Abstract
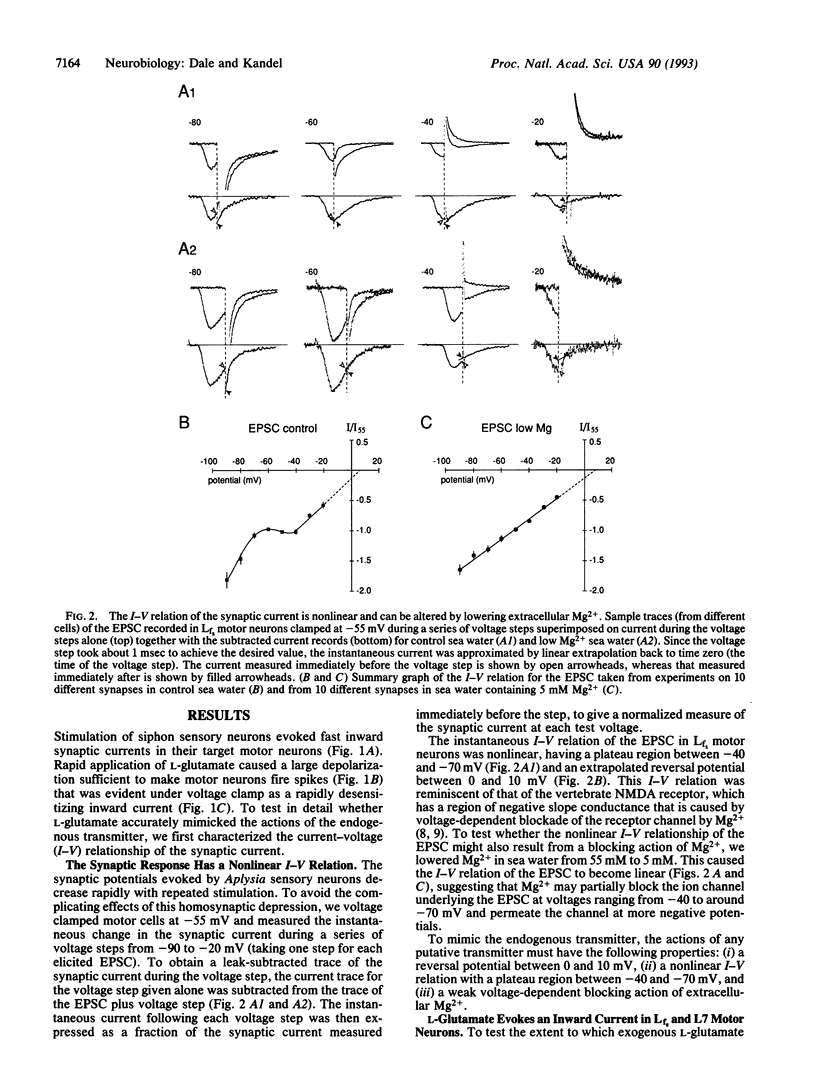
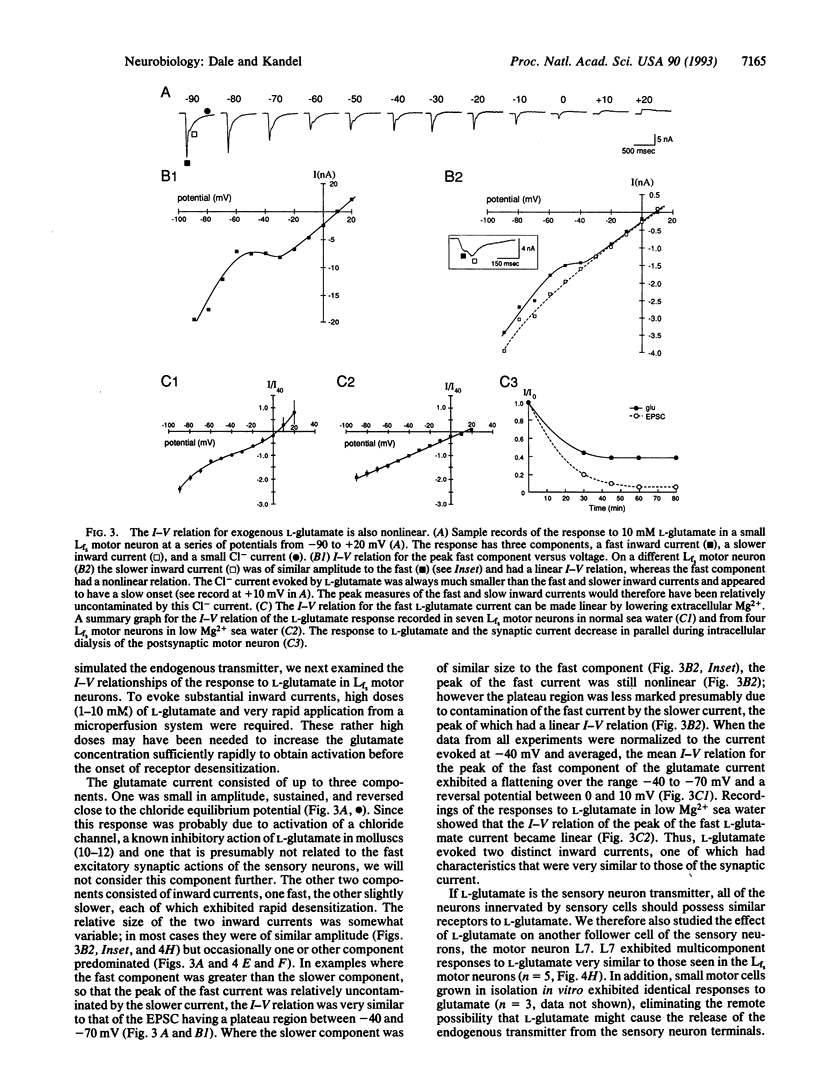
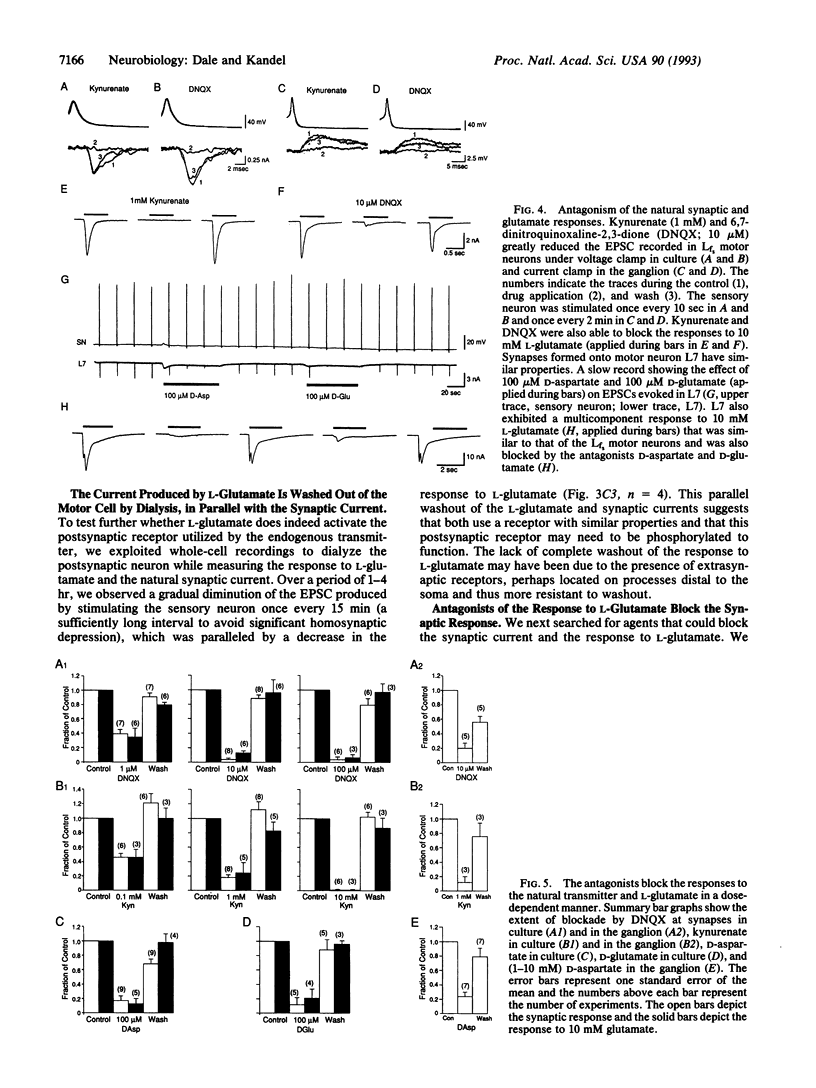
Although modulation of synaptic transmission between Aplysia mechanosensory and motor neurons has been an important model for processes thought to underlie simple forms of learning and memory, the nature of the fast excitatory transmitter utilized by the sensory neurons has remained obscure. To identify the sensory neuron transmitter, we first examined the detailed properties of the synaptic response evoked in motor neurons cocultured with pleural sensory neurons. The excitatory postsynaptic current had a nonlinear current-voltage relation with a reversal potential between 0 and 10 mV and a plateau region between -40 and -70 mV. When the concentration of Mg2+ in the artificial sea water was lowered to 5 mM, the current-voltage relation of the excitatory postsynaptic current became linear, suggesting that Mg2+ blocks the postsynaptic receptor in a voltage-dependent manner. After screening a variety of small molecules, we found that L-glutamate could mimic the actions of the sensory neuron transmitter: responses to L-glutamate also had a reversal potential between 0 and 10 mV and a nonlinear current-voltage relation that could be made linear by lowering external Mg2+. To demonstrate further similarity of action between L-glutamate and the endogenous transmitter, we utilized four antagonists (kynurenate, 6,7-dinitroquinoxaline-2,3-dione, D-aspartate, and D-glutamate) to block in a dose-dependent manner the actions of L-glutamate and the natural transmitter. We therefore suggest that the sensory neurons use a glutamate-like transmitter and favor L-glutamate itself, because no other naturally occurring amino acid that we have studied has had similar actions. As the postsynaptic receptor for the sensory neuron transmitter is weakly blocked in a voltage-dependent manner by Mg2+, the excitatory receptors innervated by the Aplysia sensory neuron may represent a distant precursor of the vertebrate N-methyl-D-aspartate receptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castellucci V. F., Kandel E. R. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V., Kandel E. R. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science. 1976 Dec 10;194(4270):1176–1178. doi: 10.1126/science.11560. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N., Kandel E. R. Facilitatory and inhibitory transmitters modulate spontaneous transmitter release at cultured Aplysia sensorimotor synapses. J Physiol. 1990 Feb;421:203–222. doi: 10.1113/jphysiol.1990.sp017941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer-Linn C., Glantz R. M. An arthropod NMDA receptor. Synapse. 1991 Sep;9(1):35–42. doi: 10.1002/syn.890090106. [DOI] [PubMed] [Google Scholar]
- Szczepaniak A. C., Cottrell G. A. Biphasic action of glutamic acid and synpatic inhibition in an identified serotonin-containing neurone. Nat New Biol. 1973 Jan 10;241(106):62–64. doi: 10.1038/newbio241062a0. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Yarowsky P. J., Carpenter D. O. Aspartate: distinct receptors on Aplysia neurons. Science. 1976 May 21;192(4241):807–809. doi: 10.1126/science.4895. [DOI] [PubMed] [Google Scholar]



