Abstract
Estrogens have profound actions on the structure of the nervous system during development and in adulthood. One of the signature actions of estradiol is to alter the morphology of neural processes. In the hippocampus, estradiol modulates spines and cellular excitability that affect cognitive behaviors. In the hypothalamus, estradiol increases spine density in mediobasal hypothalamic nuclei that regulate reproduction. The hypothalamic arcuate nucleus (ARH), an important site for modulation of female sexual receptivity, has a sexual dimorphism in dendritic spine density that favors females. In the present study, we used both β-actin immunostaining and Golgi staining to visualize estradiol-induced changes in spine density in Long–Evans rats. Golgi impregnation was used to visualize spine shape, and then β-actin immunoreactivity was used as a semiquantitative measure of spine plasticity since actin forms the core of dendritic spines. At 4 h after estradiol treatment, both β-actin immunofluorescence and filopodial spines were increased (from 70.57 ± 1.09% to 78.01 ± 1.05%, p < 0.05). Disruption of estradiol-induced β-actin polymerization with cytochalasin D attenuated lordosis behavior, indicating the importance of estradiol-mediated spinogenesis for female sexual receptivity (81.43 ± 7.05 to 35.00 ± 11.76, p < 0.05). Deactivation of cofilin, an actin depolymerizing factor is required for spinogenesis. Membrane-initiated estradiol signaling involving the metabotropic glutamate receptor 1a was responsible for the phosphorylation and thereby deactivation of cofilin. These data demonstrate that estradiol-induced spinogenesis in the ARH is an important cellular mechanism for the regulation of female sexual behavior.
Introduction
Estradiol regulates CNS functions ranging from reproduction to energy balance to cognition. Underlying these actions, estradiol has the ability to regulate plasticity by affecting dendritic structure, particularly spinogenesis. Although the actions of estradiol on dendritic spines were initially described in the hypothalamus, they have been extensively characterized in the hippocampus (Matsumoto and Arai, 1981; Frankfurt et al., 1990; Gould et al., 1990; Woolley and McEwen, 1992; Calizo and Flanagan-Cato, 2000). In regions associated with the regulation of reproduction in females, such as the ventromedial nucleus (VMH) and the arcuate nucleus (ARH) of the hypothalamus, estradiol increases spine density, suggesting a relationship between these events that has never been tested (Matsumoto and Arai, 1981; Garcia-Segura et al., 1986; Calizo and Flanagan-Cato, 2000). In the ARH, the increase of spines was assumed to mediate estrogen positive feedback, which regulates luteinizing hormone release and ovulation (Matsumoto and Arai, 1981; Parducz et al., 2002, 2006; Csakvari et al., 2007, 2008; Naftolin et al., 2007). Additionally, the ARH is also important for estradiol induction of female sexual receptivity (Mills et al., 2004; Dewing et al., 2007, 2008), actions mediated by membrane-initiated estradiol signaling (MIES), in which estrogen receptor-α (ERα) transactivates a group 1 metabotropic glutamate receptor (mGluR1a) (Dewing et al., 2007, 2008). These events activate an opioid circuit mediated by β-endorphin (β-END) that inhibits μ-opioid receptor (MOR)-expressing neurons in the medial preoptic nucleus (MPN), which project to the VMH. Within minutes of estradiol treatment, this circuit produces a transient inhibition needed to elicit the full display of lordosis behavior 48 h later (Sinchak and Micevych, 2001; Mills et al., 2004).
The present studies test the hypothesis that estradiol induction of spinogenesis in the medial basal hypothalamus (MBH) is necessary for lordosis behavior. Actin remodeling underlies the establishment and maturation of dendritic spines, and β-actin-immunoreactive levels are a measure of dendritic spine density (Matus et al., 1982; Kaech et al., 1997). To begin analyzing the mechanism of estradiol action, phosphorylated cofilin was measured (Schubert et al., 2006; Carlisle et al., 2008). Cofilin, an F-actin severing protein, is inactivated by phosphorylation. Estradiol increased both phosphorylated cofilin and spine density. To test whether p-cofilin was regulated by MIES, mGluR1a was blocked, preventing spinogenesis. Visualization of spines with Golgi impregnation revealed that the majority of spines formed within the first 4 h after estradiol treatment had an immature, filopodial morphology. After the initial increase, the total number of spines did not increase for 48 h, but after 20 h, there was an increase in the number of functional, mushroom-shaped spines (Kasai et al., 2003). Finally, a behavioral study was used to determine whether blocking spinogenesis in the MBH prevented lordosis behavior. Cytochalasin D (CD) blocked estradiol-induced actin polymerization, which significantly attenuated lordosis behavior. Together these results provide strong evidence that MIES regulates spinogenesis, which is critical for the induction of sexual receptivity in the female rat.
Materials and Methods
Animals.
Male and ovariectomized (ovx; by the supplier) female (200–250 g) Long–Evans rats were purchased (Charles River). Upon arrival, rats were housed in a climate-controlled room, two per cage in a 12 h light:12 h dark cycle room (lights on at 6:00 A.M.) and provided food and water ad libitum. All experimental procedures were approved by the Chancellor's Animal Research Committee at the University of California, Los Angeles.
Steroid priming.
Animals were allowed to survive 2–3 weeks after ovx before steroid treatment. For all experiments, 17β-estradiol benzoate (EB) dissolved in safflower oil was injected subcutaneously in a total volume of 0.1 ml per rat. For determination of spine density and morphology, ovx rats injected with either 50 μg of EB or oil vehicle were perfused at the appropriate survival time (4, 20, 30, or 48 h after injection). For cofilin immunostaining, animals were perfused 1 h after a single injection of 5 μg of EB. Females that were treated with cytochalasin D or behaviorally tested received 5 μg of EB every 4 d between 9:00 and 10:00 A.M. for three cycles to mimic the natural estrous cycle of female rats (Micevych et al., 1994). A large 50 μg bolus of EB was used initially to ascertain the effects a single dose of estrogen had in the ARH. Later experiments use a smaller dose to be more physiologically relevant.
Guide cannula implantation surgery.
Bilateral guide cannulae (22 gauge; Plastics One) directed at the ARH (coordinates from bregma: anterior −2.0 mm, lateral 0.8 mm, ventral −6.9 mm from dura; tooth bar: −3.3 mm) or single guide cannulae directed at the lateral ventricle (coordinates from bregma: anterior −1.0 mm, lateral ±1.4 mm, ventral −3.5 mm from dura; tooth bar: −3.3 mm) were implanted using standard stereotaxic procedures while female rats were anesthetized with isoflurane (2–3% in equal parts oxygen and nitrous oxide). Cannulae were secured to the skull with dental acrylic and stainless steel bone screws. Stylets were placed in the guide cannulae, which protruded <0.5 mm beyond the opening of the guide cannulae. Animals were individually housed after surgery, received oral antibiotics (trimethoprim and sulfamethoxazole, 0.4 mg/ml; Hi-Tech Pharmacal) in the drinking water, and allowed to recover 7 d before behavioral testing or infusion.
Microinjection.
For ARH site-specific microinjections 50 nmol of the β-actin polymerization inhibitor, CD (Enzo Life Sciences) was dissolved in DMSO. For infusions into the lateral ventricle, 400 nmol of CD was used. mGluR1a antagonist LY367,385 (400 nmol) was dissolved in 10 mm NaOH and aCSF (1:1, pH 7.8) and was infused into the lateral ventricle. Microinjections/infusions were performed with an infusion pump (Harvard Apparatus) at a rate of 0.5–1.0 μl/min. Microinjection needles (28 gauge) protruded 2 mm beyond the opening of the cannula and were allowed to remain in place for 1 min after infusion to allow for diffusion of drug treatment or control vehicle from the injector. After microinjection, the obturators were reinserted into the guide cannulae and animals returned to their home cage until testing.
Behavioral testing.
Animals received two cycles of EB (5 μg) injections. Thirty minutes before the third EB injection, β-actin polymerization in the ARH was blocked by site-specific injections of CD. Control animals were infused with aCSF rather than CD. Lordosis behavior was tested 30 h after the last injection EB. Sexual receptivity was measured by placing each female rat in a Plexiglas testing arena with a stud male. Sexually experienced males were acclimatized to the arenas for at least 15 min before testing. Males were allowed to mount females 10 times and the number of times the female displayed lordosis (lifting of the head, arching of the back, movement of the tail to one side) was recorded. For each female, the sexual receptivity was quantified as a lordosis quotient (LQ), the number of lordosis displays/number of mounts × 100.
Confirmation of guide cannulae placement.
Animals were anesthetized after the series of behavioral experiments and transcardially perfused with chilled 0.9% saline, followed with a fixative, 4% paraformaldehyde dissolved in 0.2 m Sorenson's phosphate buffer, pH 7.4. Brains were removed and placed in fixative overnight at 4°C and then replaced with 20% sucrose in phosphate buffer for cryoprotection. Brains were blocked, sectioned (20 μm) on a cryostat (Leica CM 1800), and collected into chambers filled with PBS. Sections were mounted onto SuperFrost/Plus slides (Fisher), stained with thionine, dehydrated, and coverslipped with Krystalon (EMD Chemicals). Injection sites were mapped and verified with bright-field illumination. Rats with cannulae that were not positioned in the ARH (i.e., located above, lateral to the ARH, or where microinjections had compromised the wall of the third ventricle) were excluded from the study.
Golgi staining.
Animals were perfused, as described above, 4, 20, 30, and 48 h after a 50 μg EB injection and immediately processed for Golgi staining using the FD Rapid Golgi Stain Kit (FD Neurotechnologies) according to the manufacturer's protocol. After staining, brains were sectioned at 120 μm and direct mounted on gelatin-coated SuperFrost/Plus slides. The sections were then stained, dehydrated, and coverslipped using Permount (Fisher).
Golgi image analysis.
In both analyses, only neurons whose cell bodies could be visualized within the ARH and whose dendrites were unobstructed along their entire visible length were chosen for analysis. Spines were morphologically characterized under 630× magnification (Axioskop 2; Zeiss) and classified as filopodial, stubby, mushroom, or cup shaped (Hering and Sheng, 2001; Bourne and Harris, 2008). Filopodial spines were defined as thin protrusions with a uniform diameter (∼1–4 μm long). Stubby spines were thicker and shorter (∼1–4 μm long with a head of ∼0.5–1 μm diameter). Spines with a thin neck that ended in a bulbous head were classified as mushroom shaped (∼1–2 μm long and 1–2 μm wide), and those with a U-shaped head were classified as cup shaped. Percentages were calculated by totaling the number of each shape and dividing by the total number of spines counted and multiplying by 100.
To determine spine density, Golgi-stained sections were analyzed using a Zeiss Axioskop 2 with at a magnification of 397× equipped with Neurolucida program (MBF Bioscience). The cell body and full length of all dendrites in a section were traced. Spines were placed where they were visualized along the dendrite. Density was normalized as the number of spines per 10 μm of dendrite length.
Immunohistochemistry.
For β-actin staining, animals were perfused 4 h after estradiol injection and immediately processed for immunohistochemistry. Rabbit primary antibody directed against β-actin (1:1000; Abcam) was used. Sections processed for fluorescence were incubated in blocking buffer (tyramide signal amplification kit; NEN Life Science Products) and then in biotin-conjugated goat anti-rabbit IgG (Vector Laboratories; 1:200) for 1 h. Tissue was then washed in Tris-buffered saline and incubated in streptavidin-horseradish peroxidase (NEN Life Science Products; 1:100) for 30 min, washed, and incubated for 5 min in fluorescein-conjugated tyramide (1:50; tyramide signal amplification kit; NEN Life Science Products). Sections were washed again in 0.1 m Tris buffer and mounted on SuperFrost/Plus slides. Mounted sections were air dried and coverslipped using Aqua Polymount mounting medium (Polysciences).
For p-cofilin staining, animals were perfused 1 h after injection and processed for immunohistochemistry. Sections were incubated overnight with an antibody directed against p-cofilin (1:250, Cell Signaling Technology). Immunoreactivity was visualized with diaminobenzidine (DAB) histochemistry kit (VECTASTAIN Elite ABC kit; Vector Laboratories). Sections from control and estradiol-treated rats were incubated in parallel to reduce variability of staining. Mounted sections were air dried and dehydrated before being coverslipped with Krystalon.
Image analysis.
All fluorescent sections were examined using a Zeiss Axioskop 2 equipped with epifluorescent illumination, Axiocam digital camera, and AxioVision digital image analysis system (Carl Zeiss North America). Fluorescein isothiocyanate was imaged with a 488 nm excitation and 550 nm emission filter. Images were adjusted for brightness and contrast using the Zeiss LSM-PC and PhotoShop (version 6.0; Adobe) programs. DAB sections were viewed with bright-field illumination. Pictures were taken using the Axiocam digital camera in grayscale using the same exposure time for all sections without further adjustment.
To determine a relative measurement of immunoreactivity, fluorescent images (×360) of the regions under study were converted to grayscale and adjusted for brightness and contrast in Adobe Photoshop. ImageJ (version 1.32j; NIH) was set to the Pixel Inverter function and calibrated. For calibration, a negative region was measured on each section. For β-actin immunostaining, an area outside the ARH and for p-cofilin the internal capsule was used as the negative control because this region did not respond to estradiol and was found in all sections in which the ARH was present. The p-cofilin optical density (OD) in the internal capsule was found to be similar in all treatment groups (A. Christensen, unpublished observation). For β-actin and p-cofilin immunostaining in the ARH, the OD within a 75 μm circle was measured. The OD measurement from the negative control areas was subtracted to determine the OD of the staining in the ARH.
Western blot.
N-38 cells were cultured in high-glucose DMEM (Invitrogen) containing 10% FBS, 0.15% NaHCO3, and 1% penicillin/streptomycin. Cells were harvested and lysed by pulling through a syringe in RIPA lysis buffer system, which included protease inhibitors (Santa Cruz Biotechnology) supplemented with a protein phosphatase inhibitor set (Millipore). The cells were then centrifuged and the pellet was discarded. Twenty micrograms of protein was loaded onto a 15% polyacrylamide gel and run for 1 h at 120 V. The protein was transferred overnight onto a PVDF membrane. The membrane was blocked for 1 h with 5% milk, then incubated overnight in primary antibody against p-cofilin (1:500) in 5% milk. The membrane was washed in 0.1% TBS-Tween and incubated with peroxidase anti-rabbit secondary for 1 h. The membrane was washed for at least 1 h in TBS-Tween before being rinsed in TBS. It was covered in GE Healthcare ECL for 1 min before being exposed to film for 30 s.
Statistical analysis.
All data are expressed as the mean ± SEM. Mean differences between groups in behavior and p-cofilin data were determined using two-way ANOVAs followed by Bonferroni post hoc analysis in which the main effect or interaction was significant at p < 0.05. Golgi and β-actin data were analyzed by two-tailed t tests and one-way ANOVAs followed by Newman–Keuls post hoc analysis in which the main effect or interaction was significant at p < 0.05. Differences were considered significant at p < 0.05. Statistical analysis was conducted using GraphPad Prism 5 (version 5.02, GraphPad Software) software. The number of animals used in each experiment is specified in Results.
Results
Estradiol induces spinogenesis in the ARH
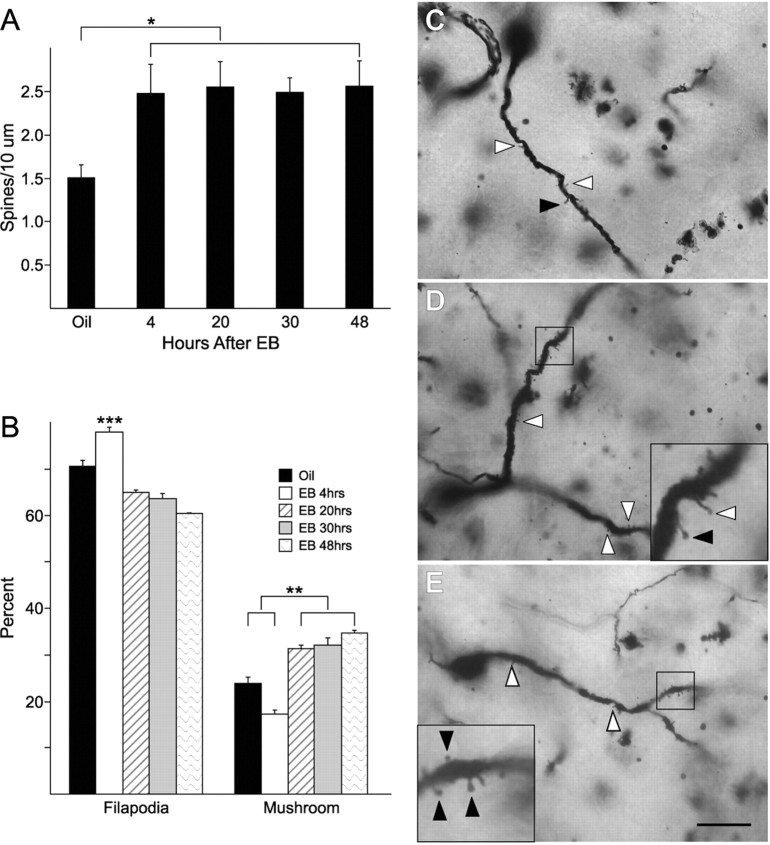
Golgi staining indicated that EB treatment significantly increased the density of spines on neurons in the ARH at all time points examined when compared to control levels (Fig. 1; two-tailed t test, oil vs 4 h EB, p = 0.05; t = 2.284; df = 8; oil vs 20 h, p = 0.02; t = 3.120; df = 6; oil vs 30 h, p = 0.003; t = 4.335; df = 7; oil vs 48 h EB, p = 0.02; t = 3.210; df = 6; n = 4–6 animals per group). After the initial increase at 4 h, the density of dendritic spines did not change at any of the time points examined. On the other hand, the morphology of the spines did change. Spine morphology has been correlated with maturation (Matsuzaki et al., 2001; Kasai et al., 2003; Smith et al., 2003). Filopodial spines are considered more immature than stubby and mushroom-shaped spines and not as likely to be contacted by a presynaptic element (Dailey and Smith, 1996; Ziv and Smith, 1996). At 4 h, a significant increase in the percentage of filopodial spines was associated with a significant decrease in the percentage of mushroom spines compared with controls and with later time points (Fig. 1; one-way ANOVA, p < 0.0001; F = 53.23; df = 4; SNK comparison test, 20 h EB vs 4 h EB, p < 0.0001; q = 13.64; 30 h EB vs 4 h EB, p < 0.0001; q = 15.95; 48 h EB vs 4 h EB, p < 0.0001; q = 18.28). At 20+ h after estradiol treatment, the number of mature mushroom-shaped spines significantly increased (one-way ANOVA, p < 0.0001; F = 40.85; df = 4; SNK comparison test, oil vs 4 h EB, p < 0.0001; q = 5.992; 20 h EB vs 4 h EB, p < 0.0001; q = 12.31; 30 h EB vs 4 h EB, p < 0.0001; q = 13.88; 48 h EB vs 4 h EB, p < 0.0001; q = 15.52). The number of stubby and cup-shaped spines observed was very low and no difference in the numbers of stubby or cup-shaped spines was measured throughout the 48 h time course of the experiment.
Figure 1.
Estradiol increases spine density and maturity. A, Ovx animals treated for 4 h with EB show an increased spine density compared to oil-treated controls. At later time points the density remains elevated (n = 4–6). B, Four hours of EB treatment significantly increases the number of spines with a filopodial appearance in the ARH compared to oil-treated animals, as well as all other EB time points. Spine morphology matured at later time points after estradiol treatment (i.e., 20, 30, and 48 h). Significantly more mushroom spines were present at time points later than 4 h or in the oil-treated controls (n = 4–6). C–E, An oil-treated animal (C), an animal treated with EB for 4 h (D), and an animal treated with EB for 48 h (E). White-filled arrowheads indicate filopodial spines, and black arrowheads indicate mushroom-shaped spines. Insets show close ups of filopodial (in D) and mushroom (in E) shaped spines. Since cup-shaped and stubby spines accounted for <5% of the population of spines in the ARH, they were not included in the figure. Scale bar measures 20 μm. Error bars represent the SEM. *p < 0.05. **p < 0.001. ***p < 0.0001.
CD inhibition of β-actin polymerization decreased spinogenesis
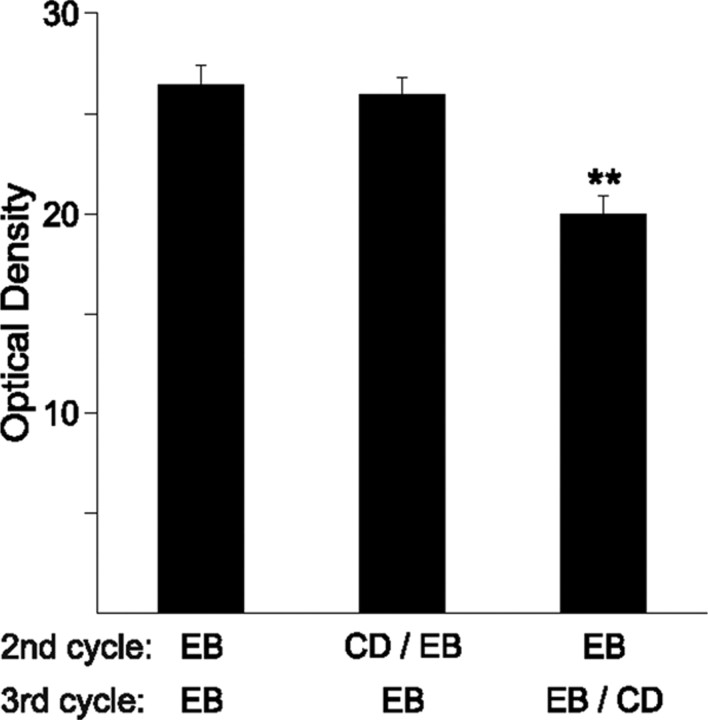
In the gonadally intact female rat, estradiol peaks every 4 d as part of the estrous cycle. Our paradigm of treating animals every 4 d with EB mimics this pattern. To determine whether the EB is inducing new ARH spines during every cycle, CD, a blocker of β-actin polymerization, was infused into the lateral ventricle to avoid damaging the arcuate nucleus. The bulk of neuronal β-actin is located in dendritic spines, where it is the major cytoskeletal component supporting dendritic spines (Hotulainen and Hoogenraad, 2010). Thus, immunohistochemical staining for β-actin was used as a semiquantitative indicator of spine density (Matus et al., 1982; Kaech et al., 1997). In our cyclic paradigm, CD was infused 1 h before the second EB injection or 1 h before the third EB injection. Inhibiting β-actin polymerization with CD during the second cycle did not decrease β-actin density when the rats were treated for a third cycle compared to control animals (Fig. 2). However, infusing CD before the third EB injection decreased the amount of β-actin density, suggesting that during the estrous cycle, EB can induce new spines every 4 d (Fig. 2; one-way ANOVA, p < 0.0001; F = 15.55; SNK comparison test, EB vs third cycle CD, p < 0.0001; q = 6.97; EB vs second cycle CD, p > 0.05; q = 0.494; second cycle CD vs third cycle CD, p < 0.0001; q = 6.79, n = 5–6 animals per group). Moreover, the data support the idea that CD inhibited spine growth acutely without toxic effects to the neurons, and that dendrites retain the ability to continue to make spines with future treatments of EB.
Figure 2.
CD inhibits β-actin polymerization. Each female was given three cycles of 5 μg of EB and perfused 4 h after their final dose. Before either the second or third cycle, some animals received a 400 nmol infusion of the β-actin polymerization inhibitor CD into the lateral ventricle. Animals given CD on the second cycle were able to recover the ability to make new spines by the third cycle and showed an increase in spinogenesis equal to those that had received three doses of EB only. In contrast, CD given on the third cycle, before the final dose of EB had significantly reduced levels of β-actin optical density indicating decreased spinogenesis (n = 5–7). Error bars represent the SEM. **p < 0.001.
Spinogenesis in the MBH is required for female sexual receptivity
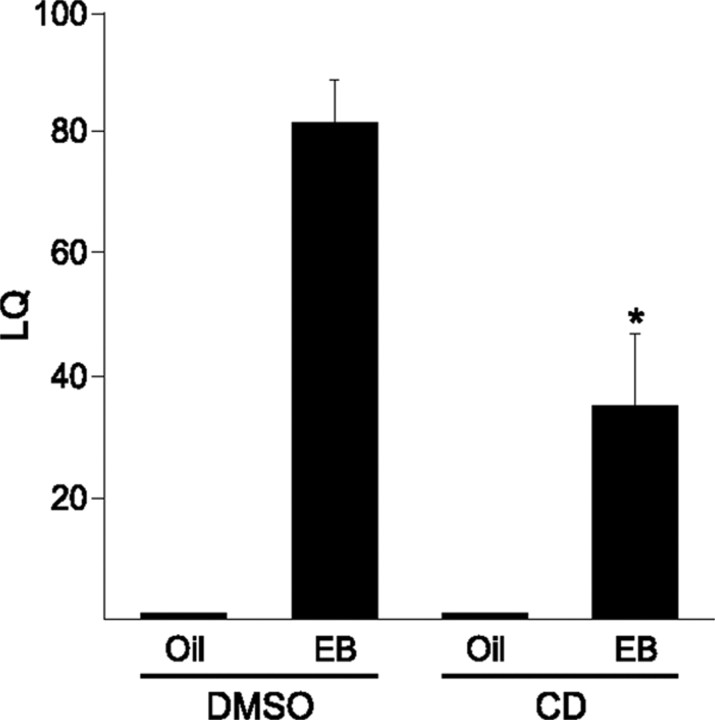
To determine the behavioral consequence of estradiol-induced spinogenesis in the MBH, we tested the lordosis reflex in ovx rats treated with estradiol for 3 cycles. Rats treated this way are maximally receptive (Dewing et al., 2007). Thirty minutes before the third injection, CD or DMSO vehicle was microinfused directly into the ARH. Animals were tested 30 h later. Animals primed with EB and treated with the DMSO vehicle were maximally receptive. Those that received CD microinfusions, however, had significantly attenuated LQs (Fig. 3; two-way ANOVA, drug, p = 0.0052; df = 1; F = 9.843; steroid, p < 0.001; df = 1; F = 61.90; drug × steroid, p = 0.0052; df = 1; F = 9.843). CD infusions without EB treatment had no affect on the receptivity of the animals. These data suggest that estradiol-induced spinogenesis is a necessary component of the neural mechanism controlling female sexual receptivity.
Figure 3.
Spinogenesis is important for female sexual behavior. Ovx animals were given 5 μg of EB every 4 d for three cycles. Thirty minutes before the final estradiol treatment, females had 50 nmol of CD microinfused into the ARH. Thirty hours later, they were tested for sexual receptivity. Those that were given CD and EB had a significantly reduced LQ compared to DMSO + EB controls (n = 4–7). Error bars represent the SEM. *p < 0.01.
MIES regulates cofilin phosphorylation
Dendritic spine development and morphology is dependent on regulating the actin cytoskeleton (Matus, 2000) (for review, see Hotulainen and Hoogenraad, 2010). An important actin regulating protein is cofilin. Cofilin disassembles filamentous actin and is inhibited by phosphorylation. In order for new spines to form, cofilin must be deactivated through phosphorylation (Bamburg, 1999; Meng et al., 2002). When ovx females were injected with estradiol, p-cofilin immunoreactivity increased in the ARH compared with oil-injected controls (Fig. 4; two-tailed t test, p = 0.01; t = 3.113; df = 9; n = 5–6 animals per group). In addition to an increase in OD, there was also an increase in the number of large cells in estradiol-treated animals. These cells were located generally in the ventral and lateral portion of the ARH. The neurons were consistent in both size and location with β-endorphin-positive cells.
Figure 4.
Cofilin is deactivated by estrogen receptor interaction with mGluR1a. A, A Western blot showing a single band for p-cofilin migrating at an apparent molecular weight of ∼19 kDa. B, Ovx animals were injected with 5 μg of EB or oil and perfused 1 h later. Animals that received the mGluR1 antagonist, LY367,385, were infused 1 h before injection. C, D, There is significantly less p-cofilin in the oil- (C) than in the EB- (D) treated animals. This increase was blocked by inhibition of mGluR1, suggesting that the interaction between ERα and mGluR1 is important for the initiation of spinogenesis (n = 5–7). The ARH is outlined by the dotted line. Scale bar measures 100 μm. Error bars represent the SEM. *p < 0.01. 3V, Third ventricle.
To determine whether MIES regulated spinogenesis was part of the mechanism of estradiol-induced lordosis, the interaction of MIES with deactivation of cofilin was examined. Previous work has demonstrated that blockade of mGluR1a with LY367,385 prevented MIES in the ARH and blocked the estradiol-induced lordosis behavior (Dewing et al., 2007). Here, LY367,385 infused 1 h before injection with EB significantly reduced estradiol-induced p-cofilin levels in the ARH (Fig. 4; two-way ANOVA, drug, p = 0.136; df = 1; F = 2.41; steroid, p = 0.0403; df = 1; F = 4.81; drug × steroid, p = 0.0075; df = 1; F = 8.84; n = 5–7 animals per group), suggesting that MIES regulated spinogenesis through a modulation of cofilin activity.
Discussion
The major finding of the present study was that MIES-mediated spinogenesis in the ARH was necessary for the induction of sexual receptivity. Using a well established cyclical administration of estradiol, once every 4 d to mimic changing estradiol levels during the estrous cycle, we observed that estradiol induced new spines every cycle, which is necessary for estradiol induction of lordosis behavior. The initial action of estradiol was to increase the percentage of spines with filopodial morphology. With increasing time after estradiol priming, the percentage of filopodial spines decreased and mushroom shaped spines increased. This shift in morphology suggested that a population of immature filopodial spines matured to mushroom shapes. This is the natural development of spines, which begin as a filopodial extension of a dendrite. If a synaptic partner is found, receptors will be recruited into the spine membrane as well as scaffold proteins that anchor these receptors at the postsynaptic specialization (Matsuzaki et al., 2001; Holtmaat et al., 2006; Knott et al., 2006; Yoshihara et al., 2009). As all of these new proteins are trafficked into the spine, the spine morphology changes—the tip becomes wider with a growing postsynaptic density. Thus, stubby and mushroom spines are considered to be functional, i.e., forming a synapse that processes incoming synaptic signals. Indeed, the present results are consistent with a model in which estradiol treatment induces spinogenesis in a relatively rapid manner, but the appearance of mature spines requires more time and coincides with the period of female sexual receptivity, which can be induced some 20 h after estradiol priming and is readily apparent 30–48 h after estradiol.
There is increasing evidence that estradiol activates cell signaling through transactivation of mGluRs. In the ARH, ERα at the plasma membrane interacts directly with mGluR1a to activate a G-protein signaling cascade that leads to the phosphorylation and activation of the novel protein kinase, PKCθ (Dewing et al., 2007, 2008). This interaction between ERα and mGluR1a has been shown to be important for the control of both female sexual receptivity and the release of neuroprogesterone from astrocytes in the hypothalamus (for review, see Micevych and Mermelstein, 2008; Micevych et al., 2009). In fact, recent work has shown that ERs may be able to interact with several different kinds of mGluRs in brain-region-specific patterns to activate many G-protein-coupled signaling cascades (Meitzen and Mermelstein, 2011).
Several factors point to MIES as the mechanism through which estradiol induced spinogenesis. These results are congruent with our previous work that demonstrated that MIES is an important component of ARH regulation of lordosis behavior (Dewing et al., 2007, 2008). First, estradiol was able to rapidly increase spinogenesis within the ARH. Only 4 h were needed to observe a significant increase in spine density. It is possible that an increase could have been seen even earlier, but we wanted to focus on time points when lordosis is expected (30–48 h) and chose only one time point during the refractory period, before sexual receptivity is expressed (i.e., 4 h). This idea of rapid estradiol action involved in spinogenesis is supported by the observation that estradiol induced p-cofilin within 1 h. Since the formation of spines is dependent on polymerization of G-actin, the primary structural component of dendritic spines, the deactivation of an actin depolymerizing factor is critical for spinogenesis. Second, the phosphorylation of cofilin is attenuated by mGluR1a antagonism. Thus, the mechanism through which estradiol is acting involves the mERα-mGluR1a complex and cell signaling that potentially activates LIM kinase-1, which in turn phosphorylates cofilin, deactivating it and allowing the establishment of new spines (Bamburg, 1999; Meng et al., 2002). Such regulation of the actin cytoskeleton provides a mechanism through which estradiol can induce the formation of filopodial dendritic spines, as observed from our studies in the ARH. Moreover, p-cofilin has been associated with the stabilization of long-term potentiation in the hippocampus synapses through the expansion of synaptic contacts (Fedulov et al., 2007). Therefore, the deactivation of cofilin provides a mechanism to explain the generation and maturation of dendritic spines associated with lordosis behavior, because it may act during both the early increase in spine density and the later maturation of spines. We did not look at later time points to see whether it was still phosphorylated.
Estradiol-stimulated spinogenesis has been well documented in multiple brain areas (Matsumoto and Arai, 1981; Frankfurt et al., 1990; Gould et al., 1990; Woolley and McEwen, 1992; Calizo and Flanagan-Cato, 2000). The increase in spine density in the hippocampus has been shown to affect hippocampus-dependent working memory (Daniel and Dohanich, 2001; Sandstrom and Williams, 2001, 2004). In the ventral striatum, estradiol decreased the spine density in the core of the nucleus accumbens (Matsumoto and Arai, 1981; Frankfurt et al., 1990; Gould et al., 1990; Woolley and McEwen, 1992; Calizo and Flanagan-Cato, 2000). In addition, estradiol was seen to shift the population of spines from a more mature to less mature morphology, suggesting a decrease in synaptic excitability. This outcome is diametrically opposed to our results but suggests that MIES in the striatum is inhibitory. In the ARH, membrane ER interacts with mGluR1a and activates stimulatory cell signaling. In the striatum, on the other hand, MIES involves the mGluR3, which is negatively coupled to adenylyl cyclase and inhibits L-type voltage-gated calcium channels (Mermelstein et al., 1996; Grove-Strawser et al., 2010). Thus, depending on the mGluR transactivated by membrane ER, MIES can either stimulate or inhibit spinogenesis.
Estradiol-regulated spinogenesis in the ARH is a well established effect, originally observed by Matsumoto and Arai (1979). However, this regulation of spine density had not been correlated with behavior. Estradiol induction of ARH spines has been proposed as a mechanism for regulating the estrogen-induced LH surge (Ojeda and Urbanski, 1994), but this has never been formally tested. Our data establish the relevance of spines to behavior by demonstrating that estradiol induction of spines is necessary for sexual receptivity as part of the ARH-MPN-VMH circuit (Mills et al., 2004; Sinchak et al., 2010). The specificity of the ARH effect was demonstrated by our site-specific infusion of CD. Estradiol activated spinogenesis throughout the brain together with all of the lordosis-regulating circuitry, including in the ARH and VMH. But inhibiting spinogenesis specifically within the ARH greatly attenuated the lordosis behavior, suggesting that morphological plasticity in the ARH is necessary for female sexual receptivity.
Our model of estradiol activation of the lordosis-regulating circuit is based on the interaction of NPY and β-endorphin through NPY-Y1 receptors in the ARH (Mills et al., 2004). At the present time, it is difficult to predict whether estradiol-induced spinogenesis occurs in NPY, β-endorphin or as yet an unidentified population of neurons. While the p-cofilin increase in large cells suggests that β-endorphin neurons are increasing spines, it does not rule out an increase in NPY or other cell types. One scenario is that new spines occur predominantly on β-endorphin-containing neurons in the ARH, which would have an enhanced response to NPY. If spines were prevented from forming and subsequently maturing, β-endorphin neurons would be more refractory to the NPY signal. Alternatively, estradiol may stimulate spinogenesis in NPY neurons, which would be more receptive to excitatory stimulation and activate β-endorphin neurons in turn. The ARH also mediates energy balance through NPY and β-endorphin. Estradiol is anorexic and has been reported to reduce NPY mRNA in vivo and inhibit NPY release from immortalized hypothalamic neurons (Silva et al., 2010; Dhillon and Belsham, 2011). In terms of feeding regulation, NPY and β-endorphin neurons appear to have a parallel organization with both sets of neurons affecting melanin-concentrating hormone neurons in the lateral hypothalamus (Schwartz and Gelling, 2002). For sexual receptivity, the NPY and β-endorphin neurons appear to be arranged in series. One likely possibility is that there are two populations of NPY and β-endorphin, one mediating energy balance and the other sexual receptivity. Further studies are needed to resolve this question.
In summary, these experiments have shown that estradiol-mediated plasticity is important for regulating female sexual receptivity. This structural plasticity occurs every 4 d coincident with the rise of estradiol during the estrous cycle, and relies on MIES.
Footnotes
This work was supported by NIH Grants DA013185 (P.M.) and HD007228, a training grant (A.C.). We are grateful to Dr. Larry Hoffman for use of his Neurolucida system.
References
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calizo LH, Flanagan-Cato LM. Estrogen selectively regulates spine density within the dendritic arbor of rat ventromedial hypothalamic neurons. J Neurosci. 2000;20:1589–1596. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle HJ, Manzerra P, Marcora E, Kennedy MB. SynGAP regulates steady-state and activity-dependent phosphorylation of cofilin. J Neurosci. 2008;28:13673–13683. doi: 10.1523/JNEUROSCI.4695-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csakvari E, Hoyk Z, Gyenes A, Garcia-Ovejero D, Garcia-Segura LM, Párducz A. Fluctuation of synapse density in the arcuate nucleus during the estrous cycle. Neuroscience. 2007;144:1288–1292. doi: 10.1016/j.neuroscience.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Csakvari E, Kurunczi A, Hoyk Z, Gyenes A, Naftolin F, Parducz A. Estradiol-induced synaptic remodeling of tyrosine hydroxylase immunopositive neurons in the rat arcuate nucleus. Endocrinology. 2008;149:4137–4141. doi: 10.1210/en.2007-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PG, McEwen BS, Pfaff DW. Localized behavioral effects of tritiated estradiol implants in the ventromedial hypothalamus of female rats. Endocrinology. 1979;104:898–903. doi: 10.1210/endo-104-4-898. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008;149:5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon SS, Belsham DD. Estrogen inhibits NPY secretion through membrane-associated estrogen receptor (ER)-alpha in clonal, immortalized hypothalamic neurons. Int J Obes (Lond) 2011;35:198–207. doi: 10.1038/ijo.2010.124. [DOI] [PubMed] [Google Scholar]
- Fedulov V, Rex CS, Simmons DA, Palmer L, Gall CM, Lynch G. Evidence that long-term potentiation occurs within individual hippocampal synapses during learning. J Neurosci. 2007;27:8031–8039. doi: 10.1523/JNEUROSCI.2003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendocrinology. 1990;51:530–535. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Baetens D, Naftolin F. Synaptic remodelling in arcuate nucleus after injection of estradiol valerate in adult female rats. Brain Res. 1986;366:131–136. doi: 10.1016/0006-8993(86)91287-4. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Fischer M, Doll T, Matus A. Isoform specificity in the relationship of actin to dendritic spines. J Neurosci. 1997;17:9565–9572. doi: 10.1523/JNEUROSCI.17-24-09565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Synaptogenic effect of estrogen on the hypothalamic arcuate nucleus of the adult female rat. Cell Tissue Res. 1979;198:427–433. doi: 10.1007/BF00234187. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Neuronal plasticity in the deafferented hypothalamic arcuate nucleus of adult female rats and its enhancement by treatment with estrogen. J Comp Neurol. 1981;197:197–205. doi: 10.1002/cne.901970203. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A. Actin-based plasticity in dendritic spines. Science. 2000;290:754–758. doi: 10.1126/science.290.5492.754. [DOI] [PubMed] [Google Scholar]
- Matus A, Ackermann M, Pehling G, Byers HR, Fujiwara K. High actin concentrations in brain dendritic spines and postsynaptic densities. Proc Natl Acad Sci U S A. 1982;79:7590–7594. doi: 10.1073/pnas.79.23.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat. 2011 doi: 10.1016/j.jchemneu.2011.02.002. Advance online publication. Retrieved May 3, 2011. doi: 10.1016/j.jchemneu.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, Jackson M, Lu WY, MacDonald JF, Wang JY, Falls DL, Jia Z. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Abelson L, Fok H, Ulibarri C, Priest CA. Gonadal steroid control of preprocholecystokinin mRNA expression in the limbic-hypothalamic circuit: comparison of adult with neonatal steroid treatments. J Neurosci Res. 1994;38:386–398. doi: 10.1002/jnr.490380404. [DOI] [PubMed] [Google Scholar]
- Micevych P, Kuo J, Christensen A. Physiology of membrane oestrogen receptor signalling in reproduction. J Neuroendocrinol. 2009;21:249–256. doi: 10.1111/j.1365-2826.2009.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Garcia-Segura LM, Horvath TL, Zsarnovszky A, Demir N, Fadiel A, Leranth C, Vondracek-Klepper S, Lewis C, Chang A, Parducz A. Estrogen-induced hypothalamic synaptic plasticity and pituitary sensitization in the control of the estrogen-induced gonadotrophin surge. Reprod Sci. 2007;14:101–116. doi: 10.1177/1933719107301059. [DOI] [PubMed] [Google Scholar]
- Ojeda S, Urbanski H. Puberty in the rat. In: Knobil E, Neill J, editors. Physiology of reproduction. New York: Raven; 1994. pp. 453–486. [Google Scholar]
- Parducz A, Hoyk Z, Kis Z, Garcia-Segura LM. Hormonal enhancement of neuronal firing is linked to structural remodelling of excitatory and inhibitory synapses. Eur J Neurosci. 2002;16:665–670. doi: 10.1046/j.1460-9568.2002.02127.x. [DOI] [PubMed] [Google Scholar]
- Parducz A, Hajszan T, Maclusky NJ, Hoyk Z, Csakvari E, Kurunczi A, Prange-Kiel J, Leranth C. Synaptic remodeling induced by gonadal hormones: neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neuroscience. 2006;138:977–985. doi: 10.1016/j.neuroscience.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Schubert V, Da Silva JS, Dotti CG. Localized recruitment and activation of RhoA underlies dendritic spine morphology in a glutamate receptor-dependent manner. J Cell Biol. 2006;172:453–467. doi: 10.1083/jcb.200506136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Gelling RW. Rats lighten up with MCH antagonist. Nat Med. 2002;8:779–781. doi: 10.1038/nm0802-779. [DOI] [PubMed] [Google Scholar]
- Silva LE, Castro M, Amaral FC, Antunes-Rodrigues J, Elias LL. Estradiol-induced hypophagia is associated with the differential mRNA expression of hypothalamic neuropeptides. Braz J Med Biol Res. 2010;43:759–766. doi: 10.1590/s0100-879x2010007500059. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinchak K, Garcia B, Bowlby R, Charukulvanich, Garcia M, Sanathara N. Mu-opioid receptor neurons and opioid receptor-like receptor neurons in the medial preoptic nucleus project to the region of the ventromedial nucleus of the hypothalamus. Soc Neurosci Abstr. 2010;36:88–3. [Google Scholar]
- Smith MA, Ellis-Davies GC, Magee JC. Mechanism of the distance-dependent scaling of Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol. 2003;548:245–258. doi: 10.1113/jphysiol.2002.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y, De Roo M, Muller D. Dendritic spine formation and stabilization. Curr Opin Neurobiol. 2009;19:146–153. doi: 10.1016/j.conb.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]