Abstract
A proteomics approach was used to reveal the up-regulated proteins involved in the targeted mitogen-activated protein kinase (MAPK) signal transduction pathway in DF-1 cells after ALV subgroup J (ALV-J) infection. Next, we found that ALV-J CHN06 strain infection of DF-1 cells correlated with extracellular signal-regulated kinase 2 (ERK2) activation, which was mainly induced within 15 min, a very early stage of infection, and at a late infection stage, from 108 h to 132 h post-infection. Infection with other ALV subgroup (A/B) strains also triggered ERK/MAPK activation. Moreover, when activating ERK2, ALV subgroups A, B and J simultaneously induced the phosphorylation of c-Jun, an AP1 family member and p38 activation but had no obvious effect on JNK activation at either 15 min or 120 h. Interestingly, only PD98059 inhibited the ALV-induced c-Jun phosphorylation while SP600125 or SB203580 had no influence on c-Jun activation. Furthermore, the viral gp85 and gag proteins were found to contribute to ERK2/AP1 activation. Additionally, the specific ERK inhibitor, PD980509, significantly suppressed ALV replication, as evidenced by extremely low levels of ALV promoter activity and ALV-J protein expression. In vivo analysis of ERK2 activation in tumor cells derived from ALV-J-infected chicken demonstrated a strong correlation between ERK/MAPK activation and virus-associated tumorigenesis.
Avian leukosis viruses (ALV), an oncogenic retrovirus, mainly induce neoplastic diseases and other reproduction problems by both vertical and horizontal transmission infection. Based on the identity of viral envelope glycoproteins, ALV is divided into subgroups A, B, C, D, E, J and the recently identified subgroup K1,2. So far, most of isolated and identified ALV in China pertain to subgroups A, B and J inducing disease in the commercial meat and egg type chickens as well as in the Chinese local breeds3,4,5,6. ALV can cause significant economic losses as a result of tumor-induced mortality, serious immunosuppression and reduction of weight gain, egg production and breeding potential. Widespread ALV-J can induce various tumors in chickens, especially myeloid leukosis and hemangioma. Our previous study demonstrated that MYC, TERT, and ZIC1genes were identified as the common insertion sites in ALV-J induced myeloid leukosis, which might be a putative “driver” for the activation of the oncogene7. However, the cellular factors involved in exogenous ALV infection and oncogenesis, especially signal transduction pathways, are yet to be revealed.
Isobaric tags for relative and absolute quantification (iTRAQ) is a stable isotope method for protein measurement by using mass spectrometry8. In the current study, we applied iTRAQ followed by liquid chromatography/tandem mass spectrometry (LC-MS/MS) to compare the protein profile of DF-1 cell line with and without ALV-J infection. Based on the proteome data and Western blot confirmation, we inferred that the up-regulation of mitogen-activated protein kinase1 (MAPK) which is closely related to ERK/MAPK signaling pathway may play a role in ALV-J propagation, cell proliferation and neoplasm formation.
MAPK cascades are composed of four prototype members, the extracellular signal-regulated kinase (ERK) 1/2, p38, the Jun-N-terminal kinase (JNK/SAPK) and ERK5, which are key signaling pathways in the control of cell proliferation, differentiation, apoptosis and immune responses9,10,11,12. Aberrant regulation of MAPK cascades incurs neoplasm and other diseases. In particular, the ERK/MAPK pathway has been the subject of intense study on treatment of cancer12. ERK1/2 is downstream of the growth-factor-stimulated Raf/MEK/ERK signaling cascade, while p38 and JNK/SAPK are activated by apoptosis signal-regulating kinase 113. Activator protein 1 (AP1) regulates a wide range of cellular processes, including cell proliferation, death, survival and differentiation, and is induced by a plethora of physiological stimuli and environmental insults. AP-1, as the downstream of MAPK cascade, can be activated by any or all three main of the MAPK pathways through phosphorylation of distinct substrates14. The AP-1 complex is consisted of heterodimers of Jun (c-Jun, JunB and JunD) and Fos (c-Fos, Fos B, Fra1 and Fra2) family members, or Jun homodimers. In particular, c-Jun has been implicated in events leading to tumor development15,16,17,18.
Maintenance of the oncogenic phenotype depends on the accumulation of genetic changes, as well as epigenetic events such as the induction of specific promotion-relevant effector genes. Certain target genes of the AP-1 transcription factor complex are thought to mediate in neoplastic transformation, although the identity of these genes remains largely unknown19. Vascular endothelial growth factor (VEGF) is a highly specific mitogen for vascular endothelial cells. The expression of VEGF is potentiated in response to hypoxia, by activated oncogenes, and by a variety of cytokines. VEGF induces endothelial cell proliferation, promotes cell migration, and inhibits apoptosis20,21. Activated oncogenes that are part of the ras/MAP-kinase signal transduction pathway can up-regulate VEGF mRNA expression22,23. Since MAPK/AP-1 signal transduction pathway is found to be associated with oncogenesis, ALV-J-induced tumor in chickens may be associated with the activation of MAPK/AP-1 pathway.
In this study, we investigated the role of MAPK/AP-1 regulation during exogenous ALV propagation in vitro and ALV-J oncogenesis in vivo. Our findings extend our current understanding of the exogenous ALV infection and oncogenecity mechanisms.
Results
MAPK protein is up-regulated in ALV-J-infected DF-1 cells
We compared the differences in the levels of the isolated proteins from ALV-J-infected and mock-infected DF-1 cells by quantitative proteomics. Using a strict cutoff value of 1.5-fold for expressed variation24 and using the GO function significant sexual enrichment analysis, we found some differentially expressed proteins involved in the MAPK signaling pathway (Fig. 1). And the up-regulated MAPK protein was further verified by Western blotting (Fig. 2).
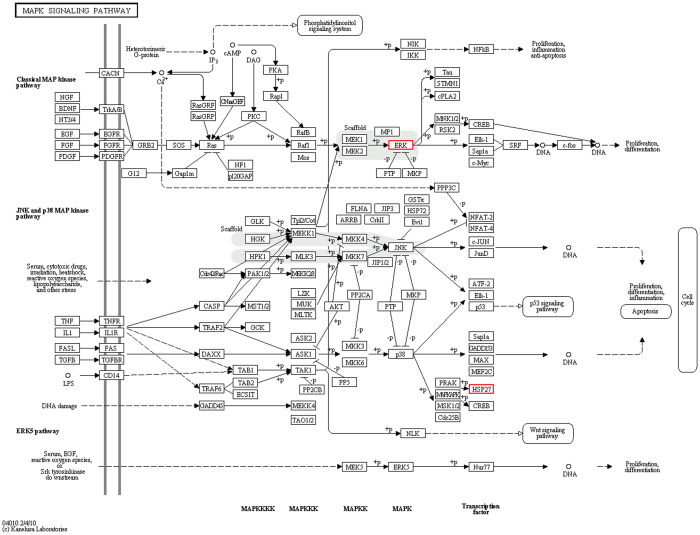
Figure 1. MAPK signaling pathway analysis of proteins that were significantly altered in ALV-J-infected DF-1 cells.
Red means up-regulated proteins. The mapped pathway of the increased proteins in ALV-J-infected DF-1 cells was obtained from the KEGG PATHWAY Database (http://www.genome.jp/kegg-bin/show_pathway?map04010)81.
Figure 2. Confirmation of MAPK protein levels by western blotting.
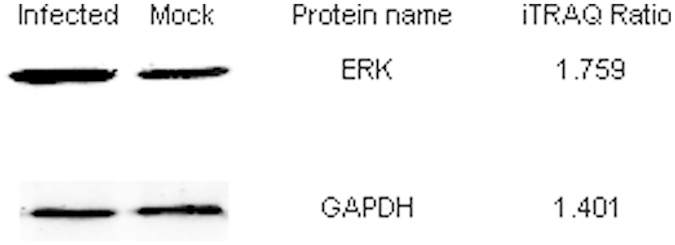
DF-1 cells were infected by live viral stocks of ALV-J CHN06 for 60 h at a multiplicity of infection (MOI) of 1. Mock-infected DF-1 cells were used as a negative control. Cell lysates were subjected to western blotting with rabbit anti-p44/42 MAPK and GAPDH antibodies. iTRAQ ratios are shown on the right side.
Exogenous ALV infection activates ERK2/AP-1 pathway in DF-1 cells
Given the significant correlation between the ERK/MAPK signaling pathway and tumorigenesis, we initially investigated whether ALV-J strain CHN06 leads to activation of this pathway during the infection process. Chickens solely express the 42-kDa ERK2 isoform, but not ERK125,26, which is consistent with our results. In contrast to the mock-treated cell lysate results, CHN06 infection led to a rapid and transient increase in ERK phosphorylation within 15 min p.i., which declined by 30 min p.i., and returned to the basal level by 2 h p.i. ERK phosphorylation increased again at 6 h p.i., subsiding by 12.5 h p.i., and then activation resumed between 108 h and 132 h p.i. (Fig. 3a). These results suggest that ALV-J-induced activation of the MAPK pathway occurred mainly at the early and late infection stages.
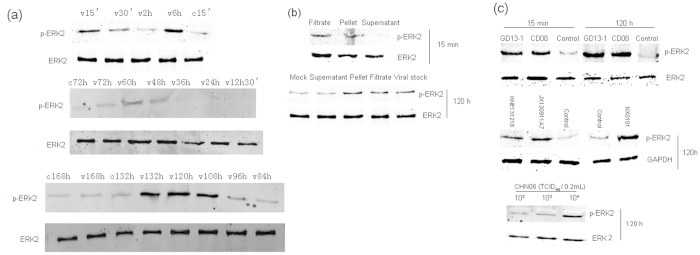
Figure 3. ALV infection activates ERK2 phosphorylation.
DF-1 cells were infected by live viral stocks of ALV-J CHN06 (104 TCID50 0.2 ml−1), ALV-J NX0101 (104 TCID50 0.2 ml−1), ALV-A GD13-1 (104 TCID50 0.2 ml−1), ALV-B CD08 (104 TCID50 0.2 ml−1) or wild strains of ALV-J KNE131218 (S/P ratio of 1.8) and JX130911A7 (S/P ratio of 2.2). Mock-infected DF-1 cells in various phases were used as a negative control. Cell lysates prepared at the indicated times p.i. were subjected to SDS-PAGE and the amounts of phosphorylated ERK1/2 (p-ERK1/2) and total ERK1/2 were evaluated by western blotting. Chickens solely express the 42-kDa ERK2 isoform. (a) The activation profile of ERK2 was explored in CHN06-infected DF-1 cells. (b) Viral stocks of CHN06 were filtered and pelleted. The filtrate, pellet and supernatant were used individually to infect DF-1 cells for 15 min and 120 h. (c) ERK2 phosphorylation was examined in DF-1 cells infected by ALV-A or B, NX0101 or wild strains of ALV-J, or various virus titers of CHN06, at 15 min or at 120 h p.i. The results are representative of two to three independent experiments.
To eliminate any secreted factors in the conditioned media associated with the viral stock that could have triggered the production of phosphorylated ERK (p-ERK), viral stocks were filtered and pelleted by centrifugation, and then suspended and inoculated onto DF-1 cells. Figure 3b demonstrates that the viral filtrate and pellet from the viral stocks of CHN06 induced p-ERK at 15 min and 120 h p.i., showing the same activation pattern as that by the viral stocks from the infected cell culture supernatants. No signal was detected in the cellular extraction of DF-1 cells inoculated with supernatant after centrifugation. These results indicate that CHN06 virus particles, but not the secreted factors associated with the viral stock, activated ERK at the stage of cell infection.
To explore whether other ALV subgroups can also activate ERK/MAPK in DF-1 cells, we examined the level of ERK phosphorylation at 15 min and 120 h p.i. with ALV-A strain GD13-1 or ALV-B strain CD08. Similar to the results of ALV-J strain infection, both GD13-1 and CD08 activated ERK phosphorylation as early as 15 min and as late as 120 h after infection of DF-1 cells. Moreover, ALV-J strain NX0101 and two strains of ALV-J isolated from clinical plasma samples, which were numbered KNE131218 and JX130911A7, increased p-ERK expression at 120 h p.i. Furthermore, the expression levels of ERK phosphorylation increased with the ALV-J strain CHN06 virus titer at 120 h p.i. (Fig. 3c). Accordingly, we concluded that there was no significant difference among the exogenous ALV subgroups in activating ERK/MAPK in DF-1 cells, and that ALV infection led to increased ERK phosphorylation, which was virus titer-dependent.
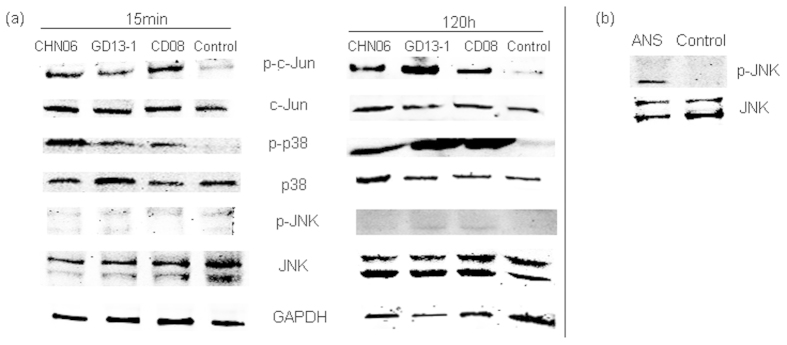
To investigate whether exogenous ALV infection can also activate the other two MAPK pathways in the same way, we examined the level of p38 and JNK phosphorylation at 15 min and 120 h p.i. with ALV-J strain CHN06, ALV-A strain GD13-1 and ALV-B strain CD08. The results showed that the virus stocks of all exogenous ALV infections could activate p-p38, but had no obvious effect on JNK activation at 15 min and 120 h, which was analogous to the mock-treated cell lysate results (Fig. 4a). And JNK activator, Anisomycin indeed activated p-JNK in DF-1 cells (Fig. 4b), which eliminated the factors of reagents and operations in the experiments. Besides, ALV infection increased the phosphorylation of c-Jun at 15 min and 120 h p.i. (Fig. 4a).
Figure 4. ALV infection significantly activates AP-1 and p-p38, but not JNK phosphorylation, at 15 min and 120 h p.i.
DF-1 cells were mock infected or infected by CHN06, GD13-1 or CD08 at 104 TCID50 0.2 ml−1. (a) After 15 min or 120 h, cell lysates were collected and the amounts of p-c-Jun, c-Jun, p-JNK, total JNK, p-p38, total p38 and GAPDH were evaluated by western blotting. (b) DF-1 cells were treated with the JNK activator, Anisomycin (20 μM) for 12 hours according to the instructions, and cell lysates were collected and the amounts of p-JNK, total JNK were evaluated by western blotting. The results are representative of two to three independent experiments. Abbreviations: ANS, Anisomycin.
To explore which one of the three MAPK pathways induced the phosphorylation of c-Jun, we examined the influences of various MAPK pathway inhibitors on c-Jun activation. Additionally, a CCK-8 assay was performed on DF-1 cells treated with different concentrations of inhibitors, to pick the right concentrations without affecting cell viability. Various inhibitors have no cell toxicity except 20 μM LY294002 (Fig. 5). Surprisingly, only PD98059 inhibited the ALV-induced c-Jun phosphorylation while SP600125 or SB203580 had no influence on c-Jun activation at 15 min p.i (Fig. 6a–c). Hence, we deduced that ALV infection activated AP-1 family members mainly through the ERK signaling pathway in DF-1 cells. More importantly, PD98059 simultaneously inhibited the ALV-induced c-Jun phosphorylation and VEGF-A expression at 120 h p.i (Fig. 6d).
Figure 5. Drug cell toxicity test.
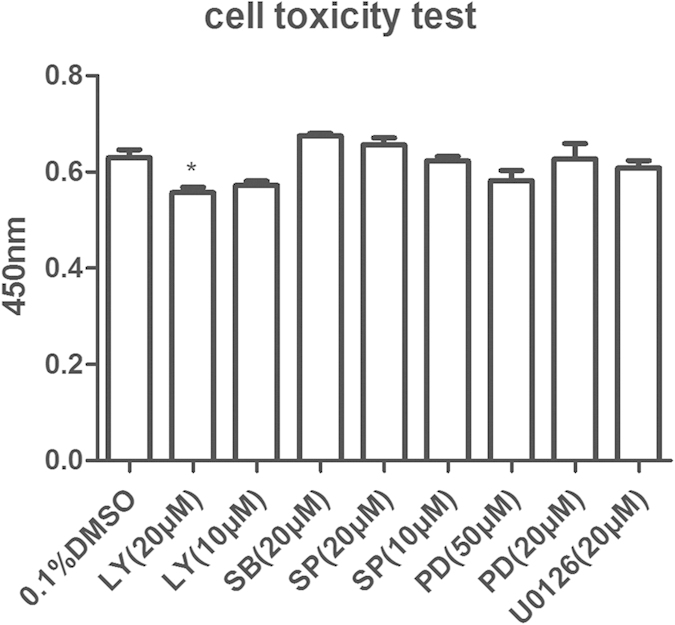
DF-1 cells were treated with LY294002 (10–20 μM), SB203580 (20 μM), SP600125 (10–20 μM), PD98059 (20–50 μM), U0126 (20 μM) or DMSO (0.1%, v/v). Cytotoxicity test was performed with Cell Counting Kit-8 dye. Data are representative of two independent experiments, both performed in triplicate. ***p < 0.001. Error bars indicate SEM. Abbreviations: LY, LY294002; SB, SB203580; SP, SP600125; PD, PD98059.
Figure 6. AP-1 is activated by ERK/MAPK pathway.
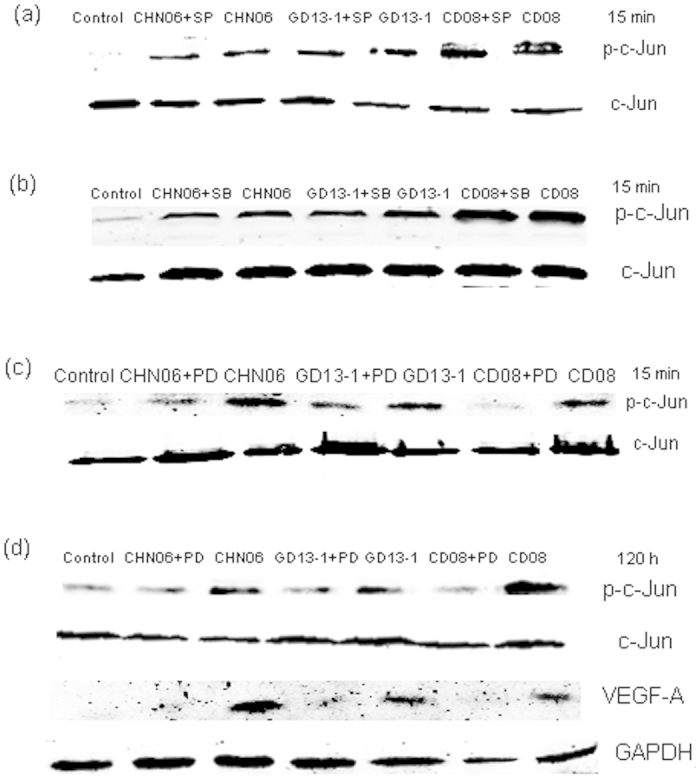
DF-1 cells were preincubated with 20 μM SP600125 (a), 20 μM SB203580 (b), 20 μM PD98059 (c) and 50 μM PD98059(d) for 1 h and subsequently infected with CHN06, GD13-1 or CD08 at 104 TCID50 0.2 ml−1. The inhibitors were in the cell culture fluid all the time. Cell lysates were collected and the amounts of p-c-Jun, c-Jun, VEGF-A and GAPDH were evaluated by western blotting. The results are representative of two independent experiments.
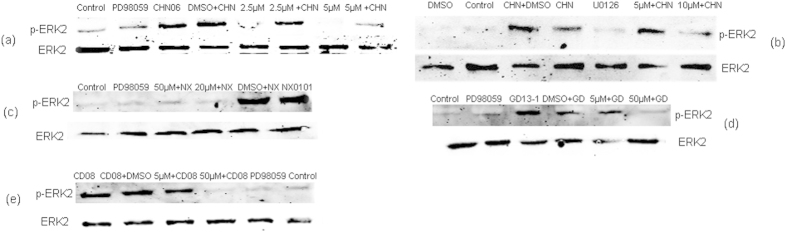
To determine whether the phosphorylation of ERK in DF-1 cells was MEK1/2-dependent or -independent, the role of MEK1/2 in ERK activation following ALV infection was investigated. As expected, ALV infection increased the phosphorylation of ERK, while PD98059 or U0126 pretreatment inhibited the ALV-induced ERK phosphorylation in a concentration-dependent manner (Fig. 7a–e).
Figure 7. ALV infection activates ERK2 phosphorylation in a MEK-dependent manner.
DF-1 cells were preincubated with PD98059 (2.5–50 μM), U0126 (5–10 μM) or DMSO (0.1%, v/v) for 1 h and subsequently infected with CHN06 (a,b), GD13-1 (d) or CD08 (e) for 15 min, or with NX0101 (c) for 120 h, at 104 TCID50 0.2 ml−1. The inhibitors or DMSO were in the cell culture fluid all the time. And the concentration of inhibitor control is the maximum concentration used in each independent experiment. Cell lysates were collected and the amounts of p-ERK2 and total ERK were evaluated by western blotting. The results are representative of two independent experiments. Abbreviations: CHN, CHN06 or HN06; NX, NX0101; GD13-1, GD; CD08, CD.
Gp85 or gag protein alone induces ERK2/AP1 activation
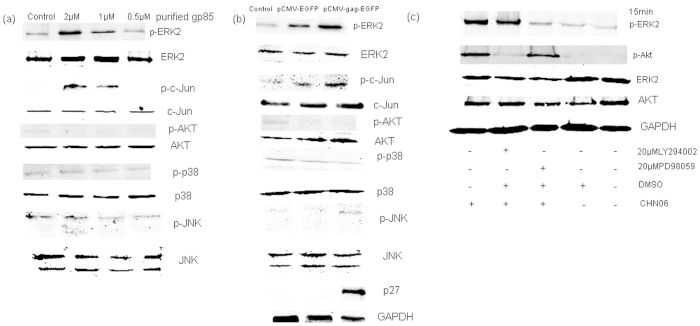
To investigate which viral proteins are involved in ALV activation of the ERK2/AP-1 pathway, we focused on gp85 protein, which is easier to mutate, and gag protein, whose gene is relatively conserved. DF-1 cells were exposed to different concentrations of purified gp85 for 10 min followed by western blot analysis of kinase activation using specific antibodies. As shown in Fig. 8a, gp85 increased the phosphorylation level of ERK2 and c-Jun, and had no obvious effect on Akt, p38 and JNK activation, which confirmed that ALV activates the ERK2/AP1 pathway via gp85 protein.
Figure 8. gp85 or gag protein alone induces ERK2/AP1activation.
Cell lysates were analyzed by western blot analysis of kinase activation using antibodies specific for the phosphorylated forms of the proteins. (a) DF-1 cells were stimulated for 10 min with different concentrations of purified gp85, followed by western blot analysis of phosphorylated proteins and total proteins. (b) DF-1 cells were electroporated with pCMV-gag-EGFP or vector. Seventy-two hours later, cell lysates were subjected to western blotting with phosphorylated proteins, total proteinsp27 or GAPDH antibodies. (c) DF-1 cells were incubated for 1 h in the presence or absence of the PI3K inhibitor, LY294002 (20 μM), the ERK/MAPK inhibitor, PD98059 (20 μM), or DMSO (0.1%, v/v), and subsequently infected with CHN06 for 15 min at 104 TCID50 0.2 ml−1, followed by western blot analysis of ERK and Akt activation. Data shown are representative of two independent experiments
We detected a strong phosphorylated ERK2 signal in both the vector control sample and the gag-expressing sample (data not shown) after transfection with cationic lipid transfection reagents, such as Lipofectamine (Invitrogen), which is consistent with another report27. Thus, we concluded that the cationic lipid reagents boosted signal transduction28, therefore we used electroporation to transiently express gag. Figure 8b shows that electroporation of DF-1 cells with the vector induced visible phosphorylation of ERK2 and c-Jun, however, gag-expressing cells showed a greater degree of ERK2 and c-Jun phosphorylation. In contrast, cells transfected with the control vector or gag-expressing vector did not affect Akt, p38 or JNK activation. This result suggests that gag protein, whose gene is conserved among ALV subgroups, also contributed to the ALV-induced activation of the ERK2/AP1 pathway in DF-1 cells. This is consistent with the findings in Fig. 3c, showing that there was no significant difference between the ALV subgroups in ERK2/AP1 pathway activation.
Lee et al.29 have reported that phosphatidylinositol-3 (PI-3) kinase is an upstream regulator of MAPK activation in monocyte-derived macrophages (MDM) following HIV-1 gp120 stimulation. In our laboratory, we have previously reported that some exogenous ALVs induced Akt phosphorylation within 15 minutes30, while ALV also activated ERK within 15 min at the early infection stage in this study. Therefore, we tested the relationship between PI3K and MAPK activation in response to ALV infection. Consistent with the observations above, CHN06-induced Akt and ERK phosphorylation were PI3K-dependent and MAPKK-dependent, respectively. However, PI3K inhibitor LY294002 had no inhibition effect on ERK activation and MAPKK inhibitor PD98059 could not suppress Akt phosphorylation (Fig. 8c). Taken together, these results suggest that the ALV could induce the activation of the ERK/AP-1 and PI3K/Akt pathways may be in parallel.
The MAPK pathway regulates viral replication and cellular vascular endothelial growth factor (VEGF) mRNA expression
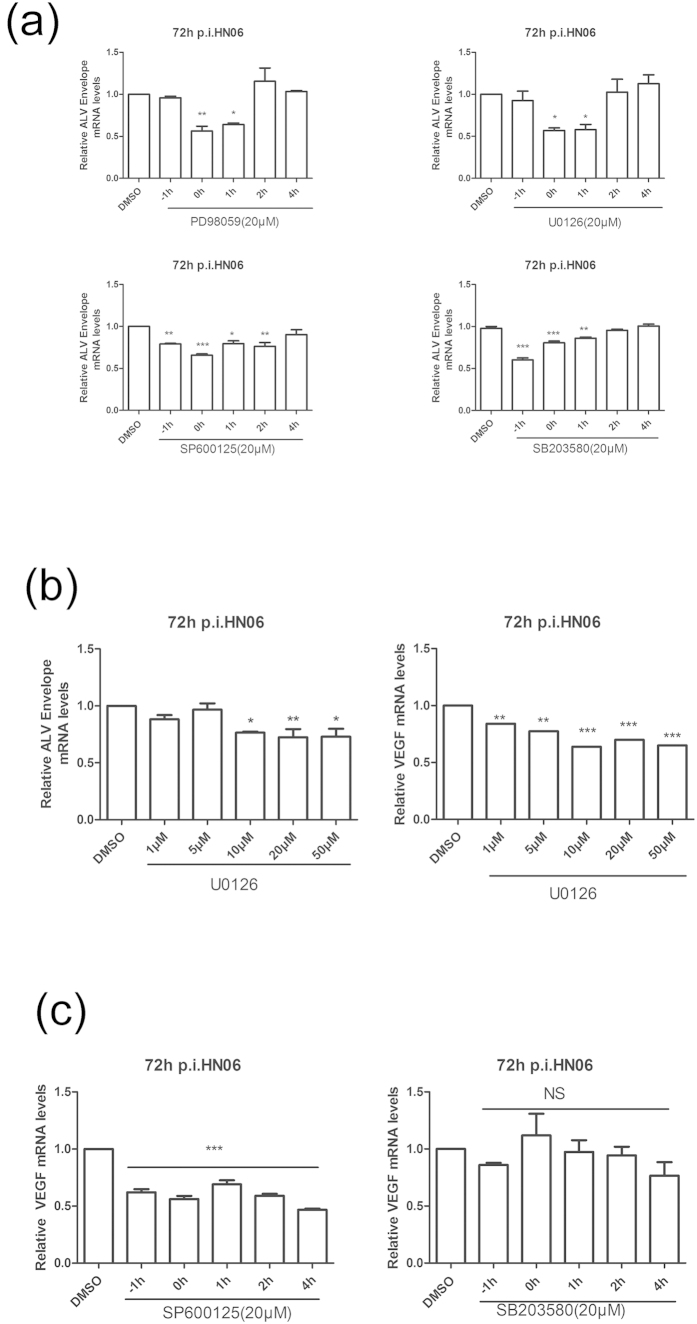
Our foregoing results indicated that ALV infection led to MAPK pathway activation. To further detemine the contribution of each MAPK pathway to ALV infection, we investigated the effect of each MAPK pathway inhibitor on viral production measured by viral RNA transcription and viral protein synthesis. As shown in Fig. 9a, addition of PD98059 or U0126 at 0 h p.i. or 1 h p.i. significantly inhibited viral transcription compared with the vehicle-treated control. Similarly, a significant decrease in viral transcription was observed when SP600125 or SB203580 was added at −1, 0 or 1 h p.i. However, no statistical differences in viral transcription were observed when either inhibitor was added at 4 h p.i. Moreover, addition of U0126 at 0 h p.i had an inhibitory effect on the viral RNA levels of ALV-J and on the ALV-J-induced VEGF mRNA expression in a dose-dependent manner (Fig. 9b). Treatment of cells with inhibitors at −1, 0, 1, 2 and 4 h p.i. with ALV-J, showed that 20 μM SP600125 treatment resulted in a remarkable decrease in VEGF mRNA expression levels, however, incubation with 20 μM SB203580 showed no significant effect (Fig. 9c). These results demonstrated that MAPK primarily acted at an early stage of ALV-J replication cycle. The ERK pathway inhibitor, U0126, and the JNK pathway inhibitor, SP600125, significantly reduced the levels of VEGF mRNA expression, whereas the p38 pathway inhibitor, SB203580, exhibited no statistical difference in VEGF mRNA expression compared with DMSO treatment before ALV-J infection.
Figure 9. MAPK pathway regulates ALV envelope mRNA and cellular VEGF mRNA expression.
(a) DF-1 cells were treated with PD98059, U0126, SP600125, SB203580 (20 μM) or DMSO (0.1%, v/v) at the indicated times during the infection with CHN06. At 72 h p.i., viral production was monitored by real-time RT-PCR with primers designed for the envelope gene. (b) DF-1 cells were simultaneously incubated with CHN06 (104 TCID50 0.2 ml−1) and increasing concentrations of U0126 or DMSO (0.1%, v/v). Viral envelope gene and cellular VEGF transcription levels were measured by real-time RT-PCR at 72 h p.i. using special primers. (c) DF-1 cells were treated with SP600125, SB203580 (20 μM) or DMSO (0.1%, v/v) at the indicated times during the infection with CHN06. At 72 h p.i., the cellular VEGF transcription level was monitored by real-time RT-PCR with special primers. Data are representative of two independent experiments, both performed in triplicate. *p < 0.05, **p < 0.01, ***p < 0.001. NS, not significant. Error bars indicate SEM.
Effects of MAPK pathway inhibitors on ALV LTR promoter activation
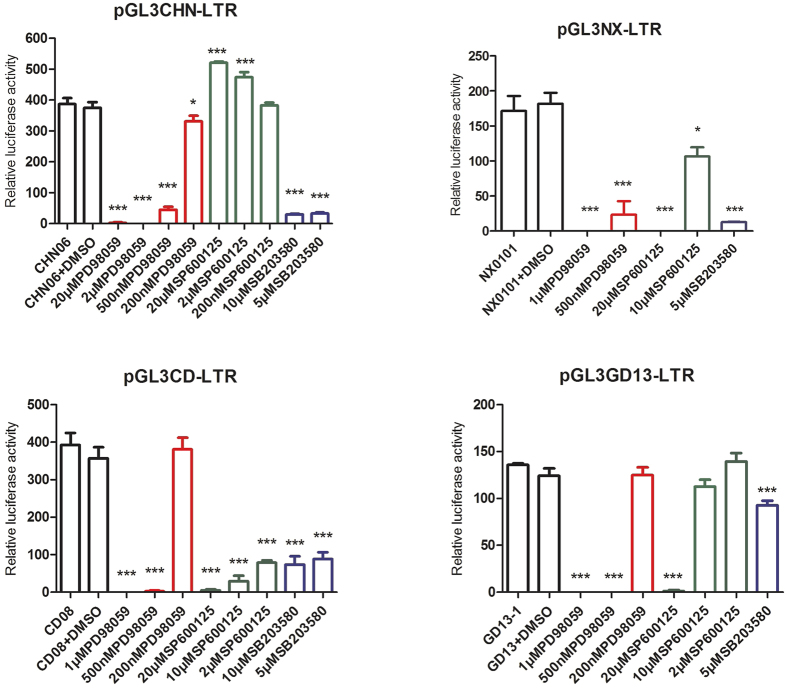
To further investigate the effects of MAPK pathway inhibitors on ALV replication, we studied the effects of the MAPK inhibitors on ALV LTR promoter activity. We found that the relative luciferase activity of the ALV LTR promoter was significantly inhibited by the ERK pathway inhibitor, PD98059, in a dose-dependent manner and with as little as 500 nM. The p38 MAPK pathway inhibitor, SB203580, also significantly inhibited ALV LTR promoter activity, but with a dose of 5 μM, hence, it was far less effective than PD98059. Interestingly, the JNK pathway inhibitor, SP600125 caused a dose-dependent increase in the levels of CHN06 LTR promoter activity and a dose-dependent decrease in the LTR promoter activity levels of NX0101, GD13-1 and CD08 strains (Fig. 10). These results suggest that the MAPK pathways play a role in the activation of the ALV LTR promoter. Moreover, of the three distinct MAPK signaling pathway inhibitors, the ERK pathway inhibitor, PD98059, contributed the most to LTR promoter activity inhibition.
Figure 10. Effects of MAPK pathway inhibitors on ALV LTR promoter activity.
DF-1 cells were seeded in 24-well dishes and co-transfected with 0.2 μg pGL3-LTR and 0.02 μg pRL-TK. At 6 h p.i., cells were treated with PD98059 (red bar, 200 nM–20 μM), SP600125 (green bar, 200 nM–20 μM), SB203580 (purple bar, 5 μM–10 μM) or DMSO (second black bar, 0.1%, v/v). At 48 h p.i., cells were lysed for luciferase detection. Data are representative of three independent experiments, performed in triplicate. *p < 0.05, ***p < 0.001. Error bars indicate SEM.
Effects of MAPK pathway inhibitors on ALV-J protein expression
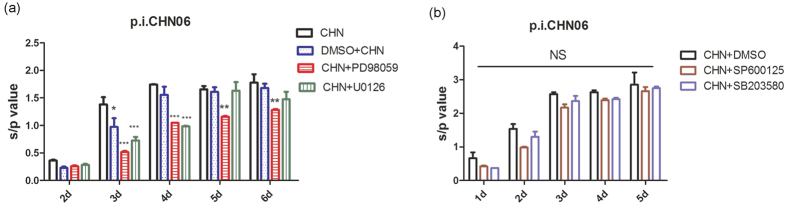
To further validate the ALV inhibition by MAPK pathway inhibitors, we studied the effects of these inhibitors on ALV-J viral protein synthesis. We found that 20 μM PD98059 significantly reduced p27 production of CHN06 strain if compared with the mock and the DMSO control at 3, 4, 5 and 6 days p.i.(DPI), while 20 μM U0126 conspicuously decreased CHN06 strain p27 production at 3 and 4 DPI (Fig. 11a). In contrast, 20 μM SP600125 or 20 μM SB203580 induced no significant CHN06 strain p27 inhibition compared with the DMSO control at different times p.i. (Fig. 11b). Furthermore, 20 μM PD98059 dramatically suppressed gp85 protein expression of ALV-J strains CHN06 and NX0101 (Fig. 12). In contrast, 20 μM SP600125 or 20 μM SB203580 did not affect the gp85 protein expression of ALV-J (data not shown). These results suggest that the ERK/MAPK signaling pathway, but not p38 and JNK signaling pathways, plays an important role in ALV-J viral protein synthesis in vitro.
Figure 11. Effects of MAPK pathway inhibitors on ALV-J p27 protein expression.
(a) DF-1 cells were simultaneously incubated with CHN06 (104 TCID50 0.2 ml−1) and PD98059, U0126 (20 μM) or DMSO (0.1%, v/v). (b) DF-1 cells were simultaneously incubated with CHN06 (104.5 TCID50 0.2 ml−1) and SP600125, SB203580 (20 μM) or DMSO (0.1%, v/v). The inhibitors or DMSO were in the cell culture fluid all the time. ALV-J production was measured by ELISA with anti-p27 antibodies. Data are representative of two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001. NS, not significant. Error bars indicate SEM.
Figure 12. Effects of the ERK pathway inhibitor on ALV-J gp85 protein expression.
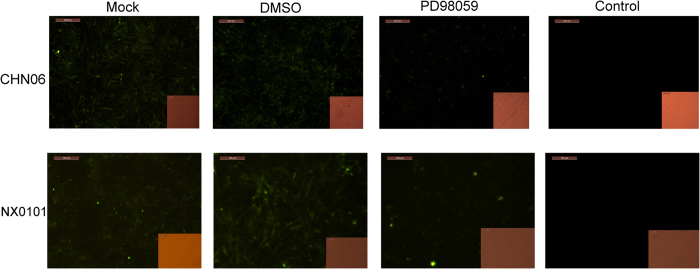
DF-1 cells were mock treated or simultaneously incubated with 50 μM PD98059 or DMSO (0.1%, v/v) during the infection with CHN06 or NX0101 (105 TCID50 0.2 ml−1). At 3 d p.i., monolayers were fixed and stained for gp85 expression, which was visualized by epifluorescent microscopy. Data are representative of two independent experiments. CHN06 group, bar = 200 μm; NX0101 group, bar = 100 μm.
MAPK is activated in tumor cells
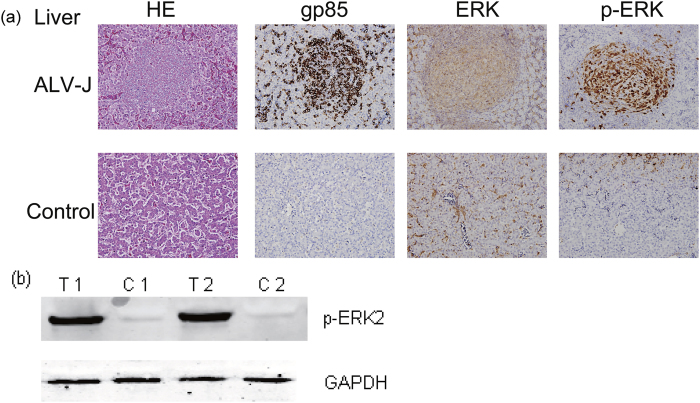
Oncogenicity is one of the classical pathologies associated with ALV-J infection31. As such, many studies are currently attempting to define the molecular determinants of the ALV-J tumorigenic mechanisms. In light of the numerous reports on the association between the ERK/MAPK pathway and cancer, in addition to our in vitro experiments demonstrating the importance of activated ERK2 to ALV replication, we next examined the activation of MAPK in tumor cells from ALV-J-infected chickens. The methods of PCR tests on the genomic DNA of tissues and viral isolation assays have been previously described32 and the results were all positive for ALV-J, and negative for ALV-A, ALV-B, REV and MDV. Under the microscope, the tumor cells were observed as relatively uniform large myeloid cells and lymphoid cell hyperplasia in the liver whose morphology are similar to eosinophils (Fig. 13a, HE). Compared with normal cells in the liver, we observed a significant up-regulation of gp85 and pMAPK expression in tumor cells from ALV-J-infected liver. In contrast, only few cells expressing pMAPK were identified in quiescent livers without tumor cells and gp85 expression (Fig. 13a, p-ERK, gp85). Of note, both the tumor cells and normal cells exhibited abundant overall MAPK expression in the liver (Fig. 13a, ERK). These results were also confirmed by western blot analysis of tissue homogenates prepared from tumor tissues and normal tissues (Fig. 13b). These findings suggest that ALV-J-induced tumorigenesis in vivo could be correlated with MAPK activation.
Figure 13. Immunohistochemical and western blot analysis of tumor cells from the liver of ALV-J-infected chickens.
(a) Fresh tissues were collected and fixed in 10% neutralized buffered formalin, dehydrated, embedded in paraffin wax, and then sliced into 6-μm sections. These sections were routinely stained with hematoxylin and eosin (HE), and examined microscopically. Tissues were further immunohistochemically labeled for gp85, ERK or p-ERK with JE9 monoclonal, anti-p44/42 MAPK or anti-phospho-p44/42 MAPK (Thr202/Tyr204) antibodies, respectively. Bar = 100 μm. (b) Western blot analysis detected high expression of p-ERK proteins in myeloid leukosis (ML) samples, but not in the normal samples. Sample identification numbers are indicated above the panel as follows: T, tumor samples; C, non-tumor samples.
Discussion
Avian retroviruses were originally identified as cancer-inducting filterable agents in chicken neoplasms at the beginning of the 20th century33. ALV-J, which originated from a recombination event between ALV and an endogenous retroviral element34,35, induces a different spectrum of tumors than ALV-A and ALV-B, primarily myeloid leukosis and hemangiomas3. Although many studies concentrate on the ALV viral replication and tumorigenesis, the molecular basis of oncogenesis in these tumors is not well understood. In our pervious study, we proposed that ALV provirus has to integrate into the particular genes with a long latency to deregulate specific regulatory pathways and lead to malignant transformation7. Exploring cellular signaling pathways involved in virus replication and oncogenesis has become a potential objective. We took advantage of proteomics to identify the differential proteins and intracellular signal transduction pathways in DF-1 infected by ALV-J. The results demonstrated that MAPK1 involved in the ERK/MAPK signaling pathway was up-regulated at 60 h p.i. According to the bioinformatics analysis, Wang et al.36 have shown that the differentially expressed miRNAs are involved in the tumorigenesis-related MAPK signaling pathway, which may represent a possible signaling pathway involved in the ALV-J-induced tumorigenesis. Hence, we examined the role of the MAPK pathway during exogenous ALV infection and pathogenesis.
The experiments presented in the current study provide evidence that exogenous ALV could induce and sustain activation of ERK2, which is, in turn, required for productive virus replication. The best characterized MAPK module contains ERK1 and ERK2, which is the unique isoform in chickens. ERK2 was found to be phosphorylated at 15 min, 6 h and from 108 h to 132 h post ALV-J infection, suggesting that it was mainly activated at the early and late infection stages. Both ALV-A and ALV-B triggered the production of phosphorylated ERK at 15 min and 120 h p.i. Meanwhile we detected the phosphorylated p38 and c-Jun but not JNK activation at these time points. Besides, ALV-induced c-Jun-phosphorylation could be inhibited by PD98059 instead of SP600125 and SB203580. Hence, it may be active ERK2 that phosphorylates the transcription factor, c-Jun. c-Jun is a member of the AP-1 transcription factor family, and is highly responsive to extracellular signals that control proliferative and apoptotic programs37. ERK has been shown to phosphorylate and activate AP-1, which binds to specific genes at binding sites in their promoters38,39. Dimerization of Jun is required for DNA binding, and DNA binding is necessary for transcriptional activation. All three properties, dimerization, DNA binding and transactivation, are essential for oncogenic transformation40. Megan et al. have shown that active ERK2 induced the activation of the transcription factors AP-1 and Elk-1, which was sufficient to induce differentiation or transformation depending on the cellular context41. MYC, TERT and ZIC1 genes are common targets of viral integration and transcriptional deregulation in ALV-J-induced myeloid leukosis7. Furthermore, tumor growth and metastasis require angiogenesis, which are regulated by VEGF20,42,43. Wang et al.44 and Li et al.45 have found that VEGF-A was overexpressed in DF-1 cells after infection with ALV-J, indicating an increased opportunity for ALV-J to further induce the expression level of VEGF-A to reach the threshold level needed to promote tumorigenesis. AP-1 present in the promoter region of the human VEGF gene has been shown to play an important role in the regulation of VEGF gene expression46,47. Intriguingly, 20 μM PD98059 or SP600125 inhibited VEGF mRNA expression in ALV-J-infected cells to approximately 50% (p < 0.001), whereas SB203580 did not affect it (p > 0.05). Actually, compared with the negative control DF-1 cells, the CHN06 strain of ALV-J caused a 1.75-fold up-regulation of VEGF mRNA at 72 h p.i. (data not shown). In this regard, both ERK and JNK pathways were involved in the regulation of VEGF gene expression after ALV-J infection. Though we did not detect JNK activation at 15min and 120h p.i, ALV infection may activate JNK at other time points. Surprisingly, PD98059 simultaneously inhibited the ALV-induced c-Jun phosphorylation and VEGF-A expression at 120 h p.i. We also found that the mRNA expression of VEGF in the tumor tissues was significantly higher than that in the control tissues. In light of our present study, we speculate that the ALV-induced oncogenic transformation was triggered by binding of active c-Jun to the promoters of candidate genes (e.g., VEGF, MYC, TERT and ZIC1). In our ongoing study, we hope to identify the promoter sequences and transcription factors’ binding sequences in these chicken candidate genes to further verify the above hypothesis.
There is mounting evidence that activation of MAPK during infection plays an important role in the multiplication of a number of RNA and DNA viruses that replicate in both the nucleus and the cytoplasm, including human immunodeficiency virus type 1 (HIV-1)48, influenza virus A and B49,50, murine coronavirus51, Borna disease virus52, coxsackievirus B353, BK virus54, human cytomegalovirus55, herpes simplex virus 256, and the orthopoxvirus vaccinia virus (VCAV)57. In this study, we took advantage of four available MAPK signal pathway inhibitors, the ERK pathway inhibitor, PD9859 and U0126, the JNK pathway inhibitor, SP600125, and the p38 pathway inhibitor, SB203580, to further investigate the contribution of each MAPK signaling pathway to ALV virus propagation. To some degree, inhibition of the three kinds of MAPK pathways resulted in a reduction in ALV-J envelope mRNA levels in DF-1 cells, without exception. As ALV transcripts are mainly initiated from the 5’LTR, generating DNA and mRNAs58,59, we further investigated whether the MAPK inhibitors affect ALV 5’LTR promoter activity. We found that PD98059 and SB203580 substantially reduced the5’LTR promoter activity of ALV subgroups J, A and B in a dose-dependent manner. Specifically, PD98059 almost completely blocked ALV 5’LTR promoter activity with a mere 500nM dose. Of note, SP600125 lead to an obvious increase in the CHN06 5’LTR promoter activity, whereas it inhibited the promoter activity of the NX0101, GD13-1 and CD08 stains. It is conceivable that the differential effect of the JNK inhibitor on the LTR promoter activity was due to the LTR sequence differences among the various strains. The unique 3′ (U3) region of retroviral LTRs has been characterized with transcriptional enhancers and promoters60,61,62,63, and multiple cis-acting enhancer elements have been verified within the avian retroviruses64. Hence, it is possible that MAPK pathways influence LTR promoter activity by interacting with transcription control sequences, further affecting the transcription rate or RNA stability. However, the discrepant effects of SP600125 between CHN06 strain viral RNA level and LTR promoter activity remain unclear. Interestingly, when the ERK pathway was effectively blocked, for example in the presence of U0126 or PD98059, the expression of p27 and gp85 proteins dramatically decreased, whereas SP600125 and SB203580 only slightly decreased the viral protein synthesis. Thus, ERK/MAPK plays active roles in posttranscriptional regulation, supporting the concept that the ERK pathway contributes the most to ALV infection if compared with the other two pathways. This is consistent with other studies on HIV-1 infection65.
Previously, we have reported that two kinds of signaling pathway play roles in some exogenous ALVs infection, the PI3K/Akt pathway which is essential for the entry of ALV30, and the autophagy pathway inhibited by ALV-J infection66. In this study, we first verified that the MAPK pathways are involved in ALV replication and that gp85 or gag protein alone contributes to ERK2/AP-1, but not Akt, p38 and JNK activation. In view of the importance of ERK/MAPK in ALV-J infection, we further explored the relevance of ERK/MAPK activation to ALV-J-associated neoplasm. We showed that the presence of activated ERK2 in ALV-J-induced tumor cells, within which classical targets (gp85 protein) were identified for ALV replication in vivo, strongly correlated with virus-induced neoplasm. In other diseases such as visna virus-induced encephalitis67 and in human reactive astrocytes in response to CNS injury68, chronic activation of ERK1/2 was observed. In this context, the requirement of activated ERK2 for ALV replication is meaningful with regard to our current understanding of cellular proteins and pathways required for avian retroviruses replication and associated oncogenic pathology.
In conclusion, we have shown that infection with exogenous subgroup J, A or B ALVs could activate the ERK/AP1 pathway in associated with viral gp85 and gag proteins, mainly at the early and late infection stages. The ERK/MAPK pathway plays an important role in ALV-J virus replication and associated myeloid leukosis. These findings provide novel insights into the mechanisms of ALV –induced neoplasm.
Materials and Methods
Ethics statement
Our animal research was conducted under the guidance of the SCAU’s Institutional Animal Care and Use Committee. The chicken sampling procedures were approved by the Animal Care and Use Committee of Guangdong Province, China.
Virus propagation and quantification of virus titer
Once DF-1 cells(American Type Culture Collection, Manassas, VA, USA), which are known to be susceptible only to exogenous ALV69, had attained 80% confluence, they were infected for 2 h at 37 °C and 5% CO2 with the following ALV strains respectively: ALV-J strains CHN06 associated with hemangiomas70 and NX0101 associated with myelocytomas71, ALV-A strain GD13-172 and ALV-B strain CD086. After the inoculum was removed, maintenance medium containing Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Life Technologies, Carlsbad, CA, USA) with 1% FBS (Gibco) was added and incubated for 6 days. After repeated freezing and thawing three times, the DF-1 cells were centrifuged at 4 °C and 5000 × g for 2 min to isolate the supernatants, which were stored as viral stocks at −80 °C until use. The virus titres were determined by serially diluting viral supernatants in DMEM and applying onto DF-1 cells for 6 days in a 96-well plate according to the method described by Reed & Muench73, and expressed as TCID50 per 0.2 mL. The infected cells were monitored by enzyme-linked immunosorbent assay (ELISA) (ALV Ag Test, IDEXX, Inc., Westbrook, MA) according to the manufacturer’s instructions.
Cell culture and virus infection
DF-1 cells were seeded in 6-well dishes at 2.0 × 106 cells/ml in DMEM with 10% FBS at 37 °C and 5% CO2 until they reached approximately 90% confluence. After starvation in serum-free medium for 6 h, virus stocks diluted in DMEM and DMEM negative control were added to the cells at 37 °C and 5% CO2 for 15 min to 168 h. Once the incubation time of the virus-containing media exceeded 4 h, the media was substituted with DMEM containing 0.05% FBS for 6 h to 168 h.
Sample preparation for proteomic analysis
Sixty hours post-infection (p.i.), mock-infected cells and CHN06-infected cells were washed with phosphate-buffered saline (PBS) and lysed in buffer containing 4% (w/v) 3-[(3-cholamidopropyl)dimethylammonium]-1-propanesulfonate (CHAPS), 7 M urea, 2 M thiourea, 10 mM Tris-HCl and 1 mM EDTA (pH 8.3). The supernatants were collected by centrifugation at 15,000 × g for 1 h at 4 °C. Protein concentrations were determined using the Bradford protein assay (Bio-Rad Laboratories, Hercules, CA, USA).
Concentration of the virus particles by differential centrifugation
As described previously74, DF-1 cells infected with ALV-J strain CHN06 for 6 days were centrifuged at 4 °C and 5000 × g for 10 min to isolate the DF-1 supernatants after repeated freezing and thawing three times. The supernatants were collected and filtered through a 0.45 μm pore size cellulose acetate filter. A portion of the filtrate was used directly, and the remaining was pelleted by centrifugation for 1 h at 16,000 × g. Both of the pellets and the supernatants after centrifugation at 16,000 × g were used.
Plasmid construction
The pGL3-LTR vectors of the different ALV subgroups were constructed by PCR amplification of the long terminal repeat (LTR) region from the genomic DNA of DF-1 cells infected with the ALV strains followed by being cloned into the pGL3-Encher vector (Promega, Madison, WI, USA) using the SmaI and BglII sites. The LTR primers for the CHN06 strain, NX0101 strain and CD08 strain have been previously described75. The LTR primers for GD13-1 were 5′-TCCCCCGGGTGTAGTCTTATGCAATACCC-3′ and 5′-GGAAGATCTAATGAAGCCTTCTGCTTCAT-3′. The pGL3-LTR vectors of the different ALV subgroups were sequenced and all had the predicted nucleotide sequences. The pRL-TK plasmid (Promega) was used as a control plasmid that expresses the Renilla luciferase reporter gene driven by the herpes simplex virus thymidine kinase promoter, and was used to monitor the transfection efficiency.
The expression of gp85 and gag protein
Recombinant PET28a-gp85 (kindly provided by Dr. Jian zhu Liu, Shandong Agricultural University, China) was expressed in Rosetta (DE3) cells and gp85 protein was purified by a Ni-NTA column and identified by western blot analysis as described previously76. pCMV-gag-EGFP was constructed by PCR amplification of the gag gene from genomic DNA of GD13-infected DF-1 cells, and subsequently cloned into pCMV-C-EGFP (Beyotime Inst Biotech, Shanghai, China) using the HindIII and SalI sites. The construct was further confirmed by sequencing and transfected into cells by electroporation. Brifely, 10 μg of pCMV-C-EGFP or pCMV-gag-EGFP were electroporated individually into 5.0 × 106 DF-1 cells. After electroporation, cells were maintained in DMEM containing 10% FBS for 24 h, which was substituted with 0.05% DMEM for 48 h.
Drug cell toxicity test
The protocol for cell toxicity has been reported elsewhere77. Cytotoxicity test was determined using Cell Counting Kit-8 dye (Beyotime Inst Biotech) according to the manufacturer’s instructions. Briefly, 5 × 103 DF-1 cells/well were seeded in a 96-well flat-bottomed plate, incubated at 37 °C for 24 h, and then placed in serum-free conditions for another 6 h. Subsequently, cells were treated with drugs at increasing concentrations in the presence of 2% FBS for 48 h. After 10 μL CCK-8 dye was add to each well, cells were incubated at 37 °C for 2 h and the absorbance was determined at 450 nm using a Multiskan FC microplate reader (Thermo Fisher, Shanghai, China).
Inhibitor treatments and time-course
Phosphoinositide 3-kinase (PI3K)-specific inhibitor LY294002, and the MAPK pathway inhibitors, PD98059, U0126, SB203580 and SP600125, were all obtained from Sigma (St Louis, MO, USA). Treatment of cells with PD98059 (200 nM–50 μM), U0126 (1–50 μM), SB203580 (200 nM–20 μM), SP600125 (200 nM–20 μM), LY294002 (2–20 μM), or solvent (0.1% DMSO, v/v) was performed at the indicated times. For the time-course experiments, PD98059, U0126, SP600125 and SB203580 were added separately to a final concentration of 20 μM at −1, 0, 1, 2 and 4 h p.i. The infection was terminated 72 h p.i. by collecting the monolayers for viral transcription analysis by real-time RT-PCR. To examine the expression level of ALV p27 and env protein, 50 μM PD98059 or DMSO was added to the DF-1 cells for 1 h, which were then infected with CHN06 strain. The cell supernatants were collected for p27 antigen detection at the indicated times, and the cells were used to examine the env protein by immunofluorescence assay (IFA). All experiments were performed in triplicate.
Luciferase reporter gene assay
DF-1 cells (~1 × 105) were seeded in 24-well dishes and transfected with pGL3-LTR the following day using Lipofectamine 3000 (Life technologies). To normalize for transfection efficiency, the thymidine kinase (TK)-Renilla luciferase reporter plasmid (pRL-TK) was added to each transfection. At 6 h post-transfection, cells were treated with the inhibitors of the MAPK signaling pathways. Reporter luciferase activity was measured at 48 h post-transfection using the Dual-Luciferase Reporter Assay System (Promega) and a Tecan Infinite M1000 luminometer (Tecan, Maennedorf, Switzerland) according to the manufacturer’s instructions. Firefly luciferase activity was normalized against the activity of Renilla luciferase. Data are representative of three independent experiments, performed in triplicate.
Western blot analysis
At the indicated times, cell monolayers were washed with PBS and lysed in Cell lysis buffer for Western and IP (Beyotime Inst Biotech). The lysates were collected and incubated on ice for 10 min. Lysates were cleared by centrifugation at 10,000 × g for 5 min at 4 °C. The supernatants were analyzed for total protein content with the BCA protein assay kit (Fermentas, Life Technologies). Total protein (20 μg) was resolved by 12% SDS-PAGE and transferred onto nitrocellulose membranes (Whatman, Maidstone, UK). Membranes were blocked with 5% (w/v) skim milk for 1 h at 37 °C, and then incubated overnight at 4 °C with specific rabbit anti-phospho-p44/42 MAPK (Thr202/Tyr204) antibody (Cell Signaling Technology, Danvers, MA, USA), rabbit anti-p44/42 MAPK antibody (Cell Signaling Technology), mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (Beyotime Inst Biotech), rabbit anti-phospho-c-jun (ser73) antibody (Millipore, Billerica, MA, USA), rabbit anti-c-Jun antibody (Abcam), rabbit anti-phospho-JNK (Thr183/Tyr185, Thr221/Tyr223) antibody (Millipore), rabbit anti-JNK1 (EPR17557) antibody (Abcam), rabbit anti-phospho-Akt (Ser473) antibody (Cell Signaling Technology), rabbit anti-Akt antibody (Bioworld Technology, Inc), rabbit anti-phospho-p38 MAPK (Thr180/Tyr182) antibody (Cell Signaling Technology), rabbit anti p38 MAPK antibody (Cell Signaling Technology), rabbit anti-VEGF-A antibody (Abbiotec, USA) or rabbit anti-p27 antibody (kindly provided by Avian Disease and Oncology Laboratory, ADOL). After three rinses with PBS Tween20 (PBST) buffer, the membranes were incubated at 37 °C for 1 h with IRDye 700DX-conjugated anti-rabbit IgG or IRDye 800-conjugated anti-mouse IgG (1:10,000; Rockland Immunochemicals, Limerick, PA, USA) diluted in PBS as the secondary antibody. Membranes were washed three times with PBST, then visualized and analyzed with an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA). The JNK activator, Anisomycin, was purchased from Selleckchem (USA).
RNA analysis
Total RNA of cells was extracted with RNAfast200 kit (Fastagen, Shanghai, China) followed by cDNA synthesis with the RevertAid First strand cDNA synthesis kit (Fermentas) according to the manufacturer’s instructions. The generated cDNA was then used for real-time PCR amplification. The detailed steps of the SYBR Green I real-time PCR assay for the detection of exogenous ALVs have been previously described78. All reactions were performed in triplicate. Finally, real-time quantitative PCR analysis was carried out using the 2−ΔΔCT method79.
ELISA and IFA analysis
At the indicated times p.i., the cell culture supernatants were collected, and the p27 expression level was examined by ELISA following the manufacturer’s instructions. At 72 h p.i. of mock, or ALV-J strains CHN06 or NX0101, DF-1 cells were washed three times with PBS and fixed with 4% paraformaldehyde for 30 min. An ALV-J-specific monoclonal antibody (JE9, kindly provided by Dr. Aijian Qin, Yangzhou University) was used to detect the env protein80. Binding of the primary antibodies was detected using FITC-labeled anti-mouse IgG (Sigma) and by fluorescent microscopy (Leica, Solms, Germany). All experiments were performed in triplicate.
Tissue sample selection and immunohistochemical staining
Suspicious and normal samples were collected from commercial broiler breeder flocks in Guangdong Province, China, in December 2014. Diagnosis of tissue samples was based on characteristic microscopic lesions, molecular analyses and virus isolation. Tissue samples were further diagnosed by immunohistochemical staining with JE9 monoclonal antibody. Anti-p44/42 MAPK antibody was used to immunohistochemically stain tissue sections from ALV-J-positive and uninfected normal chickens. To determine whether pMAPK was expressed in tumor cells, tissues were also immunohistochemically examined for pMAPK expression with anti-phospho-p44/42 MAPK (Thr202/Tyr204) antibody. Binding of the primary antibodies was detected using anti-rabbit-HRP or anti mouse-HRP (Zhongshan Goldenbridge, Beijing, China) and by optical microscopy (Olympus, Tokyo, Japan).
Statistical analysis
The significance of the differences among the trials was analyzed using the GraphPad Prism version 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). The results are presented as mean ± SEM, and the statistical significance is represented by p values of <0.05, 0.01 or 0.001.
Additional Information
How to cite this article: Dai, M. et al. Exogenous avian leukosis virus-induced activation of the ERK/AP1 pathway is required for virus replication and correlates with virus-induced tumorigenesis. Sci. Rep. 6, 19226; doi: 10.1038/srep19226 (2016).
Acknowledgments
The work was funded by a Key Project from the Agricultural Ministry [CARS-42-G09], the Key Program of Science and Technology Development of Guangdong Province [2012A0201000001], the Modern agriculture talents support program [[2012] no. 160], the Natural Science Foundation of Guangdong Province [2014A030313450] and the Public Industry Research Program of the Ministry of Agriculture, China [201203055]. We also appreciate the support from Jiangsu Coinnovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou.
Footnotes
Author Contributions M.M.D. participated in the design of the study, performed the experiments, collected and analyzed data, and drafted the manuscript. M.F. constructed the pGL3-LTR vectors of the different ALV subgroups and helped with the animal experiment. Y.Y. and X.C.W. participated in proteomic analysis. D.L. performed the statistical analysis. M.L. and W.S.C. participated the design and coordination of the study. All authors read and approved the final manuscript.
References
- Payne L. N., Howes K., Gillespie A. M. & Smith L. M. Host range of Rous sarcoma virus pseudotype RSV(HPRS-103) in 12 avian species: support for a new avian retrovirus envelope subgroup, designated J. J Gen Virol 73, (Pt 11) 2995 (1992). [DOI] [PubMed] [Google Scholar]
- Wang X., Zhao P. & Cui Z. Z. [Identification of a new subgroup of avian leukosis virus isolated from Chinese indigenous chicken breeds]. Bing Du Xue Bao 28, 609 (2012). [PubMed] [Google Scholar]
- Cheng Z., Liu J., Cui Z. & Zhang L. Tumors associated with avian leukosis virus subgroup J in layer hens during 2007 to 2009 in China. J Vet Med Sci 72, 1027 (2010). [DOI] [PubMed] [Google Scholar]
- Cui Z., Du Y., Zhang Z. & Silva R. F. Comparison of Chinese field strains of avian leukosis subgroup J viruses with prototype strain HPRS-103 and United States strains. Avian Dis 47, 1321 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang Q. C., Zhao D. M., Guo H. J. & Cui Z. Z. Isolation and identification of a subgroup A avian leukosis virus from imported meat-type grand-parent chickens. Virol Sin 25, 130 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. et al. Isolation and identification of CD08 strain of avian leukosisvirus associated with hemangioma. Chin J Vet Med 45, 8 (2009) (in Chinese). [Google Scholar]
- Li Y. et al. The MYC, TERT, and ZIC1 genes are common targets of viral integration and transcriptional deregulation in avian leukosis virus subgroup J-induced myeloid leukosis. J Virol 88, 3182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. L. et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics 3, 1154 (2004). [DOI] [PubMed] [Google Scholar]
- Pearson G. et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22, 153 (2001). [DOI] [PubMed] [Google Scholar]
- DeSilva D. R., Feeser W. S., Tancula E. J. & Scherle P. A. Anergic T cells are defective in both jun NH2-terminal kinase and mitogen-activated protein kinase signaling pathways. J Exp Med 183, 2017 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C., Davis R. J. & Flavell R. A. MAP kinases in the immune response. Annu Rev Immunol 20, 55 (2002). [DOI] [PubMed] [Google Scholar]
- Roberts P. J. & Der C. J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26, 3291 (2007). [DOI] [PubMed] [Google Scholar]
- Garrington T. P. & Johnson G. L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol 11, 211 (1999). [DOI] [PubMed] [Google Scholar]
- Shaulian E. & Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol 4, E131 (2002). [DOI] [PubMed] [Google Scholar]
- Behrens A., Jochum W., Sibilia M. & Wagner E. F. Oncogenic transformation by ras and fos is mediated by c-Jun N-terminal phosphorylation. Oncogene 19, 2657 (2000). [DOI] [PubMed] [Google Scholar]
- Ben-Ari E. T., Bernstein L. R. & Colburn N. H. Differential c-jun expression in response to tumor promoters in JB6 cells sensitive or resistant to neoplastic transformation. Mol Carcinog 5, 62 (1992). [DOI] [PubMed] [Google Scholar]
- Li J. J., Cao Y., Young M. R. & Colburn N. H. Induced expression of dominant-negative c-jun downregulates NFkappaB and AP-1 target genes and suppresses tumor phenotype in human keratinocytes. Mol Carcinog 29, 159 (2000). [DOI] [PubMed] [Google Scholar]
- Darnell J. E. Transcription factors as targets for cancer therapy. Nat Rev Cancer 2, 740 (2002). [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Jun, the oncoprotein. Oncogene 20, 2365 (2001). [DOI] [PubMed] [Google Scholar]
- Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer 2, 795 (2002). [DOI] [PubMed] [Google Scholar]
- Neufeld G., Cohen T., Gengrinovitch S. & Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 13, 9 (1999). [PubMed] [Google Scholar]
- Grugel S., Finkenzeller G., Weindel K., Barleon B. & Marme D. Both v-Ha-Ras and v-Raf stimulate expression of the vascular endothelial growth factor in NIH 3T3 cells. J Biol Chem 270, 25915 (1995). [DOI] [PubMed] [Google Scholar]
- Rak J. et al. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res 55, 4575 (1995). [PubMed] [Google Scholar]
- Lin X. C. et al. Quantitative Proteomic Profiling of Renal Tissue in Human Chronic Rejection Biopsy Samples After Renal Transplantation. Transpl P 47, 323 (2015). [DOI] [PubMed] [Google Scholar]
- Garcia S., Lopez E. & Lopez-Colome A. M. Glutamate accelerates RPE cell proliferation through ERK1/2 activation via distinct receptor-specific mechanisms. J Cell Biochem 104, 377 (2008). [DOI] [PubMed] [Google Scholar]
- Xing Z., Cardona C. J., Anunciacion J., Adams S. & Dao N. Roles of the ERK MAPK in the regulation of proinflammatory and apoptotic responses in chicken macrophages infected with H9N2 avian influenza virus. J Gen Virol 91, 343 (2010). [DOI] [PubMed] [Google Scholar]
- Shin Y. K., Liu Q., Tikoo S. K., Babiuk L. A. & Zhou Y. Influenza A virus NS1 protein activates the phosphatidylinositol 3-kinase (PI3K)/Akt pathway by direct interaction with the p85 subunit of PI3K. J Gen Virol 88, 13 (2007). [DOI] [PubMed] [Google Scholar]
- Giorgione J. R., Huang Z. & Epand R. M. Increased activation of protein kinase C with cubic phase lipid compared with liposomes. Biochemistry-US 37, 2384 (1998). [DOI] [PubMed] [Google Scholar]
- Lee C., Tomkowicz B., Freedman B. D. & Collman R. G. HIV-1 gp120-induced TNF-{alpha} production by primary human macrophages is mediated by phosphatidylinositol-3 (PI-3) kinase and mitogen-activated protein (MAP) kinase pathways. J Leukoc Biol 78, 1016 (2005). [DOI] [PubMed] [Google Scholar]
- Feng S. Z., Cao W. S. & Liao M. The PI3K/Akt pathway is involved in early infection of some exogenous avian leukosis viruses. J Gen Virol 92, 1688 (2011). [DOI] [PubMed] [Google Scholar]
- Venugopal K. Avian leukosis virus subgroup J: a rapidly evolving group of oncogenic retroviruses. Res Vet Sci 67, 113 (1999). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Isolation, identification, and phylogenetic analysis of two avian leukosis virus subgroup J strains associated with hemangioma and myeloid leukosis. Vet Microbiol 166, 356 (2013). [DOI] [PubMed] [Google Scholar]
- Justice J. & Beemon K. L. Avian retroviral replication. Curr Opin Virol 3, 664 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco M. A., Flannery D. M., Howes K. & Venugopal K. Avian endogenous retrovirus EAV-HP shares regions of identity with avian leukosis virus subgroup J and the avian retrotransposon ART-CH. J Virol 74, 1296 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M. et al. Novel endogenous retroviral sequences in the chicken genome closely related to HPRS-103 (subgroup J) avian leukosis virus. J Gen Virol 80, (Pt 1) 261 (1999). [DOI] [PubMed] [Google Scholar]
- Wang Q. et al. Differential expression of microRNAs in avian leukosis virus subgroup J-induced tumors. Vet Microbiol 162, 232 (2013). [DOI] [PubMed] [Google Scholar]
- Karin M., Liu Z. & Zandi E. AP-1 function and regulation. Curr Opin Cell Biol 9, 240 (1997). [DOI] [PubMed] [Google Scholar]
- Robinson M. J. & Cobb M. H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol 9, 180 (1997). [DOI] [PubMed] [Google Scholar]
- Harris V. K. Induction of the Angiogenic Modulator Fibroblast Growth Factor-binding Protein by Epidermal Growth Factor Is Mediated through Both MEK/ERK and p38 Signal Transduction Pathways. J Biol Chem 275, 10802 (2000). [DOI] [PubMed] [Google Scholar]
- Morgan I. M., Ransone L. J., Bos T. J., Verma I. M. & Vogt P. K. Transformation by Jun: requirement for leucine zipper, basic region and transactivation domain and enhancement by Fos. Oncogene 7, 1119 (1992). [PubMed] [Google Scholar]
- Robinson M. J., Stippec S. A., Goldsmith E., White M. A. & Cobb M. H. A constitutively active and nuclear form of the MAP kinase ERK2 is sufficient for neurite outgrowth and cell transformation. Curr Biol 8, 1141 (1998). [DOI] [PubMed] [Google Scholar]
- Plate K. H., Breier G., Weich H. A. & Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 359, 845 (1992). [DOI] [PubMed] [Google Scholar]
- Ferrara N. The Biology of Vascular Endothelial Growth Factor. Endocr Rev 18, 4 (1997). [DOI] [PubMed] [Google Scholar]
- Wang Q. et al. A 205-nucleotide deletion in the 3’ untranslated region of avian leukosis virus subgroup J, currently emergent in China, contributes to its pathogenicity. J Virol 86, 12849 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. et al. Quantitative iTRAQ LC-MS/MS Proteomics Reveals the Proteome Profiles of DF-1 Cells after Infection with Subgroup J Avian Leukosis Virus. Biomed Res Int 2015, 1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C., Saule S. & Gospodarowicz D. Transcriptional regulation of vascular endothelial growth factor gene expression in ovarian bovine granulosa cells. Growth Factors 8, 109 (1993). [DOI] [PubMed] [Google Scholar]
- Baud V. & Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 11, 372 (2001). [DOI] [PubMed] [Google Scholar]
- Jacqué J. M. et al. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. The Embo Journal 17, 2607 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig S. et al. MEK inhibition impairs influenza B virus propagation without emergence of resistant variants. Febs Lett 561, 37 (2004). [DOI] [PubMed] [Google Scholar]
- Pleschka S. et al. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat Cell Biol 3, 301 (2001). [DOI] [PubMed] [Google Scholar]
- Cai Y., Liu Y. & Zhang X. Suppression of coronavirus replication by inhibition of the MEK signaling pathway. J Virol 81, 446 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planz O., Pleschka S. & Ludwig S. MEK-specific inhibitor U0126 blocks spread of Borna disease virus in cultured cells. J Virol 75, 4871 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H. et al. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J Virol 76, 3365 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamone M. E. et al. MAP kinase activation increases BK polyomavirus replication and facilitates viral propagation in vitro. J Virol Methods 170, 21 (2010). [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Ma X. L., Yurochko A. D. & Huang E. S. The role of MKK1/2 kinase activity in human cytomegalovirus infection. J Gen Virol 82, 493 (2001). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. Distinct effects of knocking down MEK1 and MEK2 on replication of herpes simplex virus type 2. Virus Res 150, 22 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade A. A. et al. The vaccinia virus-stimulated mitogen-activated protein kinase (MAPK) pathway is required for virus multiplication. Biochem J 381, 437 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman S. A. & Coffin J. M. Differential transcription from the long terminal repeats of integrated avian leukosis virus DNA. J Virol 60, 497 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W. et al. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell 41, 719 (1985). [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Tsichlis P. & Khoury G. Multiple enhancer domains in the 3′ terminus of the Prague strain of Rous sarcoma virus. Nucleic Acids Res 12, 6427 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciw P. A., Bishop J. M., Varmus H. E. & Capecchi M. R. Location and function of retroviral and SV40 sequences that enhance biochemical transformation after microinjection of DNA. Cell 33, 705 (1983). [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I. & Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci USA 79, 6777 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B. & Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci USA 79, 6453 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo S., Yun M. & Beemon K. cis-acting regulatory elements within gag genes of avian retroviruses. Mol Cell Biol 7, 388 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Shen X. H., Chen C., Qiu H. & Yang R. G. Down-regulation of HIV-1 infection by inhibition of the MAPK signaling pathway. Virol Sin 26, 114 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. et al. Subgroup J avian leukosis virus infection inhibits autophagy in DF-1 cells. Virol J 10, 196 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber S. A. et al. Visna virus-induced activation of MAPK is required for virus replication and correlates with virus-induced neuropathology. J Virol 76, 817 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell J. W. & VandenBerg S. R. ERK/MAP kinase is chronically activated in human reactive astrocytes. Neuroreport 10, 3567 (1999). [DOI] [PubMed] [Google Scholar]
- Maas R., van Zoelen D., Oei H. & Claassen I. Replacement of primary chicken embryonic fibroblasts (CEF) by the DF-1 cell line for detection of avian leucosis viruses. Biologicals 34, 177 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. An ALV-J isolate is responsible for spontaneous haemangiomas in layer chickens in China. Avian Pathol 40, 261 (2011). [DOI] [PubMed] [Google Scholar]
- Du Y., Cui Z. & Qin A. Isolation of subgroup J avian leukosis viruses and their partial sequence comparison. Chin J Virol 16, 341 (2000) (in Chinese). [Google Scholar]
- Feng M. et al. Etiology survey and Phylogenetic analysis of avian leukosis virus subgroup A isolated from breeder chickens farm. Journal of South China Agricultural University 35, 11 (2014) (in Chinese). [Google Scholar]
- Reed L. J. & Muench H., A simple method of estimating fifty percent endpoints. Am J Hyg 27, 493 (1938). [Google Scholar]
- Dai M. M. et al. Effect of differential centrifugation on the biological activities of avian leukosis virus subgroup J isolate SCAU 11-H. Chinese Journal of Veterinary Medicine 50, 26 (2014) (in Chinese). [Google Scholar]
- Feng S., Li J., Wu X., Cao W. & Liao M. Sequence and promoter activity analysis of 5’ LTR from different subgroups of avian leukosis viruses. China animal husbandry & Veterinary medicine 38, 125 (2011) (in Chinese). [Google Scholar]
- Zhang L. et al. Liposomes containing recombinant gp85 protein vaccine against ALV-J in chickens. Vaccine 32, 2452 (2014). [DOI] [PubMed] [Google Scholar]
- Hou G. et al. An activated mTOR/p70S6K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against mTOR. Cancer Lett 253, 236 (2007). [DOI] [PubMed] [Google Scholar]
- Dai M., Feng M., Liu D., Cao W. & Liao M. Development and application of SYBR Green I real-time PCR assay for the separate detection of subgroup J Avian leukosis virus and multiplex detection of avian leukosis virus subgroups A and B. Virol J 12, 52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402 (2001). [DOI] [PubMed] [Google Scholar]
- Qin A., Lee L. F., Fadly A., Hunt H. & Cui Z. Development and characterization of monoclonal antibodies to subgroup J avian leukosis virus. Avian Dis 45, 938 (2001). [PubMed] [Google Scholar]
- Dent P., Yacoub A., Fisher B. P., Hagan P. M. & Grant S. MAPK pathways in radiation responses. Oncogene 22, 5885(2003) [DOI] [PubMed] [Google Scholar]