Abstract
Background:
Vaginal atrophy is a common complication in menopause which does not improve with time and, if untreated, can affect the quality of life for women. The aim of this study was to compare the effectiveness of the vaginal cream of hyaluronic acid and conjugated estrogen (Premarin) in treatment of vaginal atrophy.
Methods:
This study was a randomized controlled clinical trial on 56 menopausal women with symptoms of vaginal atrophy; they were randomly allocated to two groups (recipient conjugated estrogen and hyaluronic acid). The severity of each sign of atrophy was evaluated by visual analog signals (VAS) and on the basis of a four point scale. Also to recognize the cellular maturation with pap smear and the maturation degree were calculated according to the formula and scores 0-100. As to the vaginal PH, we used PH marker band, the rate of which was divided into 4 degrees. Data were analyzed using SPSS, version 20, and P≤0.05 was considered as significant.
Results:
The results of this study showed that the symptoms of vaginal atrophy compared with the baseline level were relieved significantly in both groups. Dryness, itching, maturation index, PH and composite score of the vaginal symptoms were relieved significantly in both groups (P<0.001). Dyspareunia in Premarin (P<0.05) and hyaluronic acid (P<0.001) decreased compared with pre-treatment. Urinary incontinence only showed improvement in the hyaluronic acid group (P<0.05). Improvement in urinary incontinence, dryness, maturation index (P<0.05) and composite score of vaginal symptoms (P<0.001) in the hyaluronic acid group was better than those in the Premarin group.
Conclusion:
According to the results of the present study, hyaluronic acid and conjugated estrogen improved the symptoms of vaginal atrophy. But hyaluronic acid was more effective and this drug is suggested for those who do not want to or cannot take local hormone treatment.
Trial Registration Number: IRCT2013022712644N1
KEYWORDS: Atrophic vaginitis, Estrogen, Hyaluronic acid, Menopause
INTRODUCTION
Menopause is defined as the permanent experience of long-lasting endocrinal, somatic and psychological changes.1 During these periods, women experience some symptoms which begin with vasomotor signs (like flushing, night sweat, etc.), changes in menstruation cycle, vaginal dryness, Itching and dyspareunia and continue with temper changes, memory reduction, disorders of sexual arousal reduction, stress urinary incontinence and complaint from musculo-eskeletal pains.
Even though some of the complications subside during the time, the symptoms of vasomotor, vaginal dryness and dyspareunia which are connected to disorder in sexual function related to lack of sexual hormones (especially Estrogen) irrespective of treatment will progress markedly and unfortunately will not be solved without treatment.2,3
Following the subsidence or discontinuity of this hormone, women are affected by symptomatic vaginal atrophy and basic changes will occur in their genitor-urinary mucous.4 These changes include vaginal dryness, irritation, itching, post-coital bleeding, vaginal discharge and dyspareunia and in the urinary system, urine frequency and urinary incontinence appear.3,5
As a whole, it is estimated that 10.0-40.0% of women experience the symptoms connected with atrophy and on the other hand about 16 million women (500 thousand new cases) show such symptoms every year.4 In confirmation to the prevalence of this problem, Crandall C et-al. (2004) and Mac Bride et-al. (2010) considered this matter and reported that the vaginal dryness was observed from 23.4% pre-menopause to 61.5% post-menopause among the women under the study.3,6 The results of the researches conducted by Kingerberg et-al. (2009) and Mehta and Bachman also showed that 10.0-40.0% of women at the post-menopause stage face inconvenience and problems related to vulva and vaginal atrophy that requires treatment but only 25.0% of them refer for treatment.7,8
Two hormonal and non-hormonal methods are usually used in treatment of such problems. In the studies which applied non-hormonal method, materials like lubricants and vaginal moistures,4,9,10 vitamin E oil and improving lifestyle like stopping cigarette smoking have been mentioned.5 For hormonal methods also the conjugated Estrogen in two forms of systemic (oral and parenteral) and topical are prescribed.11,12 The systemic method is useful for those women who are suffering from flushing and sleep disorder related to vaginal atrophy.13,14
On the other hand, the contraindication of this method for tumors sensitive to Estrogen, liver failure and having thromboembolization history related to Estrogen should also be considered. Also, attention should be paid to their side effects like breast sensitivity, nausea and vomiting, vaginal bleeding, mild increase in the risk of affecting the neoplasms dependent on Estrogen and in lesser amount the pain in the perineal area.13,15,16
Topical treatment in the form of cream, tablet and ring (conjugated Estrogen 0.625) which has been confirmed by FDA (Food and Drug Association) with the objective of preparing sufficient Estrogen for reducing the symptoms of atrophy and relief of its resultant complications is applied.11,17 In this regard, researches show that topical drugs have similar effect18,19 and even though the probability of general absorption is there, as to the influence and improvement of the symptoms to the rate of 80.0-90.0%, which is expected, they are similar.12
Topical hormones are also not without complication; the results of a study (2004) showed that they have similar effects in the incidence rate of hyperplasia and endometrial thickening.20 Creams are probably accompanied with more side-effects compared with ring or tablet which may be due to the application of a dose more than recommended.4
Considering the aforesaid points, for those who do not select Estrogen-therapy due to the medical prohibitions or having side-effects,1 the non-hormonal interventions which are mostly neglected for the sexual problems are propounded. In these methods, applying lubricants, moisture creams and using dilators are recommended. It was reported in a study in the year 2010 that their use will reduce the complications of vaginal atrophy.21 Also, in this connection, materials which could be applied as gel in the form of the extract of some plants like Vitex Agnus-Castus and compounds like Hyaluronic Acid alone or in combination with Vitamin E for the treatment of sexual disorders and vaginal atrophy are mentioned.22,23
Regarding Hyaluronic Acid which is a natural polysaccharide, it can be mentioned that, it forms an important part of extra-cellular matrix of the skin and cartilage. This substance is able to conserve a large amount of water molecules and due to the properties like formation and conservation of extra-cellular inflation, skin moistening in the case of inflammation and preservation of water equilibrium has a key role. Also, it is effective widely in treatment of skin diseases due to preservation of tissue consistency, facilitating the cellular emigration in the cases of inflammation and also the process of improvement and regeneration of the tissues.24
Various studies carried out regarding Hyaluronic Acid have shown that this compound has been tolerated well without side-effects among patients and the complications have been observed only when applied in the form of parenteral jelly by creating susceptibility at injection sites as mild inconveniences, redness, edema and cyanosis.25,26 It should be notified that this medicine in the form of suppositories or tablets has rarely been used in Iran for treatment of atrophy of genitor-urinary system.
The present study aimed at achieving an appropriate and uncomplicated treatment, which is accepted by those who have contraindication for hormonal drug and or desire to use non-hormonal methods. Therefore, we tried to compare the effects of conjugated Estrogen cream 0.625 mg (hormonal) and hyaluronic acid cream (non-hormonal) for the treatment of atrophy and its complications with the aim to promote the health of menopause women. In spite of the importance of the matter and effect of vaginal atrophy symptoms on women’s life, they are mostly not reported and do not go under treatment subsequently. Therefore, to remove such problems, beginning of treatment and taking care of them by physical evaluation, talking about their sexual problems and the qualitative problems of their life seem necessary.27
MATERIALS AND METHODS
This study was approved by ethics committee under number: CT-92-6681 on Oct 2013 and was carried out in multi-stages as a simple randomized controlled clinical trial on 56 menopause women referred to Shahid Motahari Clinic during 6 months (September to March 2013-2014). They were selected on the basis of the aim and by sample size determination with error of 5%, confidence of 95%, a power of 80%, an effect size of 6.0 (mean=4 and standard deviation=6), and correlation of 70% using the formula:
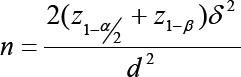
To consider the possibility of loss of 15% and the longitudinal nature of the study and sizes repeated, we used the formula:
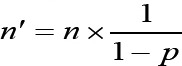
Finally, the sample size in each group was determined 28.
Inclusion Criteria
Married and menopause women, existence of moderate to severe dryness at the vaginal region, endometrial thickening with vaginal sonography maximum 5mm were among the inclusion criteria.
Exclusion Criteria
Smoking cigarette, and using anti-coagulate drugs (Heparin), topical hormonal and nonhormonal drugs one month before the study, existence of vaginal infection requiring treatment in the primary examination for Pup Smear, sensitivity (such as Rash, Erythema, Inflammation) to drug or its compounds, existence of doubtful or known history of hormone relative diseases (such as breast cancer, unknown cases of vaginal bleeding, severe Thrombophlebitis or Thromboebolism disorders related to Estrogen), existence of chronic diseases (such as cardiac diseases, hypertension, diabetes) .
At first, multi-stage sampling was done with no blinding eligible individuals. In this way, the researcher selected the existing centers in proportion to the number of referrals from 30 to 50 percent through simple sampling method. The design and protocol of the study is shown in figure 1.
Figure 1.
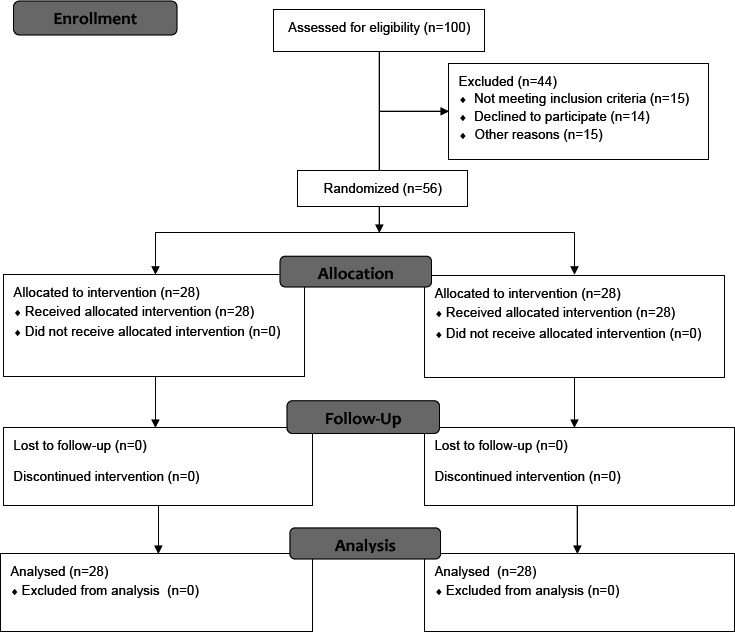
Consort flow diagram of participants
Then, the numbers from 1 to 80 were written on the same card and then put in a bag covered; then we assigned the odd numbers to one group (group A) and even numbers to the other group (group B). Groups of A and B (both groups had inclusion criteria) filled in the informed consent form. Group A received conjugated Estrogen 0.625 mg cream (production by Aborayhan Pharmaceutical Company) and group B received Hyaluronic Acid vaginal cream (containing 5 mg sodium salt), which had been prepared from Shiraz Pharmacy College.
Group A applied one applicator of drug (0.058 mg) every night before sleep for a period of two weeks and two times a week for the next six weeks and group B used one applicator (5 mg) every night before sleep for a period of 8 weeks. The manner of putting cream inside vagina, proper place and using at a specified time were explained for both groups and follow-up through telephone calls.
The study of the rate of vaginal atrophy symptoms at zero week (before treatment) and eighth week (after treatment) was carried out for both groups with a compound scale including vaginal dryness and itching, dyspareunia and urinary incontinence. Stress urinary incontinence (the urinary incontinence followed by increasing the intra-abdominal pressure at the time of sneezing, coughing, etc.) and urgency (sudden and severe feeling in urinary urgency) were considered in this study.
The severity of each sign of atrophy was evaluated by VAS (Visual Analog Signals) before and after the intervention and on the basis of four points scale in which zero=asymptomatic, one=mild (score 1 to 3), two=moderate (score 4 to 6) and three=severe (score 7 to 10) were propounded, respectively. The signs were evaluated by the researcher with attending the interview sessions.
To recognize the cellular maturation (before and after intervention) also by carrying out vaginal and cervical Pap Smear, the available rate and type of cells (para-basal, medial and surface) were determined. The Smear samples were colored with Ethyl alcohol 90.0%, studied as uni-blind by a cytologist (unaware of the type of treatment) and the maturation degree was calculated according to the formula [(percentage of surface cells x1)+(percentage of medial cells x0.5)+(percentage of Para-basal cellsx0)=maturation degree] with index: Lack 0-25, low estrogenic effect=26-49, moderate estrogenic effect=50-75, sevear estrogenic effect=76-100.9
The vaginal PH was studied before and after the intervention using PH indicator strip inserted into the vagina; its rate was divided into 4 degrees as zero (PH<5.0), one (PH=5.0-5.49), two (PH=5.5-6.49) and three (PH>6.49) (9). It is necessary to mention that the cytologist, sample taker, the type of PH marker band and the laboratory were fixed throughout the study. Finally, the collected data were analyzed through SPSS 20 software, using descriptive statistics, Chi-square, paired and independent t-tests, and retest by Wilcoxon and Mann-Whitney tests at the confidence interval of 95.0%.
RESULTS
The results showed that from the view point of age, menarche, age of menopause beginning, number of pregnancies, occupation, education, disease history and drug consumption both therapeutic groups were similar and the Chi-square test did not show any statistical significant difference (P>0.05) (table 1).
Table 1.
Demographic characteristics of the samples in the study groups
| Variables | Hyaluronic acid group (n=28) | Premarin group (n=28) | P value |
|---|---|---|---|
| Mean±SD | Mean±SD | ||
| Age (year) | 56.4±5.47 | 51.92±4.31 | 0.44 |
| Age menarche (year) | 13±1.26 | 12.5±1.40 | 0.139 |
| Age menopause (year) | 47.71±5.26 | 46.2±4.16 | 0.823 |
| Parity | 4.92±2.32 | 5±2 | 0.551 |
*Frequency Mean±SD Chi-square
As to the atrophy symptoms before and after the intervention, it was specified that vaginal dryness and itching and also dyspareunia were significantly improved after the intervention among the groups (P<0.001). The relief of vaginal dryness in the hyaluronic acid group was observed more in the intra-group comparison (P<0.05) and urinary incontinence was also improved in this group only (P<0.05).
On the other hand, studying the compound mean score from vaginal atrophy symptoms (itching, dryness, dyspareunia and urinary incontinence) after treatment showed a reduction in group A at a rate of 1.7±.62 and in group B at a rate of 3.32±0.76 (the mean after-the mean before the intervention) and a significant result was obtained (P<0.001). This reduction was more in the hyaluronic acid group; comparison of the results of both groups showed that the drug has had a better effect on group B (P<0.001) (table 2).
Table 2.
Comparison of the mean±SD within and between groups of the composite score of vaginal atrophy symptoms (vaginal dryness and itching, dyspareunia and urinary incontinence) before and after the treatment
| Composite score of vaginal atrophy symptoms | Premarin group (n=28) | P-value within groups | Hyaluronic acid group (n=28) | P-value within groups | P-value between groups |
|---|---|---|---|---|---|
| Mean±SD | Mean±SD | ||||
| Before treatment | 5.8±2.28 | *P<0.001 | 5.92±2.15 | *P<0.001 | 0.904 |
| After treatment | 4.10±1.66 | 2.60±1.39 | *P<0.001 |
* Mean±SD paired and independent t retest by Wilcoxon and Mann-Whitney tests
As to the vaginal cellular maturation, the results indicated the maturity of cells; both groups had a significant difference comparedwith the results before treatment (P<0.05) (table 3).
Table 3.
Comparison of the frequency of vaginal cell maturation index in the g roups treated before and after the intervention
| VCMIa | Premarin group N (%) | P value within groups | Hyaluronic acid group N (%) | P value within groups | P value between groups | |
|---|---|---|---|---|---|---|
| Before | None (0-25) | 10 (35.7) | *P<0.001 | 3 (10.7) | *P<0.001 | 0.593 |
| Mild (26-49) | 14 (50) | 25 (89.3) | ||||
| Moderate (50-75) | 4 (14.3) | 0 (0) | ||||
| Severe (76-100) | 0 (0) | 0 (0) | ||||
| After | None (0-25) | 0 (0) | 0 (0) | *0.018 | ||
| Mild (26-49) | 0 (0) | 1 (3.6) | ||||
| Moderate (50-75) | 25 (89.3) | 25 (89.3) | ||||
| Severe (76-100) | 3 (10.7) | 2 (7.1) | ||||
Vaginal Cell Maturation Index;
Paired and independent t test retest by Wilcoxon and Mann-Whitney tests
Also, studies showed that comparing the results of intra-group (before and after treatment), the amount of PH of both groups reduced, moving towards acidity (P<0.001) and no significant difference was obtained when the results of both groups were compared (P>0.05) (table 4).
Table 4.
Comparison of the frequency of vaginal pH in the groups treated before and after the intervention
| Vaginal pH | Premarin group N (%) | P value within groups | Hyaluronic acid group N (%) | P value within groups | P value between groups | |
|---|---|---|---|---|---|---|
| Before intervention | <5.0 | 11 (39.3) | *P<0.001 | 11 (39.3) | *P<0.001 | 0.507 |
| 5-5.49 | 1 (3.6) | 4 (14.3) | ||||
| 5.5-6.49 | 3 (10.7) | 3 (10.7) | ||||
| >6.49 | 13 (46.4) | 10 (35.7) | ||||
| After intervention | <5 | 12 (42.9) | 17 (60.7) | 0.463 | ||
| 5-5.49 | 6 (21.4) | 7 (25.1) | ||||
| 5.5-6.49 | 6 (21.4) | 2 (7.1) | ||||
| >6.49 | 4 (14.3) | 2 (7.1) | ||||
Paired and independent t-test retest by Wilcoxon and Mann-Whitney tests
DISCUSSION
The results showed that the women in both groups did not have any difference and were similar with respect to some of the demographic characteristics (P>0.05). In a study by Ziagham et-al. (2012) which compared the effect of hyaluronic acid vaginal suppository with vitamin E in the treatment of vaginal atrophy among menopause women, both groups were similar as to the age, menopause duration, occupation, educational level and economical status and had no statistical difference.28
In a study, the effect of Hyaluronic Acid vaginal tablet and Estradiol was compared on the vaginal atrophy for a period of 8 weeks; no significant difference was observed between the mean age and the age of menopause beginning among both groups.29 In another study, the researchers compared the effect of Genestine with Hyaluronic Acid on vaginal Atrophy also no significant difference was observed between age, menopause age and their effects on vaginal symptoms.30
The results of the research also indicated that both Hyaluronic Acid and Permarine improved the vaginal atrophy symptoms, cellular maturation increase and reduced PH; this improvement was sometimes more among the Hyaluronic Acid group.
We did not have access to the same study. But many researchers studied separately the effect of two aforesaid drugs on atrophy and their results were similar to those of the present study in selection of the objective group and effect of drug but there were some differences in terms of drug form and duration of the intervention.
In this respect, we reviewed some studies as Castelo-Branco C et al (2005) to study the management of post-menopausal vaginal atrophy and atrophic vaginitis with focuses on the changes involved in vaginal aging. It was shown that estrogen increased the content of the skin collagen, and hyaluronic acid to improve the skin moisture and genitourinary symptoms.31
Another researcher studied the effect of conjugated Estrogen cream in treatment of atrophy which was consumed daily or two times a week for a period of 12 weeks. The results indicated that applying both methods of using the drug caused more improvement in symptoms of atrophy, maturation index and vaginal PH significantly compared with the placebo group.32
In another study, the conjugated Estrogen vaginal cream was used twice a week for a period of 12 weeks for the treatment of vaginal atrophy. The results showed that Estrogen cream caused a reduction in the vaginal dryness, dyspareunia and PH and an increase in the vaginal cells maturation index.33 The findings of our study is similar to the results of the above studies in terms of improving atrophy symptoms after taking estrogen, but does not agree in the amount of consumption, the duration of intervention and use of a single drug without comparison with other drugs.
The effect of hyaluronic acid on treatment of vaginal atrophy was studied in other researc-, the results f which were similar to those of the present study as to improving the symptoms of atrophy in the genito-urinary system. Especially, the results of evaluating the effect of hyaluronic acid suppository of another study in Iran on the severity of the symptoms of vaginal Atrophy 2, 4 and 8 weeks after treatment became significant and more effect was observed in improvement of symptoms compared with the group receiving vitamin E.28 Our findings are similar to the mentioned study in terms of reduction of symptoms and duration of the use drugs but and doesn’t match in terms of drug form and comparison with vitamin E.
Another researcher studied the effectiveness of three drugs, i.e. hyluronic acid, vitamin E and A in the form of suppository for a period of one month firstly in continuous days and then every other day on menopaused women and the results showed that using hyaluronic acid caused a significant reduction in signs and symptoms of vaginal atrophy.25
The results of the present study showed a significant reduction in symptoms with using hyluronic acid that is similar to the results obtained by Castantino and Guaraldi’s study. But is there was a difference in terms of comparison of vitamin A and E and drug form.
It is also reported that the effect of the gel of hyaluronic acid on the symptoms of vaginal atrophy was due to the chemo-therapy started from the first week.28 Our results are in the same line with Tea et al.’s study in terms of improving the symptoms using hyaloronic acid and is different in terms of drug form, target - group and comparison with other drugs.
Another study on the effect of prescribing genestine vaginal suppository compared with hyaluronic acid suppository on the epithelium atrophy showed that using drugs for 15 continuous days in each month for a period of 3 months improved the symptoms of vaginal dryness and itching, dyspareunia, colposcopy specialties and the rate of vaginal cellular maturation.30 The results of our study were similar to those of Le Donne et-al.’s survey in term of drug effects on atrophy and drug type, but they were different as to the drug form and duration of the intervention.
To the best of our knowledge, no study was previously done in Iran that used hyaloronic acid cream for vaginal atrophy treatment. The variety of admitted patients to the clinic and finding the menopause women from different department were the limitations of this study.
CONCLUSION
This study showed the better and more relief of the symptoms of urinary incontinence, cellular maturation and vaginal dryness in menopause women in the hyaluronic acid cream therapeutic group compared with Estrogen-therapy group. Therefore, the hyaluronic acid could be a suitable alternative for those women who suffer from the complications of atrophy of the genital system and those with medical contraindications or negative experience in using hormonal drugs.
ACKNOWLEDGEMENTS
The current paper was derived from thesis written by Tayebe Davari and financially supported by the Deputy of Research and Technology, Shiraz University of Medical Sciences under grant no. 6681. Hereby, we express our gratitude and thanks to all cooperating and assisting in the performance of the research project.
Conflict of Interest: None declared.
REFERENCES
- 1.Parnan Emamverdikhan A, Golmakani N, Sharifi Sistani N, et al. Comparing Two Treatment Methods of Vitamin E Suppository and Conjugated Estrogen Vaginal Cream on the Quality of Life in Menopausal Women with Vaginal Atrophy. J MidwiferyReprod Health. 2014;2:253–61. [Google Scholar]
- 2.Jenkins MR, Sikon AL. Update on nonhormonal approaches to menopausal management. Cleveland Clinic Journal of Medicine. 2008;75:S17–24. doi: 10.3949/ccjm.75.suppl_4.s17. [DOI] [PubMed] [Google Scholar]
- 3.Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clinic Proceedings. 2010;85:87–94. doi: 10.4065/mcp.2009.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14:355–69. doi: 10.1097/gme.0b013e31805170eb. [DOI] [PubMed] [Google Scholar]
- 5.Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49:292–303. doi: 10.1016/j.maturitas.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Crandall C, Petersen L, Ganz PA, Greendale GA. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519–30. doi: 10.1097/01.gme.0000117061.40493.ab. [DOI] [PubMed] [Google Scholar]
- 7.Mehta A, Bachmann G. Vulvovaginal complaints. Clinical Obstetrics and Gynecology. 2008;51:549–55. doi: 10.1097/GRF.0b013e3181809a26. [DOI] [PubMed] [Google Scholar]
- 8.Kingsberg SA, Kellogg S, Krychman M. Treating dyspareunia caused by vaginal atrophy: a review of treatment options using vaginal estrogen therapy. International Journal of Women’s Health. 2009;1:105–11. doi: 10.2147/ijwh.s4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Society of Obstetricians and Gynaecologists of Canada. The detection and management of vaginal atrophy. Number 145, May 2004. Int J Gynaecol Obstet. 2005;88:222–8. doi: 10.1016/j.ijgo.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Lynch C. Vaginal estrogen therapy for the treatment of atrophic vaginitis. Journal of Women’s Health. 2009;18:1595–606. doi: 10.1089/jwh.2008.1281. [DOI] [PubMed] [Google Scholar]
- 11.Pandit L, Ouslander JG. Postmenopausal vaginal atrophy and atrophic vaginitis. The American Journal of the Medical Sciences. 1997;314:228–31. doi: 10.1097/00000441-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Johnston A. Estrogens–pharmacokinetics and pharmacodynamics with special reference to vaginal administration and the new estradiol formulation–Estring. Acta Obstet Gynecol Scand Suppl. 1996;163:16–25. [PubMed] [Google Scholar]
- 13.Bachmann GA, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. American Family Physician. 2000;61:3090–6. [PubMed] [Google Scholar]
- 14.Cardozo L, Lose G, McClish D, et al. A systematic review of estrogens for recurrent urinary tract infections: third report of the hormones and urogenital therapy (HUT) committee. International Urogynecology Journal and Pelvic Floor Dysfunction. 2001;12:15–20. doi: 10.1007/s001920170088. [DOI] [PubMed] [Google Scholar]
- 15.Botsis D, Kassanos D, Antoniou G, et al. Transvaginal sonography in postmenopausal women treated with low-dose estrogens locally administered. Maturitas. 1996;23:41–5. doi: 10.1016/0378-5122(95)00951-5. [DOI] [PubMed] [Google Scholar]
- 16.Lupulescu A. Estrogen Use and Cancer Incidence: A Review. Cancer Investigation. 1995;13:287–95. doi: 10.3109/07357909509094464. [DOI] [PubMed] [Google Scholar]
- 17.Stika CS. Atrophic vaginitis. Dermatologic Therapy. 2010;23:514–22. doi: 10.1111/j.1529-8019.2010.01354.x. [DOI] [PubMed] [Google Scholar]
- 18.Handa VL, Bachus KE, Johnston WW, et al. Vaginal administration of low-dose conjugated estrogens: systemic absorption and effects on the endometrium. Obstetrics and Gynecology. 1994;84:215–8. [PubMed] [Google Scholar]
- 19.Rioux JE, Devlin C, Gelfand MM, et al. 17beta-estradiol vaginal tablet versus conjugated equine estrogen vaginal cream to relieve menopausal atrophic vaginitis. Menopause. 2000;7:156–61. doi: 10.1097/00042192-200007030-00005. [DOI] [PubMed] [Google Scholar]
- 20.Johnston SL, Farrell S, Bouchard C, et al. The detection and management of vaginal atrophy. Journal of obstetrics and gynaecology Canada: JOGC. 2004;26:503–15. doi: 10.1016/s1701-2163(16)30662-4. [DOI] [PubMed] [Google Scholar]
- 21.Carter J, Goldfrank D, Schover LR. Simple strategies for vaginal health promotion in cancer survivors. The Journal of Sexual Medicine. 2011;8:549–59. doi: 10.1111/j.1743-6109.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- 22.Morali G, Polatti F, Metelitsa EN, et al. Open, non-controlled clinical studies to assess the efficacy and safety of a medical device in form of gel topically and intravaginally used in postmenopausal women with genital atrophy. Arzneimittel-Forschung. 2006;56:230–8. doi: 10.1055/s-0031-1296715. [DOI] [PubMed] [Google Scholar]
- 23.Mazaro-Costa R, Andersen ML, Hachul H, Tufik S. Medicinal plants as alternative treatments for female sexual dysfunction: utopian vision or possible treatment in climacteric women? The Journal of Sexual Medicine. 2010;7:3695–714. doi: 10.1111/j.1743-6109.2010.01987.x. [DOI] [PubMed] [Google Scholar]
- 24.Costantino D, Guaraldi C. Effectiveness and safety of vaginal suppositories for the treatment of the vaginal atrophy in postmenopausal women: an open, non-controlled clinical trial. Eur Rev Med Pharmacol Sci. 2008;12:411–6. [PubMed] [Google Scholar]
- 25.Goa KL, Benfield P. Hyaluronic acid. A review of its pharmacology and use as a surgical aid in ophthalmology, and its therapeutic potential in joint disease and wound healing. Drugs. 1994;47:536–66. doi: 10.2165/00003495-199447030-00009. [DOI] [PubMed] [Google Scholar]
- 26.Duranti F, Salti G, Bovani B, et al. Injectable hyaluronic acid gel for soft tissue augmentation. A clinical and histological study. Dermatologic Surgery. 1998;24:1317–25. doi: 10.1111/j.1524-4725.1998.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 27.Panay N, Maamari R. Treatment of postmenopausal vaginal atrophy with 10-µg estradiol vaginal tablets. Menopause International. 2012;18:15–9. doi: 10.1258/mi.2012.011120. [DOI] [PubMed] [Google Scholar]
- 28.Ziagham S, Abbaspour Z, Abbaspour MR. The comparison betweenthe effects of hyaluronic acid vaginal suppository and vitamin E on the treatment of atrophic vaginitis in menopausal women. Arak Medical University Journal. 2012;15:57–64. [In Persian] [Google Scholar]
- 29.Ekin M, Yasar L, Savan K, et al. The comparison of hyaluronic acid vaginal tablets with estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Archives of Gynecology and Obstetrics. 2010;283:539–43. doi: 10.1007/s00404-010-1382-8. [DOI] [PubMed] [Google Scholar]
- 30.Le Donne M, Caruso C, Mancuso A, et al. The effect of vaginally administered genistein in comparison with hyaluronic acid on atrophic epithelium in postmenopause. Archives of Gynecology and Obstetrics. 2011;283:1319–23. doi: 10.1007/s00404-010-1545-7. [DOI] [PubMed] [Google Scholar]
- 31.Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52:S46–52. doi: 10.1016/j.maturitas.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Bachmann G, Bouchard C, Hoppe D, et al. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause. 2009;16:719–27. doi: 10.1097/gme.0b013e3181a48c4e. [DOI] [PubMed] [Google Scholar]
- 33.Freedman M, Kaunitz AM, Reape KZ, et al. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16:735–41. doi: 10.1097/gme.0b013e318199e734. [DOI] [PubMed] [Google Scholar]


