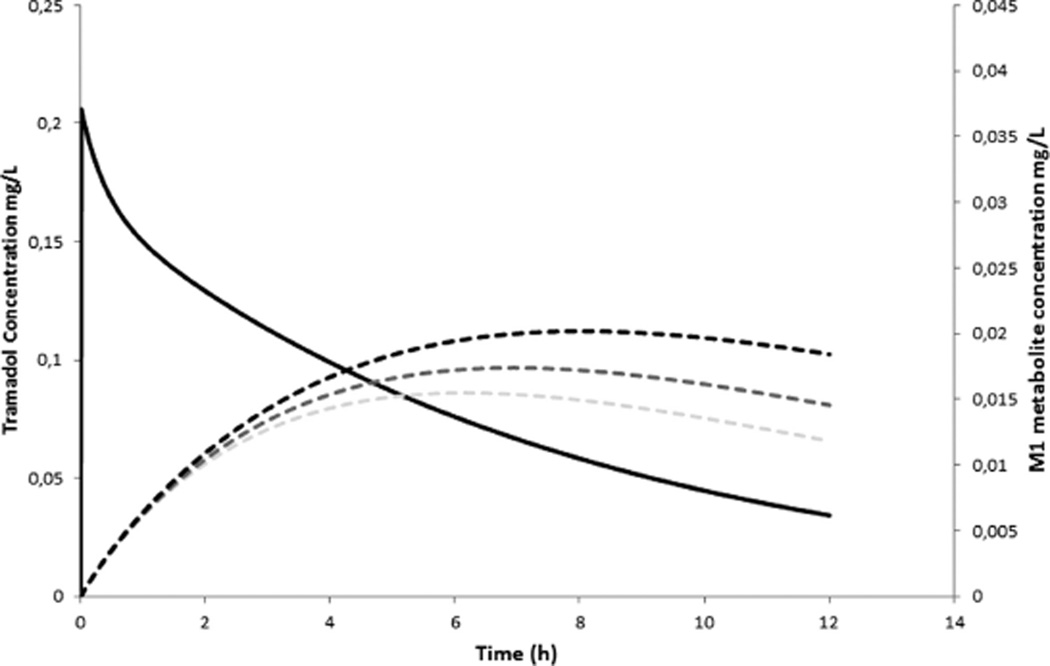
To illustrate this, we refer to published work on tramadol disposition in newborns. Tramadol (M) undergoes O-demethylation through cytochrome P450 (CYP) 2D6 to O-demethyl tramadol (M1, metabolic clearance) with subsequent GFR-driven renal elimination clearance of M1 or primary M elimination. It turned out that the ontogeny of CYP2D6 is faster compared to GFR ontogeny, resulting in relevant M1 plasma concentrations, not so much because of very high phenotypic CYP2D6 activity, but likely due to “delayed” elimination clearance capacity.6 In Figure 2, we illustrate the impact of a reduction in renal function with 20 or 40% (similar to the impact of ibuprofen or indomethacin coadministration) on the M1 metabolite profile following intravenous tramadol (1 mg·kg−1, bolus) administration.6
Figure 2.
The impact of a reduction in renal function with 20 or 40% (similar to the impact of ibuprofen or indomethacin coadministration) on the M1 metabolite profile following intravenous tramadol (1 mg.kg−1, bolus) administration in a term newborn (RF = renal function, M1 = O-demethyltramadol).6
Developmental PD can only be considered after developmental PK have sufficiently been studied. Developmental PD is the study of age-related maturation of the structure and function of biologic systems and how this affects response to pharmacotherapy. This may manifest as a change in the potency, efficacy, or therapeutic range of a drug.17 The available illustrations in neonates are limited, but, e.g., maturational toxicity of aminoglycosides likely relates to megalin and cubilin ontogeny.18 The PD of aminoglycosides are in part unrelated to age (e.g., bacterial resistance, target concentrations), while other aspects (nephro- and ototoxicity, colonizing intestinal microbiota) display infancy-specific patterns that may be either protective or make this population more vulnerable. Just like renal immaturity has an impact on drug handling, this immature kidney may undergo further impairment after early drug exposure.16,19 This is because in preterm neonates, glomerulogenesis becomes a postnatal instead of an intrauterine event. A more restricted renal development results in a lower number of nephrons that may have several long-term effects, such as hypertension, albuminuria, and renal failure.16,19 The developing brain displays enhanced susceptibility for seizures during early infancy due to receptor (e.g., gammaaminobutyric acid [GABA] excitatory/inhibitory, glutamate driven receptors) specific ontogeny and function.20
Wang et al. recently provided evidence that the accuracy of prediction of neonatal pharmacokinetics when based on prior data using population PK modeling to select a first dose in neonates indeed depends on the type of source data used during such an analysis.21 Using median average fold prediction for clearance (CLpredicted/observed), the bias for neonatal PK estimates was reduced when prior PK data also included observations in young infants as opposed to an assessment without PK data in young infants. The inclusion of infant data reduced the median biases, while the extent of range errors remained similar. The integration of data in young infants (>28 days) to predict neonatal PK resulted in a more equal probability of both over- or underprediction. In contrast, a more limited analysis (data in children >2 years) commonly resulted in overprediction of neonatal clearance. Interestingly, within-population differences in prediction errors between either preterm or term neonates were relatively small.21 This does suggest that besides maturational and weight-driven changes (“ontogeny”), other covariates like pharmacogenetics or environmental factors further affect the PK/PD variability in early infancy.
Pharmacogenetics: newborns are not just small adults
The aim to individualize pharmacotherapy using pharmacogenetics (PGx) reflects the fact that specific (side) effects are not just randomly distributed, but relate to genetic variation at the level of transporters, drug metabolizing enzymes, or receptors. This concept obviously also seems promising to further tailor neonatal pharmacotherapy.22,23 The most commonly applied approach to evaluate PGx in neonates is to search for similar signals initially reported in adults or children. This “from adult to newborn” approach turned out to be fruitful to document the impact of phase I iso-enzymes (e.g., cytochrome P450 (CYP) C219 (pantoprazole dealkylation), CYP2D6 (tramadol O-demethylation)) or phase II iso-enzymes (e.g., N-acetyl transferase (NAT)2 (isoniazide acetylation) and UDP-glucuronosyltransferase (UGT) 2B7 (morphine glucuronidation)) polymorphisms.22,24–26
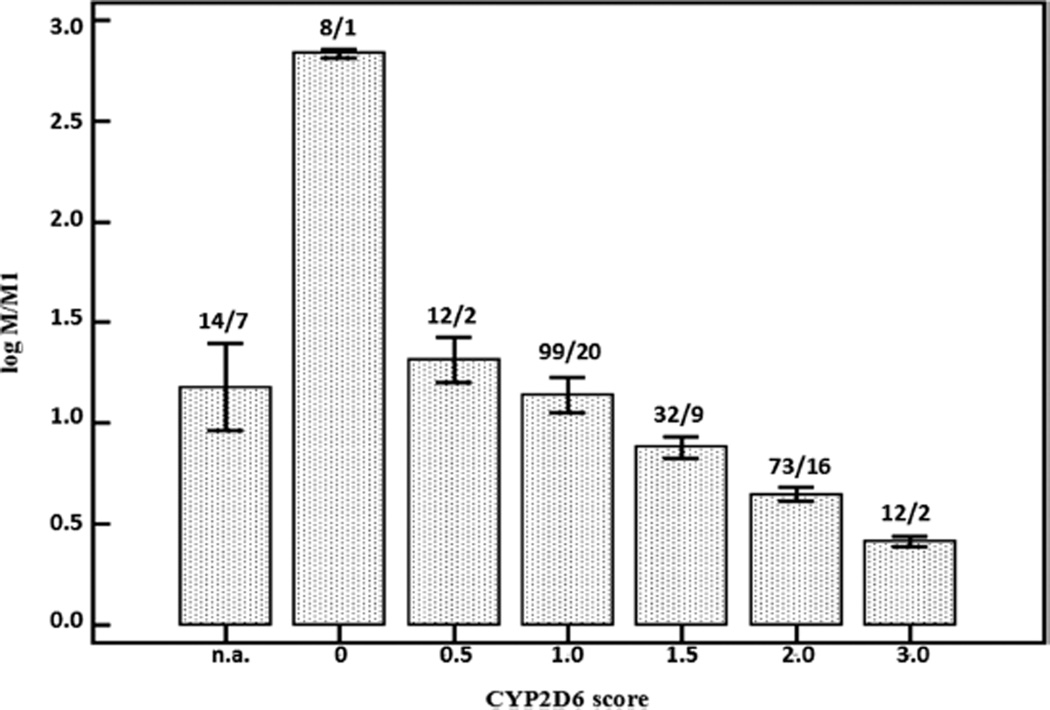
In Figure 3, we illustrate the impact of the CYP2D6 activity score on tramadol disposition in early infancy. The CYP2D6 activity score hereby aims to translate genotype information (genetic variation) into a qualitative measure of phenotype.27
Figure 3.
Plasma log M/M1 values (mean, ±SEM) in a dataset of 250 observations in 57 neonates and young infants strongly depend on the CYP2D6 activity score during continuous intravenous tramadol administration (n.a. = no data on the individual CYP2D6 activity score available).24
In a dataset of 57 cases that were treated with continuous intravenous tramadol because of medical or postoperative analgesia, there was a significant decrease in the plasma log value of tramadol/O-demethyl tramadol (log M/M1) with increasing CYP2D6 activity score, reflecting higher phenotypic CYP2D6 activity, equal to higher metabolic clearance to M1 in neonates with a higher CYP2D6 activity score.24,28 In a forward multiple regression model we concluded that postmenstrual age and CYP2D6 polymorphisms determined O-demethylation activity in (pre)term neonates and young infants.24
However, PGx should also be tailored to neonates, and not just mirror adult observations. PGx studies should go beyond confirmations of the impact of a given polymorphism on phenotypic drug metabolism. These types of studies may also provide information on the ontogeny of not yet well-described processes, like drug transporter or receptor ontogeny. A recent illustration of the first approach is the impact of polymorphisms on the severity of neonatal abstinence syndrome following maternal opioid intake.29 While the median length of stay for term neonates was 35 days, specific cathechol-O-methyltransferase (COMT, 158A>G) and µ-opioid receptor (OPRM1, 118A>G) polymorphisms were associated with a relevant reduction of this length of stay with 10.8 and 8.5 days, respectively. Finally, PGx studies may also focus very specifically on the relationship between genotype/phenotype within cohorts of newborns and young infants.22 A systematic approach for initial evaluation of the contribution of ontogeny and genetic variation to variability in PK/PD, including toxicity, has been suggested, and is based on five questions: 1) What gene products (if known) are important for the disposition of a given compound? 2) What is the developmental trajectory (if known) of functional (e.g., enzymatic, transporter) activity? 3) Does allelic variation affect functional activity? 4) Does allelic variation affect the developmental drug disposition phenotype? and finally 5) What is the developmental context in which the genes of interest are operating in?30
It may be worth exploring, e.g., the association described between acetaminophen exposure during infancy and the subsequent higher risk to develop pediatric asthma and atopy based on this methodology, or to explore the impact of genetic variation in CYP3A7, which is mainly active in perinatal life with a subsequent decreasing activity after birth. To illustrate the potential relevance of such an approach, we refer to the impact of variation in metabolic capacity on outcome (neonatal respiratory distress) after antenatal corticosteroid use. Based on observations collected in 109 women and 117 infants, associations between polymorphisms and subsequent neonatal respiratory outcome were documented for a fetal single nucleotide polymorphism CYP3A7*1E (odds ratio [OR] 23.68) and for the maternal CYP3A5 expresser status (CYP3A5*1/*1 and *1/*3 subjects, OR 1.63).31
Environmental factors
Drug choice and dosing should reflect the physiological characteristics of the population, further adapted to the specific pathophysiological characteristics of the individual patient. This means that the above-mentioned covariates (age, size/weight) driven maturational trends are further affected by environmental covariates. Such environmental covariates are either disease characteristics or the medical interventions related to these diseases (e.g., whole-body cooling following perinatal asphyxia, extracorporeal membrane oxygenation (ECMO) to treat severe circulatory or respiratory failure, critical illness, or nutritional strategy).5,32 The impact of these environmental covariates on compound-specific disposition or effects may become highly relevant and can be better quantified when integrated in a covariate analysis. We aim to highlight some of these environmental covariates to illustrate the potential interaction between treatment modality, disease, and individualized pharmacotherapy.
Whole-body cooling is a valid and effective treatment modality in (near)term neonates following moderate to severe perinatal asphyxia. The body temperature aimed at for the duration of 72 hours is 33.5°C, with subsequent stepwise rewarming towards normothermia. This treatment modality may affect physicochemical properties, and the PK/PD of specific drugs.33 These aspects are covered by thermopharmacology, a term recently introduced into neonatal pharmacology by van den Broek et al.34 However, in current practice hypothermia is an intervention restricted to neonates with peripartal asphyxia and this disease characteristic in itself also affects PK/PD. Although only still based on a limited number of observations, it seems that the primary renal clearance is not further affected when hypothermia is initiated. In contrast, drug metabolism is reduced. In addition to PK effects, there are also PD-related effects since the transition rate from a continuous normal voltage to discontinuous normal voltage electroencephalographic background level also depends on body temperature.34 Similar to the use of hypothermia, neonates who qualify for ECMO are critically ill, with circulatory and/or respiratory failure. Although it seems reasonable to assume that this critical illness in itself will affect PK/PD of drugs, the introduction of an ECMO circuit likely also affects the disposition of drugs.34,35 Most studies unveil an altered volume of distribution (due to, e.g., higher body water content, lower plasma proteins, additional extracorporeal circuit). However, clearance may even increase due to compound-specific absorption in the ECMO circuit and relates to the lipophilicity of the product (e.g., fentanyl or propofol compared to aminoglycosides or acetaminophen).35
However, it is too simple to assume a priori that critical illness or sepsis will always result in a reduced clearing capacity. At least in adults, augmented renal clearance is a common finding in critically ill patients receiving antimicrobial therapy and is associated with worse clinical outcome.36 We are unaware of any similar explorations on the presence of augmented renal clearance in neonates. In contrast, Ince et al. reported that critical illness was the major determinant of interindividual variability in midazolam clearance in children from 1 month old onwards. Compared to healthy infants or pediatric oncology patients who were treated with midazolam for procedural sedation, midazolam clearance capacity in pediatric intensive care patients was 80–90% lower.37 Inflammatory conditions associated with elevated cytokines may explain this phenomenon by “phenoconversion,” a setting with transient conversion of genotypic extensive to phenotypic poor metabolizers.38 Whenever studied in infants, this will necessitate age-dependent references on phenotypic activity to subsequently explore within-population variability and its covariates.
As a final illustration, it has been reported that the type of feeding (mother’s milk or formula) is one of the covariates that affects the intestinal bacterial flora as well as drug metabolism. The predominance of lactobacillus in the gut microflora of breast-fed infants is thought to be due to the oligosaccharides in human breast milk. In contrast, a more adult-type intestinal microbiota is found when the infant is formula-fed. Formula feeding accelerates maturation of CYP3A4 or CYP1A2.32,39 It is tempting to link both events and speculate that the intestinal microbiome affects drug metabolism and outcome during infancy. To further illustrate this, we refer to the maturational capacity to synthesize vitamin K by the intestinal flora, while there is also mounting evidence in support of early enteral supplementation of probiotics (Lactobacillus, Bifidobacterium) to prevent severe necrotizing enterocolitis and all-cause mortality in preterm infants.40 Other covariates of neonatal gut colonization are prebiotic formula feeding (diversity of bifidobacterium strains), or oligosaccharides, perinatal antibiotics or acid-blocking agents (impact on the incidence of necrotizing enterocolitis and on the developing microbiome).41,42
NEONATAL FORMULATIONS: THERE IS MORE THAN JUST THE ACTIVE COMPOUND
The overall lower clearance and the extensive variability within the neonatal age range should be translated to a clinical need for formulations with low, adjustable, and flexible dosing in early life to maintain dose accuracy. Commonly administered formulations in this population are either liquids (e.g., drops, syrup) or vials for intravenous administration. For the enteral route, specific issues in neonates are simultaneous administration with milk or interactions with the plastics of the feeding tube. Most vials for intravenous administration contain relatively high concentrations of the active compound, inadequate for neonates or infants. Tailored vials for intravenous administration, resulting in appropriate, flexible, and correct dosage of drugs in neonates are needed.43–46 Besides the fact that 10-fold errors are more common when “highly” concentrated formulations are used, dosing inaccuracy can also be reduced with more tailored formulations.44,45
Marketed formulations do not always meet the specific requirement of all patient groups, especially not for use in neonates. As a consequence, it is likely that compounding practices will remain common practice.46 Nunn et al. quantified the practice of medicines manipulation to provide accurate doses for children, including neonates in one regional children’s hospital.43 Based on 5,375 drug administration events recorded, about 10% were judged to require manipulation or needed a small dosing volume (<0.2 mL). Measured doses below 0.1 mL accounted for 25% of the manipulations, and this was most common in the neonatal intensive care unit (60%).43 Nonauthorized (i.e., information not included in the Summary of Product Characteristics (SmPC)) compounding or manipulation thereby is still very common, although the quality of compounding and excipient ingredients may vary considerably. An illustration of the need to validate such compounding practices is the evaluation of a pediatric oral formulation with a low proportion of hydrochlorothiazide, also suited for use in neonates. Santovena et al. thereby illustrated that only one of five suspensions of hydrochlorothiazide (2 mg/mL) guaranteed drug stability and correct dose administration after 3 weeks of storage in predefined conditions.47 Until tailored formulations make it to the market, compounding practices for drug formulations in neonates should be evaluated to guarantee correct dosing, product stability, safety, and to support pharmacists in their daily practice.46
An issue that warrants focused attention in neonatal formulation is the kind and extent of excipients used. The United States’ National Formulary refers to an excipient as “everything in the formulation in addition to the active compound(s).”48 It is any substance that is used as vehicle or additive for administering drugs in the suitable consistency or formulation. Excipients are added, e.g., to ensure stability over a given shelf life, to improve palatability, or to facilitate solubility or to bulk up formulations that otherwise contain highly potent active ingredients and are referred to as preservatives, sweeteners, fillers and solvents, coating materials, or coloring agents.48 The exposure to potentially toxic excipients in medicines is not rare, as they are present in many commonly used drug products in neonates. In essence, the issue of maturational PK and PD is not limited to the active compounds, but also the PK and PD of these excipients should be evaluated in neonates.
Historical observations, but also more recent observations on, e.g., Kaletra (lopinavir/ritonavir, human immunodeficiency virus treatment) syrup confronted clinicians with unanticipated, but sometimes predictable side effects of neonatal drug formulations merely because of the excipients coadministered (for Kaletra: propylene glycol and ethanol).49 A recent European observational study quantified the exposure of neonates to eight potentially harmful excipients in a single-day point prevalence study. Based on observations collected in 21 countries, 89 neonatal intensive care units, and 726 neonates, the authors confirmed the exposure to excipients in 63% of the neonates and 31% of the prescriptions.50 The available knowledge on the safety or toxicity of excipients is difficult to retrieve, but the Safety and Toxicity of Excipients for Pediatrics (STEP) database initiative is trying to improve this situation.51 The European and United States Pediatric Formulation Initiatives thereby have codeveloped this STEP database with the aim to centralize any available excipient safety and toxicity data.51 In addition, population-focused studies on aspects of clinical pharmacology of excipients in neonates have been performed and generated new information. The propylene glycol research project and the European Study for Neonatal Excipient Exposure (ESNEE) initiative illustrate the feasibility to report on PK and PD observations for specific excipients (propylene glycol, methyl-, and propyl-parabens).52–54
NEONATAL MEDICINES RESEARCH: OPTIMISM IS A MORAL DUTY
Some of the hurdles and challenges to conduct clinical research on neonatal pharmacotherapy are provided in Table 1. Despite legal initiatives taken in the US and, more recently, in Europe to stimulate pediatric drug research, the available knowledge on neonatal clinical pharmacology is lagging behind and has not resulted in a significant number of label changes. Failed labeling is even the case for drugs that may be potentially very relevant in young infants.7 These include intravenous acetaminophen, valganciclovir, caspofungin, and lansoprazole.7 The absence of label changes was mainly because a relevant portion of studies were unable to document efficacy in newborns. McCune and Mulugeta recently stressed that it is unclear whether this absence of efficacy is indeed correct and accurate, or secondary to study design issues including the study population, disease characteristics in the newborn, drug properties (e.g., dose and formulation, drug metabolism), or the inability of the selective PD endpoints to reflect clinically meaningful outcomes.55
Table 1.
Some of the hurdles and challenges to conduct clinical research in neonatal pharmacotherapy
| Circumstantial issues related to studies |
| • Ethics, parental consent (e.g., during pregnancy, information strategy) |
| • Study facilities (investigators, research facilities) |
| • Recruitment strategies, the need for multicenter collaboration |
| • Perceived risks and fear of negative outcomes, perceptions of society |
| • Drug development programs initially develop for other populations, and subsequently fitted to the neonatal population, not primary driven by neonatal diseases and needs |
| • Neonatal drug therapy development is a perceived "must," instead of an opportunity |
| Pharmacokinetics/pharmacodynamics |
| • Sample collection (limit number and volume), specific analytical techniques |
| • Population pharmacokinetic modeling (mechanism, physiology based) not always sufficiently validated to support study design and sampling strategy, and uncertainty about extrapolation |
| • Extensive variability in pharmacokinetics/pharmacodynamics within the neonatal population |
| • How to assess efficacy? Robust and relevant pharmacodynamics endpoints are needed. Neurodevelopmental outcome is most relevant, but cannot reliably be done in early infancy |
| • Data on formulation, including stability and compatibility (e.g., human milk, other drugs, parenteral nutrition) |
| • Safety: how to assess (serious) adverse reactions when overall morbidity is already high? |
The neonatal study population is by definition not a homogeneous population, with potential impact of birthweight, postnatal age, gestational age, or postmenstrual age (gestational age + postnatal age, in weeks) on drug disposition and subsequent effects. The same holds true for disease characteristics, like hyaline membrane disease, patent ductus arteriosus, retinopathy of prematurity, or hypotension. These diseases are not just dichotomous (present/absent), but display differences in severity with inter-rater variability in its assessment. Dose selection and administration based on suboptimal formulations remain challenging. In relation to pharmacodynamics, Zhang and Schmidt provided a systematic review on the primary outcomes used in neonatal randomized controlled trials. The authors documented that trials with a discrete primary outcome were often short-term and designed to detect only large risk reductions.56 There are, however, very nice examples of successful trials. The Caffeine for Apnea of Prematurity trial is such an example. Based on a randomized controlled trial in 2006 preterm infants, it was documented that caffeine therapy of apnea of prematurity indeed reduces the rates of cerebral palsy and cognitive delay at 18 months of age, but is no longer associated with a significantly improved rate of survival without disability at the age of 5 years.57 Other trials that had a major impact on neonatal pharmacotherapy are, e.g., the whole-body cooling studies to improve the neurodevelopmental outcome after peripartal asphyxia. As mentioned earlier, this generated a whole new field of thermopharmacology.5,32,34
In addition to the above-discussed study design-related issues, there is also a more fundamental aspect. Compared to or in addition to the most common approach of a product development plan, i.e., reshaping and tailoring dosing and indications to neonates of products initially developed for clinical needs in mainly adult populations, product development plans driven by a newborn-focused research agenda with specific emphasis on their needs and diseases is promising. This can be considered a drug development disconnect: the majority of drugs currently used are off-label, and very few new therapies are being developed especially for neonates, in contrast to a pediatric investigation plan that includes neonates.
Such a newborn-focused research agenda has recently been put forward at a workshop (Roadmap for Applying Regulatory Science to Neonates, October 2014) co-organized by the FDA, the Critical Path Institute (C-Path), and the Burroughs Wellcome Fund (http://www.fda.gov/drugs/NewsEvents/ucm410863.htm).58 Based on the shared opinion of different stakeholders, a neonatal research agenda should cover prevention and treatment in neonates of brain injury (asphyxia, stroke, seizures in term neonates, intraventricular hemorrhage, white matter injury in preterm neonates), lung injury (persistent pulmonary hypertension of the newborn, bronchopulmonary dysplasia and associated pulmonary hypertension), gastrointestinal injury (necrotizing enterocolitis), sepsis, retinopathy of prematurity, and neonatal abstinence syndrome.
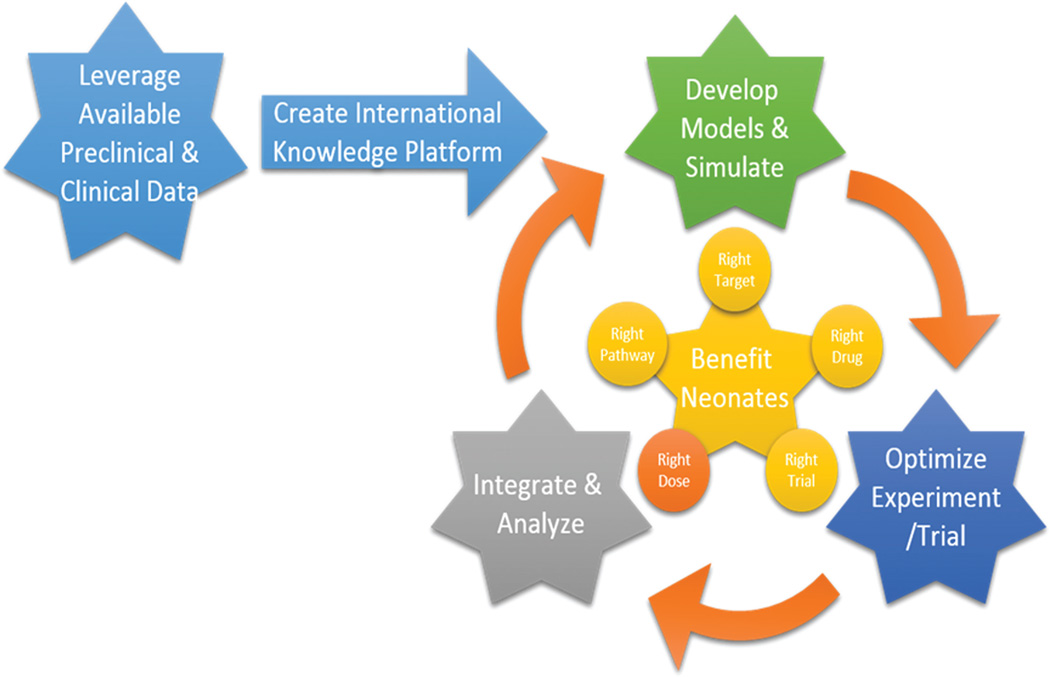
A roadmap to innovative drug development in neonates
In essence, the proposed roadmap can be summarized as a call to develop the right drug (right pathway, right target), which needs to be evaluated in the right population, based on the right dose, using the right trial design, and focusing on the right endpoints (Figure 4).
Figure 4.
A roadmap to innovative drug development in neonates: the right drug (right pathway, right target), for the right population, using the right dose, based on the best trial design, and evaluated using the right endpoints.
“Leverage Available Preclinical and Clinical Data” refers to the fact that we do not yet use the available information sufficiently well. Somewhat similar to the above-mentioned STEP database, this relates to knowledge building on, e.g., juvenile animal models, in vitro models, and in vivo observations.51 Juvenile animal models and in vitro models can be used to explore pathways and targets involved in neonatal pathophysiology. For the in vivo part, opportunistic data gathering from neonates already exposed to given drugs or compounds (e.g., PharmaCool study, excipient studies) as standard of care can be considered or databases of drug utilization, safety, and efficacy can be explored.52–54,59 Electronic registries might be of great use but this will, first of all, necessitate standardization and harmonization of, e.g., definitions or outcome criteria.
“Develop Models and Simulate” refers to the growing knowledge of modeling and simulation. Drug modeling can be considered a success when such an exercise supports a decision, e.g., initial dose, tailored individualized dosing in the clinical setting, or anticipate the extent of drug–drug interactions. Regulatory bodies also encourage the use of modeling and simulation methods for any pediatric drug development program, as also reflected in a number of regulatory guidance documents.57,60,61 The major advantage is that a study becomes confirmatory instead of exploratory, and thereby reduces the burden for every individual patient recruited and makes a robust conclusion more likely, especially when combined with an optimal experiment/trial design. Besides the issue of adaptive study designs, clinically relevant endpoints will need to be developed by the neonatal research community to avoid the issue of short-term outcome variables mentioned earlier.56 Safety monitoring is an obvious priority, but differentiation from confounding disease processes and concomitant drug exposure will necessitate the availability of standardized registries. The above-mentioned discussion on the effect/side effect profile of oxygen illustrates the complexity.4 Once clinically relevant endpoints have been defined, predictive and prognostic biomarkers (e.g., brain imaging, renal function assessment) can be developed, validated, and can subsequently be considered for regulatory acceptance.62
The above-mentioned roadmap may look ambitious but is not new, since there are some recent examples in the field of rare (pediatric) diseases that should encourage us to proceed with this strategy. Within pediatrics, important progress in the pharmacotherapy of cystic fibrosis or neuromuscular diseases have been made using a similar roadmap concept.63,64 Similar to these illustrations, this will necessitate the support by stakeholders such as regulators, clinical research networks, the Critical Path Initiative, academia, and industry, but even more relevant, the parents as representatives of the patients.
CONCLUSION
In recent years major strides have been made to improve the safe and effective use of medicines in the neonatal population. The combined efforts of the FDA and EMA, the renewed “mandated” activities by the pharmaceutical companies, old and new neonatal networks across the world, and parent/patient organizations have resulted in a revival of interest in the last therapeutic orphans in recent years. Despite these major improvements there is still a huge lack of appropriate labeling of drugs used in the neonatal period.
ACKNOWLEDGMENTS
Dr Karel Allegaert is supported by the Fund for Scientific Research, Flanders (fundamental clinical investigatorship 1800214N) and the research activities are further facilitated by the agency for innovation by Science and Technology in Flanders (IWT) through the SAFEPEDRUG project (IWT/SBO 130033). Dr John van den Anker is supported by the NIH (K24DA027992, R01HD048689, U54HD071601) and the European Commission (TINN (223614), TINN2 (260908), NEUROSIS (223060)). Special thanks goes to Dr Marc Pfister for designing Figure 4.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 2.Allegaert K, van den Anker JN. Adverse drug reactions in neonates and infants: a population-tailored approach is needed. Br. J. Clin. Pharmacol. 2014 doi: 10.1111/bcp.12430. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shinwell ES, Eventov-Friedman S. Impact of perinatal corticosteroids on neuromotor development and outcome: review of the literature and new meta-analysis. Semin. Fetal Neonatal Med. 2009;14:164–170. doi: 10.1016/j.siny.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Saugstad OD, Aune D. Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation target studies. Neonatology. 2014;105:55–63. doi: 10.1159/000356561. [DOI] [PubMed] [Google Scholar]
- 5.de Wildt S, Tibboel D, Leeder JS. Drug metabolism for the paediatrician. Arch. Dis. Child. 2014;99:1137–1142. doi: 10.1136/archdischild-2013-305212. [DOI] [PubMed] [Google Scholar]
- 6.Allegaert K, van de Velde M, van den Anker J. Neonatal clinical pharmacology. Paediatr Anaesth. 2014;24:30–38. doi: 10.1111/pan.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stiers JL, Ward RM. Newborns, one of the last therapeutic orphans to be adopted. JAMA Pediatr. 2014;168:106–108. doi: 10.1001/jamapediatrics.2013.4604. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin DK, Jr, et al. Peer-reviewed publication of clinical trials completed for pediatric exclusivity. JAMA. 2006;296:1266–1273. doi: 10.1001/jama.296.10.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hampson LV, Herold R, Posch M, Saperia J, Whitehead A. Bridging the gap: a review of dose investigations in paediatric investigation plans. Br. J. Clin. Pharmacol. 2014;78:898–907. doi: 10.1111/bcp.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pansieri C, Bonati M, Choonara I, Jacqz-Aigrain E. Neonatal drug trials: impact of EU US paediatric regulations. Arch Dis. Child. Fetal Neonatal Ed. 2014;99:F438. doi: 10.1136/archdischild-2013-305900. [DOI] [PubMed] [Google Scholar]
- 11.Abduljalil K, Jamei M, Rostami-Hodjegan A, Johnson TN. Changes in individual drug-independent system parameters during virtual paediatric pharmacokinetic trials: introducing time-varying physiology into a paediatric PBPK model. AAPS. J. 2014;16:568–576. doi: 10.1208/s12248-014-9592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smits A, Kulo A, de Hoon JN, Allegaert K. Pharmacokinetics of drugs in neonates: pattern recognition beyond compound specific observations. Curr. Pharm. Des. 2012;18:3119–3146. doi: 10.2174/1381612811209023119. [DOI] [PubMed] [Google Scholar]
- 13.Mooij MG, de Koning BA, Huijsman ML, de Wildt SN. Ontogeny of oral drug absorption processes in children. Expert Opin. Drug Metab. Toxicol. 2012;8:1293–1303. doi: 10.1517/17425255.2012.698261. [DOI] [PubMed] [Google Scholar]
- 14.Hines RN. Developmental expression of drug metabolizing enzymes: impact on disposition in neonates and young children. Int. J. Pharm. 2013;452:3–7. doi: 10.1016/j.ijpharm.2012.05.079. [DOI] [PubMed] [Google Scholar]
- 15.Rhodin MM, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr. Nephrol. 2009;24:67–76. doi: 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
- 16.Schreuder MF, Bueters RR, Allegaert K. The interplay between drugs and the kidney in premature neonates. Pediatr. Nephrol. 2014;29:2083–2091. doi: 10.1007/s00467-013-2651-0. [DOI] [PubMed] [Google Scholar]
- 17.Mulla H. Understanding developmental pharmacodynamics: importance for drug development and clinical practice. Paediatr. Drugs. 2010;12:223–233. doi: 10.2165/11319220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Tauris J, Christensen EI, Nykjaer A, Jacobsen C, Petersen CM, Ovesen T. Cubilin and megalin co-localize in the neonatal inner ear. Audiol. Neurootol. 2009;14:267–278. doi: 10.1159/000199446. [DOI] [PubMed] [Google Scholar]
- 19.Girardi A, et al. Drug-induced renal damage in preterm neonates: state of the art and methods for early detection. Drug Saf. 2015;38:535–551. doi: 10.1007/s40264-015-0288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rakhade SN, Jensen FE. Epileptogenesis in the immature brain: emerging mechanisms. Nat. Rev. Neurol. 2009;5:380–391. doi: 10.1038/nrneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Edginton AN, Avant D, Burckart GJ. Predicting neonatal pharmacokinetics from prior data using population pharmacokinetic modeling. J. Clin. Pharmacol. 2015 doi: 10.1002/jcph.524. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Leeder JS, Kearns GL. Interpreting pharmacogenetic data in the developing neonate: the challenge of hitting a moving target. Clin. Pharmacol. Ther. 2012;92:434–436. doi: 10.1038/clpt.2012.130. [DOI] [PubMed] [Google Scholar]
- 23.Leeder JS, Brown JT, Soden SE. Individualizing the use of medications in children: making Goldilocks happy. Clin. Pharmacol. Ther. 2014;96:304–306. doi: 10.1038/clpt.2014.130. [DOI] [PubMed] [Google Scholar]
- 24.Allegaert K, et al. Covariates of tramadol disposition in the first months of life. Br. J. Anaesth. 2008;100:525–532. doi: 10.1093/bja/aen019. [DOI] [PubMed] [Google Scholar]
- 25.Zhu R, et al. The pharmacogenetics of NAT2 enzyme maturation in perinatally HIV exposed infants receiving isoniazid. J. Clin. Pharmacol. 2012;52:511–519. doi: 10.1177/0091270011402826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matic M, et al. Effect of UGT2B7 −900G>A (−842G>A; rs 7438135) on morphine glucuronidation in preterm newborns: results from a pilot cohort. Pharmacogenomics. 2014;15:1589–1597. doi: 10.2217/pgs.14.115. [DOI] [PubMed] [Google Scholar]
- 27.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin. Pharmacol. Ther. 2008;83:234–342. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]
- 28.Stamer UM, Musshoff F, Kobilay M, Madea B, Hoeft A, Stuber F. Concentrations of tramadol and O-desmethyltramadol enantiomers in different CYP2D6 genotypes. Clin. Pharmacol. Ther. 2007;82:41–47. doi: 10.1038/sj.clpt.6100152. [DOI] [PubMed] [Google Scholar]
- 29.Wachman EM, et al. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA. 2013;309:1821–1827. doi: 10.1001/jama.2013.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leeder JS. Developmental pharmacogenetics: a general paradigm for application to neonatal pharmacology and toxicology. Clin. Pharmacol Ther. 2009;86:678–682. doi: 10.1038/clpt.2009.195. [DOI] [PubMed] [Google Scholar]
- 31.Haas DM, et al. The impact of drug metabolizing enzyme polymorphisms on outcomes after antenatal corticosteroid use. Am. J. Obstet. Gynecol. 2012;206:447.e17–447.e24. doi: 10.1016/j.ajog.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allegaert K. Tailored tools to improve pharmacotherapy in infants. Expert Opin. Drug Metab. Toxicol. 2014;10:1069–1078. doi: 10.1517/17425255.2014.931937. [DOI] [PubMed] [Google Scholar]
- 33.Wildschut ED, de Wildt SN, Mathot RA, Reiss IK, Tibboel D, van den Anker J. Effect of hypothermia and extracorporeal life support on drug disposition in neonates. Semin. Fetal Neonatal Med. 2013;18:23–27. doi: 10.1016/j.siny.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Van den Broek MP, et al. Pharmacokinetics and clinical efficacy of phenobarbital in asphyxiated newborns treated with hypothermia: a thermopharmacological approach. Clin. Pharmacokinet. 2012;51:671–679. doi: 10.1007/s40262-012-0004-y. [DOI] [PubMed] [Google Scholar]
- 35.Wildschut ED, Ahsman MJ, Allegaert K, Mathot RA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36:2109–2116. doi: 10.1007/s00134-010-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claus BO, Hoste EA, Colpaert K, Robays H, Decruyenaere J, de Waele JJ. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J. Crit. Care. 2013;28:695–700. doi: 10.1016/j.jcrc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Ince I, et al. Critical illness is a major determinant of midazolam clearance in children aged 1 month to 17 years. Ther. Drug Monit. 2012;34:381–389. doi: 10.1097/FTD.0b013e31825a4c3a. [DOI] [PubMed] [Google Scholar]
- 38.Shah RR, Smith RL. Inflammation-induced phenoconversion of polymorphic drug metabolizing enzymes: hypothesis with implications for personalized medicine. Drug Metab. Dispos. 2015;43:400–410. doi: 10.1124/dmd.114.061093. [DOI] [PubMed] [Google Scholar]
- 39.Blake MJ, Abdel-Rahman SM, Pearce RE, Leeder JS, Kearns GL. Effect of diet on the development of drug metabolism by cytochrome P-450 enzymes in healthy infants. Pediatr. Res. 2006;60:717–726. doi: 10.1203/01.pdr.0000245909.74166.00. [DOI] [PubMed] [Google Scholar]
- 40.Robinson J. Cochrane in context: probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid. Based Child Health. 2014;9:672–674. doi: 10.1002/ebch.1977. [DOI] [PubMed] [Google Scholar]
- 41.Neu J. Preterm infant nutrition, gut bacteria, and necrotizing enterocolitis. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:285–288. doi: 10.1097/MCO.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrett E, et al. The neonatal gut harbours distinct bifidobacterial strains. Arch. Dis. Child. Fetal Neonatal Ed. 2015 doi: 10.1136/archdischild-2014-306110. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Nunn A, et al. Estimating the requirement for manipulation of medicines to provide accurate doses for children. Eur. J. Hosp. Pharm. 2013;20:3–7. [Google Scholar]
- 44.Uppal N, Yasseen B, Seto W, Parshuram CS. Drug formulations that require less than 0.1 mL of stock solution to prepare doses for infants and children. CMAJ. 2011;183:E246–E248. doi: 10.1503/cmaj.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherwin CM, McCaffrey F, Broadbent RS, Reith DM, Medlicott NJ. Discrepancies between predicted and observed rates of intravenous gentamicin delivery for neonates. J. Pharm. Pharmacol. 2009;61:465–471. doi: 10.1211/jpp/61.04.0008. [DOI] [PubMed] [Google Scholar]
- 46.Jacqz-Aigrain E. Drug policy in Europe research and funding in neonates: current challenges, future perspectives, new opportunities. Early Hum. Dev. 2011;87(suppl. 1):S27–S30. doi: 10.1016/j.earlhumdev.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Santovena A, Hernandeze-Paiz Z, Farina JB. Design of a pediatric oral formulation with a low proportion of hydrochlorothiazide. Int. J. Pharm. 2012;423:360–364. doi: 10.1016/j.ijpharm.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 48.Hamman J, Steenekamp J. Excipients with specialized functions for effective drug delivery. Expert Opin. Drug Deliv. 2012;9:219–230. doi: 10.1517/17425247.2012.647907. [DOI] [PubMed] [Google Scholar]
- 49.Turner MA, et al. Risk assessment of neonatal excipient exposure: lessons from food safety and other areas. Adv. Drug Deliv. Rev. 2014;73:89–101. doi: 10.1016/j.addr.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Nellis G, et al. Potentially harmful excipients in neonatal medicines: a pan-European observational study. Arch. Dis. Child. 2015 doi: 10.1136/archdischild-2014-307793. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 51.Salunke S, Brandys B, Giacoia G, Tuleu C. The STEP (Safety Toxicity of Excipients for Paediatrics) database: part 2. The pilot version. Int. J. Pharm. 2013;457:310–322. doi: 10.1016/j.ijpharm.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 52.De Cock RF, et al. Developmental pharmacokinetics of propylene glycol in preterm and term neonates. Br. J. Clin. Pharmacol. 2013;75:162–171. doi: 10.1111/j.1365-2125.2012.04312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulla H, et al. An observational study of blood concentrations and kinetics of methyl- and propyl-parabens in neonates. Pharm. Res. 2015;32:1084–1093. doi: 10.1007/s11095-014-1520-2. [DOI] [PubMed] [Google Scholar]
- 54.Allegaert K, et al. Prospective assessment of short-term propylene glycol tolerance in neonates. Arch. Dis. Child. 2010;95:1054–1058. doi: 10.1136/adc.2010.190330. [DOI] [PubMed] [Google Scholar]
- 55.McCune SK, Mulugeta YA. Regulatory science needs for neonates: a call for neonatal community collaboration and innovation. Front. Pediatr. 2014;2:135. doi: 10.3389/fped.2014.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang B, Schmidt B. Do we measure the right end points? A systematic review of primary outcomes in recent neonatal randomized clinical trials. J. Pediatr. 2001;138:76–80. doi: 10.1067/mpd.2001.110299. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt B, et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA. 2012;307:275–282. doi: 10.1001/jama.2011.2024. [DOI] [PubMed] [Google Scholar]
- 58.U.S. Food and Drug Administration. [Accessed 3 May 2015]; < http://www.fda.gov/Drugs/NewsEvents/ucm410863.htmhttp://www.fda.gov/drugs/NewsEvents/ucm410863.htm>.
- 59.de Haan TR, et al. Pharmacokinetics pharmacodynamics of medication in asphyxiated newborns during controlled hypothermia. The PharmaCool multicenter study. BMC. Pediatr. 2012;12:45. doi: 10.1186/1471-2431-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manolis E, et al. Role of modeling and simulation in pediatric investigation plans. Paediatr Anaesth. 2011;21:214–221. doi: 10.1111/j.1460-9592.2011.03523.x. [DOI] [PubMed] [Google Scholar]
- 61.Zisowsky J, Krause A, Dingemanse J. Drug development for pediatric populations: regulatory aspects. Pharmaceutics. 2010;2:364–388. doi: 10.3390/pharmaceutics2040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai JP, Barrett JS, Burckart GJ, Meibohm B, Sachs HC, Yao L. Strategic biomarkers for drug development in treating rare diseases and diseases in neonates and infants. AAPS. J. 2013;15:447–454. doi: 10.1208/s12248-013-9452-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Boeck K, et al. Guideline on the design and conduct of cystic fibrosis clinical trials: the European Cystic Fibrosis Society-Clinical Trials Network (ECFS-CTN) J. Cyst. Fibros. 2011;10(suppl. 2):S67–S74. doi: 10.1016/S1569-1993(11)60010-6. [DOI] [PubMed] [Google Scholar]
- 64.Hoffman EP, Connor EM. Orphan drug development in muscular dystrophy: update on two large clinical trials of dystrophin rescue therapies. Discov. Med. 2013;16:233–239. [PubMed] [Google Scholar]