Abstract
Toll-like receptors (TLRs) are components of the innate immune system that respond to exogenous infectious ligands (pathogen-associated molecular patterns, PAMPs) and endogenous molecules that are released during host tissue injury/death (damage-associated molecular patterns, DAMPs). Interaction of TLRs with their ligands leads to activation of downstream signaling pathways that induce an immune response by producing inflammatory cytokines, type I interferons (IFN), and other inflammatory mediators. TLR activation affects vascular function and remodeling, and these molecular events prime antigen-specific adaptive immune responses. Despite the presence of TLRs in vascular cells, the exact mechanisms whereby TLR signaling affects the function of vascular tissues are largely unknown. Cardiovascular diseases are considered chronic inflammatory conditions, and accumulating data show that TLRs and the innate immune system play a determinant role in the initiation and development of cardiovascular diseases. This evidence unfolds a possibility that targeting TLRs and the innate immune system may be a novel therapeutic goal for these conditions. TLR inhibitors and agonists are already in clinical trials for inflammatory conditions such as asthma, cancer, and autoimmune diseases, but their study in the context of cardiovascular diseases is in its infancy. In this article, we review the current knowledge of TLR signaling in the cardiovascular system with an emphasis on atherosclerosis, hypertension, and cerebrovascular injury. Furthermore, we address the therapeutic potential of TLR as pharmacological targets in cardiovascular disease and consider intriguing research questions for future study.
I. Introduction
Pattern recognition receptors (PRRs) are important components of the innate immune system responsible for recognizing and responding to danger and damage. PRRs are numerous and are expressed on a wide range of immune and nonimmune cells, including tissues of the cardiovascular system (Mann, 2011). PRRs have the ability to recognize unique evolutionarily conserved motifs. As a result, distinct molecular patterns that range from pathogen-associated molecular patterns (PAMPs) to damage-associated molecular patterns (DAMPs) can activate PRRs, with unique and exclusive proinflammatory cascades (Kono and Rock, 2008). The unique signaling cascades for distinct PRRs allow for the induction of specific responses. This specificity may be attributed to the type of cell or cellular compartment where the PRR is expressed (Dauphinee et al., 2011) and/or the defense needed for that particular tissue (Matzinger and Kamala, 2011). The ability of PRRs to discriminate distinct molecular patterns and induce exclusive signaling cascades expands the defensive repertoire of the innate immune system. Characterization and knowledge of the PRRs that recognize and respond to DAMPs and PAMPs is growing exponentially. Toll-like receptors (TLRs), receptors for advanced glycation end products, and nucleotide-binding oligomerization domain-like receptors (NLRs) are all examples of PRRs of the innate immune system. Particularly, TLRs have provided important new insights with respect to our understanding of the role of inflammation in health and disease (Beutler, 2004).
The Toll receptor was first discovered in Drosophila melanogaster when researchers found that a mutation in the Toll gene resulted in abnormal development (Anderson et al., 1985). The embryos carrying the mutation were termed Toll, German for “wow.” A more closely related human homolog to Drosophila Toll was subsequently identified (Medzhitov et al., 1997), and the human Toll was then renamed TLR4 because it was “Toll-like.” Toll-like receptors are responsible for recognizing and initiating an inflammatory response to microbial components expressed by bacteria, fungi, protozoa, and viruses as well as endogenous molecules that are released by dying cells or are generated as a result of tissue injury and oxidation (Rifkin et al., 2005; Jin and Lee, 2008). The low complexity of TLR signaling, including four adapter molecules and three downstream inflammatory transcription factors (Beutler, 2004), presents an efficient means at upregulating proinflammatory genes. The inflammatory genes expressed as a result of TLR activation include cytokines, whose expression pattern guides the adaptive immune response (e.g., cell-mediated Th1 response or the humoral/antibody Th2 response), chemokines (chemotactic cytokines) that guide the migration of immune cells to target tissues, and cell adhesion molecules that promote the binding, rolling, and infiltration of immune cells into the vascular wall and translocation to end organs (Lundberg et al., 2013).
Mounting evidence demonstrates that TLRs and the innate immune system play a determinant role in the development of cardiovascular diseases, which are now recognized as chronic inflammatory conditions. Furthermore, recent studies show that in addition to pathogens, TLRs respond to circulating host-derived molecules (DAMPs) released from dying and damaged cells after hypoxia, trauma, and cell death. It has been proposed that prolonged or excessive activation of TLRs on immune and vascular cells induces chronic low-grade inflammation, leading to endothelial dysfunction and subsequent cardiovascular disease. To highlight these new findings, we review the current knowledge of TLR signaling in the cardiovascular system with an emphasis on atherosclerosis, hypertension, and cerebrovascular injury. Furthermore, we address the therapeutic potential of TLR as pharmacological targets in cardiovascular disease and consider intriguing research directions for future study.
II. Overview of the Toll-like Receptor Family
A. Structure and Function
TLRs are expressed on specialized immune cells (e.g., macrophages and dendritic cells) as well as on nonimmune cells (e.g., epithelial, fibroblast, and endothelial cells). TLRs are type I transmembrane domain glycoproteins comprised of 1) an amino (N)-terminal ectodomain domain that contains leucine rich repeats and mediates ligand recognition, 2) a single transmembrane spanning domain that determines cellular localization, and 3) a carboxyl (C)- terminal globular cytoplasmic Toll/interleukin-1 (IL-1) receptor (TIR) domain that mediates downstream signaling (Takeda and Akira, 2005).
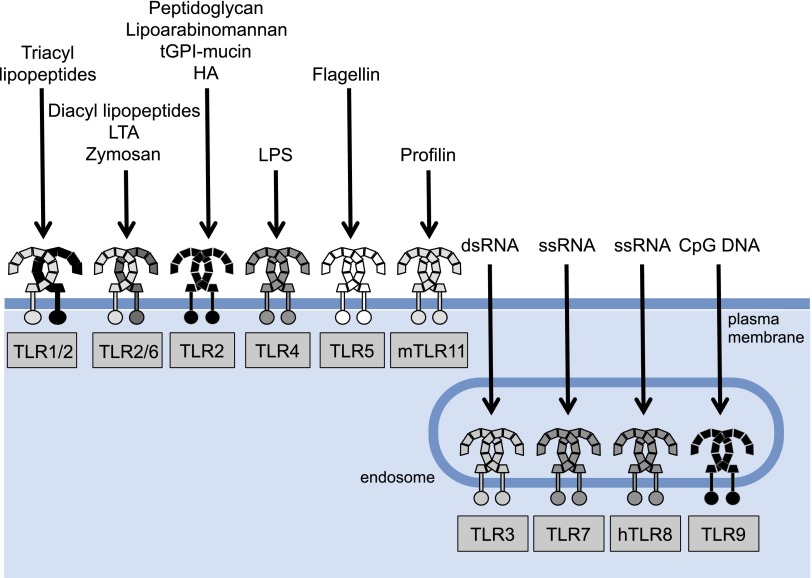
TLRs recognize viral and bacterial products (i.e., PAMPs) as well as fungi and host-derived endogenous molecules (i.e., DAMPs). This capacity of TLRs gives them both a protective role against microbial infections and a homeostatic role in response to endogenous materials. Thus far it has been shown that there are 10 TLR genes in humans (TLR1–TLR10) and 12 (TLR1–TLR9, TLR11–TLR13) in mice (Table 1) (Roach et al., 2005; Kawai and Akira, 2009). Broadly, TLRs may be divided into two groups based on their cellular localization when sensing their respective ligands. TLRs 1, 2, 4–6, and 11 localize to the cell surface (cell surface TLRs), and TLRs 3, 7–9, and 13 reside at endosomal compartments (intracellular TLRs) (O'Neill et al., 2013; Kawasaki and Kawai, 2014). Cell surface TLRs respond to microbial membrane materials such as lipids, lipoproteins, and proteins, and intracellular TLRs recognize bacteria- and virus-derived nucleic acids (Fig. 1) (Kawasaki and Kawai, 2014).
TABLE 1.
Mammalian Toll-like receptor families
Mammalian TLRs are grouped into six major families based on amino acid similarity, genomic structure and ligand properties. TLR10 is a pseudogene.
| TLR Family | |
|---|---|
| 1 | TLR1, TLR2, TLR6, TLR10 |
| 3 | TLR3 |
| 4 | TLR4 |
| 5 | TLR5 |
| 7 | TLR7, TLR8, TLR9 |
| 11 | amTLR11, mTLR12, mTLR13 |
Letter “m” preceding some TLRs indicate “mouse.” The remaining TLRs are shared by all mammals.
Fig. 1.
Cell surface and intracellular Toll-like receptors (TLRs) and their ligands. TLRs are divided into two groups based on their cellular localization when sensing their respective ligands. TLRs 1, 2, 4–6, and 11 localize to the cell surface (cell surface TLRs) and TLRs 3 and 7–9 reside at endosomal compartments (intracellular TLRs). Cell surface TLRs respond to microbial membrane materials such as lipids, lipoproteins, and proteins, whereas intracellular TLRs recognize bacteria- and virus-derived nucleic acids.
TLR4 is the receptor for the Gram-negative bacterial product lipopolysaccharide (LPS) (Poltorak et al., 1998; Hoshino et al., 1999; Qureshi et al., 1999). TLR2 responds to bacterial lipopeptides (Takeuchi et al., 2001; Jin et al., 2007; Kang et al., 2009) but can also sense nonlipopeptidic PAMPs (Kawai and Akira, 2009). TLR5 primarily senses flagelin, a structural component of flagellated bacteria (Akira et al., 2006). Human TLR10 recognizes ligands from listeria in collaboration with TLR2 (Regan et al., 2013), whereas mouse TLR10 contains a stop codon and, thus, it is a pseudogene (Kawasaki and Kawai, 2014). TLR10 also responds to influenza A virus infection (Lee et al., 2014). Mouse TLR11 detects flagelin (Madhur et al., 2010), a component of uropathogenic bacteria (Zhang et al., 2004), and binds to the Toxoplasma gondii profiling protein (Yarovinsky et al., 2005). The latter action occurs in cooperation with TLR12 (Andrade et al., 2013). Mouse TLR13 detects bacterial ribosomal RNA (Oldenburg et al., 2012) and components of vesicular stomatitis virus (Shi et al., 2011). TLR3 recognizes double-stranded RNA, which is component of many viruses (Alexopoulou et al., 2001) but also senses small interfering RNAs and self-RNAs derived from damaged cells (Zhang et al., 2007; Bernard et al., 2012). TLR9 specifically responds to cytosine—phosphate—guanine (CpG)-rich hypomethylated DNA motifs (Hemmi et al., 2000), which are primarily found in bacterial, mitochondrial, and fetal DNA, but rarely in vertebrate adult DNA (Stacey et al., 2003). More recent findings show that TLR9 also responds to herpes virus DNA (Lund et al., 2003) as well as to hemozoin, a byproduct generated by Plasmodium falciparum (Coban et al., 2010). TLR7 and TLR8 were shown to detect single-stranded viral RNA (Heil et al., 2004). TLR7 also detects RNA from streptococcus B bacteria (Mancuso et al., 2009). Collectively, TLRs provide rigorous surveillance of both intracellular and extracellular compartments, sensing most viral and bacterial molecular signatures (Fig. 2). In the last decade, however, there is supporting evidence to suggest that TLRs are components of a defense mechanism against host-derived molecules released from dying and damaged cells (Fig. 2).
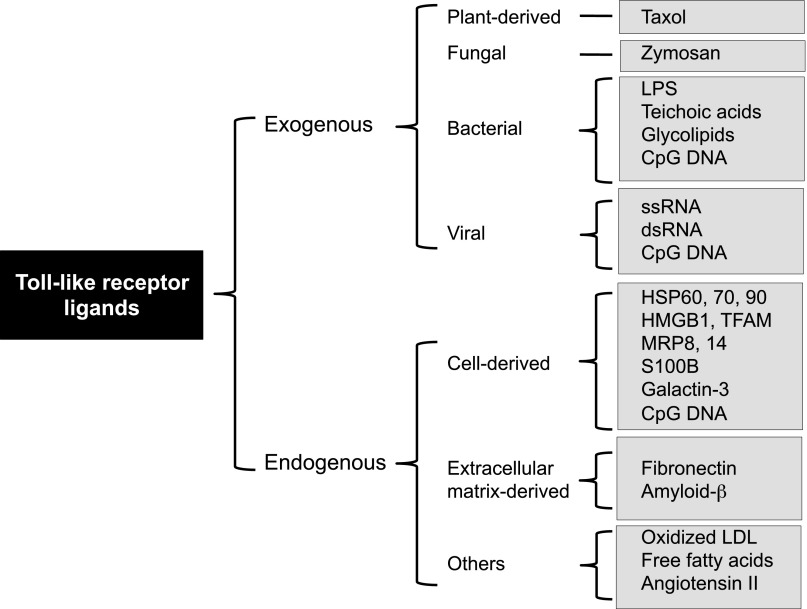
Fig. 2.
Exogenous and endogenous Toll-like receptor ligands. Categories of pathogen-associated molecular patterns (exogenous ligands) and damage-associated molecular patterns (endogenous ligands). HMBG1, high-mobility group box 1; HSP, heat shock protein; LDL, low-density lipoprotein; LPS, lipopolysaccharide; MRP, myeloid related protein; TFAM, mitochondrial transcription factor A
B. Assembly and Accessory Proteins
Most TLRs are homodimeric, but some form heterodimers. Before ligand binding, TLRs occur as dimers in a low-affinity complex [inactivated conformation (Latz et al., 2007; Tanji et al., 2013)]. Ligand binding causes conformational changes that promote engagement of the two TIR domains of the cytosolic site of each receptor. As the TIR domains come in close proximity, a new signaling platform is created (O'Neill and Bowie, 2007). The formation of this signaling platform is required for the recruitment of cytosolic TIR domain-containing adapters, a key step in TLR signaling (Fig. 3). TLR4 homodimerizes (Poltorak et al., 1998; Hoshino et al., 1999; Qureshi et al., 1999), whereas TLR2 forms either a homodimer or a heterodimer with TLR1 and TLR6 (Takeuchi et al., 2001; Jin et al., 2007; Kang et al., 2009). TLR10 has similar sequence to TLR1 and has been shown to form heterodimers with TLR1 and TLR2 (O'Neill et al., 2013). TLR12 functions as a homodimer or as a heterodimer with TLR11 (O'Neill et al., 2013).
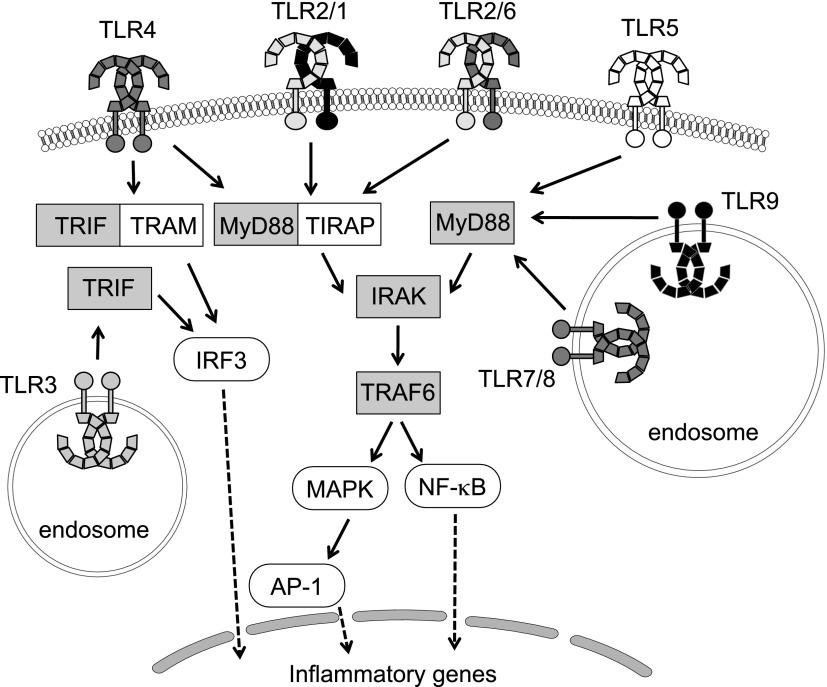
Fig. 3.
Toll-like receptors, adapter proteins, and signaling molecules. With the exception of TLR3, all TLRs recruit the adapter protein, myeloid differentiation primary response gene 88 (MyD88). In addition, TLRs 1, 2, 4, and 6 recruit the adapters cluster of differentiation 14 (CD14, not shown), which is required for lipopolysaccharide (LPS) binding, and Toll-Interleukin 1 receptor domain containing adaptor protein (TIRAP), which links the conserved C-terminal intracellular Toll/interleukin-1 receptor (TIR) domain with MyD88. In the MyD88-dependent pathway, the MyD88 recruits interleukin 1 receptor-associated kinase (IRAK), which interacts with the adapter protein tumor necrosis factor-receptor-associated factor 6 (TRAF6) and provides a link to nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) translocation. The MyD88-dependent pathway also facilitates expression of mitogen-activated protein kinase (MAPK) and transcription factors, such as interferon regulatory factors (IRFs), driving the production of proinflammatory mediators, including cytokines and chemokines. Activation of TLR3 initiates the TIR-domain-containing adapter-inducing interferon-β (TRIF)-dependent pathway, whereas TLR4 can signal via either MyD88-dependent or TRIF-dependent pathways requiring the additional linker adaptor TRIF-related adaptor molecule (TRAM) to associate with TRIF. In the TRIF-dependent pathway, TRIF interacts with TRAF3 to activate IRF3 initiating IFN-β production, which is the hallmark of the host innate response to viral infection.
Several transmembrane proteins play the role of coreceptors in TLR signaling. The ability of TLRs to cooperate with accessory proteins increases the range of ligands that TLRs can recognize. Cluster of differentiation (CD) 14 is a coreceptor of TLR4 and MD2 (lymphocyte antigen 96) and plays a role in LPS recognition (Zanoni et al., 2011). Moreover, CD14 is an accessory protein in TLR7 and TLR9 signaling (Baumann et al., 2010). CD36, a class B scavenger receptor, has a role in amyloid-β and oxidized low-density lipoprotein (LDL) recognition. Recognition of these ligands triggers the formation of a trimeric complex composed of CD36 and TLR4/TLR6 heterodimer (Stewart et al., 2010).
C. Adapter Molecules and Kinases
The recruitment of adapter proteins represents the early phase of TLR signal transduction and, consequently, the first step in innate immune system activation. To date, five adapters have been described in relation to TLR signaling: myeloid differentiation primary response gene 88 (MyD88), MyD88-adapter-like [MAL or TIRAP (Toll-Interleukin 1 receptor domain containing adapter protein)], TIR domain-containing adapter protein inducing interferon (IFN) β (TRIF or TICAM1), and sterile α- and armadillo-motif-containing protein (SARM). These proteins are involved in two main pathways, a MyD88-dependent and a TRIF-dependent pathway that lead to production of inflammatory cytokines or type I IFNs, respectively (Fig. 3).
1. Myeloid Differentiation Primary Response Gene 88-dependent Pathway.
MyD88-deficient mice revealed that MyD88 was necessary for signaling by various TLRs, such as TLR2, TLR4, TLR5, TLR7/8, and TLR9 (Kawai et al., 1999; Takeuchi et al., 2000). Currently, it is known that MyD88 is involved in signaling by all TLRs (except TLR3). In addition to its TIR domain, MyD88 has a death domain in the N terminus that interacts with a family of four death domain-containing kinases, the IL-1R-associated kinases (IRAKs) (Wesche et al., 1997). IRAK4 is the most important receptor-proximal kinase (Flannery and Bowie, 2010) and is believed to be the first kinase to engage with MyD88 (Li et al., 2002; Burns et al., 2003). Supporting the vital importance of IRAK4, studies in IRAK4-deficient human and mouse cells showed a profound reduction in TLR responses (Suzuki et al., 2002; Medvedev et al., 2003). Interaction of MyD88 with IRAK4 promotes IRAK4 autophosphorylation and subsequent recruitment of IRAK1 and IRAK2. Concomitant deficiency of IRAK1 and IRAK2 results in similar phenotype with that presented by IRAK4-deficient cells and mice (Kawagoe et al., 2008), whereas single knockout mice (either IRAK1 or IRAK2 deficient) have only partial impairments in TLR signaling (Thomas et al., 1999; Kawagoe et al., 2008). This evidence suggests that there is redundancy between IRAK1 and IRAK2. Nevertheless, both kinases are of equal importance, because it has been shown that IRAK1 has a role in the initial activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), whereas IRAK2 plays an important role in sustained activation of TLRs (Kawagoe et al., 2008). Activation of IRAKs causes recruitment of the E3 ubiquitin ligase, tumor necrosis factor (TNF)-receptor-associated factor 6 (TRAF6) to the receptor complex (Qian et al., 2001). This event leads to activation of TRAF6 and its release into the cytosol, where it forms a complex with transforming-growth-factor-β-activated kinase 1 (TAK1), TAK1-binding protein 1, and TAK1-binding protein 2/3 (Qian et al., 2001). TAK1 activation within the TRAF6 complex leads to activation of two pathways: 1) the IκB kinase (IKK) complex-NF-κB pathway (IKK complex: NF-κB essential modulator, IKKα, and IKKβ), and 2) the mitogen activated protein kinase (MAPK) pathway. IKK activation promotes the phosphorylation and proteosomic degradation of IκB, allowing NF-κB to translocate into the nucleus to promote cytokine gene transcription (Napetschnig and Wu, 2013). Concurrently, TAK1 induces activation of the MAPK family, such as extracellular-signal-regulated kinase 1/2, p38, and Jun amino-terminal kinase, mediating activation of activator protein 1 and cyclic adenosine monophosphate response element-binding protein, which also play a role in cytokine gene expression (Takeuchi and Akira, 2010). Thus, the net result of MyD88-signaling pathway is production of cytokines. However, in specialized immune cells such as plasmacytoid dendritic cells, MyD88 signaling also results in expression of type I IFNs (Honda et al., 2004; Uematsu et al., 2005).
The recent discovery of the myddosome, an oligomeric signaling platform that contains components of MyD88 and IRAKs, has provided invaluable information about the mechanisms of receptor proximal events in MyD88-dependent TLR activation (Gay et al., 2011). It is thought that the myddosome assembly occurs in a sequential hierarchical manner such as that ligand binding triggers conformational changes in TLR-TIR domains that allow the recruitment of large helical oligomers containing the death domains of MyD88, which then recruit IRAK4 and lead to its interaction with IRAK1 or IRAK2 (Motshwene et al., 2009; Lin et al., 2010). Another TIR-containing adapter protein, which is known as MAL or TIRAP, aids into the stabilization of the MyD88 oligomerization at the receptor (Valkov et al., 2011). Initially, it was suggested that MAL might represent a MyD88-independent signaling, but it is now recognized that MAL is a bridging adapter that links MyD88 to TLRs and, in particular, to TLR4 and TLR2 (Horng et al., 2002; Yamamoto et al., 2002). MAL, however, also has MyD88-independent properties. It has been proposed that MAL may participate in the recruitment of TRAF6 to a signaling complex because it contains a TRAF6-binding domain (Mansell et al., 2004). In addition to recruiting MyD88 to cell surface TLRs (TLR2, TLR4), MAL is also involved in signaling through endosomal TLRs (TLR7, TLR8, TLR9) and is present in myddosomes induced by both endosomal and cell surface TLRs (Bonham et al., 2014).
2. Toll/Interleukin-1 Receptor Domain-Containing Adapter-Inducing Interferon-β-dependent Pathway.
TRIF is a TIR-containing adapter that is used by both TLR3 and TLR4 (Yamamoto et al., 2002, 2003a; Oshiumi et al., 2003). Recruitment of TRIF by these receptors allows interaction of TRAF6 and TRAF3 with the receptor complex (Kawasaki and Kawai, 2014). TRAF6 recruits the receptor-interacting serine/threonine-protein 1 (RIP-1) kinase, and this leads to activation of the TAK1 complex, which subsequently activates NF-κB and MAPKs, leading to induction of proinflammatory genes (Sato et al., 2003; Gohda et al., 2004). TRAF3, on the other hand, recruits TRAF Family Member-associated NF-κB Activator-binding kinase 1, inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase epsilon, and NF-κB essential modulator, leading to interferon regulatory factor (IRF)3 activation and translocation to the nucleus, to induce expression of type I IFN genes. In contrast to TLR3, TLR4 does not directly interact with TRIF, but instead it requires a bridging adapter protein, TRAM, to transduce its signal (Yamamoto et al., 2003b). TRAM is considered the most restricted of the adapters, because it appears to function only in the TLR4 pathway (O'Neill and Bowie, 2007). In addition to NF-κB and IRF3 activation, TRIF is involved in TLR3-mediated apoptosis (Kaiser and Offermann, 2005). It is thought that TRIF is the only adapter protein involved in TLR signaling that has the ability to mediate apoptosis. This pathway involves RIP-1, Fas-associated protein with death domain, and caspase-8 (Han et al., 2004; Ruckdeschel et al., 2004).
SARM is a TIR-containing adapter protein that in contrast to MyD88, TRIF, MAL, and TRAF, does not induce activation of NF-κB (Liberati et al., 2004). Its importance in TLR signaling is due to its inhibitory effects on TRIF (Carty et al., 2006). SARM expression blocks TRIF-dependent transcription factor activation and gene induction but has no effect on MyD88 signaling. The exact mechanisms by which SARM inhibits TRIF are not well understood but it has been postulated that the interaction between the two proteins prevents the recruitment of downstream effector proteins such as RIP-1, TRAF6, and TANK-binding kinase 11 (O'Neill and Bowie, 2007).
D. Trafficking
1. Trafficking of Nucleic Acid-sensing Toll-like Receptors.
All TLRs are synthesized in the endoplasmic reticulum and traffic to the Golgi before they are recruited to their respective cellular compartment, where they will recognize their ligand. TLR trafficking is a tightly regulated process of high importance, because it ensures that nucleic acid-sensing TLRs do not come in contact with self-nucleic acids. UNc-93 homolog B1 (UNC93B1) is a multipass transmembrane protein that plays a role in trafficking of nucleic acid-sensing TLRs from the endoplasmic reticulum to the endosomes (Kim et al., 2008). UNC93B1 binds with nucleic acid sensing TLRs in the endoplasmic reticulum, where it loads them into coat protein complex II vesicles and transports them into intracellular compartments (endolysosomes) passing first through the Golgi complex (Chow et al., 2015; Lee et al., 2013). Mutation in one of the transmembrane domains of UNC93B1 abolishes IFN expression induced by TLR3, TLR7, and TLR9 activation, suggesting that UNC93B1 is involved in the intracellular transport of these TLRs (Kim et al., 2008). UNC93B1 also regulates excessive TLR7 signaling by recruiting TLR9 to counteract TLR7 (Fukui et al., 2009). In the case of TLR9 trafficking, interaction with the adapter protein-2 complex is also required (Lee et al., 2013; Chow et al., 2015). Protein associated with Toll-like receptor 4 is another resident protein of the endoplasmic reticulum that does not discriminate between intracellular and cell surface TLRs and plays a role in the exit of TLR1, TLR2, TLR4, TLR7, and TLR9 from the endoplasmic reticulum and their trafficking to plasma membrane and endosomes (Takahashi et al., 2007). Glycoprotein 96, a member of the heat-shock protein 90 family, is involved in the folding of TLRs (Yang et al., 2007). During their transport, the nucleic acid-sensing TLRs are cleaved by various proteases, including cathepsins B, S, L, H, and K; asparagine endopeptidase; and furin-like proprotein convertases (Ewald et al., 2008, 2011; Garcia-Cattaneo et al., 2012). Nucleic acids are able to bind to uncleaved TLRs, but this interaction cannot trigger a downstream signal (Ewald et al., 2008). Thus, in addition to TLR trafficking, proteolytic regulation is an important step in activation of endosomal TLRs.
2. Endocytosis of Toll-like Receptor 4.
TLR4 activates MyD88-dependent and TRIF-dependent pathways, which leads to the production of proinflammatory cytokines and type I interferons, respectively. The TIRAP/MyD88-dependent pathway is induced from the plasma membrane; however, TLR4 translocation into the endosomal compartments is required for TRAM/TRIF signaling to occur. CD14, a PRR that chaperones LPS molecules to the TLR4/MD-2 signaling complex (da Silva Correia et al., 2001), controls the endocytosis pathway that is responsible for TLR4 translocation from the plasma membrane to the endosomes (Zanoni et al., 2011). In resting cells, TRAM is colocalized with TLR4 at the plasma membrane and in the Golgi apparatus (Rowe et al., 2006). After TLR4 activation, TRAM is delivered to the endosomes, and this translocation is controlled by a bipartite sorting signal (Kagan et al., 2008). Translocation of both TLR4 and TRAM to endosomes is a prerequisite for the production of TLR4-induced type I interferon responses (Kagan et al., 2008).
E. Toll-like Receptor Signaling Regulators
Various negative regulatory mechanisms are in place to provide tight regulation of TLR signaling and avoid an exaggerated immune response. Loss or deficiencies of these regulatory signals may be involved in the development of immune-mediated and inflammatory diseases. Soluble decoy receptors have been described for some TLRs. Six sTLR2 isoforms have been reported to be present naturally in human plasma (LeBouder et al., 2003). In vitro studies showed that recombinant sTLR4 inhibited LPS-induced activation of NF-κB and production of TNF-α in macrophages (Iwami et al., 2000). It is thought that sTLR4 reduces TLR4 function by interrupting the interaction of the receptor with its coreceptors MD2 and CD14 (Liew et al., 2005). sTLR2 abolished bacterial lipopeptide-induced production of IL-18 and TNF-α in monocytes (Iwaki et al., 2002). In addition to naturally occurring soluble decoy receptors, intracellular molecules also play a negative regulatory role in TLR signaling. MyD88s is a splice variant of MyD88 that lacks an interdomain that interacts with IRAK4 (O'Neill and Bowie, 2007). MyD88s inhibits the recruitment of IRAK4 to MyD88 and thus it prevents activation of NF-κB (Burns et al., 2003). Overexpression of MyD88s inhibits NF-κB activation but does not affect activator protein 1 or Jun amino-terminal kinase activation (Janssens et al., 2003). IRAKM is a member of the IRAK family that has restricted expression (Wesche et al., 1999; Kobayashi et al., 2002). IRAKM does not interfere with the recruitment of IRAK1 to MyD88 (Kobayashi et al., 2002). It may inhibit, however, the disassociation of IRAKS and TRAF6 from the TLR complex (Kobayashi et al., 2002). Suppressor of cytokine signaling 1 is a nonredundant negative regulator of TLR signaling (Nakagawa et al., 2002). It primarily affects TLR4 and TLR9, whereas inhibitory effects upon other TLRs have not been reported (Kinjyo et al., 2002; Nakagawa et al., 2002). Some have suggested that the effect of suppressor of cytokine signaling 1 on TLR signaling is indirect through inhibition of type I IFN signaling, but additional studies are needed to confirm these reports (Baetz et al., 2004). Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), a member of the class IA family, is an effective negative regulator of TLR signaling. Although the exact mechanism by which PI3K inhibits TLRs is unclear, it has been reported that PI3K triggers inhibition of IL-12 synthesis and prevents the overexpression of a Th1 response (Fukao et al., 2002). Toll-interacting protein, IRAK2c, and IRAK2d have been found to selectively inhibit IRAK function (Burns et al., 2000; Zhang and Ghosh, 2002), whereas A20, a cysteine deubiquitylating enzyme, suppresses TLR signaling by deubiquitylating TRAF6, a common signal component of all TLRs (Boone et al., 2004). In addition to intracellular molecules, transmembrane proteins play a role in TLR regulation. ST2L (suppressor of tumorigenicity 2) has a negative regulatory role in TLR2, TLR4, and TLR9 signaling. Suppressor of tumorigenicity 2 interacts with MyD88 and MAL and sequestrates MyD88-induced NF-κB activation (Brint et al., 2004). Single immunoglobulin interleukin-1-related receptor interacts with TLR4 and IRAK, inhibiting transduction of their signal (Wald et al., 2003). Both ST2L and single immunoglobulin interleukin-1-related receptor are negative regulators of MyD88-dependent signaling but have no effect on MyD88-independent pathway. On the other hand, tumor-necrosis factor-related apoptosis-inducing ligand receptor, a member of the TNF family, affects both MyD88-dependent and -independent pathways (Diehl et al., 2004). Reduction of TLR expression is another mechanism that can negatively regulate TLR signaling. This may be accomplished by degradation of TLRs or inhibition of TLR expression via the action of anti-inflammatory cytokines (Liew et al., 2005).
III. The Role of Toll-like Receptor Signaling in Cardiovascular Dysfunction
Until recently, there has been limited knowledge of the role of the innate immune system in the pathogenesis of cardiovascular diseases (Mann, 2011). As a result, various components of the innate immune system, and in particular TLRs, are becoming a significant research focus in the field of cardiovascular pathophysiology, including atherosclerosis, hypertension, and stroke (McCarthy et al., 2014; Singh et al., 2014).
A. Atherosclerosis
At one time, atherosclerosis was thought to be a simple lipid storage disease. Now however, atherosclerosis is recognized as a chronic and progressive inflammatory condition, and this inflammation occurs hand-in-hand with incipient lipid accumulation in the arterial wall (Libby et al., 2002). As a result, TLRs have been implicated as significant contributors to the pathogenesis of atherosclerosis (Vink et al., 2004; Cole et al., 2013). Further implicating the contribution of TLRs to atherosclerosis pathogenesis is the knowledge that oxidized lipoproteins are DAMPs that can activate TLR signaling (Miller, 2005). TLRs have been conclusively linked with the initiation of atherosclerosis, including being expressed on local vascular cells and recruited immune cells (Cole et al., 2010). Moreover, TLRs contribute to the progression of atherosclerosis, including being expressed in atherosclerotic lesions (Edfeldt et al., 2002) and contributing to matrix degradation (Monaco et al., 2009) and plaque destabilization (Ishibashi et al., 2013).
The most well studied TLRs in atherosclerosis are the plasma membrane bound TLR2 (Curtiss and Tobias, 2007) and TLR4 (Pasterkamp et al., 2004). The contribution of TLR2 to atherosclerosis has been observed with both the TLR2-TLR1 heterodimer (Mullick et al., 2005; Qi et al., 2009; Curtiss et al., 2012) and TLR2-TLR6 heterodimer (Curtiss et al., 2012) in the two prominent animal models of atherosclerosis, apolipoprotein E (ApoE)- and LDL receptor-deficient mice. Moreover, TLR2 activation causes macrophage lipid accumulation (Kazemi et al., 2005); induces a de-differentiated, migratory, and proliferative phenotype in vascular smooth muscle cells (de Graaf et al., 2006; Lee et al., 2012); and initiates an inflammatory reaction in endothelial cells (Triantafilou et al., 2007). Similarly, TLR4 deficiency improved indices of atherosclerosis in both ApoE- (Michelsen et al., 2004) and LDL receptor-deficient mice (Ding et al., 2012). Moreover, TLR4 has an important role in the proatherogenic response of macrophages to oxidized LDL and lipid accumulation (Kazemi et al., 2005; Miller, 2005), proinflammatory and proliferative phenotype in vascular smooth muscle cells (de Graaf et al., 2006; Yang et al., 2005b; Schultz et al., 2007), and endothelial dysfunction (Yang et al., 2005a; Liang et al., 2013). On the other hand, the contribution of TLR5 to atherosclerosis is relatively sparse. Although TLR5 is expressed in the vasculature (Pryshchep et al., 2008) and macrophages (Descamps et al., 2012), the contribution of TLR5 to atherogenesis is limited at this stage (Erridge et al., 2008; Lopez et al., 2012).
Of the endosomal TLRs, TLR3 has been observed to promote atherogenic inflammation and dysfunction in endothelial cells (Zimmer et al., 2011), vascular smooth muscle cells (Yang et al., 2006), hematopoietic immune cells (Lundberg et al., 2013), and macrophages (Kazemi et al., 2005). Moreover, TLR3 plays a critical role in mediating atherosclerotic plaque instability, in part by modulation of macrophage matrix metalloproteinases-2 and -9 activities (Ishibashi et al., 2013). The contribution of endosomal TLR7 is beginning to emerge as a mediator of vascular remodeling and foam cell accumulation (Karper et al., 2012). Finally, endosomal TLR9 has been observed to propagate inflammation in response to vascular injury (Erridge et al., 2008; Lopez et al., 2012; Hirata et al., 2013), foam cell accumulation, and lesion formation (Niessner et al., 2006; Kim et al., 2009a; Sorrentino et al., 2010; Karper et al., 2012) and in LDL receptor-deficient mice (Ding et al., 2013).
In contrast to the abundance of literature suggesting a deleterious contribution of TLRs to atherosclerosis, especially TLR2 and TLR4 (Pasterkamp et al., 2004; Curtiss and Tobias, 2007), some evidence suggests that TLR can ameliorate atherogenesis. Interestingly, this has predominantly been shown for endosomal TLRs. TLR3 has been observed to have a protective effect onvthe vascular wall after mechanical and hypercholesterolemia-induced arterial injury (Cole et al., 2011), and this protective effect may have stemmed from the ability of TLR3 to induce the expression of cytoprotective and anti-inflammatory glycoprotein clusterin/apolipoprotein J (Baiersdorfer et al., 2010). Moreover, TLR7 activation constrains inflammatory macrophage activation and cytokine production (Salagianni et al., 2012). Finally, genetic deletion of TLR9 exacerbated atherosclerosis in ApoE-deficient mice fed a high-fat diet and a TLR9 agonist reduced lesion severity, collectively indicating that TLR9 constrains the atherogenic process (Koulis et al., 2014).
Overall the data suggest that TLRs participate in atherosclerosis pathogenesis (Vink et al., 2004). Inappropriate or excessive inflammation in response to PAMPs and DAMPs initiate and progress the pathogenesis of atherosclerosis (Falck-Hansen et al., 2013). Proatherogenic mechanisms of TLR activation include mediating the dysfunction of vascular cells, recruiting macrophages and other immune cells to the site of vascular injury, stimulating the formation of foam cells, and contributing to plaque destabilization. On the other hand, a few investigations have observed an antiatherogenic effect of TLRs in atherosclerosis (Baiersdorfer et al., 2010; Cole et al., 2011; Salagianni et al., 2012; Koulis et al., 2014), which fits more in line with their evolutionarily conserved function (Mann, 2011). Amplifying these positive effects, and minimizing the negative effects, should be the goal of researchers in the TLR and atherosclerosis field moving forward.
B. Hypertension
The development and maintenance of hypertension is subject to substantial debate regarding the contribution of kidneys, autonomic nervous system, and vasculature (McCarthy et al., 2014). However, uncontrolled immune system activation and inflammation have been proposed as a unifying mechanism between these three organ systems. Thus, TLRs represent potential candidates that mediate this aberrant inflammation (McCarthy et al., 2014; Singh and Abboud, 2014).
TLR4 is the most well-defined TLR involved in the etiology of hypertension (McCarthy et al., 2014). This may stem from the known association between TLR4 and angiotensin II, which some have classified as a DAMP (Erridge, 2010; McCarthy et al., 2014). TLR4 significantly contributes to the etiology of vascular dysfunction and high blood pressure in various experimental models of hypertension, such as the spontaneously hypertensive rat (SHR) (Bomfim et al., 2012, 2015) and rats with angiotensin II-induced hypertension (De Batista et al., 2014; Hernanz et al., 2015). Similarly, TLR4 plays a role in paraventricular nucleus-mediated autonomic dysfunction in SHR (Dange et al., 2015) and in rats with angiotensin II-induced hypertension (Dange et al., 2014). Less is known about the contribution of TLR4 on hypertensive kidney dysfunction; however, renal denervation can significantly decrease myocardial TLR4 expression in SHR (Jiang et al., 2012), and angiotensin II upregulates TLR4 expression on mesangial cells (Wolf et al., 2006).
Given the abundance of literature supporting the role of TLR2 in atherogenesis (Curtiss and Tobias, 2007), it is somewhat surprising that to date there are only limited data supporting the contribution of TLR2 to hypertension (McCarthy et al., 2014). Nonetheless, TLR2 has been observed to mediate the dysfunction of several cell types that contribute to the development of hypertension. In endothelial cells, high-density lipoprotein from patients with chronic kidney disease activated TLR2 (independently of TLR1 and TLR6) and reduced nitric oxide bioavailability, leading to impaired endothelial repair, enhanced inflammation, and increased arterial pressure (Speer et al., 2013). In renal tubular epithelial cells, it was observed that TLR2 upregulated NACHT, leucine rich repeats, and PYD domains containing protein 3 inflammasome, as well as its substrate IL-1β (Kasimsetty et al., 2014), and TLR2-NF-κB signaling significantly contributed to renal ischemia-reperfusion injury (Khan et al., 2012). Similarly, TLR5 has not been directly linked with hypertension, but it has been linked with metabolic syndrome, in which hypertension is one defining characteristic. Specifically, TLR5 knockout mice developed metabolic syndrome, including elevated blood pressure compared with wild-type controls (Vijay-Kumar et al., 2010).
Currently, no investigations exist on arterial hypertension for the endosomal TLRs 3, 7, or 8. However, TLR3, TLR7, and TLR8 have all been associated with maternal hypertension in rodent models with preeclampsia-like symptoms (Tinsley et al., 2009; Chatterjee et al., 2011, 2012, 2013, 2015; Ishimoto et al., 2013; Kopriva et al., 2013). Moreover TLR7/8, as well as TLR9, from SHR splenocytes evoked the greatest proinflammatory responses to angiotensin II when primed with their respective exogenous ligands (Harwani et al., 2012).
Recently, TLR9 has come to the fore as contributor to hypertension and blood pressure regulation. In addition to TLR9 activation in SHR splenocytes (Harwani et al., 2012), TLR9 inhibition in SHR lowered blood pressure. Furthermore, TLR9 activation increased blood pressure and induced vascular dysfunction in male normotensive rats (McCarthy et al., 2015) and induced maternal hypertension in pregnant rats (Goulopoulou et al., 2012). Moreover, TLR9 has been observed to be a negative regulator of cardiac vagal tone and baroreflex function (Rodrigues et al., 2015).
Overall these investigations provide evidence in support of the participation of TLRs in the etiology of hypertension or hypertension-related disorders (i.e., preeclampsia). To our knowledge, no reports in the hypertension field have suggested that activation of TLRs have beneficial or preconditioning effect on blood pressure or hallmark organ dysfunctions (i.e., kidney, autonomic nervous system, or vasculature). Thus, the therapeutic value of TLRs in hypertension remains to be explored.
C. Stroke/Cerebrovascular Injury
Cerebral ischemia triggers acute inflammation, which can exacerbate brain damage caused by stroke (Shichita et al., 2014). The regulation of inflammation after stroke is multifaceted and comprises vascular effects, distinct cellular responses, cell death, and chemotaxis (Downes and Crack, 2010). There are many cell types that are affected including neurons, astrocytes, microglia, and endothelial cells, all responding to the resultant neuroinflammation in different ways (Downes and Crack, 2010). TLRs are expressed on these endogenous brain cells, as well as the infiltrating immune cells that access lesions due to break down of the blood-brain barrier. The roles of TLRs in stroke and cerebrovascular injury can be classified into two major categories: 1) TLR activation postischemia that mediates neuroinflammation and neurodegeneration and 2) TLRs stimulation before ischemia that is neuroprotective and preconditions the brain to tolerate hypoxia and nutrient deprivation (Fadakar et al., 2014).
TLR2-deficient mice are protected against cerebral ischemia-induced cell injury and death (Lehnardt et al., 2007; Tang et al., 2007; Ziegler et al., 2011) and leukocyte and microglial infiltration into the brain (Ziegler et al., 2011). Moreover, ischemia causes an increase in TLR2 expression in neurons (Tang et al., 2007) and lesion-associated microglia (Lehnardt et al., 2007). Of clinical relevance, the amount of brain damage and neurologic deficits caused by a stroke were significantly less in mice deficient in TLR2 compared with wild-type controls (Tang et al., 2007). An interesting temporal paradox has also been reported in TLR2-deficient mice. Although the acute ischemic lesions (24 to 72 hours) have been observed to be smaller in TLR2-deficient mice, the subsequent innate immune response was reported to be more pronounced at later time points (day 7) and resulted in an exacerbation of the ischemic lesion (Bohacek et al., 2012). Despite these data suggesting a deleterious contribution of TLR2 in stroke, others have observed that preconditioning with a TLR2 ligand protected the brain from ischemia-reperfusion injury (Hua et al., 2008, 2009), possibly through a TLR2/PI3K/protein kinase B-dependent mechanism (Lu et al., 2011).
Similarly, it has been observed that TLR4-deficient mice have reduced ischemic injury (Cao et al., 2007; Caso et al., 2007; Hua et al., 2007, 2009; Sansing et al., 2011) and a TLR4 antagonist reduces neuroinflammation and neurologic deficits after intracerebral hemorrhage (Wang et al., 2013). On the other hand, TLR4-deficient mice showed less preconditioning-induced neuroprotection than wild-type controls (Pradillo et al., 2009). This neuroprotective effect may be attributed to the ability of TLR4 to promote neurogenesis after stroke by promoting neuroblasts migration and increasing the number of new neurons (Moraga et al., 2014).
Similar to the atherosclerosis and hypertension literature, less is understood about TLR5 in stroke and cerebrovascular injury. Nonetheless, TLR5 has been implicated in neuroinflammation postischemia. The anti-inflammatory agent luteolin protected the brain from the ischemic damage through downregulation of TLR5 (and TLR4) (Qiao et al., 2012).
Of the endosomal TLRs, TLR3 induces a neuroprotective tolerance against subsequent ischemia via preconditioning (Shi et al., 2013; Pan et al., 2014; Zhang et al., 2015), although this has not always been the case (Hyakkoku et al., 2010). Expression of TLR7 and TLR8 is associated with poorer outcome and greater inflammatory responses in acute ischemic stroke patients (Brea et al., 2011). A TLR8 agonist increased neuronal cell death during oxygen-glucose deprivation, and in vivo administration increased mortality, neurologic deficit, and T cell infiltration after stroke (Tang et al., 2013). On the other hand, TLR7 preconditioning mediated neuroprotection against subsequent ischemic injury through type I IFN-mediated mechanism (Leung et al., 2012). Finally, TLR9 contributed to ischemic brain injury (Hyakkoku et al., 2010; Zhang et al., 2013) and TLR9 expression showed pronounced and dynamic changes predominantly in microglia (Ji et al., 2015). On the other hand, TLR9 activation induced neuroprotection against ischemic injury by increasing serum TNF-α (Stevens et al., 2008) and activating PI3K/protein kinase B-dependent signaling (Lu et al., 2014).
Overall these data suggest a dual function of TLRs in stroke and cerebrovascular injury. Although TLRs contribute significantly to neuroinflammation and cerebral injury immediately postischemic events, controlled modulation of their signaling may precondition individuals at risk for stroke and may improve clinical outcomes, such as lesion size and neurologic deficits. Nonetheless, future studies are required to investigate the optimal dose and time course of TLR-targeted therapies.
IV. Toll-like Receptor Ligands in Cardiovascular Disease
A. Infections, Pathogens, and Cardiovascular Disease
Epidemiologic studies have reported positive associations between the risk of cardiovascular disease morbidity and mortality and markers of infection. In a clinical study, 75% of patients with coronary artery disease had been exposed to at least three of five pathogens tested, and the increasing risk of coronary artery disease was associated with the increasing aggregate number of pathogens (Zhu et al., 2000). Human cytomegalovirus has been implicated in atherosclerosis, coronary heart disease, and hypertension (Speir et al., 1994; Li et al., 2011; Haarala et al., 2012). It has been proposed that bacterial or viral infection contribute to the pathogenesis of atherosclerosis either via direct or indirect mechanisms. Direct mechanisms involve infection of vascular cells, whereas indirect mechanisms involve cytokine- and acute phase protein-induced effects on nonvascular sites (Rosenfeld and Campbell, 2011). Clinical trials have failed to demonstrate a positive effect of antibiotics in the reduction of risk for cardiovascular disease, and this evidence led to dismissal of the potential role of pathogens in the pathogenesis of atherosclerosis (O'Connor et al., 2003; Cannon et al., 2005; Grayston et al., 2005; Jespersen et al., 2006). The Oral Infections and Vascular Disease Epidemiology Study (INVEST) investigated whether periodontal bacteria were associated with prevalent hypertension and elevated continuous blood pressure measurements (Desvarieux et al., 2010). A strong positive association was found between increased subgingival colonization by Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola and prevalent hypertension, and this association was stronger among men than women (Desvarieux et al., 2010). Chronic infections, such as periodontitis, chronic bronchitis, and the pathogens Helicobacter pylori, Chlamydia pneumoniae, or cytomegalovirus all increase risk of ischemic stroke (Grau et al., 2010). Similar to studies in atherosclerosis, it has been shown that the aggregate burden of chronic and past infections rather than any one single infectious disease is associated with the risk of stroke (Grau et al., 2010). Interestingly, influenza vaccination in the previous year was associated with approximately a 50% reduction in the odds of stroke (Lavallee et al., 2002; Grau et al., 2005). Antibiotic prophylaxis has not been found effective in preventing ischemic stroke (Grau et al., 2010). Although the use of antibiotics as preventative approach for post-stroke infection has been considered, recent studies showed that did not support the use of preventive antibiotics in adults with acute stroke (Westendorp et al., 2015). Taken together, these data suggest that although there is an association between infections and cardiovascular disease, randomized controlled clinical trials do not support the hypothesis that bacterial and viral infections are causal in the development of cardiovascular disease.
B. Damage-Associated Molecular Patterns Are Novel Mediators of Sterile Inflammation
Chronic inflammation as a result of uncontrolled immune system activation contributes to cardiovascular diseases (McCarthy et al., 2014), but as discussed previously, the association between these events cannot be fully attributed to bacterial and viral infections. Innate immune system recognition and response to DAMPs is becoming an increasingly accepted mechanism. DAMPs are endogenous molecules that are normally compartmentalized within cellular membranes and protected from exposure to components of the immune system. However, when a cell is stressed or has its plasma membrane ruptured, these endogenous molecules can be expressed on cell surfaces or freely diffuse into the extracellular space, respectively. The immune system senses these molecules as danger and elicits a response (Kono and Rock, 2008). Although short-term inflammation is necessary to facilitate clearance of the danger and mediate tissue repair, inappropriate and/or chronic activation of the immune system negates its evolutionarily conserved salutary effects (Matzinger, 2002).
Dangerous molecules that can activate TLRs are varied and numerous, including cell wall components (e.g., LPS), nucleic acids, lipids, and metabolic byproducts of PAMPs (Fig. 2). Likewise, potential DAMPs include lipids, nucleic acids, and proteins from cellular sources such as extracellular matrix and organelles. Table 2 provides several examples of DAMPs specific to the cardiovascular diseases discussed in this review (atherosclerosis, hypertension, and stroke/cerebrovascular injury) and their corresponding TLRs. This list is by no means exhaustive, because many other unknown molecules may fulfill the inclusion criteria of being DAMPs and TLR ligands (e.g., neoantigens).
TABLE 2.
Damage-associated molecular patterns known to be involved in the pathogenesis of atherosclerosis, hypertension, and stroke/cerebrovascular injury
| Disease | DAMP | TLR | Cell Type/Tissue | References |
|---|---|---|---|---|
| Atherosclerosis | Apolipoprotein CIII | 2 | Monocytes | Kawakami et al., 2008 |
| C-reactive protein | 4 | VSMC | Liu et al., 2010b,c | |
| Extracellular matrix proteins | ||||
| Biglycana | 2, 4 | Macrophages | Schaefer et al., 2005; Babelova et al., 2009; Neufeld et al., 2014; Thompson et al., 2014 | |
| Decorina | 2, 4 | Macrophages, aortic valve, renal arteries | Merline et al., 2011; Neufeld et al., 2014 | |
| Fibronectin-EDA | 4 | Endothelial cells, macrophages | Tan et al., 2004; Schoneveld et al., 2008 | |
| Fibrinogen | 4 | Macrophages, monocytes | Smiley et al., 2001; Kuhns et al., 2007 | |
| Heparan sulfate | 2,4 | Macrophages | Blich et al., 2013; Goodall et al., 2014 | |
| Hyaluronan | 2,4 | Endothelial cells, macrophages | Taylor et al., 2004; Scheibner et al., 2006 | |
| Tenascin C | 4 | Macrophages | Liu et al., 2012 | |
| Versicana | 2,4,6 | Macrophages | Kim et al., 2009b; Barascuk et al., 2013; Chang et al., 2014 | |
| HMGB1 | 2,4,9 | Neutrophils, macrophages | Park et al., 2004; Hirata et al., 2013 | |
| Heat shock proteins | ||||
| HSP60 | 2,4 | Monocytes, VSMCs | de Graaf et al., 2006; Schoneveld et al., 2008 | |
| HSP70 | 2,4 | Monocytes, macrophages | Asea et al., 2002; Vabulas et al., 2002 | |
| Neutrophil elastasea | 4 | Human embryonic kidney cells | Devaney et al., 2003; Henriksen and Sallenave, 2008; Sallenave and Shapiro, 2008 | |
| Oxidized LDL | 4 | Endothelial cells, macrophages | Miller, 2005; Su et al., 2011 | |
| S100 proteinsa | ||||
| S100A8 | 4 | Macrophages | McCormick et al., 2005; Schelbergen et al., 2012 | |
| S100A9 | 4 | Macrophages | McCormick et al., 2005; Schelbergen et al., 2012 | |
| S100b | 2 | Adipocytes | Monden et al., 2013 | |
| Uric acida | 2,4 | Macrophages | Gagliardi et al., 2009; Liu-Bryan et al., 2005 | |
| Hypertension | ADMAa | 4 | Adipocytes | Matsuoka et al., 1997; Yang et al., 2009 |
| “Abnormal” HDL | 2 | Endothelial cells | Speer et al., 2013 | |
| Angiotensin IIa | 4 | Arteries, PVN | Dange et al., 2014; De Batista et al., 2014; Hernanz et al., 2015 | |
| C-reactive protein | 4 | VSMC | Liu et al., 2010b,c | |
| Mitochondrial DNA | 9 | Arteries | McCarthy et al., 2014 | |
| Fibrinogen | 4 | Cardiomyocytes | Li et al., 2009 | |
| Fibronectin-EDAa | 4 | Aorta | Takasaki et al., 1992 | |
| HMGB1 | 2,4,9 | Endothelial cells, cardiomyocytes | Kim et al., 2012; Mersmann et al., 2013 | |
| Heat shock proteins | ||||
| HSP60 | 2, 4 | VSMCs | Pockley et al., 2000; de Graaf et al., 2006 | |
| HSP70 | 2 | Kidney, cardiomyocytes | Mathur et al., 2011; Pons et al., 2013 | |
| Hyaluronan | 2,4 | Endothelial cells | Taylor et al., 2004 | |
| Uric acida | 2,4 | Macrophages | Liu-Bryan et al., 2005; Feig, 2014 | |
| Stroke & Cerebrovascular injury | Carboxyalkylpyrrolesb | 2 | Endothelial cells | West et al., 2010 |
| CRIPb | 2,4 | Macrophages | Qiang et al., 2013 | |
| HMGB1 | 2,4 | Neurons, endothelial and, glial cells | Qiu et al., 2008, 2010 | |
| Mitochondrial DAMPsb | 9 | Endothelial cells | Sun et al., 2013; Walko et al., 2014 | |
| Peroxiredoxin | 2,4 | Macrophages | Shichita et al., 2012; Kuang et al., 2014 |
DAMP has no direct link with TLR in that cardiovascular disease. However, there is link between that DAMP and TLR(s) in other pathologies and that DAMP with the cardiovascular disease in question.
Not studied specifically for the cardiovascular disease in question; however, the particular DAMP does have significant implications for the clinical features of the disease.
HDL, high-density lipoprotein.
As alluded to above, the mitochondrion has emerged as an organelle that serves as a significant source of DAMPs (Krysko et al., 2011; Wenceslau et al., 2014). Mitochondria were once prokaryotic organisms that entered into the eukaryotic cells to become organelles essential for ATP synthesis (endosymbiosis) (Sagan, 1967). This ancestry of mitochondria means they still express evolutionarily conserved similarities to bacteria, including translation of their peptides beginning with an N-formyl methionine residue and their DNA being predominately unmethylated CpG dinucleotides (Krysko et al., 2011; Wenceslau et al., 2014). Therefore, when mitochondria are released into the circulation as DAMPs, these mitochondrial components are recognized by PRRs of the innate immune system PAMPs, subsequently driving a sterile inflammatory response. As a result, mitochondrial DAMPs are emerging as significant contributors to acute (Zhang et al., 2010; Wenceslau et al., 2015) and chronic (Oka et al., 2012; McCarthy et al., 2015) sterile disease. In addition to mitochondrial DNA and N-formylated peptides, other mitochondrial DAMPs include cardiolipin (Tuominen et al., 2006; Sherer et al., 2007), ATP (Ferrari et al., 1997; Gorini et al., 2013), mitochondrial transcription factor A (Chaung et al., 2012; Julian et al., 2012), and cytochrome c (Adachi et al., 2004).
C. Mechanisms of Damage-Associated Molecular Pattern Presentation in Cardiovascular Disease
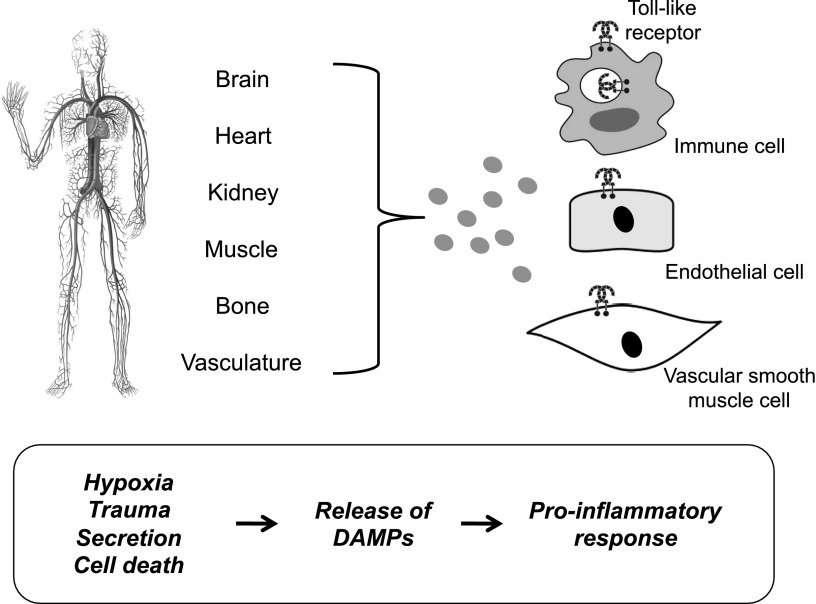
In most cases, the participation of DAMPs as inflammatory mediators in cardiovascular disease presumes that cell death is an instigating mechanism of their release. Figure 4 demonstrates the concept that circulating DAMPs released after hypoxia, trauma, and cell death lead to TLR activation in immune cells, endothelial cells, and vascular smooth muscle cells. Prolonged or excessive activation of TLRs on these cells provide a proinflammatory state leading to endothelial dysfunction and subsequent cardiovascular disease (McCarthy et al., 2014; Wenceslau et al., 2014). However, determining whether cell death primarily drives pathophysiology or is a secondary bystander is difficult. Although there are many forms of cell death, it has generally been thought that necrotic cell death was the primary source of proinflammatory DAMPs, because of disintegration of the plasma membrane and release of intracellular constituents (Miyake and Yamasaki, 2012). However, apoptosis can also be immunogenic as a result of the programmed release of immunostimulatory molecules (Krysko et al., 2011; Davidovich et al., 2014). Therefore, release of DAMPs can either be a passive secretion into the extracellular environment due to cell death or damaged extracellular matrix, an active release into the extracellular environment, or exposure on the surface of cells (e.g., neoantigens). The secretion and exposure mechanisms are results of cellular stress. This emphasizes that cell death is not an absolute precursor to participation of DAMPs in the pathophysiology of cardiovascular disease. We refer the reader to the following reviews for more information on the cell death in cardiovascular diseases (Zheng et al., 2011) and the emerging classifications of DAMPs.
Fig. 4.
Damage-associated molecular pattern (DAMP)-induced activation of Toll-like receptors (TLRs). Schematic demonstrating the concept that circulating damage-associated molecular patterns (DAMPs) released after hypoxia, trauma, and cell death lead to TLR activation in immune cells, endothelial cells, and vascular smooth muscle cells. Prolonged or excessive activation of TLRs on these cells provides a proinflammatory state, leading to endothelial dysfunction and subsequent cardiovascular disease.
V. The Therapeutic Potential of Toll-like Receptors in Cardiovascular Disease
TLRs and their associated signaling molecules have been exploited as possible targets for drug development. Both TLR agonists (inducers of protective immunity and antitumor treatment) and antagonists (suppressors of excessive inflammation) have been shown to have beneficial effects in various clinical conditions, such as cancer, microbial inflammation, autoimmunity, and allergies, by modulating the tissue inflammatory response. According to O'Neill et al. (2013), the validation criteria to determine if a TLR is a good therapeutic target for certain diseases are: 1) expression of the receptor in the disease state of study, 2) evidence showing that activation of the receptor leads to exacerbation of the disease phenotype in experimental models, 3) data demonstrating that mice deficient of a specific TLR are protected against the disease, and 4) evidence that certain TLR polymorphisms are associated with predisposition to the specific disease. The involvement of TLRs in cardiovascular diseases is a recent scientific breakthrough, and validation of the above criteria comprises exciting work in progress. As details of TLR biology and signaling pathways in cardiovascular disease are uncovered, a heightened interest in targeting of TLRs for the prevention and treatment of these diseases is emerging. In particular, TLR antagonism has been successfully employed in various experimental models of cardiovascular disease. However, these efforts are still at the experimental level.
A. Toll-like Receptor Signaling Inhibition
Cardiovascular diseases such as hypertension, stroke, and atherosclerosis are chronic inflammatory conditions. Because inflammation characterizes most TLR responses, TLR inhibition has been employed as the primary pharmacological approach in experimental models of cardiovascular disease. Pharmacological approaches of TLR signaling inhibition include TLR antagonists, neutralizing antibodies, small molecules, and agents that block protein-protein interaction. Table 3 summarizes the current applications of TLR inhibition at the experimental level.
TABLE 3.
Pharmacological inhibition of Toll-like receptor (TLR) signaling in experimental models of cardiovascular disease
| Compound | Description | Target/Mechanism | Cardiovascular Applications |
|---|---|---|---|
| Eritoran | Synthetic lipopolysaccharide–lipid A analog | TLR4 signaling/competitively binds a large pocket of MD-2 | Myocardial ischemia, heart failure, renal ischemia/reperfusion injury, cerebral infarction |
| T2.5 | Monoclonal antibody | TLR2/ abrogates TLR2 ectodomain binding to immunostimulatory lipopeptides | Cardiac fibrosis, cerebrovascular ischemia |
| OPN305 | Monoclonal antibody | TLR2 | Myocardial ischemia/reperfusion injury |
| Chloroquine, hydroxychloroquine, quinacrine | 4-aminoquinoline compound, antimalarial and amebicidal drugs | Endosomal TLRs/mask the TLR binding epitope of nucleic acids | Cerebrovascular ischemia, lupus-associated hypertension, endothelial dysfunction |
| Inhibitory oligonucleotides | Synthetic DNA sequences | TLR9/disrupt colocalization of CpG ODNs with TLR9 in endosomal vesicles | Hypertension |
| Valsartan, candesartan | Angiotensin II receptor blockers | TLR2, TLR4/unknown, angiotensin II receptor-independent | Myocardial ischemia/reperfusion injury |
| Fluvastatin, atorvastatin | 3-hydroxy-3-methylglutaryl-coenzyme (HMG-CoA) reductase inhibitors | TLR4/impair TLR4 recruitment to lipid raft, inhibits NF-κB activation | Atherosclerosis, heart failure |
| ST2825 | Small cell-permeable peptide (peptidomimetic) | MyD88/inhibits homodimerization of MyD88 | Acute myocardial infarction |
| TAK-242 | Small molecule/cyclohexene derivative | TLR4/binds to Cys747 in TLR4 TIR domain and affects the recruitment of adapters to the signaling complex | Acute kidney injury, intracerebral hemorrhage, cerebral ischemia/reperfusion injury |
| dnMyD88 | Mutated form of MyD88 | MyD88/inhibits homodimerization of MyD88 | Atherosclerosis, myocardial ischemia/reperfusion injury, cardiac hypertrophy |
Efforts to inhibit TLR4 recognition of LPS led to the development of lipid A analogs that advanced into clinical trials for sepsis (Tidswell et al., 2010; Opal et al., 2013). Eritoran (Eisai, Inc., Woodcliff Lake, NJ), an analog of the lipid A portion of bacterial-derived LPS, is a TLR4 antagonist that was developed for the treatment of severe sepsis. Eritoran does not directly inhibit TLR4, but instead, it competitively binds to a large internal pocket of MD-2 (Kim et al., 2007) and terminates TLR4/MD2-mediated signaling (Rossignol and Lynn, 2002; Mullarkey et al., 2003; Rossignol et al., 2004). In addition to sepsis, the effects of Eritoran have been investigated in some experimental models of cardiovascular diseases. Eritoran protected mice from myocardial ischemia and reduced infarct size (Shimamoto et al., 2006b), attenuated cardiac hypertrophy in a mouse model of aortic constriction (Ehrentraut et al., 2011), attenuated ischemia/reperfusion-related inflammation and improved the course of kidney ischemia/reperfusion injury (Liu et al., 2010a), and reduced inflammatory gene expression in a rat model of myocardial ischemia/reperfusion (Shimamoto et al., 2006a). The beneficial effects of Eritoran are due to its ability to inhibit TLR4-induced NF-κB activation. An advantageous role of Eritoran in cerebral infarction has also been suggested (Buchanan et al., 2010). Although this compound is too large to pass the blood-brain barrier, it is possible that after a stroke, which compromises the barrier, Eritoran and other large molecules can pass and provide their beneficial actions.
Neutralizing antibodies, such as the neutralizing antibody against the extracellular domain of TLR2, T2.5, have inhibitory effects on TLR-mediated proinflammatory signals (Meng et al., 2004). T2.5 was shown to antagonize TLR2-induced activation of mouse and human macrophages both in vitro and in vivo (Meng et al., 2004). In a mouse model of transient brain ischemia, treatment with T2.5 reduced inflammation and neural death (Ziegler et al., 2011). More recently, it was reported that inhibition of TLR2 with T2.5 protected against angiotensin II-induced cardiac fibrosis (Wang et al., 2014) by attenuating macrophage recruitment and the inflammatory response in the heart. The humanized anti-TLR2 monoclonal antibody OPN305 has shown promising results for the prevention of myocardial ischemia/reperfusion injury (Arslan et al., 2012). OPN305 is able to block both TLR2/1- and TLR2/6-mediated signaling, reducing TLR2-mediated proinflammatory cytokine production. In 2009, OPN305 was given orphan status for the prevention of ischemia/reperfusion injury associated with solid organ transplantation (Connolly and O'Neill, 2012). Preclinical studies have reported beneficial effects of OPN305 on other diseases, such as kidney ischemia/reperfusion injury (Farrar et al., 2012). In a subsequent phase I, prospective randomized, double-blind, placebo-controlled study, OPN305 fully blocked TLR2 receptor on monocytes and was well tolerated in various doses by healthy subjects (Reilly et al., 2013). Treatment with anti-TLR4 IgG antibody reduced mean arterial pressure, attenuated expression of IL-6 and production of reactive oxygen species, and reduced phosphorylation of p38MAPK and NF-κB in mesenteric resistance arteries of SHR (Bomfim et al., 2015). Similar responses were found in other experimental models of hypertension (Hernanz et al., 2015).
Chloroquine, hydroxychloroquine, and quinacrine inhibit activation of endosomal TLRs (TLR3, TLR7, TLR8, TLR9). These compounds have been primarily used as antimalaria drugs. It was thought that antimalaria drugs prevent the acidification of lysosomes, where intracellular TLRs reside. However, Kuznik et al. (2011) showed that chloroquine neither inhibited endosomal proteolysis nor increased the endosomal pH. Furthermore, antimalaria compounds (i.e., chloroquine) inhibited activation of endosomal TLRs by nucleic acids, but it increased activation of TLR8 by a small synthetic compound, R848. In a similar manner, other synthetic compounds such as imidazoquinoline and propidium iodide inhibited signaling of intracellular TLRs (Kuznik et al., 2011). A mechanism of action of antimalaria compounds and imidazoquinolines involves the ability of these compounds to mask the TLR binding epitope of nucleic acids (Kuznik et al., 2011).
Recent evidence supports the use of chloroquine in cardiovascular diseases because of its anti-inflammatory effects. Using a rat model of transient global cerebral ischemia, Cui et al. (2013) showed that cerebral ischemia reduced learning and memory capacity and increased expression of TLR3, interferon regulatory factor 3 (IRF3), and IFN-β in the hippocampus. Chloroquine pretreatment enhanced rats' short-term spatial memory capacity and attenuated the expression of TLR3, IFR3, and IFN-β in the hippocampus compared with nontreated rats. In a murine model of lupus, chronic hydroxychloroquine treatment did not alter lupus disease activity but reduced hypertension and aortic endothelial dysfunction (Gomez-Guzman et al., 2014). Furthermore, chloroquine and hydroxychloroquine had beneficial effects on experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation (Long et al., 2013).
Immunoregulatory DNA sequences [i.e., oligonucleotides (ODN)] have been used to neutralize the stimulatory effect of CpG ODNs. TTAGGG repeats that form G-tetrads and are present at high frequency in mammalian telomeres disrupt colocalization of CpG DNA with TLR9 in endosomal vesicles without affecting cellular binding and uptake (Gursel et al., 2003). This disruption suppresses the inflammatory signaling that is initiated by the interaction between CpG DNA and TLR9 (Gursel et al., 2003). ODN 2088 is another potent inhibitory antagonist of TLR9 that derives from a stimulatory ODN by replacement of three bases (Stunz et al., 2002). We previously showed that in vivo treatment with ODN 2088 reduced systolic blood pressure in spontaneously hypertensive rats, a genetic model of hypertension (McCarthy et al., 2015). These oligonucleotides have been reported to be potent in both human and mice (Duramad et al., 2005).
The effects of currently available anti-inflammatory cardiovascular drugs on TLR signaling were recently studied. Angiotensin II receptor blockers are commonly used as antihypertensive medication. In addition to its hypertensive and constrictor effects, angiotensin II has inflammatogenic properties. Interestingly, preclinical and clinical studies show that in addition to their hypotensive actions, angiotensin II receptor blockers have anti-inflammatory and antiatherosclerotic effects independent from blood pressure reductions (Navalkar et al., 2001; Sironi et al., 2004; Varagic et al., 2008). Stimulation of vascular smooth muscle cells with angiotensin II increases TLR4 mRNA levels (Otsui et al., 2007). In rodent models of ischemia/reperfusion injury, Valsartan (Beijing Novartis Pharma Ltd., China) reduced infarct size and production of inflammatory cytokines because of its effects on TLR4-mediated NF-κB activation (Yang et al., 2009a). PAM3CSK4 and LPS induced TLR2 and TLR4 mRNA and protein expression in human monocytes, and candesartan inhibited these actions (Dasu et al., 2009).
Statins, a lipid lowering class of drugs that also reduces cardiovascular events, may provide an additional level of protection against cardiovascular disease because of their inhibitory effects on TLR signaling-induced inflammation. Fluvastatin attenuated the elevated expression of TLR4 in monocytes from whole blood of patients with heart failure (Foldes et al., 2008) and inhibited the expression levels of TLR4, TNF-α, and NF-κB in a rat model of ischemia-reperfusion (Yang et al., 2011a). Simvastatin induced a reduction in TLR4 expression in human monocytes (Methe et al., 2005). Atorvastatin reduced the expression of TLR4 protein and mRNA and inhibited NF-κB activation in atherosclerotic plaques (Fang et al., 2014). Investigating the effects of anti-inflammatory effects of atorvastatin in murine pro-B cell lines, previous studies showed atorvastatin did not exert its inhibitory effect via TLR4 receptor-ligand binding mechanism, but instead, it impaired TLR4 recruitment into the lipid raft (Chansrichavala et al., 2010). Furthermore, atorvastatin inhibited NF-κB activation by stabilizing IκBα and inactivating ERK phosphorylation and reducing LPS-induced TLR4 mRNA expression in human umbilical vein endothelial cells (Wang et al., 2011b). These effects were similar to those accomplished by TLR gene silencing.
B. Toll-like Receptor Signaling Activation
The most widely explored application of TLR agonists is their use as vaccine adjuvants. Specific TLR agonists with low toxicity and high potency are preferred over other adjuvants for the development of prophylactic vaccines (Wille-Reece et al., 2005; Halperin et al., 2006; Gupta et al., 2014; Bortolatto et al., 2015). Furthermore, preclinical data support the use of TLR3, TLR4, TLR7, and TLR7/8 agonists to enhance vaccines against cancer and viral diseases (Patel et al., 2014; Zhao et al., 2014; Shirota et al., 2015). TLR agonists that induce a shift in TH2/TH1 ratio have also been developed for treatment of allergic diseases and asthma (Asai et al., 2008; Horak, 2011; Aryan et al., 2014). In addition, combination of existing modes of therapy (i.e., radiation) with TLR agonists has been suggested as promising therapy for cancer (Mason and Hunter, 2012). Because cardiovascular diseases are associated with inflammation, TLR activation seems counterintuitive. Nevertheless, TLR activation has been found to be advantageous before ischemic insults. Low doses of the TLR2 agonists Pam3CSK4 (a synthetic diacylated lipopeptide) or peptidoglycan before myocardial ischemia/reperfusion injury reduced infarct size, cardiac function, and the susceptibility of the myocardium to damage (Ha et al., 2010; Mersmann et al., 2010). Peptidoglycan stimulation increased TLR2 tyrosine phosphorylation and enhanced association of the p85 subunit of phosphoinositide 3-kinase with TLR2 (Ha et al., 2010). In a similar manner, administration of TLR ligands before brain ischemia has neuroprotective effects (as explained in section IV.C). Reprogramming of TLR signaling activity is the proposed mechanism of the beneficial effects of TLR agonism before ischemic events (Navi et al., 2013). LPS-induced TLR4 activation before myocardial ischemia has been also shown to have cardioprotective effects (Zacharowski et al., 2000). These effects have been primarily attributed to a TLR4-mediated increased production in inducible nitric oxide synthase and nitric oxide with improvements in ventricular function (Wang et al., 2011a).
C. Novel Approaches for Targeting Toll-like Receptor Signaling
Inflammation associated with cardiovascular diseases is more likely the result of activation of more than one TLR. Thus, it is reasonable to hypothesize that a pharmacological approach targeting the downstream signaling rather than the receptor ectodomains would be more advantageous in the prevention and treatment of cardiovascular disease-associated inflammation. The cytosolic TIR domain mediates TLR signaling and is the common structural feature between TLRs and TLR adapter proteins (O'Neill et al., 2003). In the early stages of TLR signaling, there are multiple interactions of TIR domains of TLRs and their adapters that mediate adapter recruitment, assembly, and stabilization of TLR signaling complex (Fekonja et al., 2012a). Interruption of these interactions has been accomplished using decoy peptides derived from TIR domains, TIRAP/MAL, and TRAM and resulted in inhibition of TLR signaling in vitro and in vivo (Jeyaseelan et al., 2005; Couture et al., 2012; Piao et al., 2013b). These efforts identified novel TLR inhibitory peptides that were able to block TLR signaling at low micromolar concentrations. Furthermore, these studies provided novel insights into the structural interactions and function of TIR domains that could potentially augment the efficacy of TLR agonists and antagonists. Fekonja et al. (2012b) showed that the addition of a strong-coiled coil dimerization domain determined the degree of inhibition against various TLRs and prevented activation of the dimeric TIR platform and Piao et al. (2013a) identified TRIF sites that are important for interaction with TLR4 and TRAM. Intense interest in the BB loop of TIR domain has guided research efforts in the development of inhibitory peptides (Toshchakov et al., 2007, 2011; Piao et al., 2013a). The BB loop peptides, however, lack specificity for a specific TLR or TLR-adapter complex because they bind to proteins containing TIR domains with variable affinity (Toshchakov et al., 2007, 2011; Fekonja et al., 2012a; Piao et al., 2013a). Other inhibitory peptides including INT peptides (derived from the N terminus of the intermediary domain of MyD88) and VIPER peptides (derived from vaccinia virus protein A46) have been also used. INT peptides inhibit MyD88-dependent signaling pathways and suppress the production of inflammatory cytokines (Avbelj et al., 2011), whereas VIPER peptides target TLR4 adapters Mal/TIRAP and TRAM (Stack et al., 2005).
Peptidomimetics have been developed with a goal to increase stability, internalization, pharmacological properties, and receptor sensitivity (Fekonja et al., 2012a). The BB loop of TIR domain was also the starting point for the development of these peptidomimetics. ST2825, EM77, and EM110 are peptidomimetic agents that inhibit the interaction between MyD88 with TLR4 (Loiarro et al., 2007). These compounds mimic the BB loop structure of MyD88 and interrupt the interaction between IL-1R and MyD88 (EM77, EM110) (Davis et al., 2006) or inhibit homodimerization of MyD88 (ST2825) (Loiarro et al., 2007). In vivo treatment with ST2825 protected against left ventricular dilation and hypertrophy in a murine model of acute myocardial infarction (Van Tassell et al., 2010). MyD88 inhibitors provide the possibility of attenuating TLR signaling rather than completely inhibiting the action of the receptors. TAK-242 is specific inhibitor of protein interactions between TLR4 and MAL/TIRAP or TRAM. Specifically, it binds TLR4 via Cys747 in the TIR domain, affecting the recruitment of adapters to the signaling complex (Matsunaga et al., 2011). TAK-242 reduced markers of acute kidney injury in endotoxemic sheep (Fenhammar et al., 2011), attenuated inflammatory injury in a mouse model of intracerebral hemorrhage (Wang et al., 2013), and reduced acute cerebral ischemia/reperfusion injury and expression of inflammatory cytokines in mice (Hua et al., 2015).
Inhibitory peptides and peptidomimetics have relatively weak potency for TLRs, and therefore, high concentrations must be used for effective blockade. To counteract this problem, protein inhibitors with greater inhibitory potency have been used. Mutated forms of TLR adapters have been shown to inhibit TLR signaling as dominant negative (dn) mutants (Fekonja et al., 2012a). A dominant-negative form of MyD88 was used in cultures of cells isolated from atherosclerotic plaques and reduced the production of cytokines and inflammatory mediators (Monaco et al., 2009). In vivo treatment with dnMyD88 prevented ischemia/reperfusion injury in rats via inhibition of NF-κB (Hua et al., 2005) and reduced cardiac hypertrophy and cardiac myocyte apoptosis in pressure overload-induced cardiac hypertrophy in mice (Ha et al., 2006).
microRNAs are key mediators of inflammation and play a significant role as negative or positive regulators of TLR signaling (Sheedy and O'Neill, 2008). They bind to messenger RNA of a target gene, promoting its degradation or inhibiting protein translation (Hennessy et al., 2010). Several microRNAs are highly expressed in the vasculature and have the ability to modulate vascular smooth muscle cell phenotype (Kang and Hata, 2012). microRNAs are dysregulated in vascular diseases (Kang and Hata, 2012; Zhao et al., 2015). Recently, Satoh et al. (2015) demonstrated that circulating TLR4-responsive microRNAs (miR-31, miR-181a, miR-16, miR-145) were lower in patients with coronary artery disease. MiR-146a directly targets TLR4 signaling by interacting with IRAK1/TRAF6 (Taganov et al., 2006) and has been shown to inhibit oxidized LDL-induced foam cell formation and inflammatory response in macrophages, suggesting a potential role of miR-146a in atherosclerosis (Yang et al., 2011b). Circulating microRNAs are currently viewed as novel biomarkers for various diseases and, because of their short length, they serve as a great platform for drug development (Hennessy et al., 2010). Manipulating TLR-regulated microRNAs with antagomirs and locked nucleic acid microRNA inhibitors has been proposed as a novel approach for targeting TLR signaling (Sheedy and O'Neill, 2008).
Ubiquitination is an essential modification within TLRs that leads to activation of NF-κB (Carpenter and O'Neill, 2009). During ubiquitination, ubiquitin covalently links to one or more lysine residues on target proteins through a ubiquitin-activating enzyme (E1), a ubiquitin-conjugating enzyme (E2), and a ubiquitin-protein ligase (E3). TRAF6 has E3 ubiquitin-ligase activity. In vitro studies have shown that inhibition of TRAF6 abolishes autoubiquitination and cell proliferation (Guedat and Colland, 2007). The ubiquitin-proteasome system plays a role in the pathogenesis and progression of atherosclerosis (Wang et al., 2015). Inhibition of ubiquitin ligases or the proteasome reduces proliferation of vascular smooth muscle cells, inhibits modulation of their synthetic phenotype, and reduces neointima (Demasi and Laurindo, 2012). Proteasome inhibitors and small molecule inhibitors targeting E3 ubiquitin ligases and deubiquitylating enzymes may also be novel approaches of suppressing TLR-induced inflammatory responses in cardiovascular diseases.
D. Challenges in Toll-like Receptor-Targeted Drug Development
The contribution of TLRs to the development of chronic inflammation in cardiovascular disease is supported by experimental and preclinical data; however, the development of TLR-targeted drugs for cardiovascular disease is still in its infancy. Recent studies from molecular immunology and pathophysiology have provided novel insights about the structural characteristics of TLRs, their ligands, their coreceptors, and associated signaling proteins. This convergence of new knowledge extends the depth and breadth of our understanding about what factors dictate TLR agonist and antagonist specificity as well as what molecular interactions should be targeted in specific clinical conditions, including cardiovascular diseases. TLR inhibition is the main focus of most research groups working on TLRs and cardiovascular disease. Designing of efficient inhibitors, however, is a challenging task that requires investigators to address issues like toxicity, specificity, and mode of delivery. Peptidomimetics and peptide and protein inhibitors may be promising therapeutics that target TLR adapters and disrupt TLR-adapter interactions. Peptidomimetics are compounds of low molecular weight that can easily translocate into the cell but they often lack specificity. Protein inhibitors, on the other hand, induce specific inhibition of the signaling but their size compromises their effective delivery into the cell. The delivery of TLR inhibitors as purified proteins has been suggested but the cost of this approach is currently prohibitory. Cell-penetrating moieties, including short cationic peptides and fatty acids, have been successfully added to inhibitory peptides to cross the cell membrane and target TLR signaling (Nelson et al., 2007; Avbelj et al., 2011). The use of microRNAs and inhibitors of ubiquitination to suppress excessive immune system activation and inflammation may be promising, but safety and efficacy as well as specificity have not yet been addressed.
There is significant progress in our understanding of TLR signaling and its role in cardiovascular diseases; however, several challenges remain before pharmacological modulation of TLRs leads to new therapeutics for cardiovascular diseases. TLRs play a vital role in host defense against endogenous and exogenous threats and are expressed in various somatic cells. Thus, systemic inhibition of these receptors may increase the risk of infection for the host. In the case of TLR agonists, systemic activation of the receptors could lead to excessive inflammation or autoimmunity. The interactions between immune cells and the microenvironment where TLRs are activated (i.e., vascular wall) are not well understood, and their role in the progression of cardiovascular disease is not known. Efficacy studies on experimental and preclinical application of potential TLR-targeted drugs should consider the interactions between tissue microenvironment and TLRs as well as the changes that occur in this microenvironment as disease progresses. For example, in atherosclerosis the cellular composition of atherosclerotic lesions changes as the disease develops and these alterations may affect drug efficacy in chronic treatments. Regardless of these challenges, the future of targeting TLRs in cardiovascular diseases remains promising. The successful efforts with TLR agonists as adjuvants/immunomodulators and the leaps made in the field of TLR ligand/receptor biology and signaling in the last decade have paved the path for successful TLR-targeted drug development to prevent and treat cardiovascular diseases.
VI. Conclusions and Future Directions
The recent and intense interest around PRRs, and especially TLRs, has revealed that the innate immune system makes important contributions to the development of cardiovascular disease, which are now recognized as chronic inflammatory conditions. Much remains to be learned, however, about the mechanisms whereby TLRs and the innate immune system contribute to the genesis and maintenance of cardiovascular disease. It is known that the effects of TLR signaling are not limited to the activation of immune cells but also affect the function of resident vascular cells. This evidence raises questions about how TLR signaling alters the different compartments of blood vessels and the interaction between the layers of the vascular wall. Previous research has focused on TLR signaling in endothelial cells as well as immune cells that infiltrate the vascular wall (Yang et al., 2005a,b, 2006; de Graaf et al., 2006; Schultz et al., 2007; Pryshchep et al., 2008; Zimmer et al., 2011; Liang et al., 2013). Currently, there is a growing interest in the adventitial compartment of blood vessels and in particular the adventitial fibroblast, which play an important role in regulating the structure and function of the vascular wall (Stenmark et al., 2013; El Kasmi et al., 2014). Adventitial fibroblasts are heterogeneous and epithelioid-like subpopulations with high sensitivity to angiotensin II (An et al., 2015). These cells are immunologically active and are able to detect TLR ligands (Vink et al., 2002) and produce chemokines, cytokines, growth factors, reactive oxygen species (Stenmark et al., 2013). Fibrocytes, a newly discovered cell type, present tissue remodeling properties of fibroblasts and inflammatory features of macrophages (Reilkoff et al., 2011). These mesenchymal cells produce large amounts of IL-6 in response to TLR2, TLR4, and TLR7 activation (Balmelli et al., 2007) and have the ability to enter sites of injury, where they mediate wound healing. Patients with hypertensive heart disease had increased circulating levels of fibrocytes and there was a strong correlation between left ventricular mass index and fibrocyte number (Keeley et al., 2012). Fibrocytes have also been implicated in the development of atherosclerosis and in myocardial fibrosis (Reilkoff et al., 2011); however, the exact mechanisms by which TLRs affect the function of these cells in the adventitial microenvironment are unclear. Figure 5 illustrates the localization of TLRs in various components of the vascular wall including the adventitial fibroblasts.
Fig. 5.
Toll-like receptor (TLR) localization in cellular components of the vascular wall. TLR activation by damage-associated molecular patterns (DAMPs) sets an inflammatory response in the vessel wall and interferes with endothelium-dependent relaxation and vasoconstriction. TLR activation on endothelial cells contributes to decreased production and bioavailability of nitric oxide, exacerbated formation of cytokines and reactive oxygen species, and the release of vasoconstrictor metabolites of arachidonic acid. TLR activation on smooth muscle cells increases formation of reactive oxygen species and reduces calcium sequestration in the sarcoplasmic reticulum. Adventitial fibroblasts and fibrocytes are newly identified components of the vascular wall that may contribute to proinflammatory events via activation of TLRs. AA, arachidonic acid; ACh, acetylcholine; cGMP, cyclic guanosine monophosphate; COX, cyclooxygenase; eNOS, endothelial nitric oxide synthase; GTP, guanosine-5′-triphosphate; l-arg, l-arginine; NE, norepinephrine; NO, nitric oxide; sGC, soluble guanylyl cyclase; TxA2; thromboxane A2. Revised image based on a previously published Figure from FASEB J; reproduced by permission.
Another question to be addressed is how TLR activation alters intracellular second messenger-induced signaling in vascular cells. Accumulating evidence indicates that TLR activation modulates calcium homeostasis. Activation of TLR3, TLR4, and TLR9 in macrophages induced intracellular calcium fluxes and activated Ca2+/calmodulin-dependent protein kinase (CaMKII)-α (Liu et al., 2008). This led to interaction of CaMKII-α with TAK1 and IRF3 promoting both MyD88- and TRIF-triggered efficient production of proinflammatory cytokines and type I IFN (Liu et al., 2008). Studies in rat cardiomyocytes reported that TLR4 signaling was involved in CaMKII-induced augmentation of Ca2+ spark-mediated sarcoplasmic reticulum Ca2+ leak and partial depletion of sarcoplasmic reticulum Ca2+ content. These events decreased systolic Ca2+ transient and impaired cardiac contractility (Zhang et al., 2014). Recently, a novel signaling transduction mechanism was observed for TLR9. This noninflammatory signaling cascade reduced sarco/endoplasmic reticulum Ca2+ ATPase pump 2 activity in cardiomyocytes and neurons, modulating Ca2+ homeostasis and its handling between the endoplasmic reticulum and mitochondria, which led to a decrease in mitochondrial ATP levels and the activation of cell survival protein 5′ AMP-activated protein kinase (Shintani et al., 2013). Calcium is a second messenger in intracellular vascular signaling having a vital role in contractile and dilatory responses of vascular smooth muscle cells and function of endothelial cells. Impaired calcium signaling is a common feature of hypertension and other vascular diseases; however, it is not clear how TLR activation alters calcium signaling and calcium sensitization mechanisms in the vasculature.
In addition to the involvement of TLR signaling in the structure and function of the compartments and cells of the blood vessels, new research directions should target questions that have immunologic basis and cardiovascular application. Research in the field of atherosclerosis has revealed that extracellular TLRs promote proatherogenic signals, whereas activation of endolysomal TLRs has atheroprotective effects (Cole et al., 2013). Delineating the importance of compartmentalization of TLRs and their ligands in vascular cells may lead to exciting opportunities for development of novel therapeutics that can target certain TLRs. Moreover, therapies should try to target the common downstream pathways of TLR signaling to their advantage, but also keep in mind the beneficial effects of the TLR in mediating acute inflammation (e.g., tissue repair or defense against an invading pathogen).
Another “immunological” question that could shed light into our understanding of the pathogenesis of cardiovascular disease resides in the newly proposed concepts of “trained immunity” and “innate immune memory.” Recent efforts in the field of immunology provided evidence of adaptive characteristics of innate immunity, challenging the dogma that innate immunity is nonspecific. Morris et al. (2014) suggested that TLR4 activation by very low doses of endotoxin may program host leukocytes into a low-grade “memory” state, contributing to the pathogenesis of chronic diseases. This is in contrast to findings described earlier in this review that endotoxin-induced activation of TLR4 prior to ischemic events has protective effects (Zacharowski et al., 2000). It is possible, however, that there is a “switch” between priming and tolerance of endotoxin-induced TLR4 signaling, and further studies are needed to delineate the associated molecular mechanisms. Interestingly, individuals with lifestyles that involve smoking and drinking (risk factors for cardiovascular disease) have increased levels of endotoxin in their circulation (Cani et al., 2008; Manco et al., 2010; Sun et al., 2010). Furthermore, low circulating levels of endotoxin may lead to enhanced pathogenesis of atherosclerosis (Stoll et al., 2004, 2006). An interesting question includes mechanisms by which TLRs mediate the interaction between innate immune memory and dysfunction of cardiovascular tissues. Furthermore, it is not well understood at which stage of cardiovascular disease the involvement of TLRs begin. Are TLRs the instigators or the propagators of cardiovascular disease? These and many more questions make research in the field of TLRs and cardiovascular disease innovative, intriguing, and promising. Most importantly, such research sets high expectations for the development of novel therapeutics for the prevention and treatment of cardiovascular disease.
Acknowledgments
The authors thank An Nguyen for technical assistance with the manuscript preparation.
Abbreviations
- ApoE
apolipoprotein E
- CD
cluster of differentiation
- CaMKII
Ca2+/calmodulin-dependent protein kinase
- DAMPs
damage-associated molecular patterns
- HSP
heat shock protein
- IFN
interferon
- IKK
IκB kinase
- IL
interleukin
- IRAK
interleukin 1 receptor-associated kinase
- IRF
interferon regulatory factor
- LDL
low-density lipoprotein
- LPS
lipopolysaccharide
- MAL
MyD88-adapter-like
- MAPK
mitogen activated protein kinase
- MyD88
myeloid differentiation primary response gene 88
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PAMPs
pathogen-associated molecular patterns
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- PRRs
pattern recognition receptors
- RIP-1
receptor-interacting serine/threonine-protein 1
- SARM
sterile α- and armadillo-motif-containing protein
- SHR
spontaneously hypertensive rats
- TAK1
transforming-growth-factor-β-activated kinase 1
- TIR
Toll/interleukin-1 receptor
- TIRAP
TIR domain containing adapter protein
- TLR
Toll-like receptors
- TNF-α
tumor necrosis factor α
- TRAF6
tumor necrosis factor-receptor-associated factor 6
- TRAM
TIR-domain-containing adapter-inducing interferon-β related adapter molecule
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- UNC93B1
Unc-93 homologue B1
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Goulopoulou, McCarthy, Webb.
Footnotes
Original research was supported by the American Heart Association [Grants 13SDG17050056 (to S.G.), 13PRE14080019 (to C.G.M.), 15GNT25700451 (to R.C.W.)]; National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases, DiaComp Pilot/Feasibility Program [Grant 14GHSU1435 to R.C.W.].
References
- Adachi N, Hirota M, Hamaguchi M, Okamoto K, Watanabe K, Endo F. (2004) Serum cytochrome c level as a prognostic indicator in patients with systemic inflammatory response syndrome. Clin Chim Acta 342:127–136. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124:783–801. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732–738. [DOI] [PubMed] [Google Scholar]
- An SJ, Liu P, Shao TM, Wang ZJ, Lu HG, Jiao Z, Li X, Fu JQ. (2015) Characterization and functions of vascular adventitial fibroblast subpopulations. Cell Physiol Biochem 35:1137–1150. [DOI] [PubMed] [Google Scholar]
- Anderson KV, Jürgens G, Nüsslein-Volhard C. (1985) Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42:779–789. [DOI] [PubMed] [Google Scholar]
- Andrade WA, Souza MdoC, Ramos-Martinez E, Nagpal K, Dutra MS, Melo MB, Bartholomeu DC, Ghosh S, Golenbock DT, Gazzinelli RT. (2013) Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe 13:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan F, Houtgraaf JH, Keogh B, Kazemi K, de Jong R, McCormack WJ, O’Neill LA, McGuirk P, Timmers L, Smeets MB, et al. (2012) Treatment with OPN-305, a humanized anti-Toll-Like receptor-2 antibody, reduces myocardial ischemia/reperfusion injury in pigs. Circ Cardiovasc Interv 5:279–287. [DOI] [PubMed] [Google Scholar]
- Aryan Z, Holgate ST, Radzioch D, Rezaei N. (2014) A new era of targeting the ancient gatekeepers of the immune system: toll-like agonists in the treatment of allergic rhinitis and asthma. Int Arch Allergy Immunol 164:46–63. [DOI] [PubMed] [Google Scholar]
- Asai K, Foley SC, Sumi Y, Yamauchi Y, Takeda N, Desrosiers M, Lavigne F, Hamid Q. (2008) Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy increases CD4+CD25+ T cells in the nasal mucosa of subjects with allergic rhinitis. Allergol Int 57:377–381. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277:15028–15034. [DOI] [PubMed] [Google Scholar]
- Avbelj M, Horvat S, Jerala R. (2011) The role of intermediary domain of MyD88 in cell activation and therapeutic inhibition of TLRs. J Immunol 187:2394–2404. [DOI] [PubMed] [Google Scholar]
- Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Gröne HJ, et al. (2009) Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem 284:24035–24048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetz A, Frey M, Heeg K, Dalpke AH. (2004) Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. J Biol Chem 279:54708–54715. [DOI] [PubMed] [Google Scholar]
- Baiersdörfer M, Schwarz M, Seehafer K, Lehmann C, Heit A, Wagner H, Kirschning CJ, Koch-Brandt C. (2010) Toll-like receptor 3 mediates expression of clusterin/apolipoprotein J in vascular smooth muscle cells stimulated with RNA released from necrotic cells. Exp Cell Res 316:3489–3500. [DOI] [PubMed] [Google Scholar]
- Balmelli C, Alves MP, Steiner E, Zingg D, Peduto N, Ruggli N, Gerber H, McCullough K, Summerfield A. (2007) Responsiveness of fibrocytes to toll-like receptor danger signals. Immunobiology 212:693–699. [DOI] [PubMed] [Google Scholar]
- Barascuk N, Genovese F, Larsen L, Byrjalsen I, Zheng Q, Sun S, Hosbond S, Poulsen TS, Diederichsen A, Jensen JM, et al. (2013) A MMP derived versican neo-epitope is elevated in plasma from patients with atherosclerotic heart disease. Int J Clin Exp Med 6:174–184. [PMC free article] [PubMed] [Google Scholar]
- Baumann CL, Aspalter IM, Sharif O, Pichlmair A, Blüml S, Grebien F, Bruckner M, Pasierbek P, Aumayr K, Planyavsky M, et al. (2010) CD14 is a coreceptor of Toll-like receptors 7 and 9. J Exp Med 207:2689–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, Martinez L, Greidinger EL, Yu BD, Gallo RL. (2012) Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med 18:1286–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B. (2004) Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430:257–263. [DOI] [PubMed] [Google Scholar]
- Blich M, Golan A, Arvatz G, Sebbag A, Shafat I, Sabo E, Cohen-Kaplan V, Petcherski S, Avniel-Polak S, Eitan A, et al. (2013) Macrophage activation by heparanase is mediated by TLR-2 and TLR-4 and associates with plaque progression. Arterioscler Thromb Vasc Biol 33:e56–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek I, Cordeau P, Lalancette-Hébert M, Gorup D, Weng YC, Gajovic S, Kriz J. (2012) Toll-like receptor 2 deficiency leads to delayed exacerbation of ischemic injury. J Neuroinflammation 9:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MH. (2012) Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond) 122:535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomfim GF, Echem C, Martins CB, Costa TJ, Sartoretto SM, Dos Santos RA, Oliveira MA, Akamine EH, Fortes ZB, Tostes RC, et al. (2015) Toll-like receptor 4 inhibition reduces vascular inflammation in spontaneously hypertensive rats. Life Sci 122:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham KS, Orzalli MH, Hayashi K, Wolf AI, Glanemann C, Weninger W, Iwasaki A, Knipe DM, Kagan JC. (2014) A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell 156:705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, et al. (2004) The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol 5:1052–1060. [DOI] [PubMed] [Google Scholar]
- Bortolatto J, Mirotti L, Rodriguez D, Gomes E, Russo M. (2015) Adsorption of Toll-like receptor 4 agonist to alum-based tetanus toxoid vaccine dampens pro-T helper 2 activities and enhances antibody responses. J Immunol Res 2015:280238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brea D, Sobrino T, Rodríguez-Yáñez M, Ramos-Cabrer P, Agulla J, Rodríguez-González R, Campos F, Blanco M, Castillo J. (2011) Toll-like receptors 7 and 8 expression is associated with poor outcome and greater inflammatory response in acute ischemic stroke. Clin Immunol 139:193–198. [DOI] [PubMed] [Google Scholar]
- Brint EK, Xu D, Liu H, Dunne A, McKenzie AN, O’Neill LA, Liew FY. (2004) ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol 5:373–379. [DOI] [PubMed] [Google Scholar]
- Buchanan MM, Hutchinson M, Watkins LR, Yin H. (2010) Toll-like receptor 4 in CNS pathologies. J Neurochem 114:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns K, Clatworthy J, Martin L, Martinon F, Plumpton C, Maschera B, Lewis A, Ray K, Tschopp J, Volpe F. (2000) Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat Cell Biol 2:346–351. [DOI] [PubMed] [Google Scholar]
- Burns K, Janssens S, Brissoni B, Olivos N, Beyaert R, Tschopp J. (2003) Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med 197:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57:1470–1481. [DOI] [PubMed] [Google Scholar]
- Cannon CP, Braunwald E, McCabe CH, Grayston JT, Muhlestein B, Giugliano RP, Cairns R, Skene AM, Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators (2005) Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med 352:1646–1654. [DOI] [PubMed] [Google Scholar]
- Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. (2007) Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun 353:509–514. [DOI] [PubMed] [Google Scholar]
- Carpenter S, O’Neill LA. (2009) Recent insights into the structure of Toll-like receptors and post-translational modifications of their associated signalling proteins. Biochem J 422:1–10. [DOI] [PubMed] [Google Scholar]
- Carty M, Goodbody R, Schröder M, Stack J, Moynagh PN, Bowie AG. (2006) The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol 7:1074–1081. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. (2007) Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation 115:1599–1608. [DOI] [PubMed] [Google Scholar]
- Chang MY, Tanino Y, Vidova V, Kinsella MG, Chan CK, Johnson PY, Wight TN, Frevert CW. (2014) A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease. Matrix Biol 34:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chansrichavala P, Chantharaksri U, Sritara P, Ngaosuwankul N, Chaiyaroj SC. (2010) Atorvastatin affects TLR4 clustering via lipid raft modulation. Int Immunopharmacol 10:892–899. [DOI] [PubMed] [Google Scholar]
- Chatterjee P, Chiasson VL, Kopriva SE, Young KJ, Chatterjee V, Jones KA, Mitchell BM. (2011) Interleukin 10 deficiency exacerbates toll-like receptor 3-induced preeclampsia-like symptoms in mice. Hypertension 58:489–496. [DOI] [PubMed] [Google Scholar]
- Chatterjee P, Chiasson VL, Seerangan G, Tobin RP, Kopriva SE, Newell-Rogers MK, Mitchell BM. (2015) Cotreatment with interleukin 4 and interleukin 10 modulates immune cells and prevents hypertension in pregnant mice. Am J Hypertens 28:135–142. [DOI] [PubMed] [Google Scholar]
- Chatterjee P, Kopriva SE, Chiasson VL, Young KJ, Tobin RP, Newell-Rogers K, Mitchell BM. (2013) Interleukin-4 deficiency induces mild preeclampsia in mice. J Hypertens 31:1414–1423, discussion 1423. [DOI] [PubMed] [Google Scholar]
- Chatterjee P, Weaver LE, Doersch KM, Kopriva SE, Chiasson VL, Allen SJ, Narayanan AM, Young KJ, Jones KA, Kuehl TJ, et al. (2012) Placental Toll-like receptor 3 and Toll-like receptor 7/8 activation contributes to preeclampsia in humans and mice. PLoS One 7:e41884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaung WW, Wu R, Ji Y, Dong W, Wang P. (2012) Mitochondrial transcription factor A is a proinflammatory mediator in hemorrhagic shock. Int J Mol Med 30:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J, Franz KM, Kagan JC. (2015) PRRs are watching you: Localization of innate sensing and signaling regulators. Virology 479-480:104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban C, Igari Y, Yagi M, Reimer T, Koyama S, Aoshi T, Ohata K, Tsukui T, Takeshita F, Sakurai K, et al. (2010) Immunogenicity of whole-parasite vaccines against Plasmodium falciparum involves malarial hemozoin and host TLR9. Cell Host Microbe 7:50–61. [DOI] [PubMed] [Google Scholar]
- Cole JE, Georgiou E, Monaco C. (2010) The expression and functions of toll-like receptors in atherosclerosis. Mediators Inflamm 2010:393946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JE, Kassiteridi C, Monaco C. (2013) Toll-like receptors in atherosclerosis: a ‘Pandora’s box’ of advances and controversies. Trends Pharmacol Sci 34:629–636. [DOI] [PubMed] [Google Scholar]
- Cole JE, Navin TJ, Cross AJ, Goddard ME, Alexopoulou L, Mitra AT, Davies AH, Flavell RA, Feldmann M, Monaco C. (2011) Unexpected protective role for Toll-like receptor 3 in the arterial wall. Proc Natl Acad Sci USA 108:2372–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly DJ, O’Neill LA. (2012) New developments in Toll-like receptor targeted therapeutics. Curr Opin Pharmacol 12:510–518. [DOI] [PubMed] [Google Scholar]
- Couture LA, Piao W, Ru LW, Vogel SN, Toshchakov VY. (2012) Targeting Toll-like receptor (TLR) signaling by Toll/interleukin-1 receptor (TIR) domain-containing adapter protein/MyD88 adapter-like (TIRAP/Mal)-derived decoy peptides. J Biol Chem 287:24641–24648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Ye X, Zuo T, Zhao H, Zhao Q, Chen W, Hua F. (2013) Chloroquine pretreatment inhibits toll-like receptor 3 signaling after stroke. Neurosci Lett 548:101–104. [DOI] [PubMed] [Google Scholar]
- Curtiss LK, Black AS, Bonnet DJ, Tobias PS. (2012) Atherosclerosis induced by endogenous and exogenous toll-like receptor (TLR)1 or TLR6 agonists. J Lipid Res 53:2126–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss LK, Tobias PS. (2007) The toll of Toll-like receptors, especially toll-like receptor 2, on murine atherosclerosis. Curr Drug Targets 8:1230–1238. [DOI] [PubMed] [Google Scholar]
- Dange RB, Agarwal D, Masson GS, Vila J, Wilson B, Nair A, Francis J. (2014) Central blockade of TLR4 improves cardiac function and attenuates myocardial inflammation in angiotensin II-induced hypertension. Cardiovasc Res 103:17–27. [DOI] [PubMed] [Google Scholar]
- Dange RB, Agarwal D, Teruyama R, Francis J. (2015) Toll-like receptor 4 inhibition within the paraventricular nucleus attenuates blood pressure and inflammatory response in a genetic model of hypertension. J Neuroinflammation 12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Correia J, Soldau K, Christen U, Tobias PS, Ulevitch RJ. (2001) Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J Biol Chem 276:21129–21135. [DOI] [PubMed] [Google Scholar]
- Dasu MR, Riosvelasco AC, Jialal I. (2009) Candesartan inhibits Toll-like receptor expression and activity both in vitro and in vivo. Atherosclerosis 202:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinee SM, Voelcker V, Tebaykina Z, Wong F, Karsan A. (2011) Heterotrimeric Gi/Go proteins modulate endothelial TLR signaling independent of the MyD88-dependent pathway. Am J Physiol Heart Circ Physiol 301:H2246–H2253. [DOI] [PubMed] [Google Scholar]
- Davidovich P, Kearney CJ, Martin SJ. (2014) Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biol Chem 395:1163–1171. [DOI] [PubMed] [Google Scholar]
- Davis CN, Mann E, Behrens MM, Gaidarova S, Rebek M, Rebek J, Jr, Bartfai T. (2006) MyD88-dependent and -independent signaling by IL-1 in neurons probed by bifunctional Toll/IL-1 receptor domain/BB-loop mimetics. Proc Natl Acad Sci USA 103:2953–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Batista PR, Palacios R, Martín A, Hernanz R, Médici CT, Silva MA, Rossi EM, Aguado A, Vassallo DV, Salaices M, et al. (2014) Toll-like receptor 4 upregulation by angiotensin II contributes to hypertension and vascular dysfunction through reactive oxygen species production. PLoS One 9:e104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf R, Kloppenburg G, Kitslaar PJ, Bruggeman CA, Stassen F. (2006) Human heat shock protein 60 stimulates vascular smooth muscle cell proliferation through Toll-like receptors 2 and 4. Microbes Infect 8:1859–1865. [DOI] [PubMed] [Google Scholar]
- Demasi M, Laurindo FR. (2012) Physiological and pathological role of the ubiquitin-proteasome system in the vascular smooth muscle cell. Cardiovasc Res 95:183–193. [DOI] [PubMed] [Google Scholar]
- Descamps D, Le Gars M, Balloy V, Barbier D, Maschalidi S, Tohme M, Chignard M, Ramphal R, Manoury B, Sallenave JM. (2012) Toll-like receptor 5 (TLR5), IL-1β secretion, and asparagine endopeptidase are critical factors for alveolar macrophage phagocytosis and bacterial killing. Proc Natl Acad Sci USA 109:1619–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvarieux M, Demmer RT, Jacobs DR, Jr, Rundek T, Boden-Albala B, Sacco RL, Papapanou PN. (2010) Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J Hypertens 28:1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaney JM, Greene CM, Taggart CC, Carroll TP, O’Neill SJ, McElvaney NG. (2003) Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Lett 544:129–132. [DOI] [PubMed] [Google Scholar]
- Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, Lenz LL, Cado D, Riley LW, Winoto A. (2004) TRAIL-R as a negative regulator of innate immune cell responses. Immunity 21:877–889. [DOI] [PubMed] [Google Scholar]
- Ding Y, Subramanian S, Montes VN, Goodspeed L, Wang S, Han C, Teresa AS, 3rd, Kim J, O’Brien KD, Chait A. (2012) Toll-like receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 32:1596–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Liu S, Wang X, Khaidakov M, Dai Y, Mehta JL. (2013) Oxidant stress in mitochondrial DNA damage, autophagy and inflammation in atherosclerosis. Sci Rep 3:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes CE, Crack PJ. (2010) Neural injury following stroke: are Toll-like receptors the link between the immune system and the CNS? Br J Pharmacol 160:1872–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duramad O, Fearon KL, Chang B, Chan JH, Gregorio J, Coffman RL, Barrat FJ. (2005) Inhibitors of TLR-9 act on multiple cell subsets in mouse and man in vitro and prevent death in vivo from systemic inflammation. J Immunol 174:5193–5200. [DOI] [PubMed] [Google Scholar]
- Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. (2002) Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 105:1158–1161. [PubMed] [Google Scholar]
- Ehrentraut H, Weber C, Ehrentraut S, Schwederski M, Boehm O, Knuefermann P, Meyer R, Baumgarten G. (2011) The toll-like receptor 4-antagonist eritoran reduces murine cardiac hypertrophy. Eur J Heart Fail 13:602–610. [DOI] [PubMed] [Google Scholar]
- El Kasmi KC, Pugliese SC, Riddle SR, Poth JM, Anderson AL, Frid MG, Li M, Pullamsetti SS, Savai R, Nagel MA, et al. (2014) Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol 193:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erridge C. (2010) Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol 87:989–999. [DOI] [PubMed] [Google Scholar]
- Erridge C, Burdess A, Jackson AJ, Murray C, Riggio M, Lappin D, Milligan S, Spickett CM, Webb DJ. (2008) Vascular cell responsiveness to Toll-like receptor ligands in carotid atheroma. Eur J Clin Invest 38:713–720. [DOI] [PubMed] [Google Scholar]
- Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. (2011) Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med 208:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, Barton GM. (2008) The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 456:658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadakar K, Dadkhahfar S, Esmaeili A, Rezaei N. (2014) The role of Toll-like receptors (TLRs) in stroke. Rev Neurosci 25:699–712. [DOI] [PubMed] [Google Scholar]
- Falck-Hansen M, Kassiteridi C, Monaco C. (2013) Toll-like receptors in atherosclerosis. Int J Mol Sci 14:14008–14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Yang S, Quan W, Jia H, Quan Z, Qu Z. (2014) Atorvastatin suppresses Toll-like receptor 4 expression and NF-κB activation in rabbit atherosclerotic plaques. Eur Rev Med Pharmacol Sci 18:242–246. [PubMed] [Google Scholar]
- Farrar CA, Keogh B, McCormack W, O’Shaughnessy A, Parker A, Reilly M, Sacks SH. (2012) Inhibition of TLR2 promotes graft function in a murine model of renal transplant ischemia-reperfusion injury. FASEB J 26:799–807. [DOI] [PubMed] [Google Scholar]
- Feig DI. (2014) Serum uric acid and the risk of hypertension and chronic kidney disease. Curr Opin Rheumatol 26:176–185. [DOI] [PubMed] [Google Scholar]
- Fekonja O, Avbelj M, Jerala R. (2012a) Suppression of TLR signaling by targeting TIR domain-containing proteins. Curr Protein Pept Sci 13:776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekonja O, Benčina M, Jerala R. (2012b) Toll/interleukin-1 receptor domain dimers as the platform for activation and enhanced inhibition of Toll-like receptor signaling. J Biol Chem 287:30993–31002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenhammar J, Rundgren M, Forestier J, Kalman S, Eriksson S, Frithiof R. (2011) Toll-like receptor 4 inhibitor TAK-242 attenuates acute kidney injury in endotoxemic sheep. Anesthesiology 114:1130–1137. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. (1997) Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol 159:1451–1458. [PubMed] [Google Scholar]
- Flannery S, Bowie AG. (2010) The interleukin-1 receptor-associated kinases: critical regulators of innate immune signalling. Biochem Pharmacol 80:1981–1991. [DOI] [PubMed] [Google Scholar]
- Földes G, von Haehling S, Okonko DO, Jankowska EA, Poole-Wilson PA, Anker SD. (2008) Fluvastatin reduces increased blood monocyte Toll-like receptor 4 expression in whole blood from patients with chronic heart failure. Int J Cardiol 124:80–85. [DOI] [PubMed] [Google Scholar]
- Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. (2002) PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol 3:875–881. [DOI] [PubMed] [Google Scholar]
- Fukui R, Saitoh S, Matsumoto F, Kozuka-Hata H, Oyama M, Tabeta K, Beutler B, Miyake K. (2009) Unc93B1 biases Toll-like receptor responses to nucleic acid in dendritic cells toward DNA- but against RNA-sensing. J Exp Med 206:1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi AC, Miname MH, Santos RD. (2009) Uric acid: A marker of increased cardiovascular risk. Atherosclerosis 202:11–17. [DOI] [PubMed] [Google Scholar]
- Garcia-Cattaneo A, Gobert FX, Müller M, Toscano F, Flores M, Lescure A, Del Nery E, Benaroch P. (2012) Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling. Proc Natl Acad Sci USA 109:9053–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay NJ, Gangloff M, O’Neill LA. (2011) What the Myddosome structure tells us about the initiation of innate immunity. Trends Immunol 32:104–109. [DOI] [PubMed] [Google Scholar]
- Gohda J, Matsumura T, Inoue J. (2004) Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domain-containing adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J Immunol 173:2913–2917. [DOI] [PubMed] [Google Scholar]
- Gómez-Guzmán M, Jiménez R, Romero M, Sánchez M, Zarzuelo MJ, Gómez-Morales M, O’Valle F, López-Farré AJ, Algieri F, Gálvez J, et al. (2014) Chronic hydroxychloroquine improves endothelial dysfunction and protects kidney in a mouse model of systemic lupus erythematosus. Hypertension 64:330–337. [DOI] [PubMed] [Google Scholar]
- Goodall KJ, Poon IK, Phipps S, Hulett MD. (2014) Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4. PLoS One 9:e109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini S, Gatta L, Pontecorvo L, Vitiello L, la Sala A. (2013) Regulation of innate immunity by extracellular nucleotides. Am J Blood Res 3:14–28. [PMC free article] [PubMed] [Google Scholar]
- Goulopoulou S, Matsumoto T, Bomfim GF, Webb RC. (2012) Toll-like receptor 9 activation: a novel mechanism linking placenta-derived mitochondrial DNA and vascular dysfunction in pre-eclampsia. Clin Sci (Lond) 123:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau AJ, Fischer B, Barth C, Ling P, Lichy C, Buggle F. (2005) Influenza vaccination is associated with a reduced risk of stroke. Stroke 36:1501–1506. [DOI] [PubMed] [Google Scholar]
- Grau AJ, Urbanek C, Palm F. (2010) Common infections and the risk of stroke. Nat Rev Neurol 6:681–694. [DOI] [PubMed] [Google Scholar]
- Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, Rogers WJ, Crouse JR, Borrowdale SL, Schron E, et al. ACES Investigators (2005) Azithromycin for the secondary prevention of coronary events. N Engl J Med 352:1637–1645. [DOI] [PubMed] [Google Scholar]
- Guédat P, Colland F. (2007) Patented small molecule inhibitors in the ubiquitin proteasome system. BMC Biochem 8 (Suppl 1):S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Bajwa P, Deb R, Chellappa MM, Dey S. (2014) Flagellin a toll-like receptor 5 agonist as an adjuvant in chicken vaccines. Clin Vaccine Immunol 21:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, Klinman DM. (2003) Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol 171:1393–1400. [DOI] [PubMed] [Google Scholar]
- Ha T, Hu Y, Liu L, Lu C, McMullen JR, Kelley J, Kao RL, Williams DL, Gao X, Li C. (2010) TLR2 ligands induce cardioprotection against ischaemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Cardiovasc Res 87:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha T, Hua F, Li Y, Ma J, Gao X, Kelley J, Zhao A, Haddad GE, Williams DL, Browder IW, et al. (2006) Blockade of MyD88 attenuates cardiac hypertrophy and decreases cardiac myocyte apoptosis in pressure overload-induced cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol 290:H985–H994. [DOI] [PubMed] [Google Scholar]
- Haarala A, Kähönen M, Lehtimäki T, Aittoniemi J, Jylhävä J, Hutri-Kähönen N, Taittonen L, Laitinen T, Juonala M, Viikari J, et al. (2012) Relation of high cytomegalovirus antibody titres to blood pressure and brachial artery flow-mediated dilation in young men: the Cardiovascular Risk in Young Finns Study. Clin Exp Immunol 167:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin SA, Dobson S, McNeil S, Langley JM, Smith B, McCall-Sani R, Levitt D, Nest GV, Gennevois D, Eiden JJ. (2006) Comparison of the safety and immunogenicity of hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide and a licensed hepatitis B vaccine in healthy young adults. Vaccine 24:20–26. [DOI] [PubMed] [Google Scholar]
- Han KJ, Su X, Xu LG, Bin LH, Zhang J, Shu HB. (2004) Mechanisms of the TRIF-induced interferon-stimulated response element and NF-kappaB activation and apoptosis pathways. J Biol Chem 279:15652–15661. [DOI] [PubMed] [Google Scholar]
- Harwani SC, Chapleau MW, Legge KL, Ballas ZK, Abboud FM. (2012) Neurohormonal modulation of the innate immune system is proinflammatory in the prehypertensive spontaneously hypertensive rat, a genetic model of essential hypertension. Circ Res 111:1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. (2004) Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526–1529. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. (2000) A Toll-like receptor recognizes bacterial DNA. Nature 408:740–745. [DOI] [PubMed] [Google Scholar]
- Hennessy EJ, Parker AE, O’Neill LA. (2010) Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov 9:293–307. [DOI] [PubMed] [Google Scholar]
- Henriksen PA, Sallenave JM. (2008) Human neutrophil elastase: mediator and therapeutic target in atherosclerosis. Int J Biochem Cell Biol 40:1095–1100. [DOI] [PubMed] [Google Scholar]
- Hernanz R, Martínez-Revelles S, Palacios R, Martín A, Cachofeiro V, Aguado A, García-Redondo L, Barrús MT, de Batista PR, Briones AM, et al. (2015) Toll-like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II-induced hypertension. Br J Pharmacol 172:3159–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Kurobe H, Higashida M, Fukuda D, Shimabukuro M, Tanaka K, Higashikuni Y, Kitagawa T, Sata M. (2013) HMGB1 plays a critical role in vascular inflammation and lesion formation via toll-like receptor 9. Atherosclerosis 231:227–233. [DOI] [PubMed] [Google Scholar]
- Honda K, Yanai H, Mizutani T, Negishi H, Shimada N, Suzuki N, Ohba Y, Takaoka A, Yeh WC, Taniguchi T. (2004) Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc Natl Acad Sci USA 101:15416–15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak F. (2011) VTX-1463, a novel TLR8 agonist for the treatment of allergic rhinitis. Expert Opin Investig Drugs 20:981–986. [DOI] [PubMed] [Google Scholar]
- Horng T, Barton GM, Flavell RA, Medzhitov R. (2002) The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420:329–333. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. (1999) Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162:3749–3752. [PubMed] [Google Scholar]
- Hua F, Ha T, Ma J, Gao X, Kelley J, Williams DL, Browder IW, Kao RL, Li C. (2005) Blocking the MyD88-dependent pathway protects the myocardium from ischemia/reperfusion injury in rat hearts. Biochem Biophys Res Commun 338:1118–1125. [DOI] [PubMed] [Google Scholar]
- Hua F, Ma J, Ha T, Kelley J, Williams DL, Kao RL, Kalbfleisch JH, Browder IW, Li C. (2008) Preconditioning with a TLR2 specific ligand increases resistance to cerebral ischemia/reperfusion injury. J Neuroimmunol 199:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Ma J, Ha T, Kelley JL, Kao RL, Schweitzer JB, Kalbfleisch JH, Williams DL, Li C. (2009) Differential roles of TLR2 and TLR4 in acute focal cerebral ischemia/reperfusion injury in mice. Brain Res 1262:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Ma J, Ha T, Xia Y, Kelley J, Williams DL, Kao RL, Browder IW, Schweitzer JB, Kalbfleisch JH, et al. (2007) Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol 190:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Tang H, Wang J, Prunty MC, Hua X, Sayeed I, Stein DG. (2015) TAK-242, an antagonist for Toll-like receptor 4, protects against acute cerebral ischemia/reperfusion injury in mice. J Cereb Blood Flow Metab 35:536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyakkoku K, Hamanaka J, Tsuruma K, Shimazawa M, Tanaka H, Uematsu S, Akira S, Inagaki N, Nagai H, Hara H. (2010) Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience 171:258–267. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Sayers S, D’Armiento JM, Tall AR, Welch CL. (2013) TLR3 deficiency protects against collagen degradation and medial destruction in murine atherosclerotic plaques. Atherosclerosis 229:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto T, Shimada M, Gabriela G, Kosugi T, Sato W, Lee PY, Lanaspa MA, Rivard C, Maruyama S, Garin EH, et al. (2013) Toll-like receptor 3 ligand, polyIC, induces proteinuria and glomerular CD80, and increases urinary CD80 in mice. Nephrol Dial Transplant 28:1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki D, Mitsuzawa H, Murakami S, Sano H, Konishi M, Akino T, Kuroki Y. (2002) The extracellular toll-like receptor 2 domain directly binds peptidoglycan derived from Staphylococcus aureus. J Biol Chem 277:24315–24320. [DOI] [PubMed] [Google Scholar]
- Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T, Yoshikai Y. (2000) Cutting edge: naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol 165:6682–6686. [DOI] [PubMed] [Google Scholar]
- Janssens S, Burns K, Vercammen E, Tschopp J, Beyaert R. (2003) MyD88S, a splice variant of MyD88, differentially modulates NF-kappaB- and AP-1-dependent gene expression. FEBS Lett 548:103–107. [DOI] [PubMed] [Google Scholar]
- Jespersen CM, Als-Nielsen B, Damgaard M, Hansen JF, Hansen S, Helø OH, Hildebrandt P, Hilden J, Jensen GB, Kastrup J, et al. CLARICOR Trial Group (2006) Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ 332:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaseelan S, Manzer R, Young SK, Yamamoto M, Akira S, Mason RJ, Worthen GS. (2005) Toll-IL-1 receptor domain-containing adaptor protein is critical for early lung immune responses against Escherichia coli lipopolysaccharide and viable Escherichia coli. J Immunol 175:7484–7495. [DOI] [PubMed] [Google Scholar]
- Ji Y, Zhou Y, Pan J, Li X, Wang H, Wang Y. (2015) Temporal pattern of Toll-like receptor 9 upregulation in neurons and glial cells following cerebral ischemia reperfusion in mice. Int J Neurosci 5:1–9. [DOI] [PubMed] [Google Scholar]
- Jiang W, Tan L, Guo Y, Li X, Tang X, Yang K. (2012) Effect of renal denervation procedure on left ventricular hypertrophy of hypertensive rats and its mechanisms. Acta Cir Bras 27:815–820. [DOI] [PubMed] [Google Scholar]
- Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. (2007) Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130:1071–1082. [DOI] [PubMed] [Google Scholar]
- Jin MS, Lee JO. (2008) Structures of the toll-like receptor family and its ligand complexes. Immunity 29:182–191. [DOI] [PubMed] [Google Scholar]
- Julian MW, Shao G, Bao S, Knoell DL, Papenfuss TL, VanGundy ZC, Crouser ED. (2012) Mitochondrial transcription factor A serves as a danger signal by augmenting plasmacytoid dendritic cell responses to DNA. J Immunol 189:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. (2008) TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol 9:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Offermann MK. (2005) Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol 174:4942–4952. [DOI] [PubMed] [Google Scholar]
- Kang H, Hata A. (2012) MicroRNA regulation of smooth muscle gene expression and phenotype. Curr Opin Hematol 19:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JY, Nan X, Jin MS, Youn SJ, Ryu YH, Mah S, Han SH, Lee H, Paik SG, Lee JO. (2009) Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 31:873–884. [DOI] [PubMed] [Google Scholar]
- Karper JC, Ewing MM, Habets KL, de Vries MR, Peters EA, van Oeveren-Rietdijk AM, de Boer HC, Hamming JF, Kuiper J, Kandimalla ER, et al. (2012) Blocking toll-like receptors 7 and 9 reduces postinterventional remodeling via reduced macrophage activation, foam cell formation, and migration. Arterioscler Thromb Vasc Biol 32:e72–e80. [DOI] [PubMed] [Google Scholar]
- Kasimsetty SG, DeWolf SE, Shigeoka AA, McKay DB. (2014) Regulation of TLR2 and NLRP3 in primary murine renal tubular epithelial cells. Nephron Clin Pract 127:119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe T, Sato S, Matsushita K, Kato H, Matsui K, Kumagai Y, Saitoh T, Kawai T, Takeuchi O, Akira S. (2008) Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol 9:684–691. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. (1999) Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115–122. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. (2009) The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol 21:317–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami A, Osaka M, Aikawa M, Uematsu S, Akira S, Libby P, Shimokado K, Sacks FM, Yoshida M. (2008) Toll-like receptor 2 mediates apolipoprotein CIII-induced monocyte activation. Circ Res 103:1402–1409. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kawasaki T, Kawai T. (2014) Toll-like receptor signaling pathways. Front Immunol 5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi MR, McDonald CM, Shigenaga JK, Grunfeld C, Feingold KR. (2005) Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler Thromb Vasc Biol 25:1220–1224. [DOI] [PubMed] [Google Scholar]
- Keeley EC, Mehrad B, Janardhanan R, Salerno M, Hunter JR, Burdick MM, Field JJ, Strieter RM, Kramer CM. (2012) Elevated circulating fibrocyte levels in patients with hypertensive heart disease. J Hypertens 30:1856–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AM, Li M, Abdulnour-Nakhoul S, Maderdrut JL, Simon EE, Batuman V. (2012) Delayed administration of pituitary adenylate cyclase-activating polypeptide 38 ameliorates renal ischemia/reperfusion injury in mice by modulating Toll-like receptors. Peptides 38:395–403. [DOI] [PubMed] [Google Scholar]
- Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, et al. (2007) Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130:906–917. [DOI] [PubMed] [Google Scholar]
- Kim JS, Park DW, Lee HK, Kim JR, Baek SH. (2009a) Early growth response-1 is involved in foam cell formation and is upregulated by the TLR9-MyD88-ERK1/2 pathway. Biochem Biophys Res Commun 390:196–200. [DOI] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. (2009b) Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Ku SK, Bae JS. (2012) Inhibitory effects of kaempferol-3-O-sophoroside on HMGB1-mediated proinflammatory responses. Food Chem Toxicol 50:1118–1123. [DOI] [PubMed] [Google Scholar]
- Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. (2008) UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452:234–238. [DOI] [PubMed] [Google Scholar]
- Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M, Yoshida H, Kubo M, Yoshimura A. (2002) SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity 17:583–591. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hernandez LD, Galán JE, Janeway CA, Jr, Medzhitov R, Flavell RA. (2002) IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110:191–202. [DOI] [PubMed] [Google Scholar]
- Kono H, Rock KL. (2008) How dying cells alert the immune system to danger. Nat Rev Immunol 8:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopriva SE, Chiasson VL, Mitchell BM, Chatterjee P. (2013) TLR3-induced placental miR-210 down-regulates the STAT6/interleukin-4 pathway. PLoS One 8:e67760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulis C, Chen YC, Hausding C, Ahrens I, Kyaw TS, Tay C, Allen T, Jandeleit-Dahm K, Sweet MJ, Akira S, et al. (2014) Protective role for Toll-like receptor-9 in the development of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 34:516–525. [DOI] [PubMed] [Google Scholar]
- Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. (2011) Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol 32:157–164. [DOI] [PubMed] [Google Scholar]
- Kuang X, Wang LF, Yu L, Li YJ, Wang YN, He Q, Chen C, Du JR. (2014) Ligustilide ameliorates neuroinflammation and brain injury in focal cerebral ischemia/reperfusion rats: involvement of inhibition of TLR4/peroxiredoxin 6 signaling. Free Radic Biol Med 71:165–175. [DOI] [PubMed] [Google Scholar]
- Kuhns DB, Priel DA, Gallin JI. (2007) Induction of human monocyte interleukin (IL)-8 by fibrinogen through the toll-like receptor pathway. Inflammation 30:178–188. [DOI] [PubMed] [Google Scholar]
- Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. (2011) Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol 186: 4794–4804. [DOI] [PubMed] [Google Scholar]
- Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DC, Monks BG, McKnight CJ, Lamphier MS, Duprex WP, et al. (2007) Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol 8:772–779. [DOI] [PubMed] [Google Scholar]
- Lavallée P, Perchaud V, Gautier-Bertrand M, Grabli D, Amarenco P. (2002) Association between influenza vaccination and reduced risk of brain infarction. Stroke 33:513–518. [DOI] [PubMed] [Google Scholar]
- LeBouder E, Rey-Nores JE, Rushmere NK, Grigorov M, Lawn SD, Affolter M, Griffin GE, Ferrara P, Schiffrin EJ, Morgan BP, et al. (2003) Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol 171:6680–6689. [DOI] [PubMed] [Google Scholar]
- Lee BL, Moon JE, Shu JH, Yuan L, Newman ZR, Schekman R, Barton GM. (2013) UNC93B1 mediates differential trafficking of endosomal TLRs. eLife 2:e00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GL, Chang YW, Wu JY, Wu ML, Wu KK, Yet SF, Kuo CC. (2012) TLR 2 induces vascular smooth muscle cell migration through cAMP response element-binding protein-mediated interleukin-6 production. Arterioscler Thromb Vasc Biol 32:2751–2760. [DOI] [PubMed] [Google Scholar]
- Lee SM, Kok KH, Jaume M, Cheung TK, Yip TF, Lai JC, Guan Y, Webster RG, Jin DY, Peiris JS. (2014) Toll-like receptor 10 is involved in induction of innate immune responses to influenza virus infection. Proc Natl Acad Sci USA 111:3793–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, Krueger C, Nitsch R, Meisel A, Weber JR. (2007) Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol 190:28–33. [DOI] [PubMed] [Google Scholar]
- Leung PY, Stevens SL, Packard AE, Lessov NS, Yang T, Conrad VK, van den Dungen NN, Simon RP, Stenzel-Poore MP. (2012) Toll-like receptor 7 preconditioning induces robust neuroprotection against stroke by a novel type I interferon-mediated mechanism. Stroke 43:1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Strelow A, Fontana EJ, Wesche H. (2002) IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci USA 99:5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhu J, Zhang W, Chen Y, Zhang K, Popescu LM, Ma X, Lau WB, Rong R, Yu X, et al. (2011) Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation 124:175–184. [DOI] [PubMed] [Google Scholar]
- Li T, Wang Y, Liu C, Hu Y, Wu M, Li J, Guo L, Chen L, Chen Q, Ha T, et al. (2009) MyD88-dependent nuclear factor-kappaB activation is involved in fibrinogen-induced hypertrophic response of cardiomyocytes. J Hypertens 27:1084–1093. [DOI] [PubMed] [Google Scholar]
- Liang CF, Liu JT, Wang Y, Xu A, Vanhoutte PM. (2013) Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler Thromb Vasc Biol 33:777–784. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. (2002) Inflammation and atherosclerosis. Circulation 105:1135–1143. [DOI] [PubMed] [Google Scholar]
- Liberati NT, Fitzgerald KA, Kim DH, Feinbaum R, Golenbock DT, Ausubel FM. (2004) Requirement for a conserved Toll/interleukin-1 resistance domain protein in the Caenorhabditis elegans immune response. Proc Natl Acad Sci USA 101:6593–6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O’Neill LA. (2005) Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 5:446–458. [DOI] [PubMed] [Google Scholar]
- Lin SC, Lo YC, Wu H. (2010) Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465:885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Gu M, Xu D, Lv Q, Zhang W, Wu Y. (2010a) Protective effects of Toll-like receptor 4 inhibitor eritoran on renal ischemia-reperfusion injury. Transplant Proc 42:1539–1544. [DOI] [PubMed] [Google Scholar]
- Liu N, Liu J, Ji Y, Lu P. (2010b) Toll-like receptor 4 signaling mediates inflammatory activation induced by C-reactive protein in vascular smooth muscle cells. Cell Physiol Biochem 25:467–476. [DOI] [PubMed] [Google Scholar]
- Liu N, Liu JT, Ji YY, Lu PP. (2010c) C-reactive protein triggers inflammatory responses partly via TLR4/IRF3/NF-κB signaling pathway in rat vascular smooth muscle cells. Life Sci 87:367–374. [DOI] [PubMed] [Google Scholar]
- Liu R, He Y, Li B, Liu J, Ren Y, Han W, Wang X, Zhang L. (2012) Tenascin-C produced by oxidized LDL-stimulated macrophages increases foam cell formation through Toll-like receptor-4. Mol Cells 34:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yao M, Li N, Wang C, Zheng Y, Cao X. (2008) CaMKII promotes TLR-triggered proinflammatory cytokine and type I interferon production by directly binding and activating TAK1 and IRF3 in macrophages. Blood 112:4961–4970. [DOI] [PubMed] [Google Scholar]
- Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. (2005) Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum 52:2936–2946. [DOI] [PubMed] [Google Scholar]
- Loiarro M, Capolunghi F, Fantò N, Gallo G, Campo S, Arseni B, Carsetti R, Carminati P, De Santis R, Ruggiero V, et al. (2007) Pivotal Advance: Inhibition of MyD88 dimerization and recruitment of IRAK1 and IRAK4 by a novel peptidomimetic compound. J Leukoc Biol 82:801–810. [DOI] [PubMed] [Google Scholar]
- Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, Morrell NW. (2013) Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res 112:1159–1170. [DOI] [PubMed] [Google Scholar]
- López J, Fernández-Pisonero I, Dueñas AI, Maeso P, San Román JA, Crespo MS, García-Rodríguez C. (2012) Viral and bacterial patterns induce TLR-mediated sustained inflammation and calcification in aortic valve interstitial cells. Int J Cardiol 158:18–25. [DOI] [PubMed] [Google Scholar]
- Lu C, Ha T, Wang X, Liu L, Zhang X, Kimbrough EO, Sha Z, Guan M, Schweitzer J, Kalbfleisch J, et al. (2014) The TLR9 ligand, CpG-ODN, induces protection against cerebral ischemia/reperfusion injury via activation of PI3K/Akt signaling. J Am Heart Assoc 3:e000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Liu L, Chen Y, Ha T, Kelley J, Schweitzer J, Kalbfleisch JH, Kao RL, Williams DL, Li C. (2011) TLR2 ligand induces protection against cerebral ischemia/reperfusion injury via activation of phosphoinositide 3-kinase/Akt signaling. J Immunol 187:1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. (2003) Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med 198:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg AM, Ketelhuth DF, Johansson ME, Gerdes N, Liu S, Yamamoto M, Akira S, Hansson GK. (2013) Toll-like receptor 3 and 4 signalling through the TRIF and TRAM adaptors in haematopoietic cells promotes atherosclerosis. Cardiovasc Res 99:364–373. [DOI] [PubMed] [Google Scholar]
- Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. (2010) Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manco M, Putignani L, Bottazzo GF. (2010) Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev 31:817–844. [DOI] [PubMed] [Google Scholar]
- Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, Teti G, Beninati C. (2009) Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol 10:587–594. [DOI] [PubMed] [Google Scholar]
- Mann DL. (2011) The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res 108:1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell A, Brint E, Gould JA, O’Neill LA, Hertzog PJ. (2004) Mal interacts with tumor necrosis factor receptor-associated factor (TRAF)-6 to mediate NF-kappaB activation by toll-like receptor (TLR)-2 and TLR4. J Biol Chem 279:37227–37230. [DOI] [PubMed] [Google Scholar]
- Mason KA, Hunter NR. (2012) CpG plus radiotherapy: a review of preclinical works leading to clinical trial. Front Oncol 2:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur S, Walley KR, Wang Y, Indrambarya T, Boyd JH. (2011) Extracellular heat shock protein 70 induces cardiomyocyte inflammation and contractile dysfunction via TLR2. Circ J 75:2445–2452. [DOI] [PubMed] [Google Scholar]
- Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. (2011) TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol 79:34–41. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Itoh S, Kimoto M, Kohno K, Tamai O, Wada Y, Yasukawa H, Iwami G, Okuda S, Imaizumi T. (1997) Asymmetrical dimethylarginine, an endogenous nitric oxide synthase inhibitor, in experimental hypertension. Hypertension 29:242–247. [DOI] [PubMed] [Google Scholar]
- Matzinger P. (2002) The danger model: a renewed sense of self. Science 296:301–305. [DOI] [PubMed] [Google Scholar]
- Matzinger P, Kamala T. (2011) Tissue-based class control: the other side of tolerance. Nat Rev Immunol 11:221–230. [DOI] [PubMed] [Google Scholar]
- McCarthy CG, Goulopoulou S, Wenceslau CF, Spitler K, Matsumoto T, Webb RC. (2014) Toll-like receptors and damage-associated molecular patterns: novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol 306:H184–H196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy CG, Wenceslau CF, Goulopoulou S, Ogbi S, Baban B, Sullivan JC, Matsumoto T, Webb RC. (2015) Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovasc Res 107:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick MM, Rahimi F, Bobryshev YV, Gaus K, Zreiqat H, Cai H, Lord RS, Geczy CL. (2005) S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J Biol Chem 280:41521–41529. [DOI] [PubMed] [Google Scholar]
- Medvedev AE, Lentschat A, Kuhns DB, Blanco JC, Salkowski C, Zhang S, Arditi M, Gallin JI, Vogel SN. (2003) Distinct mutations in IRAK-4 confer hyporesponsiveness to lipopolysaccharide and interleukin-1 in a patient with recurrent bacterial infections. J Exp Med 198:521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394–397. [DOI] [PubMed] [Google Scholar]
- Meng G, Rutz M, Schiemann M, Metzger J, Grabiec A, Schwandner R, Luppa PB, Ebel F, Busch DH, Bauer S, et al. (2004) Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J Clin Invest 113:1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhão JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, et al. (2011) Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal 4:ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersmann J, Berkels R, Zacharowski P, Tran N, Koch A, Iekushi K, Dimmeler S, Granja TF, Boehm O, Claycomb WC, et al. (2010) Preconditioning by toll-like receptor 2 agonist Pam3CSK4 reduces CXCL1-dependent leukocyte recruitment in murine myocardial ischemia/reperfusion injury. Crit Care Med 38:903–909. [DOI] [PubMed] [Google Scholar]
- Mersmann J, Iskandar F, Latsch K, Habeck K, Sprunck V, Zimmermann R, Schumann RR, Zacharowski K, Koch A. (2013) Attenuation of myocardial injury by HMGB1 blockade during ischemia/reperfusion is toll-like receptor 2-dependent. Mediators Inflamm 2013:174168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methe H, Kim JO, Kofler S, Nabauer M, Weis M. (2005) Statins decrease Toll-like receptor 4 expression and downstream signaling in human CD14+ monocytes. Arterioscler Thromb Vasc Biol 25:1439–1445. [DOI] [PubMed] [Google Scholar]
- Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. (2004) Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA 101:10679–10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller YI. (2005) Toll-like receptors and atherosclerosis: oxidized LDL as an endogenous Toll-like receptor ligand. Future Cardiol 1:785–792. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Yamasaki S. (2012) Sensing necrotic cells. Adv Exp Med Biol 738:144–152. [DOI] [PubMed] [Google Scholar]
- Monaco C, Gregan SM, Navin TJ, Foxwell BM, Davies AH, Feldmann M. (2009) Toll-like receptor-2 mediates inflammation and matrix degradation in human atherosclerosis. Circulation 120:2462–2469. [DOI] [PubMed] [Google Scholar]
- Monden M, Koyama H, Otsuka Y, Morioka T, Mori K, Shoji T, Mima Y, Motoyama K, Fukumoto S, Shioi A, et al. (2013) Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of Toll-like receptor 2. Diabetes 62:478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraga A, Pradillo JM, Cuartero MI, Hernández-Jiménez M, Oses M, Moro MA, Lizasoain I. (2014) Toll-like receptor 4 modulates cell migration and cortical neurogenesis after focal cerebral ischemia. FASEB J 28:4710–4718. [DOI] [PubMed] [Google Scholar]
- Morris MC, Gilliam EA, Li L. (2014) Innate immune programing by endotoxin and its pathological consequences. Front Immunol 5:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motshwene PG, Moncrieffe MC, Grossmann JG, Kao C, Ayaluru M, Sandercock AM, Robinson CV, Latz E, Gay NJ. (2009) An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J Biol Chem 284:25404–25411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullarkey M, Rose JR, Bristol J, Kawata T, Kimura A, Kobayashi S, Przetak M, Chow J, Gusovsky F, Christ WJ, et al. (2003) Inhibition of endotoxin response by e5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J Pharmacol Exp Ther 304:1093–1102. [DOI] [PubMed] [Google Scholar]
- Mullick AE, Tobias PS, Curtiss LK. (2005) Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest 115:3149–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, et al. (2002) SOCS-1 participates in negative regulation of LPS responses. Immunity 17:677–687. [DOI] [PubMed] [Google Scholar]
- Napetschnig J, Wu H. (2013) Molecular basis of NF-κB signaling. Annu Rev Biophys 42:443–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navalkar S, Parthasarathy S, Santanam N, Khan BV. (2001) Irbesartan, an angiotensin type 1 receptor inhibitor, regulates markers of inflammation in patients with premature atherosclerosis. J Am Coll Cardiol 37:440–444. [DOI] [PubMed] [Google Scholar]
- Navi A, Patel H, Shaw S, Baker D, Tsui J. (2013) Therapeutic role of toll-like receptor modification in cardiovascular dysfunction. Vascul Pharmacol 58:231–239. [DOI] [PubMed] [Google Scholar]
- Nelson AR, Borland L, Allbritton NL, Sims CE. (2007) Myristoyl-based transport of peptides into living cells. Biochemistry 46:14771–14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld EB, Zadrozny LM, Phillips D, Aponte A, Yu ZX, Balaban RS. (2014) Decorin and biglycan retain LDL in disease-prone valvular and aortic subendothelial intimal matrix. Atherosclerosis 233:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. (2006) Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation 114:2482–2489. [DOI] [PubMed] [Google Scholar]
- O’Connor CM, Dunne MW, Pfeffer MA, Muhlestein JB, Yao L, Gupta S, Benner RJ, Fisher MR, Cook TD, Investigators in the WIZARD Study (2003) Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA 290:1459–1466. [DOI] [PubMed] [Google Scholar]
- Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, et al. (2012) Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg M, Krüger A, Ferstl R, Kaufmann A, Nees G, Sigmund A, Bathke B, Lauterbach H, Suter M, Dreher S, et al. (2012) TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science 337:1111–1115. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Bowie AG. (2007) The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7:353–364. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Fitzgerald KA, Bowie AG. (2003) The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol 24:286–290. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Golenbock D, Bowie AG. (2013) The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol 13:453–460. [DOI] [PubMed] [Google Scholar]
- Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D, Tidswell M, et al. ACCESS Study Group (2013) Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA 309:1154–1162. [DOI] [PubMed] [Google Scholar]
- Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. (2003) TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol 4:161–167. [DOI] [PubMed] [Google Scholar]
- Otsui K, Inoue N, Kobayashi S, Shiraki R, Honjo T, Takahashi M, Hirata K, Kawashima S, Yokoyama M. (2007) Enhanced expression of TLR4 in smooth muscle cells in human atherosclerotic coronary arteries. Heart Vessels 22:416–422. [DOI] [PubMed] [Google Scholar]
- Pan LN, Zhu W, Li Y, Xu XL, Guo LJ, Lu Q, Wang J. (2014) Astrocytic Toll-like receptor 3 is associated with ischemic preconditioning-induced protection against brain ischemia in rodents. PLoS One 9:e99526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279:7370–7377. [DOI] [PubMed] [Google Scholar]
- Pasterkamp G, Van Keulen JK, De Kleijn DP. (2004) Role of Toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur J Clin Invest 34:328–334. [DOI] [PubMed] [Google Scholar]
- Patel MC, Shirey KA, Pletneva LM, Boukhvalova MS, Garzino-Demo A, Vogel SN, Blanco JC. (2014) Novel drugs targeting Toll-like receptors for antiviral therapy. Future Virol 9:811–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao W, Ru LW, Piepenbrink KH, Sundberg EJ, Vogel SN, Toshchakov VY. (2013a) Recruitment of TLR adapter TRIF to TLR4 signaling complex is mediated by the second helical region of TRIF TIR domain. Proc Natl Acad Sci USA 110:19036–19041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao W, Vogel SN, Toshchakov VY. (2013b) Inhibition of TLR4 signaling by TRAM-derived decoy peptides in vitro and in vivo. J Immunol 190:2263–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Wu R, Lemne C, Kiessling R, de Faire U, Frostegård J. (2000) Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension 36:303–307. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088. [DOI] [PubMed] [Google Scholar]
- Pons H, Ferrebuz A, Quiroz Y, Romero-Vasquez F, Parra G, Johnson RJ, Rodriguez-Iturbe B. (2013) Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. Am J Physiol Renal Physiol 304:F289–F299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradillo JM, Fernández-López D, García-Yébenes I, Sobrado M, Hurtado O, Moro MA, Lizasoain I. (2009) Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J Neurochem 109:287–294. [DOI] [PubMed] [Google Scholar]
- Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. (2008) Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation 118:1276–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LH, Wang Y, Gao F, Zhang C, Ding SF, Ni M, Feng JB, Zhang Y. (2009) Enhanced stabilization of atherosclerotic plaques in apolipoprotein E-knockout mice by combinatorial Toll-like receptor-1 and -2 gene silencing. Hum Gene Ther 20:739–750. [DOI] [PubMed] [Google Scholar]
- Qian Y, Commane M, Ninomiya-Tsuji J, Matsumoto K, Li X. (2001) IRAK-mediated translocation of TRAF6 and TAB2 in the interleukin-1-induced activation of NFkappa B. J Biol Chem 276:41661–41667. [DOI] [PubMed] [Google Scholar]
- Qiang X, Yang WL, Wu R, Zhou M, Jacob A, Dong W, Kuncewitch M, Ji Y, Yang H, Wang H, et al. (2013) Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med 19:1489–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Zhang X, Zhu C, Dong L, Wang L, Zhang X, Xing Y, Wang C, Ji Y, Cao X. (2012) Luteolin downregulates TLR4, TLR5, NF-κB and p-p38MAPK expression, upregulates the p-ERK expression, and protects rat brains against focal ischemia. Brain Res 1448:71–81. [DOI] [PubMed] [Google Scholar]
- Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. (2008) Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab 28:927–938. [DOI] [PubMed] [Google Scholar]
- Qiu J, Xu J, Zheng Y, Wei Y, Zhu X, Lo EH, Moskowitz MA, Sims JR. (2010) High-mobility group box 1 promotes metalloproteinase-9 upregulation through Toll-like receptor 4 after cerebral ischemia. Stroke 41:2077–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi ST, Larivière L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. (1999) Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med 189:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan T, Nally K, Carmody R, Houston A, Shanahan F, Macsharry J, Brint E. (2013) Identification of TLR10 as a key mediator of the inflammatory response to Listeria monocytogenes in intestinal epithelial cells and macrophages. J Immunol 191:6084–6092. [DOI] [PubMed] [Google Scholar]
- Reilkoff RA, Bucala R, Herzog EL. (2011) Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol 11:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly M, Miller RM, Thomson MH, Patris V, Ryle P, McLoughlin L, Mutch P, Gilboy P, Miller C, Broekema M, et al. (2013) Randomized, double-blind, placebo-controlled, dose-escalating phase I, healthy subjects study of intravenous OPN-305, a humanized anti-TLR2 antibody. Clin Pharmacol Ther 94:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. (2005) Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev 204:27–42. [DOI] [PubMed] [Google Scholar]
- Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. (2005) The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci USA 102:9577–9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues FL, Silva LE, Hott SC, Bomfim GF, da Silva CA, Fazan R, Jr, Resstel LB, Tostes RC, Carneiro FS. (2015) Toll-like receptor 9 plays a key role in the autonomic cardiac and baroreflex control of arterial pressure. Am J Physiol Regul Integr Comp Physiol 308:R714–R723. [DOI] [PubMed] [Google Scholar]
- Rosenfeld ME, Campbell LA. (2011) Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost 106:858–867. [DOI] [PubMed] [Google Scholar]
- Rossignol DP, Lynn M. (2002) Antagonism of in vivo and ex vivo response to endotoxin by E5564, a synthetic lipid A analogue. J Endotoxin Res 8:483–488. [DOI] [PubMed] [Google Scholar]
- Rossignol DP, Wasan KM, Choo E, Yau E, Wong N, Rose J, Moran J, Lynn M. (2004) Safety, pharmacokinetics, pharmacodynamics, and plasma lipoprotein distribution of eritoran (E5564) during continuous intravenous infusion into healthy volunteers. Antimicrob Agents Chemother 48:3233–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DC, McGettrick AF, Latz E, Monks BG, Gay NJ, Yamamoto M, Akira S, O’Neill LA, Fitzgerald KA, Golenbock DT. (2006) The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc Natl Acad Sci USA 103:6299–6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckdeschel K, Pfaffinger G, Haase R, Sing A, Weighardt H, Häcker G, Holzmann B, Heesemann J. (2004) Signaling of apoptosis through TLRs critically involves toll/IL-1 receptor domain-containing adapter inducing IFN-beta, but not MyD88, in bacteria-infected murine macrophages. J Immunol 173:3320–3328. [DOI] [PubMed] [Google Scholar]
- Sagan L. (1967) On the origin of mitosing cells. J Theor Biol 14:255–274. [DOI] [PubMed] [Google Scholar]
- Salagianni M, Galani IE, Lundberg AM, Davos CH, Varela A, Gavriil A, Lyytikäinen LP, Lehtimäki T, Sigala F, Folkersen L, et al. (2012) Toll-like receptor 7 protects from atherosclerosis by constraining “inflammatory” macrophage activation. Circulation 126:952–962. [DOI] [PubMed] [Google Scholar]
- Sallenave JM, Shapiro S. (2008) Proteases and antiproteases in development, homeostasis and disease: The old, the new, and the unknown.... Int J Biochem Cell Biol 40:1066–1067. [DOI] [PubMed] [Google Scholar]
- Sansing LH, Harris TH, Welsh FA, Kasner SE, Hunter CA, Kariko K. (2011) Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Ann Neurol 70:646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, Akira S. (2003) Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol 171:4304–4310. [DOI] [PubMed] [Google Scholar]
- Satoh M, Takahashi Y, Tabuchi T, Tamada M, Takahashi K, Itoh T, Morino Y, Nakamura M. (2015) Circulating Toll-like receptor 4-responsive microRNA panel in patients with coronary artery disease: results from prospective and randomized study of treatment with renin-angiotensin system blockade. Clin Sci (Lond) 128:483–491. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Götte M, et al. (2005) The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest 115:2223–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. (2006) Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177:1272–1281. [DOI] [PubMed] [Google Scholar]
- Schelbergen RF, Blom AB, van den Bosch MH, Slöetjes A, Abdollahi-Roodsaz S, Schreurs BW, Mort JS, Vogl T, Roth J, van den Berg WB, et al. (2012) Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum 64:1477–1487. [DOI] [PubMed] [Google Scholar]
- Schoneveld AH, Hoefer I, Sluijter JP, Laman JD, de Kleijn DP, Pasterkamp G. (2008) Atherosclerotic lesion development and Toll like receptor 2 and 4 responsiveness. Atherosclerosis 197:95–104. [DOI] [PubMed] [Google Scholar]
- Schultz K, Murthy V, Tatro JB, Beasley D. (2007) Endogenous interleukin-1 alpha promotes a proliferative and proinflammatory phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 292:H2927–H2934. [DOI] [PubMed] [Google Scholar]
- Sheedy FJ, O’Neill LA. (2008) Adding fuel to fire: microRNAs as a new class of mediators of inflammation. Ann Rheum Dis 67 (Suppl 3):iii50–iii55. [DOI] [PubMed] [Google Scholar]
- Sherer Y, Gerli R, Gilburd B, Bartoloni Bocci E, Vaudo G, Mannarino E, Shoenfeld Y. (2007) Thickened carotid artery intima-media in rheumatoid arthritis is associated with elevated anticardiolipin antibodies. Lupus 16:259–264. [DOI] [PubMed] [Google Scholar]
- Shi H, Gabarin N, Hickey E, Askalan R. (2013) TLR-3 receptor activation protects the very immature brain from ischemic injury. J Neuroinflammation 10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Cai Z, Sanchez A, Zhang T, Wen S, Wang J, Yang J, Fu S, Zhang D. (2011) A novel Toll-like receptor that recognizes vesicular stomatitis virus. J Biol Chem 286:4517–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, Sekiya T, Ooboshi H, Kitazono T, Yanagawa T, et al. (2012) Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med 18:911–917. [DOI] [PubMed] [Google Scholar]
- Shichita T, Ito M, Yoshimura A. (2014) Post-ischemic inflammation regulates neural damage and protection. Front Cell Neurosci 8:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, et al. (2006a) Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation 114(1, Suppl)I270–I274. [DOI] [PubMed] [Google Scholar]
- Shimamoto A, Pohlman TH, Shomura S, Tarukawa T, Takao M, Shimpo H. (2006b) Toll-like receptor 4 mediates lung ischemia-reperfusion injury. Ann Thorac Surg 82:2017–2023. [DOI] [PubMed] [Google Scholar]
- Shintani Y, Kapoor A, Kaneko M, Smolenski RT, D’Acquisto F, Coppen SR, Harada-Shoji N, Lee HJ, Thiemermann C, Takashima S, et al. (2013) TLR9 mediates cellular protection by modulating energy metabolism in cardiomyocytes and neurons. Proc Natl Acad Sci USA 110:5109–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota H, Tross D, Klinman DM. (2015) CpG Oligonucleotides as Cancer Vaccine Adjuvants. Vaccines (Basel) 3:390–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MV, Abboud FM. (2014) Toll-like receptors and hypertension. Am J Physiol Regul Integr Comp Physiol 307:R501–R504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MV, Chapleau MW, Harwani SC, Abboud FM. (2014) The immune system and hypertension. Immunol Res 59:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi L, Gelosa P, Guerrini U, Banfi C, Crippa V, Brioschi M, Gianazza E, Nobili E, Gianella A, de Gasparo M, et al. (2004) Anti-inflammatory effects of AT1 receptor blockade provide end-organ protection in stroke-prone rats independently from blood pressure fall. J Pharmacol Exp Ther 311:989–995. [DOI] [PubMed] [Google Scholar]
- Smiley ST, King JA, Hancock WW. (2001) Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 167:2887–2894. [DOI] [PubMed] [Google Scholar]
- Sorrentino R, Morello S, Chen S, Bonavita E, Pinto A. (2010) The activation of liver X receptors inhibits toll-like receptor-9-induced foam cell formation. J Cell Physiol 223:158–167. [DOI] [PubMed] [Google Scholar]
- Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Kränkel N, Kania G, Zewinger S, Akhmedov A, Shi Y, et al. (2013) Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38:754–768. [DOI] [PubMed] [Google Scholar]
- Speir E, Modali R, Huang ES, Leon MB, Shawl F, Finkel T, Epstein SE. (1994) Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265:391–394. [DOI] [PubMed] [Google Scholar]
- Stacey KJ, Young GR, Clark F, Sester DP, Roberts TL, Naik S, Sweet MJ, Hume DA. (2003) The molecular basis for the lack of immunostimulatory activity of vertebrate DNA. J Immunol 170:3614–3620. [DOI] [PubMed] [Google Scholar]
- Stack J, Haga IR, Schröder M, Bartlett NW, Maloney G, Reading PC, Fitzgerald KA, Smith GL, Bowie AG. (2005) Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J Exp Med 201:1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark KR, Yeager ME, El Kasmi KC, Nozik-Grayck E, Gerasimovskaya EV, Li M, Riddle SR, Frid MG. (2013) The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol 75:23–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, Lessov NS, Simon RP, Stenzel-Poore MP. (2008) Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab 28:1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, et al. (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 11:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll LL, Denning GM, Weintraub NL. (2004) Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol 24:2227–2236. [DOI] [PubMed] [Google Scholar]
- Stoll LL, Denning GM, Weintraub NL. (2006) Endotoxin, TLR4 signaling and vascular inflammation: potential therapeutic targets in cardiovascular disease. Curr Pharm Des 12:4229–4245. [DOI] [PubMed] [Google Scholar]
- Stunz LL, Lenert P, Peckham D, Yi AK, Haxhinasto S, Chang M, Krieg AM, Ashman RF. (2002) Inhibitory oligonucleotides specifically block effects of stimulatory CpG oligonucleotides in B cells. Eur J Immunol 32:1212–1222. [DOI] [PubMed] [Google Scholar]
- Su X, Ao L, Shi Y, Johnson TR, Fullerton DA, Meng X. (2011) Oxidized low density lipoprotein induces bone morphogenetic protein-2 in coronary artery endothelial cells via Toll-like receptors 2 and 4. J Biol Chem 286:12213–12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Yu Z, Ye X, Zou S, Li H, Yu D, Wu H, Chen Y, Dore J, Clément K, et al. (2010) A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care 33:1925–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Sursal T, Adibnia Y, Zhao C, Zheng Y, Li H, Otterbein LE, Hauser CJ, Itagaki K. (2013) Mitochondrial DAMPs increase endothelial permeability through neutrophil dependent and independent pathways. PLoS One 8:e59989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Suzuki S, Duncan GS, Millar DG, Wada T, Mirtsos C, Takada H, Wakeham A, Itie A, Li S, et al. (2002) Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature 416:750–756. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. (2006) NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 103:12481–12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Shibata T, Akashi-Takamura S, Kiyokawa T, Wakabayashi Y, Tanimura N, Kobayashi T, Matsumoto F, Fukui R, Kouro T, et al. (2007) A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J Exp Med 204:2963–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki I, Chobanian AV, Mamuya WS, Brecher P. (1992) Hypertension induces alternatively spliced forms of fibronectin in rat aorta. Hypertension 20:20–25. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. (2005) Toll-like receptors in innate immunity. Int Immunol 17:1–14. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. (2010) Pattern recognition receptors and inflammation. Cell 140:805–820. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Kawai T, Mühlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. (2001) Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol 13:933–940. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Takeda K, Hoshino K, Adachi O, Ogawa T, Akira S. (2000) Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int Immunol 12:113–117. [DOI] [PubMed] [Google Scholar]
- Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. (2004) Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood 104:11–18. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, et al. (2007) Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA 104:13798–13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Yeh SJ, Li YI, Wang YC, Baik SH, Santro T, Widiapradja A, Manzanero S, Sobey CG, Jo DG, et al. (2013) Evidence for a detrimental role of TLR8 in ischemic stroke. Exp Neurol 250:341–347. [DOI] [PubMed] [Google Scholar]
- Tanji H, Ohto U, Shibata T, Miyake K, Shimizu T. (2013) Structural reorganization of the Toll-like receptor 8 dimer induced by agonistic ligands. Science 339:1426–1429. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. (2004) Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem 279:17079–17084. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Allen JL, Tsen M, Dubnicoff T, Danao J, Liao XC, Cao Z, Wasserman SA. (1999) Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J Immunol 163:978–984. [PubMed] [Google Scholar]
- Thompson JC, Tang T, Wilson PG, Yoder MH, Tannock LR. (2014) Increased atherosclerosis in mice with increased vascular biglycan content. Atherosclerosis 235:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidswell M, Tillis W, Larosa SP, Lynn M, Wittek AE, Kao R, Wheeler J, Gogate J, Opal SM, Eritoran Sepsis Study Group (2010) Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med 38:72–83. [DOI] [PubMed] [Google Scholar]
- Tinsley JH, Chiasson VL, Mahajan A, Young KJ, Mitchell BM. (2009) Toll-like receptor 3 activation during pregnancy elicits preeclampsia-like symptoms in rats. Am J Hypertens 22:1314–1319. [DOI] [PubMed] [Google Scholar]
- Toshchakov VY, Fenton MJ, Vogel SN. (2007) Cutting Edge: Differential inhibition of TLR signaling pathways by cell-permeable peptides representing BB loops of TLRs. J Immunol 178:2655–2660. [DOI] [PubMed] [Google Scholar]
- Toshchakov VY, Szmacinski H, Couture LA, Lakowicz JR, Vogel SN. (2011) Targeting TLR4 signaling by TLR4 Toll/IL-1 receptor domain-derived decoy peptides: identification of the TLR4 Toll/IL-1 receptor domain dimerization interface. J Immunol 186:4819–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou M, Gamper FG, Lepper PM, Mouratis MA, Schumann C, Harokopakis E, Schifferle RE, Hajishengallis G, Triantafilou K. (2007) Lipopolysaccharides from atherosclerosis-associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2-induced inflammatory responses in human vascular endothelial cells. Cell Microbiol 9:2030–2039. [DOI] [PubMed] [Google Scholar]
- Tuominen A, Miller YI, Hansen LF, Kesäniemi YA, Witztum JL, Hörkkö S. (2006) A natural antibody to oxidized cardiolipin binds to oxidized low-density lipoprotein, apoptotic cells, and atherosclerotic lesions. Arterioscler Thromb Vasc Biol 26:2096–2102. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, Matsuda M, Coban C, Ishii KJ, Kawai T, et al. (2005) Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-alpha induction. J Exp Med 201:915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. (2002) HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277:15107–15112. [DOI] [PubMed] [Google Scholar]
- Valkov E, Stamp A, Dimaio F, Baker D, Verstak B, Roversi P, Kellie S, Sweet MJ, Mansell A, Gay NJ, et al. (2011) Crystal structure of Toll-like receptor adaptor MAL/TIRAP reveals the molecular basis for signal transduction and disease protection. Proc Natl Acad Sci USA 108:14879–14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tassell BW, Seropian IM, Toldo S, Salloum FN, Smithson L, Varma A, Hoke NN, Gelwix C, Chau V, Abbate A. (2010) Pharmacologic inhibition of myeloid differentiation factor 88 (MyD88) prevents left ventricular dilation and hypertrophy after experimental acute myocardial infarction in the mouse. J Cardiovasc Pharmacol 55:385–390. Erratum in: J Cardiovasc Pharmacol (2011) 57(2):272. Note: Dosage error in article text). [DOI] [PubMed] [Google Scholar]
- Varagic J, Frohlich ED, Susic D, Ahn J, Matavelli L, López B, Díez J. (2008) AT1 receptor antagonism attenuates target organ effects of salt excess in SHRs without affecting pressure. Am J Physiol Heart Circ Physiol 294:H853–H858. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. (2010) Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328:228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink A, de Kleijn DP, Pasterkamp G. (2004) Functional role for toll-like receptors in atherosclerosis and arterial remodeling. Curr Opin Lipidol 15:515–521. [DOI] [PubMed] [Google Scholar]
- Vink A, Schoneveld AH, van der Meer JJ, van Middelaar BJ, Sluijter JP, Smeets MB, Quax PH, Lim SK, Borst C, Pasterkamp G, et al. (2002) In vivo evidence for a role of toll-like receptor 4 in the development of intimal lesions. Circulation 106:1985–1990. [DOI] [PubMed] [Google Scholar]
- Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. (2003) SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol 4:920–927. [DOI] [PubMed] [Google Scholar]
- Walko TD, 3rd, Bola RA, Hong JD, Au AK, Bell MJ, Kochanek PM, Clark RS, Aneja RK. (2014) Cerebrospinal fluid mitochondrial DNA: a novel DAMP in pediatric traumatic brain injury. Shock 41:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Feng Y, Zhang M, Zou L, Li Y, Buys ES, Huang P, Brouckaert P, Chao W. (2011a) Toll-like receptor 4 signaling confers cardiac protection against ischemic injury via inducible nitric oxide synthase- and soluble guanylate cyclase-dependent mechanisms. Anesthesiology 114:603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Lerman A, Herrmann J. (2015) Dysfunction of the ubiquitin-proteasome system in atherosclerotic cardiovascular disease. Am J Cardiovasc Dis 5:83–100. [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li YL, Zhang CC, Cui W, Wang X, Xia Y, Du J, Li HH. (2014) Inhibition of Toll-like receptor 2 reduces cardiac fibrosis by attenuating macrophage-mediated inflammation. Cardiovasc Res 101:383–392. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang MX, Meng X, Liu FQ, Yu GS, Zhang C, Sun T, Wang XP, Li L, Wang YY, et al. (2011b) Atorvastatin suppresses LPS-induced rapid upregulation of Toll-like receptor 4 and its signaling pathway in endothelial cells. Am J Physiol Heart Circ Physiol 300:H1743–H1752. [DOI] [PubMed] [Google Scholar]
- Wang YC, Wang PF, Fang H, Chen J, Xiong XY, Yang QW. (2013) Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke 44:2545–2552. [DOI] [PubMed] [Google Scholar]
- Wenceslau CF, McCarthy CG, Szasz T, Goulopoulou S, Webb RC. (2015) Mitochondrial N-formyl peptides induce cardiovascular collapse and sepsis-like syndrome. Am J Physiol Heart Circ Physiol 308:H768–H777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenceslau CF, McCarthy CG, Szasz T, Spitler K, Goulopoulou S, Webb RC, Working Group on DAMPs in Cardiovascular Disease (2014) Mitochondrial damage-associated molecular patterns and vascular function. Eur Heart J 35:1172–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche H, Gao X, Li X, Kirschning CJ, Stark GR, Cao Z. (1999) IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J Biol Chem 274:19403–19410. [DOI] [PubMed] [Google Scholar]
- Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. (1997) MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7:837–847. [DOI] [PubMed] [Google Scholar]
- West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, Podrez EA, Salomon RG, Byzova TV. (2010) Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 467:972–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp WF, Vermeij JD, Zock E, Hooijenga IJ, Kruyt ND, Bosboom HJ, Kwa VI, Weisfelt M, Remmers MJ, ten Houten R, et al. PASS investigators (2015) The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet 385:1519–1526. [DOI] [PubMed] [Google Scholar]
- Wille-Reece U, Flynn BJ, Loré K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. (2005) HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci USA 102:15190–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G, Bohlender J, Bondeva T, Roger T, Thaiss F, Wenzel UO. (2006) Angiotensin II upregulates toll-like receptor 4 on mesangial cells. J Am Soc Nephrol 17:1585–1593. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. (2003a) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640–643. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, et al. (2002) Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 420:324–329. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. (2003b) TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol 4:1144–1150. [DOI] [PubMed] [Google Scholar]
- Yang J, Jiang H, Yang J, Ding JW, Chen LH, Li S, Zhang XD. (2009a) Valsartan preconditioning protects against myocardial ischemia-reperfusion injury through TLR4/NF-kappaB signaling pathway. Mol Cell Biochem 330:39–46. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang XD, Yang J, Ding JW, Liu ZQ, Li SG, Yang R. (2011a) The cardioprotective effect of fluvastatin on ischemic injury via down-regulation of toll-like receptor 4. Mol Biol Rep 38:3037–3044. [DOI] [PubMed] [Google Scholar]
- Yang K, He YS, Wang XQ, Lu L, Chen QJ, Liu J, Sun Z, Shen WF. (2011b) MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett 585:854–860. [DOI] [PubMed] [Google Scholar]
- Yang QW, Mou L, Lv FL, Wang JZ, Wang L, Zhou HJ, Gao D. (2005a) Role of Toll-like receptor 4/NF-kappaB pathway in monocyte-endothelial adhesion induced by low shear stress and ox-LDL. Biorheology 42:225–236. [PubMed] [Google Scholar]
- Yang X, Coriolan D, Murthy V, Schultz K, Golenbock DT, Beasley D. (2005b) Proinflammatory phenotype of vascular smooth muscle cells: role of efficient Toll-like receptor 4 signaling. Am J Physiol Heart Circ Physiol 289:H1069–H1076. [DOI] [PubMed] [Google Scholar]
- Yang X, Murthy V, Schultz K, Tatro JB, Fitzgerald KA, Beasley D. (2006) Toll-like receptor 3 signaling evokes a proinflammatory and proliferative phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 291:H2334–H2343. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrançois L, Li Z. (2007) Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity 26:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZC, Wang KS, Wu Y, Zou XQ, Xiang YY, Chen XP, Li YJ. (2009b) Asymmetric dimethylarginine impairs glucose utilization via ROS/TLR4 pathway in adipocytes: an effect prevented by vitamin E. Cell Physiol Biochem 24:115–124. [DOI] [PubMed] [Google Scholar]
- Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, et al. (2005) TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308:1626–1629. [DOI] [PubMed] [Google Scholar]
- Zacharowski K, Frank S, Otto M, Chatterjee PK, Cuzzocrea S, Hafner G, Pfeilschifter J, Thiemermann C. (2000) Lipoteichoic acid induces delayed protection in the rat heart: A comparison with endotoxin. Arterioscler Thromb Vasc Biol 20:1521–1528. [DOI] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. (2011) CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147:868–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Mo M, Ding W, Liu W, Yan D, Deng J, Luo X, Liu J. (2014) High-mobility group box 1 (HMGB1) impaired cardiac excitation-contraction coupling by enhancing the sarcoplasmic reticulum (SR) Ca(2+) leak through TLR4-ROS signaling in cardiomyocytes. J Mol Cell Cardiol 74:260–273. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. (2004) A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303:1522–1526. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ghosh S. (2002) Negative regulation of toll-like receptor-mediated signaling by Tollip. J Biol Chem 277:7059–7065. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fu B, Zhang X, Chen L, Zhang L, Zhao X, Bai X, Zhu C, Cui L, Wang L. (2013) Neuroprotective effect of bicyclol in rat ischemic stroke: down-regulates TLR4, TLR9, TRAF6, NF-κB, MMP-9 and up-regulates claudin-5 expression. Brain Res 1528:80–88. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464:104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, et al. (2007) TLR3 deficiency in patients with herpes simplex encephalitis. Science 317:1522–1527. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ha T, Lu C, Lam F, Liu L, Schweitzer J, Kalbfleisch J, Kao RL, Williams DL, Li C. (2015) Poly (I:C) therapy decreases cerebral ischaemia/reperfusion injury via TLR3-mediated prevention of Fas/FADD interaction. J Cell Mol Med 19:555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BG, Vasilakos JP, Tross D, Smirnov D, Klinman DM. (2014) Combination therapy targeting toll like receptors 7, 8 and 9 eliminates large established tumors. J Immunother Cancer 2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zhao SP, Zhao YH. (2015) MicroRNA-143/-145 in Cardiovascular Diseases. BioMed Res Int 2015:531740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Gardner SE, Clarke MC. (2011) Cell death, damage-associated molecular patterns, and sterile inflammation in cardiovascular disease. Arterioscler Thromb Vasc Biol 31:2781–2786. [DOI] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, Norman JE, Csako G, Waclawiw MA, Shearer GM, Epstein SE. (2000) Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol 85:140–146. [DOI] [PubMed] [Google Scholar]
- Ziegler G, Freyer D, Harhausen D, Khojasteh U, Nietfeld W, Trendelenburg G. (2011) Blocking TLR2 in vivo protects against accumulation of inflammatory cells and neuronal injury in experimental stroke. J Cereb Blood Flow Metab 31:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer S, Steinmetz M, Asdonk T, Motz I, Coch C, Hartmann E, Barchet W, Wassmann S, Hartmann G, Nickenig G. (2011) Activation of endothelial toll-like receptor 3 impairs endothelial function. Circ Res 108:1358–1366. [DOI] [PubMed] [Google Scholar]