Abstract
Background
Sugarcane has been used as the main crop for ethanol production for more than 40 years in Brazil. Recently, the production of bioethanol from bagasse and straw, also called second generation (2G) ethanol, became a reality with the first commercial plants started in the USA and Brazil. However, the industrial processes still need to be improved to generate a low cost fuel. One possibility is the remodeling of cell walls, by means of genetic improvement or transgenesis, in order to make the bagasse more accessible to hydrolytic enzymes. We aimed at characterizing the cell wall proteome of young sugarcane culms, to identify proteins involved in cell wall biogenesis. Proteins were extracted from the cell walls of 2-month-old culms using two protocols, non-destructive by vacuum infiltration vs destructive. The proteins were identified by mass spectrometry and bioinformatics.
Results
A predicted signal peptide was found in 84 different proteins, called cell wall proteins (CWPs). As expected, the non-destructive method showed a lower percentage of proteins predicted to be intracellular than the destructive one (33 % vs 44 %). About 19 % of CWPs were identified with both methods, whilst the infiltration protocol could lead to the identification of 75 % more CWPs. In both cases, the most populated protein functional classes were those of proteins related to lipid metabolism and oxido-reductases. Curiously, a single glycoside hydrolase (GH) was identified using the non-destructive method whereas 10 GHs were found with the destructive one. Quantitative data analysis allowed the identification of the most abundant proteins.
Conclusions
The results highlighted the importance of using different protocols to extract proteins from cell walls to expand the coverage of the cell wall proteome. Ten GHs were indicated as possible targets for further studies in order to obtain cell walls less recalcitrant to deconstruction. Therefore, this work contributed to two goals: enlarge the coverage of the sugarcane cell wall proteome, and provide target proteins that could be used in future research to facilitate 2G ethanol production.
Electronic supplementary material
The online version of this article (doi:10.1186/s12870-015-0677-0) contains supplementary material, which is available to authorized users.
Keywords: Cell wall protein, Saccharum sp, Stem, Proteomics, Second generation ethanol
Background
The use of Saccharum sp. to produce second generation (2G) ethanol can reduce waste and increase the yield without expanding the crop area, contributing to a cleaner, more efficient and more sustainable production. However, from the economic point of view, the costs of the process need to be reduced, mostly those related to the enzymes used to deconstruct plant cell walls. Therewith, research is mainly focused on the identification of new enzymes that could efficiently degrade cell walls [1]. Other studies have been developed from the biomass perspective, describing the plant cell wall components [2–5], and even altering them attempting to achieve a higher ethanol 2G yield. Since pre-treatments facilitate cell wall digestibility to increase ethanol production, when altering plant cell wall components, focus should be either on lignin- carbohydrate complex cleavage and hemicellulose removal, or lignin modification and even on redistribution and cellulose decrystallization [6].
Plant cell walls are mainly composed of polysaccharides and cell wall proteins (CWPs) [7]. Proteomics studies have revealed the large diversity of CWPs [8–10]. They have been grouped in different functional classes according to predicted functional domains and experimental data: polysaccharide modifying proteins, oxido-reductases and proteases, have been found as major classes. Structural proteins such as hydroxyproline-rich glycoproteins, namely extensins, arabinogalactan proteins and hydroxyproline/proline-rich proteins, have been estimated to account for about 10 % of the cell wall mass in dicots [11] and approximately 1 % in monocots [12]. However, only a few of them have been identified in proteomics studies. CWPs are involved in growth and development, signaling and defense against pathogens. They virtually take part in most functions of the cells [4, 11, 13]. They can affect cell fate, being able to sense stress signals and transmitting them to the cell interior [14]. They can also have tissue-specific functions , such as playing roles in cuticle formation [15]. Due to this versatility, plant cell walls are the subject of many fields of research.
In the case of grasses, type II-cell walls present specific features [7]. The cellulose microfibrils are interlocked by glucuronoarabinoxylans, instead of xyloglucans of type I-cell walls. In addition, the grass cell walls contain a substantial portion of non-cellulosic polymers ‘wired on’ the microfibrils by alkali-resistant phenolic linkages.
As mentioned above, plant cell walls contain enzymes capable of modifying the cell wall matrix [16]: endoglucanases which cleave the polysaccharide backbones; glycosidases which remove side chains; transglycosylases which cut the polysaccharides and link them together; esterases which remove methyl groups of pectins, and cleave ester bonds in polysaccharide chains; and class III peroxidases (Prxs) which form or break phenolic bonds. Altogether, these enzymes offer many possibilities to modify the structure and the mechanical properties of cell walls, and thus biomass structure [3]. Besides, the addition of plant glycosidases during the hydrolysis of corn stover could increase the ethanol yield [17]. These examples show that the repertoire of CWPs could provide interesting tools to improve the deconstruction of cell walls.
As commonly known, classical CWPs share common features. The first one is a signal peptide at the N-terminus of the protein which is responsible for their targeting to the endoplasmic reticulum (ER) [18], the first organelle of the secretory pathway [19]. The signal peptide is not formed by a consensus amino acid sequence. However, it has a positively charged n-region at its N-terminus and a central hydrophobic h-region followed by a polar c-region at its C-terminus comprising the cleavage site [20]. In addition, CWPs do not possess the canonical ER retention signal KDEL or HDEL tetrapeptide at their C-terminus [19, 20]. The third feature is that they do not present a trans-membrane domain. When passing through the secretory pathway, proteins go from ER to the Golgi complex in order to be packed into vesicles and directed to be secreted. Plasma membrane proteins show the same features as CWPs except that they have a trans-membrane domain [20, 21].
Cell wall proteomics require challenging strategies comprising several steps, from the extraction to the identification of the proteins, compared to other sub-cellular proteomics works. Despite the technical hurdles, a lot of studies have been successful [8, 9]. Several aerial organs have been studied in different plant species, such as alfalfa [22], Linum usitatissimum [23], Solanum tuberosum [24], and Arabidopsis thaliana [25]. In Brachypodium distachyon leaves and stems, different classes of proteins have been identified and it was possible to address some of them to the mechanism of 2G biofuel production [26]. It is then possible to alter their expression to improve cell walls deconstruction, such as the upregulation of a cell wall transcript in rice [27].
In a recent publication, 69 CWPs have been described from isolated cells obtained from cell suspension cultures of sugarcane [28]. However, the description of the cell wall proteome from a differentiated organ is still missing. In this work, two different strategies were developed to extract the CWPs of two month-old stems: either a destructive method (DT Method) or a non-destructive one (ND Method), i.e. vacuum infiltration [29]. Proteins were identified by mass spectrometry (MS) and bioinformatics. The results were compared regarding the number and the type of CWPs. Quantitative MS data were used to identify the most abundant CWPs in sugarcane culms.
Results
Extraction of proteins from cell walls
Two-month-old sugarcane culms were selected for presenting a soft and young material, at an early stage of development. The use of young organs could lead to the identification of proteins involved in cell wall expansion, thus clarifying the mechanisms that the plant itself uses to allow growth.
Sugarcane features four stages of development: (i) germination and emergence, (ii) tillering phase, (iii) grand growth period and (iv) ripening phase, when sugar accumulates [30]. The tillering phase begins about 40 days after planting and can last up to 120 days, being the early stage of plant development [31, 32]. In this work, plants were collected 60 days after planting, halfway from the maximum tillering, measuring around 40–50 cm in height from the bottom to the upper leaf. This age was also chosen to allow distinguishing leaves and culms visually.
The DT Method was a destructive one relying on the grinding of the material and its centrifugation in solutions of increasing sucrose concentration. On the contrary, the ND Method was a non-destructive one, since it maintained the cell structures intact while performing the extraction of CWPs by vacuum infiltration of the tissues. Thus, it was expected that the DT Method would be able to extract more wall-bound proteins than the ND one. In both protocols, protein extraction from cell walls was performed using 0.2 M CaCl2 and 2 M LiCl. The efficiency of CaCl2 to release CWPs could rely on the fact that demethylesterified homogalacturonans strongly chelate calcium [33], solubilizing weakly-bound proteins by a competition mechanism [34]. On the other hand, LiCl was used to extract mostly hydroxyproline-rich glycoproteins [35] All the experiments were performed in duplicates.
The DT Method produced around 518 μg of proteins from 35 g of culms (fresh weight). Regarding the ND Method, the yield was slightly lower: around 667 μg of proteins were recovered from about 50 g of culms (fresh weight). Figure 1 shows the patterns of the proteins extracted from sugarcane culms. The presence of thin resolved bands after staining showed the quality of the procedure with no degradation pattern. Each biological replicate, using either method, showed a pattern very similar to that of its counterpart and each method gave rise to a different pattern.
Fig. 1.
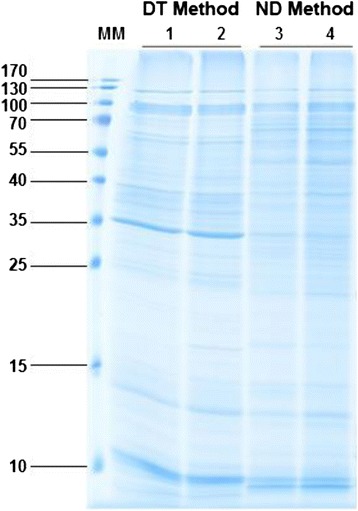
1D-electrophoresis of proteins extracted form 2-month-old sugarcane culm cell walls. Proteins have been extracted using either the DT or the ND Method. The biological repeats corresponding to each Methods are respectively numbered 1–2 and 3–4. The molecular mass markers (MM) are indicated in kDa on the left
Identification of proteins by MSE and bioinformatics analyses
Proteins were analyzed by shotgun LC-MS/MS, after tryptic digestion. The identification of proteins was performed using the translated-SUCEST database containing ESTs [36]. Homologous genes in Sorghum bicolor, the closest related species with a fully sequenced genome, were systematically searched for. Predictions of sub-cellular localization and functional domains were done on translated ESTs when they were full-length, otherwise on homologous S. bicolor coding sequences. Because of the high level of ploidy of the sugarcane genome [37], in some cases, different ESTs matched the same S. bicolor gene.
More detailed results of MS analyses, such as protein score and number of matched peptides, can be found in Additional files 1, 2, 3 and 4. About 65 % and 82 % of the proteins identified were found in both biological replicates, in the DT and ND Methods, respectively. These Methods allowed the identification of 70 and 103 different proteins from the translated-SUCEST database, respectively. From these, 39 (56 %) and 69 (67 %) proteins respectively had a predicted signal peptide, no known intracellular retention signal such as an endoplasmic retention signal and one trans-membrane domain at most (Table 1). These proteins were considered as CWPs (Additional file 5), and the others as intracellular proteins (Additional file 6). The DT and ND Methods lead to the identification of different sets of proteins.
Table 1.
CWPs identified in sugarcane young culms
| SUCEST accession numbera | Number of peptidesb | Number of unique peptidesb | Protein score | Femtomole average | S. bicolor homologues | Functional annotation | Extraction method |
|---|---|---|---|---|---|---|---|
| Proteins acting on polysaccharides | |||||||
| SCCCCL3001B10.b | 16; 16 | 3; 6 | 4368.653; 1207.559 | 87.40605 | Sb01g010840.1 | GH1 | ND |
| SCJFLR1017E03 | 7; 8 | 1; 1 | 627.7585; 389.633 | 4.65435 | Sb01g010840.1 | GH1 | ND |
| SCEQLB1066E08 | 11; 5 | 9; 2 | 1258.872; 452.9845 | 6.72165 | Sb01g010825.1 | GH1 | ND |
| SCEQHR1082B01 | 9; 7 | 8; 6 | 2256.574; 388.3896 | 28.182652 | Sb02g028400.1 | GH1 | ND |
| SCEZLB1007A09 | 18; 12 | 11; 8 | 4982.336; 2259.83 | 41.55905 | Sb01g008030.2 | GH3 | ND |
| SCEQLR1093F09* | 12; 18–20; 14 | 5; 8–6; 5 | 523.6582; 1309.333 – 3688.09; 10810.11 | 17.23065 – 50.3928 | Sb01g008040.3* | GH3 | DT - ND2 |
| SCCCCL4009F05 | 20; 14 | 16; 12 | 10955.92; 8452.891 | 156.84746 | Sb06g030270.1 | GH3 | ND |
| SCQSAM1030G04 | 3; 2 | 3; 2 | 8506.176; 6709.646 | 71.61725 | Sb06g030270.1 | GH3 | ND |
| SCQSRT2031D12 | 10; 1 | 7; 9 | 1169.301; 2716.698 | 34.5685 | Sb03g045490.1 | GH17 | ND |
| SCVPRZ3029G05 | 3; 2 | 2; 1 | 853.3968; 815.915 | 15.9117 | Sb03g040600.1 | GH18 | ND |
| SCJLLB2076C12 | 9; 8 | 4; 6 | 3324.314; 1244.686 | 40.19725 | Sb06g021220.1 | GH19 | ND |
| SCEZRZ3015E11 | 8; 5 | 6; 5 | 3106.044; 2091.409 | 55.74035 | Sb01g048140.1 | GH19 | ND |
| SCCCCL5004G07 | 8; 8 | 5; 6 | 7937.636; 2173.338 | 76.40835 | Sb10g000660.1 | GH28 | ND |
| SCJFRT1007G04 | 4; 2 | 1; 1 | 4752.977; 2164.125 | 31.240002 | Sb10g000660.1 | GH28 | ND |
| SCCCCL6004H07 | 9; 8 | 7; 7 | 633.8843; 542.2719 | 19.80175 | Sb01g040750.1 | GH35 | ND |
| SCVPRZ3029F03 | 6; 4 | 4; 4 | 1035.417; 246.8027 | 8.5248 | Sb03g029700.1 | Acyl esterase (homologous to AtPMR5) | ND |
| SCSGLR1025E03 | 5; 2–5; 8 | 4; 2–4; 8 | 460.239; 887.9516- 1045.544; 226,6407 | 19.8042 – 13.1516 | Sb02g042780.1 | Pectin methylesterase (carbohydrate esterase family 8, CE8) | DT – ND |
| Oxido-reductases | |||||||
| SCCCRZ1002B03 | 8; 1 | 3; 2 | 708.6577; 447.837 | 12.9346 | Sb01g041770.1 | Prx homologous to SbPrx20 | DT |
| SCCCRT1001G12 | 9; 9–14; 9 | 5; 6–7; 5 | 2689.641; 2574.119 – 10308.06; 9888.154 | 65.36725 – 77.029495 | Sb04g008590.1 | Prx homologous to SbPrx71 | DT - ND |
| SCCCLB1004B09* | 9; 16 | 4; 4 | 690.8371; 1079.03 | 26.908451 | Sb10g027490.1* | Prx homologous to SbPrx139 | DT |
| SCEQRT2030A04* | 7; 12 | 1; 3 | 306.9711; 658.3382 | 7.35725 | Sb10g027490.1 | Prx homologous to SbPrx139 | DT |
| SCCCLR1C03A09 | 12; 11 | 8; 7 | 845.2605; 759.2087 | 46.113102 | Sb09g004650.1* | Prx homologous to SbPrx115 | DT |
| SCCCLR1C05G08* | 11; 11 | 5; 8 | 1494.011; 1461.387 | 66.7759 | Sb03g024460.1* | Prx homologous to SbPrx65 | DT |
| SCRLAD1042E05 | 6; 4–5; 2 | 1; 2–1; 1 | 2528.694; 933.4598 – 873.3041; 1444.467 | 17.7972 – 10.54785 | Sb09g002740.1* | Prx homologous to SbPrx108 | DT – ND |
| SCVPRZ2035F03* | 11; 8–9; 5 | 6; 6–4; 3 | 2417.947; 1151.436 – 1401.302; 1033.822 | 42.28915 – 17.264 | Sb09g002740.1 | Prx homologous to SbPrx108 | DT - ND |
| SCVPLB1020D03 | 2; 8 | 2; 7 | 372.5895; 854.9581 | 23.364399 | Sb03g046760.1 | Prx homologous to SbPrx68 | DT |
| SCEPRZ1011A06* | 7; 11–12; 6 | 3; 5–4; 3 | 866.9835; 940.5853 – 5970.014; 1026.785 | 17.7399 – 45.46655 | Sb03g010250.1* | Prx homologous to SbPrx54 | DT - ND |
| SCCCAD1001B08 | 3; 3 | 1; 1 | 9547.608; 3981.619 | Identified | Sb03g010740.1 | Prx homologous to SbPrx55 | ND |
| SCJFRZ2013F04 | 7; 1 | 1; 1 | 23916.97; 9892.667 | 7.78455 | ND | ||
| SCJLRT1019B02 | 9; 6 | 1; 1 | 16559.67; 6310.499 | 14.36725 | ND | ||
| SCEQRT1024D03 | 1; 1 | 3; 2 | 16709.58; 4972.862 | 60.093697 | Sb03g010740.1 | Prx homologous to SbPrx55 | ND |
| SCCCAD1001C08 | 6; 5 | 3; 4 | 7855.956; 13571.95 | 28.358952 | Sb02g042860.1 | Prx homologous to SbPrx47 | ND |
| SCQSST3114C09 | 5; 8 | 5; 4 | 2082.782; 583.5138 | 16.338501 | Sb01g031740.1 | Prx homologous to SbPrx14 | ND |
| SCBFFL4112F05 | 2; 3 | 1; 2 | 1435.365; 4620.795 | 35.5025 | Sb06g018350.1 | Blue copper binding protein (plastocyanin) | DT |
| SCRFHR1006G03 | 3; 2 | 2; 1 | 391.4637; 1712.47 | 1.22305 | Sb01g010510.1 | Blue copper binding protein (plastocyanin) | DT |
| SCJLLR1104H07 | 3; 3 | 3; 3 | 912.4164; 417.4785 | 15.684 | Sb07g011870.1 | blue copper binding protein (plastocyanin) | ND |
| SCEPAM1021H07 | 8; 3 | 3; 3 | 924.3445; 347.9209 | 12.423151 | Sb10g027270.1 | Multicopper oxidase | ND |
| Proteins related to lipid metabolism | |||||||
| SCCCAM2002F12 | 4; 5–4; 5 | 1; 1–1; 1 | 716.9828; 1069.318 – 5961.475; 9251.332 | 19.3443 – 115.4035 | Sb03g038280.1 | LTP | DT – ND |
| SCBFLR1046E09 | 5; 6–4; 5 | 1; 1–1; 1 | 816.9942; 1488.674 – 14868.01; 16290.19 | 34.07175 – 242.60735 | Sb03g038280.1 | LTP | DT - ND |
| SCVPRZ2039B03 | 5; 6–4; 5 | 1; 1–1; 1 | 816.9942; 2065.907 – 14868.01; 16290.19 | Identified - identified | DT - ND | ||
| SCVPRZ2041C11 | 5; 6–4; 5 | 1; 1–1; 1 | 902.0069; 1488.674 – 18617.13; 24758.37 | 8.77335 - identified | DT - ND | ||
| SCCCLR1072C06 | 3; 2–6; 5 | 1; 1–1; 1 | 243.1648; 2495.928 – 35001.54; 23317.92 | 6.5461 – 132.9761 | Sb08g002700.1 | LTP | DT - ND |
| SCRFLR1012A10 | 3; 2–7; 5 | 1; 1–1; 1 | 345.6155; 2314.583 – 35158.61; 23317.92 | Identified - identified | DT – ND | ||
| SCEPRT2047G01 | 10; 5 | 1; 1 | 35204.81; 23919.76 | 250.87096 | ND | ||
| SCEZLR1031G07 | 5; 4 | 1; 1 | 34256.59; 23296.33 | Identified | ND | ||
| SCRUSB1064D08 | 9; 5 | 1; 1 | 35280.34; 23317.92 | Identified | ND | ||
| SCEPLB1044H04 | 3; 4–3; 4 | 1; 1–1; 1 | 3924.14; 3159.724 – 1548.66; 926.7365 | 189.36455 – 46.1548 | Sb01g049830.1 | LTP | DT – ND |
| SCEZLB1006F09 | 3; 4–3; 2 | 1; 1–1; 1 | 6772.019; 11919.76 – 8845.361; 13343.73 | 194.30121 – 146.9349 | Sb08g002670.1 | Protease inhibitor/seed storage/LTP family | DT - ND |
| SCCCLR1048F06 - SCCCLR1048F06 | 10; 13–5; 4 | 1; 1–1; 2 | 91432.66; 77846.23 – 176534.4; 124470.6 | 318.6974 – 352.27365 | Sb08g002690.1 | Protease inhibitor/seed storage/LTP family | DT - ND |
| SCBGLR1114E07 | 5; 5 | 2; 2 | 145690.4; 125905.7 | 588.4425 | ND | ||
| SCCCCL3004H07.b | 3; 3 | 2; 2 | 145392.5; 124459 | Identified | ND | ||
| SCVPHR1092G06 | 4; 4 | 2; 2 | 145392.5; 124470.6 | Identified | ND | ||
| SCUTST3131G03 | 3; 6–4; 3 | 2; 1–1; 1 | 6793.345; 3745.457 – 21630.89; 20854.45 | 150.85635 – 109.76019 | Sb08g002690.1 | Protease inhibitor/seed storage/LTP family | DT – ND |
| SCCCCL3001E03.b* | 5; 7 | 2; 3 | 3263.44; 1945.298 | 39.72605 | Sb01g033830.1* | LTP | ND |
| SCJFRZ2033G07 | 4; 3 | 1; 1 | 26826.32; 13939.5 | 8.15625 | Sb08g002700.1 | LTP | ND |
| SCRUFL4024B04 | 4; 3 | 1; 1 | 26805.06; 13987.92 | 202.91615 | ND | ||
| SCCCRZ1001H02 | 3; 3 | 1; 1 | 7741.618; 3045.282 | 70.59645 | Sb03g039880.1 | LTP | ND |
| SCCCRZ2002G09 | 5; 5 | 2; 2 | 20532.6; 6676.209 | 49.378 | Sb06g016170.1 | LTP | ND |
| SCQSFL3039E08.b | 5; 5 | 2; 2 | 22572.43; 7334.285 | 20.2533 | ND | ||
| SCCCLR1024C05* | 6; 3 | 1; 1 | 11552.57; 5130.042 | 5.59605 | Sb08g002660.1* | Protease inhibitor/seed storage/LTP family | ND |
| SCCCLR1076D05 | 6; 5 | 1; 1 | 16880.65; 8523.134 | 129.08115 | ND | ||
| SCEPLB1044H11* | 7; 3 | 1; 1 | 11788.22; 5527.095 | 13.30225 | ND | ||
| SCCCLR2C03F01 | 3; 3 | 1; 1 | 9426.873; 6414.531 | 78.86415 | Sb08g002670.1 | Protease inhibitor/seed storage/LTP family | ND |
| SCCCRT1003B03 | 6; 4 | 2; 3 | 722.451; 408.6039 | 26.09375 | Sb10g003930.1 | GDSL lipase | ND |
| Proteases | |||||||
| SCBGLR1023G11 | 6; 8 | 5; 8 | 553.142; 705.4267 | 24.15155 | Sb04g029670.1 | Asp protease, peptidase A1 | DT |
| SCBGLR1097G03 | 4; 6 | 3; 3 | 1475.072; 7045.55 | 168.19795 | Sb05g027510.1 | Asp protease, peptidase A1 | DT |
| SCMCLR1123H12 | 6; 7–3; 2 | 3; 3–2; 1 | 1722.723; 4799.767 – 598.7939; 1717.224 | 122.60135 – 52.145752 | Sb05g027510.1 | Asp protease, peptidase A1 | DT - ND |
| SCQGST1032H01 | 11; 14 | 8; 7 | 653.9818; 997.1608 | 45.5457 | Sb05g027510.1 | Asp protease, peptidase A1 | DT |
| SCQGSB1083B11 | 8; 5 | 5; 4 | 4851.335; 4946.649 | 47.901802 | Sb02g041760.1 | Asp protease, peptidase A1 | ND |
| SCRLRZ3042B09 | 9; 6 | 5; 3 | 390.5894; 367.0505 | 7.24305 | Sb03g026970.1 | Asp protease, peptidase A1 | ND |
| SCVPLR2012E01 | 3; 3–4; 3 | 2; 2–2; 2 | 1075.957; 4197.95 – 23533.5; 11935.54 | 147.1347 – 160.93646 | Sb01g044790.1 | Asp protease/Taxi _N/Taxi_C | DT - ND |
| SCVPRZ2038B09 | 3; 4–4; 2 | 2; 3–4; 2 | 1194.285; 2283.918 – 6719.425; 1320.476 | 61.6057 – 103.41875 | Sb01g044790.1 | Asp protease/Taxi _N/Taxi_C | DT - ND |
| SCCCST1004B07 | 11; 8 | 11; 8 | 4096.934; 3149.51 | 44.2912 | Sb01g013970.1 | Ser protease (subtilisin family, peptidase S8/S53) | ND |
| SCJFRZ2011B07 | 5; 4 | 4; 3 | 2390.547; 1211.642 | 25.90875 | Sb06g016860.1 | Ser protease (subtilisin family, peptidase S8/S53) | ND |
| SCCCLR1022B11* | 7; 5 | 6; 6 | 1017.185; 492.4018 | 20.1544 | Sb06g030800.1* | Cys protease, (papain family, peptidase C1A) | ND |
| Proteins with interaction domains (with proteins or polysaccharides) | |||||||
| SCJFLR1013A04 | 4; 4 | 1; 1 | 3741.598; 4709.589 | 31.54085 | Sb05g026650.1 | Ser protease inhibitor (Bowman-Birk) | DT |
| SCRUFL3062D08 - SCRUFL3062D08 | 5; 5–4; 4 | 1; 1–1; 1 | 2784.365; 4868.514 – 11731.47; 8790.486 | 45.9138 – 44.6385 | DT - ND | ||
| Signaling | |||||||
| SCRUAD1063C06 | 4; 2 | 4; 4 | 5824.59; 1313.647 | 55.09195 | Sb09g000430.1 | Leucine-rich repeat (LRR) receptor kinase | ND |
| Miscellaneous proteins | |||||||
| SCEZRZ1014C04* | 6; 5–4; 8 | 2; 2–1; 1 | 6025.488; 9344.188 – 18335.89; 4427.775 | 79.66205 – 67.5792 | Sb03g039330.1* | Thaumatin | DT - ND |
| SCCCLR2003G06 | 4; 4 | 1; 2 | 1364.424; 533.9335 | 18.352499 | Sb08g018720.1 | Thaumatin | ND |
| SCUTLR1037F02 | 3; 4 | 1; 2 | 1083.055; 546.6533 | Identified | ND | ||
| SCCCSD1003E02 | 3; 2 | 1; 1 | 2326.563; 4054.495 | 18.28735 | Sb08g022410.1 | Thaumatin | ND |
| SCRUHR1076B06 | 3; 2 | 1; 1 | 3641.288; 5434.818 | 2.588 | Sb08g022410.1 | Thaumatin | ND |
| SCVPRT2073B04 | 4; 4 | 2; 2 | 2289.087; 18490.86 | 87.17195 | Sb08g022420.1 | Thaumatin | ND |
| SCBGRT1047G10 | 6; 7 | 4; 6 | 3131.626; 2684.819 | 52.720253 | Sb02g004500.1 | Germin (cupin domain) | ND |
| SCCCLR2C02D04 | 3; 4 | 3; 3 | 9021.729; 14936.96 | 149.43965 | Sb09g004970.1 | Germin (cupin domain) | ND |
| SCCCRZ1C01H06 | 13; 1–12; 14 | 4; 3–7; 6 | 3376.122; 3105.723 – 10082.07; 3506.265 | 55.5037 – 33.9362 | Sb08g001950.1 | Nucleoside phosphatase | DT - ND |
| SCJLRT3078H06 | 6; 2 | 3; 1 | 1286.289; 1236.944 | 45.866447 | Sb05g025670.1 | Dirigent protein | DT |
| SCVPRT2073B08 | 6; 4 | 4; 1 | 333.12; 1046.494 | 19.9186 | Sb10g001940.1 | SCP-like extracellular protein | ND |
| Unknown function | |||||||
| SCCCCL4009G04* | 11; 1–8; 8 | 4; 4–3; 3 | 1383.823; 3928.575 – 15270.52; 16670.59 | 126.7531 – 141.14679 | Sb01g004270.1* | Unknown function (DUF642) | DT - ND |
| SCSGLR1084A12* | 12; 14–10; 9 | 6; 6–6; 5 | 6772.538; 5277.542 – 18024.07; 13067.63 | 158.43881 – 150.2402 | Sb01g004270.1 | Unknown function (DUF642) | DT - ND |
| SCCCLB1001G04 | 7; 3 | 5; 3 | 314.0911; 273.5209 | 9.64495 | Sb03g027650.1 | Unknown function (DUF642) | DT |
| SCVPLR2027A11 | 5; 4–5; 3 | 5; 4–2; 1 | 2870.491; 1609.635 | 34.981 | Sb07g026630.1 | Unknown function (DUF568) | ND |
| SCCCRZ3002G10* | 4; 7–6; 5 | 1; 1–1; 2 | 844.0264; 313.8415 – 4025.449; 1307.088 | 3.07575 – 35.7293 | Sb01g031470.1* | Homologous to phloem filament protein 1 (Cucurbita phloem) | DT - ND |
| SCEZRT2018F03* | 4; 6 | 1; 1 | 675.4673; 446.4631 | 5.89995 | Sb01g031470.1 | Homologous to phloem filament protein 1 (Cucurbita phloem) | DT |
| SCEZLB1013B06 | 14; 15 | 8; 11 | 5254.302; 4812.086 | 137.18835 | Sb10g001440.1 | Homologous to phloem filament protein 1 (Cucurbita phloem) | DT |
| SCSFST1066G10 | 5; 5–6; 5 | 1; 1–1; 1 | 718.6985; 2383.583 – 15310.67; 5735.458 | 102.53975 – 192.7034 | Sb08g018710.1 | Expressed protein | DT - ND |
| SCRUFL4024B08.b | 3; 6 | 1; 2 | 5551.887; 12727.94 – 52010.09; 28346.53 | 120.5459 – 288.07706 | Sb08g018710.1 | Expressed protein | DT - ND |
| SCCCRZ2004B02* | 8; 8 | 1; 1 | 9551.813; 4751.091 | 43.9473 | Sb03g000700.1* | Expressed protein | ND |
| SCCCLR1079C11 | 7; 4 | 6; 4 | 3527.088; 4063.023 | 33.617348 | Sb04g011100.1 | Expressed protein | ND |
| SCAGLR2011E04/SCEPAM2057B02 | 3; 3 | 1; 1 | 11178.66/11961; 8954.521/7215.72 | identified | Sb08g003040.1 | Expressed protein (stress responsive alpha/beta barrel) | ND |
| SCEPLR1051E09 | 3; 3 | 1; 1 | 11178.66; 7215.72 | 28.171349 | ND | ||
aBold letters indicate that the ESTs share common sequences. Full length ESTs are in italics. Stars (*) indicate the proteins also identified in the cell wall proteome of sugarcane cell suspension cultures [15]
bSemicolons separate data from different biological repeats. Dashes separate data from different extraction methods (DT, then ND)
Altogether, 84 different CWPs were identified and distributed into eight functional classes (Fig. 2 and Table 1): proteins acting on carbohydrates, proteins possibly related to lipid metabolism; proteins with interaction domains; oxido-reductases; proteases; miscellaneous proteins; signaling and proteins of unknown function. From these 84 CWPs, 24 (29 %) were identified using both the DT and ND Methods. It should be noted that no structural protein was identified. Besides, 16 CWPs (18 %) were previously identified in the cell wall proteome of sugarcane cell suspension cultures [28]. Consequently, 68 sugarcane CWPs were newly identified in this study.
Fig. 2.
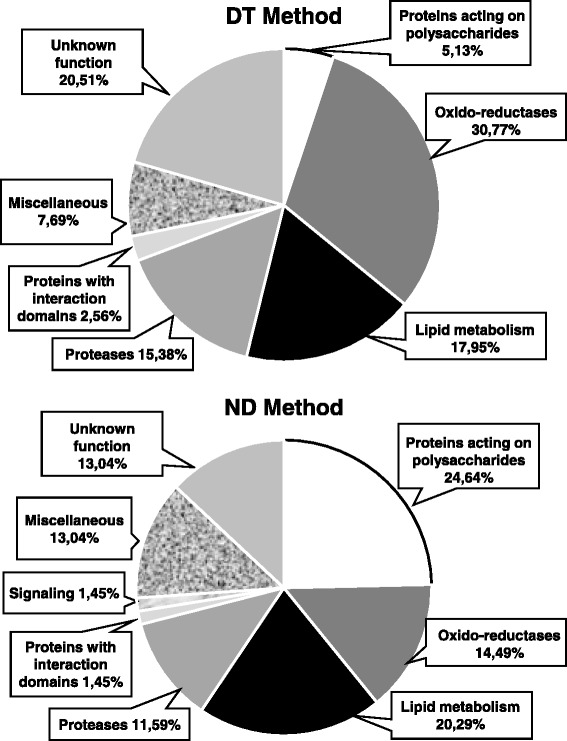
Distribution of CWPs identified in 2-month-old sugarcane culms. Proteins were distributed in functional classes according to bioinformatics predictions: PAC stands for proteins acting on carbohydrates; OR, for oxido-reductases; LM, for proteins possibly involved in lipid metabolism; P, for proteases; ID, for proteins with interaction domains (with proteins or polysaccharides); S, for proteins possibly involved in signaling; M, for miscellaneous; UF, for unknown function
Regarding the DT Method, the oxido-reductases (31 %), mainly peroxidases (Prxs) and two blue copper binding proteins, constituted the most represented class, followed by proteins related to lipid metabolism (18 %), all being lipid transfer proteins (LTPs). Asp proteases (16 %) and miscellaneous proteins (7.5 %), comprising thaumatin, germins and dirigent protein, were also identified (Table 1). Surprisingly, only one glycoside hydrolase (GH) of the GH3 family, as well as a single pectin methylesterase (PME) were identified from the proteins acting on carbohydrates class (5 %). Proteins with interaction domains (2.5 %) were represented by one serine protease inhibitor. Proteins of yet unknown function (20 %) were numerous and it was possible to highlight the presence of proteins with DUF642 domains, already found in other cell wall proteomes [38, 39], and proteins homologous to phloem filament protein 1.
The most represented functional class using the ND Method was that of proteins acting on carbohydrates (25 %), mostly GHs (families 1, 3, 19, 28, 17, 18, 35) and two carbohydrate esterases. Proteins related to lipid metabolism (20 %) comprised LTPs and one GDSL-lipase. Oxido-reductases (14 %) were mostly Prxs. Miscellaneous proteins (13 %) were mainly represented by thaumatins and germins. Proteases (12 %) were Asp, Ser or Cys proteases. Proteins with interaction domains were represented by one Ser protease inhibitor and signaling proteins by one leucine-rich repeat receptor kinase. Finally, proteins of unknown function comprised proteins with DUF642 and DUF568 domains.
We have also performed a quantitative analysis of the CWPs identified by both methods (Table 1). Only the proteins present in amounts higher than 100 femtomoles, calculated by averaging the results of the two biological repeats, have been listed in Table 2. When a protein has been identified using both methods, its quantification could be the same or different if either of the two methods could extract it more efficiently. These differences could, (i) result from the loss of proteins during the washings steps required to purify cell walls using the DT Method or, (ii) due to different types of interactions with cell wall components. Among the proteins present in high amount in culm cell walls, LTPs are well represented with 10 out of 17 proteins. One GH3, three Asp proteases and two DUF642 proteins were also found in the top17 list.
Table 2.
Most abundant CWPs in the cell wall proteome of sugarcane young stems. Proteins with average amounts between the two biological repeats higher than 100 femtomols using either method are listed (see Table 1)
| SUCEST accession number | Functional annotation | Methoda |
|---|---|---|
| Proteins acting on carbohydrates | ||
| SCCCCL4009F05 | GH3 | ND |
| Proteins related to lipid metabolism | ||
| SCCCAM2002F12 | LTP | DT < < ND |
| SCBFLR1046E09 | LTP | DT < < ND |
| SCEPLB1044H04 | LTP | DT > > ND |
| SCEZLB1006F09 | LTP | DT > ND |
| SCCCLR1048F06 | LTP | DT ~ ND |
| SCUTST3131G03 | LTP | DT > ND |
| SCRUFL4024B04 | LTP | ND |
| SCCCLR1076D05 | LTP | ND |
| Proteases | ||
| SCBGLR1097G03 | Asp protease | DT |
| SCMCLR1123H12 | Asp protease | DT > > ND |
| SCVPLR2012E01 | Asp protease | DT ~ ND |
| Unknown function | ||
| SCCCCL4009G04 | Expressed protein (DUF642) | DT ~ ND |
| SCSGLR1084A12 | Expressed protein (DUF642) | DT ~ ND |
| SCEZLB1013B06 | Homologous to phloem filament protein 1 | DT |
| SCSFST1066G10 | Expressed protein | DT < < ND |
| SCRUFL4024B08.b | Expressed protein | DT < < ND |
aThe relative amount of proteins quantified using either method is indicated (see Table 1)
Two approaches were used to statistical analysis: a multivariate analysis, the Scores plot and Vip scores (Fig. 3b, c, respectively), and a univariate one, the Volcano plot, as shown in Fig. 3a. In Fig. 3a, three proteins could be considered as those contributing the most to the distinction between the DT and ND Methods. Figure 3b indicates that the DT and ND Methods differ statistically from each other, since it is possible to separate two distinct groups of proteins regarding the quantity of proteins extracted in each technique. In addition, the two first components (vectors) contributed positively to the model (value of Q2 positive = 66.5 %), and the variation of the proteins was 97.5 % (R2). Values of Q2 > 0.08 indicates that a model is better than chance, and scores of 0.7 or higher, demonstrate a very robust trend or separation [40]. The protein SCCCRZ3002G10 of unknown function was the one that contributed the most to the separation of the groups, being found in higher amount using the ND Method (Fig. 3a, c). The SCCCAM2002F12 and SCEPLB1044H04 LTPs, in turn, were the third and the fourth proteins that contributed to the separation of the two groups in Partial-Least Squares Discriminant Analysis - PLS-DA2, being found in higher amount in the ND and DT Methods, respectively.
Fig. 3.
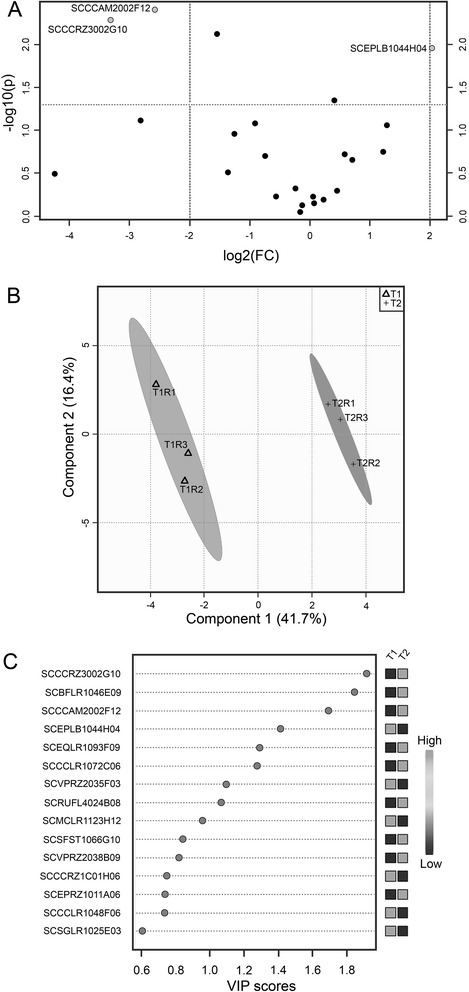
a. Volcano plot: Univariate Statistical analysis of the quantified proteins in both methods. Axis x: Fold Change. Axis y: p value. b. Scores plot: separation of two groups based on the statistical analysis of the amount of the proteins. c. VIP scores. Multivariate Statistical analysis showing the 15 proteins that contributed the most to the separation of the two groups: DT (T1) and ND Method (T2), regarding quantitative data. Black squares mean higher amounts of proteins and gray ones lower amounts. Since two replicates were used for each treatment, the median was calculated from both of them and named T1R3 and T2R3 for DT and ND Methods, respectively
As presented in Fig. 3c, using the average of the quantitative data obtained for each method, the statistical analysis showed that from the 15 proteins that most contributed to distinguish the DT and ND Methods, nine of them showed a much higher amount using the ND Method. Additional file 7 shows important features identified by Volcano Plot.
Comparison of the CWPs of sugarcane young culms to those of stems of other plants
Previous cell wall proteomics studies were performed on B. distachyon basal and apical internodes [26], Medicago sativa basal and apical stems [22] and Linum usitatissimum young stems [23]. All these data have been collected in the WallProtDB database [39] and annotated in the same way, thus allowing comparisons [41]. These CWPs were compared to the newly identified CWPs of sugarcane stems (Fig. 4). In B. distachyon, a protocol very similar to the DT Method was used, but the LC-MS/MS analysis were done with 1-D gel pieces [26]. L. usitatissimum stem CWPs were extracted using a protocol similar to the DT Method and 1-D gel pieces corresponding to stained protein bands were used as starting material for FT-ICR MS analysis [23]. On the other hand, in alfalfa stems, EGTA tretament and LiCl were used for protein extraction, and 1-D gel pieces were digested prior to analysis using a nanoAcquity UPLC system [22]. Although different strategies for protein extraction and MS analyses have been used, all the protocols used the same salts to extract proteins from cell walls: CaCl2 and/or LiCl.
Fig. 4.
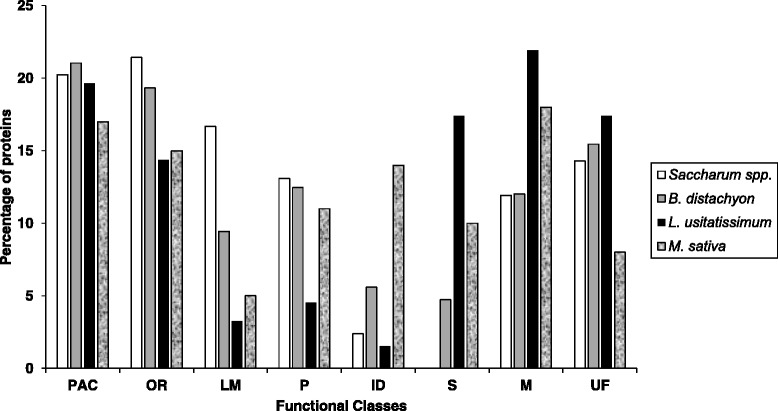
Comparison of the percentage of proteins identified. CWPs present in this study were compared with known cell wall proteomes of stems from different species: B. distachyon [26], L. usitatissimum [23], and M. sativa [22]. Proteins were distributed in functional classes – according to the legend of Fig. 2
The stem cell wall proteomes of all the above species showed very similar percentages of proteins acting on carbohydrates. An outstanding observation was that sugarcane had a much higher percentage of proteins related to lipid metabolism (17 %) than all the other species (0–9 %). The dicot M. sativa presented a much higher proportion of proteins with interaction domains in comparison with the monocots (14 % vs less than 5 %). The monocots showed a higher proportion of oxido-reductases in comparison with the dicots (about 20 % vs about 15 %). A much smaller proportion of proteases was found in L. usitatissimum stems [23].
Discussion
In this work, 84 different sugarcane CWPs were identified in young culms using two different strategies. Together with the cell wall proteome of cell suspension cultures [28], 137 different CWPs of sugarcane have been identified. In this study alone, 68 CWPs were newly identified and 16 CWPs were identified in both culms and cell suspension cultures, among which 5 Prxs. Besides, the proportion of proteins predicted to be intracellular in culm extracts (33 % and 44 %) was lower than in sugarcane cell suspension culture extracts (81.6 %) [28], being quite the same as in B. distachyon young internodes [26]. This is probably inherent to the type of material, since a lot of cell debris are present in the culture medium [28].
Interestingly, the proportion of intracellular proteins was higher in leaves than in stems in B. distachyon [26]; the same case has been observed for sugarcane (unpublished observations). The ND Method has lead to the identification of about 75 % more CWPs than the DT Method (69 CWPs vs 39), and around 81 % of the CWPs (68 CWPs out of 84) have been identified using one method of extraction only. These results show the importance of using different strategies to enlarge the coverage of a cell wall proteome. The ND Method has allowed the recovery of more CWPs of sugarcane culms, and much more GHs than the DT method. If the objective of the study is to get an overview of CWPs or of glycosidases, this strategy should be considered. In addition, if the goal is especifically to recover GHs, perhaps a total protein extraction followed by affinity chromatography on Concanavalin A is the best option [25]. However, if the aim is to go deeper into Prxs, the DT Method looks more appropriate. Besides, both methods showed a good reproducibility since between 65 % and 82 % of CWPs were identified in both biological replicates. Although rarely discussed in cell wall proteomics paper, this result is consistent with those of previous studies [26].
The ND Method could recover both a higher number of CWPs and a higher amount of those contributing to the discrimination between the two methods through the statistical analysis. Additionally, the three proteins highlighted in the univariate analysis were also present in the multivariate analysis, being numbers 1, 3 and 4 from the 15 CWPs considered to be the most important for the discrimination between the two methods. The major difference between the two ND and DT methods regards proteins acting on carbohydrates: only one CWP has been identified using the DT Method whereas one fourth of the CWPs belongs to this class using the ND Method. Since the same organs were analyzed, this difference has to be related to the strategy used for protein extraction. Some proteins could have been lost during the washing steps required to clean cell wall fragments in the case of the DT Method [9]. This could explain why more CWPs were found using the ND Method. However, the use of the DT Method with sugarcane cell suspension cultures allowed the recovery of several GHs [28]. Then, the low number of GH identified in this study using the DT Method could be related to the structure of the sugarcane culm cell walls. In the case of grasses, cell walls contain different matrix polysaccharides and protein components, when compared to dicot cell walls. As an example, grass cell walls present cellulose microfibrils interlocked by glucuronoarabinoxylans instead of xyloglucans. In addition, they contain a substantial proportion of non-cellulosic polymers wired on cellulose microfibrils by alkali-resistant phenolic linkages [7].
As found with the ND Method, most previous cell wall proteomics studies showed that proteins acting on carbohydrates were the most represented [29, 42]. The role of such proteins in cell walls points to the rearrangements of polysaccharides during development [11, 43–45]. These modifications can occur through the hydrolysis of glycosidic bonds within polysaccharides or between a carbohydrate and a non-carbohydrate moiety [46]. Not surprisingly, they can play important roles during germination [47], defense against herbivory [48], lignification [49] and regulation of phytohormones [50]. In this functional class, all but two were GHs, represented by one acyl-esterase and one PME. GH1, 3, 17, 19 and 28 were also found as the major GH families present in the cell wall proteomes of B. distachyon, Oryza sativa and A. thaliana [26, 51]. One member of the A. thaliana GH1 family has been shown to degrade β-mannosides, suggesting that it could hydrolyze mannans, galactomannans, or glucogalactomannans in muro [46]. Proteins of the GH3 family could have α-L-arabinofuranosidase and/or β-xylosidase activities [52]. One GH3 is among the most abundant CWPs identified in sugarcane culms. GH1 and GH3 were also identified in termite stomach, being characterized as β-glucosidases, i.e. cellulases that preferentially hydrolyze β-1-4 glycosidic linkages [53]. However, the overexpression of a rice β-glucosidase and an endo-glucanase was null and led to deleterious effects, respectively [54]. This may indicate that perhaps these enzymes should not be altered if the goal is to achieve a less recalcitrant plant. However, by altering the expression of exo-glucanases, it was possible to increase saccharification in rice, besides negative effects on plant development [54]. In B. distachyon culms, no GH35 was identified and a higher proportion of GH17 and 18 were found in comparison to GH1 and 3 [26], an opposite finding to sugarcane culms. Another CWP-to-watch is the PME, since the expression of a fungal pectin methylesterase inhibitor (PMEI) in wheat and Arabidopsis could increase the efficiency of enzymatic saccharification [55].
The proportion of oxido-reductases was almost the same as that found in the cell wall proteome of sugarcane cell suspension cultures [28]. In B. distachyon culms, the percentage of oxido-reductases was closer to that found using the ND Method [26], although the work was performed with a protocol very similar to the DT Method. So it is not possible to conclude that the method itself was more likely to extract these proteins. As found in B. distachyon [26], Prxs and blue copper binding proteins were more numerous in the sugarcane than in the A. thaliana cell wall proteome. Different populations of Prxs were extracted by the ND and DT Methods. This could be related to their different abilities to interact with pectins as shown for a zucchini and an A. thaliana Prxs [56, 57]. Although Prxs are numerous (14 out of 84 CWPs) in the sugarcane culm cell wall proteome, none of them was found amongst the most abundant CWPs. Prxs are well-known cell wall enzymes, identified in many cell wall proteomics studies [58]. They could be involved in cell wall polysaccharide rearrangements during development, defense reactions or signaling [58]. Their activity is versatile. During the hydroxylic cycle, Prxs can produce ROS that break cell wall polysaccharides in a non-enzymatic way, promoting wall extension, whereas during the peroxidative cycle, Prxs can favor cross-linking of cell wall components such as structural proteins or lignins [59]. So, Prxs are also a class of proteins to be watched when searching for proteins that could potentially facilitate the production of cellulosic ethanol. The blue copper binding proteins have already been found in cell wall proteomes [42, 60]. They have been associated to redox processes such as electron transfer proteins with small molecular mass compounds [61]. Blue copper binding proteins were not found in the cell wall proteome of sugarcane cell suspension culture [28].
LTPs were already identified in many cell wall proteomes [29, 60]. They have been assumed to bind hydrophobic molecules in cell walls which could be essential for cell wall loosening, thus facilitating wall extension [62]. LTPs could also be involved in cuticle formation [63]. Since sugarcane culms have a thick cuticle, this could explain the high number of identified LTPs in their cell wall proteome. This number is much higher than in any other species studied before [10, 22, 25, 26]. This explanation is consistent with the fact that a much lower number of lipid-related proteins was found in sugarcane cell suspension cultures which are undifferentiated cells [28]. LTPs are also the family that embraced the highest number of proteins with an average quantity higher than 100 femtomoles (8 out of 17 proteins). Additionally, five LTPs were among the 15 proteins who contributed the most to the discrimination between the ND and DT Methods.
Proteases can participate in various processes of the plant life cycle, such as development, defense, stress response and adaptation to the environment [64]. In sugarcane culms, mostly Asp proteases have been identified. Asp proteases were also numerous in the B. distachyon cell wall proteome [26]. Three Asp protease were found among the 17 most abundant CWPs of the sugarcane culm cell wall proteome. Asp proteases may be linked to disease resistance signaling, being accumulated in the extracellular matrix under pathogen attack [65]. Besides, two Ser proteases of the subtilisin family were identified. Proteins of this family have been shown to display various functions in plant development and signaling [14, 64, 66, 67]. Finally, one Cys protease was identified, a type of protease that can be related to the regulation of senescence and seed germination, as well as to defense roles [65, 68]. Cys proteases are known to be secreted in the apoplast [65]. It should be noted that Ser and Cys proteases were only found using the ND Method.
Several thaumatins have been identified, mainly using the ND Method. Thaumatins are pathogenesis-related proteins. Several of them have been shown to be β-1,3-glucanases showing anti-fungal activity [69]. However, one thaumatin has been shown to exhibit a polyphenol oxidase activity [70]. Finally, some proteins of unknown function were found, especially members of the DUF642 and DUF568 families. DUF642 proteins present a conserved region found in a number of plant proteins [71], and have been identified in all the cell wall proteomes studied so far [63]. One A. thaliana DUF642 protein has been shown to interact with cellulose in vitro [38]. In sugarcane culms, two DUF642 proteins were among the most abundant proteins. Thus, these proteins probably take part in important processes in the cell wall. On the other hand, one DUF568 is known as an auxin-responsive protein, AIR12, that may interact with other redox partners within the plasma membrane to constitute a redox link between the cytoplasm and the apoplast [72].
Some protein families were under-represented in the cell wall proteome of sugarcane culms when compared to other cell wall proteomes. Only one protease inhibitor has been identified. It belonged to the Bowman-Birk family. It has been characterized as a trypsin inhibitor associated with the regulation of endogenous seed proteinases, storage of sulfur amino acids and defense against insects and pathogens [73]. In sugarcane cell suspension cultures, different families of proteins with interaction domains have been identified, and in B.distachyon, proteins of the Bowman-Birk family were found both in leaves and internodes [26]. Regarding proteins possibly involved in signaling, the LRR receptor kinase family was commonly found in other cell wall proteomics studies [23, 26, 28]. Such proteins probably play roles in signal perception during development or in response to environmental cues [74]. One dirigent protein has been identified in sugarcane culms. Such proteins have been assumed to play a role in lignification through the control of monolignol coupling affecting wall flexibility and its mechanical strength [75]. Members of this family have been identified in B. distachyon stems [26]. No structural protein has been found in the sugarcane cell wall proteome, as in previous studies [23, 25, 26, 28, 29, 42]. This is probably because these proteins are difficult to extract when they are covalently cross-linked [59]. Usually, they cannot be extracted by salts [35], thus, different strategies should be used if structural proteins, such as extensins, are the focus [76].
Conclusions
This work has contributed to three main aspects: (i) characterize CWPs from sugarcane young stems, (ii) compare the CWPs found, regarding type and amount, using two different methods of extraction and (iii) point at candidate CWPs to be used in future research to enhance 2G ethanol production. This study also offered a glimpse to the quantification of CWPs, providing help for the decision of which method is more suitable for the efficient extraction of different types of CWPs from sugarcane culms. If the focus is on GHs or getting an overview of the cell wall proteome, then the ND Method could be used. Otherwise, if looking for Prxs, the DT Method is the more adequate. Our results highlight the importance of using different strategies to isolate CWPs.
Future studies that could explain how these proteins interact with cell wall components, and use these GHs to obtain a custom-made plant to enhance 2G ethanol production will bring new perspectives to an old problem: the viability of this biofuel. In addition to GHs, attention should be paid to other proteins such as Prxs and dirigent proteins, since Prxs can favor cross-linking of the cell wall components such as proteins or lignins [58]. Therefore, they could be used in genetic engineering since lignin is a cell wall barrier preventing the access of cellulose to enzyme attack in order to break these sugars into fermentable ones [77]. Lowering the lignin content or modifying lignin linkages to facilitate its removal are two possible ways to enhance the efficiency of biomass deconstruction [1]. Finally, some proteins of yet unknown function could be interesting candidates.
Methods
Plant material
Sugarcane plants from variety SP80-3280 were used in all the experiments, provided by Dr. Maria Cristina Falco from the Sugarcane Technology Center (CTC, http://www.ctcanavieira.com.br/). This sugarcane variety was chosen as the one having available sequenced ESTs [36]. Pieces of culms of 7 cm each containing lateral buds were planted in pots, containing a mixture of vermiculite 1:1 compost (Plantmax, Eucatex Indústria e Comércio SA, São Paulo, Brazil) and acclimated in a greenhouse at 26 °C. Sugarcane plants were watered daily and nutrient solution (Plant-Prod 4 g/L, Master Plant-Prod Inc, Brampton, ON, Canada) was added every 15 days. Since the plants were obtained after only two months of growth, all the portions of the culms were collected. For both methods, the plants were collected and the proteins were immediately extracted.
Extraction of proteins from cell walls and separation by 1D-electrophoresis
Two different strategies were used, respectively called DT and ND Method. Two biological replicates were performed in each case. For each experiment, material from 2 different plants randomly picked was used. The DT Method was a destructive one [35], whereas the ND Method was a non-destructive one [29].
To perform the extraction of proteins with the DT Method, culms were collected and cut into small pieces, washed with Ultra High Quality (UHQ) water and transferred to a blender containing 500 mL of a sodium acetate buffer 5 mM, pH 4.6, with 0.4 M sucrose, polyvinylpolypyrrolidone (PVPP) (1 g per 10 g of fresh tissue, Sigma Chemical, St Louis, MO, USA) and 3.3 % (v/v) anti-protease cocktail (P9599, Sigma). The plant material was ground in the blender for 8 min at maximum speed. Cell walls were separated from the soluble cytoplasmic fluid through centrifugation for 15 min, at 1000 g and 4 °C. The resulting pellet was submitted to two successive centrifugations in 500 mL of sodium acetate buffer 5 mM, pH 4.6, plus 0.6 M and 1 M sucrose, respectively. The final pellet was washed with 3 L of 5 mM sodium acetate buffer, pH 4.6, on a Nylon net (pore size = 50 μm) (Nitex, Dominique Dutscher, Brumath, France). The resulting cell wall fraction was ground with liquid nitrogen, and freeze dried for 48 h. The extraction of proteins from purified cell walls with 0.2 M CaCl2 and 2 M LiCl solutions was conducted as described [59].
In the case of the ND Method, the culms were collected, washed with UHQ water, cut in small pieces (about 7 cm in length) and then immersed in a beaker with a buffer solution containing 5 mM sodium acetate, pH 4.6, 0.3 M mannitol, 0.2 M CaCl2 and 0.1 % (v/v) anti-protease cocktail (P9599, Sigma). The beaker was placed in a desiccator attached to a vacuum pump and the culm pieces were infiltrated under vacuum for 10 min. Thereafter, the infiltrated material was centrifuged (200 g for 20 min at 4 °C) in swinging buckets (CTR429, Jouan centrifuge). The resulting fluids were collected at the bottom of the tubes. The processes of vacuum infiltration and centrifugation were repeated once. Finally, the pieces of culms were infiltrated again and centrifuged, as in the previous step, in a solution containing 2 M LiCl instead of CaCl2. The protein extracts were desalted on EconoPac® 10DG column (BIO-RAD, Hercules, CA, USA) as described [42]. Proteins were then solubilized in UHQ water and quantified by the CooAssay Protein Assay kit (Interchim, Montluçon, France) according to a modified Bradford method [78].
In order to verify the quality of the extractions, 40 μg of proteins were separated by 1D-electrophoresis as described [79]. After that, the staining of the bands was carried out with a Coomassie Brilliant Blue (CBB)-based method [80]. The image of the gel was obtained through a scanner (GE-III Image scanner, GE Healthcare, Ramonville Saint-Agne, France).
MSE analysis
Sample preparation was performed as described [28]. However, after increasing pH by adding 5 μL of 1 N NH4OH, an additional step was performed: the addition of phosphorylase B-rabbit (Waters, Manchester, UK) as an internal standard (2.5 μL of 1 pmol.μL−1) to the digested aliquot (80 μL). Consequently, 17.5 μL of 20 mM ammonium formate was added to the vials, reaching a final volume of 100 μL.
For each extract, 5 μL of the total protein digest (containing 3 μg of proteins) were fractionated by reverse-phase ultraperformance liquid chromatography (2D- nanoACQUITY UPLC®, Waters®, Manchester, UK). Separation in two dimensions, elution and trapping were performed as described [28]. Acquisition of MS data used a Synapt G2 HDMS equipped with an ion mobility cell and a NanoLockSpray source in the positive ion and ‘V’mode (Waters®), with the same parameters as described [28]. MS experiments were performed by switching between low (3 eV) and high collision energies (15–50 eV) applied to the ‘T-wave’ cell trap, filled with argon. The low and high energy scans from m/z 50 to 2000 used a scan time of 0.8 s. The intensities of the spectra were calculated using the stoichiometric method during MS experiments, according to the internal standard, to identify and quantify the proteins [81].
The doubly-charged ion ([M + 2H] 2+) was used for initial single-point calibration and MS/MS fragment ions of GFP [Glu 1]-Fibrinopeptide B m/z 785,84,206 ([M + 2H] 2+) (Waters, Corp., Milford, USA) were used as lock masses and instrument calibration. Data-independent scanning (MSE) experiments were performed by switching between low (3 eV) and elevated collision energies (15–50 eV), applied to the trap ‘T-wave’ cell filled with argon. Scan time of 0.8 s were used for low and high energy scans from m/z 50 to 2000 [81].
Identification and annotation of proteins
The bioinformatics analysis was performed as described [28]. However, since phosphorylase B-rabbit (Waters) was used as an internal standard to quantify peptides in the present study, its sequence was added to the SUCEST-translated EST database. The quantification of the proteins, in femtomoles, was obtained as an average from the biological replicates. Proteins were noted as “identified” when quantification was not possible due to low abundance (Table 1). The PGLS 2.5.1 expression data values of p < 0.05 and p > 0.95 were considered as statistically significant for down or up-regulation, respectively, considering the quantitative protein ratio DT method/ND Method.
Two biological replicates were performed in this study, and only proteins presented in both replicates were considered. Proteins were considered to be secreted and named CWPs when it was possible to predict a signal peptide with at least two bioinformatics programs, when no intracellular retention signal was predicted and when no more than one trans-membrane domain was predicted [28]. This work was done either manually for sugarcane translated ESTs or using ProtAnnDB for S. bicolor sequences [82]. In order to find S. bicolor (the closest species with a fully sequenced genome) homologous genes for the identified sugarcane ESTs, a blastp search was performed [83], as described [28]. Only proteins showing at least one specific peptide were considered.
CWPs were distributed into eight functional classes according to their annotation using InterPro [84] and PFAM [85]. All the data have been included in the WallProtDB database [43].
The median of the quantified proteins identified was calculated, being considered as T1R3 and T2R3, for the DT and ND Methods, respectively. Statistical processing was performed with MetaboAnalyst software 2.0 [86]. The quantitative data were normalized by the median, followed by a logarithmic transformation (Log2) and Pareto Scaling. The Partial-Least Squares Discriminant Analysis (PLS-DA) was used for the data analysis. In PLS-DA, R2 values were observed, which indicate how much of the total variation in the dataset is described by the analysis components, and Q2 values, which indicate how accurately the model can predict class membership. Both of them, therefore, are performance indicators [87]. The PLS-DA models were constructed and the importance of the variable in the projection (VIP) was used to identify the 15 ions that had a higher discrimination between the groups in the component with the highest power projection.
Besides the multivariate approaches, the univariate method (Student’s t- test and fold change) was performed to measure the significance of each protein in distinguishing the DT and ND Methods groups. The fold change threshold (x 4) and t-tests threshold 0.05 were adopted. To assess whether the proteins highlighted in the loading scores were statistically significant, a Volcano analysis was performed.
Availability of supporting data
The proteomics data have been included in the WallProtDB public database (http://www.polebio.lrsv.ups-tlse.fr/WallProtDB/).
Acknowledgements
Financial support was provided by a USP-COFECUB project (Process number: 10.1.1947.11.9), a BIOEN/FAPESP PRONEX project (Process number: 2008/56100-5) as well as by INCT - Instituto Nacional de Ciência e Tecnologia do Bioetanol, CNPq (Process number: 142784/2007-9) and FAPESP (Process number: 2007/59327-8). EJ is thankful to Paul Sabatier University (Toulouse, France) and CNRS for supporting the research work of her team. The authors are thankful to Dr MC Falco and CTC for providing the sugarcane plants. They thank GM Souza and Dr M Nishiyama, for kindly providing sugarcane protein sequences from SUCEST. MJCR thanks LM Franceschini for the bioinformatics assistance, ER Alexandrino for the help with the figures and S Guidetti for handling the mass spectrometer. The authors are grateful to Dr C Albenne for stimulating discussions.
Abbreviations
- CWP
cell wall protein
- DT
Destructive
- EST
expressed sequenced tag
- FC
fold change
- GH
glycoside hydrolase
- LRR
leucine-rich repeat
- LTP
lipid transfer protein
- MS
mass spectrometry
- ND
non-destructive
- PME
pectin methylesterase
- Prx
class III peroxidase
- UHQ
ultra high quality
- UPLC
ultra Performance Liquid Chromatography
Additional files
Raw MS data from sugarcane culms using Method 1, biological replicate1. (XLS 2.26 MB)
Raw MS data from sugarcane culms using Method 1, biological replicate 2. (XLS 3.02 MB)
Raw MS data from sugarcane culms using Method 2, biological replicate1. (XLS 2.33 MB)
Raw MS data from sugarcane culms using Method 2, biological replicate2. (XLS 1.39 MB)
CWPs identified in 2 month-old sugarcane culms. (XLSX 30.3 kb)
Proteins of sugarcane young culms identified using Method 1 or 2 and predicted to be intracellular. (XLSX 21.2 kb)
Important features identified by Volcano plot. (XLSX 10.0 kb)
Footnotes
Rafael Pont-Lezica Deceased.
Competing interests
The authors have declared no conflict of interest.
Authors’ contributions
MJCR participated in the conception of the study, performed the extractions, the preparation for sequencing, data analysis and drafted the manuscript. EJ participated in the design of the study, data analysis, discussion and revision of the manuscript. TD gave supervision for protein extractions and data analysis. MBCR assisted in the data analysis and draft of the manuscript. TRC assisted in protein quantification and revision of the manuscript. JFG assisted in data analysis and revision of the manuscript. HSC assisted in the bioinformatics analysis and updating of WallProtDB. RPL assisted in the conception of the study, the extraction procedures and discussion of the results. CAL participated in the conception of the study, data analysis, discussion and revision of the manuscript. All authors have read and approved the final version of the manuscript.
Contributor Information
Maria Juliana Calderan-Rodrigues, Email: phdjuliana@gmail.com.
Elisabeth Jamet, Email: jamet@lrsv.ups-tlse.fr.
Thibaut Douché, Email: thibaut.douche@pasteur.fr.
Maria Beatriz Rodrigues Bonassi, Email: beatrizcalderan@gmail.com.
Thaís Regiani Cataldi, Email: thais.cataldi@usp.br.
Juliana Guimarães Fonseca, Email: j.g.fonseca@usp.br.
Hélène San Clemente, Email: sancle@lrsv.ups-tlse.fr.
Carlos Alberto Labate, Phone: +55-19-3429-4248, Email: calabate@usp.br.
References
- 1.Boaretto LF, Mazzafera P. The proteomes of feedstocks used for the production of second-generation ethanol: a lacuna in the biofuel era. Ann Appl Biol. 2013;163:12–22. doi: 10.1111/aab.12031. [DOI] [Google Scholar]
- 2.Sorek N, Yeats TH, Szemenyei H, Youngs H, Somerville C. The implications of lignocellulosic biomass chemical composition for the production of advanced biofuels. BioScience. 2014;64:192–201. doi: 10.1093/biosci/bit037. [DOI] [Google Scholar]
- 3.Abramson M, Shoseyov O, Shani Z. Plant cell reconstruction toward improved lignocellulosic production and processability. Plant Sci. 2010;178:61–72. doi: 10.1016/j.plantsci.2009.11.003. [DOI] [Google Scholar]
- 4.Carpita N, Tierney M, Campbell M. Molecular biology of the plant cell wall: searching for the genes that define structure, architecture and dynamics. Plant Mol Biol. 2001;47:1–5. doi: 10.1023/A:1010603527077. [DOI] [PubMed] [Google Scholar]
- 5.Somerville C, Bauer S, Brininstool G, Facette M, Hamman T, Milne J, et al. Toward a systems approach to understanding plant cell walls. Science. 2004;306:2206–11. doi: 10.1126/science.1102765. [DOI] [PubMed] [Google Scholar]
- 6.Chundawat SPS, Beckham GT, Himmel ME, Dale BE. Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu Rev Chem Biomol Eng. 2011;2:121–145. doi: 10.1146/annurev-chembioeng-061010-114205. [DOI] [PubMed] [Google Scholar]
- 7.Carpita NC, Gibeaut DM. Structural models of primary-cell walls in flowering plants - consistency of molecular-structure with the physical-properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313X.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 8.Albenne C, Canut H, Jamet E. Plant cell wall proteomics: the leadership of Arabidopsis thaliana. Front Plant Sci. 2013;4:111. doi: 10.3389/fpls.2013.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albenne C, Canut H, Hoffmann L, Jamet E. Plant cell wall proteins: A large body of data, but what about runaways? Proteomes. 2014;2:224–42. doi: 10.3390/proteomes2020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose JKC, Lee SJ. Straying off the highway: Trafficking of secreted plant proteins and complexity in the plant cell wall proteome. Plant Physiol. 2010;153:433–36. doi: 10.1104/pp.110.154872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassab G, Varner JE. Cell wall proteins. Ann Rev Plant Phys. 1988;39:321–53. doi: 10.1146/annurev.pp.39.060188.001541. [DOI] [Google Scholar]
- 12.Vogel J. Unique aspects of the grasses cell wall. Curr Opin Plant Biol. 2008;11(3):301–7. doi: 10.1016/j.pbi.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Jamet E, Canut H, Boudart G, Pont-Lezica RF. Cell wall proteins: a new insight through proteomics. Trends Plant Sci. 2006;11:33–9. doi: 10.1016/j.tplants.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Von Groll U, Berger D, Altmann T. The subtilisin-like serine protease SDD1 mediates cell-to-cell signaling during Arabidopsis stomatal development. Plant Cell. 2002;14:1527–39. doi: 10.1105/tpc.001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeBono A, Yeats TH, Rose JKC, Bird D, Jetter R, Kunst L, et al. Arabidopsis LTPG is a glycosylphosphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface. Plant Cell. 2009;21:1230–38. doi: 10.1105/tpc.108.064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fry SC. Primary cell wall metabolism: tracking the careers of the wall polymers in living plant cells. New Phytol. 2004;161:641–75. doi: 10.1111/j.1469-8137.2004.00980.x. [DOI] [PubMed] [Google Scholar]
- 17.Han Y, Chen H. Improvement of corn stover bioconversion efficiency by using plant glycosidase hydrolase. Bioresour Technol. 2011;102:4787–92. doi: 10.1016/j.biortech.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Rapoport TA. Transport of proteins across the endoplasmic reticulum membrane. Science. 1992;258:931–36. doi: 10.1126/science.1332192. [DOI] [PubMed] [Google Scholar]
- 19.Vitale A, Denecke J. The endoplasmic reticulum - Gateway of the secretory pathway. Plant Cell. 1999;11:615–28. doi: 10.1105/tpc.11.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chivasa S, Ndimba BK, Simon WJ, Robertson D, Yu XL, Knox JP, et al. Proteomic analysis of the Arabidopsis thaliana cell wall. Electrophoresis. 2002;23:1754–65. doi: 10.1002/1522-2683(200206)23:11<1754::AID-ELPS1754>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 21.Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300:1005–16. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- 22.Verdonk JC, Hatfield RD, Sullivan ML. Proteomic analysis of cell walls of two developmental stages of alfafa stems. Front Plant Sci. 2012;3:279. doi: 10.3389/fpls.2012.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Day A, Fénart S, Neutelings G, Hawkins S, Rolando C, Tokarski C. Identification of cell wall proteins in the flax (Linum usitatissimum) stem. Proteomics. 2013;13:812–25. doi: 10.1002/pmic.201200257. [DOI] [PubMed] [Google Scholar]
- 24.Lim S, Crisholm K, Coffin RH, Peters RD, Al-Mughrabi KI, Wang-Pruski G, et al. Protein profiling in potato (Solanum tuberosum L.) leaf tissues by differential centrifugation. J Proteome Res. 2012;11:2594–601. doi: 10.1021/pr201004k. [DOI] [PubMed] [Google Scholar]
- 25.Minic Z, Jamet E, Negroni L, der Garabedian PA. A subproteome of Arabidopsis thaliana trapped on Concanavalian A is enriched in cell wall glycoside hydrolases. J. Exp Bot. 2007;58:2503–12. doi: 10.1093/jxb/erm082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douché T, San Clemente H, Burlat V, Roujol D, Valot B, Zivy M, et al. Brachypodium distachyon as a model plant toward improved biofuel crops: Search for secreted proteins involved in biogenesis and disassembly of cell wall polymers. Proteomics. 2013;13:2438–54. doi: 10.1002/pmic.201200507. [DOI] [PubMed] [Google Scholar]
- 27.Núñez-López L, Aguirre-Cruz A, Barrera-Figueroa B, Peña-Castro JM. Improvement of enzymatic saccharification yield in Arabidopsis thaliana by ectopic expression of the rice SUB1A-1 transcript factor. PeerJ. 2015;3:e817. doi: 10.7717/peerj.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calderan-Rodrigues MJ, Jamet E, Bonassi MBCR, Guidetti-Gonzalez S, Begossi AC, Setem LV, et al. Cell wall proteomics of sugarcane cell suspension culture. Proteomics. 2014;14:738–49. doi: 10.1002/pmic.201300132. [DOI] [PubMed] [Google Scholar]
- 29.Boudart G, Jamet E, Rossignol M, Lafitte C, Borderies G, Jauneau A, et al. Cell wall proteins in apoplastic fluids of Arabidopsis thaliana rosettes: Identification by mass spectrometry and bioinformatics. Proteomics. 2005;5:212–21. doi: 10.1002/pmic.200400882. [DOI] [PubMed] [Google Scholar]
- 30.Bakker H. Sugar cane cultivation and management. 1. Horsham: Springer Science & Business Media; 2012. [Google Scholar]
- 31.Diola V, Santos F. Fisiologia. In: Santos F, Borém A, Caldas C, editors. Cana-de-açúcar: bioenergia, açúcar e álcool: tecnologias e perspectivas. Viçosa: Editora UFV; 2010. pp. 25–49. [Google Scholar]
- 32.Marafon AC. Análise Quantitativa de Crescimento em Cana-de-açúcar: uma Introdução ao Procedimento Prático. Embrapa Tabuleiros Costeiros. 2012. http://www.cpatc.embrapa.br/publicacoes_2012/doc_168.pdf. Accessed 28 Oct 2015.
- 33.Angyal SJ, Craig DC, Kuszmann J. The composition and conformation of D-Threo-3,4-Hexodiulose in solution, and the X-ray crystal-structure of its beta-anomer. Carbohydr Res. 1989;194:21–29. doi: 10.1016/0008-6215(89)85002-5. [DOI] [Google Scholar]
- 34.Jamet E, Canut H, Albenne C, Boudart G, Pont-Lezica R. Cell wall. In: Agrawal GK, Rakwal R, editors. Plant Proteomics: Technologies, Strategies, and Applications. London: Wiley; 2008. pp. 293–307. [Google Scholar]
- 35.Feiz L, Irshad M, Pont-Lezica RF, Canut H, Jamet E. Evaluation of cell wall preparations for proteomics: a new procedure for purifying cell walls from Arabidopsis hypocotyls. Plant Methods. 2006;2:10–23. doi: 10.1186/1746-4811-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vettore AL. Analysis and functional annotation of an expressed sequence tag collection for tropical crop sugarcane. Genome Res. 2003;13:2725–35. doi: 10.1101/gr.1532103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ming R, Liu S, Lin Y, da Silva J, Wilson W, Braga D, et al. Detailed alignment of saccharum and sorghum chromosomes: comparative organization of closely related diploid and polyploid genomes. Genetics. 1998;150:1663–82. doi: 10.1093/genetics/150.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vázquez-Lobo A, Roujol D, Zuñiga-Sánchez E, Albenne C, Piñero D, Gamboa de Buen A, et al. The highly conserved spermatophyte cell wall DUF642 protein family: phylogeny and first evidence of interaction with cell wall polysaccharides in vitro. Mol Phylogenet Evol. 2012;63:510–20. doi: 10.1016/j.ympev.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 39.WallProtDB Laboratoire de Recherche en Sciences Végétales, Toulouse. http://www.polebio.lrsv.ups-tlse.fr/WallProtDB/. Accessed 21 Aug 2015.
- 40.Salek RM, Xia J, Innes A, Sweatman BC, Adalbert R, Randle S, et al. A metabolomic study of the CRND8 transgenic mouse model of Alzheimer’s disease. Neurochem Int 2010, 56:937–47. [DOI] [PubMed]
- 41.San Clemente H, Jamet E. WallProtDB, a database resource for plant cell wall proteomics. Plant Methods. 2015;11:2. doi: 10.1186/s13007-015-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ligat L, Lauber E, Albenne C, San Clemente H, Valot B, Zivy M, et al. Analysis of the xylem sap proteome of Brassica oleracea reveals a high content in secreted proteins. Proteomics. 2011;11:1798–813. doi: 10.1002/pmic.201000781. [DOI] [PubMed] [Google Scholar]
- 43.Freshour G, Clay RP, Fuller MS, Albersheim P, Darvill AG, Hahn MG. Developmental and tissue-specific structural alterations of the cell-wall polysaccharides of Arabidopsis thaliana roots. Plant Physiol. 1996;110:1413–29. doi: 10.1104/pp.110.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicol F, Hofte H. Plant cell expansion: scaling the wall. Cur Opin Plant Biol. 1998;1:12–7. doi: 10.1016/S1369-5266(98)80121-0. [DOI] [PubMed] [Google Scholar]
- 45.Rose JC, Bennett AB. Cooperative disassembly of the cellulose-xyloglucan network of plant cell walls: Parallels between cell expansion and fruit ripening. Trends Plant Sci. 1999;4:176–83. doi: 10.1016/S1360-1385(99)01405-3. [DOI] [PubMed] [Google Scholar]
- 46.Xu Z, Escamilla-Treviño LL, Zeng L, Lalgondar M, Bevan DR, Winkel BSJ, et al. Functional genomic analysis of Arabidopsis thaliana glycoside hydrolase family 1. Plant Mol Biol. 2004;55:343–67. doi: 10.1007/s11103-004-0790-1. [DOI] [PubMed] [Google Scholar]
- 47.Leah R, Kigel J, Svendsen I, Mundy J. Biochemical and molecular characterization of a barley seed β-glucosidase. J Biol Chem. 1995;270:15789–97. doi: 10.1074/jbc.270.26.15789. [DOI] [PubMed] [Google Scholar]
- 48.Rask L, Andréasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J. Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Mol Biol. 2000;42:93–113. doi: 10.1023/A:1006380021658. [DOI] [PubMed] [Google Scholar]
- 49.Dharmawardhana DP, Ellis BE, Carlson JE. A β-glucosidase from lodgepole pine specific for the lignin precursor coniferin. Plant Physiol. 1995;107:331–39. doi: 10.1104/pp.107.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falk A, Rask L. Expression of a zeatin-O-glucosidedegrading β-glucosidase in Brassica napus. Plant Physiol. 1995;108:1369–77. doi: 10.1104/pp.108.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darley CP, Forrester A, McQueen-Mason S. The molecular basis of plant cell wall extension. Plant Mol Biol. 2001;47:179–95. doi: 10.1023/A:1010687600670. [DOI] [PubMed] [Google Scholar]
- 52.Tateishi A, Kamiyoshihara Y, Matsuno J, Miyohashi F, Shiba H, Kanayama Y, et al. Heterologous expression of tomato glycoside hydrolase family 3 α-L-arabinofuranosidase/β-xylosidases in tobacco suspension cultured cells and synergic action of a family 51 isozyme under antisense suppression of the enzyme. Physiol Plantarum. 2013;150:238–51. doi: 10.1111/ppl.12079. [DOI] [PubMed] [Google Scholar]
- 53.Franco-Cairo JPL, Leonardo FC, Alvarez TM, Ribeiro DA, Bϋchli F, Costa-Leonardo AM, et al. Functional characterization and target discovery of glycoside hydrolases from the digestome of the lower termite Coptotermes gestroi. Biotechnol Biofuels. 2011;4:50. doi: 10.1186/1754-6834-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nigorikawa M, Watanabe A, Furukawa K, Sonoki T, Ito Y. Enhanced saccharification of rice straw by overexpression of rice exo-glucanase. Rice J. 2012;5:14. doi: 10.1186/1939-8433-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lionetti V, Francocci F, Ferrari S, Volpi C, Bellincampi D, Galletti R, et al. Engineering the cell wall by reducing de-methyl-esterified homogalacturonan improves saccharification of plant tissues for bioconversion. PNAS. 2010;107(2):617–21. doi: 10.1073/pnas.0907549107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carpin S, Crèvecoeur M, de Meyer M, Simon P, Greppin H, Penel C. Identification of a Ca2 + −pectate binding site on an apoplastic peroxidase. Plant Cell. 2001;13:511–20. doi: 10.1105/tpc.13.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shah K, Penel C, Gagnon J, Dunand C. Purification and identification of a Ca 2 + −pectate binding peroxidase from Arabidopsis leaves. Phytochemistry. 2004;65:307–12. doi: 10.1016/j.phytochem.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 58.Francoz E, Ranocha P, Nguyen-Kim H, Jamet E, Burlat V, Dunand C. Roles of cell wall peroxidases in plant development. Phytochemistry. 2015;112:15–21. doi: 10.1016/j.phytochem.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 59.Irshad M, Canut H, Borderies G, Pont-Lezica R, Jamet E. A new picture of cell wall protein dynamics in elongating cells of Arabidopsis thaliana: Confirmed actors and newcomers. BMC Plant Biol. 2008;8:16–32. doi: 10.1186/1471-2229-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen XY, Kim ST, Cho WK, Rim Y, Kim S, Kim SW, et al. Proteomics of weakly bound cell wall proteins in rice calli. J Plant Physiol. 2009;166:675–85. doi: 10.1016/j.jplph.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 61.Nersissian AM, Shipp EL. Blue copper-binding domains. Adv Protein Chem. 2002;60:271–340. doi: 10.1016/S0065-3233(02)60056-7. [DOI] [PubMed] [Google Scholar]
- 62.Nieuwland J, Feron R, Huisman BAH, Fasolino A, Derksen J, Mariani C. Lipid transfer proteins enhance cell wall extension in tobacco. Plant Cell. 2005;17:2009–19. doi: 10.1105/tpc.105.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeats TH, Rose JKC. The formation and function of plant cuticles. Plant Physiol. 2013;163:5–20. doi: 10.1104/pp.113.222737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Figueiredo A, Monteiro F, Sebastiana M. Subtilisin-like proteases in plant–pathogen recognition and immune priming: a perspective. Front Plant Sci. 2014;5:739. doi: 10.3389/fpls.2014.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Hoorn RAL. Plant proteases: from phenotypes to molecular mechanisms. Ann Rev Plant Biol. 2008;59:191–223. doi: 10.1146/annurev.arplant.59.032607.092835. [DOI] [PubMed] [Google Scholar]
- 66.Rautengarten C, Steinhauser D, Bussis D, Stintzi A, Schaller A, Kopka J, et al. Inferring hypotheses on functional relationships of genes: analysis of the Arabidopsis thaliana subtilase gene family. PLoS Comput Biol. 2005;1:297–312. doi: 10.1371/journal.pcbi.0010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanaka H, Onouchi H, Kondo M, Hara-Nishimura I, Nishimura M, Machida C, et al. A subtilisin-like serine protease is required for epidermal surface formation in Arabidopsis embryos and juvenile plants. Development. 2001;128:4681–89. doi: 10.1242/dev.128.23.4681. [DOI] [PubMed] [Google Scholar]
- 68.Martinez M. Plant protein-coding gene families: emerging bioinformatics approaches. Trends Plant Sci. 2011;16:558–67. doi: 10.1016/j.tplants.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 69.Liu JJ, Sturrock R, Ekramoddoullah AK. The superfamily of thaumatin-like proteins: its origin, evolution, and expression towards biological function. Plant Cell Rep. 2010;29:419–36. doi: 10.1007/s00299-010-0826-8. [DOI] [PubMed] [Google Scholar]
- 70.Li R, Wang Y, Hu W, Liao X. Changes in the activity, dissociation, aggregation, and the secondary and tertiary structures of a thaumatin-like protein with a high polyphenol oxidase activity induced by high pressure CO2. Innov Food Sci Emerg Technol. 2014;23:68–78. doi: 10.1016/j.ifset.2014.02.013. [DOI] [Google Scholar]
- 71.Bayer EM, Bottrill AR, Walshaw J, Vigouroux M, Naldrett MJ, Thomas CL, et al. Arabidopsis cell wall proteome defined using multidimensional protein identification technology. Proteomics. 2006;6:301–11. doi: 10.1002/pmic.200500046. [DOI] [PubMed] [Google Scholar]
- 72.Preger V, Tango N, Marchand C, Lemaire SD, Carbonera D, Di Valentin M, et al. Auxin-responsive genes AIR12 Ccde for a new family of plasma membrane b-type cytochromes specific to flowering plants. Plant Physiol. 2009;150:606–20. doi: 10.1104/pp.109.139170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mello MO, Tanaka AS, Silva-Filho MC. Molecular evolution of Bowman-Birk type proteinase inhibitors in flowering plants. Mol Phylogenet Evol. 2003;27:103–12. doi: 10.1016/S1055-7903(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 74.Park S, Moon J-C, Park YC, Kim JH, Kim DS, Jang CS. Molecular dissection of the response of a rice leucine-rich repeat receptor-like kinase (LRR-RLK) gene to abiotic stresses. J Plant Physiol. 2014;171:1645–53. doi: 10.1016/j.jplph.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Wu R, Wang L, Wang Z, Shang H, Liu X, Zhu Y, et al. Cloning and expression analysis of a dirigent protein gene from the resurrection plant Boea hygrometrica. Prog Nat Sci. 2009;19:347–52. doi: 10.1016/j.pnsc.2008.07.010. [DOI] [Google Scholar]
- 76.Chen Y, Ye D, Held MA, Cannon MC, Ray T, Saha P, et al. Identification of the abundant hydroxyproline-rich glycoproteins in the root walls of wild-type Arabidopsis, an ext3 mutant line, and its phenotypic revertant. Plants. 2015;4:85–111. doi: 10.3390/plants4010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang T-Y, Chen H-L, Li W-H, Sung H-M, Shih M-C. Omics applications to biofuel research. In: Hou CT, Shaw J-F, editors. Biocatalysis and Biomolecular Engineering. Hoboken: Wiley; 2010. pp. 265–76. [Google Scholar]
- 78.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 79.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–93. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 80.Scheler C, Lamer S, Pan Z, Li XP, Salnikow J, Jangblut P. Peptide mass fingerprint sequences coverage from differentially stained proteins in two-dimensional electrophoresis patterns by matrix assisted laser desorption/ionization-mass spectrometry (MALDI-MS) Electrophoresis. 1998;19:918–27. doi: 10.1002/elps.1150190607. [DOI] [PubMed] [Google Scholar]
- 81.Silva JC, Gorenstein MV, Li GZ, Vissers JP, Geromanos SJ. Absolute quantification of proteins by LCMSE: a virtue of parallel MS acquisition. Mol Cell Proteomics. 2006;5:144–56. doi: 10.1074/mcp.M500230-MCP200. [DOI] [PubMed] [Google Scholar]
- 82. ProtAnnDB. Laboratoire de Recherche em Sciences Végétales. 2015. http://www.polebio.lrsv.ups-tlse.fr/ProtAnnDB/index.php/searchseq. Accessed 15 Aug 2015.
- 83.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;1990(215):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 84.Mitchell A, Hsin-Yu C, Daugherty L, Fraser M, Hunter S, Lopez R, et al. The InterPro protein families database: the classification resource after 15 years. Nucleic Acids Res. 2015;D43(Database issue):D213–21. doi: 10.1093/nar/gku1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, et al. The Pfam protein families database. Nucleic Acids Res. 2014;D42(Database issue):D222–30. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xia J, Sinelnikov I, Han B,Wishart DS. MetaboAnalyst 3.0 - making metabolomics more meaningful. Nucl Acids Res (doi:10.1093/nar/gkv380). [DOI] [PMC free article] [PubMed]
- 87.Salek RM, Xia J, Innes A, Sweatman BC, Adalbert R, Randle S, et al. A metabolomics study of the CRND8 transgenic mouse model of Alzheimer’s disease. Neurochem Int. 2010;56:937–47. doi: 10.1016/j.neuint.2010.04.001. [DOI] [PubMed] [Google Scholar]


