Abstract
Background
Coronary artery disease (CAD) continues to be one of the top public health burden. Perfusion cardiovascular magnetic resonance (CMR) is generally accepted to detect CAD, while data on its cost effectiveness are scarce. Therefore, the goal of the study was to compare the costs of a CMR-guided strategy vs two invasive strategies in a large CMR registry.
Methods
In 3’647 patients with suspected CAD of the EuroCMR-registry (59 centers/18 countries) costs were calculated for diagnostic examinations (CMR, X-ray coronary angiography (CXA) with/without FFR), revascularizations, and complications during a 1-year follow-up. Patients with ischemia-positive CMR underwent an invasive CXA and revascularization at the discretion of the treating physician (=CMR + CXA-strategy). In the hypothetical invasive arm, costs were calculated for an initial CXA and a FFR in vessels with ≥50 % stenoses (=CXA + FFR-strategy) and the same proportion of revascularizations and complications were applied as in the CMR + CXA-strategy. In the CXA-only strategy, costs included those for CXA and for revascularizations of all ≥50 % stenoses. To calculate the proportion of patients with ≥50 % stenoses, the stenosis-FFR relationship from the literature was used. Costs of the three strategies were determined based on a third payer perspective in 4 healthcare systems.
Results
Revascularizations were performed in 6.2 %, 4.5 %, and 12.9 % of all patients, patients with atypical chest pain (n = 1’786), and typical angina (n = 582), respectively; whereas complications (=all-cause death and non-fatal infarction) occurred in 1.3 %, 1.1 %, and 1.5 %, respectively. The CMR + CXA-strategy reduced costs by 14 %, 34 %, 27 %, and 24 % in the German, UK, Swiss, and US context, respectively, when compared to the CXA + FFR-strategy; and by 59 %, 52 %, 61 % and 71 %, respectively, versus the CXA-only strategy. In patients with typical angina, cost savings by CMR + CXA vs CXA + FFR were minimal in the German (2.3 %), intermediate in the US and Swiss (11.6 % and 12.8 %, respectively), and remained substantial in the UK (18.9 %) systems. Sensitivity analyses proved the robustness of results.
Conclusions
A CMR + CXA-strategy for patients with suspected CAD provides substantial cost reduction compared to a hypothetical CXA + FFR-strategy in patients with low to intermediate disease prevalence. However, in the subgroup of patients with typical angina, cost savings were only minimal to moderate.
Electronic supplementary material
The online version of this article (doi:10.1186/s12968-015-0222-1) contains supplementary material, which is available to authorized users.
Background
Coronary artery disease (CAD) continues to be a major source of public health burden particularly in industrialized countries [1]. For the European Union, the estimated costs for CAD management were 60 billion Euros in 2009, of which approximately 20 billion Euros were attributed to direct health care costs [2]. Similarly, the total direct costs of CAD in the United States were estimated to be 107 billion dollars in the same time period [3]. Patients with myocardial ischemia benefit most from revascularizations, as the presence of myocardial ischemia is a strong predictor of major adverse cardiovascular outcomes. Accordingly, current guidelines recommend revascularizing patients with stable CAD if substantial myocardial ischemia is confirmed by either non-invasive ischemia testing or fractional flow reserve (FFR) [4–7]. Fearon and coworkers demonstrated that such an FFR-, i.e. ischemia-guided approach, was not only safe and effective in improving patient outcomes, but reduced costs during the first year after percutaneous coronary interventions (PCI) comparing to a luminal anatomy-guided approach [8]. Invasive CXA, particularly when combined with FFR, is an alternative to non-invasive testing and should be considered in intermediate to high risk patients, i.e. with an annual mortality ≥1 % according to ESC guidelines [5]. The AHA/ACC guidelines on ischemic heart disease justify the use of CXA as first line test to define the extent and severity of CAD in patients with a high likelihood of severe disease (based on clinical assessment and/or exercise ECG testing) [9]. Cardiovascular magnetic resonance (CMR) is now well established as a reliable and safe technique to evaluate ischemia in patients with known or suspected CAD [10–16] and it is recommended as a class I or IIa test in European and US guidelines for stable CAD, respectively [4–7]. However, few data are available to estimate the potential cost savings using the CMR-based approach [17].
Assessment of patients with suspected CAD aiming at effective clinical decision-making needs to not only consider patient factors including cardiovascular risk factor profile, presenting sign and symptoms, but the presence of myocardial ischemia and the coronary arterial anatomy. The aim of this study was to compare the costs of a CMR-guided strategy vs two invasive strategies. The costs of these strategies were assessed from a health care payer perspective for the German, United Kingdom, Swiss, and United States health care systems.
Methods
Definitions of strategies
In the CMR-based strategy (CMR + CXA, Fig. 1), only patients positive for ischemia on CMR were referred to a CXA examination with potential revascularization performed at the discretion of the treating physician. For the CMR + CXA strategy costs included those for the initial CMR, for the CXA in the ischemia-positive patients, and for revascularizations and complications (as shown in Fig. 1). For details on cost calculations of the strategies, see also section below (costs of the different procedures and strategies). For comparison, a hypothetical CXA + FFR strategy was designed which starts with a CXA examination and in case of ≥50 % stenosis in a coronary artery, an FFR testing is added. In the registry population the revascularization rate was 6.2 % and we assumed FFR to be positive with the same proportion in the CXA + FFR strategy. Extrapolating from the correlation between FFR and diameter stenosis from the literature [18, 19], and assuming 6.2 % positive FFR tests (FFR ≤ 0.80) we calculate that approximately 35 % coronary arteries have ≥50 % diameter stenosis (for formulas see Additional file 1). Accordingly, 35 % of patients were assumed to undergo FFR testing to yield 6.2 % ischemia positive findings (Fig. 2). We applied the annual hard event rate observed from the European CMR registry, 0.38 % annually of cardiac death and non-fatal MI, to both the CMR + CXA and CXA + FFR strategies. This assumption is supported by strong evidence that both, FFR [20–22] and CMR [23–25] provide accurate prognostic information at a similar level. We also calculated the costs for a third CXA-only strategy (Fig. 3) which includes costs for the initial CXA and those for revascularizations in all patients with ≥50 % coronary stenoses as described by Moschetti and coworkers [19]. Finally, these calculations were also performed for the 2 sub-groups of patients with typical angina and atypical chest pain.
Fig. 1.
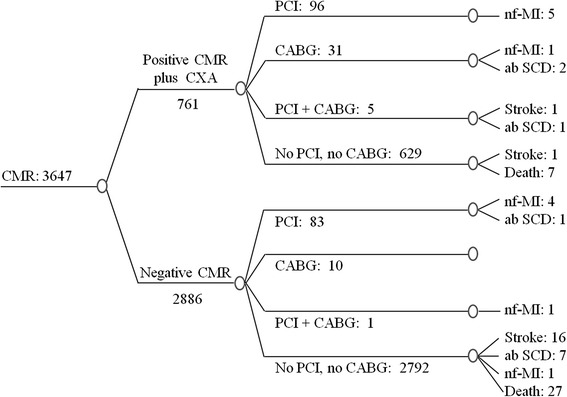
Decision tree and outcome in the study population – CMR + CXA strategy. Diagnostic pathway, treatment, and outcomes are shown for the CMR + CXA strategy in the 3’647 patients of the European CMR Registry. nf-MI: non-fatal MI; ab SCD: aborted SCD
Fig. 2.
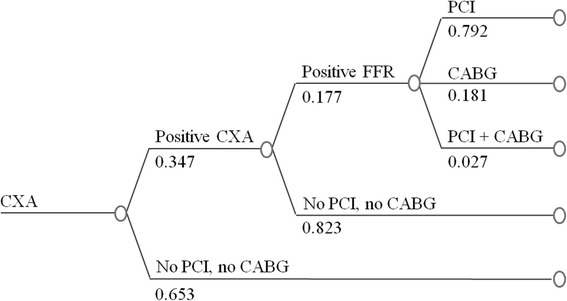
The CXA + FRR guided strategy. A hypothetical invasive CXA + FFR strategy is applied to the patients of the European CMR Registry
Fig. 3.
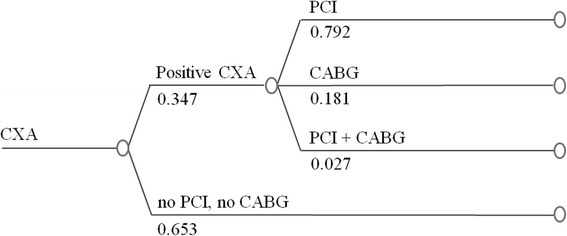
The CXA-only strategy. With this hypothetical strategy, anatomy as defined by invasive x-ray coronary angiography is the only test for decision making, no ischemia testing is used. Revascularizations are performed in patients with ≥50 % coronary stenosis
Patient population
Data from the European CMR registry were used for this analysis [24]. The prospective “Suspected CAD” cohort aims to assess the prognostic value of CMR in a clinical routine setting by collecting data on subsequent treatment and major adverse cardiac events (MACE) during a follow-up of 1 year after the CMR examination [26]. The primary combined end-point of MACE included all-cause death, aborted sudden cardiac death (SCD), non-fatal MI, and stroke. The protocol was approved by the ethics committee at each participating institution and all study participants provided written informed consent. The present analysis includes 3’647 patients with suspected CAD who underwent CMR for ischemia testing and for whom the one-year follow-up was completed (Table 1). For ischemia testing, a first-pass perfusion approach was used [10–14] with vasodilation induced by adenosine (dipyridamole was used in one center; n = 10). Patients were classified as ischemia-positive, if ≥1 segment was ischemic by visual reading (using a 16-segment model) [26].
Table 1.
Baseline characteristics
| Total population | Atypical chest pain | Typical angina | p-values a | |
|---|---|---|---|---|
| Demographics | ||||
| n (%) | 3’647 (100 %) | 1’786 (49.0 %) | 582 (16.0 %) | - |
| Male (%)† | 58.7 % | 45.7 % | 42.8 % | <0.001 |
| Age at baseline (y); mean (range) ‡ | 61.6 (14–92) | 61.1 (14–92) | 62.6 (26–88) | <0.05 |
| Weight (kg); mean (range) § | 80.4 (28–183) | 80.2 (30–183) | 79.7 (28–182) | ns |
| Risk profile | ||||
| Hypertension § | ns | |||
| - none | 38.1 % | 39.1 % | 35.4 % | |
| - treated | 58.1 % | 56.7 % | 60.3 % | |
| - untreated | 3.9 % | 4.3 % | 4.3 % | |
| Dyslipidemia ǁ | 42.2 % | 40.5 % | 45.9 % | 0.059 |
| Diabetes mellitus ǁ | 13.3 % | 10.8 % | 15.0 % | <0.001 |
| Smoker ǁ | ns | |||
| - No | 74.5 % | 73.9 % | 73.9 % | |
| - Current | 12.9 % | 12.9 % | 13.8 % | |
| - Previous | 12.7 % | 13.2 % | 12.4 % | |
| Family history of CAD § | 27.0 % | 28.3 % | 29.4 % | <0.05 |
| Reasons for CAD work-up | ||||
| - Patient complaints | 72.7 % | 89.7 % | 89.7 % | <0.001 |
| - Presence of cardiovascular risk factors | 55.9 % | 53.4 % | 53.1 % | <0.001 |
| - Ambiguous Stress ECG | 20.2 % | 17.4 % | 14.4 % | <0.001 |
| - Ambiguous Stress Echocardiography | 1.9 % | 1.4 % | 1.2 % | <0.01 |
| - Ambiguous Stress SPECT | 0.3 % | 0.2 % | 0.5 % | ns |
| - Ambiguous Cardiac CT | 0.9 % | 0.7 % | 0.5 % | ns |
| Treatment: n (%) | ||||
| Revascularizations | 226 (6.2 %) | 81 (4.5 %) | 75 (12.9 %) | <0.001 |
| - PCI only | 179 (4.9 %) | 70 (3.9 %) | 53 (9.1 %) | <0.001 |
| - CABG only | 41 (1.1 %) | 10 (0.6 %) | 19 (3.3 %) | <0.001 |
| - PCI and CABG | 6 (0.2 %) | 1 (0.1 %) | 3 (0.5 %) | 0.059 |
| Outcome (complications): n (%) | ||||
| Primary endpoint | 75 (2.1 %) | 33 (1.9 %) | 11 (1.9 %) | ns |
| - Mortality : all cause | 34 (0.9 %) | 15 (0.8 %) | 7 (1.2 %) | ns |
| - Cardiac death | 7 (0.2 %) | 6 (0.4 %) | 0 (0.0 %) | ns |
| - Cardiac death and unknown cause | 23 (0.6 %) | 13 (0.7 %) | 2 (0.3 %) | ns |
| - Non-fatal myocardial infarction | 11 (0.3 %) | 5 (0.2 %) | 2 (0.3 %) | ns |
| - Aborted sudden cardiac death | 8 (0.3 %) | 4 (0.2 %) | 1 (0.2 %) | ns |
| - Stroke | 18 (0.5 %) | 10 (0.6 %) | 1 (0.2 %) | ns |
a Differences for age and weight were assessed by one-way ANOVA and for the other parameters by the Chi-square statistic. P-values >0.10 are reported as ns. Reasons for CAD work-up may add up to >100 % as several reasons per patient may apply. † n = 3’643; ‡ n = 3’646; § n = 3’642; ǁ n = 3’641
Costs of the different procedures and strategies
The analysis was performed from a health care payer perspective using 2014 unit costs data in Euros (€) for Germany, in pounds (£) for the United Kingdom, in Swiss Francs (CHF) for Switzerland, and in US Dollars (US$) for the United States (for details on health care systems, see reference [19]). We used reimbursement rates (tariffs) to assess the costs of procedures. See Additional file 2 for details on the sources of information used to derive the costs of the different tests in every country.
The average costs per patient for the 3 strategies were calculated by multiplying the proportion of patients in the different branches of the diagram (Figs 1, 2 and 3) with the unit costs of the different tests, revascularizations, and/or treatments of complications (Table B1 in Additional file 2).
In patients with revascularizations, the costs of one-year treatment with clopidogrel and aspirin were included. By contrast, costs of drugs associated with the management of risk factors were not taken into account, as risk factors should be treated in all patients irrespective of the presence or absence of ischemia. Finally, death was not associated with any costs. Given the time horizon of the analysis of 12 months, no discount rate was applied.
Sensitivity analysis
To assess the influence of various cost parameters on the results, one-way deterministic sensitivity analyses were performed where input parameters were varied one at a time while the remaining values were held at their baseline values. Thus, the model was re-run with changes in the costs of the diagnostic tests of CMR, FFR, and CXA. As the CMR + CXA strategy and the CXA + FFR strategy were assumed to yield the same proportion of ischemia-positive patients, the revascularization procedures would not differ for the 2 arms. Accordingly, costs for treatment were not varied. As costs for the various tests may differ considerably in various geographical regions of the 4 countries and as costs for FFR testing are not (yet) well defined in all 4 health care systems, a break-even analysis was also performed to illustrate the magnitude of reimbursement changes needed to result in equal costs for the 3 strategies.
Statistics
Categorical data are reported as frequencies and continuous data as mean ± SD. Differences between patient groups were assessed using one-way ANOVA and Chi-square statistics where appropriate (Table 1). A p-value <0.05 was considered statistically significant.
Results
Demographics
Based on CMR testing, 20.9 % of patients were diagnosed with ischemia and 17.4 % of these patients were revascularized (72.7 % by PCI, 23.5 % by CABG, and 3.8 % underwent both, PCI and CABG). Additionally, 3.3 % of the CMR-negative patients were revascularized. In the sub-group of patients with typical angina, ischemia was diagnosed by CMR in 34.9 % and revascularizations were performed in 23.2 % of these patients. For outcomes, see Table 1. During the CMR examination, no major complications occurred (for details, see Additional file 3, Table C).
Cost analysis
The average costs per patient for the 3 strategies in the 4 countries with all diagnostic tests (CMR and CXA with/without FFR) performed as outpatient procedures are given in Table 2. The cost reductions by the CMR + CXA strategy in the 4 countries are summarized in Fig. 4a. Costs reductions of CMR + CXA vs CXA + FFR were highest in the UK (34 %) and lowest in Germany (14 %), with US and Switzerland positioned in between with reductions of 24 % and 27 %, respectively.
Table 2.
Costs of the 3 strategies per health care system (n = 3’647)
| Costs CMR + CXA | Costs CXA + FFR | Costs CXA-only | % Cost reduction of CMR + CXA versus CXA + FFR | % Cost reduction of CMR + CXA versus CXA-only | % Cost reduction of CXA + FFR versus CXA-only | |
|---|---|---|---|---|---|---|
| German context (€) | ||||||
| Main analysis (n = 3'647) | 932 | 1'090 | 2'298 | 14.5 | 59.4 | 52.6 |
| Main analysis (n = 3'647) without rehab. & without cardiologist's visit | 919 | 1'082 | 2'290 | 15.1 | 59.9 | 52.8 |
| - Atypical chest pain (n = 1'786) | 787 | 971 | 1'990 | 19.0 | 60.5 | 51.2 |
| - Atypical chest pain (n = 1'786) without rehab. & without cardiologist's visit | 780 | 966 | 1'985 | 19.3 | 60.7 | 51.3 |
| - Typical angina pectoris (n = 582) | 1'466 | 1'500 | 2'690 | 2.3 | 45.5 | 44.2 |
| - Typical angina pectoris (n = 582) without rehab. & without cardiologist's visit | 1'456 | 1'514 | 2'704 | 3.8 | 46.2 | 44.0 |
| UK context (£) | ||||||
| Main analysis (n = 3'647) | 1'075 | 1'623 | 2'224 | 33.8 | 51.7 | 27.0 |
| - Atypical chest pain (n = 1'786) | 968 | 1'552 | 2'052 | 37.6 | 52.8 | 24.4 |
| - Typical angina pectoris (n = 582) | 1'513 | 1'866 | 2'444 | 18.9 | 38.1 | 23.7 |
| Swiss context (CHF) | ||||||
| Main analysis (n = 3'647) | 3'252 | 4'451 | 8'399 | 26.9 | 61.3 | 47.0 |
| Main analysis (n = 3'647) without rehab. & without cardiologist's visit | 3'191 | 4'420 | 8'368 | 27.8 | 61.9 | 47.2 |
| - Atypical chest pain (n = 1'786) | 2'783 | 4'044 | 7'520 | 31.2 | 63.0 | 46.2 |
| - Atypical chest pain (n = 1'786) without rehab. & without cardiologist's visit | 2'733 | 4'017 | 7'493 | 32.0 | 63.5 | 46.4 |
| - Typical angina pectoris (n = 582) | 5'074 | 5'816 | 9'511 | 12.8 | 46.7 | 38.9 |
| - Typical angina pectoris (n = 582) without rehab. & without cardiologist's visit | 4'983 | 5'784 | 9'479 | 13.8 | 47.4 | 39.0 |
| US context ($) | ||||||
| Main analysis (n = 3'647) | 1'740 | 2'292 | 6'022 | 24.1 | 71.1 | 61.9 |
| Main analysis (n = 3'647) with cardiologist's visit | 1'759 | 2'294 | 6'024 | 23.3 | 70.8 | 61.9 |
| - Atypical chest pain (n = 1'786) | 1'429 | 1'996 | 5'588 | 28.4 | 74.4 | 64.3 |
| - Atypical chest pain (n = 1'786) with cardiologist's visit | 1'444 | 1'997 | 5'589 | 27.7 | 74.2 | 64.3 |
| - Typical angina pectoris (n = 582) | 2'947 | 3'335 | 6'592 | 11.6 | 55.3 | 49.4 |
| - Typical angina pectoris (n = 582) with cardiologist's visit | 2'983 | 3'336 | 6'593 | 10.6 | 54.8 | 49.4 |
Fig. 4.
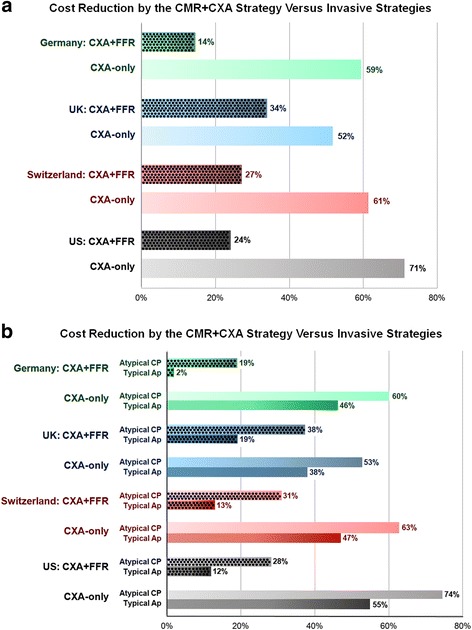
Percentage of cost reductions of the CMR + CXA strategy in comparison to the CXA + FFR and CXA-only strategies for the German, UK, Swiss, and US health care systems. 4a Cost reductions for the CMR + CXA strategy in the entire study population of 3’647 patients. 4b Cost reductions for the CMR + CXA strategy in the subgroups of patients with atypical chest pain (n = 1’786) and with typical angina (n = 582)
As expected, in the study population with typical angina, the rate of revascularizations was higher than in the total population (Table 1). Also, in the population with typical angina, the CMR + CXA approach yielded lower cost savings vs the CXA + FFR strategy ranging from 2.3 % for Germany to 18.9 % for UK and with cost savings for US and Switzerland in between with 11.6 % and 12.8 %, respectively (see Table 2 and Fig. 4b). Cost savings in the population with atypical chest pain symptoms were comparable to the entire study population (see Table 2 and Fig. 4b).
Inclusion or exclusion of costs for rehabilitation after a non-fatal MI (for Germany and Switzerland) or of costs for a visit to see a cardiologist in the first year after revascularization (for Germany, Switzerland, and US) did not significantly influence the differences between strategies (difference of <1 percentage point, Table 2).
Sensitivity analysis
Results of the sensitivity analyses are given in the Additional file 4 (Tables D1-D4). Generally, a 10 % change in the costs of a diagnostic test (changes introduced into the model one by one) leads to variations ranging from 1 % to 8 % in final costs of the 3 strategies. A 10 % increase in the CMR costs leads to a reduction of 0.7 to 4.3 percentage points on the cost savings of the CMR + CXA strategy vs CXA + FFR in the 4 health care systems. A 10 % decrease in the CXA costs would reduce the cost savings of the CMR + CXA strategy vs CXA + FFR by 2.4 to 4.5 percentage points.
When varying the proportion of patients which undergo FFR in the CXA + FFR strategy from 30 % to 55 %, the sensitivity analysis yields changes in costs in favor of the CMR + CXA strategy for all health care systems and all populations studied with the only exception for the patients with typical angina in the German system, where costs savings were minor ranging from 0.8 % to 6.1 % (Additional file 4, Tables D1-D4).
Discussion
Cost minimization by the CMR-guided strategy to manage patients with suspected CAD
Our study represents an effort of cost-benefits analysis across systems from 4 countries extrapolated from “real-world” multinational data from the European CMR registry. Using the CMR + CXA strategy cost savings ranging from 14 % to 34 % can be expected compared to an invasive CXA + FFR strategy when applied on a population of low to intermediate prevalence of disease. By the same token, substantial cost savings ranging from 19 % to 38 % can be anticipated in the patients with atypical chest pain. However, in the population with typical angina, cost savings were minimal in the German health care system and only moderate in the US and Swiss systems with 12 %-13 %, while they remained substantial in the UK with 19 % cost savings. This sub-population analysis underscores the importance of pre-test probabilities when searching for the most cost-effective work-up strategy. Thus, in a low pre-test probability population, which dominates those referred to non-invasive testing, cost savings might be substantial with a CMR + CXA strategy, even if the costs of an invasive CXA is added to all patients with a positive CMR. With an increasing pre-test probability, as e.g. in the typical angina population, substantial cost savings may persist in some countries but may decline in others.
The potential cost savings were observed if all interventions were calculated as out-patient procedures and by taking into account the costs for revascularizations and complications during the first year after PCI and/or CABG. This favorable cost profile of the CMR-based strategy in a population with low to intermediate disease prevalence is in line with a cost-effectiveness analysis performed in the setting of the CE-MARC trial [27]. CMR as a first line test followed by CXA in patients with a positive or inconclusive CMR study was found cost-effective in this population at the higher end of the National Institute for Health and Clinical Excellence (NICE) threshold range [27]. Of note, the European CMR registry data are collected from a network of 59 centers representing 18 countries and are therefore highly likely to reflect broad CMR performance achievable in current routine cardiology practice. This study design also accounted for costs of mis-classifications (i.e. for false negative CMR studies), as costs of invasive tests, revascularizations, and costs for complication management in CMR-negative patients (=false negatives) were added to the overall costs of the CMR + CXA strategy. Interestingly, European and US guidelines recommend to consider CXA as a first test, if the annual mortality is relatively high, i.e. ≥1 % [5], and/or if results of noninvasive testing (exclusive of stress imaging) indicate a high likelihood of CAD (as e.g. in long-standing diabetes or in patients with electrocardiography with diffuse ischemic changes in multiple territories) [9]. The presented results indicate, that current guidelines on invasive CXA utilization in stable CAD [5, 9] are also valid when economic aspects are taken into account as cost savings of the CMR + CXA strategy were relatively small (or almost absent) in the studied population with typical angina and an annual mortality >1 %.
If the CMR + CXA strategy is compared with a CXA-only strategy, cost savings can be as high as 52–71 %, most likely due to the fact that ischemia testing reduces the need for revascularizations. Similar results have been shown in the past in prospective trials comparing a combined CXA and FFR approach vs a CXA-only approach [8, 28]. In a simulation, Moschetti et al. included the FFR ischemia testing in the model and showed that a CMR-based strategy was more cost-effective than CXA combined with FFR when applied to a population of low to intermediate pretest likelihood of CAD [19]. The results of the current study derived from a real patient population with an ischemia prevalence of 21 % now confirm these model simulations of Moschetti [19], which predicted cost-effectiveness in the German, UK, Swiss, and US health care systems in populations with an ischemia prevalence below 62 % to 83 %.
The European CMR registry design requires indications for CMR to be in accordance with appropriate use criteria established by recognized professional organizations [26, 29]. Interestingly, the prevalence of ischemia on stress perfusion CMR in a population fulfilling the appropriate use criteria was 18.8 % (vs 4.8 % in the rarely appropriate group) [30], which is close to the prevalence of 20.9 % in the current study. This finding may indirectly support the notion that indications in the registry were following current appropriate use criteria in most cases.
Prognostic power of stress perfusion CMR in a real-world multi-center setting
Since false negative tests are likely to decrease quality of life and to increase costs by the management of complications of unrecognized disease, it is important to assess the prognostic performance of non-invasive methods. In this large unselected patient population with suspected CAD, a normal perfusion-CMR predicted an excellent outcome with an annual event rate for cardiac death and non-fatal MI of 0.38 %, which increased to 1.11 %, if deaths of unknown cause and aborted SCD were added. These registry outcome data match well with previous prospective CMR single center studies reporting annual event rates of cardiac death and non-fatal MI in ischemia-negative patients of 0.7 % per year [23, 25].
In the CMR negative patients revascularizations occurred in 3.3 % and in the sub-group with typical angina, they occurred in 7.4 %, which might represent false negative CMR examinations. However, no FFR proof was required to guide these revascularizations of CMR negative patients. In addition, in the FFR-negative population of FAME 2, a similar revascularization rate was observed with 12.0 % over 2 years [22]. It might be speculated that progression of disease over 1 year post-testing in ischemia-negative patients could partly account for these revascularizations. On the other hand, only 17.4 % of the CMR-positive patients were revascularized and only 23.2 % of the patients with typical angina. This is most likely explained by the fact, that patients by definition were categorized as “ischemic” with at least one segment positive on CMR [26], while it is recommended to revascularize patients with 2 or more ischemic segments [5].
Limitations
The costs in the 2 invasive strategies were calculated based on the relationship between the stenosis degree and FFR-positive findings as reported in the literature [18, 19]. This relationship was not verified in the study population. Sensitivity analyses, however, demonstrated cost savings for the CMR + CXA strategy even when this relationship was modified. The fact that the invasive strategies were modeled in this study is certainly a limitation and the 3 different strategies should be assessed in future prospective randomized cost-effectiveness trials. We believe that this study still yields useful results as it is based on real-world data in a patient population of low to intermediate disease prevalence.
In patients with confirmed ischemia, the treatment of symptoms might be more aggressive and consequently more costly. This situation, however, would equally affect costs in the CMR + CXA and CXA + FFR arm. The knowledge of anatomical stenoses could also lead to more aggressive treatment of symptoms and higher costs in the invasive groups. These potential mechanisms could not be taken into account in the current study and could cause underestimations of costs for the invasive strategies.
The registry structure did not allow collecting data to ensure that patients received optimum medical treatment before revascularizations as is recommended by guidelines [5–7, 9, 31] which could influence the outcome. As the outcomes of the CMR + CXA and CXA + FFR strategies were assumed to match, this aspect would not affect the difference of calculated costs for the two strategies. Also, in a recent cost-effectiveness model, for CMR (followed by invasive CXA) and for invasive CXA (including FFR) the quality-adjusted life years gained varied by only 0.08 % (for both, the UK and US systems), while costs varied by 4.7 % and 9.2 %, respectively, in favor of CMR [32].
In general, assigning costs to the various procedures and to hospital stays is a demanding task as some tariff systems are heterogeneous and in addition, differences between geographical regions within a system also exist. This fact has to be considered when interpreting the study results. Costs for FFR were not coded in all tariff systems and therefore, the costs for FFR were calculated as the difference between two tariff positions (for Germany, UK, and Switzerland) or by estimating costs for material [8] and physician payment [33] (for the US). A low reimbursement of this FFR position could disadvantage the CXA-only approach. However, the break-even analysis indicates, that a 6–12 fold increase in the FFR reimbursement would be required to match the costs of the CXA-only strategy.
The CMR + CXA strategy was not compared with other non-invasive imaging stress tests. This aspect warrants testing in future studies.
Conclusions
A CMR + CXA-guided strategy to manage patients with suspected CAD is less costly than an invasive CXA + FFR strategy when applied in a real-world patient population of low to intermediate prevalence of disease and when assuming same outcomes for the strategies. This finding was observed for the German, UK, Swiss, and US health care systems. However, in the subgroup of patients with typical angina, cost savings were only minimal to moderate. The costs of tests, but also the patient characteristics represent important factors that determine the cost-effectiveness of various work-up strategies. These findings warrant further confirmation in prospective cost-effectiveness trials.
Additional files
The stenosis-FFR relationship. (DOC 38 kb)
Sources of cost calculations. (DOC 74 kb)
Complictions during CMR. (DOC 37 kb)
Sensitivity analysis. (DOC 687 kb)
Acknowledgments
Disclosures
S.E. Petersen, MD, served as a consultant to Circle Cardiovascular Imaging Inc. (Calgary, Canada). J. Schwitter, MD, received support from Bayer Healthcare, Germany. The other authors report no conflicts.
The EuroCMR Registry is supported by unrestricted educational grants from the following companies (in alphabetic order):
-Life Sciences GE Healthcare, Munich, Germany
-Medtronic Inc., Minneapolis MN, USA
-Novartis International AG, Basel, Switzerland
-Siemens Health Care, Erlangen, Germany
Importantly, industry sponsoring was exclusively used for registry data management and analysis. All CMR scans reported in this registry were clinically indicated according to the actual appropriateness criteria [18], and thus completely funded by the regular health care providers.
Funding sources
The study was funded in part by EU Grant 005-GW02-067D (H.M. and O.B.) and the Swiss Heart Foundation (J.S.). This work forms part of the research areas contributing to the translational research portfolio of the Cardiovascular Biomedical Research Unit at Barts which is supported and funded by the National Institute for Health Research.
Abbreviations
- CAD
Coronary artery disease
- CMR
Cardiovascular magnetic resonance
- CXA
X-ray coronary angiography
- FFR
Fractional flow reserve
- PCI
Percutaneous coronary intervention
- CABG
Coronary artery bypass grafting
- European CMR registry
European Cardiovascular Magnetic Resonance registry
- MACE
Major adverse cardiac events
- SCD
sudden cardiac death
- MI
Myocardial infarction
- nf-MI
non-fatal MI
- ab SCD
aborted SCD
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12968-015-0222-1) contains supplementary material, which is available to authorized users.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KM is responsible for the conception and design of the cost analysis, she performed the costs analysis and participated to the drafting process of the manuscript. SP, GP, RYK, JBW, ML, GK, ACR were involved in the analysis and the interpretation of the data. In addition, SP, GP, RYK provided cost data specific for UK, Germany, and US, respectively. OB and HM are responsible for the conception and design of the study as well as analysis and interpretation of the data. JS is responsible for the conception and design of the study and the costs analysis, was involved in the interpretation of the data and drafted the manuscript. All authors provided helpful comments to revise the manuscript critically for important intellectual content; all authors approved the submitted manuscript.
Contributor Information
Karine Moschetti, Email: karine.moschetti@chuv.ch.
Steffen E. Petersen, Email: s.e.petersen@qmul.ac.uk
Guenter Pilz, Email: pilz@khagatharied.de.
Raymond Y. Kwong, Email: rykwong@partners.org
Jean-Blaise Wasserfallen, Email: Jean-Blaise.Wasserfallen@chuv.ch.
Massimo Lombardi, Email: Massimo.Lombardi@grupposandonato.it.
Grigorios Korosoglou, Email: gkorosoglou@hotmail.com.
Albert C. Van Rossum, Email: ac.vrossum@vumc.nl
Oliver Bruder, Email: o.bruder@contilia.de.
Heiko Mahrholdt, Email: heiko.mahrholdt@rbk.de.
Juerg Schwitter, Phone: +41 (0)21 314 0012, Email: jurg.schwitter@chuv.ch.
References
- 1.McGillion M, O’Keefe-McCarthy S, Carroll SL, Victor JC, Cosman T, Cook A, Hanlon JG, Jolicoeur EM, Jamal N, McKelvie R, Arthur HM. Impact of self-management interventions on stable angina symptoms and health-related quality of life: a meta-analysis. BMC Cardiovasc Disord. 2014;14:14. doi: 10.1186/1471-2261-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leal, J., R. Luengo-Fernandez, A. Gray, European Cardiovascular Disease Statistics, European Heart Network and European Society of Cardiology, Edition 2012. ISBN 987-2-9537898-1-2
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the AHA. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMurray, J.J.V., S. Adamopoulos, S.D. Anker, A. Auricchio, M. Böhm, K. Dickstein, V. Falk, G. Filippatos, C. Fonseca, M.A.G. Sanchez, T. Jaarsma, L. Køber, G.Y.H. Lip, A.P. Maggioni, A. Parkhomenko, B.M. Pieske, B.A. Popescu, P.K. Rønnevik, F.H. Rutten, J. Schwitter, P. Seferovic, J. Stepinska, P.T. Trindade, A.A. Voors, F. Zannad, A. Zeiher. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012;33(14):1787–1847. [DOI] [PubMed]
- 5.Montalescot G, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Budaj CAA, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, Van Der Wall EE VCJMESC. Guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht310.P4876. [DOI] [PubMed] [Google Scholar]
- 6.Windecker, S., P. Kolh, F. Alfonso, J.-P. Collet, J. Cremer, V. Falk, G. Filippatos, C. Hamm, S.J. Head, P. Jüni, A.P. Kappetein, A. Kastrati, J. Knuuti, U. Landmesser, G. Laufer, F.-J. Neumann, D.J. Richter, P. Schauerte, M. Sousa Uva, G.G. Stefanini, D.P. Taggart, L. Torracca, M. Valgimigli, W. Wijns, A. Witkowski. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541-619. [DOI] [PubMed]
- 7.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King Iii SB, Kligfield PD, Krumholz HM, Kwong RYK, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Jr, Smith SC, Jr, Spertus JA, Williams SV. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: A Report of the ACC Foundation/AHA Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Fearon WFB, Bornschein PA, Tonino RM, Gothe BD, Bruyne NH, Pijls U, Siebert I. Fractional Flow Reserve Versus Angiography for Multivessel Evaluation Study. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation. 2010;122:2545–2550. doi: 10.1161/CIRCULATIONAHA.109.925396. [DOI] [PubMed] [Google Scholar]
- 9.Fihn S, Blankenship J, Alexander K, Bittl J, Byrne J, Fletcher B, Fonarow G, Lange R, Levine G, Maddox T, Naidu S, Ohman E, Smith P. ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the ACC/AHA Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014;130:1749–1767. doi: 10.1161/CIR.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 10.Schwitter J, Nanz D, Kneifel S, Bertschinger K, Buchi M, Knusel PR, Marincek B, Luescher TF, von Schulthess GK. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation. 2001;103:2230–2235. doi: 10.1161/01.CIR.103.18.2230. [DOI] [PubMed] [Google Scholar]
- 11.Nagel E, Klein C, Paetsch I, Hettwer S, Schnackenburg B, Wegscheider K, Fleck E. Magnetic Resonance Perfusion Measurements for the Noninvasive Detection of Coronary Artery Disease. Circulation. 2003;108:432–437. doi: 10.1161/01.CIR.0000080915.35024.A9. [DOI] [PubMed] [Google Scholar]
- 12.Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H, Dill T, Larsson HBW, Flamm SD, Marquardt M, Johansson L. MR-IMPACT: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial: Comparison of perfusion CMR with Single Photon Emission Computed Tomography for the Detection of Coronary Artery Disease in a Multicenter, Multivendor, Randomized Trial. Eur Heart J. 2008;29:480–489. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 13.Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, Schönberg SO, Debl K, Strohm O, Ahlstrom H, Dill T, Hoebel N, Simor T. Superior diagnostic performance of perfusion-CMR versus SPECT to detect coronary artery disease: The secondary endpoints of the multicenter multivendor MR-IMPACT II. J Cardiovasc Magn Reson. 2012;14:61–71. doi: 10.1186/1532-429X-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, Bijsterveld P, Ridgway JP, Radjenovic A, Dickinson CJ, Ball SG, Plein S. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–460. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plein SJ, Schwitter D, Suerder JP, Greenwood P, Boesiger S, Kozerke K. Space and time sensitivity encoding-accelerated myocardial perfusion MR imaging at 3.0 T: comparison with 1.5 T. Radiology. 2008;249:493–500. doi: 10.1148/radiol.2492080017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruder O, Schneider S, Nothnagel D, Pilz G, Lombardi M, Sinha A, Wagner A, Dill T, Frank H, van Rossum A, Schwitter J, Nagel E, Senges J, Sabin G, Sechtem U, Mahrholdt H. Acute adverse reactions to gadolinium-based contrast agents in CMR: multicenter experience with 17,767 patients from the EuroCMR Registry. JACC Cardiovasc Imaging. 2011;4:1171–1176. doi: 10.1016/j.jcmg.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Francis S, Daly C, Heydari B, Abbasi S, Shah R, Kwong R. Cost-effectiveness analysis for imaging techniques with a focus on cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:52. doi: 10.1186/1532-429X-15-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toth G, Hamilos M, Pyxaras S, Mangiacapra F, Nelis O, De Vroey F, Di Serafino L, Muller O, Van Mieghem C, Wyffels E, Heyndrickx GR, Bartunek J, Vanderheyden M, Barbato E, Wijns W, De Bruyne B. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J. 2014;35:2831–2838. doi: 10.1093/eurheartj/ehu094. [DOI] [PubMed] [Google Scholar]
- 19.Moschetti K, Favre D, Pinget C, Pilz G, Petersen SE, Wagner A, Wasserfallen JB, Schwitter J. Comparative cost-effectiveness analyses of cardiovascular magnetic resonance and coronary angiography combined with fractional flow reserve for the diagnosis of coronary artery disease. J Cardiovasc Magn Reson. 2014;16:13. doi: 10.1186/1532-429X-16-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–2111. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 21.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Juni P, Fearon WF. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 22.De Bruyne B, Fearon WF, Pijls NHJ, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius-Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd K, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Limacher A, Nüesch E, Jüni P. Fractional Flow Reserve–Guided PCI for Stable Coronary Artery Disease. N Engl J Med. 2014;371:1208–1217. doi: 10.1056/NEJMoa1408758. [DOI] [PubMed] [Google Scholar]
- 23.Jahnke C, Nagel E, Gebker R, Kokocinski T, Kelle S, Manka R, Fleck E, Paetsch I. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–1776. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 24.Bruder O, Wagner A, Lombardi M, Schwitter J, van Rossum A, Pilz G, Nothnagel D, Steen H, Petersen S, Nagel E, Prasad S, Schumm J, Greulich S, Cagnolo A, Monney P, Deluigi CC, Dill T, Frank H, Sabin G, Schneider S, Mahrholdt H. European Cardiovascular Magnetic Resonance (EuroCMR) registry--multi national results from 57 centers in 15 countries. J Cardiovasc Magn Reson. 2013;15:9. doi: 10.1186/1532-429X-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coelho-Filho OR, Seabra LF, Mongeon FP, Abdullah SM, Francis SA, Blankstein R, Di Carli MF, Jerosch-Herold M, Kwong RY. Stress myocardial perfusion imaging by CMR provides strong prognostic value to cardiac events regardless of patient’s sex. JACC Cardiovasc Imaging. 2011;4:850–861. doi: 10.1016/j.jcmg.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner A, Bruder O, Schneider S, Nothnagel D, Buser P, Pons-Lado G, Dill T, Hombach V, Lombardi M, van Rossum A, Schwitter J, Senges J, Sabin S, Sechtem U, Mahrholdt H, Nagel E. Current variables, definitions and endpoints of the European Cardiovascular Magnetic Resonance Registry. J Cardiovasc Magn Reson. 2009;11:43–55. doi: 10.1186/1532-429X-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker S, Girardin F, McKenna C, Ball SG, Nixon J, Plein S, Greenwood JP, Sculpher M. Cost-effectiveness of cardiovascular magnetic resonance in the diagnosis of coronary heart disease: an economic evaluation using data from the CE-MARC study. Heart. 2013;99:873–881. doi: 10.1136/heartjnl-2013-303624. [DOI] [PubMed] [Google Scholar]
- 28.Di Serafino L, De Bruyne B, Mangiacapra F, Bartunek J, Agostoni P, Vanderheyden M, Scognamiglio G, Heyndrickx GR, Wijns W, Barbato E. Long-term clinical outcome after fractional flow reserve– versus angio-guided percutaneous coronary intervention in patients with intermediate stenosis of coronary artery bypass grafts. Am Heart J. 2013;166:110–118. doi: 10.1016/j.ahj.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, Pohost GM, Williams KA. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine Endorsed by the American College of Emergency Physicians. J Am Coll Cardiol. 2009;53:2201–2229. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 30.McGraw S, Mirza O, Bauml M, Rangarajan V, Farzaneh-Far A. Downstream clinical consequences of stress cardiovascular magnetic resonance based on appropriate use criteria. J Cardiovasc Magn Reson. 2015;17:35. doi: 10.1186/s12968-015-0137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes H-P, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen M-R, Tendera M, Tuomilehto J, Valensi P, Zamorano JL. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 32.Genders TSS, Petersen SE, Pugliese F, Dastidar AG, Fleischmann KE, Nieman K, Hunink MGM. The Optimal Imaging Strategy for Patients With Stable Chest Pain. A Cost-Effectiveness Analysis. Ann Int Med. 2015;162:474–484. doi: 10.7326/M14-0027. [DOI] [PubMed] [Google Scholar]
- 33.Procedural Reinbursement Guide Medicare 2012, BostonScientifc, available at http://www.bostonscientific.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The stenosis-FFR relationship. (DOC 38 kb)
Sources of cost calculations. (DOC 74 kb)
Complictions during CMR. (DOC 37 kb)
Sensitivity analysis. (DOC 687 kb)


