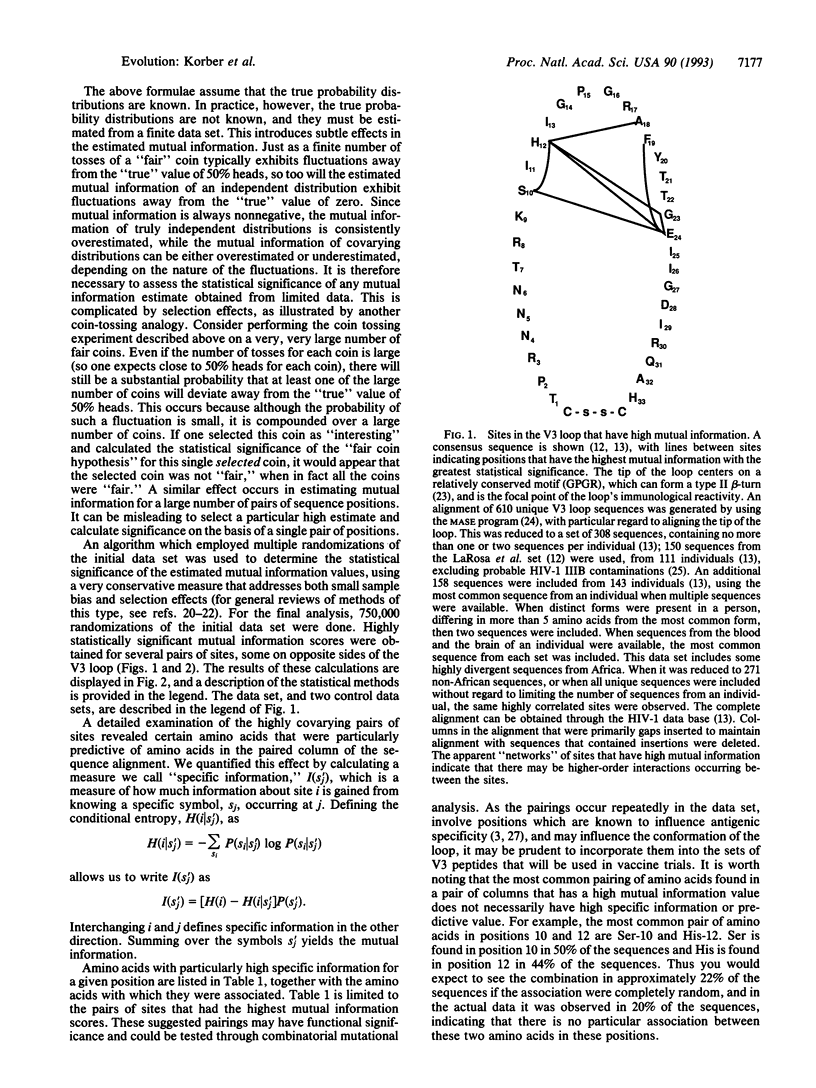
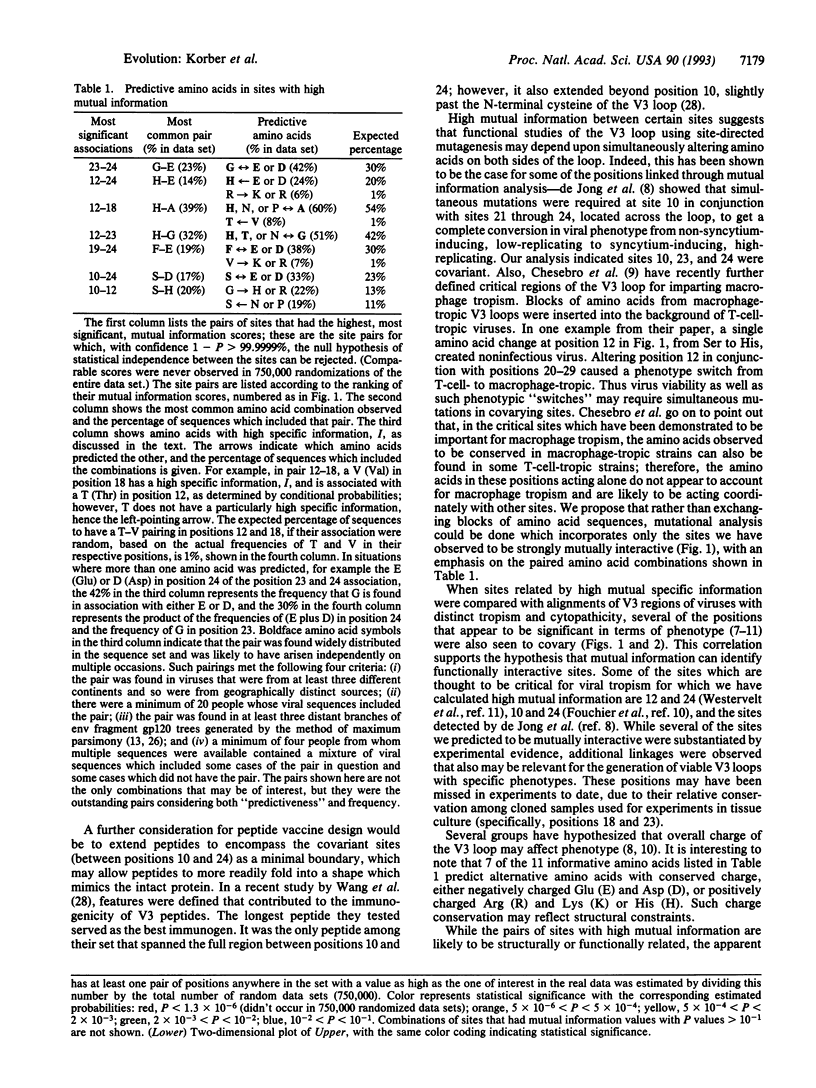
Abstract
The V3 loop of the human immunodeficiency virus type 1 (HIV-1) envelope protein is a highly variable region that is both functionally and immunologically important. Using available amino acid sequences from the V3 region, we have used an information theoretic quantity called mutual information, a measure of covariation, to quantify dependence between mutations in the loop. Certain pairs of sites, including non-contiguous sites along the sequence, do not have independent mutations but display considerable, statistically significant, covarying mutations as measured by mutual information. For the pairs of sites with the highest mutual information, specific amino acids were identified that were highly predictive of amino acids in the linked site. The observed interdependence between variable sites may have implications for structural or functional relationships; separate experimental evidence indicates functional linkage between some of the pairs of sites with high mutual information. Further specific mutational studies of the V3 loop's role in determining viral phenotype are suggested by our analyses. Also, the implications of our results may be important to consider for V3 peptide vaccine design. The methods used here are generally applicable to the study of variable proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandrasekhar K., Profy A. T., Dyson H. J. Solution conformational preferences of immunogenic peptides derived from the principal neutralizing determinant of the HIV-1 envelope glycoprotein gp120. Biochemistry. 1991 Sep 24;30(38):9187–9194. doi: 10.1021/bi00102a009. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Nishio J., Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992 Nov;66(11):6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B., Tibshirani R. Statistical data analysis in the computer age. Science. 1991 Jul 26;253(5018):390–395. doi: 10.1126/science.253.5018.390. [DOI] [PubMed] [Google Scholar]
- Farber R., Lapedes A., Sirotkin K. Determination of eukaryotic protein coding regions using neural networks and information theory. J Mol Biol. 1992 Jul 20;226(2):471–479. doi: 10.1016/0022-2836(92)90961-i. [DOI] [PubMed] [Google Scholar]
- Faulkner D. V., Jurka J. Multiple aligned sequence editor (MASE). Trends Biochem Sci. 1988 Aug;13(8):321–322. doi: 10.1016/0968-0004(88)90129-6. [DOI] [PubMed] [Google Scholar]
- Fouchier R. A., Groenink M., Kootstra N. A., Tersmette M., Huisman H. G., Miedema F., Schuitemaker H. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J Virol. 1992 May;66(5):3183–3187. doi: 10.1128/jvi.66.5.3183-3187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J., Debouck C., Meloen R. H., Smit L., Bakker M., Asher D. M., Wolff A. V., Gibbs C. J., Jr, Gajdusek D. C. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M. K., Palker T. J., Matthews T. J., Langlois A. J., Lerche N. W., Martin M. E., Scearce R. M., McDanal C., Bolognesi D. P., Haynes B. F. Synthetic peptides containing T and B cell epitopes from human immunodeficiency virus envelope gp120 induce anti-HIV proliferative responses and high titers of neutralizing antibodies in rhesus monkeys. J Immunol. 1990 Oct 15;145(8):2677–2685. [PubMed] [Google Scholar]
- Holley L. H., Goudsmit J., Karplus M. Prediction of optimal peptide mixtures to induce broadly neutralizing antibodies to human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6800–6804. doi: 10.1073/pnas.88.15.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., LaRosa G. J., Profy A. T., Bolognesi D. P., Herlihy W. C., Putney S. D., Matthews T. J. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990 Dec 14;250(4987):1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- Korber B., Wolinsky S., Haynes B., Kunstman K., Levy R., Furtado M., Otto P., Myers G. HIV-1 intrapatient sequence diversity in the immunogenic V3 region. AIDS Res Hum Retroviruses. 1992 Aug;8(8):1461–1465. doi: 10.1089/aid.1992.8.1461. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Davide J. P., Weinhold K., Waterbury J. A., Profy A. T., Lewis J. A., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science. 1990 Aug 24;249(4971):932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Weinhold K., Profy A. T., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P., Bolognesi D. P., Matthews T. J., Emini E. A. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant: further clarifications. Science. 1991 Sep 6;253(5024):1146–1146. doi: 10.1126/science.1887238. [DOI] [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Nakagawa Y., Pendleton C. D., Houghten R. A., Yokomuro K., Germain R. N., Berzofsky J. A. Induction of broadly cross-reactive cytotoxic T cells recognizing an HIV-1 envelope determinant. Science. 1992 Jan 17;255(5042):333–336. doi: 10.1126/science.1372448. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Looney D. J., Li M. L., Walfield A. M., Ye J., Hosein B., Tam J. P., Wong-Staal F. Long-term high-titer neutralizing activity induced by octameric synthetic HIV-1 antigen. Science. 1991 Oct 11;254(5029):285–288. doi: 10.1126/science.254.5029.285. [DOI] [PubMed] [Google Scholar]
- Westervelt P., Gendelman H. E., Ratner L. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westervelt P., Trowbridge D. B., Epstein L. G., Blumberg B. M., Li Y., Hahn B. H., Shaw G. M., Price R. W., Ratner L. Macrophage tropism determinants of human immunodeficiency virus type 1 in vivo. J Virol. 1992 Apr;66(4):2577–2582. doi: 10.1128/jvi.66.4.2577-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs T. F., Zwart G., Bakker M., Valk M., Kuiken C. L., Goudsmit J. Naturally occurring mutations within HIV-1 V3 genomic RNA lead to antigenic variation dependent on a single amino acid substitution. Virology. 1991 Nov;185(1):195–205. doi: 10.1016/0042-6822(91)90767-6. [DOI] [PubMed] [Google Scholar]
- de Jong J. J., Goudsmit J., Keulen W., Klaver B., Krone W., Tersmette M., de Ronde A. Human immunodeficiency virus type 1 clones chimeric for the envelope V3 domain differ in syncytium formation and replication capacity. J Virol. 1992 Feb;66(2):757–765. doi: 10.1128/jvi.66.2.757-765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]