Abstract
Swertia chirayita (Gentianaceae), a popular medicinal herb indigenous to the temperate Himalayas is used in traditional medicine to treat numerous ailments such as liver disorders, malaria, and diabetes and are reported to have a wide spectrum of pharmacological properties. Its medicinal usage is well-documented in Indian pharmaceutical codex, the British, and the American pharmacopeias and in different traditional medicine such as the Ayurveda, Unani, Siddha, and other conventional medical systems. This ethnomedicinal herb is known mostly for its bitter taste caused by the presence of different bioactive compounds that are directly associated with human health welfare. The increasing high usage of Swertia chirayita, mostly the underground tissues, as well as the illegal overharvesting combined with habitat destruction resulted in a drastic reduction of its populations and has brought this plant to the verge of extinction. The increasing national and international demand for Swertia chirayita has led to unscrupulous collection from the wild and adulteration of supplies. The aim of this review is to provide a synthesis of the current state of scientific knowledge on the medicinal uses, phytochemistry, pharmacological activities, safety evaluation as well as the potential role of plant biotechnology in the conservation of Swertia chirayita and to highlight its future prospects. Pharmacological data reported in literature suggest that Swertia chirayita shows a beneficial effect in the treatment of several ailments. However, there is lack of adequate information on the safety evaluation of the plant. The pharmacological usefulness of Swertia chirayita requires the need for conservation-friendly approaches in its utilization. Providing high-quality genetically uniform clones for sustainable use and thereby saving the genetic diversity of this species in nature is important. In this regard, plant biotechnological applications such as micropropagation, synthetic seed production, and hairy root technology can play a significant role in a holistic conservation strategy. In addition to micropropagation, storage of these valuable genetic resources is equally important for germplasm preservation. However, more advanced research is warranted to determine the activities of bioactive compounds in vitro and in vivo, establish their underlying mechanisms of action and commence the process of clinical research.
Keywords: biological activity, conservation, medicinal plant, Swertia chirayita, traditional medicine
Introduction
One of the prerequisites for the success of primary health care is the availability and use of suitable drugs. Traditional medicine is still the most affordable and easily accessible source of treatment in the primary healthcare system. Medicinal plants have always been a potential source to cure different diseases, either in the form of traditional preparations or as pure active principles, and they are frequently the only source of medicine for the majority of people in the developing world.
Swertia, a genus in the family Gentianaceae include a large group of annual and perennial herbs, representing approximately 135 species. Swertia species are common ingredients in a number of herbal remedies. In India, 40 species of Swertia are recorded (Clarke, 1885; Kirtikar and Basu, 1984), of which, Swertia chirayita is considered the most important for its medicinal properties. S. chirayita was first described by Roxburgh under the name of Gentiana chyrayta in 1814 (Scartezzini and Speroni, 2000). S. chirayita, common name: “Chiretta” (Figure 1) is a critically endangered medicinal herb that grows at high altitudes in the sub-temperate regions of the Himalayas between 1200 and 2100 m altitudes from Kashmir to Bhutan (Bentley and Trimen, 1880; Clarke, 1885) on the slopes of moist shady places (Gaur, 1999; Figure 2). Its widespread uses in traditional medicine have resulted in over-exploitation from the natural habitat and it is now on the verge of extinction in the wild. S. chirayita is also known by an array of names such as Anaryatikta, Bhunimba, Chiratitka, Kairata in Sanskrit, Qasabuzzarirah in Arab and Farsi, Chiaravata in Urdu, Sekhagi in Burma, and Chirrato or Chiraita in Nepal (Joshi and Dhawan, 2005). Some authors have described S. chirayita as an annual (Anon, 1982; Kirtikar and Basu, 1984) and others as a biennial or pluri-annual (Edwards, 1993). This ethnomedicinal herb is known mostly for its bitter taste caused by the presence of different chemical constituents such as amarogentin (most bitter compound isolated till date), swerchirin, swertiamarin, and other bioactive compounds that are directly associated with human health welfare (Joshi and Dhawan, 2005). Due to its excessive over-exploitation from the natural habitat, narrow geographic occurrence (Bhat et al., 2013) and unresolved inherent problems of seed viability and seed germination (Badola and Pal, 2002; Joshi and Dhawan, 2005), alternative approaches for propagation and conservation are urgently required to avoid the possible extinction of this important species. Consequently, S. chirayita has been receiving increasing attention from a wide range of researchers as evident from the number of publications appearing in the literature (Chen et al., 2011; Nagalekshmi et al., 2011; Ghosh et al., 2012; Kumar and Chandra, 2013, 2014, 2015; Fan et al., 2014; Kumar et al., 2014; Sharma et al., 2014, 2015; Padhan et al., 2015; Zhou et al., 2015). However, a comprehensive review detailing the documented ethnomedicinal uses, pharmacological properties and safety evaluation carried out on S. chirayita and identifying the existing knowledge gap is lacking. In this review, we document the medicinal uses and phytochemical properties of S. chirayita. Future prospects including the potential conservation approaches to ensure a continuous supply for both local and international expanding markets and safety evaluation on uses of the species for medicinal purposes are highlighted.
Figure 1.
Swertia chirayita. (A) Seeds, (B) Plant in nature, (C) Root of a mature plant, (D) Dry plant material, (E) High shoot multiplication in a plant tissue culture system.
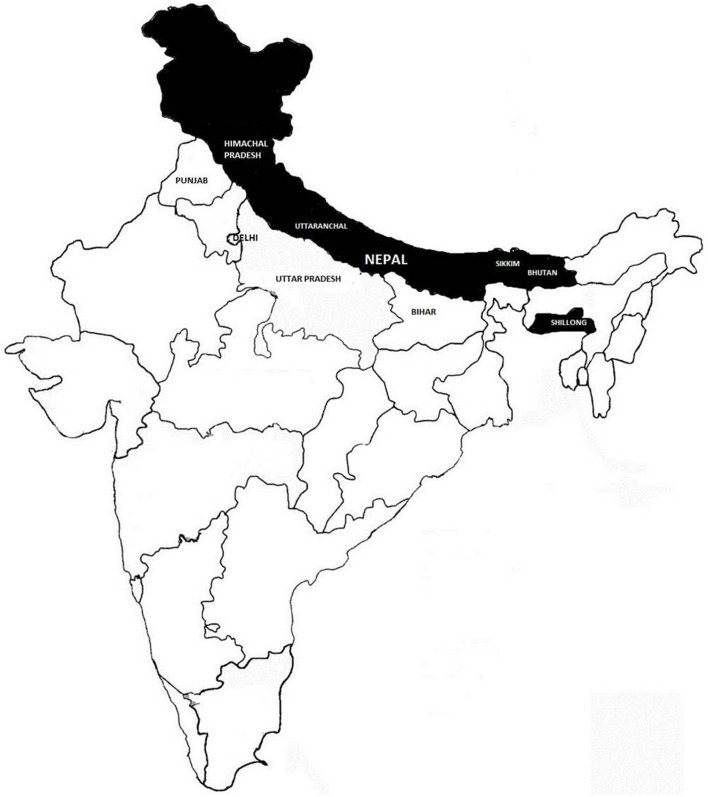
Figure 2.
Natural distribution of Swertia chirayita. The shaded area represents the natural habitat of Swertia chirayita in the Himalayan Region.
Botanical description
S. chirayita is an annual/biennial herb 0.6–1.5 m tall. It has an erect, around 2–3 ft long stem, the middle portion is cylindrical, while the upper is quadrangular, with a prominent decurrent line at each angle. Its stem is orange brown or purplish in color with large continuous yellowish pith (Bentley and Trimen, 1880; Joshi and Dhawan, 2005). Leaves are lanceolate, in opposite pairs, no stalks, acuminate, cordate at the base, sessile, five to seven nerved and 4 cm long (Scartezzini and Speroni, 2000). The root is simple, yellowish, somewhat oblique, or geniculate, tapering and short, almost 7–8 cm long and usually half an inch thick (Bentley and Trimen, 1880; Scartezzini and Speroni, 2000). Flowers are small, numerous, tetramerous, large leafy panicles, green-yellow, and tinged with purple and green or white hairs (Scartezzini and Speroni, 2000; Joshi and Dhawan, 2005). The calyx is gamophyllous with four lobes, corolla-lobes four twisted and superimposed, united at the base where they have pairs of nectaries on each lobe covered with long hairs. Stamens 4, opposite the corolla lobe, at the base of the corolla. Ovary unilocular with ovules laminal placentation parietale; two stigmas. Capsules are egg-shaped, 2-valved with a transparent yellowish pericarp. Seeds are numerous, very small and dark brownish in color (Chandra et al., 2012). Multi-colored corolla and the presence of nectaries support cross-pollination in S. chirayita.
Medicinal uses
S. chirayita a traditional Ayurvedic herb is used by different indigenous population groups in multiple ways for several medicinal purposes (Table 1). The whole plant is widely used by local people for the treatment of hepatitis, inflammation, and digestive diseases (Bhatt et al., 2006). The wide range of medicinal uses include the treatment of chronic fever, malaria, anemia, bronchial asthma, hepatotoxic disorders, liver disorders, hepatitis, gastritis, constipation, dyspepsia, skin diseases, worms, epilepsy, ulcers, scanty urine, hypertension, melancholia, and certain types of mental disorders, secretion of bile, blood purification, and diabetes (Karan et al., 1999; Banerjee et al., 2000; Rai et al., 2000; Saha et al., 2004; Chen et al., 2011). Recently, S. chirayita extracts showed anti-hepatitis B virus (anti-HBV) activities (Zhou et al., 2015). Traditionally, decoctions of this species are used for anthelmintic, hepatoprotective, hypoglycemic, antimalarial, antifungal, antibacterial, cardiostimulant, antifatigue, anti-inflammatory, antiaging, antidiarrheal, as protectant of the heart and also help in lowering blood pressure and blood sugar (Schimmer and Mauthner, 1996). Herbal formulations such as Ayush-64, Diabecon, Mensturyl syrup, and Melicon V ointment (Edwin and Chungath, 1988; Mitra et al., 1996) contain S. chirayita extract in different concentrations for its antipyretic, hypoglycaemic, antifungal, and antibacterial properties. Furthermore, the curative value of this herb has also been recorded in ancient Ayurveda medicine systems and other conventional medical systems.
Table 1.
Ethnobotanical uses of Swertia chirayita in traditional medicine.
| Plant part used | Traditional uses | References |
|---|---|---|
| Whole plant | Used in several traditional and indigenous systems of medicines, such as Ayurveda, Unani, and Siddha | Mukherji, 1953; Kirtikar and Basu, 1984; Joshi and Dhawan, 2005; |
| Whole plant | Used in British and American pharmacopeias as tinctures and infusions | Joshi and Dhawan, 2005 |
| Root | Serves as a drug and an effective tonic for general weakness, fever, cough, joint pain, asthma, and the common cold | Kirtikar and Basu, 1984; Joshi and Dhawan, 2005 |
| Whole plant | For headaches and blood pressure, the leaves and chopped stems are soaked overnight in water. A paste is prepared and filtered with 1 glass of water. The preparation is consumed once a day for 2–3 days | de Rus Jacquet et al., 2014; Malla et al., 2015 |
| Whole plant | For Tremor fever, whole S. chirayita plants are cut into small pieces and boiled in 1/2 L of water until the volume is reduced to less than half glass. The filtered water is stored in a glass bottle and half spoon is given to children once a day for 2 days. For adult, the posology is 1 spoon once in a day for 2 days and varies to three times a day until cured | de Rus Jacquet et al., 2014 |
| Whole plant | Boiled in water and one cup of decoction is taken orally to cure malaria | Shah et al., 2014 |
| Whole plant | Paste of the plant is applied to treat skin diseases such as eczema and pimples | Joshi and Dhawan, 2005; Malla et al., 2015 |
| Whole plant | Liver disorders; stomach disorders like dyspepsia and diarrhea, intestinal worms | Mukherji, 1953; Joshi and Dhawan, 2005 |
| Whole plant | Hiccups and vomiting, ulcers, gastrointestinal infections, and kidney diseases | Kirtikar and Basu, 1984 |
| Whole plant | Used in combination with other drugs in cases of scorpion bite | Nandkarni, 1976 |
| Whole plant | Used in excessive vaginal discharge | Jadhav and Bhutani, 2005 |
The widespread uses of S. chirayita in traditional drugs have resulted in considerable chemical analysis of the plant, and active principles which attribute the plant its medicinal properties. S. chirayita is also used in British and American pharmacopeias as tinctures and infusions (Joshi and Dhawan, 2005). The whole plant is used in traditional remedies but the root is mentioned to be the most bioactive part (Kirtikar and Basu, 1984).
Pharmacological activity
The varied ethnobotanical uses of S. chirayita have led to the initiation of various pharmacological investigations. Previous research demonstrates that the S. chirayita extracts exhibit a wide range of biological activities, such as antibacterial, antifungal, antiviral, anticancer, anti-inflammatory, and others like antidiabetic and antioxidant activities (Verma et al., 2008; Alam et al., 2009; Arya et al., 2011; Chen et al., 2011; Laxmi et al., 2011). Concurrently, a diverse range of in vitro and in vivo test systems has been used to evaluate the pharmacological properties of S. chirayita. Evidence-based laboratory investigations indicate that aqueous, alcoholic and methanolic extracts of S. chirayita possess a number of promising pharmacological properties. The whole plant of S. chirayita have been reported to be used for the treatment of antibacterial and antifungal activity (Alam et al., 2009; Laxmi et al., 2011; Rehman et al., 2011). Anti-hepatitis B virus activity of S. chirayita extracts was also studied on HepG 2.2.15 cells line (Zhou et al., 2015). The whole plant of S. chirayita has been reported for the anti-inflammatory and hypoglycemic activity (Banerjee et al., 2000; Kar et al., 2003; Alam et al., 2011; Das et al., 2012; Verma et al., 2013). Chen et al. (2011) investigated the 70% ethanolic extract of S. chirayita for antioxidant activities by using antioxidant tests including reducing power and beta-carotene assay. The results showed that 70% ethanolic extracts exhibited high DPPH scavenging activity (IC50 = 267.80 μg/mL). Table 2 presents a summary focusing on the pharmacological evaluations using in vitro and in vivo systems whereas Table 3 provides antioxidant potential of S. chirayita.
Table 2.
Evaluation of the biological activities of Swertia chirayita.
| Bioactivity evaluated | Plant part(s) tested | Test system | aExtracting solvent | Test Organism/Models | Control | Toxicity test | References |
|---|---|---|---|---|---|---|---|
| Antibacterial | Whole plant | In vitro | EtOH | Escherichia coli ATCC 26922 | Ciprofloxacin | None | Rehman et al., 2011 |
| Klebsiella pneumonia ATCC 15380 | |||||||
| Pseudomonas aeruginosa ATCC 25619 | |||||||
| Proteus vulgaris ATCC 6380 | |||||||
| Antibacterial | Stem | In vitro | MeOH | Bacillus subtilis ATCC 6633 | Ceftriaxone, Ceftriaxone sodium, Cefuroxine, Ciprofloxacin, Gentamycine, Levofloxacin, Metronidazole, Tranexamicacid | None | Khalid et al., 2011 |
| Enterococcus faecalis (ATCC 14506) | |||||||
| Staphylococcus aureus (ATCC 6538) | |||||||
| Pseudomonas aeruginosa (ATCC 27853) | |||||||
| Salmonella typhi (ATCC 14028) | |||||||
| Antibacterial | Whole plant | In vitro | MeOH | Bacillus subtilis MTCC 736 | Gentamycin | None | Laxmi et al., 2011 |
| Bacillus polymyxa | |||||||
| Staphylococcus aureus MTCC 3160 | |||||||
| Escherichia coli MTCC 723 | |||||||
| Salmonella typhi MTCC 3216 | |||||||
| Vibria cholera MTCC 3906 | |||||||
| Streptococcus pyogenes MTCC 1927 | |||||||
| Proteus mirabilis MTCC 1429 | |||||||
| Providentia alkalifaciens | |||||||
| Pseudomonas aeruginosa MTCC 7837 | |||||||
| Antibacterial | Whole plant | In vitro | DCM; EtOH | Staphylococcus aureus | Kanamycin 30 μg/disc | None | Alam et al., 2009 |
| Antibacterial | Stem | In vitro | EtOH | Staphylococcus aureus | Chloramphenicol 30 μg/disc | Brine shrimp assay–positive | Sultana et al., 2007 |
| Bacillus subtilis | |||||||
| Salmonella typhi | |||||||
| Shigella flexeneriae | |||||||
| Sarcina lutea | |||||||
| Bacillus megaterium | |||||||
| Antifungal | Whole plant | In vitro | MeOH | Aspegillus niger MTCC 1881 | Amphotericin | None | Laxmi et al., 2011 |
| Aspergillus flavus MTCC 1883 | |||||||
| Cladosporium oxysporum MTCC 1777 | |||||||
| Antileishmanial | Aerial part | In vitro | 95% EtOH | Leishmania donovani UR6 | – | None | Ray et al., 1996 |
| Antileishmanial | Whole plant | In vitro | MeOH | Leishmania donovani AG83 | – | Cytotoxicity test-negative | Medda et al., 1999 |
| Antihelmintic | Whole plant | In vitro | Water; MeOH | Haemonchus contortus | Levamisole 0.55 mg/ml | None | Iqbal et al., 2006 |
| Antimalarial | Leaves/Stem | In vitro | MeOH; PE; Water; EtOH | Plasmodium falciparum FCK 2 | Parasitized red blood cells and 10 μCi of [35S]-methionine | None | Bhat and Surolia, 2001 |
| Egg hatchability and larvicidal | Whole plant | In vitro | HEX; EA; MeOH | Aedes aegypti | Tween-80 | None | Balaraju et al., 2009b |
| Culex quinquefasciatus | |||||||
| Anti-hepatitis B virus | Whole plant | In vitro | 50% EtOH | HepG 2.2.15 cells line | Tenofovir | None | Zhou et al., 2015 |
| Antiinflammatory | Aerial parts | In vivo | Petroleum | N/A | Mice treated with vehicle or Diclofenac (10 mg/kg) | None | Banerjee et al., 2000 |
| Antiinflammatory | Root | In vivo | 95% EtOH | N/A | Diclofenac (25 mg/kg) | None | Das et al., 2012 |
| Hypoglycemic | Whole plant | In vivo | 95% EtOH | N/A | Mice treated with vehicle | None | Kar et al., 2003 |
| Hypoglycemic | Leaves | In vivo | EtOH | N/A | Glibenclamide (5 mg/kg) | None | Alam et al., 2011 |
| Hypoglycemic | Whole plant | In vivo | EA; EtOH | N/A | Glibenclamide (5 mg/kg) | Cytotoxicity test-negative | Verma et al., 2013 |
| Antidiabetic | Whole plant | In vitro | 95% EtOH; HEX | STZ-NAD(streptozotocin- nicotinamide) induced diabetic albino mice | Metformin (100 μg/kg) | None | Grover et al., 2002 |
| Antidiabetic | Whole plant | In vitro | EtOH; HEX; Chloroform | STZ-NAD(streptozotocin- nicotinamide) induced diabetic albino mice | Metformin (100 μg/kg) | None | Arya et al., 2011 |
| Antipyretic | Root | In vitro | Water | Brewer's yeast induced pyrexia Typhoid-Paratyphoid A, B vaccine induced Hyperexia | Paracetamol (150 mg kg−1) | None | Bhargava et al., 2009 |
| Anticarcinogenic | Whole plant | In vivo | HEX | N/A | 9,10-dimethyl benz(a)anthracene (DMBA) | None | Saha et al., 2004 |
| Analgesic | Leaves/Stem | In vivo | EtOH | N/A | Diclofenac sodium (25 mg/kg) | None | Alam et al., 2010 |
| Analgesic | Root | In vivo | EtOH | N/A | Aminopyrine (50 mg/kg) | None | Das et al., 2012 |
| Hepatoprotective | Aerial parts | In vivo | 70% EtOH | N/A | Paracetamol (150 mg/kg) | None | Nagalekshmi et al., 2011 |
| CNS | Whole plant | In vivo | EtOH | N/A | Mice treated with vehicle | None | Bhattacharya et al., 1976 |
| Antiviral | Leaves/Stem | In vitro | Water | Herpes simplex virus type-1 | Acyclovir (1 mg/mL) | Cytotoxicity test-negative | Verma et al., 2008 |
Extracting solvent: EtOH, ethanol; EA, ethyl acetate; HEX, hexane; MeOH, methanol; N/A, not applicable; PE, petroleum ether.
Table 3.
Antioxidant potential of different solvent extracts of S. chirayita.
| Plant part tested | aExtracting solvent | Test system | Control used and result | Toxicity test | References |
|---|---|---|---|---|---|
| Whole plant | 70% EtOH | In vitro | BHT and Vitamin C | None | Chen et al., 2011 |
| IC50 = 267.80 μg/mL (DPPH) | |||||
| IC50 = 1.502 ± 0.200 μg/mL (β-carotene) | |||||
| IC50 = 6.50 μg/mL (ABTS) | |||||
| Whole plant | 70% EtOH | In vivo | NA | Cytotoxicity test-negative | Chen et al., 2011 |
| Whole plant | MeOH | In vitro | BHT | None | Sharma et al., 2013b |
| EC50 = 27.70 μg/ml (DPPH) | |||||
| Whole plant | MeOH | In vitro | BHA | None | Ahirwal et al., 2014 |
| IC50 = 222.74 μg/mL (DPPH) | |||||
| Whole plant | Water | In vitro | Gallic acid | None | Kumar et al., 2011 |
| EC50 = 315.83 μg/mL (DPPH) | |||||
| Leaves | Water | In vitro | BHA; BHT | None | Ghosh et al., 2012 |
| IC50 = 86 μg/mL (DPPH) | |||||
| 900 ± 11(4 min) and 2070 ± 110 (30 min) μM Fe (II)/g sample DW (FRAP) | |||||
| Whole plant | 12% EtOH | In vitro | Ascorbic acid | None | Phoboo et al., 2013 |
| IC50 = 156.62 μg/mL (DPPH) | |||||
| Whole plant | In vitro | Gallic acid | None | Kshirsagar et al., 2015 | |
| MeOH | IC50 = 551.26 μg/mL (DPPH) | ||||
| EtOH | IC50 = 557.61 μg/mL (DPPH) | ||||
| ACE | IC50 = 551.96 μg/mL (DPPH) | ||||
| Water | IC50 = 559.05 μg/mL (DPPH) |
ABTS, 2,2-azino-bis (3-ethylebenzthiazoline-6-sulphonicacid); BHA, Butylated hydroxy anisole; BHT, Butylated hydroxytoluene; DPPH, 2,2-Diphenyl-1-picrylhdrazyl; DW, Dry weight; FRAP, Ferric Reducing Antioxidant Power
Extracting solvent: ACE, acetone; EtOH, ethanol; MeOH, methanol
Phytochemistry
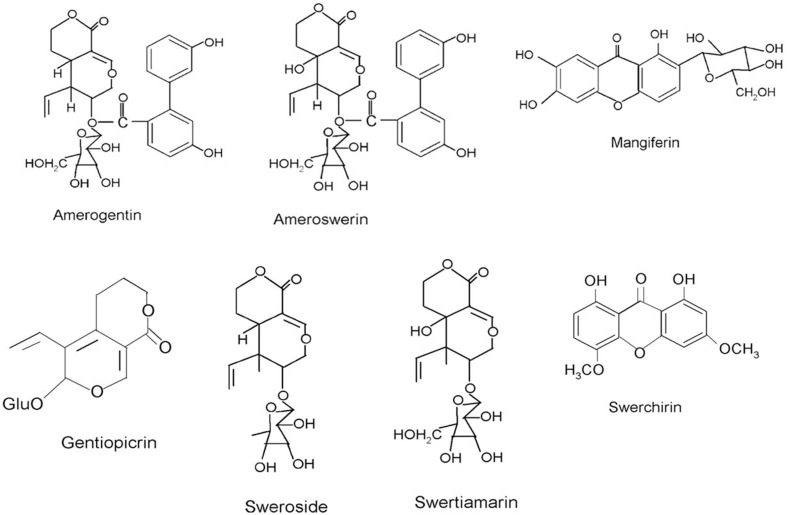
The widespread uses of S. chirayita as a traditional drug and its commercialization in modern medical systems have led to a rise in scientific exploration of its phytochemistry in order to identify the active phytochemicals. This has resulted in a considerable body of literature exploring the chemical constituents of this plant (Mandal and Chatterjee, 1987; Chakravarty et al., 1991, 1994; Mandal et al., 1992; Chatterjee and Pakrashi, 1995; Pant et al., 2000). The wide-range biological activities of S. chirayita are attributed to the presence of a diverse group of pharmacologically bioactive compounds belonging to different classes such as xanthones and their derivatives, lignans, alkaloids, flavonoids, terpenoids, iridoids, secoiridoids, and other compounds such as chiratin, ophelicacid, palmitic acid, oleic acid, and stearic acid (Pant et al., 2000; Patil et al., 2013). The first isolated dimeric xanthone was chiratanin present in different parts of S. chirayita. The pharmacological efficacy of S. chirayita has been partly attributed to the biological activity of major phytoconstituents including amarogentin, swertiamarin, mangiferin, swerchirin, sweroside, amaroswerin, and gentiopicrin (Figure 3). Amarogentin is reported to be anti-diabetic (Phoboo et al., 2013), anticancerous (Saha et al., 2006; Pal et al., 2012), and antileishmanial (Ray et al., 1996; Medda et al., 1999), whereas swertiamarin has been tested for its anti-hepatitis (Wang et al., 2001), anticancer (Kavimani and Manisenthlkumar, 2000), anti-arthritic activities (Saravanan et al., 2014). It has been shown to exhibit anti-diabetic (Vaidya et al., 2013) properties. Mangiferin is also reported to have anti-diabetic, antiatherosclerotic (Pardo-Andreu et al., 2008), anticancer, anti-HIV (Guha et al., 1996), antiparkinson (Kavitha et al., 2013), and chemopreventive (Yoshimi et al., 2001) activities. Swerchirin is known to be antimalarial, hypoglycemic (Bajpai et al., 1991; Saxena et al., 1996), hepatoprotective, pro-heamatopoietic (Ya et al., 1999), with blood glucose lowering activity (Sekar et al., 1987; Saxena et al., 1991) and weak chemo preventive pharmacological effects (Hirakawa et al., 2005). Swerchirin at different concentrations (1, 10, and 100 μM) significantly enhanced glucose stimulated insulin release from isolated islets (Saxena et al., 1993). Sweroside is reported to be antibacterial (Siler et al., 2010), hepatoprotective (Liu et al., 1994; Luo et al., 2009), preventative in treatment for hyperpigmentation (Jeong et al., 2015), and is also suggested as a promising osteoporosis therapeutic natural product (Sun et al., 2013). Amaroswerin is known for its gastroprotective effects of the bitter principles (Niiho et al., 2006). Table 4 provides a summary focusing on the biological activity of the phytochemicals present in S. chirayita.
Figure 3.
Chemical structures of important phytoconstituents found in Swertia chirayita.
Table 4.
Important bioactive compounds isolated from Swertia chirayita.
| Phytochemical | Biological activity | References |
|---|---|---|
| Amarogentin | Antileishmanial | Ray et al., 1996; Medda et al., 1999 |
| Topoisomerase inhibitor | Ray et al., 1996 | |
| Anticancer | Saha et al., 2006; Pal et al., 2012 | |
| Anti-diabetic | Phoboo et al., 2013 | |
| Gastroprotective | Niiho et al., 2006 | |
| Swertiamarin | CNS depressant | Bhattacharya et al., 1976 |
| Anticholinergic | Suparna et al., 1998 | |
| Anticancer | Kavimani and Manisenthlkumar, 2000 | |
| Anti-hepatitis | Wang et al., 2001 | |
| Antibacterial | Kumarasamy et al., 2003 | |
| Cardio-protective, anti-atherosclerotic | Vaidya et al., 2009 | |
| anti-diabetic | Vaidya et al., 2013 | |
| Anti-arthritic | Saravanan et al., 2014 | |
| Mangiferin | Antiviral | Zheng and Lu, 1990 |
| Immunomodulatory, antitumor, anti-HIV | Guha et al., 1996 | |
| Antioxidant | Sanchez et al., 2000 | |
| Chemopreventive | Yoshimi et al., 2001 | |
| Antiinflammatory | Kumar et al., 2003 | |
| Hypoglycemic | Muruganandan et al., 2005 | |
| Anti-diabetic, Antiatherosclerotic | Pardo-Andreu et al., 2008 | |
| Antiparkinson | Kavitha et al., 2013 | |
| Swerchirin | Hypoglycemic | Bajpai et al., 1991; Saxena et al., 1996 |
| Hepatoprotective, pro-heamatopoietic | Ya et al., 1999 | |
| Blood glucose lowering activity | Sekar et al., 1987; Saxena et al., 1993 | |
| Chemopreventive | Hirakawa et al., 2005 | |
| Sweroside | Antibacterial | Siler et al., 2010 |
| Hepatoprotective | Liu et al., 1994; Luo et al., 2009 | |
| Hyperpigmentation | Jeong et al., 2015 | |
| Osteoporosis | Sun et al., 2013 | |
| Amaroswerin | Gastroprotective | Niiho et al., 2006 |
| Gentianine | Antipsychotic | Bhattacharya et al., 1974 |
| Antimalarial | Natarajan et al., 1974 | |
| Oleanolic acid | Antimicrobial | Jesus et al., 2015 |
| Antitumor | Soica et al., 2014 | |
| Antiinflamatory, antioxidant | Liu, 1995 | |
| Ursolic acid | Antimicrobial | Jesus et al., 2015 |
| Antitumor | Bonaccorsi et al., 2008; Soica et al., 2014 | |
| Swertanone | Antiinflammatory | Kumar et al., 2003; Tabassum et al., 2012 |
| Syringaresinol | Hepatoprotective | Chakravarty et al., 1994 |
| Bellidifolin | Hypoglycemic | Basnet et al., 1995 |
| Isobellidifolin | Hypoglycemic | Basnet et al., 1995 |
| 1-Hydroxy-3,5,8-trimethoxyxanthone | Antimalarial | Mandal and Chatterjee, 1994 |
| 1-Hydroxy-3,7,8-trimethoxyxanthone | Spasmogenic agent | Ateufack et al., 2007 |
| Antiulcerogenic | Ateufack et al., 2014 | |
| 1,5,8-trihydroxy-3-methoxyxanthone | Blood sugar lowering | Ghosal et al., 1973 |
| β-Amyrin | Anti-inflammatory | Holanda et al., 2008 |
| Antimicrobial, antifungal | Vázquez et al., 2012 | |
| Chiratol | Anti-inflammatory | Banerjee et al., 2000 |
Safety evaluation
Concerns regarding safety of conventional drugs are vital issues of pharmaceutical industries. Studies have indicated that some commonly used medicinal plants may be mutagenic or cytotoxic especially over a long period of use (Verschaeve and Van Staden, 2008). There is increasing evidence on the toxicity of crude extracts and isolated compounds from different plant species (Koorbanally et al., 2006). However, despite its long history of use in traditional medicine, there is still a lack of scientific information concerning the safety evaluation of S. chirayita. It can be traced through the medicinal history as a nontoxic and safe ethnomedicinal herb and has been mentioned in medical papyri to expel fever, relieve headache, inflammation, and to stimulate the central nervous system. S. chirayita extracts, did not cause obvious toxic effects in mice as there were no significant differences in body weight and body temperature between the treated and control groups (Alam et al., 2011; Das et al., 2012). A clinical study by Medda et al. (1999) concluded that S. chirayita revealed no evidence of toxicity in both liposomal and niosomal forms. Furthermore, stringent efforts are required to further delineate the well-documented toxicological properties involving toxicity and mutagenic tests to evaluate the safety of this plant. Nevertheless, rigorous clinical studies involving different mechanisms are still needed to confirm the safety of S. chirayita in traditional medicine so that it can be used safely and effectively. Despite the fact that the benefits of medicinal plants is globally acknowledged, the need for better insight on the safety evaluation remains essential, so as to differentiate between toxic effects and pharmacological importance of plant extracts (Aremu and Van Staden, 2013).
Swertia chirayita conservation
Destruction of plant resources is a normal occurrence. The current speed of extinction through human interferences is estimated to be approximately 100–1000 times faster than the natural speed of extinction (Chapin et al., 2000). Due to developmental activities in the Himalayan region, wild populations of many medicinal plants, including S. chirayita are reduced to the verge of extinction. S. chirayita is traded and used mostly as a traditional drug. Due to its multiple uses the demand is on the rise by both national and international trading leading to increasing over harvesting of wild populations. This has resulted in drastic reductions of its populations. Lack of comprehensive data on annual harvested and traded plants of S. chirayita is also a major concern. According to the International Union of Conservation of Nature (IUCN) criteria, S. chirayita conservation status has been categorized as “critically endangered” (Joshi and Dhawan, 2005). S. chirayita is among the 32 most highly prioritized medicinal plants of India as identified by The National Medicinal Plant Board, Government of India (http://www.nmpb.nic.in).
The implication of losing this plant species due to extinction lies not only in the loss of genes useful for plant development or in the biosynthesis of new compounds but also the loss of potentially novel compounds of pharmaceutical or nutraceutical benefit. In order to meet the escalating demand in national and international trade markets of raw plants, cultivation must be escalated. There are limitations in the use of seed propagation, due to low viability, and low germination percentages (Badola and Pal, 2002; Chandra et al., 2012). Biotechnology offers new means of improving biodiversity and biotechnological approaches such as micropropagation techniques (Figure 1E) has received more attention and may play a vital role in the establishment of genetically uniform plants for the Swertia industry. It is believed that the development of efficient micropropagation protocols, can guarantee an adequate supply of S. chirayita plants (devoid of environmental-imposed constraints) with subsequent reduction in uncontrolled harvesting pressure on wild populations. Several studies reported on micropropagation, somatic embryogenesis and acclimatization procedures with the capacity to produce many uniform S. chirayita clones throughout the year (Kumar and Chandra, 2013, 2014; Kumar et al., 2014). As shown in Table 5, micropropagation protocols have successfully been established for S. chirayita using different explants.
Table 5.
Micropropagation data for Swertia chirayita.
| Tissue culture Study | Explant type | Optimum concentrations | Major observations | References |
|---|---|---|---|---|
| Regeneration | Seeds | 3.0 μM BA | Adventitious shoot regeneration from root explants | Wawrosch et al., 1999 |
| Micropropagation | In vivo axillary bud/shoot apices | 0.5 mg/l BA + 1.0 mg/l GA3 | Methods and compositions for rapid in vitro propagation | Ahuja et al., 2003 |
| Axillary multiplication | Seedling-derived nodal explants | 4.0 μM BA + 1.5 μM 2iP | Improved shoot proliferation | Joshi and Dhawan, 2007 |
| Regeneration | In vivo stem with node | 0.44 μM BA + 4.65 μM KN | Improved regeneration from the nodal explants | Chaudhuri et al., 2007 |
| Direct shoot multiplication | In vitro leaves | 2.22 μM BA + 11.6 μM KN + 0.5 μM NAA | Improved protocol for propagation | Chaudhuri et al., 2008 |
| Regeneration | Seeds | 2.22 μM BA + 2.22 μM KN + 0.54 μM NAA | Regeneration from immature seed culture | Chaudhuri et al., 2009 |
| Direct shoot regeneration | In vivo leaves | 13.32 μM BA + 0.54 μM NAA | In vitro shoot regeneration | Wang et al., 2009 |
| Micropropagation | In vitro shoot tip | 1.0 mg/l BA + 0.1 mg/l KN | Improved shoot proliferation | Balaraju et al., 2009a |
| In vitro regeneration | Node | 2 mg/l BA | Rapid in vitro propagation system | Koul et al., 2009 |
| Shoot Organogenesis | In vitro root | 4.44 μM BA + 1.07 μM NAA | Improved protocol for plant regeneration | Pant et al., 2010 |
| Somatic embryogenesis | In vivo leaves | 1.0 mg/l 2,4-D and 0.5 mg/l 2,4-D + 0.5 mg/l BA | Rapid system for micropropagation | Balaraju et al., 2011 |
| Callus culture | In vitro root | 13.32 μM BA + 0.90 μM 2,4-D | Plant regeneration via indirect organogenesis | Pant et al., 2012 |
| Efficient Regeneration | In vivo shoot tip | 0.5 mg/l BA + 1.0 mg/l GA3 | An efficient shoot proliferation | Kumar and Chandra, 2013 |
| In vitro flower production | Axillary bud | 1.0 mg/l BA + 70 mg/l Adenine sulfate | In vitro flowering and effective protocol for regeneration | Sharma et al., 2014 |
| Somatic embryogenesis | In vivo leaves | 0.5 mg/l 2,4-D + 0.5 mg/l KN | An efficient protocol for plant regeneration through somatic embryogenesis | Kumar and Chandra, 2014 |
| Direct and Indirect regeneration | In vivo leaves | 1.0 mg/l BA + 100 mg/l Adenine sulfate + 0.1 mg/l IAA | An efficient protocol of plant regeneration through direct and indirect organogenesis | Kumar et al., 2014 |
2,4-D, 2,4-Dicholorophenoxyacetic acid; BA, 6-benzyl-adenine; GA3, Gibberellic acid; IAA, Indole-3-acetic acid; KN, Kinetin; NAA, Naphthalene Acetic Acid.
Synthetic seed technology is also an applied application of modern plant biotechnology which offers tremendous potential for easy handling, micropropagation and plant germplasm conservation through cryopreservation (Ara et al., 2000; Sharma et al., 2013a; Perveen and Anis, 2014; Gantait et al., 2015). Successful implementation of synthetic seed technology for mass propagation and short-term storage of genetically uniform clones require manipulation of in vitro tissue culture systems that are able to transform into complete plantlets (Ara et al., 2000). Recently, Kumar et al. (2014) reported on synthetic seed production and plant regeneration of S. chirayita from somatic embryos. However, further studies are required to improve technology so that it can be used on a commercial scale.
Many plant secondary metabolites accumulate in roots (Flores et al., 1999) but harvesting of these organs is destructive. Therefore, in the recent past Agrobacterium rhizogenes induced hairy root technology has received attention and engaged a new platform of applied research in generating pharmaceutical lead compounds. The large scaling-up of hairy root cultures is of importance for biotechnological applications (Guillon et al., 2006). Attempts have been made to standardize A. rhizogenes transformed root cultures for production of active secondary metabolites under in vitro conditions of S. chirayita (Keil et al., 2000). For commercialization of S. chirayita adventitious roots and to elucidate the feasibility for commercial application, hairy root technology is required along with various factors affecting the production of root biomass and bioactive compounds. Overall, micropropagation which is conducted under a controlled environment will help to prevent the current plant biodiversity conservation problems arising from over harvesting practices of wild populations and can profoundly improve the quality of bioactive secondary metabolites of this age old medicinal plant S. chirayita.
Conclusions and future perspectives
S. chirayita offers many promising prospects for both traditional and modern medicine. S. chirayita is apparently a potential herbal therapy for many ailments. This review summarized the existing ethnobotanical uses, phytochemistry, pharmacological activities, safety evaluation, and conservation status on S. chirayita.
So far no serious side effects or toxicity of S. chirayita have been reported, but further toxicological studies are still needed to confirm the safety of S. chirayita in humans. Efforts are required for further studies, especially evaluating its biological activities in vivo and toxicological and mutagenic properties in order to better validate the safety of these different plant-derives compounds. In all probability there is a need for clinical trials to establish the efficacy of using S. chirayita in medicine. Due to its multiple uses the demand in both national and international markets is constantly on the rise. Overexploitation combined with habitat destruction has resulted in the drastic reduction of its population. For the successful commercialization of this critically endangered medicinal plant any proposed research must be viewed in a wider context that includes conservation practices and sustainable supply of raw plants. This will require innovative tools, which utilize biotechnological interventions, including micropropagation, cryopreservation, and bioreactors for the conservation, as well as for raising commercial production. In synthetic seed technology more detailed research is required mainly for improvement in germination frequency of synthetic seeds and subsequent plantlet growth in soil so that it can be used on a commercial scale. Additionally, in the near future, hairy root technology can be used as a model system and will also provide plant biotechnologists with powerful tools to improve the valuable phytochemicals of S. chirayita. Although efficient micropropagation protocols have been established, further studies focusing on seed biology and ways of improving bioactive secondary metabolites in cultivated S. chirayita would be beneficial for their commercialization. Quality control protocols to prevent misidentification and possible adulteration of S. chirayita are also needed. In summary, S. chirayita have been studied extensively in terms of taxonomy, ethnobotany, phytochemistry, biological activities, and conservation. However, new findings may increase the present therapeutic importance of S. chirayita and promote their future use in modern medicine, while novel biotechnological approaches are required for further conservation.
Author contributions
VK conducted the research and wrote the paper. JVS supervised the work and proof read the paper.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
VK is grateful to the National Research Foundation and the University of KwaZulu-Natal, South Africa for the financial support. Dr. M. Moyo and Dr. A. O. Aremu are thanked for reading the manuscript.
Glossary
Abbreviations
- ABTS
2,2-azino-bis (3-ethylebenzothiazoline-6-sulphonic acid)
- ACE
Acetone
- BA
6-benzyl-adenine
- BHA
Butylated hydroxy anisole
- BHT
Butylated hydroxytoluene
- 2, 4-D
2,4-Dicholorophenoxyacetic acid
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- DW
Dry weight
- EtOH
Ethanol
- EA
Ethyl acetate
- FRAP
Ferric Reducing Antioxidant Power
- GA3
Gibberellic acid
- HEX
Hexane
- IAA
Indole-3-acetic acid
- KN
Kinetin
- MeOH
Methanol
- NAA
Naphthalene Acetic Acid
- PE
Petroleum ether.
References
- Ahirwal L., Singh S., Dubey M. K., Bharti V., Mehta A. (2014). Investigation of antioxidant potential of methanolic extract of Swertia chirata Buch. Ham. Eur. J. Med. Plants. 4, 1345–1355. 10.9734/EJMP/2014/8933 [DOI] [Google Scholar]
- Ahuja A., Koul S., Kaul B. L., Verma N. K., Kaul M. K., Raina R. K., et al. (2003). Media Composition for Faster Propagation of Swertia chirayita. US Patent No. W0 03/045132 A1. [Google Scholar]
- Alam K. D., Ali M. S., Mahjabeen S., Hassan M. R., Rahman M. F., Chowdhury R. M. A. A. (2011). Potential hypoglycemic effect of Swertia chirayita—An Indian subcontinent herb with important medicinal value. Pharmacologyonline 2, 642–647. [Google Scholar]
- Alam K. D., Ali M. S., Mahjabeen S., Parvin S., Akbar M. A., Ahamed R. (2010). Report: analgesic activities of ethanol extract of leaf, stem and their different fractions of Swertia chirata. Pak. J. Pharm. Sci. 23, 455–457. [PubMed] [Google Scholar]
- Alam K. D., Ali M. S., Parvin S., Mahjabeen S., Akbar M. A., Ahamed R. (2009). In vitro antimicrobial activities of different fractions of Swertia chirata ethanolic extract. Pak. J. Biol. Sci. 12, 1334–1337. 10.3923/pjbs.2009.1334.1337 [DOI] [PubMed] [Google Scholar]
- Anon (1982). In The Wealth of India: Raw Materials, Publication and Information Directorate, Vol X. New Delhi: CSIR, 78–81. [Google Scholar]
- Ara H., Jaiswal U., Jaiswal V. S. (2000). Synthetic seed: prospects and limitations. Curr. Sci. 78, 1438–1444. [Google Scholar]
- Aremu A. O., Van Staden J. (2013). The genus Tulbaghia (Alliaceae)—A review of its ethnobotany, pharmacology, phytochemistry and conservation needs. J. Ethnopharmacol. 149, 387–400. 10.1016/j.jep.2013.06.046 [DOI] [PubMed] [Google Scholar]
- Arya R., Sharma S. K., Singh S. (2011). Antidiabetic effect of whole plant extract and fractions of Swertia chirayita Buch.-Ham. Planta Med. 77, 138 10.1055/s-0031-1273667 [DOI] [Google Scholar]
- Ateufack G., Nguelefack T. B., Mbiantcha M., Tane P., Kamanyi A. (2007). Spasmogenic activity of 1-hydroxy-3,7,8– trimethoxyxanthone isolated from the methanol extract of the stem bark of Anthocleista vogelii planch (loganiaceae) in rats. Pharmacologyonline 3, 374–384. [Google Scholar]
- Ateufack G., Nguelefack T. B., Wabo H. K., Tane P., Kamanyi A. (2014). Antiulcerogenic activity of 1-Hydroxy-3,7,8-trimethoxyxanthone isolated from the methanol extract of Anthocleista vogelii PLANCH. in Rats. Ulcers 2014:172096 10.1155/2014/172096 [DOI] [Google Scholar]
- Badola H. K., Pal M. (2002). Endangered medicinal plant species in Himachal Pradesh. Curr. Sci. 83, 797–798. [Google Scholar]
- Bajpai M. B., Asthana R. K., Sharma N. K., Chatterjee S. K., Mukherjee S. K. (1991). Hypoglycemic effect of swerchirin from the hexane fraction of Swertia chirayita. Planta Med. 57, 102–104. 10.1055/s-2006-960041 [DOI] [PubMed] [Google Scholar]
- Balaraju K., Agastian P., Ignacimuthu S. (2009a). Micropropagation of Swertia chirata Buch.-Hams. exWall.:a critically endangered medicinal herb. Acta Physiol. Plant. 31, 487–494. 10.1007/s11738-008-0257-0 [DOI] [Google Scholar]
- Balaraju K., Maheswaran R., Agastian P., Ignacimuthu S. (2009b). Egg hatchability and larvicidal activity of Swertia chirata Buch.—Hams. ex Wall. against Aedes aegypti L. and Culex quinquefasciatus Say. Indian J. Sci. Technol. 2, 46–49. [Google Scholar]
- Balaraju K., Saravanan S., Agastian P., Ignacimuthu S. (2011). A rapid system for micropropagation of Swertia chirata Buch.-Ham. ex Wall.: an endangered medicinal herb via direct somatic embryogenesis. Acta Physiol. Plant. 33, 1123–1133. 10.1007/s11738-010-0640-5 [DOI] [Google Scholar]
- Banerjee S., Sur T. P., Das P. C., Sikdar S. (2000). Assessment of the antiinflammatory effects of Swertia chirata in acute and chronic experimental models in male albino rats. Indian J. Pharmacol. 32, 21–24. [Google Scholar]
- Basnet P., Kadota S., Shimizu M., Takata Y., Kobayashi M., Namba T. (1995). Bellidifolin stimulates glucose-uptake in rat-1 fibroblasts and ameliorates hyperglycemia in streptozotocin (stz)-induced diabetic rats. Planta Med. 61, 402–405. 10.1055/s-2006-958124 [DOI] [PubMed] [Google Scholar]
- Bentley R., Trimen H. (eds.). (1880). Medicinal Plants. London: J and A Churchill. [Google Scholar]
- Bhargava S., Rao P., Bhargava P., Shukla S. (2009). Antipyretic potential of Swertia chirata Buch Ham. Sci. Pharm. 77, 617–623. 10.3797/scipharm.0812-10 [DOI] [Google Scholar]
- Bhat A. J., Kumar M., Negi A. K., Todaria N. P. (2013). Informants' consensus on ethnomedicinal plants in Kedarnath Wildlife Sanctuary of Indian Himalayas. J. Med. Plants Res. 7, 148–154. [Google Scholar]
- Bhat G. P., Surolia N. (2001). In vitro antimalarial activity of extracts of three plants used in thetraditional medicine of India. Am. J. Trop. Med. Hyg. 65, 304–308. [DOI] [PubMed] [Google Scholar]
- Bhatt A., Rawal R. S., Dhar U. (2006). Ecological features of a critically rare medicinal plant, Swertia chirayita, in Himalaya. Plant Species Biol. 21, 49–52. 10.1111/j.1442-1984.2006.00150.x [DOI] [Google Scholar]
- Bhattacharya S. K., Ghosal S, Chaudhuri, R. K., Singh A. K., Sharma P. V. (1974). Letter: chemical constituents of gentianaceae. XI. antipsychotic activity of gentianine. J. Pharm. Sci. 63, 1341–1342. 10.1002/jps.2600630850 [DOI] [PubMed] [Google Scholar]
- Bhattacharya S. K., Reddy P. K., Ghosal S., Singh A. K., Sharma P. V. (1976). Chemical constituents of Gentianaceae. XIX. CNS-depressant effects of swertiamarin. Indian J. Pharm. Sci. 65, 1547–1549. 10.1002/jps.2600651037 [DOI] [PubMed] [Google Scholar]
- Bonaccorsi I., Altieri F., Sciamanna I., Oricchio E., Grillo C., Contartese G., et al. (2008). Endogenous reverse transcriptase as a mediator of ursolic acid's antiproliferative and differentiating effects in human cancer cell lines. Cancer Lett. 263, 130–139. 10.1016/j.canlet.2007.12.026 [DOI] [PubMed] [Google Scholar]
- Chakravarty A. K., Mukhopadhyay S., Das B. (1991). Swertane triterpenoids from Swertia chirata. Phytochemistry 30, 4087–4092. 10.1016/0031-9422(91)83473-X [DOI] [Google Scholar]
- Chakravarty A. K., Mukhopadhyay S., Moitra S. K., Das B. (1994). Syringareinol, a hepatoprotective agent and other constituents from Swertia chirata. Indian J. Chem. B 33, 405–408. [Google Scholar]
- Chandra S., Kumar V., Bandopadhyay R., Sharma M. M. (2012). SEM and elemental studies of Swertia chirayita: a critically endangered medicinal herb of temperate Himalayas. Curr. Trends. Biotechnol. Pharm. 6, 381–388. [Google Scholar]
- Chapin F. S., III, Zavaleta E. S., Eviner V. T., Naylor R. L., Vitousek P. M., Reynolds H. L., et al. (2000). Consequences of changing biodiversity. Nature 405, 234–242. 10.1038/35012241 [DOI] [PubMed] [Google Scholar]
- Chatterjee A., Pakrashi S. C. (1995). The Treatise on Indian Medicinal Plants, Vol. 4. New Delhi: Publication Information Directorate, CSIR; 92. [Google Scholar]
- Chaudhuri R. K., Pal A., Jha T. B. (2007). Production of genetically uniform plants from nodal expants of Swertia chirata Buch. Ham. Ex wall- a critically endangered medicinal herb. In Vitro Cell. Dev. Biol. Plant 43, 467–472. 10.1007/s11627-007-9095-9 [DOI] [Google Scholar]
- Chaudhuri R. K., Pal A., Jha T. B. (2008). Conservation of Swertia chirata Buch. –Ham through direct shoot multiplication from leaf explants. Plant Biotechnol. Rep. 2, 213–218. 10.1007/s11816-008-0064-5 [DOI] [Google Scholar]
- Chaudhuri R. K., Pal A., Jha T. B. (2009). Regeneration and characterization of Swertia chirata Buch. –Ham ex wall. plants from immature seed cultures. Sci. Hort. 120, 107–114. 10.1016/j.scienta.2008.09.022 [DOI] [Google Scholar]
- Chen Y., Huang B., He J., Han L., Zhan Y., Wang Y. (2011). In vitro and in vivo antioxidant effects of the ethanolic extract of Swertia chirayita. J. Ethnopharmacol. 136, 309–315. 10.1016/j.jep.2011.04.058 [DOI] [PubMed] [Google Scholar]
- Clarke C. B. (1885). Verbenaceae, in The Flora of British India, Vol. IV, ed Hooker J. D. (London: L. Reeve and Co; ), 560–604. [Google Scholar]
- Das S. C., Bhadra S., Roy S., Saha S. K., Islam M. S., Bachar S. C. (2012). Analgesic and anti-inflammatory activities of ethanolic root extract of Swertia chirata (Gentianaceae). Jordan J. Biol. Sci. 5, 31–36. [Google Scholar]
- de Rus Jacquet A., Subedi R., Ghimire S. K., Rochet J. C. (2014). Nepalese traditional medicine and symptoms related to Parkinson's disease and other disorders: patterns of the usage of plant resources along the Himalayan altitudinal range. J. Ethnopharmacol. 153, 178–189. 10.1016/j.jep.2014.02.016 [DOI] [PubMed] [Google Scholar]
- Edwards D. M. (1993). The marketing of non-timber forest product from the Himalayas: the trade between East Nepal and India. Rural Dev. For. Netw. 15b, 1–21. [Google Scholar]
- Edwin R., Chungath J. I. (1988). Studies in Swertia chirata. Indian Drugs 25, 143–146. [Google Scholar]
- Fan G., Luo W. Z., Luo S. H., Li Y., Meng X. L., Zhou X. D., et al. (2014). Metabolic discrimination of Swertia mussotii and Swertia chirayita known as “Zangyinchen” in traditional Tibetan medicine by (1)H NMR-based metabolomics. J. Pharm. Biomed. Anal. 98, 364–370. 10.1016/j.jpba.2014.06.014 [DOI] [PubMed] [Google Scholar]
- Flores H. E., Vivanco J. M., Loyola-Vargas V. M. (1999). “Radicle” biochemistry: the biology of root specific metabolism. Trends Plant Sci. 4, 220–226. 10.1016/S1360-1385(99)01411-9 [DOI] [PubMed] [Google Scholar]
- Gantait S., Kundu S., Ali N., Sahu N. C. (2015). Synthetic seed production of medicinal plants: a review on influence of explants, encapsulation agent and matrix. Acta Physiol. Plant 37, 98 10.1007/s11738-015-1847-2 [DOI] [Google Scholar]
- Gaur R. D. (1999). Flora of the District Garhwal, North West Himalaya (with Ethnobotanical Notes). Srinagar: Transmedia. [Google Scholar]
- Ghosal S., Sharma P. V., Chaudhuri R. K., Bhattacharya S. K. (1973). Chemical constituents of the gentianaceae V: tetraoxygenated xanthones of Swertia chirata buch.-ham. J. Pharm. Sci. 62, 926–930. 10.1002/jps.2600620614 [DOI] [PubMed] [Google Scholar]
- Ghosh D., Bandyopadhyay S. S., Chatterjee U. R., Capek P., Ray B. (2012). Carbohydrate polymers of chirata (Swertia chirata) leaves: structural features, in vitro anti-oxidant activity and fluorescence quenching study. Food Sci. Biotechnol. 21, 409–417. 10.1007/s10068-012-0052-y [DOI] [Google Scholar]
- Grover J. K., Yadav S., Vats V. (2002). Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 81, 81–100. 10.1016/S0378-8741(02)00059-4 [DOI] [PubMed] [Google Scholar]
- Guha S., Ghosal S., Chattopadhyay U. (1996). Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a naturally occurring glucosylxanthone. Chemotherapy 42, 443–451. 10.1159/000239478 [DOI] [PubMed] [Google Scholar]
- Guillon S., Trémouillaux-Guiller J., Pati P. K., Rideau M., Gantet P. (2006). Hairy root research: recent scenario and exciting prospects. Curr. Opin. Plant Biol. 9, 341–346. 10.1016/j.pbi.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Hirakawa K., Yoshida M., Nagatsu A., Mizukami H., Rana V., Rawat M. S. M., et al. (2005). Chemopreventive action of xanthone derivatives on photosensitized DNA damage. Photochem. Photobiol. 81, 314–319. 10.1111/j.1751-1097.2005.tb00189.x [DOI] [PubMed] [Google Scholar]
- Holanda S. A., Pinto L. M., Cunha G. M., Chaves M. H., Santos F. A., Rao V. S. (2008). Antiinflammatory effect of alpha, beta-Amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology 16, 48–52. 10.1007/s10787-007-1609-x [DOI] [PubMed] [Google Scholar]
- Iqbal Z., Lateef M., Khan M. N., Jabbar A., Akhtar M. S. (2006). Anthelmintic activity of Swertia chirata against gastrointestinal nematodes of sheep. Fitoterapia 77, 463–465. 10.1016/j.fitote.2006.05.010 [DOI] [PubMed] [Google Scholar]
- Jadhav A. N., Bhutani K. K. (2005). Ayurveda and gynecological disorders. J. Ethnopharmacol. 97, 151–159. 10.1016/j.jep.2004.10.020 [DOI] [PubMed] [Google Scholar]
- Jeong Y. T., Jeong S. C., Hwang J. S., Kim J. H. (2015). Modulation effects of sweroside isolated from the Lonicera japonica on melanin synthesis. Chem. Biol. Interact. 238, 33–39. 10.1016/j.cbi.2015.05.022 [DOI] [PubMed] [Google Scholar]
- Jesus J. A., Lago J. H. G., Laurenti M. D., Yamamoto E. S., Passero L. F. D. (2015). Antimicrobial activity of oleanolic and ursolic acids: an update. Evid. Based Complement. Alternat. Med. 2015:620472. 10.1155/2015/620472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P., Dhawan V. (2005). Swertia chirayita—an overview. Curr. Sci. 89, 635–640. [Google Scholar]
- Joshi P., Dhawan V. (2007). Axillary multiplication of Swertia chirayita (Roxb. Ex Fleming) H. Karst., a critically endangered medicinal herb of temperate Himalayas. In Vitro Cell. Dev. Biol. Plant 43, 631–638. 10.1007/s11627-007-9065-2 [DOI] [Google Scholar]
- Kar A., Choudhary B. K., Bandyopadhyay N. G. (2003). Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J. Ethnopharmacol. 84, 105–108. 10.1016/S0378-8741(02)00144-7 [DOI] [PubMed] [Google Scholar]
- Karan M., Vasisht K., Handa S. S. (1999). Morphological and chromatographic comparison of certain Indian species of Swertia. J. Med. Aromat. Plant Sci. 19, 995–963. [Google Scholar]
- Kavimani S., Manisenthlkumar K. T. (2000). Effect of methanolic extract of Enicostemma littorale on Dalton's ascitic lymphoma. J. Ethnopharmacol. 71, 349–352. 10.1016/S0378-8741(00)00190-2 [DOI] [PubMed] [Google Scholar]
- Kavitha M., Nataraj J., Essa M. M., Memon M. A., Manivasagam T. (2013). Mangiferin attenuates MPTP induced dopaminergic neurodegeneration and improves motor impairment, redox balance and Bcl-2/Bax expression in experimental Parkinson's disease mice. Chem. Biol. Interact. 206, 239–247. 10.1016/j.cbi.2013.09.016 [DOI] [PubMed] [Google Scholar]
- Keil M., Hartle B., Guillaume A., Psiorz M. (2000). Production of amarogentin in root cultures of Swertia chirata. Planta Med. 66, 452–457. 10.1055/s-2000-8579 [DOI] [PubMed] [Google Scholar]
- Khalid A., Waseem A., Saadullah M., Rehman U., Khiljee S., Sethi A., et al. (2011). Antibacterial activity analysis of extracts of various plants against gram -positive and -negative bacteria. Afr. J. Pharm. Pharmacol. 5, 887–893. [Google Scholar]
- Kirtikar K. R., Basu B. D. (1984). Indian Medicinal Plants, Vol. III. Allahabad: LM Basu Publishers. [Google Scholar]
- Koorbanally C., Crouch N. R., Mulholland D. A. (2006). The phytochemistry and ethnobotany of the southern African genus Eucomis (Hyacinthaceae:Hya- cinthoideae), in Phytochemistry: Advances in Research, ed Imperato F. (Trivandrum: Research Signpost; ), 69–85. [Google Scholar]
- Koul S., Suri K. A., Dutt P., Sambyal A., Ahuja A., Kaul M. K. (2009). Protocol for in vitro regeneration and marker glycoside assessment in Swertia chirata Buch-Ham. Methods Mol. Biol. 547, 139–153. 10.1007/978-1-60327-287-2_12 [DOI] [PubMed] [Google Scholar]
- Kshirsagar P., Chavan J., Nimbalkar M., Yadav S., Dixit G., Gaikwad N. (2015). Phytochemical composition, antioxidant activity and HPLC profiles of Swertia species from Western Ghats. Nat. Prod. Res. 29, 780–784. 10.1080/14786419.2014.986124 [DOI] [PubMed] [Google Scholar]
- Kumar I. V., Paul B. N., Asthana R., Saxena A., Mehrotra S., Rajan G. (2003). Swertia chirayita mediated modulation of interleukin-1beta, interleukin-6, interleukin-10, interferon-gamma, and tumor necrosis factor-alpha in arthritic mice. Immunopharmacol. Immunotoxicol. 25, 573–583. 10.1081/IPH-120026442 [DOI] [PubMed] [Google Scholar]
- Kumar V., Chandra S. (2013). Efficient regeneration and antioxidant activity of the endangered species Swertia chirayita. Int. J. Pharma Biol. Sci. 4, 823–833. [Google Scholar]
- Kumar V., Chandra S. (2014). High frequency somatic embryogenesis and synthetic seed production of the endangered species Swertia chirayita. Biologia 69, 186–192. 10.2478/s11756-013-0305-0 [DOI] [Google Scholar]
- Kumar V., Chandra S. (2015). LC-ESI/MS determination of xanthone and secoiridoid glycosides from in vitro regenerated and in vivo Swertia chirayita. Physiol. Mol. Biol. Plants 21, 51–60. 10.1007/s12298-014-0276-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. R., Kumar S., Shashidhara S., Anitha S., Manjula M. (2011). Comparison of the antioxidant capacity of an important hepatoprotective plants. Int. J. Pharm. Sci. Drug Res. 3, 48–51. [Google Scholar]
- Kumar V., Singh S. K., Bandopadhyay R., Sharma M. M., Chandra S. (2014). In vitro organogenesis secondary metabolite production and heavy metal analysis in Swertia chirayita. Cent. Eur. J. Biol. 9, 686–698. 10.2478/s11535-014-0300-7 [DOI] [Google Scholar]
- Kumarasamy Y., Nahar L., Cox P. J., Jaspars M., Sarker S. D. (2003). Bioactivity of secoiridoid glycosides from Centaurium erythraea. Phytomedicine 10, 344–347. 10.1078/094471103322004857 [DOI] [PubMed] [Google Scholar]
- Laxmi A., Siddhartha S., Archana M. (2011). Antimicrobial screening of methanol and aqueous extracts of Swertia chirata. Int. J. Pharm. Pharm. Sci. 3, 142–146. [Google Scholar]
- Liu J. (1995). Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 49, 57–68. 10.1016/0378-8741(95)90032-2 [DOI] [PubMed] [Google Scholar]
- Liu J., Liu Y. P., Klaassen C. D. (1994). The effect of Chinese hepatoprotective medicines on experimental liver-injury in mice. J. Ethnopharmacol. 42, 183–191. 10.1016/0378-8741(94)90084-1 [DOI] [PubMed] [Google Scholar]
- Luo Y. D., Chen J., Cao J., Wen X. D., Li P. (2009). Determination of sweroside in rat plasma and bile for oral bioavailability and hepatobiliary excretion. Chem. Pharm. Bull. 57, 79–83. 10.1248/cpb.57.79 [DOI] [PubMed] [Google Scholar]
- Malla B., Gauchan D. P., Chhetri R. B. (2015). An ethnobotanical study of medicinal plants used by ethnic people in Parbat district of western Nepal. J. Ethnopharmacol. 165, 103–117. 10.1016/j.jep.2014.12.057 [DOI] [PubMed] [Google Scholar]
- Mandal S., Chatterjee A. (1987). Structure of chiratanin, a novel dimeric xanthone. Tetrahedron Lett. 28, 1309–1310. 10.1016/S0040-4039(00)95355-3 [DOI] [Google Scholar]
- Mandal S., Chatterjee A. (1994). Seminar on Research in Ayurveda and Siddha. New Delhi: CCRAS; 58–59. [Google Scholar]
- Mandal S., Das P. C., Joshi P. C. (1992). Anti-inflammatory action of Swertia chirata. Fitoterapia 63, 122–128. [Google Scholar]
- Medda S., Mukhopadhyay S., Basu M. K. (1999). Evaluation of the in-vivo activity and toxicity of amarogentin, an antileishmanial agent, in both liposomal and niosomal forms. J. Antimicrob. Chemother. 44, 791–794. 10.1093/jac/44.6.791 [DOI] [PubMed] [Google Scholar]
- Mitra S. K., Gopumadhavan S., Muralidhar T. S. (1996). Effect of D-400, an ayurvedic herbal formulation on experimentally-induced diabetes mellitus. Phytother. Res. 10, 433–435. [Google Scholar]
- Mukherji B. (1953). Indian Pharmaceutical Codex, Indigenous Drugs, Vol. I. New Delhi: CSIR; 64–65. [Google Scholar]
- Muruganandan S., Srinivas K., Gupta S., Gupta P. K., Lal J. (2005). Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J. Ethnopharmacol. 97, 497–501. 10.1016/j.jep.2004.12.010 [DOI] [PubMed] [Google Scholar]
- Nagalekshmi R., Menon A., Chandrasekharan D. K., Nair C. K. K. (2011). Hepatoprotective activity of Andrographis paniculata and Swertia chirayita. Food Chem. Toxicol. 49, 3367–3373. 10.1016/j.fct.2011.09.026 [DOI] [PubMed] [Google Scholar]
- Nandkarni K. M. (1976). Indian Materia Medica, Bombay Popular Prakashan, Vol. I. Bombay: Elsevier; 1184–1186. [Google Scholar]
- Natarajan P. N., Wan A. S, Zsaman, V. (1974). Antimalarial, antiamobeic and toxicity tests on gentianine. Planta Med. 25, 258–260. 10.1055/s-0028-1097940 [DOI] [PubMed] [Google Scholar]
- Niiho Y., Yamazaki T., Nakajima Y., Yamamota T., Ando H., Hirai Y. (2006). Gastroprotective effects of bitter principles isolated from Gentian root and Swertia herb on experimentally-induced gastric lesions in rats. J. Nat. Med. 60, 82–88. 10.1007/s11418-005-0014-2 [DOI] [Google Scholar]
- Padhan J. K., Kumar V., Sood H., Singh T. R., Chauhan R. S. (2015). Contents of therapeutic metabolites in Swertia chirayita correlate with the expression profiles of multiple genes in corresponding biosynthesis pathways. Phytochemistry 116, 38–47. 10.1016/j.phytochem.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Pal D., Sur S., Mandal S., Das A., Roy A., Das S., et al. (2012). Prevention of liver carcinogenesis by amarogentin through modulation of G1/S cell cycle check point and induction of apoptosis. Carcinogenesis 33, 2424–2431. 10.1093/carcin/bgs276 [DOI] [PubMed] [Google Scholar]
- Pant M., Bisht P., Gusain M. P. (2010). De novo shoot organogenesis from cultured root explants of Swertia chirata Buch.-Ham.ex Wall.: an endangered medicinal plant. Nat. Sci. 8, 244–252. [Google Scholar]
- Pant M., Bisht P., Gusain M. P. (2012). in vitro propagation through root-derived callus cultures of Swertia chirata Buch.-Ham ex Wall. Afr. J. Biotechnol. 11, 7408–7416. [Google Scholar]
- Pant N., Jain D. C., Bhakuni R. S. (2000). Phytochemicals from genus Swertia and their biological activities. Indian J. Chem. 39, 565–586. [Google Scholar]
- Pardo-Andreu G. L., Paim B. A., Castilho R. F., Velho J. A., Delgado R., Vercesi A. E., et al. (2008). Mangifera indica L. extract (Vimang®) and its main polyphenol mangiferin prevent mitochondrial oxidative stress in atherosclerosis-prone hypercholesterolemic mouse. Pharmacol. Res. 57, 332–338. 10.1016/j.phrs.2008.03.005 [DOI] [PubMed] [Google Scholar]
- Patil K., Dhande S., Kadam V. (2013). Therapeutic Swertia chirata—an overview. Res. J. Pharmacogn. Phytochem. 5, 199–207. [Google Scholar]
- Perveen S., Anis M. (2014). Encapsulation of internode regenerated adventitious shoot buds of Indian Siris in alginate beads for temporary storage and twofold clonal plant production. Acta Physiol. Plant. 36, 2067–2077. 10.1007/s11738-014-1584-y [DOI] [Google Scholar]
- Phoboo S., Pinto M. D. S., Barbosa A. C. L., Sarkar D., Bhowmik P. C., Jha P. K., et al. (2013). Phenolic-linked biochemical rationale for the anti-diabetic properties of Swertia chirayita (Roxb. ex Flem.) Karst. Phytother. Res. 27, 227–235. 10.1002/ptr.4714 [DOI] [PubMed] [Google Scholar]
- Rai L. K., Prasad P., Sharma E. (2000). Conservation threats to some medicinal plants of the Sikkim Himalaya. Biol. Cons. 93, 27–33. 10.1016/S0006-3207(99)00116-0 [DOI] [Google Scholar]
- Ray S., Majumder H. K., Chakravarty A. K., Mukhopadhyay S., Gil R. R., Cordell G. A. (1996). Amarogentin, a naturally occurring secoiridoid glycoside and a newly recognized inhibitor of topoisomerase I from Leishmania donovani. J. Nat. Prod. 59, 27–29. 10.1021/np960018g [DOI] [PubMed] [Google Scholar]
- Rehman S., Latif A., Ahmad S., Khan A. U. (2011). In vitro antibacterial screening of Swertia chirayita Linn. against some gram negative pathogenic strains. Int. J. Pharm. Res. Dev. 4, 188–194. [Google Scholar]
- Saha P., Mandal S., Das A., Das P. C., Das S. (2004). Evaluation of the anticarcinogenic activity of Swertia chirata Buch. Ham, an Indian medicinal plant, on DMBA-induced mouse skin carcinogenesis model. Phytother. Res. 18, 373–378. 10.1002/ptr.1436 [DOI] [PubMed] [Google Scholar]
- Saha P., Mandal S., Das A., Das S. (2006). Amarogentin can reduce hyperproliferation by downregulation of Cox-II and upregulation of apoptosis in mouse skin carcinogenesis model. Cancer Lett. 244, 252–259. 10.1016/j.canlet.2005.12.036 [DOI] [PubMed] [Google Scholar]
- Sanchez G. M., Re L., Giliani A., Nunez-Selles A. J., Davision G. P., Leon-Fernandez O. S. (2000). Protective effects of Mangifera indica L. extract, magiferin and selected antioxidant against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharm. Res. 42, 565–573. 10.1006/phrs.2000.0727 [DOI] [PubMed] [Google Scholar]
- Saravanan S., Hairul Islam V. I., Prakash Babu N., Pandikumar P., Thirugnanasambantham K., Chellappandian M., et al. (2014). Swertiamarin attenuates inflammation mediators via modulating NF-kB/I kB and JAK2/STAT3 transcription factors in adjuvant induced arthritis. Eur. J. Pharm. Sci. 56, 70–86. 10.1016/j.ejps.2014.02.005 [DOI] [PubMed] [Google Scholar]
- Saxena A. M., Bajpai M. B., Mukherjee S. K. (1991). Swerchirin induced blood sugar lowering of streptozotocin treated hyperglycemic rats. Indian J. Exp. Biol. 29, 674–675. [PubMed] [Google Scholar]
- Saxena A. M., Bajpai M. B., Murthy P. S., Mukherjee S. K. (1993). Mechanism of blood sugar lowering by a Swerchirin-containing hexane fraction (SWI) of Swertia chirayita. Indian J. Exp. Biol. 31, 178–181. [PubMed] [Google Scholar]
- Saxena A. M., Murthy P. S., Mukherjee S. K. (1996). Mode of action of three structurally different hypoglycemic agents: a comparative study. Indian J. Exp. Biol. 34, 351–355. [PubMed] [Google Scholar]
- Scartezzini P., Speroni E. (2000). Review on some plants of Indian traditional medicine with antioxidant activity. J. Ethnopharmacol. 71, 23–42. 10.1016/S0378-8741(00)00213-0 [DOI] [PubMed] [Google Scholar]
- Schimmer O., Mauthner H. (1996). Polymetoxylated xanthones from the herb of Centaurium erythraea with strong antimutagenic properties in Salmonella typhimurium. Planta Med. 62, 561–564. 10.1055/s-2006-957973 [DOI] [PubMed] [Google Scholar]
- Sekar B. C., Mukherjee B., Chakravarti R. B., Mukherjee S. K. (1987). Effect of different fractions of Swertia chirayita on the blood sugar level of albino rats. J. Ethnopharmacol. 21, 175–181. 10.1016/0378-8741(87)90127-9 [DOI] [PubMed] [Google Scholar]
- Shah G. M., Abbasi A. M., Khan N., Guo X., Khan M. A., Hussain M., et al. (2014). Traditional uses of medicinal plants against malarial disease by the tribal communities of Lesser Himalayas–Pakistan. J. Ethnopharmacol. 155, 450–462. 10.1016/j.jep.2014.05.047 [DOI] [PubMed] [Google Scholar]
- Sharma N., Varshney V. K., Kala R. P., Bisht B., Sharma M. (2013a). Antioxidant capacity and total phenolic content of Swertia chirayita (Roxb. ex Fleming) H. Karst. in Uttarakhand. Int. J. Pharm. Sci. Rev. Res. 23, 259–261. [Google Scholar]
- Sharma S., Shahzad A., Teixeira da Silva J. A. (2013b). Synseed technology–a complete synthesis. Biotechnol. Adv. 31, 186–207. 10.1016/j.biotechadv.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Sharma V., Kamal B., Srivastava N., Dobriyal A. K., Jadon V. S. (2014). In vitro flower induction from shoots regenerated from cultured axillary buds of endangered medicinal herb Swertia chirayita H. Karst. Biotechnol. Res. Int. 2014:264690. 10.1155/2014/264690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V., Kamal B., Srivastava N., Negi Y., Dobriyal A. K., Jadon V. S. (2015). Enhancement of in vitro growth of Swertia chirayita Roxb. Ex Fleming co-cultured with plant growth promoting rhizobacteria. Plant Cell Tiss. Org. Cult. 121, 215–225. 10.1007/s11240-014-0696-9 [DOI] [Google Scholar]
- Siler B., Misić D., Nestorović J., Banjanac T., Glamoclija J., Soković M, et al. (2010). Antibacterial and antifungal screening of Centaurium pulchellum crude extracts and main secoiridoid compounds. Nat. Prod. Commun. 5, 1525–1530. [PubMed] [Google Scholar]
- Soica C., Oprean C., Borcan F., Danciu C., Trandafirescu C., Coricovac D., et al. (2014). The Synergistic biologic activity of oleanolic and ursolic acids in complex with Hydroxypropyl-γ-Cyclodextrin. Molecules 19, 4924–4940. 10.3390/molecules19044924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana M. J., Molla M. T. H., Alam M. T., Ahmed F. R. S. (2007). Investigation of antimicrobial activities of the plant Swertia chirayita ham. J. Life Earth Sci. 2, 31–34. [Google Scholar]
- Sun H., Li L., Zhang A., Zhang N., Lv H., Sun W., et al. (2013). Protective effects of sweroside on human MG-63 cells and rat osteoblasts. Fitoterapia 84, 174–179. 10.1016/j.fitote.2012.11.010 [DOI] [PubMed] [Google Scholar]
- Suparna M., Ranjana J., Sibabrata M. (1998). Naturally occurring with pharmacological acitvitiy. Indian J. Pharm. Sci. 60, 123–127. [Google Scholar]
- Tabassum S., Mahmood S., Hanif J., Hina M., Uzair B. (2012). An overview of medicinal importance of Swertia chirayita. Int. J. Appl. Sci. Tec. 2, 298–304. [Google Scholar]
- Vaidya H., Goyal R. K., Cheema S. K. (2013). Anti-diabetic activity of swertiamarin is due to an active metabolite, gentianine, that upregulates PPAR-γ gene expression in 3T3-L1 cells. Phytother. Res. 27, 624–627. 10.1002/ptr.4763 [DOI] [PubMed] [Google Scholar]
- Vaidya H., Rajani M., Sudarshan V., Padh H., Goyal R. (2009). Swertiamarin: a lead from Enicostemma littorale Blume. for anti-hyperlipidaemic effect. Eur. J. Pharm. Biopharm. 617, 108–112. 10.1016/j.ejphar.2009.06.053 [DOI] [PubMed] [Google Scholar]
- Vázquez L. H., Palazon J., Navarro-Ocaña A. (2012). The pentacyclic triterpenes α, β-amyrins: a review of sources and biological activities, in Phytochemicals—A Global Perspective of Their Role in Nutrition and Health, ed Venketeshwer Rao (In Tech), 487–502. [Google Scholar]
- Verma H., Patil P. R., Kolhapure R. M., Gopalkrishna V. (2008). Antiviral activity of the Indian medicinal plant extract, Swertia chirata against herpes simplex viruses: a study by in-vitro and molecular approach. Indian J. Med. Microbiol. 26, 322–326. 10.4103/0255-0857.43561 [DOI] [PubMed] [Google Scholar]
- Verma V. K., Sarwa K. K., Kumar A., Zaman M. K. (2013). Comparison of hepatoprotective activity of Swertia chirayita and Andrographis paniculata plant of Northe East India against CCl4 induced hepatotoxic rats. J. Pharm. Res. 7, 647–653. 10.1016/j.jopr.2013.07.008 [DOI] [Google Scholar]
- Verschaeve L., Van Staden J. (2008). Mutagenic and antimutagenic properties of extracts from South African traditional medicinal plants. J. Ethnopharmacol. 119, 575–587. 10.1016/j.jep.2008.06.007 [DOI] [PubMed] [Google Scholar]
- Wang C. Z., Maier U. H., Eisenreich W., Adam P., Obersteiner I. (2001). Unexpected biosynthetic precursors of amarogentin a retrobiosynthetic 13C NMR study. Eur. J. Org. Chem. 2001, 1459–1465. [DOI] [Google Scholar]
- Wang L., Lizhe A., Yanping H., Lixin W., Yi L. (2009). Influence of phytohormones and medium on the shoot regeneration from the leaf of Swertia chirayita Buch.-Ham. Ex wall. in vitro. Afr. J. Biotechnol. 8, 2513–2517. 10.4314/ajb.v8i11.60746 [DOI] [Google Scholar]
- Wawrosch C., Maskay N., Kopp B. (1999). Micropropagation of the threatened Nepatese medicinal plant Swertia chirata Buch.-Ham.ex wall. Plant Cell Rep. 18, 997–1001. 10.1007/s002990050697 [DOI] [Google Scholar]
- Ya B. Q., Nian L. C., Li C., Gen X. P. (1999). Protective effect of swerchirin on hematopoiesis in 60Co-irradiated mice. Phytomed. 6, 85–88. 10.1016/S0944-7113(99)80040-3 [DOI] [PubMed] [Google Scholar]
- Yoshimi N., Matsunaga K., Katayama M., Yamada Y., Kuno T., Qiao Z., et al. (2001). The inhibitory effects of mangiferin, a naturally occurring glucosylxanthone, in bowel carcinogenesis of male F344 rats. Cancer Lett. 163, 163–170. 10.1016/S0304-3835(00)00678-9 [DOI] [PubMed] [Google Scholar]
- Zheng M. S., Lu Z. Y. (1990). Antiviral effect of mangiferin and iso-mangiferin on herpes simplex virus. Chinese Med. J. 103, 160–165. [PubMed] [Google Scholar]
- Zhou N. J., Geng C. A., Huang X. Y., Ma Y. B., Zhang X. M., Wang J. L., et al. (2015). Anti-hepatitis B virus active constituents from Swertia chirayita. Fitoterapia 100, 27–34. 10.1016/j.fitote.2014.11.011 [DOI] [PubMed] [Google Scholar]