Abstract
Purpose
The goal of this study was to investigate the long-term efficacy and safety of a mixture of polymethyl methacrylate (PMMA) and cross-linked dextran Lipen-10® used for penile augmentation under the physical impact generated during sexual intercourse.
Materials and Methods
From March 2010 to October 2011, a total of 20 patients with a mean age of 44 years (interquartile range, 20~70 years) who requested penile augmentation participated in this study. Lipen-10® filler is a mixture of 75% cross-linked dextran, 15% PMMA, and 10% hypromellose solution. With the patient in the supine position, Lipen-10® was injected into the subcutaneous tissue between the dartos fascia and Buck's fascia of the penis using a fanning technique. Penile length and circumference were measured before the procedure and six, 12, and 18 months after the procedure. Values were compared using the Student's t-test and the paired t-test.
Results
A total of 15 patients completed this study. The increases in circumference and length observed six months after the procedure were found to have been maintained without change at 12 and 18 months of follow-up. At 12 and 18 months of follow-up, no abnormal findings were observed. Pelvic magnetic resonance imaging conducted at 18 months of follow-up showed no trace of the injected filler having migrated to other sites, and the volume was well maintained.
Conclusions
Lipen-10®, a mixture of PMMA and cross-linked dextran, showed good durability and tolerability over 18 months of follow-up during which the participants were sexually active.
Keywords: Cross-linked dextran, Filler, Penile enhancement, Polymethyl methacrylate
INTRODUCTION
Since the early 1980s, when bovine collagen products were first introduced as dermal fillers, various formulations have been developed and utilized in the clinical setting [1]. Such dermal fillers have been used with increasing frequency because they have fewer adverse effects than surgical cosmetic procedures, are less invasive, are less expensive, and result in satisfactory patient outcomes [2]. However, despite these advantages, bovine collagen products trigger hypersensitivity reactions in 3% of patients, which warrants the inconvenience of an allergen test prior to the procedure [3]. Moreover, since collagenand hyaluronic acid-based dermal fillers are naturally absorbed by the body within a short period of time, the further inconvenience of regular procedures is required [4,5]. Therefore, long-lasting products such as calcium hydroxyapatite, injectable poly-L-lactic acid, and polymethyl methacrylate (PMMA) fillers have been developed, but each filler has distinct advantages and disadvantages.
A recently developed dermal filler consisting of PMMA and cross-linked dextran (Lipen-10; Chungwha Medipower Corporation, Seoul, Korea) is known not to cause allergic reactions. Furthermore, PMMA microspheres are not subjected to phagocytosis due to their large molecular size, while some are encapsulated by connective tissue consisting of fibroblasts, which inhibit their migration to other tissues and subsequent volume loss. Dextran also stimulates fibroblasts after being absorbed by macrophages, and is therefore replaced by internal tissue, resulting in no loss of volume [2,6]. In a previous study, we established the tolerability and short-term efficacy of a mixture of PMMA and cross-linked dextran for penile augmentation [7]. In this study, we report full efficacy and safety data over 18 months of follow-up.
MATERIALS AND METHODS
1. Subjects
A total of 20 patients with a mean age of 44 years (interquartile range, 20~70 years) who requested penile augmentation from March 2010 to October 2011 participated in this study. The patients were informed about the test material, procedure, and possible complications prior to participating. The patients underwent a physical examination and an evaluation of their medical history. Those who had a history of psychological/psychiatric disorders, congenital or acquired defects on their external genitalia, a history of previous penile plastic surgery, or history of systemic chronic diseases such as cancer or diabetes were excluded from this study.
This study was conducted in two medical institutions (n=10 subjects each) and approved by the Institutional Review Board of the Kangdong Sacred Heart Hospital (IRB No. 09-113).
2. Material
Lipen-10® filler is a mixture of 75% cross-linked dextran and 15% PMMA, and its use for soft tissue augmentation was approved by the Korean Food and Drug Administration in 2010. Cross-linked dextran and PMMA are microspheres of 45 to 120 µm and 30 to 120 µm, respectively. These microspheres account for 90% of the volume and are suspended in hypromellose solution, which comprises the other 10%.
3. Injection method
Injections were performed by one surgeon at each institution, and all of the procedures were conducted externally. With the patient in the supine position, the penis was sterilized with povidone-iodine and a penile dorsal block was performed using 0.2% lidocaine.
Using a 20-gauge needle, Lipen-10® was injected into the space between the dartos fascia and Buck's fascia of the penis using a fanning technique. While the penis was pulled with constant force, the Lipen-10® was injected parallel to the corpus cavernosum, so that a constant amount was inserted between the distal shaft and the root. The average injected amount was 23.73 mL (interquartile range, 17~30 mL), and four to six needle punctures were made in each patient. The tissue was massaged gently to evenly redistribute the Lipen-10® throughout the injection site.
After the procedure, an elastic penile support bandage was applied to ensure that the Lipen-10® would remain within the injection site and prevent edema within the penis.
4. Evaluation
Penile length and circumference were measured before the procedure and six, 12, and 18 months after the procedure. Penis circumference was measured at the base, mid-shaft, and distal shaft in the flaccid state. Penis length was defined as the distance from the pubic-penile skin junction to the coronal sulcus of the penis with the patient in the supine position and the penis either flaccid or held upright without stretching. Magnetic resonance imaging (MRI) was performed in four patients at the six-month and 18-month follow-up appointments in order to monitor the migration of the injected material.
Adverse effects were divided into mild, moderate, and severe, and recorded after consultation and a physical examination at outpatient visits. All predicted and unpredicted adverse effects were collected from all participants, and their type, frequency, level, and association with the injected material were evaluated. Adverse effects were divided into local adverse effects, including injected site pain, edema, cyst formation, necrosis, serous discharge due to a foreign body reaction, infection, nodule, and asymmetry, and systemic adverse effects, including fever, allergic reaction, and hemodynamic changes.
5. Statistics
Penis circumference and length were compared using the Student's t-test and the paired t-test, and the occurrence of adverse effects was analyzed using McNemar's test. IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA) was used for all analyses. p<0.05 were considered to indicate statistical significance.
RESULTS
A total of 20 patients completed the baseline evaluation and underwent penile augmentation using Lipen-10®. Follow-up was conducted in a total of 17 patients for one year (two patients withdrew their consent and one was lost to follow-up). At 18 months, two more patients had been lost to follow-up; therefore, a total of 15 patients completed the full extent of follow-up.
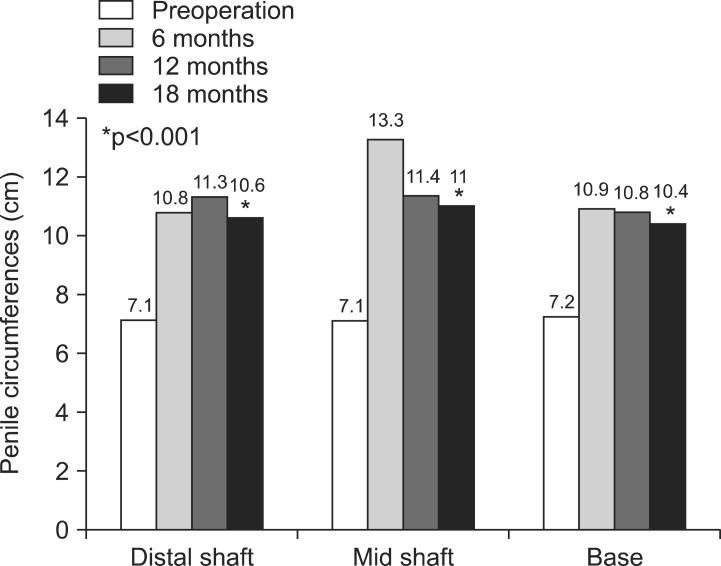
At 12 and 18 months of follow-up after the procedure, the increases in circumference observed six months after the procedure were maintained without change (Fig. 1). In the flaccid state 18 months after the procedure, the base, mid-shaft, and distal shaft circumference measurements were 10.4±0.9 cm, 11.0±0.8 cm, and 10.6±1.4 cm, respectively, which were significant increases compared to the baseline values (Fig. 2; all p<0.001). The 18-month measurements were not significantly different from those obtained six and 12 months after the procedure. The average length of the flaccid penis increased between before the procedure (3.6±1.8 cm) and 12 months after the procedure (6.6±1.6 cm). The increased length was maintained at 6.6±1.2 cm at 18 months, with no statistically significant differences compared to the six-month or 12-month values (p=0.103, p=0.897, respectively).
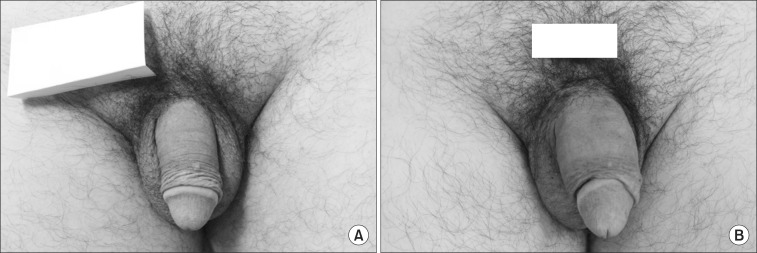
Fig. 1. Girth enhancement was maintained over 18 months after the injection (B) in comparison with the pre-injection baseline (A).
Fig. 2. Pre- and post-procedural penile circumference (cm), with a comparison of mean penile size before the penile injection and six, 12, and 18 months after the procedure. The increases in circumference observed at six months were maintained over 18 months of follow-up (preoperation vs. 18 months, all p<0.001).
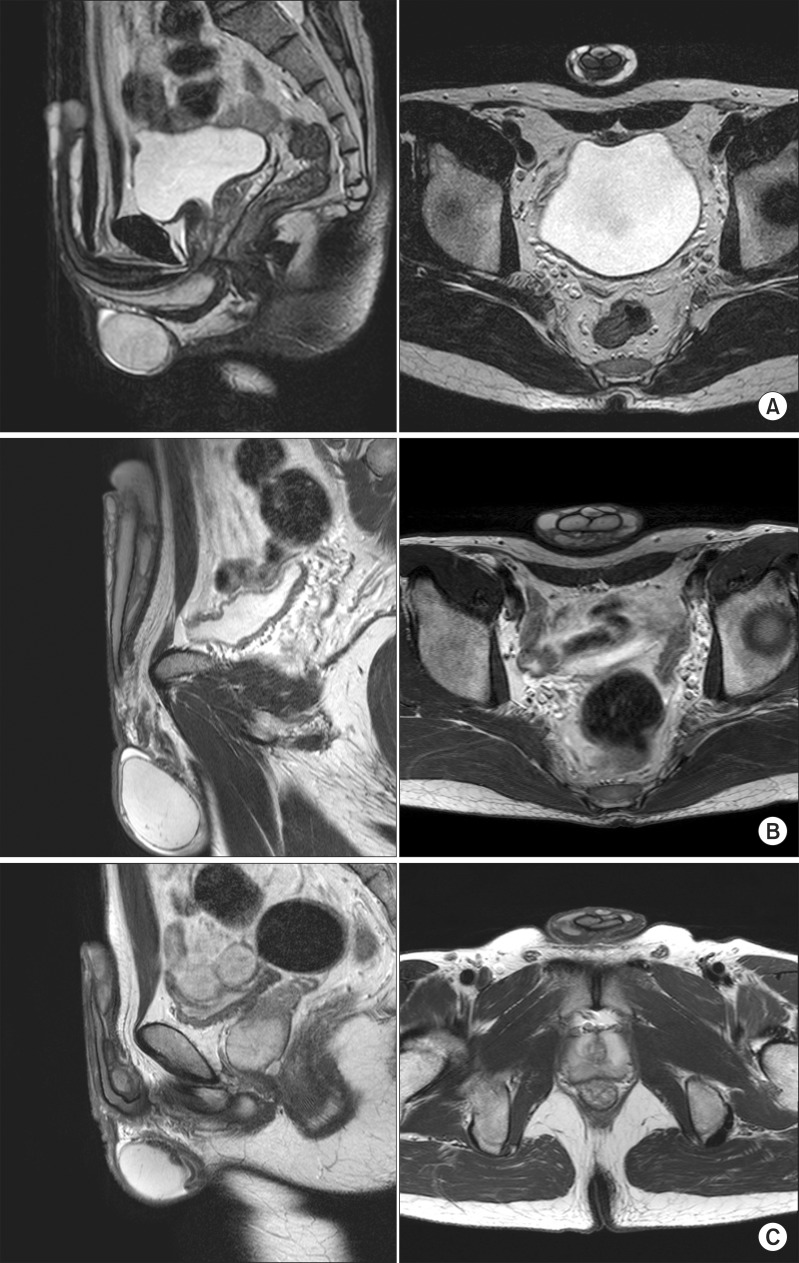
At 12 months of follow-up after the procedure, no local symptoms, such as penile edema, that were observed immediately after the procedure remained present. At 12 and 18 months of follow-up, no abnormal findings were observed, even in a patient who required an additional procedure to correct mild penile shape asymmetry observed one month after the initial procedure. In one patient, a 5-mm nodule that was observed at the injection site in the six-month follow-up examination was still visible at 12 and 18 months of follow-up, with no changes, but the patient did not complain of pain or discomfort during sexual intercourse. On a pelvic MRI conducted during the 18-month follow-up visit, no traces were observed of the injected filler having migrated to the scrotum or the abdominal wall, and the volume appeared well maintained, based on a comparison with pelvic MRI findings immediately and six months after the procedure (Fig. 3).
Fig. 3. Pelvic magnetic resonance images. No demonstrable migration of the injected Lipen-10® was seen in the scrotum or abdominal walls immediately (A), six months (B), or 18 months (C) after the procedure.
DISCUSSION
Man's desire for a larger penis has persisted over time [8], despite the range of objections that have been articulated against penile augmentation. Doctors who are against penile augmentation have expressed concerns that patients may be negatively affected by unforeseen complications, such as penile deformity, paradoxical penile shortening, unpleasant scarring, granuloma formation, migration of the injected material, and sexual dysfunction. However, the use of penile decorations throughout history, including piercings and the intentional biting of the glans of the penis by poisonous snakes, highlights man's continual desire for penile augmentation [9].
Patients seek out penile augmentation to boost their self-esteem and satisfy their partners [10]. Interestingly, most patients consider the size of their penis in the flaccid state more important than its size when erect [11,12]. This may be due to the castration complex, in which a young boy compares his penis with that of his healthy father during the course of normal psychological development [13]. It may also be due to a persistent feeling of inferiority regarding penis size based on comparisons with friends of the same age whose secondary sexual characteristics developed earlier, given that the timing of the development of secondary sexual characteristics during puberty varies among individuals [14].
A study of penis size and self-esteem of Korean males in their twenties found that the average penis length of Korean males was 6.1±1.3 cm, and that most subjects who considered their penis unusually small or large were within the range of one standard deviation, indicating that they had incorrect views regarding their penis size [12]. The penis length data of that study and those of the present study on Korean men in their forties could not be directly compared due to differences in the measurement methods. However, the average circumference at the mid-shaft of the penis, where similar measurement methods were used, was 8.9±0.8 cm for men in their twenties, which was approximately 1 cm larger than observed in men in their forties (7.3±0.9 cm). However, 18 months after penile augmentation, the mean penile circumference of the men in their forties was 11.0±0.8 cm, showing an average increase to 2 cm more than that of men in their twenties. Despite the fact that this involved an indirect comparison between different numbers and groups of patients, this finding suggests that the augmentation effect of Lipen-10® was stable.
In this study, a mean 3-cm increase in the length of the flaccid penis was observed after penile augmentation using Lipen-10®. This penile length enhancement effect was consistently observed over 18 months of follow-up, and was likely due to the splint effect, which inhibits physiological contraction of the penis once the Lipen-10® becomes stably fixed after its injection into the site between Buck's fascia and the dartos fascia [7]. This result is significant, since it shows that the injection material alone enabled long-term penile lengthening.
Although dermal filler injections are minimally invasive compared to surgical cosmetic procedures, their safety issues have been the subject of significant debate. Autologous fat injection has been reported to have only a 10% survival rate, while a number of complications have been reported to be related to ischemia due to an insufficient blood supply to the injected fat [15]. A volume of over 100 mL of silicone is required for penile augmentation, and the injected silicone migrates with gravity, causing penile distortion and a granulomatous reaction [16,17]. The collagen products that were first used as dermal fillers also exhibited problems related to rapid degradation and hypersensitivity [18]. Hyaluronic acid is a natural component within the human body and has no issues with biocompatibility and immune reaction, but it does have the disadvantage of requiring regular injections every three to six months [2,5]. In contrast, Lipen-10®, which is a mixture of cross-linked dextran and PMMA, did not show volume changes over 18 months of follow-up and no adverse effects were observed, with the exception of a tiny nodule that resulted from a technical mistake during the injection.
Although Artefill® (Artes Medical, San Diego, CA, USA), a PMMA-based dermal filler, was approved by the United States Food and Drug Administration [19], it remains unclear whether PMMA, which is composed of non-degradable microspheres, subcutaneously grafted to the penis can migrate to a site distant from the graft site due to the physical impact generated during sexual intercourse. In fact, for materials such as polyacrylamide that cannot produce their own collagen, migration due to external impacts is possible and related adverse effects have been reported [20,21]. Our results over 18 months of follow-up demonstrate that Lipen-10® maintained its volume and location despite normal sexual activity. This is likely due to the neocollagenesis characteristic of PMMA, which has been conclusively observed in histological analyses in animal experiments using rats. After one year, most of the cross-linked dextran disappears after degradation and the site becomes filled with a large amount of self-produced collagen, which encapsulates the PMMA and allows it to withstand physical impacts [6].
Of the dermal fillers that are currently in use, autologous fat, collagen, and hyaluronic acid are non-permanent options that are retained for a maximal duration of three to twelve months [2,21]. Hence, studies with 18 months of follow-up are rare. Even with the development of permanent fillers such as PMMA and polyacrylamide, long-term follow-up of injections of these materials has not been reported to date. Long-term follow-up is especially important for permanent fillers, since fatal adverse effects can occur, as with silicone. In this study, Lipen-10® successfully maintained its volume and location for 18 months, and no significant adverse effects were observed.
Despite its prospective design, this study has several limitations. First, due to the nature of the clinical experiment, no control group was included. As such, further comparative experiments with other novel dermal fillers might be beneficial. Second, no data were collected regarding patients' subjective satisfaction. Since penile augmentation is performed for personal reasons, patients' subjective satisfaction might be more important than objectively measured data.
CONCLUSIONS
Penile injections of a mixture of cross-linked dextran and PMMA demonstrated significant efficacy in enhancing penile girth, showed good durability, and were welltolerated, without serious adverse events over 18 months of follow-up.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Nicolle FV. Use of Zyderm in the aging face. Aesthetic Plast Surg. 1982;6:193–195. doi: 10.1007/BF01570645. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg DJ. Breakthroughs in US dermal fillers for facial soft-tissue augmentation. J Cosmet Laser Ther. 2009;11:240–247. doi: 10.3109/14764170903341731. [DOI] [PubMed] [Google Scholar]
- 3.Baumann L. Collagen-containing fillers: alone and in combination. Clin Plast Surg. 2006;33:587–596. doi: 10.1016/j.cps.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Rostan E. Collagen fillers. Facial Plast Surg Clin North Am. 2007;15:55–61. doi: 10.1016/j.fsc.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Monheit GD, Coleman KM. Hyaluronic acid fillers. Dermatol Ther. 2006;19:141–150. doi: 10.1111/j.1529-8019.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 6.Jang MW, Jin BK, Lee SH, Park JH, Ryu JM, Yun SP, et al. Effect of PMMA and cross-linked dextran mixture on biosafety and volume in rat. Tissue Eng Regen Med. 2010;7:57–63. [Google Scholar]
- 7.Yang DY, Lee WK, Kim SC. Tolerability and efficacy of newly developed penile injection of cross-linked dextran and polymethylmethacrylate mixture on penile enhancement: 6 months follow-up. Int J Impot Res. 2013;25:99–103. doi: 10.1038/ijir.2012.41. [DOI] [PubMed] [Google Scholar]
- 8.Vardi Y, Har-Shai Y, Gil T, Gruenwald I. A critical analysis of penile enhancement procedures for patients with normal penile size: surgical techniques, success, and complications. Eur Urol. 2008;54:1042–1050. doi: 10.1016/j.eururo.2008.07.080. [DOI] [PubMed] [Google Scholar]
- 9.Talalaj J, Talalaj S. The strangest human sex, ceremonies and customs. 1st ed. Melbourne: Hill of Content; 1994. [Google Scholar]
- 10.Francoeur R, Perper T, Scherzer N. A descriptive dictionary and atlas of sexology. 1st ed. New York, NY: Greenwood Press; 1991. [Google Scholar]
- 11.Vardi Y, Lowenstein L. Penile enlargement surgery: fact or illusion? Nat Clin Pract Urol. 2005;2:114–115. doi: 10.1038/ncpuro0120. [DOI] [PubMed] [Google Scholar]
- 12.Son H. Normal penile size and self esteem about penile size of the third decade men in Korea. Korean J Urol. 1999;40:1037–1042. [Google Scholar]
- 13.Schwartz BJ. The measurement of castration anxiety and anxiety over loss of love. J Personal. 1955;24:204–219. [Google Scholar]
- 14.Reinsch JM, Beasley R. The kinsey institute new report on sex: what you must know to be sexually literate. 1st ed. New York, NY: St Martin's press; 1990. pp. 43–65. [Google Scholar]
- 15.Nguyen A, Pasyk KA, Bouvier TN, Hassett CA, Argenta LC. Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast Reconstr Surg. 1990;85:378–386. [PubMed] [Google Scholar]
- 16.Vardi Y, Gruenwald I. The status of penile enhancement procedures. Curr Opin Urol. 2009;19:601–605. doi: 10.1097/MOU.0b013e3283318f31. [DOI] [PubMed] [Google Scholar]
- 17.Wassermann RJ, Greenwald DP. Debilitating silicone granuloma of the penis and scrotum. Ann Plast Surg. 1995;35:505–509. doi: 10.1097/00000637-199511000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Comper WD, Laurent TC. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978;58:255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- 19.Artefill prescribing information [Internet] Sandiego, CA: Artes Medical, Inc; c2007. [cited 2013 Jan 7]. Available from: http://www.artefil.com. [Google Scholar]
- 20.Xiaoling F, Yi C, Zhang Y, Wang Y, Li W, Yang M, et al. Analysis of the complications induced by polyacrylamide hydrogel injection. Plast Reconstr Surg. 2004;114:261–262. doi: 10.1097/01.prs.0000127232.07433.b7. [DOI] [PubMed] [Google Scholar]
- 21.Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003;27:354–366. doi: 10.1007/s00266-003-3022-1. [DOI] [PubMed] [Google Scholar]