Abstract
Background
It is not known whether morphological abnormalities of the hip are compatible with lifelong hip function and avoidance of osteoarthritis (OA). Our purpose was to investigate the prevalence of radiographic findings consistent with femoroacetabular impingement (FAI) and dysplasia (DDH) in senior athletes with well-functioning hips.
Questions/purposes
(1) What is the prevalence of FAI and DDH in senior athletes with well-functioning hips? (2) Are radiographic findings of FAI and DDH associated with OA? (3) Is a history of longer duration or more intense activity associated with hip pathomorphology? (4) Were the modified Harris hip scores and the Hip Outcome Scores lower (legacy scales) in patients with evidence of hip pathomorphology than those without?
Methods
Five hundred forty-seven individuals (55% men, 45% women; 1081 hips, 534 bilateral and 13 unilateral), mean age 67 years (SD 8 years), gave consent and qualified for this institutional review board-approved cross-sectional study of senior athletes. Hips were independently evaluated for radiographic signs of FAI, DDH, and OA. Additionally, a lifetime activities questionnaire and outcome instruments were used to assess pain and function. Hips that had previously undergone arthroplasty or fracture surgery were excluded.
Results
Eighty-three percent (898 of 1081) of hips had radiographic abnormalities consistent with FAI, of which 67% (599 of 898) were cam-type FAI. Ten percent (103 of 1081) of hips had radiographic evidence for dysplasia. Radiographic findings of FAI were not predictive of OA after controlling for age and sex (odds ratio [OR], 1.79; 95% confidence interval [CI], 0.48–6.62; p = 0.390). Similarly, radiographic findings of DDH were not predictive of OA (OR, 1.48; 95% CI, 0.31–7.21; p = 0.62). Our data suggest an increased risk of FAI-type morphologies in athletes who participated in competitive sporting events during early adult years (OR, 1.49; 95% CI, 1.04–2.11; p = 0.020). Additionally, participants who reported lifetime participation in competitive sports were at an increased risk of OA compared with those who did not (OR, 1.75; 95% CI, 1.14–2.69; p = 0.007). There were no differences in outcome scores between athletes with and without morphologic abnormalities.
Conclusions
Radiographic findings consistent with FAI in these senior athletes were common and were not associated with the presence of OA. These data suggest that the need to screen for asymptomatic young athletes for radiographic evidence of FAI and DDH may not be necessary.
Level of Evidence
Level III, prognostic study.
Introduction
Abnormal bony morphology of the femur and/or acetabulum is believed to initiate damage to the articular cartilage and acetabular labrum and may predispose the hip to early osteoarthritis (OA) [3, 14, 26, 31]. Radiographic findings consistent with hip pathomorphology, including femoroacetabular impingement (FAI) and developmental dysplasia of the hip (DDH), are present not only in symptomatic patients, but also in asymptomatic individuals. In the general population, previous estimates of radiographic prevalence are 15% to 30% for FAI and 6% to 7% for DDH [12, 17, 18, 24] with ages ranging from 23 to 93 years [12], 20 to 79 years [17], 22 to 93 years [18], and 17 to 20 years [24]. A recent study assessing a population of “community-dwelling” men ≥ 65 years old, using a single AP view, reported the prevalence of FAI at 38% [32]. Regardless of the prevalence reported in the literature, the degree to which these radiographic findings predict the development of OA is controversial [4, 11, 32, 37, 39, 40].
The prevalence of radiographic findings of morphologic abnormalities consistent with FAI, DDH, and OA in older athletes who have been active throughout life is unknown. Additionally, it is unclear what morphologic abnormalities can be present in lifelong athletes without progression to symptomatic hip arthritis. Therefore, quantifying the radiographic prevalence of these abnormalities in senior athletes has direct relevance to the study of hip pathomorphology as a whole. Data collected in this study may demonstrate correlations between specific activities (sports and/or labor) and an increased risk for underlying abnormalities. It may confirm or refute the need to screen younger athletes for FAI and DDH.
To further our understanding of hip pathomorphology, we sought to determine the following: (1) What is the prevalence of radiographic findings consistent with FAI and DDH in senior athletes with well-functioning hips? (2) Are these radiographic abnormalities associated with OA? (3) Is a history of longer duration or more intense activity associated with morphologic abnormalities? (4) Were patient-reported outcomes (modified Harris hip score and the Hip Outcome Score) lower in patients with these abnormalities than those without?
Patients and Methods
Approximately 10,000 senior athletes competing in the 2012 Huntsman World Senior Games in St George, UT, USA, were given the opportunity to participate in this cross-sectional study approved by our institutional review board. Recruitment occurred at the senior games health screenings hall, where a booth for the study was present for 10 days during the games. Like with other health screenings on offer at this event, the study procedures were provided at no cost. Senior athletes who were participating in the games and who presented to the booth were consented to participate on a voluntary basis. Athletes were unable to participate if they reported prior bilateral THA. Furthermore, individual hips were excluded if they had been replaced or had a history of trauma.
A total of 560 athletes from various sporting events provided informed consent. The athletes underwent radiographic evaluation of both hips and completed a questionnaire regarding their current hip function and their lifetime participation in athletics. Thirteen athletes had radiographs that were too difficult to read as a result of artifact and were withdrawn from the study. Thirteen athletes had prior THA (n = 12) or fracture (n = 1) to one hip and that hip was excluded. This resulted in 1081 hips (534 bilateral and 13 unilateral) available for review from 547 senior athletes. Forty-five percent of the athletes were women and 55% were men. The mean age was 67 years (SD 8). In regard to race, 91% were white (498 of 547), 1.6% black (nine of 547), 1.5% Asian (eight of 547), 2.7% other (15 of 547), and 2.9% (16 of 547) did not respond to this question.
For the radiographic evaluation, two orthopaedic surgeons (LAA, JC) assessed both hips independently for radiographic signs of FAI, DDH, and OA using a mobile digital radiograph system (Viztek, Garner, NC, USA, and Source-Ray, Inc, Ronkonkoma, NY, USA). For the ease of the interpretation of this study by the reader, throughout this article we refer to radiographic findings consistent with FAI morphology as FAI and similarly radiographic findings of DDH as DDH. We do not intend this to be confused with a clinical diagnosis but rather representing the radiographic morphologies commonly associated with those diagnoses. Pelvic AP and frog-leg lateral radiographs were obtained in the supine position. Femoral sphericity was assessed with the alpha angle [9]. The alpha angle, assessed on the frog-leg lateral view, is between the longitudinal axis of the femoral neck and a line connecting the femoral head center and the point where the head exceeds the radius of the best-fit sphere. Similarly, the gamma angle was measured on the AP view. Acetabular coverage was evaluated on the AP films; lateral center-edge angle (LCEA) was measured by a vertical line referenced off the horizontal pelvis (teardrops or obturator foramenae) and a line from the center of the femoral head to the lateral edge of the sourcil (sclerotic weightbearing aspect of the acetabulum) for hips on the dysplastic end of the spectrum and lateral-most rim for hips displaying an angle greater than 35° on the overcovered end of the spectrum, because these would reflect the pathology at risk [43]. The acetabular index (AI) was the angle measured by a horizontal line referenced from the pelvis and a line connecting the medial and lateral edges of the sourcil on undercovered and normal covered hips and the lateral edge of the acetabulum on overcovered hips [43]. Acetabular orientation was also evaluated on the AP films. The crossover sign was considered positive when the posterior wall of the acetabulum crosses the anterior wall (ie, acetabular retroversion) below the level of the superior femoral head. We divided the distance from the lateral acetabular rim to the center of the femoral head into thirds and graded crossover as Grades I through IV with I being a crossover near the rim in the top one-third, II being the middle third, and III being the bottom third of this distance; Grade IV was reserved for a crossover below the center of the femoral head. Crossover is sensitive to pelvic tilt and cranial crossover is likely a normal variant. We agree with Zaltz et al. [45] as well as Larson et al. [25] and so graded crossover critically and only considered the caudal Grades II to IV as diagnostic for radiographic pincer FAI while excluding cranial crossover.
To determine the radiographic prevalence of FAI and DDH, data (eg, alpha angle) were averaged between the first reads of both observers (LAA, JC). The crossover sign was considered present only if both observers detected it. We used the grading system, described by Tönnis and Heinecke [44], to assess the osteoarthritic condition of each hip. For the Tönnis grade, the highest score reported by either observer was used, representing the worst-case evaluation.
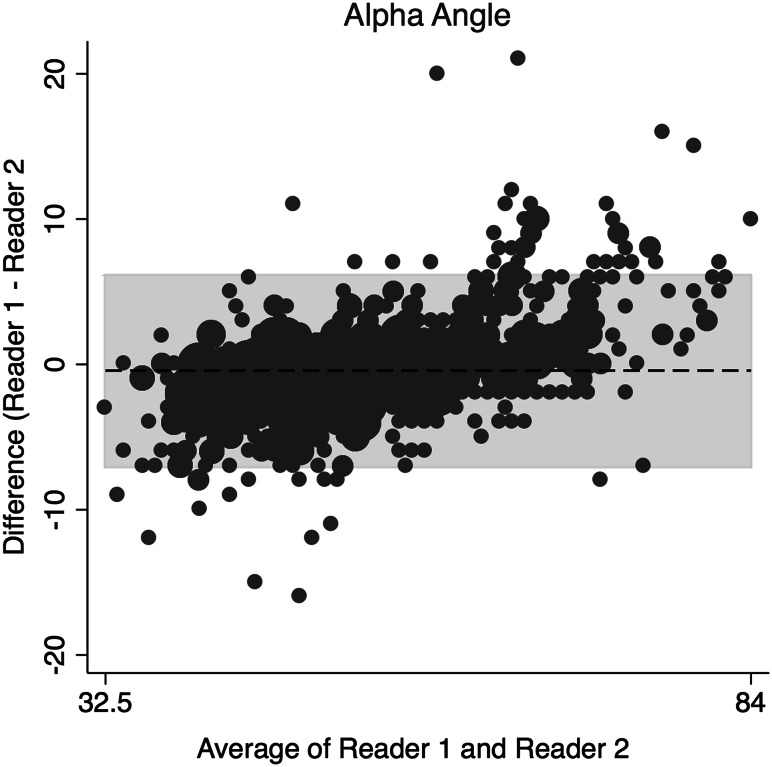
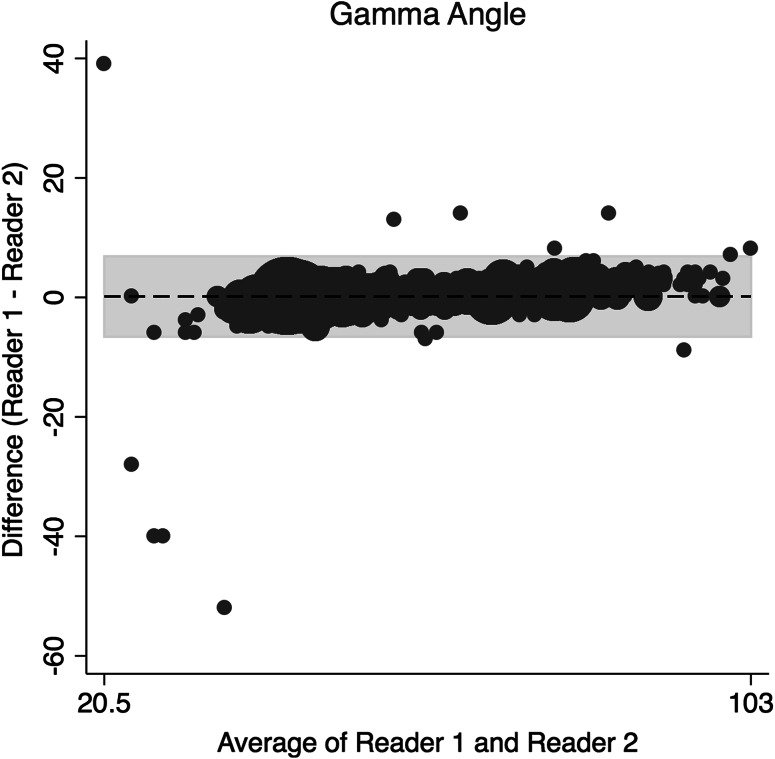
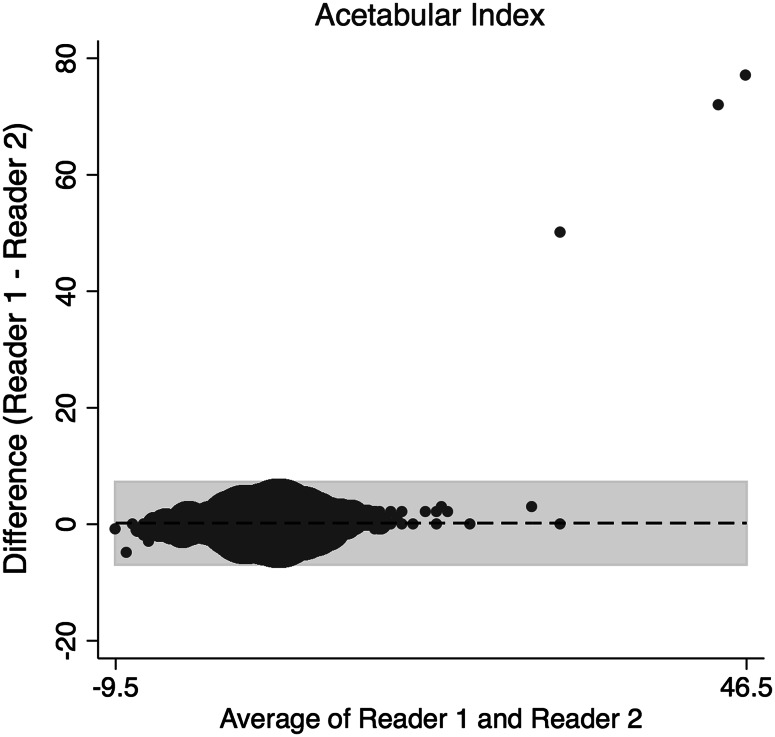
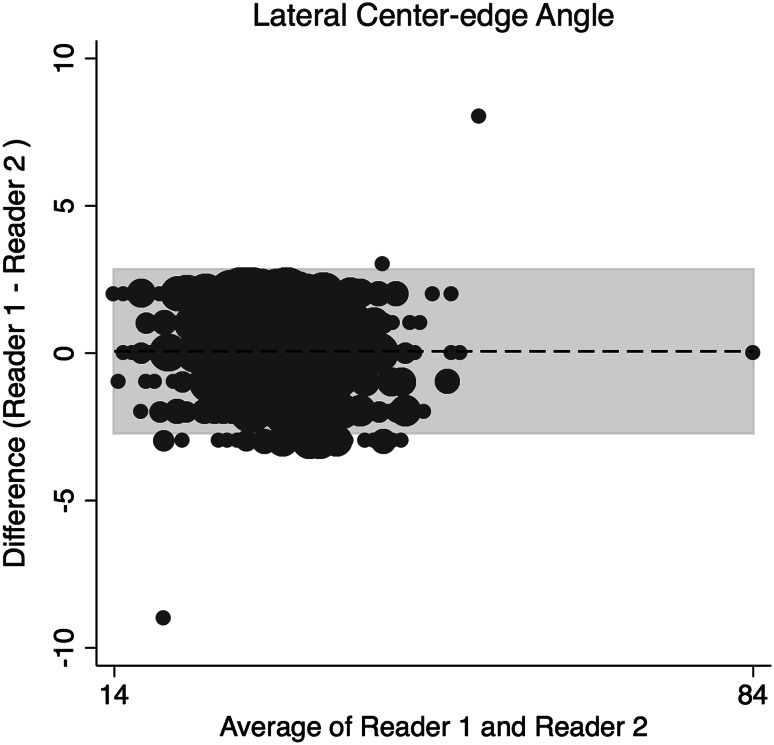
The concordance correlation coefficient (rc) and a Bland-Altman Analysis were used to assess interrater reliability and interrater agreement between the two readers for continuous data [5, 27]. The amount of agreement between readers for the rc was classified as minimal, < 0.2; poor, 0.2 to < 0.4; moderate 0.4 to < 0.6, strong; 0.6 to ≤ 0.8; and almost perfect > 0.8 [38]. Binary data were assessed using Cohen’s kappa (weighted). The two orthopaedic surgeons assessing the radiographs demonstrated strong and almost perfect agreement in the radiographic measures (Table 1) of alpha angle on the frog-leg lateral (Fig. 1), gamma angle on AP view (Fig. 2), acetabular index (Fig. 3), and LCEA (Fig. 4). They also showed almost perfect agreement in the crossover sign (κ = 0.99; 95% confidence interval [CI], 0.995–0.998) and Tönnis grade (κ = 0.997; 95% CI, 0.992–0.998).
Table 1.
Interrater agreement on radiographic reads
| Variable | rc | 95% CI | Bland-Altman average difference (SD) |
95% limits of agreement | Pearson’s r |
|---|---|---|---|---|---|
| Frog alpha angle | 0.94 | 0.94–0.95 | −0.43 (3.37) | −7.04 to 6.19 | 0.96 |
| AP alpha angle | 0.98 | 0.98–0.98 | 0.16 (3.40) | −6.5 to 6.8 | 0.98 |
| Acetabular index | 0.77 | 0.75–0.79 | −0.21 (3.61) | −6.8 to 7.30 | 0.81 |
| LCEA | 0.98 | 0.97–0.98 | 0.07 (1.42) | −2.71 to 2.84 | 0.98 |
rc = concordance correlation coefficient; CI = confidence interval; LCEA = lateral center-edge angle.
Fig. 1.
This is a Bland-Altman plot demonstrating the interrater agreement of the alpha angle on frog-leg lateral radiographs.
Fig. 2.
This is a Bland-Altman plot demonstrating the interrater agreement of the gamma angle on AP radiographs.
Fig. 3.
This Bland-Altman plot shows the interrater agreement of the AI.
Fig. 4.
This Bland-Altman plot shows the interrater agreement of LCEA.
Activity Questionnaires
Each participant was asked to complete a questionnaire on a computer tablet through the assessment center website (www.assessmentcenter.net). The questionnaire consisted of an extensive history of physical activity throughout the lifetime. The questionnaire asked which sports were the primary and secondary sports of the athlete, how many days per month they participated in each sport, and if they played competitively or recreationally. Life periods were divided as follows: (1) youth (≤ 18 years old); (2) early adult (19–35 years old); (3) adult (36–65 years old); and (4) senior (> 65 years old).
Characterization and time periods of sports participation were analyzed to determine associations with prevalence of hip pathomorphology and OA. Sports that were considered to have a high hip-impact included basketball, football, gymnastics, ice hockey, lacrosse, track and field, cross country running, soccer, racquet sports, and volleyball. Participation in competitive sports was defined as playing in organized sports in the athlete’s primary sport.
Outcome Questionnaires
We collected the modified Harris hip score (mHHS) which was modified by Byrd and Jones [6] in 2010 and subsequently used by them to assess outcomes in athletes undergoing hip arthroscopy [7]. Additionally, we collected the Hip Outcomes Score (HOS) [28–30]. The HOS was originally developed to assess self-reported functional status of patients with musculoskeletal hip disorders [28]. With the HOS, individuals are queried on 19 separate activities of daily living (ADL) and nine sports-related items. A score is generated separately for the ADL and sports subscales. The response to 17 items on the ADL subscale is scored from 4 to 0 with 4 being “no difficulty” and 0 being “unable to do.” A higher score represents a higher level of physical function for both the ADL and sports subscales. Both the mHHS and the HOS have been used in several studies to document the outcomes of surgical interventions for the treatment of symptomatic FAI [2, 8, 15, 19, 33, 34]. These instruments were used to assess the current self-reported function of the athletes and no exclusions were made regarding the results, because all athletes were able to participate in the games regardless of the function they reported.
Statistical Analysis
We used a mixed-effects multiple logistic regression analysis to assess for associations between radiographic findings of FAI and OA, DDH and OA, and FAI and age group. The Wilcoxon rank-sum analysis was used to compare average days per month of sports participation for each diagnosis (FAI, DDH, and OA) and to compare the mHHS and HOS in those with each diagnosis and those without. Fisher’s exact test was used to compare the sports impact analysis for intensity (competitive versus recreational) and impact (high or low hip-impact) levels for each diagnosis as well as provide odds ratios. Significance was assessed at the 0.05 level. Statistical analysis was performed using Stata® Version 13.1 (College Station, TX, USA).
Results
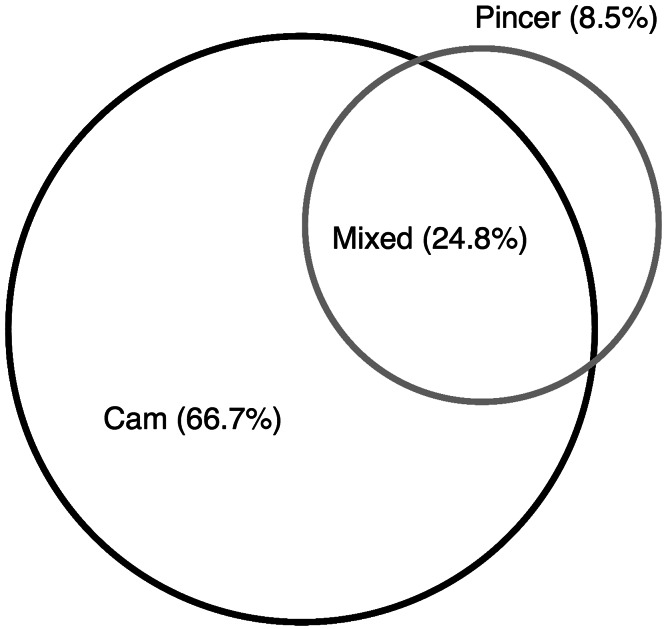
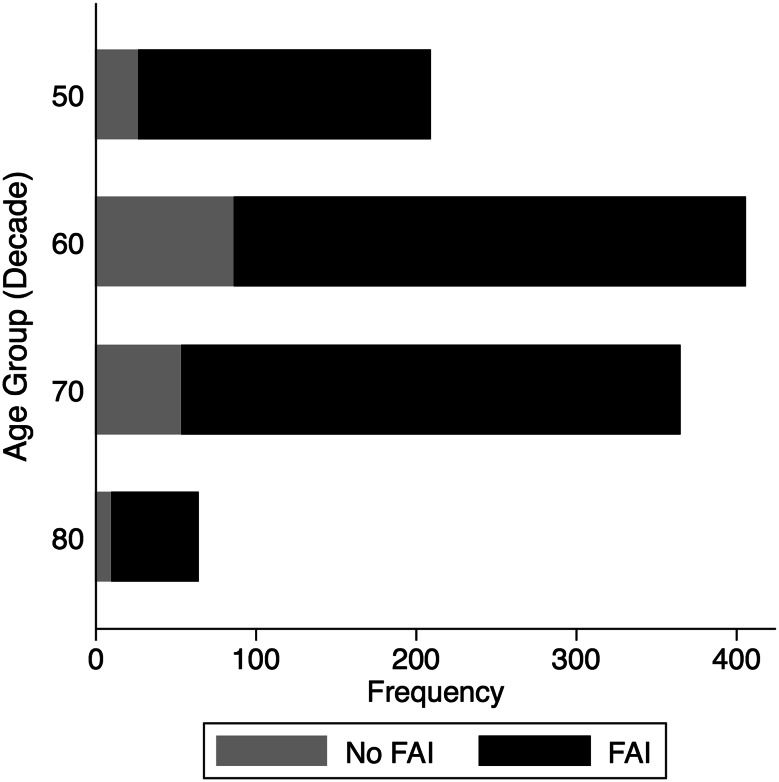
There was radiographic evidence of FAI in 83% of hips (898 of 1081); 66.7% (599 of 898) had isolated cam and 8.5% (76 of 898) isolated pincer impingement; 24.8% had mixed (Fig. 5). Histograms demonstrate the distribution of LCEA (Fig. 6) and alpha angles (Fig. 7). Ten percent of hips (103 of 1081) had radiographic evidence of dysplasia; 3% (30 of 1081) had a LCEA that was < 20° and 9% (93 of 1081) had an acetabular index that was > 10°. FAI was more prevalent in men than women (odds ratio [OR], 4.28; 95% CI, 2.13–8.59; p < 0.001) and was not associated with age (OR, 0.99; 95% CI, 0.95–1.03; p = 0.69). Subcategorizing FAI into cam, pincer, and mixed revealed that men were more likely to have cam (OR, 1.97; 95% CI, 1.13–3.4; p = 0.02) and mixed-type FAI (OR, 2.03; 95% CI, 1.13–3.64; p = 0.02), whereas women were more likely to have findings of pincer (OR, 2.00; 95% CI, 1.11–3.60; p = 0.02) on radiographs. Our data show no difference between ages grouped by decade (Fig. 8) and the proportion of FAI prevalent in those groups (OR, 0.96; 95% CI 0.95 – 1.03, p = 0.491). Of the hips with DDH, 78% (n = 80) also had radiographic abnormalities consistent with cam FAI. There was no association between age (OR, 1.00; 95% CI, 0.96–1.05; p = 0.83) or sex (OR, 0.50; 95% CI, 0.25–1.00; p = 0.05) and DDH.
Fig. 5.
This Venn diagram demonstrates the overlap of cam and pincer FAI in senior athletes.
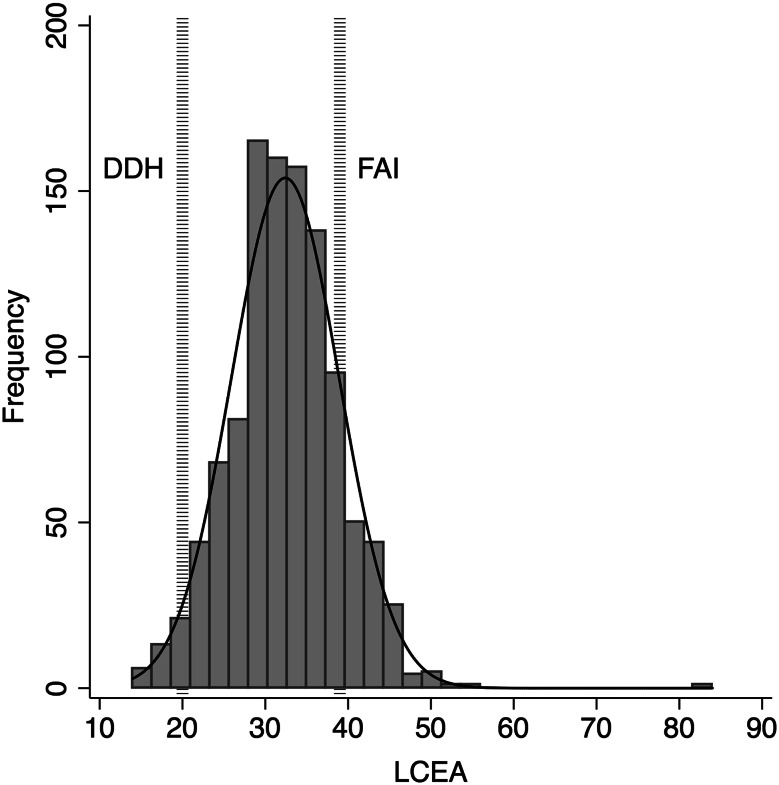
Fig. 6.
A histogram showing the distribution of participants by LCEA with reference lines indicating DDH (LCEA < 20°) or FAI (LCEA > 39°).
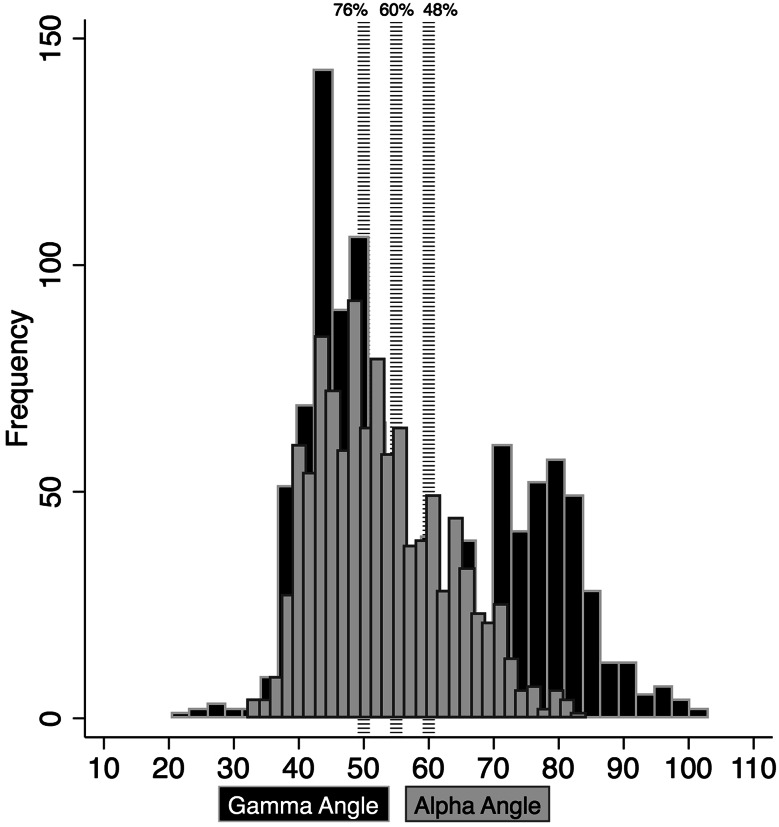
Fig. 7.
A histogram showing the distribution of participants by gamma angle on AP and alpha angle on frog lateral radiographs for the senior athletes with cutoff (dashed lines) levels at 50°, 55°, and 60°.
Fig. 8.
This graph represents the proportion of patients with radiographic evidence of FAI-type morphologies per each decade of life.
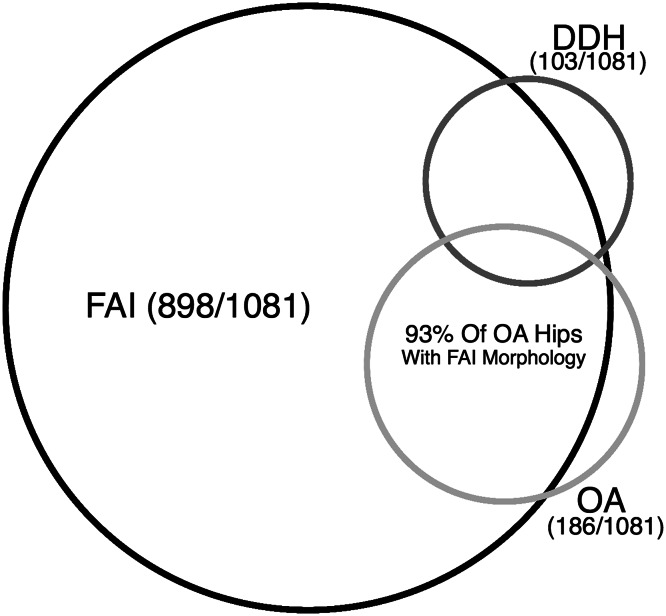
Radiographic findings of FAI were not predictive of OA after controlling for age and sex (OR, 1.79; 95% CI, 0.48–6.62; p = 0.390). Similarly, radiographic findings of DDH were not predictive of OA (OR, 1.48; 95% CI, 0.31–7.21; p = 0.62). Furthermore, OA (Tönnis Grade 2–3) was present in 17% of hips (186 of 1081); 93% (173 of 186) of hips with OA had radiographic evidence of FAI and 10% (18 of 186) had evidence of DDH. Patients with OA were more likely to have radiographic signs of FAI (OR, 3.84; 95% CI, 1.50–9.81; p = 0.005); however, 81% of the hips with findings of FAI (725 of 898) showed little to no evidence of OA (Tönnis Grade 0–1) despite the athletes’ age and lifelong activity levels (Fig. 9).
Fig. 9.
This Venn diagram shows the overlap of OA and morphologic abnormalities consistent with FAI and DDH.
Twenty-four athletes did not complete the questionnaire and were dropped from the activity history analysis resulting in 1033 hips in 523 athletes. There was no difference in the amount of days in which the athletes participated in physical activity between those with radiographic evidence of FAI or DDH and those without (Table 2). Participation in competitive sports as a young adult was associated with an increased prevalence of radiographic FAI (OR, 1.49; 95% CI, 1.04 – 2.11; p = 0.02), and lifelong participation in competitive sports was associated with an increased prevalence of OA (OR, 1.75; 95% CI, 1.14–2.69; p = 0.007). Otherwise, we found no association between participation in competitive sport and radiographic findings (Table 3). Persons who participated in high-level activity when younger than 18 years were less likely to have radiographic findings of OA (OR, 0.70; 95% CI, 0.50–0.99; p = 0.04). DDH was more prevalent in athletes who participated in a high-level activity in early adult (19–35 years old; OR,1.64; 95% CI, 1.04–2.59; p = 0.02) or adult (36–65 years old; OR, 1.86; 95% CI, 1.17–2.95; p = 0.005) years. Seniors participating in high-level activity were less like to have OA (OR, 0.67; 95% CI, 0.48–0.94; p = 0.02). We found no association between high-level activity at any age and FAI (Table 4).
Table 2.
Association between reported activity frequency and radiographic findings of morphologic changes and osteoarthritis
| Morphology type/OA | With evidence | Without evidence | p value |
|---|---|---|---|
| Youth ≤ 18 years old | |||
| FAI (m) (range) | 12 (0–30) | 12 (0–31) | 0.44 |
| DDH (m) (range) | 11 (0–31) | 12 (0–31) | 0.49 |
| OA (m) (range) | 12 (0–29) | 12(0–31) | 0.53 |
| Early adult 19–35 years old | |||
| FAI (m) (range) | 8 (0–30) | 7 (0–30) | 0.11 |
| DDH (m) (range) | 7 (0–20) | 8 (0–30) | 0.48 |
| OA (m) (range) | 8 (0–23) | 8 (0–30) | 0.33 |
| Adult 36–65 years old | |||
| FAI (m) (range) | 9 (0–29) | 8 (0–30) | 0.35 |
| DDH (m) (range) | 9 (0–23) | 9 (0–30) | 0.69 |
| OA (m) (range) | 8 (0–24) | 9 (0–30) | 0.36 |
| Senior > 65 years old | |||
| FAI (m) (range) | 5 (0–23) | 4 (0–20) | 0.52 |
| DDH (m) (range) | 5 (0–18) | 4 (0–23) | 0.29 |
| OA (m) (range) | 5 (0–23) | 4 (0–20) | 0.12 |
OA = osteoarthritis; FAI = femoroacetabular impingement; DDH = developmental dysplasia of the hip.
Table 3.
Associations between participation in competitive sports and radiographic evidence of morphologic changes and osteoarthritis
| Morphology type/OA | Odds ratio | 95% CI | p value |
|---|---|---|---|
| Youth ≤ 18 years old | |||
| FAI | 1.1 | 0.76–1.56 | 0.55 |
| DDH | 0.97 | 0.61–1.53 | 0.88 |
| OA | 1.05 | 0.75–1.48 | 0.77 |
| Early adult 19–35 years old | |||
| FAI | 1.49 | 1.04–2.11 | 0.02 |
| DDH | 0.84 | 0.53–1.33 | 0.44 |
| OA | 1.00 | 0.71–1.40 | 0.99 |
| Adult 36–65 years old | |||
| FAI | 1.02 | 0.73–1.44 | 0.88 |
| DDH | 0.98 | 0.62–1.54 | 0.92 |
| OA | 0.88 | 0.63–1.22 | 0.42 |
| Senior > 65 years old | |||
| FAI | 1.13 | 0.77–1.66 | 0.52 |
| DDH | 1.05 | 0.64–1.72 | 0.84 |
| OA | 1.11 | 0.77–1.60 | 0.56 |
| Lifetime | |||
| FAI | 0.95 | 0.59–1.54 | 0.84 |
| DDH | 0.88 | 0.47–1.64 | 0.69 |
| OA | 1.75 | 1.14–2.69 | 0.007 |
OA = osteoarthritis; CI = confidence interval; FAI = femoroacetabular impingement; DDH = developmental dysplasia of the hip.
Table 4.
Associations between high hip impact physical activity and radiographic evidence of morphologic changes and osteoarthritis
| Morphology type/OA | Odds ratio | 95% CI | p value |
|---|---|---|---|
| Youth ≤ 18 years old | |||
| FAI | 0.97 | 0.69–1.37 | 0.88 |
| DDH | 0.87 | 0.55–1.37 | 0.53 |
| OA | 0.70 | 0.50–0.99 | 0.04 |
| Early adult 19–35 years old | |||
| FAI | 1.19 | 0.84–1.68 | 0.30 |
| DDH | 1.64 | 1.04–2.59 | 0.02 |
| OA | 0.91 | 0.65–1.27 | 0.55 |
| Adult 36–65 years old | |||
| FAI | 1.22 | 0.86–1.72 | 0.25 |
| DDH | 1.86 | 1.17–2.95 | 0.005 |
| OA | 1.06 | 0.76–1.49 | 0.70 |
| Senior > 65 years old | |||
| FAI | 1.03 | 0.74–1.45 | 0.83 |
| DDH | 1.46 | 0.92–2.34 | 0.09 |
| OA | 0.67 | 0.48–0.94 | 0.02 |
| Lifetime participation | |||
| FAI | 0.94 | 0.60–1.46 | 0.77 |
| DDH | 1.24 | 0.70–2.16 | 0.42 |
| OA | 0.67 | 0.41–1.08 | 0.08 |
OA = osteoarthritis; CI = confidence interval; FAI = femoroacetabular impingement; DDH = developmental dysplasia of the hip.
Both the mHHS and HOS were analyzed to compare those with evidence of hip pathology and those without. Neither the mHHS nor the HOS (Table 5) exhibited any difference between the athletes for those with or without radiographic evidence of hip pathomorphology.
Table 5.
Comparison of the mHHS and the HOS between patients with and without evidence of hip pathomorphology
| Morphology type/OA | With morphology | Without morphology | p value |
|---|---|---|---|
| Modified Harris hip scores | |||
| FAI, median (IQR) | 98 (94–100) | 100 (94–100) | 0.457 |
| DDH, median (IQR) | 96 (96–100) | 100 (94–100) | 0.073 |
| OA, median (IQR) | 100 (96–100) | 98 (94–100) | 0.296 |
| Hip Outcome Scores | |||
| FAI, median (IQR) | 97 (90–100) | 98 (88–100) | 0.964 |
| DDH, median (IQR) | 96 (79–100) | 97 (90–100) | 0.097 |
| OA, median (IQR)) | 97 (90–100) | 97 (88–100) | 0.908 |
mHHS = modified Harris hip score; HOS = Hip Outcome Score; OA = osteoarthritis; FAI = femoroacetabular impingement; DDH = developmental dysplasia of the hip.
Discussion
The natural history of FAI and dysplasia in the asymptomatic population is not well understood. If hips with radiographic pathology are at high risk to develop early OA, prophylactic intervention of prominent abnormalities may be warranted even in asymptomatic patients. On the contrary, if a significant number of hips with radiographic FAI and dysplasia were found to survive into the senior years without symptoms or evidence of OA, there would be a strong argument against the concept of prophylactic surgical intervention as well as screening of asymptomatic hips. We therefore assessed a large group of elite senior athletes, both on radiographic and clinical grounds. We asked the following four questions: (1) What is the prevalence of FAI and DDH in senior athletes with well-functioning hips? (2) Are there radiographic findings of FAI and DDH that correlate with OA? (3) What is the relationship between radiographic measures of hip pathomorphology and activity history (type and intensity of activity from teens to the present)? (4) Were patient-reported outcomes, mHHS and the HOS, different in patients with evidence of hip pathomorphology and those without?
There were several limitations in this study that warrant discussion. First, although three-dimensional abnormities of FAI are best characterized on CT and MRI, we performed the measurements using plain film radiographs [10, 23, 35]. However, we used both AP and frog-leg lateral radiographs, visualizing two different regions of the head-neck junction [9]. This allowed for the visualization of cam deformities on both of films, and cam deformity was established by surpassing the cutoff levels for either the alpha or gamma angle. Additionally, MRI would be a better modality to evaluate chondrolabral injuries as well as joint degeneration associated with DDH and FAI but was not used as a result of financial and time constraints. A second limitation is that the normal range of radiographic parameters has yet to be established, and the prevalence of hip pathomorphology is a function of the radiographic cutoff values. For example, radiographic interpretation of FAI has varied between studies from 20% to 80% [12, 32] including the results from this study. This is likely related to limitations in reader reliability, cutoff values, and variations intrinsic to the populations examined. Thus, histograms of LCEA (Fig. 6) and alpha angle (Fig. 7) are provided so readers can interpret the results using their preferred cutoff. Although a control population was not used in this study, we compared our findings with previously reported values from the general population, leading to the conclusion that these senior athletes have a much higher occurrence of pathomorphology consistent with FAI than the general population. This was a narrow population of senior athletes; it is unknown what percentage of athletes were excluded as a result of bilateral THAs had DDH, FAI, or otherwise, nor do we have a perspective on the percentage of senior athletes who develop symptomatic hip pathology that excludes them from participating in these types of senior athlete events. Furthermore, we were unable to obtain the demographics of the entire Senior Games population and thus are not able to rule out a potential self-selection bias or demonstrate that our group of athletes resembles the entire population. Furthermore, these athletes may have had questions regarding complications with their hips and therefore were seeking out medical advice. However, the athletes were informed that the study would not be providing any medical advice and their legacy functional scores suggest a rather high-functioning population. Regardless, as a result of the possibility of self-selection bias, it is possible that our results represent the high end of prevalence for FAI, DDH, and OA in an active senior population. We understand that this study population, by definition and design, was a narrow selection of well-functioning or coping athletes, which is both a limitation and strength of this study. Finally, the use of the HHS and HOS to evaluate participant function in this study is subject to the natural limitations of these legacy scores including ceiling effects and the inability to distinguish impact of knee, ankle, contralateral limb, etc, on functional scores [16].
The radiographic prevalence of morphologies consistent with FAI and DDH in asymptomatic members of the general population ranges from 15% to 30% for FAI and 6% to 7% for dysplasia [12, 13, 17, 18, 24, 36]. In one study of 200 asymptomatic volunteers, 25% of male and 5% of female subjects had at least one hip with cam deformity (> 51°) [13]. In the Copenhagen Osteoarthritis Study, 1332 male and female participants demonstrated a prevalence of pincer deformities in 15% and 19%, respectively (LCEA > 45°), and 20% and 5% had a pistol grip deformity consistent with cam FAI, whereas 4% of males and females had evidence of DDH [12]. In trauma-related abdominal CT scans on 100 presumably asymptomatic patients, 31% of female and 48% of male participants had at least one pincer or cam abnormality [21]. Consistent with the Copenhagen Osteoarthritis Study [12], we found cam deformity to be associated with the male sex with almost a two to one male-to-female ratio. We also had a similar distribution of DDH between males and females (ratio = 1.1:1).
Although the vast majority of athletes with OA had radiographic findings of FAI, our data did not demonstrate this to be a predictive of OA (OR, 1.79; p = 0.39). Consistent with the Copenhagen study, we also found that DDH was not associated with OA [18].
We found no associations between activity levels including high hip impact activities and intense versus recreational sports participation during youth and FAI, DDH, or OA. We did note an association between competitive sports participation during young adult years and FAI, but not between high hip impact activities and FAI. We are not sure what to make of the association noted (Table 4) between participation in high hip impact activities and radiographic evidence of DDH in the adult years.
In our study, the senior athletes with radiographic evidence of FAI morphology demonstrated excellent outcome scores with a mean mHHS of 98 and a mean HOS of 97 for FAI. Patients with DDH and OA had similar scores and there was no difference in scores between those with or without morphological or osteoarthritic findings. We are not aware of any other prevalence study that assessed current hip function with the use of outcome scores. However, when studying the prevalence of FAI in asymptomatic older males, Nardo et al. [32] report that patients with a cam FAI morphology were less likely to have hip pain. Similarly, Allen et al. [1] found that only 26% of patients with bilateral cam FAI morphology had bilateral hip pain. Regarding DDH, Kapron et al. [22] reported the prevalence of DDH in female collegiate athletes to be 21%. They found no difference in radiographic measurements between hips that were painful during impingement examination and those that were not. Our data, along with the previous literature, suggest that other factors aside from morphology may play a role in the symptomology of structural hip deformities.
The prevalence of morphologic deformities found in our study was substantially higher than previous reports on athletes and the general public. Some studies have postulated that the increased prevalence in athletes is the result of vigorous sports activity in adolescence, predisposing the growing hip to the development of altered morphology [41, 42]. Another possibility is that remodeling of the mature hip as a result of activity-related stresses is neither pathological nor unexpected [20]. We do not have additional insight into the etiology of cam FAI based on our methods (a single radiograph in time). Our data undermine the argument for screening young athletes or subsequent prophylactic treatment. Finally, they demonstrate the need for prospective longitudinal studies following individuals with currently asymptomatic radiographic morphologies consistent with FAI and DDH. Furthermore, future studies should focus on factors such as genotype or variability in molecular biology of cartilage and bone over time, which may play more prominent roles in the survival or degeneration of the hip compared with radiographic pathomorphology [3, 11].
Acknowledgments
We thank the Huntsman World Senior Games for granting us access to their athletes and for their support of our research study. We also thank the athletes for their dedication to sport, the inspiration and advice they gave us, and for their ageless enthusiasm for life.
Footnotes
Funding was provided by a grant from the LS Peery Foundation (LAA, CP).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Allen D, Beaule PE, Ramadan O, Doucette S. Prevalence of associated deformities and hip pain in patients with cam-type femoroacetabular impingement. J Bone Joint Surg Br. 2009;91:589–594. doi: 10.1302/0301-620X.91B5.22028. [DOI] [PubMed] [Google Scholar]
- 2.Bardakos NV, Vasconcelos JC, Villar RN. Early outcome of hip arthroscopy for femoroacetabular impingement: the role of femoral osteoplasty in symptomatic improvement. J Bone Joint Surg Br. 2008;90:1570–1575. doi: 10.1302/0301-620X.90B12.21012. [DOI] [PubMed] [Google Scholar]
- 3.Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87:1012–1018. doi: 10.1302/0301-620X.87B7.15203. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein J. The myths of femoroacetabular impingement. Clin Orthop Relat Res. 2014;472:3623–3624; discussion 3624–3628. [DOI] [PMC free article] [PubMed]
- 5.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 6.Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2010;468:741–746. doi: 10.1007/s11999-009-0841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrd JW, Jones KS. Arthroscopic management of femoroacetabular impingement in athletes. Am J Sports Med. 2011;39(Suppl):7S–13S. doi: 10.1177/0363546511404144. [DOI] [PubMed] [Google Scholar]
- 8.Byrd JW, Jones KS. Arthroscopic management of femoroacetabular impingement: minimum 2-year follow-up. Arthroscopy. 2011;27:1379–1388. doi: 10.1016/j.arthro.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Clohisy JC, Nunley RM, Otto RJ, Schoenecker PL. The frog-leg lateral radiograph accurately visualized hip cam impingement abnormalities. Clin Orthop Relat Res. 2007;462:115–121. doi: 10.1097/BLO.0b013e3180f60b53. [DOI] [PubMed] [Google Scholar]
- 10.Dudda M, Albers C, Mamisch TC, Werlen S, Beck M. Do normal radiographs exclude asphericity of the femoral head-neck junction? Clin Orthop Relat Res. 2009;467:651–659. doi: 10.1007/s11999-008-0617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop Relat Res. 2008;466:264–272. doi: 10.1007/s11999-007-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92:1162–1169. doi: 10.2106/JBJS.H.01674. [DOI] [PubMed] [Google Scholar]
- 13.Hack K, Di Primio G, Rakhra K, Beaule PE. Prevalence of cam-type femoroacetabular impingement morphology in asymptomatic volunteers. J Bone Joint Surg Am. 2010;92:2436–2444. doi: 10.2106/JBJS.J.01280. [DOI] [PubMed] [Google Scholar]
- 14.Harris WH. Etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 1986;213:20–33. [PubMed] [Google Scholar]
- 15.Haviv B, Singh PJ, Takla A, O’Donnell J. Arthroscopic femoral osteochondroplasty for cam lesions with isolated acetabular chondral damage. J Bone Joint Surg Br. 2010;92:629–633. doi: 10.1302/0301-620X.92B5.23667. [DOI] [PubMed] [Google Scholar]
- 16.Hung M, Hon SD, Cheng C, Franklin J, Aoki SK, Anderson MB, Kapron A, Peters CL, Pelt CE. Psyhometric evaluation of the Lower Extremity Computerized Adaptive Test, the modified Harris Hip Score, and the Hip Outcome Score. Orthop J Sports Med. 2014;2. DOI: 10.1177/2325967114562191. [DOI] [PMC free article] [PubMed]
- 17.Inoue K, Wicart P, Kawasaki T, Huang J, Ushiyama T, Hukuda S, Courpied J. Prevalence of hip osteoarthritis and acetabular dysplasia in french and japanese adults. Rheumatology (Oxford). 2000;39:745–748. doi: 10.1093/rheumatology/39.7.745. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen S, Sonne-Holm S, Soballe K, Gebuhr P, Lund B. Hip dysplasia and osteoarthrosis: a survey of 4151 subjects from the Osteoarthrosis Substudy of the Copenhagen City Heart Study. Acta Orthop. 2005;76:149–158. doi: 10.1080/00016470510030517. [DOI] [PubMed] [Google Scholar]
- 19.Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93:326–331. doi: 10.1302/0301-620X.93B3.25262. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JK, Renner JB, Dahners LE. Anteroposterior thickening of the femoral neck with aging decreases the ‘offset’ in men. Am J Sports Med. 2012;40:2213–2217. doi: 10.1177/0363546512457158. [DOI] [PubMed] [Google Scholar]
- 21.Kang AC, Gooding AJ, Coates MH, Goh TD, Armour P, Rietveld J. Computed tomography assessment of hip joints in asymptomatic individuals in relation to femoroacetabular impingement. Am J Sports Med. 2010;38:1160–1165. doi: 10.1177/0363546509358320. [DOI] [PubMed] [Google Scholar]
- 22.Kapron AL, Peters CL, Aoki SK, Beckmann JT, Erickson JA, Anderson MB, Pelt CE. The prevalence of radiographic findings of structural hip deformities in female collegiate athletes. Am J Sports Med. 2015 Mar 31 [Epub ahead of print]. [DOI] [PubMed]
- 23.Konan S, Rayan F, Haddad FS. Is the frog lateral plain radiograph a reliable predictor of the alpha angle in femoroacetabular impingement? J Bone Joint Surg Br. 2010;92:47–50. doi: 10.1302/0301-620X.92B1.22359. [DOI] [PubMed] [Google Scholar]
- 24.Laborie LB, Lehmann TG, Engesaeter IO, Eastwood DM, Engesaeter LB, Rosendahl K. Prevalence of radiographic findings thought to be associated with femoroacetabular impingement in a population-based cohort of 2081 healthy young adults. Radiology. 2011;260:494–502. doi: 10.1148/radiol.11102354. [DOI] [PubMed] [Google Scholar]
- 25.Larson CM, Moreau-Gaudry A, Kelly BT, Byrd JW, Tonetti J, Lavallee S, Chabanas L, Barrier G, Bedi A. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473:1247–1254. doi: 10.1007/s11999-014-4055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavigne M, Parvizi J, Beck M, Siebenrock KA, Ganz R, Leunig M. Anterior femoroacetabular impingement: part I. Techniques of joint preserving surgery. Clin Orthop Relat Res. 2004;418:61–66. doi: 10.1097/00003086-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. doi: 10.2307/2532051. [DOI] [PubMed] [Google Scholar]
- 28.Martin RL, Kelly BT, Philippon MJ. Evidence of validity for the hip outcome score. Arthroscopy. 2006;22:1304–1311. doi: 10.1016/j.arthro.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 29.Martin RL, Philippon MJ. Evidence of validity for the hip outcome score in hip arthroscopy. Arthroscopy. 2007;23:822–826. doi: 10.1016/j.arthro.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Martin RL, Philippon MJ. Evidence of reliability and responsiveness for the hip outcome score. Arthroscopy. 2008;24:676–682. doi: 10.1016/j.arthro.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Murphy S, Tannast M, Kim YJ, Buly R, Millis MB. Débridement of the adult hip for femoroacetabular impingement: indications and preliminary clinical results. Clin Orthop Relat Res. 2004;429:178–181. doi: 10.1097/01.blo.0000150307.75238.b9. [DOI] [PubMed] [Google Scholar]
- 32.Nardo L, Parimi N, Liu F, Lee S, Jungmann PM, Nevitt MC, Link TM, Lane NE, Osteoporotic fractures in men research g. femoroacetabular impingement: prevalent and often asymptomatic in older men: the Osteoporotic Fractures in Men Study. Clin Orthop Relat Res. 2015 Mar 4 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 33.Nho SJ, Magennis EM, Singh CK, Kelly BT. Outcomes after the arthroscopic treatment of femoroacetabular impingement in a mixed group of high-level athletes. Am J Sports Med. 2011;39(Suppl):14S–19S. doi: 10.1177/0363546511401900. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen TG, Miller LL, Lund B, Christiansen SE, Lind M. Outcome of arthroscopic treatment for symptomatic femoroacetabular impingement. BMC Musculoskelet Disord. 2014;15:394. doi: 10.1186/1471-2474-15-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84:556–560. doi: 10.1302/0301-620X.84B4.12014. [DOI] [PubMed] [Google Scholar]
- 36.Raynor CM, Bryant D, Spouge A, Birmingham TB, Willits K. Presence of markers of femoroacetabular impingement in the asymptomatic population. FASEB J. 2009;23:822–827. [Google Scholar]
- 37.Reiman MP, Thorborg K. Femoroacetabular impingement surgery: are we moving too fast and too far beyond the evidence? Br J Sports Med. 2015 Feb 12 [Epub ahead of print]. [DOI] [PubMed]
- 38.Rosenkrantz AB, Lim RP, Haghighi M, Somberg MB, Babb JS, Taneja SS. Comparison of interreader reproducibility of the prostate imaging reporting and data system and Likert scales for evaluation of multiparametric prostate MRI. AJR Am J Roentgenol. 2013;201:W612–W618. doi: 10.2214/AJR.12.10173. [DOI] [PubMed] [Google Scholar]
- 39.Rubin DA. Femoroacetabular impingement: fact, fiction, or fantasy? AJR Am J Roentgenol. 2013;201:526–534. doi: 10.2214/AJR.13.10913. [DOI] [PubMed] [Google Scholar]
- 40.Sankar WN, Nevitt M, Parvizi J, Felson DT, Agricola R, Leunig M. Femoroacetabular impingement: defining the condition and its role in the pathophysiology of osteoarthritis. J Am Acad Orthop Surg. 2013;21(Suppl 1):S7–S15. doi: 10.5435/JAAOS-21-07-S7. [DOI] [PubMed] [Google Scholar]
- 41.Siebenrock KA, Behning A, Mamisch TC, Schwab JM. Growth plate alteration precedes cam-type deformity in elite basketball players. Clin Orthop Relat Res. 2013;471:1084–1091. doi: 10.1007/s11999-012-2740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siebenrock KA, Ferner F, Noble PC, Santore RF, Werlen S, Mamisch TC. The cam-type deformity of the proximal femur arises in childhood in response to vigorous sporting activity. Clin Orthop Relat Res. 2011;469:3229–3240. doi: 10.1007/s11999-011-1945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis–what the radiologist should know. AJR Am J Roentgenol. 2007;188:1540–1552. doi: 10.2214/AJR.06.0921. [DOI] [PubMed] [Google Scholar]
- 44.Tönnis D, Heinecke A. Acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am. 1999;81:1747–1770. doi: 10.2106/00004623-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471:2463–2470. doi: 10.1007/s11999-012-2689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]