Abstract
Background
Apolipoprotein E (ApoE) is a major cholesterol carrier and plays an important role in maintaining lipid homeostasis both in the periphery and brain. Human APOE gene is polymorphic at two single nucleotides (rs429358 and rs7412) resulting in three different alleles (ε2, ε3 and ε4). ApoE isoforms modulate the risk for a variety of vascular and neurodegenerative diseases; thus, APOE genotyping is crucial for predicting disease risk and designing individualized therapy based on APOE genotype.
Results
We have developed an APOE genotyping method that is based on allele-specific PCR methodology adapted to Real Time PCR monitored by TaqMan probe. Rather than using TaqMan probes specific for the two polymorphic sites, only one TaqMan probe is used as the polymorphic alleles are recognized by site-specific PCR primers. Each genotyping assay can be completed within 90 minutes and is applicable to high-throughput analysis. Using this protocol, we genotyped a total of 1158 human DNA samples and obtained a 100 % concordance with the APOE genotype determined by sequencing analysis.
Conclusion
The APOE genotyping assay we have developed is accurate and cost-effective. In addition, our assay can readily be applied to genotyping large sample numbers. Therefore, our APOE genotyping method can be used for assessing the risk for a variety of vascular and neurodegenerative diseases that have been reported to be associated with APOE polymorphism.
Keywords: Apolipoprotein E, APOE genotype, Real Time PCR, Sequencing, TaqMan, Polymorphism
Background
The human apolipoprotein E (apoE) gene is mapped to chromosome 19q13.2, which consists of four exons and three introns [1]. APOE gene is polymorphic at two single nucleotides (rs429358 and rs7412), resulting in three different alleles (ε2, ε3 and ε4) and six APOE genotypes (ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4 and ε4/ε4) [2]. The worldwide frequency of ε2, ε3 and ε4 allele is 8.4 %, 77.9 % and 13.7 %, respectively [3, 4]. However, the APOE allele frequencies vary widely among different ethnic populations [5]. Differences among the three apoE isoforms reside in the amino acid residues 112 and 158, where either cysteine or arginine is present: E2 (Cys 112, Cys 158), E3 (Cys 112, Arg 158), and E4 (Arg 112, Arg 158) [6]. Despite differences by only one or two amino acids, the structural and functional differences among the three apoE isoforms can be profound to affect disease risk [7, 8].
ApoE is a major cholesterol carrier and plays an important role in maintaining lipid homeostasis both in the periphery and brain [3, 6]. In the periphery, apoE is synthesized predominantly by liver and macrophages. In the brain, apoE is produced primarily by astrocytes and delivers cholesterol and other essential lipids to neurons through members of the low-density lipoprotein receptor (LDLR) family [9–12]. The single amino acid differences among the three apoE isoforms alter the protein’s structure and influence its lipid association and receptor binding; therefore apoE modulates cholesterol homeostasis in an isoform-dependent manner [13]. Notably, apoE2 binds to LDLR with ~50-fold weaker affinity than apoE3 and apoE4. As a result, apoE2 transports lipids less efficiently, and ε2 homozygosity is associated with an increased risk for type III hyperlipoproteinemia [14–16]. ApoE4 preferentially binds to large lipoprotein particles and is associated with increased risk for hypercholesterolemia and atherosclerosis, faster HIV disease progression, and accelerated telomere shortening [3, 17, 18].
Most importantly, genome-wide association studies have confirmed that the ε4 allele of APOE is the strongest genetic risk factor for late-onset Alzheimer’s disease (LOAD) [9, 19–21]. Compared with those with an ε3/ε3 genotype, the risk of AD was increased in individuals with one copy (ε2/ε4, OR 2.6; ε3/ε4, OR 3.2) or two copies (ε4/ε4, OR 14.9) of the ε4 allele [4, 22, 23]. Conversely, the ε2 allele of APOE has a protective effect against AD [24]. The risk of AD in individuals carrying an ε2/ε2 (OR 0.6) or ε2/ε3 (OR 0.6) genotype is lower than those carrying ε3/ε3 [4]. Additionally, the ε4 allele of APOE was found to be a risk factor for other neurodegenerative diseases including cerebral amyloid angiopathy (CAA) [25], dementia with Lewy bodies [26, 27] and multiple sclerosis [28].
As APOE genotype predicts the risk for a variety of vascular and neurodegenerative diseases, it is critical to develop rapid and cost-effective methods to analyze APOE gene polymorphism. Several different APOE genotyping methods have been developed. Among them, the PCR-Restriction Fragment Length Polymorphism (PCR-RFLP) analysis is a conventional method applied to genotype APOE polymorphism [29, 30]. However, this method is complex and labor-intensive as it involves multiple steps including restriction enzyme digestion. PCR plus sequencing or mass spectrometry is an effective method, but requires expensive detection equipment and is also labor-intensive [31]. Several Real Time PCR-based techniques have been developed to genotype APOE gene, including HRM (high resolution melt) [32, 33], TaqMan probe [34] and FRET (Fluorescent Resonance Energy Transfer) [35]. However, the formation of primer-dimers may complicate the melting curves interpretation, and the use of FRET and multiple TaqMan probes is in general costly.
In view of the importance of APOE genotyping in predicting individual risk for a variety of vascular and neurodegenerative diseases, we have developed a rapid and cost-effective method for analyzing APOE polymorphism. Using this protocol, we genotyped a total of 1158 human DNA samples and obtained a 100 % concordance with the APOE genotype determined by sequencing analysis. Therefore, the method we have developed for APOE genotyping is precise and suitable for genotyping large sample cohorts.
Results
APOE genotyping assay development and validation
Our assay was based on allele-specific PCR methodology adapted to Real Time PCR monitored by TaqMan probe. Initial PCR primers were designed according to the nucleotide differences located at the two SNPs within exon 4 of the APOE gene, rs429358 and rs7412 (Fig. 1a). We screened a group of oligonucleotide primers and obtained three pairs that gave specific amplifications of ε2, ε3 and ε4 allele, respectively (Table 1). In order to monitor real-time DNA amplification products, one double-dye oligonucleotide TaqMan probe was included in all reactions. The probe has a FAM fluorophore attached to its 5’ end and a BHQ quencher molecule attached to its 3’ end (Fig. 1b). The probe initially hybridizes to the template strand via its complementary sequences. Upon PCR amplification, Taq polymerase will degrade the probe during strand extension, resulting in the separation of fluorophore and the quencher which allows the excitation of the fluorophore by laser [36].
Fig. 1.
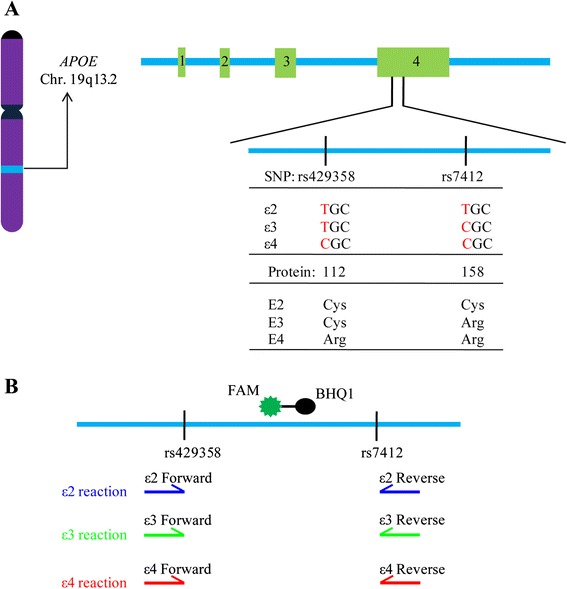
Schematic diagram of the human APOE gene and APOE genotyping method. a The APOE gene is located on chromosome 19, and is polymorphic at two single nucleotides (rs429358 and rs7412) resulting in three different alleles (ε2, ε3 and ε4). b Amplifications of the APOE ε2, ε3 and ε4 alleles are initiated by allele-specific PCR primers. One double-dye oligonucleotide TaqMan probe was included in all reactions to monitor real-time DNA amplification products
Table 1.
Sequences of primers and probes for the APOE genotyping assay
| Name | Sequence (5’-3’) |
|---|---|
| ε2-Forward | GCGGACATGGAGGACGTGT |
| ε2-Reverse | CCTGGTACACTGCCAGGCA |
| ε3-Forward | CGGACATGGAGGACGTGT |
| ε3-Reverse | CTGGTACACTGCCAGGCG |
| ε4-Forward | CGGACATGGAGGACGTGC |
| ε4-Reverse | CTGGTACACTGCCAGGCG |
| APOE probe | FAM-CAGCTCCTCGGTGCTCTGGC-BHQ1 |
| ACTB-Forward | GACGTGGACATCCGCAAAGAC |
| ACTB-Reverse | CAGGTCAGCTCAGGCAGGAA |
| ACTB probe | HEX-TGCTGTCTGGCGGCACCACCATGTACC-BHQ1 |
To validate the efficiency and specificity of our assay, we synthesized three 197-bp DNA sequences covering the two SNPs for ε2, ε3 or ε4 allele and a 179-bp DNA sequence of beta-actin gene (ACTB) which serve as positive controls for APOE genotyping. The TaqMan probe for ACTB was designed similar to APOE but the former has a HEX fluorophore attached to its 5’ end. Genotyping of each sample was carried out in parallel reactions, namely ε2, ε3 or ε4 reaction. Each reaction contains allele-specific primers for APOE in combination with the primers for ACTB. Specific amplification curve appeared only in ε2 reaction when ε2-positive DNA was used (Fig. 2). Similar results were observed in ε3 or ε4 reaction, demonstrating the specificity of our detecting system.
Fig. 2.
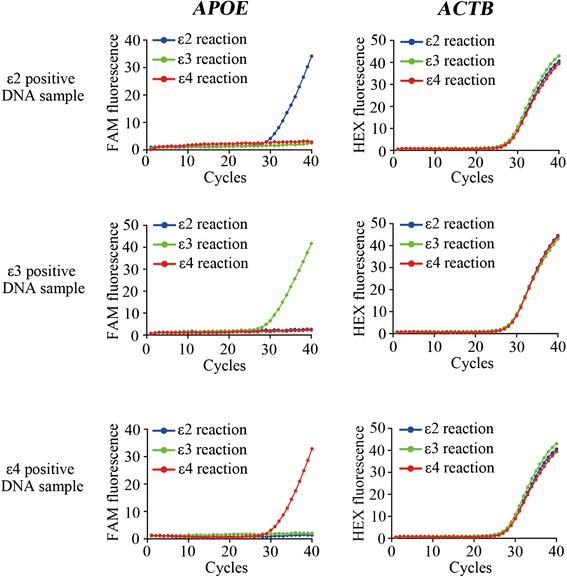
APOE genotyping on ε2-, ε3- or ε4-positive plasmid DNA by Real Time PCR. Representative amplification curves for APOE and ACTB are shown. 12000 copies of each plasmid DNA are used as templates. Blue: ε2 reaction; Green: ε3 reaction; Red: ε4 reaction. FAM fluorescence: APOE gene; HEX fluorescence: ACTB gene
APOE genotyping of clinical DNA samples
We further validated our APOE genotyping assay using human DNA samples. Genomic DNA was extracted from 1158 clinical blood samples and undergone subsequent APOE genotyping analysis. The presence/absence of haplotypes was either determined by differential amplification with the three specific amplification setups for ε2, ε3 or ε4 allele, or by DNA sequencing performed by Sangon Biotech using the ABI 3730XL DNA Sequencer. In the clinical sample testing, our APOE genotyping assay has been efficient such that a single Real Time PCR reaction took approximately 90 minutes, and the Roche LightCycler 480 II system has the potential to run 384 reactions at one time. Typical results are presented in Fig. 3, with genotyping results and allele frequencies summarized in Table 2. APOE genotyping using our assay showed 100 % concordance with DNA sequencing results, demonstrating the accuracy and reliability of our protocol. The APOE allele frequency for ε2, ε3 and ε4 in our Chinese Han population was 7.90 %, 83.94 %, 8.16 %, respectively. Thus, the frequency of the ε4 allele was lower than that for the world-wide population (8.4 %, 77.9 % and 13.7 % for ε2, ε3 and ε4, respectively), but was similar to previous studies in Chinese population [4, 5, 37]. To provide guidance on evaluating the accuracy of our APOE genotyping method, receiver operating characteristic (ROC) curve analysis was performed to assess the cut-off ΔCt values for each Real Time PCR reaction (Table 3). A sample is considered to be negative for the corresponding genotype analysis if the ΔCt value is higher than the cut-off value.
Fig. 3.
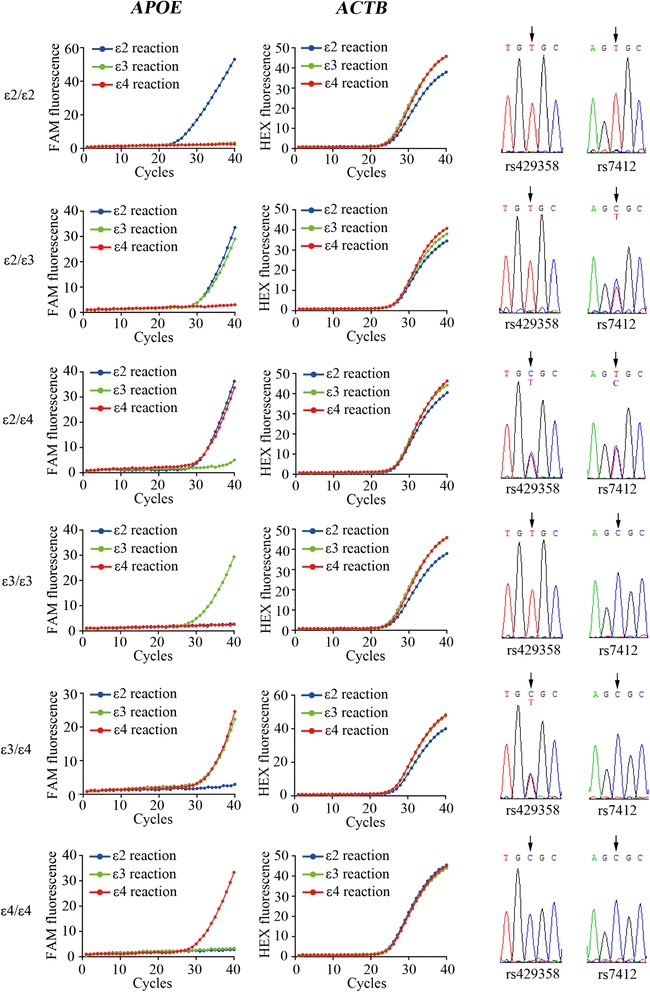
APOE genotyping on clinical DNA samples by Real Time PCR and DNA sequencing. Representative amplification curves for APOE and ACTB, and representative sequencing results for the two SNPs (rs429358 and rs7412) are shown. Blue: ε2 reaction; Green: ε3 reaction; Red: ε4 reaction. FAM fluorescence: APOE gene; HEX fluorescence: ACTB gene
Table 2.
Analysis of APOE genotypes and allele frequency in Chinese population
| Cohort | No. | APOE genotypes (No.) | Accuracy (%) | Alleles (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ε2/ε2 | ε2/ε3 | ε2/ε4 | ε3/ε3 | ε3/ε4 | ε4/ε4 | ε2 | ε3 | ε4 | |||
| Zhongshan | 399 | 2 | 60 | 6 | 278 | 51 | 2 | 100 | 8.77 | 83.58 | 7.65 |
| Fujian | 390 | 2 | 50 | 5 | 271 | 60 | 2 | 100 | 7.56 | 83.59 | 8.85 |
| Huadong | 369 | 3 | 43 | 5 | 265 | 52 | 1 | 100 | 7.32 | 84.69 | 7.99 |
| Total | 1158 | 7 | 153 | 16 | 814 | 163 | 5 | 100 | 7.90 | 83.94 | 8.16 |
Genomic DNA was extracted from peripheral blood samples obtained from three hospitals (399 samples from Zhongshan Hospital Affiliated to Xiamen University, 390 samples from Fujian Medical University Union Hospital, and 369 samples from Huadong Hospital Affiliated to Fudan University). The APOE genotypes and allele frequency were analyzed by both Real Time PCR and DNA sequencing. The 100 % accuracy was defined when APOE genotyping using the Real Time PCR assay showed 100 % concordance with DNA sequencing results
Table 3.
Cut-off values for ΔCt calculated by ROC curve analysis
| Reactions | Cut-off values |
|---|---|
| ε2 reaction | 9.2 |
| ε3 reaction | 10.4 |
| ε4 reaction | 11.1 |
The cut-off ΔCt values for the three reactions were calculated from ROC curve analysis, which represent the threshold cycle above which a sample is considered to be negative for the corresponding genotype analysis
Discussion
We have developed an APOE genotyping assay that is based on allele-specific PCR methodology adapted to Real Time PCR monitored by a common TaqMan probe. Each genotyping analysis can be accomplished within 90 minutes and is applicable to high-throughput analysis. We validated the specificity and robustness of our assay in 1158 clinical DNA samples by comparing the results with those from DNA sequencing. All samples genotyped using our assay showed perfect concordance with the APOE genotypes determined by sequencing analysis. Therefore, our method for APOE genotyping is rapid, precise and cost-effective, with the potential for high-throughput application.
APOE gene polymorphism modulates the risk for a variety of vascular and neurodegenerative diseases; thus, APOE genotyping is crucial for predicting disease risk and designing individualized therapy based on APOE genotype. Recent studies have suggested that therapeutic interventions applied earlier in the course of AD might be more likely to achieve disease modification. Indeed, there is a growing recognition that the pathophysiological process of AD begins many years prior to the onset of clinical symptoms [38]. APOE ε4 allele is the strongest genetic risk factor for AD. The mean age of onset and frequency of AD are 68 years and 91 % in ε4 homozygotes, 76 years and 47 % in ε4 heterozygotes, 84 years and 20 % in ε4 non-carriers [39]. Intriguingly, apoE4 has been associated with greater efficacy in at least two clinical trials on mild cognitive impairment [40, 41]. Taken together, APOE genotype status may add predictive value to the clinical diagnosis and evaluation of treatment efficacy [8, 42]. Developing an accurate and reliable method for APOE genotyping is therefore crucial for AD diagnosis and therapy.
PCR-RFLP analysis is a conventional method for APOE genotyping, but is relatively error-prone and labor-intensive due to a number of reaction steps [29, 30]. Compared with the PCR-RFLP method, the accuracy of our APOE genotyping method was improved by reducing the steps to one PCR reaction in closed PCR tubes and with no post-PCR sample handling. DNA sequencing is an accurate method for APOE genotyping, but it is labor-intensive and not suitable for high-throughput analysis. Our assay was based on allele-specific PCR methodology and possesses the potential for high-throughput application. Several Real Time PCR-based techniques have been developed to genotype APOE alleles, but the formation of primer-dimers makes the interpretation of the melting curves at times difficult [32, 33]. In our protocol, we provide guidance on threshold selection to evaluate the performance of PCR amplification by ROC analysis; therefore, the sensitivity and specificity of our protocol are well defined. TaqMan systems for APOE genotyping have been developed for the single nucleotide polymorphisms at rs429358 and rs7412 [34, 43]. However, most protocols require the use of four costly TaqMan probes. Only one TaqMan probe is used in our method as the polymorphic alleles are recognized by site-specific PCR primers. Thus, our method has the potential to design high-throughput application in a way that is cost-effective.
Conclusions
In this work, we present an APOE genotyping method that is accurate and cost-effective. In addition, our assay is based on allele-specific PCR methodology; therefore, can readily be applied to high-throughput APOE genotyping. Our APOE genotyping protocol can be used in addressing the impact of APOE polymorphism on disease risk, and notably in clinical assessments that predict the risk for a variety of vascular and neurodegenerative diseases.
Methods
Subjects
A total of 1158 peripheral blood samples were collected from the clinical laboratories in three hospitals (399 samples from Zhongshan Hospital Affiliated to Xiamen University, 390 samples from Fujian Medical University Union Hospital, and 369 samples from Huadong Hospital Affiliated to Fudan University). The study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committees of the three hospitals.
DNA constructs and reagents
All PCR primers and TaqMan probes were synthesized and purified by Life Technologies (Table 1). Three 197-bp DNA sequences covering the two SNPs for APOE ε2, ε3 or ε4 allele and a 179-bp DNA sequence of beta-actin gene (ACTB) were synthesized and cloned into pUC57 vector, which served as positive control DNA templates (Sangon Biotech, Shanghai, China). All constructs were verified by DNA sequencing (Sangon Biotech, Shanghai, China). Premix PrimeSTAR HS (R040A) was purchased from TAKARA; TaqMan® Genotyping Master Mix (4371357) was purchased from Applied Biosystems; DMSO (D2650) was purchased from Sigma; and blood DNA extraction kit (DP348-03) was purchased from TIANGEN (TIANGEN, Beijing, China).
Human genomic DNA isolation
Genomic DNA was extracted from 400 μL peripheral blood by using the blood DNA extraction kit (TIANGEN) according to the manufacturer’s instructions. DNA was diluted with nuclease free water to 8 ng/μL for APOE genotyping analysis.
APOE genotyping by Real Time PCR
APOE genotyping by Real Time PCR includes three reactions: ε2 reaction (primers ε2-Forward and ε2-Reverse), ε3 reaction (primers ε3-Forward and ε3-Reverse) and ε4 reaction (primers ε4-Forward and ε4-Reverse). Each PCR reaction mixture (15 μL) contained the following reagents: 1 × TaqMan® Genotyping Master Mix, 0.5 μM of each APOE primer and APOE probe, 0.1 μM of each ACTB primer and ACTB probe, 40 ng of genomic DNA. Positive control DNA template (ε2, ε3, ε4 plasmid DNA) and negative control (DNA/RNA-free water) were included in each panel of genotyping. The PCR amplification protocol was as follows: Initial activation of AmpliTaq Gold DNA Polymerase at 95 °C for 10 min, followed by 40 cycles with denaturation at 95 °C for 15 sec, and annealing/extension at 64 °C for 1 min. The fluorescence signals were collected during the annealing/extension step. FAM signal indicates APOE alleles and HEX signal indicates ACTB gene (internal control). The amplification was performed by using the Roche LightCycler 480 II system (Roche).
APOE genotyping by sequencing
For validation purpose, results by the above-mentioned APOE genotyping assay were compared with those from DNA sequencing analysis. Briefly, APOE gene fragments encompassing the two SNPs were amplified. The amplification reaction was carried out in a volume of 50 μL, which cotains 1 × Premix PrimeSTAR HS, 0.2 μM of each primer (Forward primer: 5’-AGCCCTTCTCCCCGCCTCCCACTGT-3’ and Reverse primer: 5’-CTCCGCCACCTGCTCCTTCACCTCG-3’), 5 % DMSO and 40 ng genomic DNA. The PCR cycling conditions were as follows: Initial denaturation at 98 °C for 4 min followed by 35 cycles with denaturation at 98 °C for 10 sec, annealing at 60 °C for 30 sec, extension at 72 °C for 40 sec; then a final extension at 72 °C for 10 min. All PCR products were purified and sequenced by Sangon Biotech using the ABI 3730XL DNA Sequencer (Applied Biosystems).
Ct cut-off values calculated by ROC curve analysis
The APOE genotypes of 114 human genomic DNA samples were determined either by our APOE genotyping method or by DNA sequencing. Receiver operating characteristic (ROC) curve analysis was performed to calculate the cut-off values for ΔCt (calculated by subtracting the Ct value of HEX signal from the Ct value of FAM signal) in our APOE genotyping assay. If no amplification curve appeared in the APOE allele-specific reaction, the Ct value was considered as 40 for the calculation of ΔCt. The ΔCt values of the ε2/ε3/ε4 reaction were 9.2, 10.4 and 11.1, respectively, as calculated by ROC curve analysis using SPSS software (Table 3).
Acknowledgments
The authors are grateful to all subjects for participation in our study. This study was supported by grants (81370459, 31400914) from the National Natural Science Foundation of China (to X.C.), the Fundamental Research Funds for the Central Universities (20720150051, to X.C.), NIH grants (R01AG027924, R01AG035355, R01AG046205, to G.B.), and grant from Xiamen Science and Technology Bureau (2014S0505, to HX).
Abbreviations
- AD
Alzheimer’s disease
- ApoE
Apolipoprotein E
- CAA
cerebral amyloid angiopathy
- Ct
cycle threshold
- FAM
carboxyfluorescein
- FRET
fluorescent resonance energy transfer
- HEX
hexachloro fluorescein
- HRM
high resolution melt
- LDLR
low-density lipoprotein receptor
- LOAD
late-onset Alzheimer’s disease
- RFLP
restriction fragment length polymorphism
- ROC
receiver operating characteristic
- SNP
single nucleotide polymorphism
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: GB, XC, LZ and HX. Performed the experiments and analyzed the data: LZ, YX, TC, ZW, TW, XL and RS. Wrote the paper: XC, LZ and GB. All authors read and approved the final manuscript.
Contributor Information
Li Zhong, Email: zhongli1103@163.com.
Yong-Zhuang Xie, Email: xyzh1984@163.com.
Tian-Tian Cao, Email: caott@memorigen.com.
Zongqi Wang, Email: wangzongqionline@126.com.
Tingting Wang, Email: wtt987654321wtt@163.com.
Xinxiu Li, Email: lixinxiu40@aliyun.com.
Rui-Chi Shen, Email: xmsrc@163.com.
Huaxi Xu, Email: hxxu@xmu.edu.cn.
Guojun Bu, Email: bu.guojun@mayo.edu.
Xiao-Fen Chen, Email: chenxf@xmu.edu.cn.
References
- 1.Das HK, McPherson J, Bruns GA, Karathanasis SK, Breslow JL. Isolation, characterization, and mapping to chromosome 19 of the human apolipoprotein E gene. J Biol Chem. 1985;260:6240–6247. [PubMed] [Google Scholar]
- 2.Mahley RW, Nathan BP, Pitas RE. Apolipoprotein E. Structure, function, and possible roles in Alzheimer’s disease. Ann N Y Acad Sci. 1996;777:139–145. doi: 10.1111/j.1749-6632.1996.tb34412.x. [DOI] [PubMed] [Google Scholar]
- 3.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 4.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. doi: 10.1001/jama.1997.03550160069041. [DOI] [PubMed] [Google Scholar]
- 5.Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann Hum Genet. 1999;63:301–310. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- 6.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 7.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- 11.Grehan S, Tse E, Taylor JM. Two distal downstream enhancers direct expression of the human apolipoprotein E gene to astrocytes in the brain. J Neurosci. 2001;21:812–822. doi: 10.1523/JNEUROSCI.21-03-00812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917:148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- 13.Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Breslow JL, Zannis VI, SanGiacomo TR, Third JL, Tracy T, Glueck CJ. Studies of familial type III hyperlipoproteinemia using as a genetic marker the apoE phenotype E2/2. J Lipid Res. 1982;23:1224–1235. [PubMed] [Google Scholar]
- 15.Feussner G, Feussner V, Hoffmann MM, Lohrmann J, Wieland H, Marz W. Molecular basis of type III hyperlipoproteinemia in Germany. Hum Mutat. 1998;11:417–423. doi: 10.1002/(SICI)1098-1004(1998)11:6<417::AID-HUMU1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Civeira F, Pocovi M, Cenarro A, Casao E, Vilella E, Joven J, et al. Apo E variants in patients with type III hyperlipoproteinemia. Atherosclerosis. 1996;127:273–282. doi: 10.1016/S0021-9150(96)05969-2. [DOI] [PubMed] [Google Scholar]
- 17.Burt TD, Agan BK, Marconi VC, He W, Kulkarni H, Mold JE, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci U S A. 2008;105:8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs EG, Kroenke C, Lin J, Epel ES, Kenna HA, Blackburn EH, et al. Accelerated cell aging in female APOE-epsilon4 carriers: implications for hormone therapy use. PLoS One. 2013;8:e54713. doi: 10.1371/journal.pone.0054713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 20.Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/WNL.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 21.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMurdo L, Lidbury PS, Corder R, Thiemermann C, Vane JR. Heterogeneous receptors mediate endothelin-1-induced changes in blood pressure, hematocrit, and platelet aggregation. J Cardiovasc Pharmacol. 1993;22(Suppl 8):S185–S188. doi: 10.1097/00005344-199322008-00050. [DOI] [PubMed] [Google Scholar]
- 23.Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 24.Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 26.Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, et al. APOE epsilon4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70:223–228. doi: 10.1001/jamaneurol.2013.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bras J, Guerreiro R, Darwent L, Parkkinen L, Ansorge O, Escott-Price V, et al. Genetic analysis implicates APOE, SNCA and suggests lysosomal dysfunction in the etiology of dementia with Lewy bodies. Hum Mol Genet. 2014;23:6139–6146. doi: 10.1093/hmg/ddu334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masterman T, Hillert J. The telltale scan: APOE epsilon4 in multiple sclerosis. Lancet Neurol. 2004;3:331. doi: 10.1016/S1474-4422(04)00763-X. [DOI] [PubMed] [Google Scholar]
- 29.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 30.Zivelin A, Rosenberg N, Peretz H, Amit Y, Kornbrot N, Seligsohn U. Improved method for genotyping apolipoprotein E polymorphisms by a PCR-based assay simultaneously utilizing two distinct restriction enzymes. Clin Chem. 1997;43:1657–1659. [PubMed] [Google Scholar]
- 31.Srinivasan JR, Kachman MT, Killeen AA, Akel N, Siemieniak D, Lubman DM. Genotyping of apolipoprotein E by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 1998;12:1045–1050. doi: 10.1002/(SICI)1097-0231(19980831)12:16<1045::AID-RCM281>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 32.Zhan XH, Zha GC, Jiao JW, Yang LY, Zhan XF, Chen JT, et al. Rapid identification of apolipoprotein E genotypes by high-resolution melting analysis in Chinese Han and African Fang populations. Exp Ther Med. 2015;9:469–475. doi: 10.3892/etm.2014.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poulson MD, Wittwer CT. Closed-tube genotyping of apolipoprotein E by isolated-probe PCR with multiple unlabeled probes and high-resolution DNA melting analysis. Biotechniques. 2007;43:87–91. doi: 10.2144/000112459. [DOI] [PubMed] [Google Scholar]
- 34.Koch W, Ehrenhaft A, Griesser K, Pfeufer A, Muller J, Schomig A, et al. TaqMan systems for genotyping of disease-related polymorphisms present in the gene encoding apolipoprotein E. Clin Chem Lab Med. 2002;40:1123–1131. doi: 10.1515/cclm.2002.197. [DOI] [PubMed] [Google Scholar]
- 35.Calero O, Hortiguela R, Bullido MJ, Calero M. Apolipoprotein E genotyping method by real time PCR, a fast and cost-effective alternative to the TaqMan and FRET assays. J Neurosci Methods. 2009;183:238–240. doi: 10.1016/j.jneumeth.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 36.Holland PM, Abramson RD, Watson R, Gelfand DH. Detection of specific polymerase chain reaction product by utilizing the 5’----3’ exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci U S A. 1991;88:7276–7280. doi: 10.1073/pnas.88.16.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seet WT, Mary Anne TJ, Yen TS. Apolipoprotein E genotyping in the Malay, Chinese and Indian ethnic groups in Malaysia-a study on the distribution of the different apoE alleles and genotypes. Clin Chim Acta. 2004;340:201–205. doi: 10.1016/j.cccn.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 40.Poirier J, Delisle MC, Quirion R, Aubert I, Farlow M, Lahiri D, et al. Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci U S A. 1995;92:12260–12264. doi: 10.1073/pnas.92.26.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 42.Farlow MR. Should the ApoE genotype be a covariate for clinical trials in Alzheimer disease? Alzheimers Res Ther. 2010;2:15. doi: 10.1186/alzrt39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi L, Wu T, Luo W, Zhou W, Wu J. A non-invasive, rapid method to genotype late-onset Alzheimer’s disease-related apolipoprotein E gene polymorphisms. Neural Regen Res. 2014;9:69–75. doi: 10.4103/1673-5374.125332. [DOI] [PMC free article] [PubMed] [Google Scholar]


