Abstract
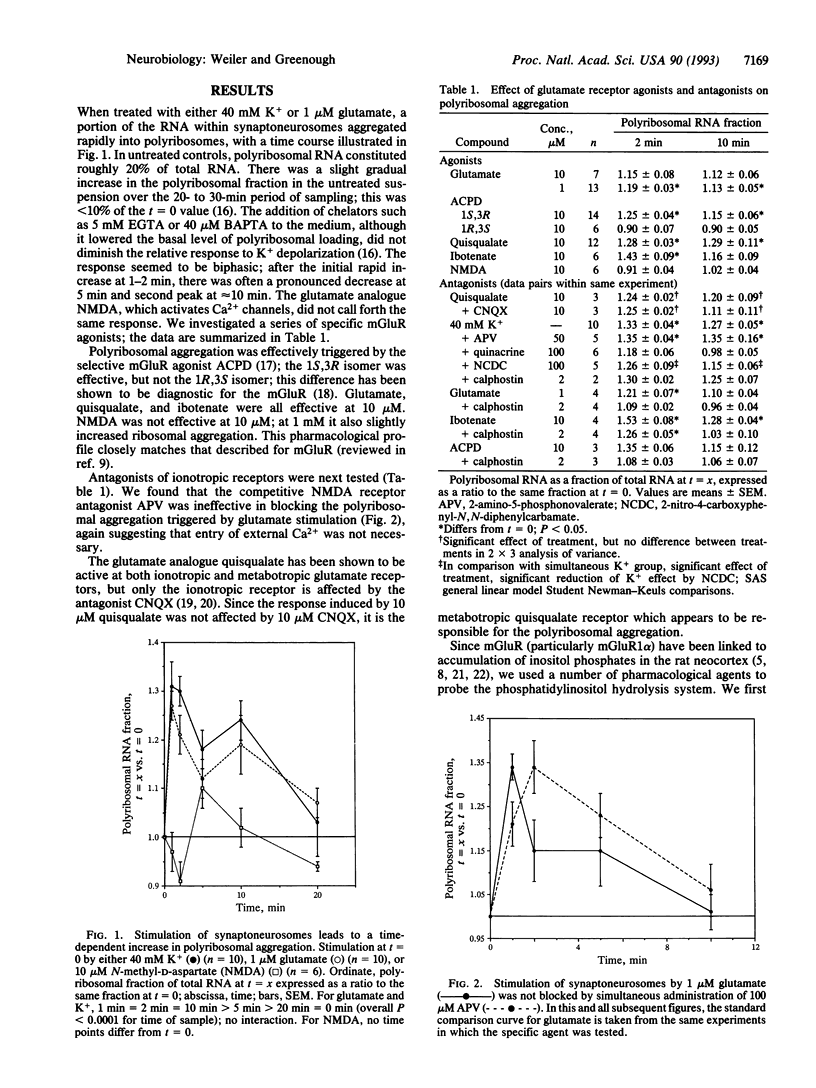
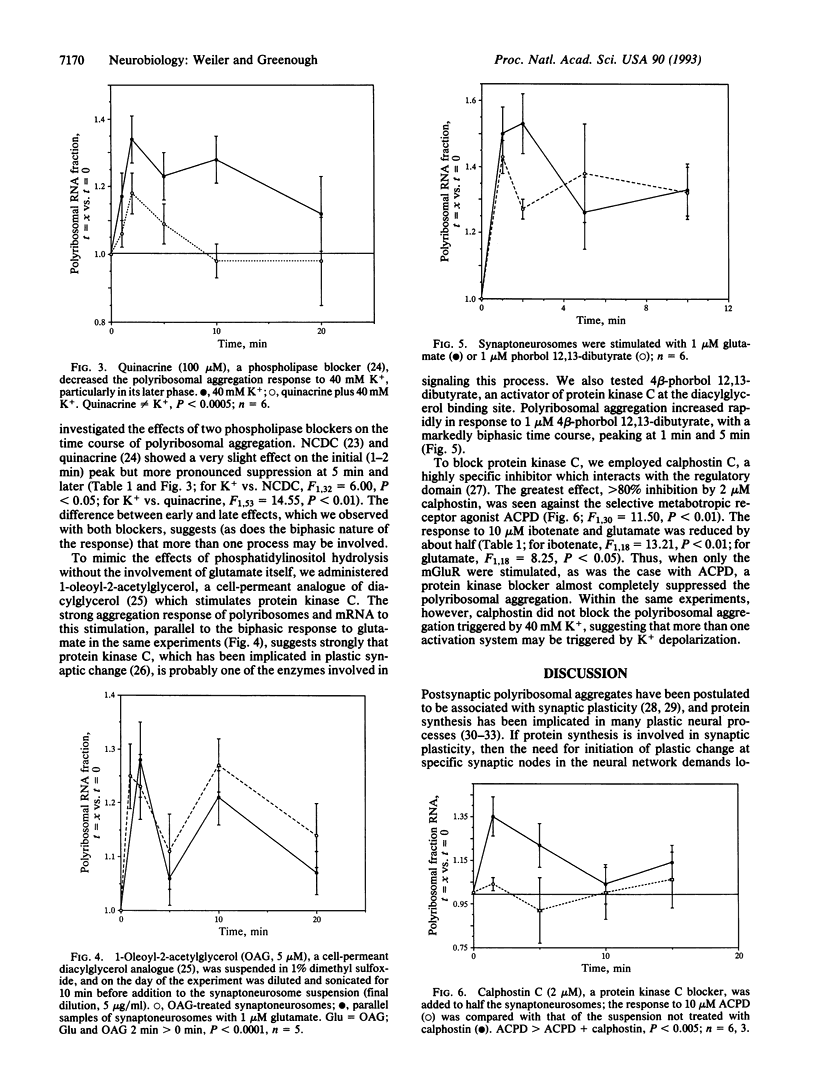
K+ depolarization or addition of glutamate to a synaptoneurosome preparation triggers a rapid increase in size of polyribosomal aggregates isolated by centrifugation of lysate through 1 M sucrose. The profile of response to the glutamate analogues quisqualate, ibotenate, and 1-aminocyclopentane-1,3-dicarboxylate corresponds to that of metabotropic receptors. Glutamate stimulation is mimicked by the diacylglycerol analogue 1-oleoyl-2-acetylglycerol and by the protein kinase C activator phorbol dibutyrate. The phospholipase blockers 2-nitro-4-carboxyphenyl-N,N-diphenylcarbamate and quinacrine reduce the late phase of the response. The protein kinase C inhibitor calphostin C suppresses the response to 1-aminocyclopentane-1,3-dicarboxylate. These data indicate that glutamatergic synapses upregulate postsynaptic protein synthesis via metabotropic glutamate receptors coupled to the phosphatidylinositol second-messenger system. This mechanism could underlie the reported involvement of metabotropic glutamate receptors in long-term potentiation and other forms of neural plasticity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akers R. F., Lovinger D. M., Colley P. A., Linden D. J., Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986 Feb 7;231(4738):587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- Aniksztejn Laurent, Otani Satoru, Ben-Ari Yehezkel. Quisqualate Metabotropic Receptors Modulate NMDA Currents and Facilitate Induction of Long-Term Potentiation Through Protein Kinase C. Eur J Neurosci. 1992;4(6):500–505. doi: 10.1111/j.1460-9568.1992.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Anwyl R. The role of the metabotropic receptor in synaptic plasticity. Trends Pharmacol Sci. 1991 Sep;12(9):324–326. doi: 10.1016/0165-6147(91)90588-j. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Blake J. F., Brown M. W., Collingridge G. L. CNQX blocks acidic amino acid induced depolarizations and synaptic components mediated by non-NMDA receptors in rat hippocampal slices. Neurosci Lett. 1988 Jun 29;89(2):182–186. doi: 10.1016/0304-3940(88)90378-3. [DOI] [PubMed] [Google Scholar]
- Deadwyler S. A., Dunwiddie T., Lynch G. A critical level of protein synthesis is required for long-term potentiation. Synapse. 1987;1(1):90–95. doi: 10.1002/syn.890010112. [DOI] [PubMed] [Google Scholar]
- Dudek S. M., Bear M. F. A biochemical correlate of the critical period for synaptic modification in the visual cortex. Science. 1989 Nov 3;246(4930):673–675. doi: 10.1126/science.2573152. [DOI] [PubMed] [Google Scholar]
- Dudek S. M., Bowen W. D., Bear M. F. Postnatal changes in glutamate stimulated phosphoinositide turnover in rat neocortical synaptoneurosomes. Brain Res Dev Brain Res. 1989 May 1;47(1):123–128. doi: 10.1016/0165-3806(89)90114-4. [DOI] [PubMed] [Google Scholar]
- Fields R. D., Yu C., Nelson P. G. Calcium, network activity, and the role of NMDA channels in synaptic plasticity in vitro. J Neurosci. 1991 Jan;11(1):134–146. doi: 10.1523/JNEUROSCI.11-01-00134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifková E., Anderson C. L., Young S. J., Van Harreveld A. Effect of anisomycin on stimulation-induced changes in dendritic spines of the dentate granule cells. J Neurocytol. 1982 Apr;11(2):183–210. doi: 10.1007/BF01258243. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Hwang H. M., Gorman C. Evidence for active synapse formation or altered postsynaptic metabolism in visual cortex of rats reared in complex environments. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4549–4552. doi: 10.1073/pnas.82.13.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S. L., Prescott S. M., Majerus P. W. The effects of mepacrine and p-bromophenacyl bromide on arachidonic acid release in human platelets. Arch Biochem Biophys. 1982 Apr 15;215(1):237–244. doi: 10.1016/0003-9861(82)90300-9. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 15;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Krug M., Lössner B., Ott T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull. 1984 Jul;13(1):39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Martin L. J., Blackstone C. D., Huganir R. L., Price D. L. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992 Aug;9(2):259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- Means A. R., Lagace L., Guerriero V., Jr, Chafouleas J. G. Calmodulin as a mediator of hormone action and cell regulation. J Cell Biochem. 1982;20(4):317–330. doi: 10.1002/jcb.240200402. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Iadarola M. J., Wroblewski J. T., Costa E. Excitatory amino acid recognition sites coupled with inositol phospholipid metabolism: developmental changes and interaction with alpha 1-adrenoceptors. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1931–1935. doi: 10.1073/pnas.83.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S., Ben-Ari Y. Metabotropic receptor-mediated long-term potentiation in rat hippocampal slices. Eur J Pharmacol. 1991 Dec 3;205(3):325–326. doi: 10.1016/0014-2999(91)90920-l. [DOI] [PubMed] [Google Scholar]
- Otani S., Marshall C. J., Tate W. P., Goddard G. V., Abraham W. C. Maintenance of long-term potentiation in rat dentate gyrus requires protein synthesis but not messenger RNA synthesis immediately post-tetanization. Neuroscience. 1989;28(3):519–526. doi: 10.1016/0306-4522(89)90001-8. [DOI] [PubMed] [Google Scholar]
- Palmer E., Monaghan D. T., Cotman C. W. Glutamate receptors and phosphoinositide metabolism: stimulation via quisqualate receptors is inhibited by N-methyl-D-aspartate receptor activation. Brain Res. 1988 Sep;464(2):161–165. doi: 10.1016/0169-328x(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Palmer E., Monaghan D. T., Cotman C. W. Trans-ACPD, a selective agonist of the phosphoinositide-coupled excitatory amino acid receptor. Eur J Pharmacol. 1989 Aug 3;166(3):585–587. doi: 10.1016/0014-2999(89)90383-x. [DOI] [PubMed] [Google Scholar]
- Récasens M., Sassetti I., Nourigat A., Sladeczek F., Bockaert J. Characterization of subtypes of excitatory amino acid receptors involved in the stimulation of inositol phosphate synthesis in rat brain synaptoneurosomes. Eur J Pharmacol. 1987 Sep 2;141(1):87–93. doi: 10.1016/0014-2999(87)90413-4. [DOI] [PubMed] [Google Scholar]
- Schoepp D. D., Johnson B. G., True R. A., Monn J. A. Comparison of (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (1S,3R-ACPD)- and 1R,3S-ACPD-stimulated brain phosphoinositide hydrolysis. Eur J Pharmacol. 1991 Aug 14;207(4):351–353. doi: 10.1016/0922-4106(91)90010-f. [DOI] [PubMed] [Google Scholar]
- Schoepp D., Bockaert J., Sladeczek F. Pharmacological and functional characteristics of metabotropic excitatory amino acid receptors. Trends Pharmacol Sci. 1990 Dec;11(12):508–515. doi: 10.1016/0165-6147(90)90052-a. [DOI] [PubMed] [Google Scholar]
- Shigemoto R., Nakanishi S., Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992 Aug 1;322(1):121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Sladeczek F. Putative role of inositol phospholipid metabolism in neurons. Biochimie. 1987 Apr;69(4):287–296. doi: 10.1016/0300-9084(87)90019-8. [DOI] [PubMed] [Google Scholar]
- Steward O., Levy W. B. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982 Mar;2(3):284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Masu M., Ishii T., Shigemoto R., Nakanishi S. A family of metabotropic glutamate receptors. Neuron. 1992 Jan;8(1):169–179. doi: 10.1016/0896-6273(92)90118-w. [DOI] [PubMed] [Google Scholar]
- Verhage M., Besselsen E., Lopes Da Silva F. H., Ghijsen W. E. Evaluation of the Ca2+ concentration in purified nerve terminals: relationship between Ca2+ homeostasis and synaptosomal preparation. J Neurochem. 1988 Dec;51(6):1667–1674. doi: 10.1111/j.1471-4159.1988.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Zheng F., Gallagher J. P. Metabotropic glutamate receptors are required for the induction of long-term potentiation. Neuron. 1992 Jul;9(1):163–172. doi: 10.1016/0896-6273(92)90231-2. [DOI] [PubMed] [Google Scholar]



