Abstract
Use of natural products is increasingly popular. In fact, many patients with liver diseases self-medicate with herbal supplements. Resveratrol (RSV), in particular, is a common natural product that can reduce injury in experimental models of liver disease. Xenobiotic hepatotoxicity is a particularly important area-of-need for therapeutics. Drug-induced liver injury, for example, is the most common cause of acute liver failure (ALF) and ALF-induced deaths in many countries. Importantly, RSV protects against hepatotoxicity in animal models in vivo caused by several drugs and chemicals and may be an effective intervention. Although many mechanisms have been proposed to explain the protection, not all are consistent with other data. Furthermore, RSV suffers from other issues, including limited bioavailability due to extensive hepatic metabolism. The purpose of this article is to summarize recent findings on the protective effects of RSV in xenobiotic-induced liver injury and other forms of liver injury and to provide a critical review of the underlying mechanisms. New mechanisms that are more consistent with data emerging from the toxicology field are suggested. Efforts to move RSV into clinical use are also considered. Overall, RSV is a promising candidate for therapeutic use, but additional studies are needed to better understand its effects.
Keywords: Acetaminophen, Liver injury, Mitochondria, Inflammation, Oxidative stress, Natural products
1. INTRODUCTION
Hepatotoxicity is a major problem in the development and use of drugs. Before a drug reaches the market, the potential for intrinsic hepatotoxicity is thoroughly tested. In fact, liver injury is one of the major reasons for the discontinuation of a drug at multiple stages in the discovery and development pipeline (US FDA, 2009). Clinically, drug-induced liver injury is the most frequent cause of acute liver failure (ALF) in the West (Lee, 2008). Although acetaminophen (APAP) overdose accounts for most of these cases, idiosyncratic hepatotoxicity caused by other drugs is also a significant problem (Lee, 2008). Hepatotoxicity can also be caused by a number of non-drug xenobiotics as well.
Many traditional medicines and other natural products are thought to have beneficial effects in the liver and to protect the liver against various insults (Girish and Pradhan, 2011; Zhao et al., 2014; Seeff et al., 2015). Despite the fact that clinical trials of many such compounds in the U.S. have been unsuccessful (Seeff et al., 2015), self-medication with herbal products is common among patients with chronic liver diseases (Strader et al., 2009; Seeff et al., 2008). Among the natural products that have been studied for their potential hepatoprotective effects, one of the most popular is 3,5,4'-trihydroxy-trans-stilbene, otherwise known as resveratrol (RSV) (Bishayee et al., 2010a). RSV is a naturally-occurring polyphenol that was first isolated from Veratrum grandiflorum (Takaoka, 1939). It was initially characterized as a phytoalexin produced by plants (particularly berries) in response to injury or stress (Langcake et al., 1976; Burns et al., 2002). It was postulated to explain the French Paradox, or the cardiovascular effect of red wine, in the early 1990s (Renaud et al., 1992). Since then, numerous biological effects of RSV have been reported, including anti-oxidant, anti-inflammatory and anti-tumorigenic effects (Baur and Sinclair, 2006). It has been shown to be beneficial in many models of disease, including cell culture and multiple in vivo rodent models of cardiovascular diseases (Bradamante et al., 2004), cell culture and multiple in vivo rodent models of neurodegenerative diseases (Sun et al., 2010), ischemic injury in the brain in gerbils (Wang et al., 2002; Sun et al., 2010), metabolic changes associated with aging in cultured cells (Park et al., 2012) and in both in vitro and in vivo mouse models of cancer (Jang et al., 1997).
Importantly, RSV has been shown to protect against numerous in vitro and in vivo rodent models of liver injury, including hepatotoxicity caused by drugs and other xenobiotics (Bishayee et al., 2010a). However, the mechanisms that have been proposed for some of the beneficial effects of RSV in xenobiotic hepatotoxicity are questionable due to poor study design or the failure to account for fundamental aspects of the known pathophysiology induced by these chemicals. Thus, the purpose of this review is to summarize the major effects of RSV in the liver and to discuss the mechanisms of RSV-mediated protection in drug hepatotoxicity. In addition, the effects of RSV in other forms of acute liver injury are summarized and the clinical use of RSV is discussed.
2. KNOWN EFFECTS OF RESVERATROL IN THE LIVER AND DURING LIVER INJURY
The most commonly proposed mechanism of RSV-mediated protection in liver injury is that RSV acts as an anti-oxidant. It is well known that reactive oxygen species (ROS) have a critical role in the initiation and progression of a number of liver pathologies, such as those caused by hepatitis C, alcohol, drugs or endotoxemia (Jaeschke et al., 2002; Muriel, 2009). RSV appears to decrease oxidative stress by directly scavenging free radicals or by upregulating cellular antioxidant enzymes, such as superoxide dismutase (SOD), catalase and glutathione peroxidases.
Data supporting the latter have been reported from several in vivo rodent models (Bishayee et al., 2010a), including hepatic ischemia-reperfusion, ethanol toxicity, and carbon tetrachloride toxicity, and these mechanisms may account for some of the protective effects of RSV in liver disease (Bishayee et al., 2010a).
Another commonly cited mechanism is an anti-inflammatory effect of RSV. Inflammation often accompanies the liver injury process, and in some cases, may exaggerate the liver damage (Jaeschke et al., 2002; Jaeschke, 2011). For example, it has been shown in naphthalene hepatotoxicity in mice that pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 are greatly increased (Şehirli et al., 2008). It is known from the galactosamine/LPS model in mice that TNF-α is responsible for directly inducing apoptotic cell death (Leist et al., 1994; 1995) and neutrophil activation and recruitment (Schayler et al., 1988), which causes additional liver injury (Jaeschke et al., 1998). RSV administration appeared to prevent these effects in mice and protected against the liver damage caused by naphthalene (Şehirli et al., 2008).
RSV could also have an effect on metabolism in the liver, which is the effect that has received the most attention of late (Kulkarni and Cantó, 2015). The AMP-related kinase (AMPK) and sirtuin 1 (SIRT1) are 2 key targets of RSV (Kulkarni and Cantó, 2015). As fuel sensors, they can activate the peroxisome-proliferator-activated receptor γ co-activator-1α (PGC-1α), and diverse downstream transcriptional regulators, such as peroxisome proliferator-activated receptors (PPARs), estrogen-related receptors (ERRs) and nuclear respiratory factors (NRFs), all of which have been shown to be critical in regulation of liver function by modulating mitochondrial biogenesis, mitophagy, gluconeogenesis or lipid metabolism (Kulkarni and Cantó, 2015; Jornayez and Shulman, 2010). Indeed, it has been shown that RSV can improve energy metabolism in alcohol- or high fat diet-induced liver disease in mice in vivo by targeting metabolism in the liver (Lagouge et al., 2006; Baur et al., 2006; Heebøll et al., 2014).
Beside these mechanisms, RSV also affects both Phase I and II drug metabolizing enzymes. RSV itself is primarily eliminated through phase II metabolism, forming multiple glucuronide and sulfate conjugates (Wenzel and Simoza, 2005). There is limited evidence for biotransformation of RSV by cytochrome P450 enzymes in vitro (Steenwyk and Tan, 2010). Furthermore, RSV seems to inhibit CYP3A4 in a mechanism-based fashion (Chan and Delucchi, 2000). However, there is no evidence for oxidation products in humans so it is not yet clear if P450s play a role in metabolism of RSV in vivo (Walle, 2011), and even the positive in vitro results have been challenged in other studies (Yu et al., 2002). Nevertheless, both in vitro studies and animal models have demonstrated that RSV inhibits the activity of various CYP enzymes as well as their expression through various nuclear factors (Baur and Sinclair, 2006). In humans, RSV induced CYP1A2 activity and inhibited CYP3A4, CYP2D6, and CYP2C9 (Wenzel and Somoza, 2005). These results suggest the possibility that RSV may alter the metabolism of other drugs in the liver, and also affect activation or detoxification of xenobiotics and carcinogens. Phase II enzymes have also been shown to be induced or inhibited by RSV (Baur and Sinclair, 2006; Chow et al., 2010).
Overall, RSV has been suggested to have a large number of biological effects. In particular, RSV is commonly believed to have anti-inflammatory and anti-oxidant effects. However, the mechanisms by which RSV has been proposed to protect in certain models of hepatotoxicity and other liver diseases are not always consistent with fundamental data from the experimental models used. A summary of our conclusions is available in table 1.
Table 1.
Proposed and likely mechanisms of RSV-mediated protection in various models.
| Model | Proposed Mechanisms | Refs. | Likely Mechanisms* |
|---|---|---|---|
| Xenobiotic hepatotoxicity | |||
| Acetaminophen overdose |
Anti-inflammatory, anti- LPO, anti-ONOO−, prevents endonuclease release |
Sener et al., 2006; Masubuchi et al., 2009; Du et al., 2015 |
Anti-ONOO− and prevents endonuclease release |
| Carbon tetrachloride | Anti-inflammatory |
Chávez et al., 2008; Roy et al., 2011; |
Unclear; anti-LPO? |
| Chronic alcohol | Anti-LPO, increased β- oxidation, decreased lipogenesis, increased autophagy |
Kasdallah-Grissa et al., 2006; You et al., 2008; Ajmo et al., 2008; Ni et al., 2013 |
Increased β-oxidation and decreased lipogenesis |
| Other xenobiotics | Anti-inflammatory, anti- LPO |
Şehirli et al., 2008; Tunali-Akbay et al., 2010; Lee et al., 2010; Ahmad and Ahmad, 2014 |
Unclear |
| Other liver diseases | |||
| Cancer | Reduced P450 expression, cell cycle arrest, cell death, decreased metastasis |
Ciolino et al., 1998; Kuo et al., 2002; Notas et al., 2006; Bishayee and Dhir, 2009; Yu et al., 2008; 2010; Zhang et al., 2014 |
Unclear; possibly all proposed mechanisms? |
| Cholestasis | Anti-inflammatory, increased mitochondrial biogenesis, increased autophagy, decreased cell death |
Chan et al., 2011; Hussein, 2013; Ara et al., 2005; Lin et al., 2012 |
Unclear |
| Ischemia-reperfusion | Anti-inflammatory, anti- oxidant |
Hassan-Khabbar et al., 2008; 2010; Nivet-Antoine et al., 2010 |
Anti-inflammatory, anti- oxidant, neutrophil inhibition? |
| Most commonly proposed protective effects in all models | |||
| Anti-inflammatory, anti-LPO, anti-oxidant, mitochondrial effects | |||
LPO, lipid peroxidation. ONOO−, peroxynitrite.
The most likely mechanism(s) of RSV-mediated protection based on the relevance of the models used and the quality of the studies reviewed here. Question marks indicate that these mechanisms are plausible, but have not yet been investigated.
3. RESVERATROL AND DRUG HEPATOTOXICITY
3.1 Resveratrol in acetaminophen hepatotoxicity
Among the hepatotoxicants that RSV has been shown to protect against, APAP is arguably the most important. As mentioned, APAP overdose is the most common cause of acute liver failure in many Western countries (Larson et al., 2005; Lee, 2008). In mice, the liver injury is initiated by the formation of a reactive metabolite that depletes liver glutathione (GSH) and binds to proteins (Jollow et al., 1973; Mitchell et al., 1973; McGill et al., 2013) (Fig. 1A). Protein binding appears to cause mitochondrial dysfunction and oxidative stress. Mitochondrial respiration is decreased (Meyers et al., 1988) and evidence of oxidative stress is dramatically increased in the livers of mice treated with toxic doses of APAP (Jaeschke, 1990). The major ROS in APAP toxicity is thought to be superoxide (O2−). There is strong evidence that O2− production within damaged mitochondria is increased and that it reacts with nitric oxide (NO) to form peroxynitrite (ONOO−). ONOO− can then react with amino acid residues in proteins, particularly tyrosine. Increased nitrotyrosine adducts have repeatedly been measured in the livers of mice after APAP overdose (Hinson et al., 2000; Knight et al., 2001; Cover et al., 2005; Ishii et al., 2006; Burke et al., 2010). Furthermore, scavenging ONOO− with GSH reduces APAP-induced liver injury (Knight et al., 2002; Bajt et al., 2003), and a ONOO− decomposition catalyst has also been shown to protect against APAP (LoGuidice and Boelsterli, 2011). The initial oxidative stress appears to activate the c-Jun N-terminal kinases (JNK) 1/2 via several other kinases (Nakagawa et al., 2008; Sharma et al., 2012; Ramachandran et al., 2013; Xie et al., 2015). The activated JNK then translocates to mitochondria (Hanawa et al., 2008; Win et al., 2011), where it enhances mitochondrial dysfunction. Eventually, the mitochondrial membrane permeability transition (MPT) pore forms and mitochondrial membrane potential is lost (Kon et al., 2004; Reid et al., 2005; Masubuchi et al., 2005; Ramachandran et al., 2011).
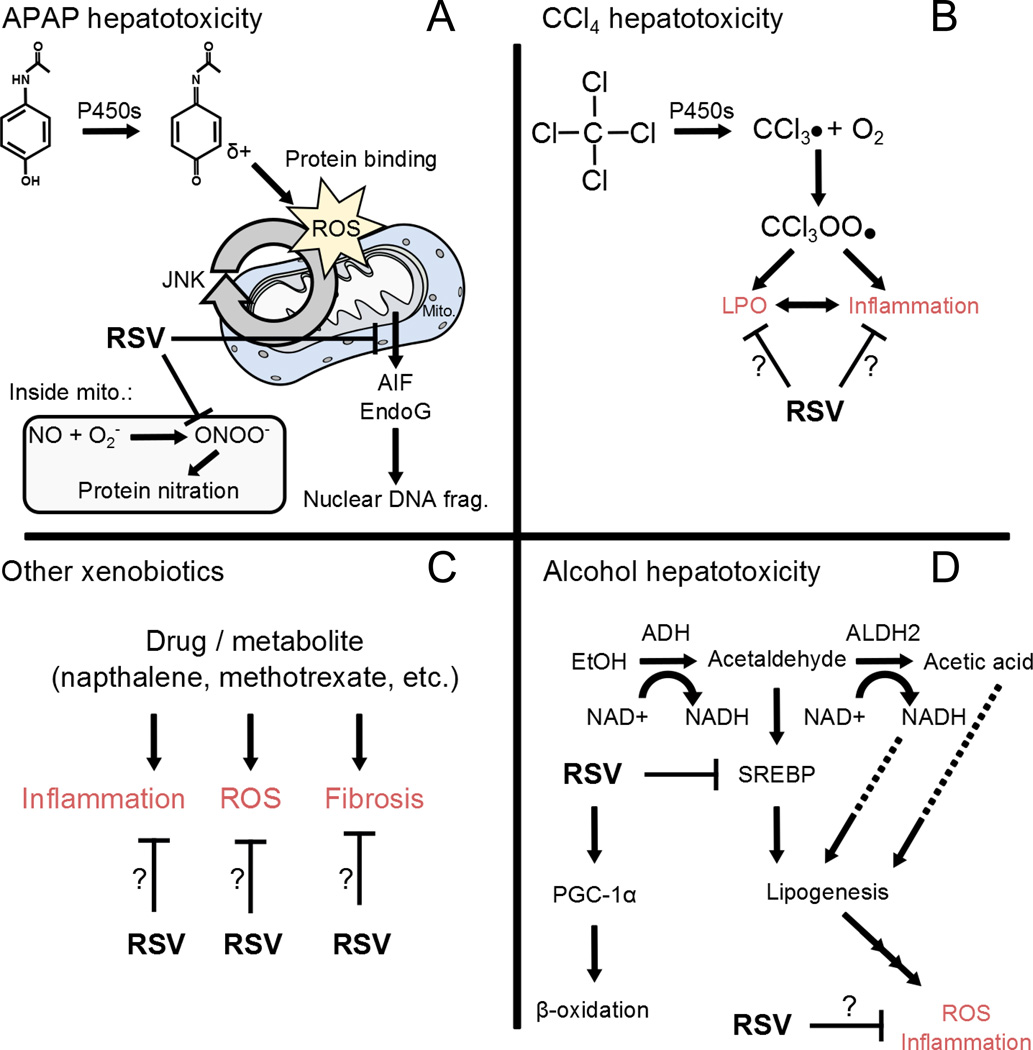
Figure 1.
Possible mechanisms of RSV-mediated protection in xenobiotic hepatotoxicity. (A) Acetaminophen (APAP) hepatotoxicity involves cytochrome P450 enzyme (P450s)-mediated conversion of APAP to a reactive intermediate that binds to proteins. Binding to mitochondrial (Mito.) proteins leads to oxidative stress that is exacerbated by the c-Jun N-terminal kinases (JNK) 1/2. Protein nitration by peroxynitrite contributes to the mitochondrial injury. Eventually, the mitochondria release endonucleases (AIF, EndoG) that damage DNA. RSV appears to prevent both protein nitration and endonuclease release. (B) Carbon tetrachloride is converted to a reactive intermediate that initiates lipid peroxidation (LPO), which may lead to inflammation. RSV may inhibit both LPO and inflammation. (C) Various xenobiotics seem to cause inflammation, oxidative stress, and even fibrosis. RSV appears to interfere with all of those processes. (D) Alcohol is metabolized to acetaldehyde by alcohol dehydrogenase (ADH). Acetaldehyde is further metabolized by aldehyde dehydrogenase (ALDH2), but can also stimulate sterol regulatory binding element protein (SREBP) to induce expression of lipogenic genes. Furthermore, production of NADH in alcohol metabolism may also stimulate lipogenesis. Accumulation of lipids seems to lead to oxidative stress, injury and inflammation. RSV has been shown to inhibit SREBP and activate peroxisome proliferator-activated receptor gamma-coactivator 1α (PGC-1α). The latter leads to enhanced fatty acid oxidation to reduce lipids and downstream oxidative stress and inflammation.
Swelling of the mitochondria and lysis of the outer mitochondrial membrane results in release of endonucleases that translocate to the nucleus and damage nuclear DNA (Bajt et al., 2006). Mitochondrial Bax also plays a role in endonuclease release at early time points (Bajt et al., 2008). The final result is oncotic necrosis of the hepatic parenchyma (Gujral et al., 2002). Importantly, the mechanisms of toxicity in humans seem to mimic these mechanisms in mice (Antoine et al., 2012; McGill et al., 2012; 2014; Xie et al., 2014).
Three studies of the mechanism of protection of RSV against APAP hepatotoxicity have been published. In the first study, the authors reported that malondialdehyde (MDA), a marker of lipid peroxidation (LPO), and TNF-α were increased in mice after APAP treatment and that these effects were attenuated by co-treatment with RSV (Sener et al., 2006). Based on these data, the authors concluded that RSV protected against APAP hepatotoxicity by reducing LPO and inflammation. In the second study, it was found that C57Bl/6 mice had higher levels of TNF-α in the liver and lower levels of the anti-inflammatory cytokine IL-6 than BALB/c mice, which are less susceptible to APAP-induced liver injury (Masubuchi et al., 2009). They also showed that that RSV treatment could protect against APAP in C57Bl/6 mice and that this protection was associated with a shift toward lower TNF-α expression and higher IL-6 expression (Masubuchi et al., 2009). The authors concluded that inflammation plays an important role in APAP hepatotoxicity and that RSV protected by reducing inflammation. However, the mechanistic conclusions from these two studies are questionable. First, neither study included a detailed analysis of NAPQI formation and protein binding, which is necessary to preclude an upstream effect on metabolism (Jaeschke et al., 2013). Any effect on formation of the reactive metabolite of APAP and its binding to proteins will alter the toxicity (Jaeschke et al., 2013). Moreover, it has been shown that the amount of LPO that occurs during APAP-induced liver injury is not sufficient to cause cell death, and a diet high in vitamin E does not protect against APAP (Knight et al., 2003). Finally, although early studies found that pre-treatment of mice with antisera against TNF-α protected against APAP toxicity (Blazka et al., 1995), there is strong evidence that treatment with antibodies can cause significant off-target effects (Jaeschke and Liu, 2007). Importantly, TNF-deficiency does not protect against APAP-induced liver injury (Boess et al., 1998) and it has also been extensively demonstrated that sterile inflammation does not aggravate APAP hepatotoxicity in mice or humans (Jaeschke et al., 2012), though it may be important for regeneration (Dambach et al., 2002; You et al., 2013; Williams et al., 2014). Thus, it is clear that the RSV-mediated reduction of LPO and TNF expression during APAP toxicity must be the consequence of protection instead of the cause. Rather, emerging evidence suggests that RSV protects against APAP-induced liver injury by reducing protein nitration and inhibiting release of endonucleases from mitochondria (Du et al., 2015) (Fig. 1A). It was recently reported that post-treatment with RSV does not interfere with protein binding, JNK activation or mitochondrial damage in the livers of mice treated with a hepatotoxic dose of APAP, but it does reduce nitrotyrosine adducts and nuclear DNA damage caused by mitochondrial endonucleases (Du et al., 2015). Consistent with this, it has been shown that RSV can directly scavenge ONOO− and prevent nitrotyrosine formation in renal tubule cells treated with a ONOO− donor (Holthoff et al., 2010). Although the reason for the reduced endonuclease release from mitochondria is unclear, it is known that RSV can partition into lipid membranes and it is possible that it stabilizes the membrane structure and prevents release in that way (Fabris et al., 2008).
3.2 Resveratrol in carbon tetrachloride hepatotoxicity
Unlike APAP, toxic doses of carbon tetrachloride (CCl4) do cause LPO in the liver (Weber et al., 2003). CCl4 is converted to trichloromethyl radical (CCl3·) and trichloromethyl peroxy radical (CCl3OO·) (Fig. 1B), which can abstract hydrogen from polyunsaturated fatty acids thereby initiating the chain reaction of LPO that destroys lipid membranes and disturbs lipid homeostasis in cells (Weber et al., 2013). RSV has been shown to protect against CCl4-induced liver injury (Rivera et al., 2008; Vitaglione et al., 2009; Chan et al., 2014). Similar to the case of APAP, it has occasionally been suggested that the mechanism of RSV-mediated protection against CCl4 is through its anti-inflammatory effects (Chávez et al., 2008; Roy et al., 2011); however, this is usually based on a reduction in expression of one or more pro-inflammatory cytokines in the liver at a single time point. As such, it is difficult to determine whether or not the reduced inflammation is a reason for or simply a result of the protection. Nevertheless, an effect on inflammation cannot be entirely ruled out at this point, particularly in models of chronic CCl4 treatment. RSV has been shown to inhibit NFκB activity (Chávez et al., 2008). It has also been shown to reduce activation and function of stellate and Kupffer cells in vitro (Kawada et al., 1998) and to prevent fibrosis in vivo (Chávez et al., 2008). The fact that RSV tends to partition into lipid membranes and can directly inhibit LPO and membrane damage in simplified model systems (Tadolini et al., 2000; Megli et al., 2004; Fabris et al., 2008) suggests that RSV also acts as a direct chain breaking antioxidant in membranes, similar to vitamin E. However, Knockaert et al. (2012) reported that inhibition of LPO in mice by known antioxidants did not fully protect against CCl4 hepatotoxicity, despite the fact that it prevented the early mitochondrial damage caused by the xenobiotic. Overall, the mechanism of RSV-mediated protection against CCl4 is not well understood. Additional research is needed.
3.3 Resveratrol in alcohol-induced liver injury
RSV has also been shown to protect against alcohol-induced liver injury. Kasdallah-Grissa et al. (Kasdallah-Grissa et al., 2006) initially reported that ethanol feeding modestly increased MDA in the liver and RSV ameliorated this effect. Although they did not measure ALT or perform any histological analysis, follow-up studies showed that RSV could reduce liver injury and mortality caused by chronic ethanol exposure (Bujanda et al., 2006). Together, these data suggested that RSV protected against ethanol by inhibiting LPO. Interestingly, it was later demonstrated that RSV-mediated SIRT1 activation results in increased deactylation and degradation of SREBP1 and activation of PGC-1α in vitro, leading to decreased expression of lipogenic genes and increased fatty acid oxidation in a mouse model of chronic ethanol feeding (You et al., 2008). It was also shown that RSV co-feeding reduces steatosis caused by ethanol in mice (Ajmo et al., 2008). In light of this, it was surprising that another group found that RSV enhances fibrosis in a continuous gastric infusion rat model of ethanol exposure (Oliva et al., 2008), since steatosis is an important part of the pathogenesis and may contribute to the development of fibrosis. Unfortunately, the relevance of either model of alcohol consumption (feeding or continuous infusion) is unclear. While continuous gastric infusion results in more severe changes in liver pathology than ad libitum ethanol feeding, and certain pathological features of the model more closely resemble what is observed in humans with advanced alcoholic liver disease, it is an extreme approach that generally does not reflect human patterns of regular but intermittent alcohol consumption. It seems likely that ethanol feeding is a better model, at least for the early stages of alcoholic liver disease. Thus, RSV probably protects against ethanol hepatotoxicity by reducing steatosis through increased fatty acid oxidation and decreased lipogenesis (Fig. 1D). RSV has also been shown to enhance autophagy in primary human hepatocytes exposed to ethanol, possibly through SIRT1-mediated deacetylation and activation of FoxO3a (Ni et al., 2013). However, the significance of the latter effect of RSV has not been tested in vivo.
3.4 Resveratrol in hepatotoxicity caused by other xenobiotics
RSV has also been shown to protect against hepatotoxicity caused by naphthalene in mice (Şehirli et al., 2008), methotrexate in rats (Tunali-Akbay et al., 2010) and dimethylnitrosamine in rats (Lee et al., 2010; Ahmad and Ahmad, 2014). Various mechanisms were proposed in these studies to explain the protection afforded by RSV in these models, including antioxidant, anti-inflammatory, and antifibrotic signaling (Fig. 1C). However, each study included only one or a few late time points, making it difficult to differentiate between what caused the protection and what was merely a consequence of it.
4. RESVERATROL AND OTHER LIVER DISEASES
4.1 Resveratrol in liver cancer
Liver cancer ranks among the most common types of cancer worldwide and approximately 90% of all liver cancers are hepatocellular carcinoma (HCC) (EASL-EORTC, 2012). The potential beneficial effect of RSV on liver cancer has been studied extensively and it appears that RSV may have benefits at multiple stages of carcinogenesis. Several in vitro studies have shown that RSV can affect growth and proliferation of hepatoma cell lines, such as HepG2 (Delmas et al., 2000; Kozuki et al., 2001; Kuo et al., 2002). Some rodent studies suggest that RSV also protects against liver cancer in vivo (Bishayee and Dhir, 2009; Bishayee et al., 2010b). Limited data from induced tumorigenesis studies in rodents suggest that RSV limits cancer through its anti-inflammatory effects (Bishayee et al., 2010c; Mbimba et al., 2012). However, the latter is largely based on the fact that treatment with RSV reduces expression of inflammatory markers. Without more detailed mechanistic investigation, the possibility that the reduction in inflammation markers was the result of protection by RSV rather than the cause of protection cannot be ruled out.
The most common causes of liver cancer in humans are chronic liver diseases including viral hepatitis, alcohol hepatotoxicity, non-alcoholic fatty liver disease (NAFLD), and exposure to carcinogens such as aflatoxin (Bishayee et al., 2010b). Interestingly, there is some evidence that RSV can protect against these insults as well. Alcohol hepatotoxicity and fatty liver disease are discussed elsewhere in this manuscript. Like alcohol and high-fat diet, transgenic expression of hepatitis C virus core protein in mice has been shown to cause steatosis and this can be reduced by RSV treatment (Jiang et al., 2012). It is well known that many carcinogens, particularly aryl hydrocarbons, require metabolic activation for their carcinogenic properties, and one of the most important mechanisms of this activation is via P450-mediated metabolism. It has been documented that RSV can act as a chemopreventative agent against liver cancer by inhibiting aryl hydrocarbon-mediated induction of CYP1A1 expression and activity (Ciolino et al., 1998). The mechanism behind this seems to be inhibition of the recruitment of both the aryl-hydrocarbon receptor and RNA-polymerase complex to the regulatory region of the CYP1A1 gene, ultimately leading to decreased expression (Beedanagari et al., 2009). Thus, RSV may play a preventative role in the initiation of tumorigenesis caused by carcinogens by modulating expression of drug metabolizing enzymes.
Once a tumor is established, malignant cancers are characterized by unregulated cell growth, tissue invasion, and metastasis. In previous studies, RSV has been shown to be protective against these processes, leading to a dual inhibitory effect of RSV on cell growth as a result of G1 cell-cycle phase arrest and induction of cell death, ultimately leading to decreased proliferation and decreased cell survival, both in cell culture and in rats (Kuo et al., 2002; Notas et al., 2006; Bishayee and Dhir, 2009). Finally, RSV has been shown to cause decreased matrix metalloprotease-9 (MMP-9) expression by inhibition of NF-kB, leading to decreased migration of HepG2 cells in culture (Yu et al., 2008). Interestingly, RSV inhibition of NF-kB was also shown to decrease VEGF expression and angiogenesis in both HepG2 cell lines and mice (Yu et al., 2010; Zhang et al., 2014), a process critical for metastasis (Stetler-Stevenson, 1999). That RSV has been shown to be protective at both early and late stages of carcinogenesis (Rajasekaran et al., 2011) underscores the complexity of the effects RSV in HCC.
4.2 Resveratrol in cholestatic liver disease
Cholestasis is defined as a reduction of bile flow and can have several etiologies. In patients with end-stage liver disease secondary to cholestatic liver disease, transplantation is required (Carrion and Bhamidimarri, 2013). During acute cholestasis, damage to the bile ducts leads to release of osteopontin and subsequent initiation of an inflammatory cascade (Yang et al., 2014; Woolbright and Jaeschke, 2012). Over the past decade, several studies have demonstrated a beneficial effect of the antioxidant properties of RSV on the pathophysiology of cholestasis. To date, most studies demonstrate a less robust inflammatory response following RSV treatment. In both the bile duct ligation and ethinylestradiol models of cholestasis, RSV treatment led to decreased expression of pro-inflammatory molecules such as TNFα, IL6, IL-1α, and NO (Ara et al., 2005; Chan et al., 2011; Hussein, 2013) with a concurrent increase in anti-inflammatory molecules such as SOD, GR, GPx, and catalase (Hussein, 2013). In addition, these findings correlated with decreased KC activation and leukocyte infiltration of the liver (Chan et al., 2011), decreased liver injury, and a decrease in ductal proliferation and fibrogenesis (Chan et al., 2011; Hussein, 2013). These data confirm the importance of inflammation in the progression of cholestatic liver injury and are in line with antioxidant properties of RSV. Perhaps a more interesting finding, however, is that by Lin, et al. (2012) demonstrating that the protective effects of RSV stem from a combination of anti-apoptotic activity, mitochondrial biogenesis, and induction of autophagy. However, it is unclear whether the involvement of these processes is a direct effect of RSV activity or secondary to other effects of RSV. In addition, recent studies indicate that necrosis, rather than apoptosis, predominates during cholestasis (Gujral et al., 2004; Fickert et al., 2005; Woolbright et al., 2013) thus, the mechanisms of RSV-mediated protection against cholestasis need further investigation.
4.3 Resveratrol in ischemia-reperfusion-induced liver injury
Ischemia-reperfusion injury (IRI) is the process by which re-introduction of oxygen to a previously ischemic organ, leads to exacerbation of injury to that organ. Clinically, IRI is observed during resection surgery (due to the Pringle maneuver), transplantation, or periods of severe hypotension followed by fluid resuscitation (Eltzschig and Eckle, 2011). In this context, a protective role of systemic administration of RSV following IRI has been described on the basis of decreased plasma ALT and AST activities and reduced necrosis early (≤3 h) after reperfusion in rats (Hassan-Khabbar et al., 2008; 2010; Nivet-Antoine et al., 2010). However, these studies examining the role of RSV during IRI have neglected to take into account the late stage, which is mediated by the influx of neutrophils to the hepatic parenchyma (Jaeschke et al., 1990). These studies focus only on the early reperfusion period, a time point at which RSV has been shown to ameliorate the oxidative stress responsible for initial injury, decrease neutrophil recruitment, and down-regulate thioredoxin-interacting protein, a protein that inhibits the function of thioredoxin (Hassan-Khabbar et al., 2008; 2010; Nivet-Antoine et al., 2010). Kupffer cells but not neutrophils are responsible for the early oxidant stress and cell death during reperfusion (Jaeschke and Farhood, 1991). However, this early reperfusion injury determines the degree of neutrophil recruitment during the later stages of the pathophysiology (Jaeschke, 2003). Neutrophil-induced oxidant stress is the main cause of neutrophil cytotoxicity (Hasegawa et al., 2005). It is therefore likely that RSV attenuated the effect of the early Kupffer cell- and later neutrophil-induced oxidant stress by either acting as antioxidant and/or modulated the capacity of the inflammatory cells to generate these reactive oxygen species. Thus, more detailed mechanistic studies involving prolonged reperfusion time points are necessary before drawing any conclusions about the possible beneficial effect of RSV during IRI.
5. CLINICAL USE OF RESVERATROL FOR LIVER DISEASES
The mounting preclinical evidence of beneficial effects of RSV has prompted a few clinical trials of its use in human diseases. Preclinical and clinical studies have demonstrated that RSV is relatively safe, well-tolerated and lacks serious adverse effects (Novelle et al., 2015). Importantly, promising results have been achieved in some clinical trials for diabetes, obesity and cardiovascular diseases, and cancer (Smoliga et al., 2011; Novelle et al., 2015).
Although the effect of RSV in drug hepatotoxicity has not been tested in humans, it has been tested in certain liver diseases. In particular, RSV may be beneficial in the treatment of fatty liver in humans. Daily treatment with RSV has been shown to modestly reduce hepatic lipid content and other evidence of liver stress in obese men (Timmers et al., 2011), although these results were not reproducible in a non-obese female population (Yoshino et al., 2012). Another study found that RSV decreased plasma levels of hepatic lipoproteins in overweight subjects (Dash et al., 2013). RSV has also been shown to decrease evidence of apoptosis in patients with NAFLD (Faghihzadeh et al., 2014), though another clinical study failed to reproduce these health benefits (Chachay et al., 2014). Currently, more than ten clinical trials investigating the effects of RSV on liver diseases, NAFLD (and related metabolic diseases) and liver cancer are ongoing, according to the US federal database of clinical trials. Nevertheless, the number and scope of human clinical trials regarding the therapeutic benefits of RSV in liver disease are still very limited, especially when compared to the >7000 publications on RSV listed in Pubmed and the various benefits in preclinical studies for liver diseases (Bishayee et al., 2010a).
Several factors may account for the small number of clinical trials of RSV for liver diseases. The most important might be the limited decisive evidence of health benefits in humans. Some completed clinical trials on RSV have obtained compelling results with a focus on its physiologic benefits. Although physiologic effects of RSV might not reflect its true potential as a therapeutic agent for pathologies, a neutral or negative result from such trials often prevents its further use into clinical patients (Smoliga et al., 2011; 2012). The second important factor might be the nature of RSV. The compound appears to have multiple molecular targets, which renders the interpretation of clinical data difficult. This is further complicated by the use of different formulations of RSV in different trials (Smoliga et al., 2012). Another issue is that clinical trials are expensive and most natural products like RSV cannot be protected by patents. Finally, RSV has poor bioavailability, undergoing rapid and extensive metabolism in the liver (Smoliga and Blanchard, 2014). Only trace amounts of free RSV (<5 ng/ml) could be detected in plasma after a 25 mg oral dose and most of the compound was converted to sulphate and glucuronide conjugates (Walle et al., 2004). Therefore, new formulations with improved RSV bioavailability will be needed for clinical trials (Amri et al., 2012). A number of strategies have been tried in animal models to improve the bioavailability of RSV, such as applying nano-emulsifying drug delivery systems, controlled release devices, or consumption with other phytochemicals (Amri et al., 2012; Smoliga and Blanchard, 2014). Interestingly, co-administration of vinegar-baked Radix bupleuri has been shown to selectively improve hepatic distribution of RSV in mice (Zhao et al., 2009), though its use in clinical studies needs further evaluation.
Another critical factor for therapeutic use of RSV is the determination of its clinical dosage, duration and route of administration. Species differences, inter-individual variation in human subjects and the type and severity of liver disease could all render the translation of data from in vitro and animal studies into clinical use difficult, and the potential confounding variables could affect the accurate interpretation of clinical studies (Novelle et al., 2015). It was also suggested that most studies investigating RSV in humans so far have been limited by their sample sizes (Novelle et al., 2015). Therefore, well-designed clinical studies are needed to bridge the gap between the large number of published preclinical studies and the paucity of information concerning the use of RSV as a therapeutic agent in liver disease.
6. CONCLUSIONS
A large number of studies exploring the effects of RSV in various liver diseases have been published. As discussed, there is compelling evidence from preclinical studies in rodents that RSV can protect against xenobiotic-induced hepatotoxicity in particular. However, the mechanisms by which this protection occurs are poorly understood. Most of the mechanisms that have been proposed to date are, at best, questionable due to poor study design. An improved understanding of the effects of RSV in liver disease and hepatotoxicity will help us to identify human diseases that might benefit from RSV therapy. The poor bioavailability of RSV also continues to be a major obstacle to clinical use. Overall, while RSV appears to be a promising therapeutic candidate, additional work is clearly needed before it is likely to be clinically useful.
RESEARCH HIGHLIGHTS.
Resveratrol has been shown to protect against many hepatoxicants
The most commonly proposed mechanisms are antioxidant and anti-inflammatory effects
The proposed mechanisms are not always consistent with data from related studies
Where possible, we suggest the most likely mechanisms of protection based on all available data
Resveratrol may be a promising candidate for clinical use to treat liver diease, but the mechanisms are not always clear and bioavailability is an issue
ACKNOWLEDGEMENTS
This work was supported in part by the U.S. National Institutes of Health grants R01 DK102142 (H.J.) and R01 DK070195 (H.J.), National Center for Research Resources grant 5P20RR021940 (H.J.), and the National Institute of Environmental Health Sciences “Training Program in Environmental Toxicology” grant T32 ES007079 (to M.R.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts.
REFERENCES
- Ahmad A, Ahmad R. Resveratrol mitigates structural changes and hepatic stellate cell activation in N’-nitrosodimethylamine-induced liver fibrosis via restraining oxidative damage. Chem Biol Interact. 2014;221:1–12. doi: 10.1016/j.cbi.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G833–G842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol: what formulation solutions to bioavailability limitations? J Control Release. 2012;158(2):182–193. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56(5):1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ara C, Kirimlioglu H, Karabulut A, Coban S, Ay S, Harputluoglu M, Kirimlioglu V, Yilmaz S. Protective effect of resveratrol against oxidative stress in cholestasis. J Surg Res. 2005;127(2):112–117. doi: 10.1016/j.jss.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94(1):217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324(1):8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Farhood A, Jaeschke H. Scaveging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther. 2003;307(1):67–73. doi: 10.1124/jpet.103.052506. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kaira A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. vol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Beedanagari SR, Bebenek I, Bui P, Hankinson O. Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicol Sci. 2009;110(1):61–67. doi: 10.1093/toxsci/kfp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayee A, Darvesh AS, Politis T, McGory R. Resveratrol and liver disease: from bench to bedside and community. Liver Int. 2010a;30(8):1103–1114. doi: 10.1111/j.1478-3231.2010.02295.x. [DOI] [PubMed] [Google Scholar]
- Bishayee A, Dhir N. Resveratrol-mediated chemoprevention of dimethylnitrosamine-initiated hepatocarcinogenesis: inhibition of cell proliferation and induction of apoptosis. Chem Biol Interact. 2009;179(2–3):131–144. doi: 10.1016/j.cbi.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Bishayee A, Politis T, Darvesh AS. Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treat Rev. 2010b;36(1):43–53. doi: 10.1016/j.ctrv.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Bishayee A, Waghray A, Barnes KF, Mbimba T, Bhatia D. Suppression of the inflammatory cascade is implicated in resveratrol chemoprevention of experimental hepatocarcinogenesis. Pharm Res. 2010c;27(6):1080–1091. doi: 10.1007/s11095-010-0144-4. [DOI] [PubMed] [Google Scholar]
- Blazka ME, Wilmer JL, Holladay SD, Wilson RE, Luster MI. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 1995;133(1):43–52. doi: 10.1006/taap.1995.1125. [DOI] [PubMed] [Google Scholar]
- Boess F, Bopst M, Althaus R, Polsky S, Cohen SD, Eugster HP, Boelsterli UA. Acetaminophen hepatotoxicity in tumor necrosis factor/lymphotoxin-alpha gene knockout mice. Hepatology. 1998;27(4):1021–1029. doi: 10.1002/hep.510270418. [DOI] [PubMed] [Google Scholar]
- Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004;22(3):169–188. doi: 10.1111/j.1527-3466.2004.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Bujanda L, García-Barcina M, Gutiérrez-de Juan V, Bidaurrazaga J, de Luco MF, Gutiérrez-Stampa M, Larzabal M, Hijona E, Sarasqueta C, Echenique-Elizondo M, Arenas JI. Effect of resveratrol on alcoholic-induced mortality and liver lesions in mice. BMC Gastroenterol. 2006;6:35. doi: 10.1186/1471-230X-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AS, MacMillan-Crow LA, Hinson JA. Reactive nitrogen species in acetaminophen-induced mitochondrial damage and toxicity in mouse hepatocytes. Chem Res Toxicol. 2010;23(7):1286–1292. doi: 10.1021/tx1001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50(11):3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- Carrion AF, Bhamidimarri KR. Liver transplant for cholestatic liver diseases. Clinics Liver Dis. 2013;17(2):245–359. doi: 10.1016/j.cld.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O’Moore TM-Lee Sullivan P, Franklin M, Klein K, Taylor PJ, Ferguson M, Coombes JS, Thomas GP, Cowin GJ, Kirkpatrick CM, Prins JB, Hickman IJ. Resveratrol does not benefit patients with non-alcoholic fatty liver disease. Clin Gastroenterol and Hepatol. 2014;12(12):2092–2103. doi: 10.1016/j.cgh.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Chan CC, Cheng LY, Lin CL, Huang YH, Lin HC, Lee FY. The protective role of natural phytoalexin resveratrol on inflammation, fibrosis and regeneration in cholestatic liver injury. Mol Nutr Food Res. 2011;55(12):1841–1849. doi: 10.1002/mnfr.201100374. [DOI] [PubMed] [Google Scholar]
- Chan CC, Lee KC, Huang YH, Chou CK, Lin HC, Lee FY. Regulation by resveratrol of the cellular factors mediating liver damage and regeneration after acute toxic liver injury. J Gastroenterol Hepatol. 2014;29(3):603–613. doi: 10.1111/jgh.12366. [DOI] [PubMed] [Google Scholar]
- Chan WK, Delucchi AB. Resveratrol, a red wine constituent, is a mechanism-based inactivator of cytochrome P450 3A4. Life Sci. 2000;67(25):3103–3112. doi: 10.1016/s0024-3205(00)00888-2. [DOI] [PubMed] [Google Scholar]
- Chávez E, Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. Resveratrol prevents fibrosis, NF-kappaB activation and TGF-beta increases induced by chronic CCl4 treatment in rats. J Appl Toxicol. 2008;28(1):35–43. doi: 10.1002/jat.1249. [DOI] [PubMed] [Google Scholar]
- Chow HS, Garland LL, Hsu CH, Vining DR, Chew WM, Miller JA, Perloff M, Crowell JA, Alberts DS. Resveratrol modulates drug-and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res. 2010;3(9):1168–1175. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998;58(24):5707–5712. [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315(2):879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35(5):1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- Dash S, Xiao C, Morgantini C, Szeto L, Lewis GF. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler Thromb Vasc Biol. 2013;33(12):2895–2901. doi: 10.1161/ATVBAHA.113.302342. [DOI] [PubMed] [Google Scholar]
- Delmas D, Jannin B, Cherkaoui Malki M, Latruffe N. Inhibitory effect of resveratrol on the proliferation of human and rat hepatic derived cell lines. Oncol Rep. 2000;7(4):847–852. doi: 10.3892/or.7.4.847. [DOI] [PubMed] [Google Scholar]
- Du K, McGill MR, Bajt ML, Xie Y, Jaeschke H. Resveratrol prevents protein nitration and release of endonucleases from mitochondria during acetaminophen hepatotoxicity. Food Chem Toxicol. 2015;81:62–70. doi: 10.1016/j.fct.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Eckle T. Ischemia and reperfusion - from mechanism to translation. Nature Med. 2011;17(11):1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer, EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Fabris S, Momo F, Ravagnan G, Stevanato R. Antioxidant properties of resveratrol and piceid on lipid peroxidation in micelles and monolamellar liposomes. Biophys Chem. 2008;135(1–3):76–83. doi: 10.1016/j.bpc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34(10):837–843. doi: 10.1016/j.nutres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Farghali H, Černý D, Kameníková L, Martinek J, Horínek A, Kmoníckova E, Zidek Z. Resveratrol attenuates lipopolysaccharide-induced hepatitis in D-galactosamine sensitized rats: role of nitric oxide synthase 2 and heme oxygenase-1. Nitric Oxide. 2009;21(3):216–225. doi: 10.1016/j.niox.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Fickert P, Trauner M, Fuchsbichler A, Zollner G, Wagner M, Marschall HU, Zatloukal K, Denk H. Oncosis represents the main type of cell death in mouse models of cholestasis. J Hepatol. 2005;42(3):378–385. doi: 10.1016/j.jhep.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Girish C, Pradhan SC. Indian herbal medicines in the treatment of liver diseases: problems and promises. Fund Clin Pharmacol. 2011;26(2):180–189. doi: 10.1111/j.1472-8206.2011.01011.x. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67(2):322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in Fas receptor-deficient C57Bl/6J–lpr mice after bile duct ligation. Hepatology. 2004;40(4):998–1007. doi: 10.1002/hep.20380. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283(20):13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am J Physiol Gastrointest Liver Physiol. 2005;289(4):G760–G767. doi: 10.1152/ajpgi.00141.2005. [DOI] [PubMed] [Google Scholar]
- Hassan-Khabbar S, Cottart CH, Wendum D, Vibert F, Clot JP, Savouret J, Conti M, Nivet-Antoine V. Postischemic treatment by trans-resveratrol in rat liver ischemia-reperfusion: a possible strategy in liver surgery. Liver Transpl. 2008;14(4):451–459. doi: 10.1002/lt.21405. [DOI] [PubMed] [Google Scholar]
- Hassan-Khabbar S, Vamy M, Cottart CH, Wendum D, Vibert F, Savouret JF, Thérond P, Clot JP, Waligora AJ, Nivet-Antoine V. Protective effect of post-ischemic treatment with trans-resveratrol on cytokine production and neutrophil recruitment by rat liver. Biochimie. 2010;92(4):405–410. doi: 10.1016/j.biochi.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Heebøll S, Thomsen KL, Pederson SB, Vilstrup H, George J, Grøbæk H. Effects of resveratrol in experimental and clinical non-alcoholic fatty liver disease. World J Hepatol. 2014;6(4):188. doi: 10.4254/wjh.v6.i4.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Michael SL, Ault SG, Pumford NR. Western blot analysis for nitrotyrosine protein adducts in livers of saline-treated and acetaminophen-treated mice. Toxicol Sci. 2000;53(2):467–473. doi: 10.1093/toxsci/53.2.467. [DOI] [PubMed] [Google Scholar]
- Holthoff JH, Woodling KA, Doerge DR, Burns ST, Hinson JA, Mayeux PR. Resveratrol, a dietary polyphenolic phytoalexin, is a functional scavenger of peroxynitrite. Biochem Pharmacol. 2010;80(8):1260–1265. doi: 10.1016/j.bcp.2010.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein MA. Prophylactic effect of resveratrol against ethinylestradiol-induced liver cholestasis. J Med Food. 2013;16(3):246–254. doi: 10.1089/jmf.2012.0183. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Iijima M, Umemura T, Nishikawa A, Iwasaki Y, Ito R, Saito K, Hirose M, Nakazawa H. Determination of nitrotyrosine and tyrosine by high-performance liquid chromatography with tandem mass spectrometry and immunohistochemical analysis in livers of mice administered acetaminophen. J Pharm Biomed Anal. 2006;41(4):1325–1331. doi: 10.1016/j.jpba.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260(3 pt 1):G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Smith CV. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4(15):3355–3359. [PubMed] [Google Scholar]
- Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parechymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160(7):3480–3486. [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255(3):935–941. [PubMed] [Google Scholar]
- Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65(2):166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Liu J. Neutrophil depletion protects against murine acetaminophen hepatotoxicity: another perspective. Hepatology. 2007;45(6):1588–1589. doi: 10.1002/hep.21549. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284(1):G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, McGill MR, Xie Y, Ramachandran A. Models of drug-induced liver injury for evaluation of phytotherapeutics and other natural products. Food Chem Toxicol. 2013;55:279–289. doi: 10.1016/j.fct.2012.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;32(1):8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol. 2011;26(s1):173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fond HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187(1):195–202. [PubMed] [Google Scholar]
- Jornayvez FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, Gharbi N, Kamoun A, El Fazaa S. Protective effect of resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol Alcohol. 2006;41(3):236–239. doi: 10.1093/alcalc/agh256. [DOI] [PubMed] [Google Scholar]
- Kawada N, Seki S, Inoue M, Kuroki T. Effect of antioxidant, resveratrol, quercetin, and N-acetylcysteine, on the function of cultured rat hepatic stellate cells and Kupffer cells. Hepatology. 1998;27(5):1265–1274. doi: 10.1002/hep.510270512. [DOI] [PubMed] [Google Scholar]
- Knight TR, Fariss MW, Farhood A, Jaeschke H. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol Sci. 2003;76(1):229–236. doi: 10.1093/toxsci/kfg220. [DOI] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 2002;303(2):468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci. 2001;62(2):212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- Knockaert L, Berson A, Ribault C, Prost PE, Fautrel A, Pajaud J, Lepage S, Lucas-Clerc C, Bégué JM, Fromenty B, Robin MA. Carbon tetrachloride-mediated lipid peroxidation induces early mitochondrial alterations in mouse liver. Lab Invest. 2012;92(3):396–410. doi: 10.1038/labinvest.2011.193. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40(5):1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Kuo PL, Chiang LC, Lin CC. Resveratrol-induced apoptosis is mediated by p53-dependent pathway in HepG2 cells. Life Sci. 2002;72(1):23–34. doi: 10.1016/s0024-3205(02)02177-x. [DOI] [PubMed] [Google Scholar]
- Kozuki Y, Miura Y, Yagasaki K. Resveratrol suppresses hepatoma cell invasion independently of its anti-proliferative action. Cancer Lett. 2001;167(2):151–156. doi: 10.1016/s0304-3835(01)00476-1. [DOI] [PubMed] [Google Scholar]
- Kulkarni SS, Cantó C. The molecular targets of resveratrol. Biochim Biophys Acta. 2015;1852(6):1114–1123. doi: 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Kuo PL, Chiang LC, Lin CC. Resveratrol-induced apoptosis is mediated by p53-dependent pathway in Hep G2 cells. Life Sci. 2002;22(1):23–34. doi: 10.1016/s0024-3205(02)02177-x. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines H, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Langcake P, Pryce RJ. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol Mol Plant Pathol. 1976;9(1):77–86. [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Lee ES, Shin MO, Yoon S, Moon JO. Resveratrol inhibits dimethylnitrosamine-induced hepatic fibrosis in rats. Arch Pharm Res. 2010;33(6):925–932. doi: 10.1007/s12272-010-0616-4. [DOI] [PubMed] [Google Scholar]
- Lee WM. Etiologies of acute liver failure. Semin Liver Dis. 2008;28(2):142–152. doi: 10.1055/s-2008-1073114. [DOI] [PubMed] [Google Scholar]
- Leist M, Gantner F, Bohlinger I, Germann PG, Tiegs G, Wendel A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J Immunol. 1994;153(4):1778–1788. [PubMed] [Google Scholar]
- Leist M, Gantner F, Jilg S, Wendel A. Activation of the 55kDa TNF receptor is necessary and sufficient for TNF-induced acute liver failure, hepatocyte apoptosis, and nitrite release. J Immunol. 1995;154(3):1307–1316. [PubMed] [Google Scholar]
- Lin TK, Huang LT, Huang YH, Tiao MM, Tang KS, Liou CW. The effect of the red wine polyphenol resveratrol on a rat model of biliary obstructed cholestasis: involvement of anti-apoptotic signaling, mitochondrial biogenesis and the induction of autophagy. Apoptosis. 2012;17(8):871–879. doi: 10.1007/s10495-012-0732-3. [DOI] [PubMed] [Google Scholar]
- LoGuidice A, Boelsterli UA. Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin D-regulated permeability transition. Hepatology. 2011;54(3):969–978. doi: 10.1002/hep.24464. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42(11):110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Sugiyama S, Horie T. Th1/Th2 cytokine balance as a determinant of acetaminophen-induced liver injury. Chem Biol Interact. 2009;179(2–3):273–279. doi: 10.1016/j.cbi.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Mbimba T, Awale P, Bhatia D, Geldenhuys WJ, Darvesh AS, Carroll RT, Bishayee A. Alteration of hepatic proinflammatory cytokines is involved in the resveratrol-mediated chemoprevention of chemically-induced hepatocarcinogenesis. Curr Phar, Biotechnol. 2012;13(1):229–234. doi: 10.2174/138920112798868575. [DOI] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269(3):240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122(4):1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Staggs VS, Sharpe MR, Lee WM, Jaeschke H Acute Liver Failure Study Group. Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology. 2014;60(4):1336–1345. doi: 10.1002/hep.27265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megli FM, Sabatini K. Mitochondrial phospholipid bilayer structure is ruined after liver oxidative injury in vivo. FEBS Letters. 2004;573(1–3):68–72. doi: 10.1016/j.febslet.2004.07.057. [DOI] [PubMed] [Google Scholar]
- Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol Appl Pharmacol. 1988;93(3):378–387. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187(1):211–217. [PubMed] [Google Scholar]
- Muriel P. Role of free radicals in liver diseases. Hepatol Int. 2009;3(4):526–536. doi: 10.1007/s12072-009-9158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135(4):1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Ni HM, Du K, You M, Ding WX. Critical role of FoxO3a in alcohol-induced autophagy and hepatotoxicity. Am J Pathol. 2013;183(6):1815–1825. doi: 10.1016/j.ajpath.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivet-Antoine V, Cottart CH, Lemaréchal H, Vamy M, Margaill I, Beaudeux JL, Bonnefont-Rousselot D, Borderie D. trans-resveratrol downregulates Txnip overexpression occurring during liver ischemia-reperfusion. Biochimie. 2010;92(12):1766–1771. doi: 10.1016/j.biochi.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Notas G, Nifli AP, Kampa M, Vercauteren J, Kouroumalis E, Castanas E. Resveratrol exerts its antiproliferative effect on HepG2 hepatocellular carcinoma cells, by inducing cell cycle arrest, and NOS activation. Biochim Biophys Acta. 2006;1760(11):1657–1656. doi: 10.1016/j.bbagen.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Novelle MG, Wahl D, Diéguez C, Bernier M, de Cabo R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res Rev. 2015;21:1–15. doi: 10.1016/j.arr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva J, French BA, Li J, Bardag-Gorce F, Fu P, French SW. Sirt1 is involved in energy metabolism: the role of chronic ethanol feeding and resveratrol. Exp Mol Pathol. 2008;85(3):155–159. doi: 10.1016/j.yexmp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philip A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148(3):421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran D, Elavarasan J, Sivalingam M, Ganapathy E, Kumar A, Kalpana K, Sakthisekaran D. Resveratrol interferes with N-nitrosodiethylamine-induced hepatocellular carcinoma at early and advanced stages in male Wistar rats. Mol Med Rep. 2011;4(6):1211–1217. doi: 10.3892/mmr.2011.555. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Rad Res. 2011;45(2):156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, McGill MR, Xie Y, Nie HM, Ding WX, Jaeschke H. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58(6):2099–2108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther. 2005;312(2):509–516. doi: 10.1124/jpet.104.075945. [DOI] [PubMed] [Google Scholar]
- Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary artery disease. Lancet. 1992;339(8808):1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- Rivera H, Shibayama M, Tsutsumi V, Perez-Alvarez V, Muriel P. Resveratrol and trimethylated resveratrol protect from acute liver damage induced by CCl4 in the rat. J Appl Toxicol. 2008;28(2):147–155. doi: 10.1002/jat.1260. [DOI] [PubMed] [Google Scholar]
- Roy S, Sannigrahi S, Majumdar S, Ghosh B, Sarkar B. Resveratrol regulates antioxidant status, inhibits cytokine expression and restricts apoptosis in carbon tetrachloride induced rat hepatic injury. Oxid Med Cell Longev. 2011:703676. doi: 10.1155/2011/703676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schayler HJ, Laaff H, Peters T, Woort-Menker M, Estler HC, Karck U, Decker K. Involvement of tumor necrosis factor in endotoxin-triggered neutrophil adherence to sinusoidal endothelial cells of mouse liver and its modulation in acute phase. Hepatology. 1988;7(2):239–249. doi: 10.1016/s0168-8278(88)80488-4. [DOI] [PubMed] [Google Scholar]
- Seeff LB, Bonkovsky HL, Navarro VJ, Wang G. Herbal products and the liver: a review of adverse effects and mechanisms. Gastroenterology. 2015;148(3):517–532. doi: 10.1053/j.gastro.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Seeff LB, Curto TM, Szabo G, Everson GT, Bonkovsky HL, Dienstag JL, Shiffman ML, Lindsay KL, Lok AS, Di Bisceglie AM, Lee WM, Chany MG HALT-C Trial Group. Herbal product use by persons enrolled in the hepatitis C antiviral long-term treatment against cirrhosis (HALT-C) trial. Hepatology. 2008;47(2):605–612. doi: 10.1002/hep.22044. [DOI] [PubMed] [Google Scholar]
- Şehirli O, Tozan A, Omurtag GZ, Cetinel S, Contuk N, Gedik N, Sener G. Protective effect of resveratrol against naphthalene-induced oxidative stress in mice. Ecotoxicol Environ Saf. 2008;71(1):301–308. doi: 10.1016/j.ecoenv.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Sener G, Toklu HZ, Sehirli AO, Velioğlu-Oğünç A, Cetinel S, Gedik N. Protective effects of resveratrol against acetaminophen-induced toxicity in mice. Hepatol Res. 2006;35(1):62–68. doi: 10.1016/j.hepres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82(5):1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health-a comprehensive review of human clinical trials. Mol Nutr Food Res. 2011;55(8):1129–1141. doi: 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- Smoliga JM, Blanchard O. Enhancing the Delivery of resveratrol in Humans: If Low Bioavailability is the Problem, What is the Solution? Molecules. 2014;19(11):17154–17172. doi: 10.3390/molecules191117154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoliga JM, Vang O, Baur JA. Challenges of translating basic research into therapeutics: resveratrol as an example. J Gerontol A Biol Sci Med Sci. 2012;67(2):158–167. doi: 10.1093/gerona/glr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenwyk RC, Tan B. In vitro evidence for the formation of reactive intermediates of resveratrol in human liver microsomes. Xenobiotica. 2010;40(1):62–71. doi: 10.3109/00498250903337384. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest. 1999;103(9):1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader DB, Bacon BR, Lindsay KL, La Breque DR, Morgan T, Wright EC, Allen J, Khokar MF, Hoofnagle JH, Seeff LB. Use of complementary and alternative medicine in patients with liver disease. Am J of Gastroenterol. 2002;97(9):2391–2397. doi: 10.1111/j.1572-0241.2002.05993.x. [DOI] [PubMed] [Google Scholar]
- Sun AY, Wang Q, Simonyi A, Sun YG. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41(2–3):375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadolini B, Juliano C, Piu L, Franconi F, Cabrini L. Resveratrol inhibition of lipid peroxidation. Free Rad Res. 2000;33(1):105–114. doi: 10.1080/10715760000300661. [DOI] [PubMed] [Google Scholar]
- Takaoka M. Resveratrol, a new phenolic compound from Veratrum grandiflorum. Nippon Kagaku Kaishi. 1939;60:1090–1100. [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van T, Weijer de, Goossens GH, Hoeks J, van S, Krieken der, Ryu D, Kersten S, Moonen E-Hesselink Kornips MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Shrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunali-Akbay T, Şehirli O, Ercan F, Sener G. Resveratrol protects against methotrexate-induced hepatic injury in rats. J Pharm Pharm Sci. 2010;13(2):303–310. doi: 10.18433/j30k5q. [DOI] [PubMed] [Google Scholar]
- United State Food and Drug Administration (U.S. F.D.A.) Guidance for industry: drug-induced liver injury: premarketing clinical evaluation. Silver Spring, MD: United States Food and Drug Administration; 2009. [Google Scholar]
- Vitaglione P, Ottanelli B, Milani S, Morisco F, Caporaso N, Fogliano V. Dietary trans-resveratrol bioavailablitity and effect on CCl4-induced liver lipid peroxidation. J Gastroenterol Hepatol. 2009;24(4):618–622. doi: 10.1111/j.1440-1746.2008.05598.x. [DOI] [PubMed] [Google Scholar]
- Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32(12):1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- Walle T. Bioavailability of resveratrol. Ann NY Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY. Resveratrol protects against gloval cerebral ischemic injury in gerbils. Brain Res. 2002;958(2):439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33(2):105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- Wenzel E, Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res. 2005;49(5):472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Sharpe MR, McGill MR, Farhood A, Jaeschke H. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol. 2014;275(2):122–133. doi: 10.1016/j.taap.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286(40):35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Antoine DJ, Jenkins R, Bajt ML, Park BK, Jaeschke H. Plasma biomarkers of liver injury and inflammation demonstrate a lack of apoptosis during obstructive cholestasis in mice. Toxicol Appl Pharmacol. 2013;273(3):524–531. doi: 10.1016/j.taap.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World J Gastroenterol. 2012;18(36):4985–4993. doi: 10.3748/wjg.v18.i36.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol. 2014;279(3):266–274. doi: 10.1016/j.taap.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Ramachandran A, Breckenridge DG, Liles JT, Lebofsky M, Farhood A, Jaeschke H. Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 2015;286(1):1–9. doi: 10.1016/j.taap.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Ramachandran A, Yan HM, Woolbright BL, Copple BL, Fickert P, Trauner M, Jaeschke H. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol Lett. 2014;224(2):186–195. doi: 10.1016/j.toxlet.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi F, Patterson Fanelli BW, Klein S. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16(5):658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008;294(4):G892–G898. doi: 10.1152/ajpgi.00575.2007. [DOI] [PubMed] [Google Scholar]
- You Q, Holt M, Yin H, Li G, Hu CJ, Ju C. Role of hepatic resident and infiltrating macrophages in liver repair after acute injury. Biochem Pharmacol. 2013;86(6):836–843. doi: 10.1016/j.bcp.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Shin YG, Chow A, Li Y, Kosmeder JW, Lee YS, Hirschelman WH, Pezzuto JM, Mehta RG, van Breemen RB. Human, rat, and mouse metabolism of resveratrol. Pharm Res. 2002;19(12):1907–1914. doi: 10.1023/a:1021414129280. [DOI] [PubMed] [Google Scholar]
- Yu H, Pan C, Zhao S, Wang Z, Zhang H, Wu W. Resveratrol inhibits tumor necrosis factor-alpha-mediated matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. Biomed Pharmacother. 2008;62(6):366–372. doi: 10.1016/j.biopha.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Yu HB, Zhang HF, Zhang X, Li DY, Xue HZ, Pan CE, Zhou SH. Resveratrol inhibits VEGF expression of human hepatocellular carcinoma cells through a NF-kappa B-mediated mechanism. Hepatogastroenterol. 2010;57(102–103):1241–1246. [PubMed] [Google Scholar]
- Zhang H, Yang R. Resveratrol inhibits VEGF gene expression and proliferation of hepatocarcinoma cells. Hepatogastroenterol. 2014;61(130):410–412. [PubMed] [Google Scholar]
- Zhao CQ, Zhou Y, Ping J, Xu LM. Traditional Chinese medicine for the treatment of liver diseases. J Integr Med. 2014;12(5):401–408. doi: 10.1016/S2095-4964(14)60039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Liu S, Mao S, Wang Y. Study on liver targeting effect of vinegar-baked Radix Bupleuri on resveratrol in mice. J Ethnopharmacol. 2009;126(3):415–420. doi: 10.1016/j.jep.2009.09.023. [DOI] [PubMed] [Google Scholar]