Abstract
Background and aim
At present, there is no consensus regarding how to medically manage chronic insomnia in the long term. The unstated standard of practice is for patients to use hypnotics intermittently. The present study aimed to compare a partial reinforcement strategy with nightly and intermittent dosing strategies for its potential as a maintenance therapy.
Methods
A mixed model was used in the study. One between-subjects factor: group (n = 4). One repeated-measures factor: time (12 weekly assessments). A total of 74 subjects with chronic Insomnia were treated with 10 mg zolpidem for 4 weeks. Treatment respondents were randomized to nightly dosing with 10 mg or 5 mg (QHS-10 and QHS-5), intermittent dosing with 10 mg (IDS-10 [3–5 days weekly]), or partial reinforcement dosing with 10 mg (PRS-10 [nightly pill use with 50% active medication and 50% placebos]) for 12 weeks.
Results
It was found, in compliant subjects (n = 55), that all four strategies evaluated maintained treatment response over time (ie, prevented or delayed relapse). For the subjects that remained in remission, the subjects in the intermittent dosing group (IDS-10) group exhibited poorer sleep continuity.
Conclusions
While best considered a preliminary study, the present findings suggest that the partial reinforcement strategy may be a viable means toward maintaining treatment gains over time with less active medication.
Keywords: Insomnia, Zolpidem, Maintenance therapy, Placebos, Partial reinforcement
1. Introduction
While it has long been the case that insomnia has been classified as both an acute and a chronic disorder [1,2], the medical treatment of the chronic disorder has always been fraught with difficulties [1,3–5]. This is true for both general and specific reasons. The general reasons include: (1) the characterization of insomnia as a symptom versus a disorder [1,6], and (2) the implicit analogy that chronic insomnia is “like chronic pain” and that sleep medications are “like analgesics.” [4] The former suggests that insomnia should not be the focus of treatment (rather, the focus should be on the primary disorder). The latter suggests that medical option is, at best, palliative and that hypnotics should be used sparingly. The specific reasons include the concern that hypnotics cannot be used for maintenance therapy as their clinical effects cannot be maintained ad infinitum (for years and decades) and that with long-term use there is an increased risk of psychological dependence and/or adverse events.
With the advent of the International Classification of Sleep Disorders, Third Edition (ICSD-3) [7] and the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [8] and the elimination of the distinction between primary and secondary insomnia, it is now clear (at least from a nosologic point of view) that insomnia should be the focus of targeted treatment. The question moving forward is “What constitutes the best practice for maintenance therapy for insomnia?” In the absence of a specified regimen, it would be whatever approach yields the best initial treatment response, the most durable efficacy, and lowest levels of dose escalation and side effects. At present, the approach that appears to best fit this profile is that of intermittent dosing (prn use of medication on 3–5 nights per week). When evaluated week to week, the sleep continuity effects with intermittent dosing appear to be comparable to, and at least as durable as, those obtained with nightly dosing [9,10]. This said, there are no data to suggest that such a strategy results in similar time to treatment response, extends the “efficacy half-life” of hypnotics, or results in less dose escalation and/or side effects. What is clear from the given data is that there are no treatment effects on non-medication nights [11]. The recurrence of insomnia on non-medication nights is problematic, and for at least two reasons. First, the insomnia is not treated on 2–4 nights a week. Second, the recurrence of insomnia on 2–4 nights per week may lead to a form of chronic insomnia that is especially persistent and may enhance the likelihood that patients become psychologically dependent on the medication.
One yet untested alternative to intermittent dosing is to provide placebos on non-medication nights. This approach, by virtue of expectancy alone, would be expected to provide for better outcomes on non-medication nights. This approach may also serve to extend the therapeutic response to non-medication nights based on the principles of conditioning and reinforcement. That is, it is possible that placebos (the medication vehicle) may become a conditioned stimulus for the pharmacotherapeutic effects of hypnotics on non-medication nights [12–14], and that periodic reexposure to active medication via intermittent dosing may serve as partial reinforcement. In order to evaluate whether a partial reinforcement strategy (nightly pill use with 50% active medication and 50% placebos) may be used for maintenance therapy, this approach was compared to two nightly dosing strategies (5 mg and 10 mg zolpidem) and an intermittent dosing approach (3–5 nights per week with 10 mg zolpidem). The outcome measures included relapse, latency to relapse, rate of relapse per unit time, and average sleep continuity prior to relapse. It was hypothesized that the partial reinforcement strategy would not significantly differ from nightly dosing with 10 mg but would be superior to nightly dosing with 5 mg and intermittent dosing with 10 mg.
2. Methods
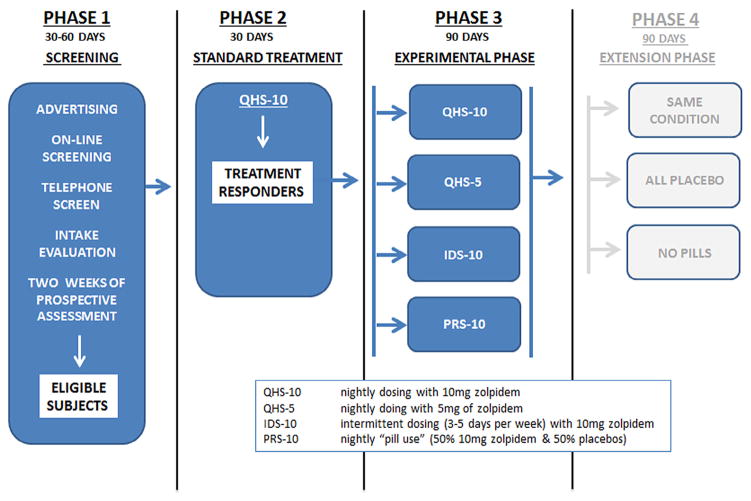
2.1. Study overview (Fig. 1)
Fig. 1.
Study diagram.
The data presented here are from a larger study on the role of partial reinforcement in the long-term management of insomnia. The parent study (which was funded as an R01 by the National Center for Complementary and Alternative Medicine [NCCAM]) had a mixed model design with four phases and four groups. The study design (as can be seen in Fig. 1) and the conduct of the investigation were overseen by the Internal Review Board of the University of Pennsylvania and by a Data Safety and Monitoring Board (DSMB). The DSMB consisted of a statistician and two board-certified Sleep Medicine physicians who were not affiliated with the investigators’ program (The Penn Behavioral Sleep Medicine Program). The four phases were: Phase 1 (baseline assessment), Phase 2 (standard treatment), Phase 3 (randomization of subjects to one of four maintenance groups), and Phase 4 (re-randomization to one of three extension conditions). This report focuses on the data obtained from the first three phases of the study. Phase 1 (baseline assessment) included an in-person interview, a history and physical examination, one overnight polysomnography (PSG) study, and two weeks of evaluation with sleep diaries to ensure that each subject’s retrospective characterization of his or her sleep continuity met inclusion criteria. Phase 2 was conducted over four weeks and entailed nightly treatment with 10 mg of zolpidem 30 min before bedtime. No behavioral interventions were provided during Phase 2 or for the remainder of the study. Subjects that exhibited a treatment response during Phase 2 (≥37.5% improvement on sleep latency [SL] or wake after sleep onset [WASO] [15]) were randomized during Phase 3 to one of four groups: (1) Nightly dosing with 10 mg (QHS-10); (2) nightly dosing with 5 mg (QHS-5); (3) an intermittent dosing strategy with 10 mg zolpidem (IDS-10); and (4) a partial reinforcement strategy (PRS-10 [nightly pill use with 50% active medication and 50% placebos]). During Phase 2 and Phase 3 of the study, all subjects completed a daily online sleep/pill-use diary and a weekly side-effect survey. Medication was disbursed and collected on a monthly basis (foil packs). All subjects were assessed for their clinical status on a bi-weekly basis and continued in each phase of the study for three months or until they exhibited a relapse. Note that Phase 2 was considered not only a standard treatment phase but also as the opportunity for the pharmacotherapeutic effects of zolpidem (sleepiness and sleep) to be conditioned to the delivery system (green and yellow capsule [green top, yellow bottom]). Phase 3 was intended to assess the durability of each of the maintenance strategies, with the primary focus being how the partial reinforcement strategy (PRS-10) compared to the other approaches.
2.2. Subjects
Subjects were recruited from the local sleep disorders center and from advertisements in local newspapers and on local TV and radio stations. Interested individuals were referred to www.sleeplessinphilly.com for initial online screening. Subjects that continued to be eligible were further screened with an in-person interview, a history and physical examination, one overnight PSG study, and two weeks of sleep diaries. Medically stable subjects between the ages of 25 and 55 years having a preferred sleep phase between 9:00 PM and 9:00 AM and meeting the criteria for DSM-4-TR [16] criteria for chronic insomnia and ICSD-2 [17] and Research Diagnostic Criteria (RDC) [18] criteria for psychophysiologic insomnia were eligible for the study.
The lower age limit was restricted to minimize circadian rhythm influences on the diagnoses of insomnia. The upper age limit was adopted to ensure that maintenance therapy with nightly dosing at 5 mg would be a reasonable “ineffective dose comparator,” that is, a means to control for cumulative amount of drug per unit time (e.g., PRS-10 = 50 mg per 10 days and QHS-5 = 50 mg per 10 days). As the 5-mg dose was only recommended as an efficacious dose for older adults, and because the goal was to have a “no efficacy” dose, older adults were excluded from the study. In 2013, the Food and Drug Administration (FDA) recommended that the standard prescribing practice for zolpidem for non-elderly women be lowered from 10 to 5 mg. This label change had two profound implications for our study. First, assuming that 5 mg is an efficacious dose for non-elderly women, it suggested that the PRS-10 group was likely to be effective, given the cumulative dose in this group was equal to the new recommended dose (for at least the women in the sample). Second, the QHS-5 group as an “on-label” dose could no longer serve as an “ineffective dose comparator” but instead now represented a low-dose alternative approach to maintenance therapy.
In addition to the age limits and the ICSD-2 [17] and RDC [18] criteria for psychophysiologic insomnia, all subjects were required to have a sleep initiation complaint (>30 min to fall asleep) with a problem frequency ≥3 nights/week and a problem duration ≥6 months. Middle and late insomnia were free to vary. This profile had to be evident at both intake (based on retrospective reports) and as an average profile from the two weeks of baseline diaries.
Exclusionary criteria (based on an intake interview, an administration of the Mini-International Neuropsychiatric Interview [M.I.N.I], a history and physical examination, and on a single night of PSG) were as follows:
Inadequate language comprehension
Unstable medical or psychiatric illness
Symptoms suggestive of sleep disorders other than insomnia
Past history of treatment failure with zolpidem or discontinuation of zolpidem owing to side effects
Past history of parasomnias (no more than one incident in last 10 years)
PSG data indicating sleep disorders other than insomnia (e.g., apnea–hypopnea index [AHI] or periodic limb movement index [PLMI] > 10)
Evidence of active illicit substance use or alcohol abuse and/or dependence
Current use of central nervous system (CNS) active medications (e.g., antidepressants and hypnotics other than zolpidem)
Pregnancy or the intention to become pregnant within the study period (8–10 months)
Any first-degree relatives with bipolar disorder or schizophrenia
2.3. Study medication
Zolpidem tartrate (trade name Ambien) is a non-benzodiazepine hypnotic whose primary indication is for sleep initiation problems. The standard dose for non-elderly adults (1992–2013) was 10 mg for oral administration at bedtime. Zolpidem interacts with the GABA-BZ receptor complex, appears to bind selectively to the BZ1 receptor, is metabolized by the human liver cytochrome P450, and has a Tmax of 1.6 h and a T1/2 of 2.6 h.
2.4. Study medication acquisition, formulation and dispensation
Zolpidem was purchased through, and managed by, the Investigational Drug Services of the University of Pennsylvania. An over-encapsulation technique was used to ensure that the drug doses and placebo formulations appeared identical. Over-encapsulation was accomplished by using green and yellow capsules. Placebos were composed of non-active ingredients. Medication was packaged in foil packs that contained 30 pills per pack. Blister packs were returned once a month. A nurse practitioner or the study coordinator dispensed and received the foil packs and queried subjects about compliance and side effects. Randomization to condition was accomplished by the Investigational Drug Services of the University of Pennsylvania. The investigators and study subjects were blind to condition for the duration of the study.
2.5. Instructions regarding study medication
Subjects in the QHS-10, PRS-10, and QHS-5 groups were told to take each and every pill, and to do so in sequence. Subjects in the IDS-10 group were told to take between three and five pills weekly and to take the pill that corresponded to the particular day for that month. All subjects were told to take the pills approximately 30 min before bed. The medication protocol was explained in the informed consent document as follows: “…you will be randomly assigned (like choosing numbers from a hat) to treatment. The treatment groups will differ in the amount of zolpidem received at any given time over the course of the 7 month study (28 weeks). The 28 weeks of treatment will include 4 weeks of treatment with a stable dose of medication and then 24 weeks with a stable and/or a variable amount of medication (range from 0 mg to 10 mg per night).” Subjects were not re-informed or reminded regarding the switch from Phase 2 to Phase 3 of the study at the fifth week of the investigation.
2.6. Compliance with the medication regimens
Compliance was assessed on a nightly basis for each individual subject. This was accomplished in two ways. First (as noted earlier in the study overview), the AM sleep diary contained a question regarding whether or not study medication was taken on the previous night. Second, the foil pack containers were retained for all subjects and each night was coded for whether the pill had been removed from the packet. The foil pack data were used as a backup for the instances where subjects had not indicated on the AM diaries whether medication had been used. Taken together, these data were used to tabulate the number of pills taken per week and percent compliance per month. Operationally, compliance for Phase 3 for the nightly dosing and partial reinforcement groups (QHS-10, QHS-5, or PRS-10) was an average weekly pill use that was between six and seven pills per week/month. Thus, the threshold for compliance was (for the whole of Phase 3) no less than one pill fewer than prescribed per week. Operationally, compliance for Phase 3 for the intermittent dosing group (IDS-10) was an average weekly pill use that was between three and six pills per week/month. Thus, the threshold for compliance was (for the whole of Phase 3) no more than one additional pill than prescribed per week. These compliance rules were used to aggregate the set of subjects for whom efficacy would be reported. The rationale for this strategy was that low adherence can, in and of itself, account for the outcomes (relapse, latency to relapse, and poor sleep continuity effects) and mask the efficacy that may, or may not, be attributed to each of the maintenance treatment approaches. Thus, as a proof of concept, the efficacy analyses are only reported for compliant subjects.
2.7. Data acquisition and measures
All questionnaire data were acquired via an on-line system (internet data portal [IDP]). Examples of contemporary IDPs may be found at www.penn-nites.com, and at www.dims-and-does.com. Several instruments were administered via the IDP on a daily, weekly, monthly, or pre-post basis to track relapse (primary outcome), assess sleep continuity (secondary outcome), and to quantify the incidence of medical symptoms (tertiary outcome). The questionnaires included a set of validated measures and a set of measures that are specific to our laboratory. The validated measures included seven questionnaires measuring insomnia severity (ISI) [19]; sleepiness (ESS) [20,21]; anxiety (STAI) [22,23], depression (QIDS) [24]; menstrual cycle regularity, menses-related symptoms, and contraception practices (MCQ) [25]; and alcohol use and abuse (AUDIT-CAGE) [26,27]. At intake, psychiatric status was assessed with the MINI International Neuropsychiatric Interview (MINI) [28]. The measures that are specific to our laboratory include eight questionnaires measuring subject demographics (DEMO); sleep disorders symptoms (SDS-CL); sleep medication history (SMHF); insomnia history (IHF); types of insomnia (TIF); medical history (MHF); medical symptom check list (MS-CL); and daily sleep continuity (AM sleep diaries).
The MS-CL (acquired weekly) contained 52 medical symptom items over 11 categories of symptoms. Subjects completed the MS-CL weekly and were instructed to “check” whatever symptoms were experienced during the last week and to rate the particular symptom for number of days per week where the symptom was experienced and to rate the average severity of each symptom. The variables constructed from these data included the following for Phase 3 of the study: (1) average number of symptoms per week; (2) average number of days per week with one or more symptoms; and (3) average symptom severity per week.
The AM sleep diaries (acquired daily) included questions pertaining to time to bed (TTB), SL, number of awakenings (NWAK), WASO, time out of bed (TOB), total sleep time (TST), and whether or not study medication was taken. In addition to these self-reported variables, time in bed (TIB), total sleep time (TST-C), and sleep efficiency (SE%-C) were auto-calculated.
2.8. Outcome variables
Traditional sleep continuity variables as assessed with daily sleep diaries were used in two ways. First, bi-weekly profiles of sleep continuity were used to assess each subject for “response to treatment” during Phase 2 of the study (four weeks) and for “relapse” during Phase 3. Treatment response was defined a priori based upon meta-analytic values for sleep diary-assessed SL and WASO [15]. Specifically, treatment response was defined as an average of ≥37.5% improvement on SL and/or WASO and/or an SE% of ≥90% per week for 2 weeks. Note that the SE% of ≥90 benchmark was adopted because this represents a common standard for what constitutes significant clinical gains within the context of Cognitive Behavioral Therapy for Insomnia (CBT-I) [29]. Relapse was also defined a priori. In this case, as there is no standard for insomnia research, the common benchmark of 50% loss of gains was adopted. Specifically, relapse was defined as the first day of a two-week average of SL and/or WASO where the subject showed a ≥50% loss of SL and/or WASO gains (where the SL and WASO were >30 min) and/or an SE of <80%.
Second, upon completion of the study, sleep continuity was assessed by group for additional evidence (beyond the categorical assessment of relapse) of the viability of each treatment approach. Specifically, the groups were compared for their average values (before relapse or for the whole 12 weeks of Phase 3 of the study) for SL, WASO, NWAK, TST-C, TIB, and SE%-C. In addition to the standard sleep continuity variables, the groups were also compared for latency to relapse and average medical symptomatology for the duration of the remission period. Latency to relapse was defined as the time elapsed from the start of Phase 3 to relapse (as defined previously). Medical symptomatology was calculated in terms of average number of symptoms per week, average number of days per week with one or more symptoms, and average symptom severity per week.
2.9. Compliance with study measures
As all but one of the outcome variables critically depended on the regular acquisition of the AM sleep diaries, subjects were required to complete at least four diaries per week. Failure to comply with this minimum resulted in up to three contacts per month by the study coordinator. Subjects were contacted by email or phone and were counseled regarding (1) the need for complete data, (2) how to resolve any issues regarding Internet access or use of the internet data portal, and/or (3) the possibility of being withdrawn from the study for non-compliance. If a subject’s sleep diary data fell below 16 diary entries per month, the subject was disenrolled. Data regarding disenrollment for non-compliance with measures are presented in the subject attrition section of the results and in Fig. 2 (consort diagram).
Fig. 2.
Consort diagram.
2.10. Phase 3 data analyses
Contingency analysis was used to assess rate of relapse per group. One-way analyses of variance (ANOVAs) were used to assess group differences with respect to demographics (sex and age) and, among only those who relapsed, time to relapse. A Cox proportional hazards model was used to evaluate time to relapse among all compliers and to estimate hazard ratios for relapse for the PRS-10, IDS-10, and QHS-5 groups compared to QHS-10, the reference group. Survival curves estimated by the model were also plotted. Generalized estimating equation (GEE) models were used to compare average sleep continuity profiles (SL, WASO, NWAK, TST-C, TIB, and SE-C%), average weekly number of pills taken, and average weekly medical symptomatology for the time before relapse or for the whole 12 weeks. An exchangeable correlation structure was used in all GEE models to account for the repeated measurements within subjects. Finally, adjusted GEE models were also used to compare SL, WASO, TST-C, and SE-C% across groups after adjusting for baseline values.
3. Results
3.1. Subject attrition
As can been seen in Fig. 2, 318 interested individuals completed a screening survey. Of these, 129 individuals were eligible for, and were enrolled into, Phase 1 (2-week assessment period) of the study, and 82 individuals exhibited a treatment response and were advanced to Phase 3 (randomization to one of the four treatment maintenance groups). Seventy-four subjects completed Phase 3. Of these subjects, 55 were identified as compliant with the medication regimens (PRS-10 = 65% [n = 13/20]), QHS-10 = 81% [n = 13/16], IDS-10 = 75% [n = 15/20], and QHS-5 = 78% [n = 14/18]). As can be seen in Table 1, the attrition and compliance rates did not significantly differ among the treatment conditions.
Table 1.
Summary statistics and group differences.*
| PRS-10 | QHS-10 | IDS-10 | QHS-5 | Test Statistic (df) | p value | |
|---|---|---|---|---|---|---|
| Whole Sample, N | 20 | 16 | 20 | 18 | ||
| Compliant, N (%) | 13 (65.0) | 13 (81.3) | 15 (75.0) | 14 (77.8) | 1.43 (3) | 0.698 |
| Compliant Subjects | ||||||
| Age, years (se) | 39.2 (2.8) | 39.5 (2.8) | 34.0 (2.6) | 34.5 (2.7) | 1.15 (3.51) | 0.337 |
| Gender, N (%) female | 11 (84.6) | 10 (76.9) | 11 (73.3) | 11 (78.6) | exact* | 0.969 |
| Pills per week (se) | 6.87 (0.06) | 6.58 (0.25) | 5.77 (0.43) | 6.81 (0.11) | 6.56 (3) | 0.087 |
| Relapse, N (%) | 3 (23.1) | 3 (23.1) | 4 (26.7) | 0 (0.0) | exact* | 0.189 |
| Time to Relapse**, days (se) | 40.7 (13.5) | 69.3 (13.5) | 36.0 (11.7) | n/a | 1.92 (2.7) | 0.217 |
| Hazard Ratio of Relapse | 1.08 (0.9) | (reference) | 1.30 (1.0) | 0.00 (0.0) | 0.13 (3) | 0.988 |
| Time in Bed, mins (se) | 504.6 (15.0) | 513.2 (18.7) | 488.8 (8.3) | 517.0 (10.4) | 5.01 (3) | 0.171 |
| Sleep Latency, mins (se) | 21.2 (4.1) | 28.6 (3.8) | 32.9 (5.7) | 21.0 (2.1) | 6.37 (3) | 0.095 |
| Wake After Sleep Onset, mins (se) | 20.3 (5.0) | 21.7 (3.0) | 27.6 (5.7) | 10.9 (4.1) | 6.95 (3) | 0.074 |
| Number of Awakenings, # (se) | 0.99 (0.27) | 1.04 (0.38) | 1.38 (0.29) | 0.71 (0.21) | 3.36 (3) | 0.339 |
| Calculated Total Sleep Time, mins (se) | 462.6 (13.1) | 463.2 (17.3) | 428.6 (11.4) | 485.2 (9.6) | 14.38 (3) | 0.002 |
| Sleep Efficiency from Calculated TST, % (se) | 91.6 (1.4) | 90.1 (0.8) | 87.8 (1.9) | 93.8 (0.8) | 14.74 (3) | 0.002 |
| Med Symptoms, #/week (se) | 1.98 (0.50) | 2.76 (0.54) | 2.74 (0.87) | 2.28 (0.60) | 1.28 (3) | 0.734 |
| Med Symptoms, avg # days/week (se) | 2.17 (0.32) | 2.98 (0.28) | 3.45 (0.37) | 2.85 (0.53) | 7.15 (3) | 0.067 |
| Severe Side Effects, avg severity/week (se) | 1.94 (0.25) | 2.17 (0.18) | 2.44 (0.26) | 2.19 (0.24) | 1.98 (3) | 0.576 |
Abbreviations: N = sample size; Se = Standard Error; mins = minutes; # = Number; TST = Total Sleep Time.
For χ2 test statistic and degrees of freedom (df), or “exact” when Fisher’s exact test was used.
Time to relapse was calculated only among the subjects that relapsed.
3.2. Subjects
Of the sample, 78% was female. The mean age of the sample was about 37 years. Means and standard errors per group and univariate tests of significance can be found in Table 1. The groups did not significantly differ with respect to sex or age.
3.3. Frequency of medication use by group
As noted earlier, the QHS-10, QHS-5, and PRS-10 groups were all instructed to take one pill per night. The IDS-10 group was instructed to take three to five pills per week. After accounting for compliance, the three nightly groups took between six and seven pills per week and the IDS-10 group took between five and six pills per week. This difference was not significant but trended at p < 0.09, with the IDS-10 group taking medication on the fewest nights and the PRS-10 group taking medication on the most nights. Means and standard errors per group and tests of significance can be found in Table 1.
3.4. Percent of subjects that exhibit a relapse by group
Approximately 18% of the sample exhibited a relapse during the 12 weeks of maintenance therapy. Means and standard errors per group and tests of significance can be found in Table 1. The groups did not significantly differ with respect to relapse rates. The trend was that the IDS-10 group exhibited the most relapses and the QHS-5 group the fewest.
3.5. Time to relapse
The mean latency to relapse, for the subjects that exhibited relapses, was about 48 days. Means and standard errors per group and tests of significance can be found in Table 1. The groups did not significantly differ with respect to time to relapse. The trend was that the IDS-10 group exhibited the shortest time to relapse and the QHS-10 group the longest time to relapse.
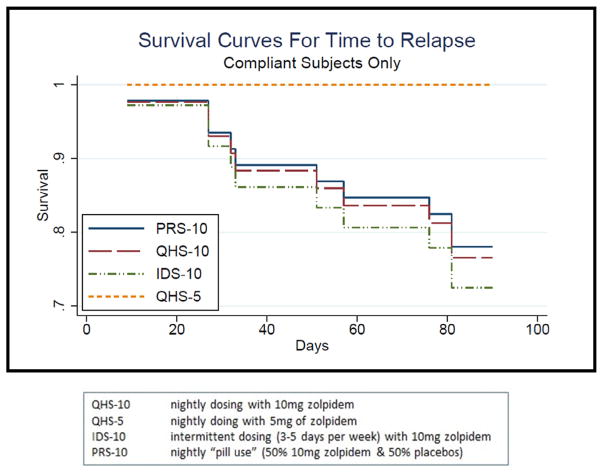
3.6. Rate of relapse over time per group (survival analysis)
The four groups did not exhibit significantly different survival patterns over time. As evident in Table 1 and Fig. 2, the trend was for the IDS-10 group to show the highest risk of relapse and the QHS-5 group the lowest risk of relapse.
3.7. Average sleep continuity
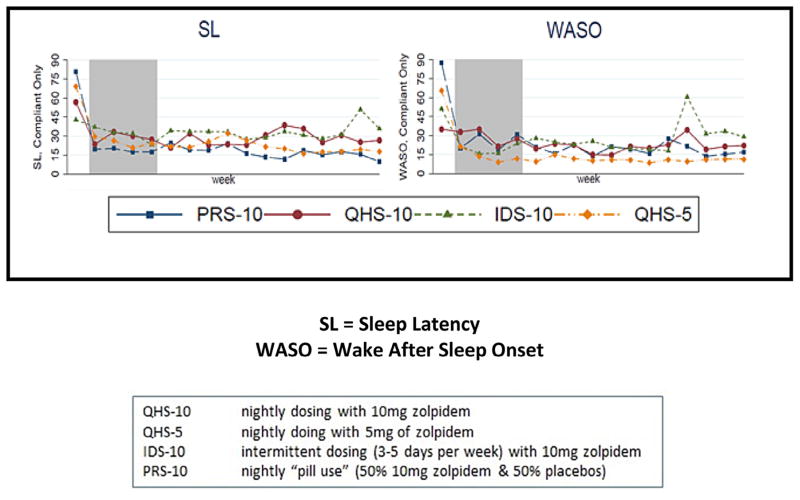
The overall average sleep continuity profile for Phase 3 (for the whole 12 weeks of Phase 3 or for the weekly averages prior to relapse) was consistent with continued treatment response. Overall, average SL and WASO times were <30 min, and SE was better than 85%. Means and standard errors per group and tests of significance can be found in Table 1. Figure 3 shows the group means over time from Phase 1 to Phase 3 for SL and WASO. Average sleep continuity for Phase 3 in the remitted subjects tended to vary by group for SL (IDS-10 > QHS-10 > PRS-10 = QHS-5) and WASO (IDS-10 > QHS-10 > PRS-10 > QHS-5) and significantly differed for TST (IDS-10 < QHS-10 = PRS-10 < QHS-5) and SE (IDS-10 < QHS-10 < PRS-10 < QHS-5). These trends toward group differences do not consider the initial differences for sleep continuity at baseline (see Fig. 4, e.g., the PRS-10 group exhibited the worst initial severity with respect to SL and WASO). When this is taken into account, the group sleep continuity differences were significantly different for all of these variables with the IDS-10 group tending to exhibit the worst outcome for all four sleep continuity variables (see Table 2).
Fig. 3.
Survival curves for time to relapse.
Fig. 4.
Average sleep continuity prior to relapse.
Table 2.
Only compliant subjects, adjusted for baseline.
| IDS-10 | QHS-10 | PRS-10 | QHS-5 | F/χ2 Stat (df) | p | |
|---|---|---|---|---|---|---|
| Sleep Latency, mins (se) | 33.8 (4.2) | 28.8 (3.4) | 19.1 (3.8) | 20.7 (2.0) | 9.95 (3) | 0.019 |
| Wake After Sleep Onset, mins (se) | 28.4 (5.2) | 21.8 (2.6) | 20.0 (4.3) | 11.3 (3.6) | 12.45 (3) | 0.006 |
| Calculated Total Sleep Time, mins (se) | 426.1 (9.4) | 465.0 (14.4) | 463.6 (12.7) | 486.6 (11.1) | 18.52 (3) | <0.001 |
| Sleep Efficiency from Calculated TST, % (se) | 87.5 (1.6) | 90.1 (0.8) | 92.0 (1.2) | 93.8 (0.9) | 20.06 (3) | <0.001 |
Significantly different from QHS-10, p < 0.0167 (0.05/3 with Bonferroni correction).
Abbreviations: QHS-10 = nightly dosing with 10 mg zolpidem; QHS-5 = nightly doing with 5 mg of zolpidem; IDS-10 = intermittent dosing (3–5 days per week) with 10 mg zolpidem; PRS-10 = nightly “pill use” (50% 10 mg zolpidem & 50% placebos).
3.8. Medical symptoms during treatment
As can be seen in Table 1, the three groups did not differ for average number of symptoms per week, average number of days per week with one or more symptoms, and/or average symptom severity per week. Means and standard errors per group and tests of significance can be found in Table 1. The clear trend across the symptom measures is for the PRS-10 group to exhibit the least severe of the profiles (less frequent symptom endorsements and less severe symptom ratings).
4. Discussion
The present study evaluated whether nightly dosing (10 or 5 mg), intermittent dosing (10 mg, 3–5 nights per week), and partial reinforcement (50% 10 mg, 50% 0 mg) differed regarding their utility as maintenance dosing strategies for chronic insomnia. It was found, in compliant subjects, that any of the four strategies evaluated may be used to maintain treatment response over time (i.e., prevent or delay relapse). For the subjects that remained in remission, the subjects in the IDS-10 group exhibited poorer sleep continuity and tended to exhibit more medical symptoms than the subjects in the QHS-5, PRS-10, and QHS-10 groups. The poorer outcomes with the IDS-10 group is particularly interesting given that (1) the average frequency of active medication use for this group was higher than the PRS-10 group (>5 doses vs. ≤3.5 doses per week) and (2) the cumulative weekly dose was higher than either the QHS-5 or PRS-10 groups (50 mg for the IDS-10 group vs. 35 mg per week for PRS-10 and QHS-5 groups). The finding that the nightly use of 5 mg of zolpidem (QHS-5) was effective is consistent with the FDA’s new guidelines regarding dosing. The finding that the partial reinforcement condition (PRS-10) was effective suggests that this approach may be used to secure the gains of the intermittent dosing while providing a bridge for non-medication nights. Finally, it should be noted that the present approach is precisely the opposite of what is presently the standard of practice; rather than “start low and go slow”, three of the four conditions evaluated in the present study “start high and go low” (start with 10 mg nightly and switch to either a lower nightly dose or an intermittent dosing strategy with or without placebos on non-medication nights). One clear value of these alternative approaches is that its trajectory is not toward, but rather away from, dose escalation.
4.1. Limitations
There are five primary limitations to the present study. First, the use of a “joint hypothesis” approach (standard nightly dosing and partial reinforcement dosing will not differ with respect to efficacy and both will be superior to intermittent dosing) may be too simplistic and too confounded by sample size. In the future, non-inferiority designs and statistics may be a more appropriate approach testing “the sameness” of treatment approaches. Second, the change in the indication for zolpidem (from 10 to 5 mg for non-elderly women) made the proof-of-concept component of the study less rigorous. That is, had it been known that 5 mg was considered an adequate dose for any non-elderly subject, the Phase-1 dose would have been lowered to either 5 mg for all subjects (or for at least the women in the sample) and the Phase-2 frequency rate for medication use for the “low-no efficacy” condition (PRS-10) would have been reduced by 50%. That is, instead of using 50% medication and 50% placebos the strategy would have been 25% medication and 75% placebos (ie, one to two active doses per week as opposed to three to four active doses per week). Third, as implied earlier, the design would have taken into account gender issues and either limited recruitment to one gender or used block randomization to ensure adequate sampling of both genders. Fourth, the sample size per group was too small. Having more subjects would (1) increase our confidence regarding treatment similarities and differences, and (2) allowed for analyses by gender. The latter is of interest not only because of dose issues but also because of the possibility that one gender may be more predisposed to benefit from one strategy as opposed to the others. Fifth, the compliant subjects are a self-selected group and the influence of unmeasured confounders may have an impact on the present results.
4.2. Conclusions
The present data clearly support the perspective that the long-term treatment of insomnia can be accomplished with less medication. In the present case, this means that, following the obtention of a treatment response, lower doses and/or less frequent dosing may be used to prevent or delay relapse. The critical questions that emerge from this study are as follows. First, can the effects of this investigation be replicated with a starting dose of 5 mg and/or maintained with either 5 or 2.5 mg, regardless of age or gender? Second, can the PRS approach (as compared with the intermittent dosing strategy) be effective at substantially lower rates of medication use (eg, nightly pill use with one to two pills per week containing active medication vs. placebo)? Third, if it is found that “partial reinforcement” is more effective than low-frequency intermittent dosing, to what extent does the use of placebos specifically confer an advantage over non-medication nights (non-pill nights in the IDS condition)? Fourth, to what extent are the observed effects with the PRS strategy because of expectancy and/or conditioning and/or partial reinforcement? Answers to these questions may not only alter the standard of practice for the management of chronic insomnia, but they may also well open the door to the regular application of behavioral principles and practices to medical management of many disorders and diseases. If successful, in the future (when using a behavioral pharmatherapeutic approach) the questions one asks regarding medical management may not only be what medication, what dose, and what time of day, but also what conditioning dose, for how long, and what schedule of partial reinforcement. Further, this approach may not only facilitate long-term maintenance therapy while minimizing medication exposure, but it may also provide a method to manage medications with narrow therapeutic indices.
Final Comment. As interesting as the partial reinforcement approach may be, there is the concern that the prospects for the real-world application of this approach (placebo use along with active therapy) are limited. This presumes that the current standard of practice is that “outside the setting of clinical trials, there is no justification for the use of placebos” [30]. Assuming this is the current standard of practice, one would hope that if there are sufficient data to support the use of the partial reinforcement strategy (and clear procedures for when it can and cannot be used effectively), then the guidelines would change. Practically, the implementation of partial reinforcement would require that pharmacies distribute medication in foil packs where matching placebos are placed in accordance with prespecified schedules. In contrast to the absolute prohibition against placebo use, the current guideline is that placebos may be used “if the patient is informed of and agrees to [their] use” [31]. Thus, it is possible that the paradigm being proposed could be adopted with patient consent. This raises an important question, “if the patient knows that some of the pills prescribed (if not specifically which pills) are placebos, would this undermine the efficacy of the approach?” Preliminary work by Ader and colleagues suggest that “knowledge of the use of placebos in the pharmacotherapeutic protocol may attenuate but will not obviate the acquisition of the conditioned responses” [32,33]. Finally, while of less theoretical interest, it is entirely possible that a partial reinforcement paradigm could be designed and deployed without placebos. That is, exceptionally low-dose administrations of active drug could be substituted for placebos. In the case of chronic insomnia and maintenance treatment with zolpidem, this could be accomplished by using 1-mg doses of zolpidem instead of placebos.
Acknowledgments
Funding source
This project was supported by a grant from NCCAM, R01AT003332.
Abbreviations
- TIB
Time in bed
- SL
Sleep latency
- WASO
Wake after sleep onset
- NWAK
Number of awakenings
- TST
Total sleep time
- TST-C
Total sleep time calculated as (TIB − [SL + WASO]=TST)
- SE%-C
(TST-C/TIB) × 100
- QHS-10
Nightly dosing with 10 mg of zolpidem
- QHS-5
Nightly dosing with 5 mg of zolpidem with 10 mg of zolpidem
- PRS-10
Nightly “pill use” (50% 10 mg of zolpidem and 50% placebos)
- IDS-10
intermittent dosing (3–5 days per week)
Footnotes
Performance Site: University of Pennsylvania, Philadelphia, PA, USA.
Conflict of interest
The authors declare no financial conflicts of interests.
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: http://dx.doi.org/10.1016/j.sleep.2015.06.015.
References
- 1.National Institute of Health. NIH State-of-the-Science Conference on manifestations and management of chronic insomnia in adults; Bethesda, MD. 2005. [Google Scholar]
- 2.Ellis JG, Gehrman P, Espie CA, Riemann D, Perlis ML. Acute insomnia: current conceptualizations and future directions [Review] Sleep Med Rev. 2012;16(1):5–14. doi: 10.1016/j.smrv.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Consensus conference. Drugs and insomnia. The use of medications to promote sleep. JAMA. 1984;251(18):2410–14. [PubMed] [Google Scholar]
- 4.Perlis M, Gehrman P, Riemann D. Intermittent and long-term use of sedative hypnotics [Review] Curr Pharm Des. 2008;14(32):3456–65. doi: 10.2174/138161208786549290. [DOI] [PubMed] [Google Scholar]
- 5.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 6.Lacks P. Psychology practitioner guidebooks. London: Pergamon; 1987. Behavioral treatment for persistent insomnia. [Google Scholar]
- 7.American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 3. Westchester, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: 2013. [Google Scholar]
- 9.Hajak G, Cluydts R, Allain H, et al. The challenge of chronic insomnia: is non-nightly hypnotic treatment a feasible alternative? [Review] Eur Psychiatry. 2003;18(5):201–8. doi: 10.1016/s0924-9338(03)00089-0. [DOI] [PubMed] [Google Scholar]
- 10.Roth T, Franklin M, Bramley TJ. The state of insomnia and emerging trends [Review] Am J Manag Care. 2007;13(5 Suppl):S117–20. [PubMed] [Google Scholar]
- 11.Perlis ML, McCall WV, Krystal AD, Walsh JK. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65(8):1128–37. doi: 10.4088/jcp.v65n0816. [DOI] [PubMed] [Google Scholar]
- 12.Ader R. Conditioned immunopharmacological effects in animals: implications for a conditioning model of pharmacotherapy. In: White L, Tursky B, Schwartz GE, editors. Placebo: theory, research and mechanisms. New York: Guilford; 1985. pp. 306–23. [Google Scholar]
- 13.Ader R. The role of conditioning in pharmacotherapy. In: Harrington A, editor. The placebo effect: an interdisciplinary exploration. Cambridge: Harvard University Press; 1997. pp. 138–65. [Google Scholar]
- 14.Ader R, Mercurio MG, Walton J, et al. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom Med. 2010;72(2):192–7. doi: 10.1097/PSY.0b013e3181cbd38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: APA; 1994. DSM-IV. [Google Scholar]
- 17.American Academy of Sleep Medicine. International classification of sleep disorders. 2. American Academy of Sleep Medicine; 2005. [Google Scholar]
- 18.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 19.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 22.Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 23.Ramanaiah NV, Franzen M, Schill T. A psychometric study of the State-Trait Anxiety Inventory. J Pers Assess. 1983;47(5):531–5. doi: 10.1207/s15327752jpa4705_14. [DOI] [PubMed] [Google Scholar]
- 24.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–83. doi: 10.1016/s0006-3223(02)01866-8. Erratum in: Biol Psychiatry 2003, 54, (5), 585. [DOI] [PubMed] [Google Scholar]
- 25.Baker FC, Driver HS. Self-reported sleep across the menstrual cycle in young, healthy women. J Psychosom Res. 2004;56:239–43. doi: 10.1016/S0022-3999(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 26.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 27.Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA. 1984;252:1905–7. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 29.Perlis M, Smith M, Jungquist C, Posner D. The cognitive-behavioral treatment of insomnia: a session by session guide. New York: Springer Publishing; 2005. [Google Scholar]
- 30.Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344(21):1594–602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- 31.Bostick NA, Sade R, Levine MA, Stewart DM, Jr American Medical Association Council on Ethical and Judicial Affairs. Placebo use in clinical practice: report of the American Medical Association Council on Ethical and Judicial Affairs. J Clin Ethics. 2008;19(1):58–61. [PubMed] [Google Scholar]
- 32.Giang DW, Goodman AD, Schiffer RB, et al. Conditioning of cyclophosphamide-induced leukopenia in humans. J Neuropsychiatry Clin Neurosci. 1996;8(2):194–201. doi: 10.1176/jnp.8.2.194. [DOI] [PubMed] [Google Scholar]
- 33.Olness K, Ader R. Conditioning as an adjunct in the pharmacotherapy of lupus erythematosus. J Dev Behav Pediatr. 1992;13(2):124–5. doi: 10.1097/00004703-199204000-00008. [DOI] [PubMed] [Google Scholar]