Abstract
Background: Anterograde amnesia is a hallmark effect of volatile anesthetics. Isoflurane is known to affect both the translation and transcription of plasticity-associated genes required for normal memory formation in many brain regions. What is not known is whether isoflurane anesthesia prevents the initiation of transcription or whether it halts transcription already in progress. We tested the hypothesis that general anesthesia with isoflurane prevents learning-induced initiation of transcription of several memory-associated immediate-early genes (IEGs) correlated with amnesia; we also assessed whether it stops transcription initiated prior to anesthetic administration.
Methods: Using a Tone Fear Conditioning paradigm, rats were trained to associate a tone with foot-shock. Animals received either no anesthesia, anesthesia immediately after training, or anesthesia before, during, and after training. Animals were either sacrificed after training or tested 24 h later for long-term memory. Using Cellular Compartment Analysis of Temporal Activity by Fluorescence in situ Hybridization (catFISH), we examined the percentage of neurons expressing the IEGs Arc/Arg3.1 and Zif268/Egr1/Ngfi-A/Krox-24 in the dorsal hippocampus, primary somatosensory cortex, and primary auditory cortex.
Results: On a cellular level, isoflurane administered at high doses (general anesthesia) prevented initiation of transcription, but did not stop transcription of Arc and Zif268 mRNA initiated prior to anesthesia. On a behavioral level, the same level of isoflurane anesthesia produced anterograde amnesia for fear conditioning when administered before and during training, but did not produce retrograde amnesia when administered immediately after training.
Conclusion: General anesthesia with isoflurane prevents initiation of learning-related transcription but does not stop ongoing transcription of two plasticity-related IEGs, Arc and Zif268, a pattern of disruption that parallels the effects of isoflurane on memory formation. Combined with published research on the effects of volatile anesthetics on memory in behaving animals, our data suggests that different levels of anesthesia affect memory via different mechanisms: general anesthesia prevents elevation of mRNA levels of Arc and Zif268 which are necessary for normal memory formation, while anesthesia at lower doses affects the strength of memory by affecting levels of plasticity-related proteins.
Keywords: isoflurane, Arc, Zif268, immediate early gene, catFISH
Introduction
Inhaled anesthetics have been used for general anesthesia for more than 160 years (Long, 1849); however, the mechanisms of action of anesthetic agents have remained largely unknown until the past several decades. Of the two basic endpoints of general anesthesia, immobility to noxious stimuli and a reversible state of amnesia (Eger et al., 1997), anesthetic-induced amnesia is of particular importance. Patients anesthetized for surgery sometimes become aware during surgery and have horrific memories of their experience, despite the appearance of being under adequate anesthesia (Kent et al., 2013; Rule and Reddy, 2014; Mashour and Avidan, 2015). A better understanding of the molecular mechanisms of anesthetics on memory-forming processes can lead not only to academic enlightenment, but also to improved patient outcomes. To better understand these molecular mechanism, we examined the effect of general anesthesia with isoflurane on behavioral training in rats and the corresponding transcription of plasticity-related immediate-early genes (IEGs; Alkire et al., 2007; Alkire and Guzowski, 2008). Synaptic plasticity is the physical basis of memory, and the expression of certain plasticity-related IEGs, including Arc and Zif268, is required to form long-term memory (Dragunow, 1996; Bozon et al., 2002, 2003; Davis et al., 2003; Plath et al., 2006; Bramham et al., 2008, 2010; Nakayama et al., 2015). In a neurobiological context, learning is the formation of long-term memory and amnesia is the loss of previously acquired memory (retrograde amnesia) or the failure to form new long-term memories (anterograde amnesia). General anesthesia with isoflurane produces anterograde amnesia, but it is not clear at which stage of memory formation this occurs: acquisition of sensory data, processing of sensory input into short-term memory, or consolidation of long-term memory (Izquierdo et al., 1999). In other words, anterograde amnesia could be the result of failing to consolidate long-term memory or the failure to form any memory at all.
Isoflurane is known to suppress activity in neurons of the sensory thalamus in a dose dependent manner, but does not completely abolish neuronal activity (Vahle-Hinz et al., 2007). It is possible that amnesia occurs as a result of suppression of sensory input to the cerebral cortex and to the memory system in the medial temporal lobe, including the amygdala, hippocampus, and associated entorhinal and perirhinal cortex. However, simply suppressing neural activity does not necessarily reduce the number of cells engaged in activity-dependent plasticity-related IEG transcription. Recent research demonstrates that neuronal activity and IEG expression are not always coupled during learning (Guzowski et al., 2006; Carpenter-Hyland et al., 2010). Because not all neuronal firing induces activity-dependent IEG transcription, simply reducing neural activity does not necessarily reduce the size of neuronal ensembles engaged in this transcription. It is possible, however unlikely, that sensory input could result in neuronal activity that may induce synaptic plasticity and possibly lead to memory formation during the anesthetized state.
Knowing that general anesthesia with isoflurane induces anterograde amnesia, we wanted to delve deeper into the effects of isoflurane on biochemical markers of learning and memory. More importantly, we wanted to examine these effects in a temporally specific manner that would allow us to examine the formation of a specific memory, controlling for effects of sensory input and neuronal activity related to the induction of anesthesia. We developed a testable hypothesis that examines isoflurane’s effects downstream of its effect on neuronal firing, the most likely point at which anesthesia effects activity-dependent IEG transcription. We directly tested the hypothesis that general anesthesia with isoflurane will suppress learning-initiated transcription of the plasticity-related IEGs Arc and Zif268 in brain regions involved in auditory fear conditioning: dorsal hippocampus, primary auditory cortex and primary somatosensory cortex (Romanski and LeDoux, 1992; Quirk et al., 1997; Bushnell et al., 1999; Maren and Holt, 2000; Anagnostaras et al., 2001; Kim and Jung, 2006). We further differentiated between the effect of isoflurane on initiation of transcription and interruption of transcription that is already underway. This dissociation is possible using the powerful molecular imaging and analysis technique known as Cellular Compartment Analysis of Temporal Activity by Fluorescence in situ Hybridization (catFISH; Guzowski et al., 2001) in conjunction with isoflurane anesthesia induced either before or immediately after Pavlovian fear conditioning.
The catFISH method allows us to examine gene expression with cellular resolution and temporal specificity when used with genes which have a short duration of transcription: Arc and Zif268 (Guzowski et al., 1999; Vazdarjanova et al., 2002b). Transcription of Arc and Zif268 mRNA follows a distinctive activity-induced time course following plasticity-inducing events, such as learning or maximal electroconvulsive shock (MECS), allowing for high temporal resolution when visualized using Fluorescence in situ Hybridization (FISH) with full-length antisense RNA probes. Intranuclear foci of transcription for Arc and Zif268 mRNA are first detectable 2 min after transcription is initiated; these foci appear first as tiny specks that grow into easily identifiable, intensely stained dots by 5 min. After 10 min, the intranuclear RNA no longer appears as foci; it diffuses into the nucleoplasm and at 15 min appears as perinuclear staining along the nuclear membrane as it migrates into the cytoplasm. By 30 min, Arc and Zif268 mRNA appear as diffuse cytoplasmic staining which fills the soma. After 30 min, Zif268 remains in the soma while Arc is transported into dendrites for targeted, synapse-specific local translation (Steward et al., 1998; Guzowski et al., 1999; Vazdarjanova et al., 2002a,b). Therefore, by identifying individual cells with clearly visible foci of transcription 6 min after fear conditioning, we can accurately evaluate the size of IEG-expressing neuronal ensembles activated by the training.
Materials and Methods
Animals
After obtaining approval from the Institutional Animal Care and Use Committee (Augusta University, Augusta, GA, USA), 25 male Sprague-Dawley rats (2 months old, 250–300 g) were obtained from Charles River Laboratories (Wilmington, MA, USA) and housed in a temperature controlled colony room with food and water freely available. The animals were maintained on a 12 h reverse light cycle (lights on at 7 PM, lights off at 7 AM); testing was performed during the dark (active) phase.
Behavioral Procedures
The tone fear conditioning (TFC) training apparatus consisted of a 10 × 50 cm acrylic box with two opaque walls and transparent lid. The other two walls and floor were lined with two metal plates separated at the floor by a 1 cm gap. The animals were habituated to the apparatus 3 min per day for 5 days prior to training. On the training day, animals were placed into the apparatus for a total of 70 s and presented with two tones (10 s, 5 kHz, 80 dB) at 15 and 40 s, each co-terminating with a foot-shock (1 s, 0.7 mA AC). Foot-shock was delivered using a custom-made constant-current generator designed to deliver the full current across a single circuit, not a grid floor as used in commercially available fear conditioning units. At the current used, the shock is intense and unpleasant but not painful. Figure 1 illustrates the behavioral parameters of each treatment group. Animals in the TFC group (n = 8) did not receive anesthesia, animals in the TFC-Iso group (n = 7) were anesthetized for 6 min immediately after training, and animals in the Iso-TFC group (n = 8) were anesthetized continuously from 10 min prior to training until 6 min after training. Freezing, defined as immobility except for respiration, was scored in non-anesthetized animals during training.
FIGURE 1.
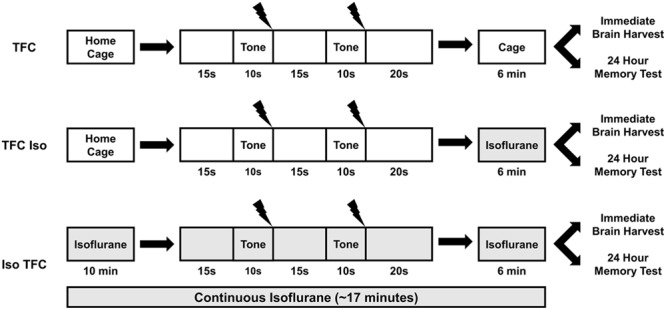
Tone fear conditioning (TFC) and isoflurane administration. This illustration depicts isoflurane administration (gray shaded areas) relative to TFC for each behavioral group. The TFC group was not anesthetized, the TFC-Iso group was anesthetized after TFC, and the Iso-TFC group was anesthetized before, during, and after TFC. Please see text for detailed experimental procedures.
Three animals from each treatment group were sacrificed by decapitation 6 min after TFC training, while two caged controls were taken from their home cages and immediately sacrificed. The remaining rats (4–5 per group) were tested 24 h after training for long-term memory for fear conditioning. Fear conditioning to context and tone were tested separately 5 h apart. To assess contextual fear conditioning, animals were placed in the training apparatus for 5 min and freezing was scored. To assess conditioned fear to the tone alone, animals were placed in a novel context, a 46 × 37 cm box, for 2 min and then subjected to six 20 s presentations of the conditioned tone (5 kHz, 80 dB) played 10 s apart; animals were removed 2 min after the last tone. Freezing was scored prior to and during the tone presentation. The order of testing for fear conditioning to tone and fear conditioning to context was randomly assigned in a counterbalanced design. Freezing was scored by an observer blinded to each animal’s group assignment.
Anesthetic Procedures
Anesthesia was induced by exposing animals to a vessel containing gauze saturated with isoflurane for 10–15 s, until loss of righting reflex was observed, and then maintained with 2% isoflurane delivered by a vaporizer with oxygen at a flow rate of 1.5–2 l/min via nose cone. The desired state of general anesthesia was defined as: loss of righting reflex, areflexia to a toe-pinch stimulus, and lack of purposeful movement except for spontaneous breathing. Animals were continuously observed while anesthetized and tested for toe-pinch response at regular intervals. Anesthetic was delivered at the minimum level required to maintain these criteria.
This induction method was designed to meet two criteria: first, to prevent the unwanted stimulation of learning pathways in the brain caused by exploration in a standard induction chamber; and second, to emulate the rapid induction of anesthesia practiced before euthanasia by decapitation. The study of time-sensitive IEG transcription necessitates tight control over the period several minutes before the subject’s brain is flash frozen.
Fluorescence in Situ Hybridization
Animals were sacrificed, their brains quickly removed and immediately frozen. The frozen brains were sectioned using a cryostat microtome into 20 μm thick sections and mounted on microscope slides. The mounted tissue was fixed in 4% paraformaldehyde, permeabilized in a 1:1 solution of acetone and methanol, and then hybridized with digoxigenin-labeled Arc or Zif268 full-length antisense RNA probes. These probes were synthesized using MAXIscript® T3 and T7 (Ambion®, Austin, TX) and AmpliScribeTM T7 (Epicentre Biotechnologies, Madison, WI, USA) RNA in vitro transcription kits. After peroxidase quenching and blocking, the hybridized tissue was incubated with peroxidase-conjugated anti-digoxigenin antibody (Roche, Indianapolis, IN, USA) and revealed using SuperGloTM Immunofluorescence Amplification Kits (Fluorescent Solutions, Augusta, GA, USA) or Tyramide Signal Amplification kits (Perkin Elmer, Waltham, MA, USA) with Cyanine 3 or Fluorescein. Nuclei were stained with SYTOX® Green (Invitrogen, Carlsbad, CA, USA), DAPI, or 7-AAD.
Imaging and Stereology
Stained slides were imaged using a Zeiss Axio Imager fluorescent microscope with ApoTome; multi-channel z-stack images were collected using a 20X/NA 0.8 objective.
Each 447 μm × 335 μm image stack consisted of 13–17 planes of 1.2 μm-thick optical sections. For each animal, at least two slides containing one section per slide were selected for each brain region; two image stacks were collected from each section. On average, four image stacks were analyzed per brain region for each animal; the total number of cells counted for each brain region by condition and probe are shown in Supplementary Table S1. Unbiased stereological cell counting was performed as follows: cells were classified as either neuron-like or glia-like based on their nuclear morphology; glia-like cells with small, intensely, and uniformly stained nuclei, were excluded from the analysis. Neuron-like cells in the regions of interest were counted using an optical dissector method, which minimizes sampling errors attributable to partial cells, as cell volumes do not influence sampling frequencies (West, 1999). Pairs of adjacent “lookup” and “sample” sections were stacked so that the first optical section in a stack was the first “lookup section,” and the second optical section was the first “sample section” as well as the “lookup section” for the next “sample section.” The dissector (the total number of “sample sections”) included the top 60% of a given stack, excluding the first “lookup section”. The dissector counting rule instructed that all neuron-like cells with leading edges present in each dissector should be included for further classification. This rule ensures that all neurons have equal probabilities of being included in the samples regardless of their size, because each of them is defined by their leading edge rather than their volume. It also minimizes type I classification errors where a positive partial cell is classified as negative because the portion of the cell containing the foci of transcription is missing. Neurons were classified as either positive or negative for intranuclear Arc or Zif268 intranuclear foci visible on at least three optical planes. Results are expressed as the percentage of total neurons containing intranuclear foci of Arc or Zif268 mRNA.
Anatomical regions were defined as follows (Paxinos and Watson, 2007): dorsal hippocampal CA1, Bregma -2.92 to -4.20 mm; primary auditory cortex A1, bregma -4.8 to -6.84 mm; primary somatosensory cortex S1, bregma -1.8 to -3.72 mm.
Statistical Analysis
Data are reported as the mean ± the standard error of the mean (SEM). Because all data sets passed the test for normality, they were subjected to one-way analysis of variance (ANOVA) using the Tukey-Kramer post hoc test to compare differences between individual groups, or mixed-design Repeated-Measures ANOVA (RM-ANOVA) for the contextual fear conditioning data. The experiment wide alpha value was set at 0.05 and significant post hoc differences are reported when the mean difference between groups exceeded the critical difference calculated by the Tukey-Kramer test. Statistical analysis was performed using StatView software (SAS Institute, Cary, NC, USA).
Results
Behavior
Freezing behavior was used as a measure of memory for fear conditioning, as it indicates intense fear in rats (Blanchard et al., 1975, 1986). Pre-training freezing during the habituation period (Figure 2A) was low in all groups [mean = 1.85% ± 1.54, range = 0–21.67%, F(2,11) = 0.72, ns] showing that the training context by itself, before the tone-shock pairing, did not elicit fear. Pairing a tone with the foot-shocks resulted in conditioned fear to both the tone and the context in which the pairing occurred, as evidenced by elevated freezing in non-anesthetized animals (TFC group) during tone and context tests 24 h after training (50% ± 8.39 and 27.87% ± 6.33, respectively, Figures 2B,C). There was a significant effect of training on the RM-ANOVA for context [F(1,11) = 16.11, p = 0.002].
FIGURE 2.
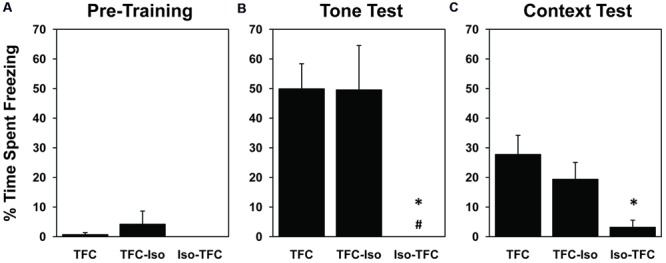
Pre-training and post-training freezing. During habituation to the foot-shock context prior to training (A), all groups exhibited negligible freezing behavior. 24 h after TFC, animals were tested for freezing to tone in a novel context (B) and freezing in the foot-shock context without tone (C). Both the TFC and TFC-Iso groups showed significantly higher levels of freezing to the tone compared with the Iso-TFC group (B); while only the TFC group showed significantly higher levels of freezing to context compared with the Iso-TFC group (C). These results show that animals anesthetized during training demonstrated anterograde amnesia for TFC, while animals anesthetized immediately after training showed no retrograde amnesia. ∗Statistical significance vs. TFC, #statistical significance vs. TFC-Iso.
Significant differences were seen between groups in the post-training tone test [F(2,11) = 6.69, p = 0.01] and context test [F(2,11) = 5.02, p = 0.03]. Rats that received the same training as TFC rats while anesthetized (Iso-TFC group), showed significantly less freezing to tone and context (Figures 2B,C) when compared to the TFC group, indicating robust anterograde amnesia. There was no evidence of retrograde amnesia in rats anesthetized immediately after training (TFC-Iso group); these animals showed freezing comparable to the non-anesthetized TFC group in both tone and context testing (Figures 2B,C).
Gene Expression
The percentage of neurons actively transcribing Arc and Zif268 mRNA induced during TFC in three brain regions involved in tone and contextual fear conditioning is shown in Table 1 and Figure 5. Representative images taken from analyzed stacks are shown for Arc in Figure 3 and Zif268 in Figure 4. For each gene, data for the CA1 region of the dorsal hippocampus was collected from an average of 491 neurons per rat (range: 351–822), 588 neurons per rat (range: 340–774) in primary auditory cortex (A1), and 636 neurons per rat (range: 356–998) in primary somatosensory cortex (S1).
Table 1.
catFISH data – active Arc and Zif268 transcription.
|
Arc |
Zif268 |
|||||
|---|---|---|---|---|---|---|
| Group | CA1 | A1 | S1 | CA1 | A1 | S1 |
| Cg | 4.95% ± 1.05* | 2.38% ± 1.16*# | 1.75% ± 0.67* | 7.22% ± 4.18* | 3.43% ± 2.76*# | 3.37% ± 1.64*# |
| TFC | 22.36% ± 3.99 | 27.32% ± 2.45 | 29.19% ± 4.62 | 24.08% ± 3.12 | 29.41% ± 1.06 | 29.13% ± 3.80 |
| TFC-Iso | 15.60% ± 2.31 | 39.75% ± 4.86 | 22.46% ± 8.22 | 13.55% ± 3.18 | 30.66% ± 5.56 | 31.31% ± 1.00 |
| Iso-TFC | 0.79% ± 0.19*# | 0.97% ± 0.09*# | 0.53% ± 0.19* | 1.58% ± 0.09* | 0.84% ± 0.28*# | 0.90% ± 0.55*# |
Data for each behavioral group is presented as the percentage of neurons expressing intranuclear foci of transcription ± SEM. Data for each immediate-early gene is shown by brain region: dorsal hippocampal CA1, primary auditory cortex A1, and primary somatosensory cortex S1. Group sizes: Cg = 2, TFC = 3, TFC-Iso = 3, Iso-TFC = 3. ∗Statistically significant vs. TFC and #statistically significant vs. TFC-Iso using Tukey-Kramer post hoc tests.
FIGURE 5.
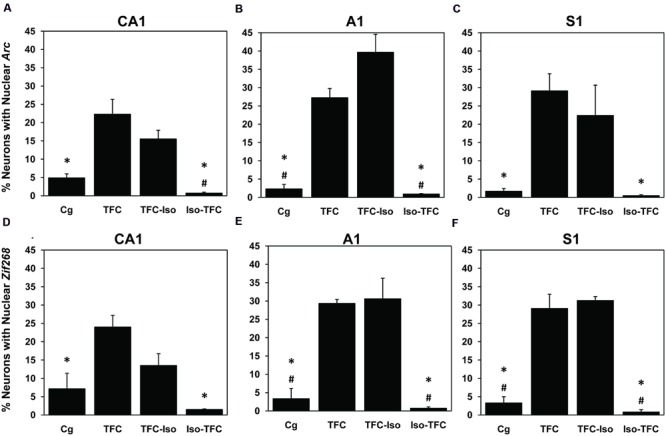
Arc and Zif268 mRNA transcription. Using the catFISH method, mRNA expression induced by TFC was measured and is reported as the percentage of neurons showing active transcription of the plasticity-related immediate-early genes (IEGs) Arc and Zif268. Arc transcription in Dorsal Hippocampal CA1 (A), Primary Auditory Cortex A1 (B), and primary somatosensory Cortex S1 (C) shows that the Iso-TFC group and caged controls both show significantly lower Arc expression than the TFC group. Zif268 transcription in Dorsal Hippocampal CA1 (D), Primary Auditory Cortex A1 (E), and primary somatosensory Cortex S1 (F) shows the same pattern of significantly lower expression in the Iso-TFC and caged control groups compared with the TFC group. Compared with the TFC-Iso group, the Iso-TFC group showed significantly lower Arc expression in CA1 and A1, as well as significantly lower Zif268 expression in A1 and S1. There was no significant difference between the TFC and TFC-Iso groups. The low levels of Arc and Zif268 expression seen in the Iso-TFC group compared with both the TFC and TFC-Iso groups correlates with the lack of post-training freezing behavior observed in this group (Figure 2). General anesthesia administered during training prevented initiation of transcription of the learning-induced IEGs Arc and Zif268 and caused anterograde amnesia, but general anesthesia administered immediately after training had no significant effect on learning or transcription of these IEGs initiated prior to anesthesia. General anesthesia with isoflurane does not directly interfere with ongoing IEG transcription. ∗Statistical significance vs. TFC, #statistical significance vs. TFC-Iso.
FIGURE 3.
Arc catFISH. Fluorescence photomicrographs of in situ hybridization with antisense riboprobes for Arc mRNA. Arc is shown in red, cell nuclei are stained with DAPI and psuedocolored green. Scale bar is 50 μm.
FIGURE 4.
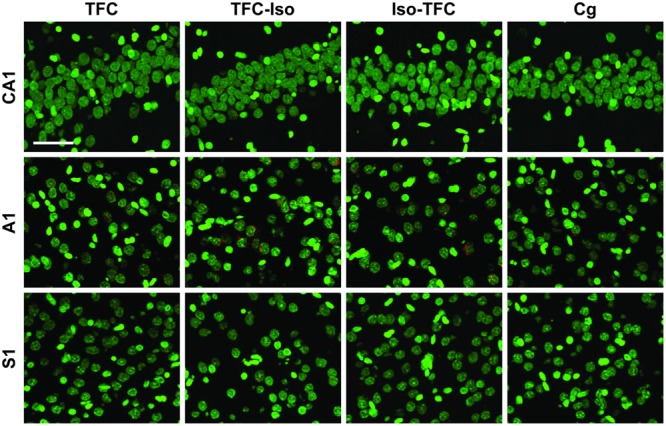
Zif268 catFISH. Fluorescence photomicrographs of in situ hybridization with antisense riboprobes for Zif268 mRNA. Zif268 is shown in red, cell nuclei are stained with DAPI and psuedocolored green. Scale bar is 50 μm.
Consistent with previous findings (Vazdarjanova et al., 2002b, 2006; Carpenter-Hyland et al., 2010), the percentage of neurons expressing Arc at baseline (Cg group) was low in all examined regions (CA1 = 4.95% ± 1.05, A1 = 2.38% ± 1.64, S1 = 1.75% ± 0.67, Figures 5A–C). Figure 5A shows that Arc expression in dorsal hippocampal CA1 varied dramatically across groups [F(3,7) = 14.98, p < 0.01]. The TFC group showed significantly higher Arc expression than Cg or Iso-TFC groups, but no significant difference compared with the TFC-Iso group. A similar pattern of Arc expression was observed in primary auditory cortex, A1 [F(3,7) = 39.19, p < 0.0001, Figure 5B] and primary somatosensory cortex, S1 [F(3,7) = 7.63, p = 0.013, Figure 5C]. Thus, general anesthesia with isoflurane prior to and during training prevented Arc expression in all of the examined brain regions, while, significantly, post-training general anesthesia did not stop transcription that was already initiated.
To test whether the observed effects of isoflurane are specific to Arc expression, we investigated the expression of another activity-induced IEG, Zif268, in the same tissue. ZIF268 protein is a transcription factor known to show an activity-induced pattern of mRNA expression and is also linked to plasticity (Tischmeyer and Grimm, 1999; Hall et al., 2001). In all brain regions there were significant group differences in Zif268 expression [CA1: F(3,7) = 12.36, p < 0.01; A1: F(3,7) = 24.41, p < 0.001; and S1: F(3,7) = 51.61, p < 0.001]. As with Arc, in all three regions we observed low levels of Zif268 transcription in the Cg group (CA1 = 7.22% ± 4.18, A1 = 3.42% ± 3.90, S1 = 2.31% ± 1.64). Also like Arc, the percentage of Zif268 positive cells was elevated in the TFC group compared to the caged and the Iso-TFC groups, but not compared to the TFC-Iso group (Figures 5D–F). General anesthesia with isoflurane affected Zif268 and Arc expression similarly: it prevented initiation of learning-related IEG transcription while not interfering with transcription that was already initiated by fear conditioning.
Discussion
This study was designed to examine the effect of general anesthesia with isoflurane on learning-induced IEG transcription by assessing the size of neuronal ensembles involved in learning a TFC task. We made two important observations: first, we saw a lack of significant observable effects of isoflurane on either plasticity-related IEG transcription (Figure 5) or learning (Figures 2B,C) in animals placed under general anesthesia immediately after training. Isoflurane anesthesia did not cause retrograde amnesia for the TFC task in the TFC-Iso group, nor did it stop the transcription of ongoing learning-induced IEG expression. The second finding was that animals who received TFC training while under general anesthesia (Iso-TFC group) exhibited anterograde amnesia (Figures 2B,C), a finding consistent with previous studies (Dutton et al., 2002). More importantly, however, these animals showed a lack of learning-induced IEG expression (Figure 5). Together, these two observations make an important contribution to understanding the actions of isoflurane on learning and memory at the cellular level. Our findings show that isoflurane does not interfere with ongoing IEG transcription initiated prior to anesthetic administration and, under these conditions, does not cause retrograde amnesia for memory corresponding to this gene expression. In contrast, our results suggest that surgical levels of isoflurane anesthesia prevent the initiation of plasticity-related IEG transcription and subsequently cause anterograde amnesia.
Isoflurane does Not Stop Ongoing IEG Transcription
To determine whether or not general anesthesia with isoflurane interferes directly with ongoing activity-induced IEG transcription, we examined the effects of general anesthesia administered immediately after Tone Fear Conditioning (TFC-Iso group), once learning-induced Arc and Zif268 transcription was already initiated. If isoflurane affected transcription initiated by fear conditioning performed less than 1 min prior to the induction of anesthesia, then we should see a decrease in the number of neurons with Arc and Zif268 intranuclear foci of transcription. Even if transcription was initiated and prematurely terminated in some neurons within the first few minutes after TFC, our application of the catFISH technique would not count these neurons as IEG positive for two reasons: first, Arc and Zif268 mRNA is not detectable until approximately 2 min following initiation of transcription; second, our counting method excludes very small foci (appearing on less than three planes) that can represent prematurely halted transcription. We did not observe a significant difference in either freezing behavior or the size of neuronal ensembles actively transcribing Arc and Zif268. This suggests that the basic mechanisms of transcription for these IEGs are not directly affected by isoflurane. The finding that isoflurane administered after learning did not alter IEG transcription is of particular importance for animal-based research. This study was designed, in part, to determine whether the accepted method of anesthetic administration prior to decapitation as a means of euthanasia in animal research could interfere with the study of IEGs. Our findings show that a single administration of isoflurane prior to euthanasia does not interfere with the IEG transcription initiated prior to anesthesia and, for this behavioral task, does not interfere with IEG-dependent learning. While we did not see any differences between the TFC and TFC-Iso groups there may still be differences in gene expression beyond the scope of this study. Isoflurane does not interfere with ongoing transcription, but could still have effects on translation and, in the case of Arc, targeting of mRNA to synapses.
Isoflurane Prevents Activity-Induced Initiation of IEG Transcription
We observed that animals maintained at a surgical level of anesthesia prior to and during the fear conditioning procedure (Iso-TFC group) did not show active transcription of Arc and Zif268 in brain regions involved in TFC (Figure 5), nor did they acquire a conditioned fear response to either the tone or the foot-shock context. While the behavioral effects of isoflurane have been previously reported, the findings describing the effects of isoflurane on transcription of these IEGs is novel. Because Arc and Zif268 proteins are required for the formation of long-term memory (Bozon et al., 2003; Plath et al., 2006), and their mRNA is short-lived (Waters et al., 1990; Lyford et al., 1995), if mRNA transcription is not initiated as a result of behavioral training, then Arc and Zif268 proteins are not available to enable memory consolidation and learning will not occur. Isoflurane alters the functional connectivity of the CNS to the PNS via the sensory thalamus by decreasing, but not preventing, neuronal firing (Vahle-Hinz et al., 2007; Ying et al., 2009). As Arc and Zif268 are both activity-induced IEGs, the dramatic reduction in transcription we observed is most likely caused by decreased neuronal activity rather than interference with the mechanisms of transcription, especially because we also observed that the same level of anesthesia did not prevent transcription that was already initiated.
Isoflurane has Persistent Effects on Gene Expression: Implications for Lasting Effects on Memory
In addition to the immediate effects of isoflurane on transcription of IEGs, it has an indirect effect on the transcription, as well as the translation, of many other genes. Isoflurane administration results in prolonged upregulation and down-regulation of mRNA and protein expression seen across many genes in various organs (Hamaya et al., 2000; Culley et al., 2006; Rampil et al., 2006; Pan et al., 2008; Kalenka et al., 2010). These effects persist for weeks to months beyond its clearance from the body and therefore cannot be caused by the direct effects of isoflurane binding with cell surface receptors or interacting with cell membranes (Franks and Lieb, 1994; Campagna et al., 2003; Ying et al., 2009; Garcia et al., 2010). Epigenetic changes induced by isoflurane account for some of these changes and can also contribute to anesthesia-induced memory impairment by affecting the same pathways as Arc and Zif268 (Levenson et al., 2004; Chwang et al., 2006; Ahn et al., 2008; Zhong et al., 2014). The proper formation and maintenance of memory requires several rounds of gene expression, making both new and old memories vulnerable to the effects of isoflurane.
Our finding that general anesthesia with isoflurane prevents initiation of transcription of both effector IEGs (Arc) and transcription factors (Zif268) is of particular significance, because it sheds light on how isoflurane, and possibly other anesthetics, may indirectly alter gene expression in the brain over a period of days to months following anesthesia. If an activity-dependent transcription factor such as Zif268 is effectively prevented from being transcribed for a period of several hours, during surgery for example, then there will likely be less Zif268 protein available to act as a transcription factor at some time point after anesthetic administration is discontinued. This could lead to a “ripple effect” of diminished transcription (and thus a diminished availability of mRNA for translation) of a wide variety of genes which use Zif268 as a transcription factor. Zif268 is also a transcription factor for itself, so it is plausible that transcription of new Zif268 mRNA will be affected by the previous decrease in activity and transcription during anesthesia. It is conceivable that a single, discrete period of drastically reduced neuronal activity could have persistent effects throughout multiple rounds of transcription and translation. Arc, for example, undergoes a second round of transcription during memory consolidation (Ramirez-Amaya et al., 2005; Nakayama et al., 2015); this second round of Arc transcription could be impaired by a decrease in available Zif268 protein due to a decrease in Zif268 mRNA during anesthesia. A period of drastically decreased neuronal activity has the potential to effect any cellular mechanism that is effected at any stage by the availability of activity-induced gene products. This proposed process for explaining delayed effects from anesthesia may deserve further research.
Conclusion
We found that isoflurane did not stop ongoing transcription of IEG mRNA but it did prevent the initiation of new activity-induced IEG transcription. These findings correlate well with the observed effects on long-term memory: lack of retrograde amnesia with marked anterograde amnesia. Together, they contribute to understanding the molecular mechanisms of the action of isoflurane. Our findings are important for two reasons: first, this suggests that isoflurane affects protein levels of plasticity-related genes, to a large degree, by decreasing mRNA transcription, and second, brief isoflurane administration prior to euthanasia should not interfere with studies of gene expression. The fact that isoflurane does not interfere with gene transcription when administered immediately before euthanasia is reassuring to those of us who routinely use this method when studying IEGs and may encourage researchers who use live decapitation to employ this method for humane euthanasia.
Author Contributions
KB, RN, and AV each participated in experimental design, conducted experiments, analyzed data, and helped prepare the manuscript.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. Funding for this study was provided by Augusta University and the National Institute of Mental Health (R21MH08318). The study was supported in part by the U.S. Department of Veterans Affairs.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fnbeh.2015.00363
References
- Ahn H. J., Hernandez C. M., Levenson J. M., Lubin F. D., Liou H.-C., Sweatt J. D. (2008). c-Rel, an NF-κB family transcription factor, is required for hippocampal long-term synaptic plasticity and memory formation. Learn. Mem. 15 539–549. 10.1101/lm.866408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire M. T., Beydoun T., Miyashita T., McReynolds J. R., Guzowski J. F. (2007). Toward the mechanism of anesthetic-induced amnesia: anesthetics shut down memory consolidation by inhibiting hippocampal Arc-protein synthesis in the rat. Anesth. Analg. 104:S213. [Google Scholar]
- Alkire M. T., Guzowski J. F. (2008). Hypothesis: suppression of memory protein formation underlies anesthetic-induced amnesia. Anesthesiology 109 768–770. 10.1097/ALN.0b013e31818aa6f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras S. G., Gale G. D., Fanselow M. S. (2001). Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus 11 8–17. [DOI] [PubMed] [Google Scholar]
- Blanchard R. J., Flannelly K. J., Blanchard D. C. (1986). Defensive behavior of laboratory and wild Rattus norvegicus. J. Comp. Psychol. 1983 101–107. 10.1037/0735-7036.100.2.101 [DOI] [PubMed] [Google Scholar]
- Blanchard R. J., Mast M., Blanchard D. C. (1975). Stimulus control of defensive reactions in the albino rat. J. Comp. Physiol. Psychol. 88 81–88. 10.1037/h0076213 [DOI] [PubMed] [Google Scholar]
- Bozon B., Davis S., Laroche S. (2002). Regulated transcription of the immediate-early gene Zif268: mechanisms and gene dosage-dependent function in synaptic plasticity and memory formation. Hippocampus 12 570–577. 10.1002/hipo.10100 [DOI] [PubMed] [Google Scholar]
- Bozon B., Kelly A., Josselyn S. A., Silva A. J., Davis S., Laroche S. (2003). MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358 805–814. 10.1098/rstb.2002.1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham C. R., Alme M. N., Bittins M., Kuipers S. D., Nair R. R., Pai B., et al. (2010). The Arc of synaptic memory. Exp. Brain Res. 200 125–140. 10.1007/s00221-009-1959-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham C. R., Worley P. F., Moore M. J., Guzowski J. F. (2008). The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J. Neurosci. 28 11760–11767. 10.1523/JNEUROSCI.3864-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell M. C., Duncan G. H., Hofbauer R. K., Ha B., Chen J. I., Carrier B. (1999). Pain perception: is there a role for primary somatosensory cortex? Proc. Natl. Acad. Sci. U.S.A. 96 7705–7709. 10.1073/pnas.96.14.7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna J. A., Miller K. W., Forman S. A. (2003). Mechanisms of actions of inhaled anesthetics. N. Engl. J. Med. 348 2110–2124. 10.1056/NEJMra021261 [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland E. P., Plummer T. K., Vazdarjanova A., Blake D. T. (2010). Arc expression and neuroplasticity in primary auditory cortex during initial learning are inversely related to neural activity. Proc. Natl. Acad. Sci. U.S.A. 107 14828–14832. 10.1073/pnas.1008604107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwang W. B., O’Riordan K. J., Levenson J. M., Sweatt J. D. (2006). ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn. Mem. 13 322–328. 10.1101/lm.152906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culley D. J., Yukhananov R. Y., Xie Z., Gali R. R., Tanzi R. E., Crosby G. (2006). Altered hippocampal gene expression 2 days after general anesthesia in rats. Eur. J. Pharmacol. 549 71–78. 10.1016/j.ejphar.2006.08.028 [DOI] [PubMed] [Google Scholar]
- Davis S., Bozon B., Laroche S. (2003). How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav. Brain Res. 142 17–30. 10.1016/S0166-4328(02)00421-7 [DOI] [PubMed] [Google Scholar]
- Dragunow M. (1996). A role for immediate-early transcription factors in learning and memory. Behav. Genet. 26 293–299. 10.1007/BF02359385 [DOI] [PubMed] [Google Scholar]
- Dutton R. C., Maurer A. J., Sonner J. M., Fanselow M. S., Laster M. J., Eger E. I. (2002). Isoflurane causes anterograde but not retrograde amnesia for pavlovian fear conditioning. Anesthesiology 96 1223–1229. 10.1097/00000542-200205000-00027 [DOI] [PubMed] [Google Scholar]
- Eger E. I., Koblin D. D., Harris R. A., Kendig J. J., Pohorille A., Halsey M. J., et al. (1997). Hypothesis: inhaled anesthetics produce immobility and amnesia by different mechanisms at different sites. Anesth. Analg. 84 915–918. 10.1213/00000539-199704000-00039 [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. (1994). Molecular and cellular mechanisms of general anaesthesia. Nature 367 607–614. 10.1038/367607a0 [DOI] [PubMed] [Google Scholar]
- Garcia P. S., Kolesky S. E., Jenkins A. (2010). General anesthetic actions on GABAA receptors. Curr. Neuropharmacol. 8 2–9. 10.2174/157015910790909502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski J. F., McNaughton B. L., Barnes C. A., Worley P. F. (1999). Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 2 1120–1124. 10.1038/16046 [DOI] [PubMed] [Google Scholar]
- Guzowski J. F., McNaughton B. L., Barnes C. A., Worley P. F. (2001). Imaging neural activity with temporal and cellular resolution using FISH. Curr. Opin. Neurobiol. 11 579–584. 10.1016/S0959-4388(00)00252-X [DOI] [PubMed] [Google Scholar]
- Guzowski J. F., Miyashita T., Chawla M. K., Sanderson J., Maes L. I., Houston F. P., et al. (2006). Recent behavioral history modifies coupling between cell activity and Arc gene transcription in hippocampal CA1 neurons. Proc. Natl. Acad. Sci. U.S.A. 103 1077–1082. 10.1073/pnas.0505519103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J., Thomas K. L., Everitt B. J. (2001). Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J. Neurosci. 21 2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaya Y., Takeda T., Dohi S., Nakashima S., Nozawa Y. (2000). The effects of pentobarbital, isoflurane, and propofol on immediate-early gene expression in the vital organs of the rat. Anesth. Analg. 90 1177–1183. 10.1097/00000539-200005000-00034 [DOI] [PubMed] [Google Scholar]
- Izquierdo I., Medina J. H., Vianna M. R., Izquierdo L. A., Barros D. M. (1999). Separate mechanisms for short- and long-term memory. Behav. Brain Res. 103 1–11. 10.1016/S0166-4328(99)00036-4 [DOI] [PubMed] [Google Scholar]
- Kalenka A., Gross B., Maurer M. H., Thierse H.-J., Feldmann R. E. (2010). Isoflurane anesthesia elicits protein pattern changes in rat hippocampus. J. Neurosurg. Anesthesiol. 22 144–154. 10.1097/ANA.0b013e3181cb7cb8 [DOI] [PubMed] [Google Scholar]
- Kent C. D., Mashour G. A., Metzger N. A., Posner K. L., Domino K. B. (2013). Psychological impact of unexpected explicit recall of events occurring during surgery performed under sedation, regional anaesthesia, and general anaesthesia: data from the Anesthesia Awareness Registry. Br. J. Anaesth. 110 381–387. 10.1093/bja/aes386 [DOI] [PubMed] [Google Scholar]
- Kim J. J., Jung M. W. (2006). Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci. Biobehav. Rev. 30 188–202. 10.1016/j.neubiorev.2005.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J. M., O’Riordan K. J., Brown K. D., Trinh M. A., Molfese D. L., Sweatt J. D. (2004). Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 279 40545–40559. 10.1074/jbc.M402229200 [DOI] [PubMed] [Google Scholar]
- Long C. W. (1849). An account of the first use of sulphuric ether by inhalation as an anesthetic. South. Med. Surg. J. 5 705–713. [Google Scholar]
- Lyford G. L., Yamagata K., Kaufmann W. E., Barnes C. A., Sanders L. K., Copeland N. G., et al. (1995). Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 14 433–445. 10.1016/0896-6273(95)90299-6 [DOI] [PubMed] [Google Scholar]
- Maren S., Holt W. (2000). The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav. Brain Res. 110 97–108. 10.1016/S0166-4328(99)00188-6 [DOI] [PubMed] [Google Scholar]
- Mashour G. A., Avidan M. S. (2015). Intraoperative awareness: controversies and non-controversies. Br. J. Anaesth. 115 (Suppl 1) i20–i26. 10.1093/bja/aev034 [DOI] [PubMed] [Google Scholar]
- Nakayama D., Iwata H., Teshirogi C., Ikegaya Y., Matsuki N., Nomura H. (2015). Long-delayed expression of the immediate early gene Arc/Arg3.1 refines neuronal circuits to perpetuate fear memory. J. Neurosci. 35 819–830. 10.1523/JNEUROSCI.2525-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J. Z., Xi J., Eckenhoff M. F., Eckenhoff R. G. (2008). Inhaled anesthetics elicit region-specific changes in protein expression in mammalian brain. Proteomics 8 2983–2992. 10.1002/pmic.200800057 [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. (2007). The Rat Brain in Stereotaxic Coordinates 6th Edn. Amsterdam: Elsevier. [Google Scholar]
- Plath N., Ohana O., Dammermann B., Errington M. L., Schmitz D., Gross C., et al. (2006). Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron 52 437–444. 10.1016/j.neuron.2006.08.024 [DOI] [PubMed] [Google Scholar]
- Quirk G. J., Armony J. L., LeDoux J. E. (1997). Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron 19 613–624. 10.1016/S0896-6273(00)80375-X [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V., Vazdarjanova A., Mikhael D., Rosi S., Worley P. F., Barnes C. A. (2005). Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J. Neurosci. 25 1761–1768. 10.1523/JNEUROSCI.4342-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampil I. J., Moller D. H., Bell A. H. (2006). Isoflurane modulates genomic expression in rat amygdala. Anesth. Analg. 102 1431–1438. 10.1213/01.ane.0000202384.96269.51 [DOI] [PubMed] [Google Scholar]
- Romanski L. M., LeDoux J. E. (1992). Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala circuits in auditory fear conditioning. J. Neurosci. 12 4501–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule E., Reddy S. (2014). Awareness under general anaesthesia. Br. J. Hosp. Med. (Lond.) 2005 573–577. 10.12968/hmed.2014.75.10.573 [DOI] [PubMed] [Google Scholar]
- Steward O., Wallace C. S., Lyford G. L., Worley P. F. (1998). Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron 21 741–751. 10.1016/S0896-6273(00)80591-7 [DOI] [PubMed] [Google Scholar]
- Tischmeyer W., Grimm R. (1999). Activation of immediate early genes and memory formation. Cell. Mol. Life Sci. 55 564–574. 10.1007/s000180050315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahle-Hinz C., Detsch O., Siemers M., Kochs E. (2007). Contributions of GABAergic and glutamatergic mechanisms to isoflurane-induced suppression of thalamic somatosensory information transfer. Exp. Brain Res. 176 159–172. 10.1007/s00221-006-0604-6 [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A., Houston F. P., Worley P. F., Barnes C. A., Guzowski J. F. (2002a). Similar activity-dependent immediate-early gene transcriptional responses in hippocampal pyramidal neurons and dentate gyrus granule cells. Abstr. Soc. Neurosci. 28 678.672. [Google Scholar]
- Vazdarjanova A., McNaughton B. L., Barnes C. A., Worley P. F., Guzowski J. F. (2002b). Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J. Neurosci. 22 10067–10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A., Ramirez-Amaya V., Insel N., Plummer T. K., Rosi S., Chowdhury S., et al. (2006). Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J. Comp. Neurol. 498 317–329. 10.1002/cne.21003 [DOI] [PubMed] [Google Scholar]
- Waters C. M., Hancock D. C., Evan G. I. (1990). Identification and characterisation of the egr-1 gene product as an inducible, short-lived, nuclear phosphoprotein. Oncogene 5 669–674. [PubMed] [Google Scholar]
- West M. J. (1999). Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 22 51–61. 10.1016/S0166-2236(98)01362-9 [DOI] [PubMed] [Google Scholar]
- Ying S.-W., Werner D. F., Homanics G. E., Harrison N. L., Goldstein P. A. (2009). Isoflurane modulates excitability in the mouse thalamus via GABA-dependent and GABA-independent mechanisms. Neuropharmacology 56 438–447. 10.1016/j.neuropharm.2008.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong T., Qing Q. J., Yang Y., Zou W. Y., Ye Z., Yan J. Q., et al. (2014). Repression of contexual fear memory induced by isoflurane is accompanied by reduction in histone acetylation and rescued by sodium butyrate. Br. J. Anaesth. 113 634–643. 10.1093/bja/aeu184 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


