Abstract
Poly(ADP-ribose) polymerase 1 (PARP1) interacts genetically with the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) to suppress early-onset T-lineage lymphomas in the mouse, but the underlying mechanisms have remained unknown. To address this question, we analyzed a series of lymphomas arising in PARP1 −/− /DNA-PKcs−/− (P1 −/−/D−/−) mice. We found that, despite defective V(D)J recombination, P1−/− /D−/− lymphomas lacked clonal reciprocal translocations involving antigen-receptor loci. Instead, tumor cells were characterized by aneuploidy driven by two main mechanisms: p53 inactivation and abnormal chromosome disjunction due to telomere fusions (TFs). Aberrant accumulation of p53 was observed in 13/19 (68.4%) lymphomas. Sequence analysis revealed five p53 mutations: three missense point mutations (one transition in exon 8 and two transversions in exons 5 and 8, respectively), one in-frame 5–11 microindel in exon 7 and a 410-bp deletion encompassing exons 5–8, resulting in a truncated protein. Analysis of tumor metaphases using sequential telomere fluorescent in-situ hybridization and spectral karyotyping revealed that nine out of nine lymphomas contained TFs. Mutant but not wild-type p53 status was associated with frequent clonal and nonclonal TFs, suggesting that p53 normally limits the extent of telomere dysfunction during transformation. Chromosomes involved in TFs were more likely to be aneuploid than chromosomes not involved in TFs in the same metaphases, regardless of the p53 status, indicating that TFs promote aneuploidy via a mechanism that is distinct from p53 loss. Finally, analysis of radiation responses in P1 −/−/D−/−, and control primary cells and tissues indicates that loss of PARP1 increases in vivo radiosensitivity and genomic instability in DNA-PKcs-deficient mice without impairing p53 stabilization and effector functions, suggesting a more severe defect in double-strand break (DSB) repair in double mutants. Together, our findings uncover defective DSB repair leading to tumor suppressor inactivation and abnormal segregation of fused chromosomes as two novel mechanisms promoting tumorigenesis in thymocytes lacking PARP1 and DNA-PKcs.
Keywords: telomere, DNA-PKcs, PARP1, p53, lymphoma, mutation
INTRODUCTION
Poly(ADP-ribose) polymerase 1 (PARP1), the founding member of a family of 22 proteins containing a PARP domain,1 has evolved pleiotropic functions in DNA repair and other cellular processes2,3(see http://parplink.u-strasbg.fr/index.html for a comprehensive web-based resource). In the context of DNA double-strand breaks (DSBs), PARP1 modifies histones and DNA repair factors to relax chromatin and promote the recruitment of the MRE11/RAD50/ NBS1 complex and other factors.4–6 Consistent with this notion, PARP1 −/− cells show a modest defect in the DNA DSB repair by homologous recombination.6–8
Among numerous substrates, PARP1 modifies the DNA-dependent protein kinase catalytic subunit (DNA-PKcs),9,10a phosphoinositide-3 kinase-like serine/threonine kinase that associates to DNA-bound Ku to promote repair by ligase IV-dependent nonhomologous end-joining.11,12 Consistent with defective DSB repair, mice deficient for either DNA-PKcs or PARP1 share phenotypes of radiosensitivity and chromosomal instability, particularly after exposure to DNA-damaging agents.13–16 Moreover, PARP1 suppresses mutation in vitro and in vivo,17–19 and loss of PARP1 accelerates tumorigenesis in p53 +/− mice.20 Similarly, the subset of human lung adenocarcinomas harboring mutations in DNA-PKcs show a marked increase in genome-wide mutation,21 a finding that correlates with worse clinical outcome.21 However, despite increased chromosomal instability, PARP1−/− and DNA-PKcs−/− mice are only modestly tumor prone,22–25 in line with mostly preserved cell cycle checkpoint functions.14,26 In contrast, mice lacking PARP1 in a scid background (harboring a truncated inactive DNA-PKcs27,28) develop aggressive lymphomas early in life with high penetrance.29These findings suggest that combined deficiency confers a novel phenotype leading to thymocyte transformation, but specific mechanisms remain unknown. In this context, DNA-PKcs is required for the processing of recombination-activating gene (RAG)-dependent DSBs at antigen-receptor loci,30 and loss of PARP1 may promote their aberrant repair to form oncogenic translocations.31
In addition to their roles at DSBs, PARP1 and DNA-PKcs cooperate with the shelterin complex to maintain telomeres.32In this context, chromosome ends lacking DNA-PKcs may be aberrantly rejoined to each other to form telomere fusions (TFs).33–37 In addition, ‘uncapped’ telomeres may be rejoined to DSBs elsewhere in the genome. These events are rare in primary scid or DNA-PKcs−/− cells,16,33,34 but frequent in passage-immortalized scid cells,33 suggesting that p53 and possibly other factors normally suppress them. However, telomere dysfunction was not reported in previous studies of lymphomas arising in scid, scid/p53−/− or PARP1−/− /scid mice,23,24,29,39 and therefore, its contribution to in vivo tumorigenesis remains to be established. Moreover, PARP1 binds to the shelterin factor TRF240 and suppresses telomere instability in primary and lymphoma cells,20,40,41 suggesting that PARP1 loss may modulate telomere dysfunction in DNA-PKcs-deficient cells.
Here we set to test the notion that PARP1−/− /DNA-PKcs−/− (P1−/− /D−/−) lymphomas arise from aberrant rearrangements of RAG-dependent DSBs at antigen-receptor loci to form oncogenic translocations. Our studies below disproved that hypothesis and demonstrate for the first time a synergism between PARP1 and DNA-PKcs in the suppression of p53 mutation and telomere dysfunction in developing lymphocytes.
RESULTS
P1 −/−/D−/− tumors lack chromosomal translocations with breakpoints at antigen receptor loci and are markedly aneuploid In agreement with previous analyses of PARP1−/−/scid mice,29P1−/−/D−/− mice are viable, but severely growth retarded (Supplementary Figure S1a) and succumb early in life to aggressive thymic lymphomas (Supplementary Figure S1b). Phenotypic analysis of lymphomas revealed large lymphoblasts (Supplementary Figure S1c) that typically expressed T-cell surface markers CD4 and/or CD8 (Supplementary Figure S1d).
DNA-PKcs−/− pro-T cells fail to process RAG-dependent hairpin-sealed coding ends at antigen-receptor loci.30 In a checkpoint-deficient background, these persistent DNA ends represent a potential substrate for oncogenic translocation.42,43To investigate a role for defective V(D)J recombination in the transformation of P1−/−/D−/− thymocytes, we painted chromosomes 14, 6 and 13 (containing the RAG targets T-cell receptor (TCR)α/δ, TCRβ and TCRγ loci, respectively) in metaphase spreads of P1−/−/D−/− lymphomas (Supplementary Table S1 and Supplementary Figure S2). These analyses indicated lack of clonal reciprocal translocations involving these chromosomes in five P1−/−/D−/− tumors (Supplementary Table S1 and Supplementary Figure S2a). Instead, chromosome painting revealed frequent trisomies and/or tetrasomies of chromosomes 14, 12, 6 and 13 in several P1−/−/D−/− tumors (Supplementary Figure S2a and b). Aneuploidy was not the result of our brief in vitro culturing, because analysis of DNA content by flow cytometry of propidium-iodide-stained fresh tumor cells similarly revealed several discrete ‘peaks’ in several tumors (Supplementary Figure S2c). Aneuploidy was in some cases associated with centrosome amplification (Supplementary Figure S2d) and appeared specific to tumor cells, as we failed to observe numerical abnormalities in metaphases spreads of primary P1−/−/D−/− B cells (Supplementary Figure S2e and f).
PARP1 deficiency leads to frequent mutation at the p53 locus in DNA-PKcs−/− thymocytes
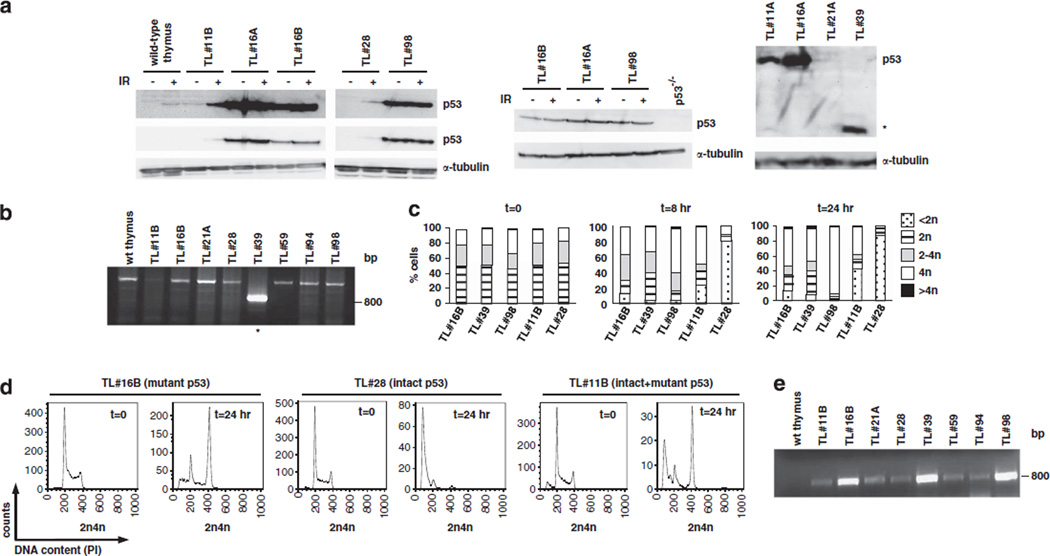
Lymphomas arising in scid mice show intact p53 function.23 Our observations above of aneuploidy and centrosome amplification, often associated to p53 deficiency,44 led us to investigate whether loss of PARP1 may result in a novel phenotype of acquired p53 inactivation during lymphomagenesis. Indeed, we detected high baseline p53 expression in 13/19 (68.4%) P1−/−/D−/− lymphomas by immunoblotting (Figure 1a and Supplementary Figure S3a). Consistent with a mutant form, p53 expression was not inducible by irradiation in 5/5 tumors with abnormal baseline expression (TL#16A, TL#16B, TL#98 (Figure 1a, left and middle blots) and TL#46 and TL#48 (Supplementary Figure S3a, right blot). In contrast, p53 was detected in TL#28 only after irradiation (Figure 1a, left blot), suggesting that this tumor maintains wild-type (wt) p53. Finally, TL#11B showed low-baseline p53 expression that was further induced upon irradiation (Figure 1a, left blot), suggesting that subpopulations containing wt and mutant p53 may coexist in this tumor (see below).
Figure 1.
Frequent mutation at the p53 locus in P1−/−/D−/− tumors. (a) Immunodetection of p53 in extracts from early-passage tumor cells (left and middle blot) or fresh tumor tissue (right blot). Extracts from a lymphoma arising in a p53 knock-out mouse were used as a negative control. For irradiated samples, cells were exposed to 5Gy of IR and harvested after 4h. Alpha-tubulin was detected as a loading control. (b) Reverse-transcriptase PCR for p53 (exons 2–11) on cDNA from wt thymocytes and eight P1−/−/D−/− lymphomas. (c, d) Early-passage tumor cells were exposed to 5Gy of IR, stained with propidium iodide at the indicated time points and analyzed by flow cytometry. The percentage of cells in each phase of the cell cycle was calculated using FloJo software and is plotted in c. Representative plots are shown in (d). (e) Reverse-transcriptase PCR for p19/Arf (exons 1–3) on cDNA from wt thymus and eight P1−/−/D−/− lymphomas.
In human and mouse cells, p53 is often inactivated by missense mutations in exons 5–9, encoding its DNA-binding domain.45Sequencing of exons 5–10 of tumor genomic DNA, as well as exons 2–11 of p53 in tumor cDNA, revealed alterations in five lymphomas (Supplementary Table S2). Three tumors contained a missense point mutation (one transition and two transversions). Of these, the mutations in TL#16B and TL#5 resulted in abnormal p53 accumulation (Figure 1a and Supplementary Figure 3Sa), whereas the mutant in TL#2 was not detectable by immunoblotting (Supplementary Figure S3a), consistent with previous findings that this mutation yields an unstable protein.46 The remaining two mutations were more complex. TL#98 harbored an in-frame 5–11 microindel in exon 7 (net change, 6-bp loss; Supplementary Table S2) that resulted in the loss of C235 and C239 (C238 and C241 in human p53), two cysteines that mediate protein dimerization in response to DNA damage. The second tumor (TL#39) contained a 410-bp deletion encompassing exons 5–8 that resulted in a truncated cDNA (Figure 1b, marked by an asterisk) and protein (Figure 1a, right blot, marked by asterisk; Supplementary Table S2).
Consistent with our molecular analysis, TL#16B, TL#39 and TL#98 with mutant p53 failed to undergo G1/S arrest in response to ionizing radiation (IR; Figure 1c and d). In contrast, TL#28 with IR-inducible p53 underwent rapid G1/S arrest and death after irradiation (notice sub-G1 peak in Figure 1d). Irradiation induced both sub-G1 and G2/M peaks in TL#11B (Figure 1c and d), consistent with the presence of subclones with differing p53 status within this tumor.
Deletion of p19/Arf results in inactivation of the p53 pathway in immortalized mouse cells.47 As expected,48 p19/Arf transcription was repressed in wt thymocytes (Figure 1e, lane 1). In contrast, p19/Arf cDNA of the expected size was present in 17/17 lymphomas examined (Figure 1e and data not shown), excluding a large deletion of this locus and indicating that p19 expression is dysregulated in P1−/−/D−/− lymphomas. Moreover, significantly higher p19/Arf expression was observed in TL#16B, TL#98 and TL#39 with p53 alterations (Figure 1e and Supplementary Figure S3b), in line with previous observations that p53 is a negative regulator of p19/Arf transcription.49 We conclude that PARP1 normally suppresses p53 mutations (and other alterations) that lead to p53 inactivation in DNA-PKcs-deficient thymocytes.
Marked defects in chromosome end capping in P1−/−/D−/− lymphomas
Both DNA-PKcs and PARP1 function in telomere maintenance.33,34,40,41 To investigate a genetic interaction in this chromatin context, we analyzed nine P1−/−/D−/− lymphomas by telomere fluorescent in-situ hybridization (T-FISH) (Figure 2). Expected patterns for TFs in the absence of DNA-PKcs are diagrammed in Figure 2a. These analyses revealed frequent p-to-p arm ‘chromosome-type’ (involving the two sister chromatids) TFs in eight out of nine tumors analyzed (Figure 2b for quantification; Figure 2c, white arrow, for an example). Although tumor cells typically harbored 1–2 TF, some contained multiple (>4) TFs per cell (see Supplementary Figure S4 for a representative example). In contrast, we observed no q-to-q or p-to-q ‘chromosome-type’ TFs or ‘chromatid-type’ TFs, likely because they are unstable upon division. However, we documented rare chromosomal rearrangements containing interstitial telomeric sequences (Figure 2d), which may have originated during breakage-fusion-bridge cycles of dicentrics, or by other mechanisms. Furthermore, TFs coexisted with Robertsonian translocation and reciprocal translocations in some tumor cells (see Figure 2c, yellow arrow, for a complex aberration resulting from the fusion of a TF and a Roberstonian translocation). Spectral karyotyping (SKY) analysis of tumor metaphases (n = 143 independent TFs in 9 tumors) demonstrated that all mouse chromosomes are represented (Figure 2e), consistent with previous findings that acrocentric chromosomes are preferentially involved in TFs.50
Figure 2.
Marked telomere instability in P1−/−/D−/− lymphomas. (a) Schematic representation of chromosomal aberrations due to TFs in the absence of DNA-PKcs. (b) Early-passage tumor metaphases were stained with a Cy3-labeled (TTAGGG)3 probe, counterstained with DAPI, and the number of p-to-p arm TFs per metaphase scored and plotted in histograms. (c) A p-to-p TF (white arrow) and a complex aberration involving a TF fused to a Roberstonian translocation (yellow arrow). (d) Examples of chromosomes with interstitial telomeric DNA in P1−/−/ D−/− lymphoblasts. (e) Tumor metaphases (n = 9 tumors) were analyzed by T-FISH/SKY to identify and catalog all TFs, and the frequency for each chromosome plotted. Clonal TFs were counted once. (f) To quantify telomere length in P1−/−/D−/− lymphomas, metaphase spreads from primary B cells obtained from a healthy P1−/−/D−/− mouse and from seven P1−/−/D−/− lymphomas were stained with a Cy3-labeled telomere probe, and telomere signal intensity quantified using TFL-Telo and normalized to kilobase using reference cell lines (see Materials and Methods for details). Histograms were generated by pooling data from 10 metaphases. For each sample, the average and s.d. of the telomere length and the total number of telomeres measured are indicated.
Finally, we assessed the effect of combined deficiency on average telomere length and length distribution using quantitative T-FISH (Figure 2f). We observed a wide range of average telomere length across individual tumors (from 29.7–96.7 kbp). As previously described for thymic lymphomas arising in Atm−/− mice,51 telomeres of P1−/−/D−/− lymphoblasts were in some cases longer than those of control primary P1−/−/D−/− cells of the same genetic background, possibly related to variable levels of telomerase expression in the transforming thymocytes. Telomere length followed in all cases an approximately normal distribution, suggesting that, like PARP1−/− /p53−/− lymphomas,20 P1−/−/ D−/− lymphomas likely maintain their telomeres via telomerase. Specifically, we did not observe subsets of very long or very short (‘signal free’) telomeres, a hallmark of telomere maintenance via telomerase-independent alternative lengthening of telomere.52
Clonal TFs in tumors with wt p53
TFs may promote tumorigenesis by interfering with normal segregation, leading to chromosome gains and/or losses and, if advantageous to the tumor, clonal selection. To start investigating the relationship between TFs and aneuploidy, we next performed sequential T-FISH/SKY analysis on nine P1−/−/D−/− lymphomas (Table 1, Figure 3 and Supplementary Figure S5 for additional examples). We observed two main patterns of TFs. Three tumors (TL#28, TL#11B and TL#45) contained one clonal TF and no or rare random TFs. Of these, TL#28 and TL#11B showed IR-inducible p53 expression, consistent with a functional 53 pathway. The p53 status of TL#45 is undetermined. This tumor had normal p53 DNA and cDNA sequence, and did not express an abnormal protein. However, it did not grow in culture and therefore, we were not able to perform a functional assay for p53 activation. A second group of tumors (TL#16A, TL#98, TL#21A, TL#16B, TL#39 and TL#11A) contained several clonal/subclonal fusions and frequent random fusions; five out of six tumors in this group harbored a mutant p53. Altogether, these findings suggest that p53 limits, but does not completely suppress TFs in transformed thymocytes.
Table 1.
Analysis of P/D thymic lymphomas by sequential T-FISH and SKY
| Tumor ID |
p53 status | Age at death (weeks) |
No. of cells analyzed |
Clonal rearrangementsa (no. of cells) |
Nonclonal rearrangementsa (no. of cells) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Reciprocal translocation |
End-to-end fusionsb |
Reciprocal translocation |
End-to-end fusionsb |
||||||
| TF | RT | TF | RT | ||||||
| TL#28 | Wild-type | 15 | 10 | None | f(14:7)10 | None | t(14:1)1; t(14:3)1 | None | r(2:15)4 |
| TL#45 | n.d. | 15 | 8 | None | f(4:15)7 | None | None | None | None |
| TL#11B | Wild-typec | 17 | 9 | t(12:15)9 | f(15:15)9,d | r(16:16)9 | None | f(17:17)1 | |
| TL#16A | Mutant | 21 | 10 | None | f(18:19)10; f(4:15)5; f(4:1)5 |
None | None | f(7:14)1; f(16:19)1; f(14:13)1; f(14:1)1; f(11:7)1; f(14:3)1; f(10:15)1; f(1:6)1 |
None |
| TL#16B | Mutant | 23 | 20 | None | None | None | t(1:15)1; t(8:7)1; t(18:14)1 |
f(2:10)8; f(1:17)7; f(4:14)1; f(14:8)1; f(15:9)1; f(7:11)1; f(10:17)1; f(8:7)1; f(Y:Y)1; f(12:5)1; f(16:2)1; f(3:12)1; f(18:14)1; f(16:5)1; f(19:6)1 |
None |
| TL#39 | Mutant | 32 | 11 | None | f(14:7)11 | None | None | f(15:18)5; f(1:4)3; f(4:4)2; f(10:2)1; f(15:16)1; f(19:14)1; f(11:2)1; f(11:4)1 |
None |
| TL#98 | Mutant | 24 | 12 | None | f(15:15)6 | None | t(14:7)3; t(12:7)1; t(14:12)1 |
f(15:14)4; f(4:4)2; f(15:Y)1; f(14:14)1; f(14:8)1; f(14:7)1; f(1:1)1; f(12:2)1 |
None |
| TL#11A | Mutant | 31 | 11 | None | f(6:10)10; f(15:15)11 |
None | None | f(19:7)2; f(10:X)2; f(10:2)2; f(10:7)1; f(4:8)1; f(10:19)1; f(12:15)1; |
None |
| TL#21A | n.d. | 26 | 22 | None | None | None | t(X:12)3; t(4:10)3; t(17:10)2; t(4:14)2; t(18:10)1; t(16:12)1; t(3:17)1; t(10:14)1; t(3:14)1 |
f(15:15)6; f(4:Y)3; f(15:18)6;f(15:Y)2; f(14:10)3; f(4:4)2f(9:10)1; f(13:2)1; f(4:17)1; f(14:14)1; f(7:9)1; f(3:14)1; f(15:6)1; f(11:6)1 |
None |
Abbreviations: TF, telomere fusion; n.d., not done; RT, Robertsonian translocation; P/D, PARP1 −/−/DNA-PKcs−/−; SKY, spectral karyotyping; T-FISH, telomere fluorescent in-situ hybridization.
Rearrangements present in ≥ 50% of the cells analyzed are shown as ‘clonal’(left side of the table); rearrangements present in <50% of the cells analyzed are shown as ‘nonclonal’ (right side of the table). This classification is arbitrary and the exact number of cells containing each rearrangement is indicated between parentheses.
Chromosomal end-to-end fusions were subdivided as TF or RT when telomere signals were present or absent at the fusion point, respectively.
This tumor also contained a subpopulation of p53 mutant cells (see text for details).
Clonal ‘hybrid’ fusion between a dysfunctional telomere in one chromosome 15 and a chromosomal break in another chromosome 15.
Figure 3.
Analysis of P1−/−/D−/− lymphomas by sequential T-FISH/SKY. (a) Representative metaphases of two tumors containing a single clonal TF; TL#28 was wt for p53. (b) Representative metaphases of two tumors containing several subclonal and random TFs. Both tumors are mutant for p53. (c) Distribution of individual chromosome gains (trisomy/tetrasomy) in nine P1−/−/D−/− lymphomas.
TFs result in aneuploidy
We next examined whether defective disjunction of fused chromosomes may contribute to alterations in chromosome copy number. Consistent with our chromosome painting studies above, the SKY analysis revealed aneuploidy in all P1−/−/D−/− lymphomas (Figure 3c for chromosome gains, Supplementary Figure S6a for chromosome losses), with chromosomes 14 and 15 involved most frequently (seven out of nine and nine out of nine tumors, respectively). As expected, aneuploidy was more marked in tumors with mutant p53, and chromosome gains were overall more frequent than chromosome losses.
To investigate whether chromosomes involved in TFs are more likely to be aneuploid, we determined, for each chromosome involved in a TF, whether it had normal or abnormal copy number (Table 2). As a control, we performed the same analysis on chromosomes not involved in TFs from the same metaphases (Table 2). These analyses demonstrated a strong correlation between participation in TF and trisomy. For example, clonal chromosome 14 trisomy observed in TL#28 (Figure 3c) correlated with a clonal f(7:14; Table 1 and Figure 3a); clonal chromosome 15 trisomy in TL#45 (Figure 3c) correlated with a clonal f(4:15; Table 1 and Figure 3a); and clonal chromosome 15 trisomy in TL#11B (Figure 3c) correlated with a clonal fusion of the telomeres of chromosome 15 to a DSB within another chromosome 15 (Table 1, Supplementary Figure S6b for an example). This tumor also displayed clonal 16 trisomy (Figure 3c) correlating with a Robertsonian translocation involving two chromosomes 16 (Table 1; Supplementary Figure S6b for an example), suggesting that both TFs and Robertsonian translocations cooperate in the generation of aneuploidy during tumor evolution. Similar analysis of p53 mutant tumors (n = 5 lymphomas, Table 2) further supported a strong correlation between TFs and aneuploidy. Although an average of 8.7% of nonfused chromosomes were aneuploid (range, 4–16%), an average of 46% of fused chromosomes in the same metaphases were aneuploid (range, 8–70.8%).This difference was statistically significant (P = 0.02) and suggests that the effects of p53 loss and TFs in the promotion of aneuploidy are separable.
Table 2.
Relationship between TFs and aneuploidy in P/D lymphomas. After SKY analysis, chromosomes were subdivided as ‘fused’ (engaged in a TF) or ‘nonfused’ (Schultz et al.,8)
| Tumor ID |
p53 status | No. of chr analyzed |
No. of fused chr |
No. of aneuploid (%) |
No. of nonfused chr |
No. of aneuploid (%) |
Fold- increaseb |
|---|---|---|---|---|---|---|---|
| TL#28 | Wild-type | 414 | 20 | 10 (50.00) | 394 | 21 (5.33) | 9.3 |
| TL#45 | n.d. | 332 | 14 | 9 (64.29) | 318 | 14 (4.40) | 14.5 |
| TL#11B | Mixeda | 380 | 20 | 20 (100.00) | 360 | 9 (2.50) | 40 |
| TL#16A | Mutant | 544 | 56 | 31 (55.36) | 488 | 79 (16.19) | 3.4 |
| TL#16B | Mutant | 826 | 56 | 5 (8.93) | 770 | 31 (4.03) | 2.2 |
| TL#39 | Mutant | 487 | 52 | 22 (42.31) | 435 | 29 (6.67) | 6.3 |
| TL#98 | Mutant | 553 | 36 | 19 (52.78) | 517 | 46 (8.90) | 5.9 |
| TL#11A | Mutant | 537 | 72 | 51 (70.83) | 466 | 37 (7.94) | 8.9 |
Abbreviations: chr, chromosome; TF, telomere fusion; n.d., not done; P/D, PARP1 −/−/DNA-PKcs−/−; SKY, spectral karyotyping.
This tumor contained subpopulations of p53 proficient and mutant cells.
Percentage of chromosomes fused and aneuploid/percentage of chromosomes nonfused and aneuploid in the same metaphases.
Loss of PARP1 aggravates the repair defect in DNA-PKcs-deficient primary cells
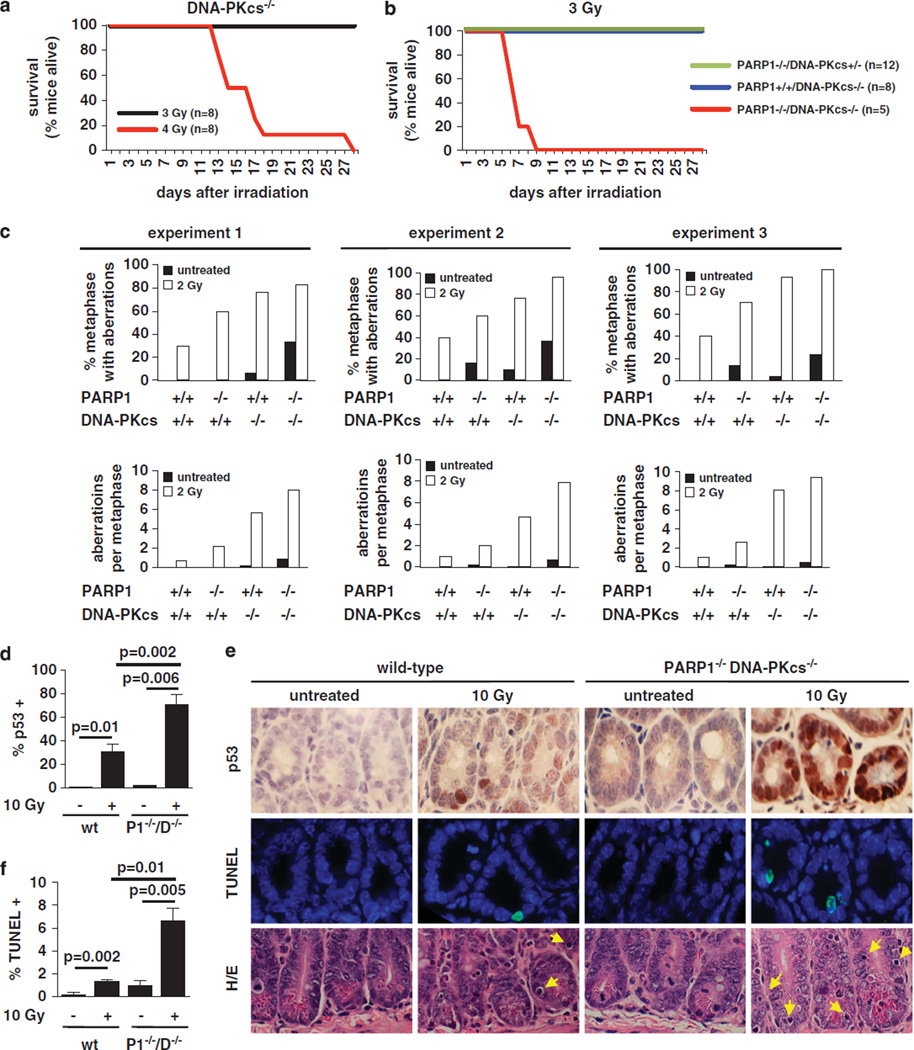
Our finding of p53 mutation is a subset of P1−/−/D−/− lymphomas led us to hypothesize that PARP1 and DNA-PKcs have nonoverlapping roles in DSB repair. In this context, some previous studies reported that PARP1 loss/inhibition increases genomic instability in DNA-PKcs-deficient cells,53,54 whereas others found otherwise.29,55 To address this question in our genetic model we first compared organismal radiosensitivity in P1−/−/D−/− and control mutants (Figure 4a and b). The lethal dose50 of our PARP1−/− strain is above 5Gy,56 whereas that of our DNA-PKcs−/− strain is approximately 3.5 Gy (Figure 4a). Consistent with these data, P1−/− and D−/− mice recovered from a single exposure to 3Gy (Figure 4b). In contrast, all P1 −/−/ D−/− mice succumbed within 9 days after IR (Figure 4b).
Figure 4.
PARP deficiency aggravates organismal radiosensitivity and genomic instability observed in a DNA-PKcs-deficient background. (a, b) Mice of the indicated genotypes were exposed to a single dose of IR and monitored clinically for 4 weeks. (c) In-vitro-activated wt, P1−/−, D−/− and P1−/−/D−/− B cells were exposed to 2Gy of IR, and after 24 h, fixed for T-FISH analysis. The percentage of metaphases containing at least one aberration and the average number of aberrations per metaphase is indicated. Three independent experiments are shown; n = 30 metaphases per experiment. (d–f) To assess the integrity of the p53-dependent checkpoint, wt and P1−/−/D−/− mice were exposed to 10Gy, and after 4 h, organs were fixed and embedded in paraffin. The percentage of p53+ cells was quantified by immunohistochemistry with a rabbit polyclonal anti-p53 antibody (CM5; d); representative examples are shown in e, top row. The percentage of apoptotic cells was quantified using the TUNEL assay (f); representative examples are shown in e, middle row. Hematoxylin eosin-stained sections of the same organ are shown in e, bottom row. Yellow arrows point to apoptotic figures. Bars represent the average and s.d. of three mice.
To determine whether PARP1 deficiency increases radiosensitivity in DNA-PKcs−/− via its functions in the maintenance of genomic integrity, we next quantified chromosomal aberrations on metaphases of primary P1−/−/D−/− and control lymphocytes via T-FISH, as described57 (see Materials and Methods, and for the generation of B cells in a DNA-PKcs-deficient background). The frequency of metaphases containing aberrations was 0.0 ±0.0, 10 ±8.8, 6.6 ± 3.3 and 31.1 ± 6.9 for wt, P1−/−, D−/− and P1−/−/ D−/− cultures, respectively, (average and s.d. of 3 independent cultures per genotype; n = 30 metaphases per culture; Figure 4c; Supplementary Table S3 for data in individual cultures; Supplementary Figure S7a for examples of spontaneous aberrations in P1−/−/D−/− cells). These differences were statistically significant (P = 0.03 for P1−/−/D−/− vs P1−/−; P = 0.01 for P1−/−/D−/− vs D−/− cultures). Similarly, the number of chromosomal aberrations per metaphase in the same cultures was 0±0, 0.13 ±0.13, 0.07 ±0.03 and 0.64 ±0.15 (Figure 4c, Supplementary Table S3; P = 0.01 for P1−/−/D−/− vs P1−/−; P = 0.02 for P1−/−/D−/− vs D−/− cultures). In addition, the number of metaphases containing aberrations and the frequency of aberrations per metaphase were also increased in double mutants exposed to 2Gy of IR, relative to single mutants analyzed in parallel (Figure 4c, Supplementary Table S3 and Supplementary Figure S7b for representative examples). As in D−/− B cells,16 TFs were observed rarely in primary P1−/−/D−/− cells (10 TFs in a total of 360 P1−/−/D−/− primary B-cell metaphases analyzed, or 0.02 TFs per metaphase; not shown).
In activated B-cell cultures deficient for repair factors, a subset of broken DNA ends is aberrantly rejoined to a DSB in another chromosome to form a translocation.57 To investigate the effect of combined deficiency for PARP1 and DNA-PKcs on aberrant rejoining, we also classified DNA ends in wt, P1−/−, D−/− and P1−/−/D−/− B-cell metaphases as either ‘free (nontranslocated) ends’ or ‘translocated ends’. These studies were done in metaphases obtained 24 h after exposure to 2Gy, to increase the number of translocation acceptors. We found that approximately one in every four broken DNA ends is aberrantly rejoined to form a translocation in these cultures, regardless of genotype (Supplementary Table S4 and Supplementary Figure 7Sa, for example, of nontranslocated and translocated broken DNA ends). Thus, primary lymphocytes can rejoin broken DNA ends aberrantly to form a translocation in the absence of both PARP1 and DNA-PKcs.
The increased radiosensitivity and genomic instability observed above may reflect on a greater defect in repair per se and/or deficient checkpoint activation. To assess p53 activation in double mutant cells, we quantified p53 stabilization and apoptosis in intestinal crypt epithelial cells of wt and P1−/−/D−/− mice 4h after exposure to 10Gy (Figure 4d–f). Similar to previous findings in scid crypts26 and our own analysis of D−/− crypts (not shown), the number of p53+ cells was markedly increased in double mutant cells relative to their wt counterparts (Figure 4d and e). Furthermore, the frequency of apoptotic cells was similarly increased (Figure 4e and f). These observations were not specific to intestinal crypt epithelial cells, because residual lymphoid populations in P1−/−/D−/− thymi (Supplementary Figure S8) and spleens (Supplementary Figure S9) showed markedly enhanced p53 stabilization and apoptosis after irradiation. Altogether, these findings suggest that loss of PARP1 aggravates the DSB repair defect in DNA-PKcs−/− cells.
DISCUSSION
PARP1 and DNA-PKcs show weak tumor suppressor activity in the mouse. Yet, their combined deficiency leads to aggressive lymphomagenesis. Our analysis of chromatin context-dependent functions has now elucidated two main molecular mechanisms underlying this phenotype. First, loss of PARP1 markedly increases the selective pressure for inactivating mutations at the p53 locus (and possibly other loci) in DNA-PKcs-deficient cells, effectively disabling cellular checkpoints and making them tolerant to genomic instability and aneuploidy. Second, telomere dysfunction-induced structural and numerical abnormalities fuel genomic instability and clonal evolution in this model.
Clonal reciprocal translocations, involving DSBs at TCR loci and/or ‘random’ DSBs, are the hallmark of thymic lymphomas arising in DSB-repair-deficient backgrounds.43,58–60 However, despite well-known roles for DNA-PKcs in the processing of RAG-dependent coding ends and a subset of ‘random’ DNA ends,16,61 we find that P1−/−/D−/− lymphomas typically lack clonal reciprocal translocations involving antigen-receptor loci or DSBs elsewhere. Instead, these tumors were invariably aneuploid. At least two independent mechanisms contributed to aneuploidy: mutations in p53 and defects in disjunction due to TFs.
In contrast to intact p53 status in scid lymphomas,23 we find that most P1−/−/D−/− lymphomas display abnormal p53 accumulation and/or sequence alterations. In line with previous studies in human cancers,62 p53 mutations clustered in exons 5–8. Moreover, three out of five tumors contained missense point mutations previously shown to disrupt p53 function in human cancers of diverse lineages. Significantly, p53 inactivation was caused by a different mechanism in the remaining two lymphomas. For TL#98, we observed an in-frame microindel, whereas TL#39 harbored a 410-bp deletion that resulted in a truncated protein lacking the DNA-binding domain. These alterations are rare in human tumors with p53 mutation.63Although the number of mutations analyzed is small, these findings suggest that the spectrum of p53 mutations may differ in tumors arising in DSB-repair-proficient and -deficient backgrounds. In the latter case, two or more persistent DSBs may result in repair by deletional recombination or by a deletion/ insertion mechanism using nearby sequences.63 In the future, we will develop alternative experimental systems to quantify the frequency of these complex alterations and the components of the pathway that mediates them.
P1−/−/D−/− tumors also show marked telomere dysfunction, including frequent p-to-p arm TFs. This observation indicates that PARP1 is dispensable for the rejoining of DNA-PKcs-deficient telomeres, although PARP1 deficiency may modulate the frequency of fusions or the nature of the pathway mediating the fusions, a notion that will be addressed in future studies. All chromosomes participated in these fusion events, as expected from their acrocentric structure.50 Our finding that tumors with mutant p53 tend to have more frequent TFs suggests that p53 may have a major role in limiting telomere dysfunction in P1−/−/ D−/− cells during in vivo tumorigenesis, as previously observed in mice with critically short telomeres.64 Nevertheless, tumors with functional p53 and/or modest aneuploidy harbored one clonal TF, indicating that TFs can occur and be propagated even in p53-competent cells. More importantly, chromosomes involved in TFs were typically trisomic in these tumors, suggesting that they represent a main mechanism in the generation of aneuploidy. Similarly, we found that chromosomes involved in TFs in p53 mutant tumors have a significantly higher probability of being aneuploid relative to nonfused chromosomes in the same cells, suggesting that TFs and loss of p53 represent independent mechanisms leading to aneuploidy. Finally, p53 status may modulate telomere function in P1−/−/D−/− tumors by alternative mechanisms, including the suppression of breakage-fusion-bridge cycles,65 the suppression of polyploidization66 or others.
In summary, loss of PARP1 in a DNA-PKcs-deficient background results in novel molecular phenotypes not observed in single mutants. Although mice deficient for DNA-PKcs develop lymphoma at low frequency,24,67 these lymphomas are proficient for p53,23 in marked contrast to frequently acquired p53 inactivation observed in double mutants. Moreover, our findings that PARP1 and DNA-PKcs cooperate to suppress deletional mutation in a subset of tumors and chromosomal breaks and translocations in primary cells point to nonoverlapping roles in DSB repair and rearrangement suppression. Future studies will identify the cellular pathways that mediate aberrant repair in cells deficient for PARP1 and DNA-PKcs, and establish the relative contribution of abnormal repair in cis versus in trans to genomic instability in their absence. Finally, our finding that clonal TFs strongly correlate with clonal trisomies of the fused chromosomes, regardless of p53 status, strongly suggests that TFs represent an ongoing source of aneuploidy, a mechanism that may contribute to tumor clonal evolution and resistance to therapy.
MATERIALS AND METHODS
Animal breeding and irradiation
Mice deficient for PARP115 and DNA-PKcs30 were previously described. All strains are in pure 129/Sv background. To bypass developmental block at the pro-B cells stage in the absence of DNA-PKcs and obtain mature B cells, PARP1−/− mice were bred to DNA-PKcs−/− mice containing pre-rearranged ‘knocked-in’ immunoglobulin heavy chain and kappa light chain transgenes, as described.16 For irradiation experiments, mice were exposed to a 137Cs source (GammaCell40, Theratronics, Otatawa, ON, Canada; rate, 0.5Gy/min) and monitored clinically for 28 days after irradiation. All studies were conducted in accordance with the NIH guidelines and Institutional Animal Care and Use Committee-approved protocols.
Cell culture
For tumor metaphase preparation, fresh tumor tissue was disaggregated mechanically and cultured briefly in the presence of interleukin-2 (100IU/ml) and interleukin-7 (5ng/ml). B cells were purified and activated in vitro as previously described.57
Telomere FISH
To prepare metaphases, cells were incubated in 0.1 µg/ml colcemid (KaryoMAX, Gibco, Gaithersburg, MD, USA) for 4h, swollen in 30 mm sodium citrate for 25min at 37 °C and fixed in methanol/acetic acid (3/1). Metaphases were hybridized with a telomere probe as described57 and mounted in Vectashield with DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA, USA). Images were obtained using a Zeiss Axioplan Imager Z.1 microscope (Zeiss, Gottingen, Germany) equipped with a Zeiss AxioCam and an HXP120 mercury lamp (Jena GmbH, Gottingen, Germany) and dedicated software (Zeiss Axiovision Rel 4.6). For quantitative analysis of telomere length, we employed TFL-Telo software (kind gift of Dr Peter Lansdorp) and normalized arbitrary units of fluorescence to kilobase, using two mouse lymphoma cell lines with known telomere length68 (kind gift of Dr Predrag Slijepcevic).
Chromosome paint analysis
Paints to mouse chromosomes 12 and 14 were purchased from Cambio (Cambridge, UK), and paints to chromosomes 6 and 13 were purchased from Applied Spectral Imaging (Applied Spectral Imaging, Carlsbad, CA, USA). Hybridization was performed following manufacturer’s instructions and images were acquired using a Zeiss Axioplan Imager Z.1 microscope.
SKY analysis
Metaphases were first hybridized with a telomere probe as described above, and after analysis, rehybridized with Sky Paint probes (Applied Spectral Imaging), following the manufacturer’s instructions. Images were acquired using a Zeiss Axioskop microscope and analyzed using dedicated software (Applied Spectral Imaging). T-FISH and SKY images were directly compared. End-to-end chromosomal fusions were classified as Robertsonian translocation or TF according to the absence or presence of telomere signals at the fusion point, respectively.
Immunoblotting
Cells were washed in phosphate-buffered saline and resuspended in RIPA buffer supplemented with protease inhibitors, sodium orthovanadate and [β-glycerophosphate. Extracts were resolved by SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes and incubated with antibodies to mouse p53 (pAb240, Santa Cruz, Santa Cruz, CA, USA) and a-tubulin (Sigma, Saint Louis, MO, USA) following standard procedures.
Amplification and sequencing of genomic DNA and cDNA
Genomic DNA was obtained after phenol/chloroform extraction of tumor tissue and exons 5–10 of p53, containing mutational hotspots, were amplified using published primers69 and the following conditions: 94 °C 30 s, 55 °C 30 s, 72 °C 45 s, for 40 cycles. RNA was extracted from tumor tissues using Trizol, and reverse transcribed using T17 primer and Superscript III (Invitrogen, Carlsbad, CA, USA), followed by incubation with RNAse H for 20 min at 37 °C. p53 cDNA (exons 2–11) or p19/Arf cDNA (exons 1–3) were amplified using published primers69 and the following PCR conditions: 94 °C 20s, 65 °C 60s, 72 °C 120s, for 40 cycles. PCR products were purified (Qiagen, Valencia, CA, USA) and sequenced at the Johns Hopkins University DNA Analysis Facility.
Cell cycle analysis
Cells were fixed in cold 70% ethanol, permeabilized in Triton-X, digested with RNAse A and stained with propidium iodide. Data was acquired using a FACSCalibur (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo software (Tree Star Inc, Ashland, OR, USA).
Detection of p53 by immunohistochemistry
Mice were exposed to 10Gy of IR using a 137Cs source (GammaCell40). After 4 h, organs were fixed in buffered formalin and embedded in paraffin, following standard procedures. Immunostaining was carried out with a rabbit polyclonal antibody to p53 (CM5, Novocastra, Newcastle upon Tyne, UK; 1:500) following the manufacturer’s instructions, except that antigen retrieval was performed by boiling samples in 10mm sodium citrate pH = 6.0for 15min in a microwave oven. Tissues were counterstained with hematoxylin, and the number of p53+ and p53− cells in 5–15 crypts per slide were counted. At least three mice per genotype and condition were analyzed.
Detection of apoptotic cell by the terminal deoxynucleotidyl transferase dUTP nick-end-labeling (TUNEL) assay
Deparaffinized sections were digested with proteinase K, incubated with TdT and fluorescein-labeled dUTP (In situ Cell Death Detection Kit, Roche, Branford, CT, USA) and mounted in Vectashield with DAPI. Images were captured using a Zeiss Axioplan Imager Z.1 microscope and analyzed with Zeiss Axiovision Rel 4.6 software.
Statistical analysis
Statistical significance was calculated using Student’s t-test. At least three data points obtained from at least three independent experiments were used for each calculation.
Supplementary Material
ACKNOWLEDGEMENTS
We are most grateful to Carol Greider and Margaret Strong for assistance with the SKY analysis; Fred Alt, Ted Dawson and Klaus Rajewsky for mouse models; Bonnie Gambichler at the Histopathology Core and Ada Tam at the Flow Cytometry Core for excellent technical assistance; the Sidney Kimmel Comprehensive Cancer Center (SKCCC) Animal Care Facility for continued support; and Alan Meeker and Susan Bailey for critical reading of the manuscript.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Hottiger MO, Hassa PO, Luscher B, Schuler H, Koch-Nolte F. Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends Biochem Sci. 2010;35:208–219. doi: 10.1016/j.tibs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 3.Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5:387–393. doi: 10.1016/j.molonc.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pleschke JM, Kleczkowska HE, Strohm M, Althaus FR. Poly(ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J Biol Chem. 2000;275:40974–40980. doi: 10.1074/jbc.M006520200. [DOI] [PubMed] [Google Scholar]
- 5.Ahel D, Horejsi Z, Wiechens N, Polo SE, Garcia-Wilson E, Ahel I, et al. Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science. 2009;325:1240–1243. doi: 10.1126/science.1177321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant HE, Petermann E, Schultz N, Jemth AS, Loseva O, Issaeva N, et al. PARP is activated at stalled forks to mediate Mre11-dependent replication restart and recombination. EMBO J. 2009;28:2601–2615. doi: 10.1038/emboj.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YG, Cortes U, Patnaik S, Jasin M, Wang ZQ. Ablation of PARP-1 does not interfere with the repair of DNA double-strand breaks, but compromises the reactivation of stalled replication forks. Oncogene. 2004;23:3872–3882. doi: 10.1038/sj.onc.1207491. [DOI] [PubMed] [Google Scholar]
- 8.Schultz N, Lopez E, Saleh-Gohari N, Helleday T. Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res. 2003;31:4959–4964. doi: 10.1093/nar/gkg703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ariumi Y, Masutani M, Copeland TD, Mimori T, Sugimura T, Shimotohno K, et al. Suppression of the poly(ADP-ribose) polymerase activity by DNA-dependent protein kinase in vitro. Oncogene. 1999;18:4616–4625. doi: 10.1038/sj.onc.1202823. [DOI] [PubMed] [Google Scholar]
- 10.Ruscetti T, Lehnert BE, Halbrook J, Le Trong H, Hoekstra MF, Chen DJ, et al. Stimulation of the DNA-dependent protein kinase by poly(ADP-ribose) polymerase. J Biol Chem. 1998;273:14461–14467. doi: 10.1074/jbc.273.23.14461. [DOI] [PubMed] [Google Scholar]
- 11.Smith GC, Jackson SP. The DNA-dependent protein kinase. Genes Dev. 1999;13:916–934. doi: 10.1101/gad.13.8.916. [DOI] [PubMed] [Google Scholar]
- 12.Meek K, Dang V, Lees-Miller SP. DNA-PK: the means to justify the ends? Adv Immunol. 2008;99:33–58. doi: 10.1016/S0065-2776(08)00602-0. [DOI] [PubMed] [Google Scholar]
- 13.Taccioli GE, Amatucci AG, Beamish HJ, Gell D, Xiang XH, Torres Arzayus MI, et al. Targeted disruption of the catalytic subunit of the DNA-PK gene in mice confers severe combined immunodeficiency and radiosensitivity. Immunity. 1998;9:355–366. doi: 10.1016/s1074-7613(00)80618-4. [DOI] [PubMed] [Google Scholar]
- 14.de Murcia JM, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M, et al. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci USA. 1997;94:7303–7307. doi: 10.1073/pnas.94.14.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZQ, Auer B, Stingl L, Berghammer H, Haidacher D, Schweiger M, et al. Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev. 1995;9:509–520. doi: 10.1101/gad.9.5.509. [DOI] [PubMed] [Google Scholar]
- 16.Franco S, Murphy MM, Li G, Borjeson T, Boboila C, Alt FW. DNA-PKcs and Artemis function in the end-joining phase of immunoglobulin heavy chain class switch recombination. J Exp Med. 2008;205:557–564. doi: 10.1084/jem.20080044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata A, Kamada N, Masumura K, Nohmi T, Kobayashi S, Teraoka H, et al. Parp-1 deficiency causes an increase of deletion mutations and insertions/rearrangements in vivo after treatment with an alkylating agent. Oncogene. 2005;24:1328–1337. doi: 10.1038/sj.onc.1208289. [DOI] [PubMed] [Google Scholar]
- 18.Shibata A, Maeda D, Ogino H, Tsutsumi M, Nohmi T, Nakagama H, et al. Role of Parp-1 in suppressing spontaneous deletion mutation in the liver and brain of mice at adolescence and advanced age. Mutat Res. 2009;664:20–27. doi: 10.1016/j.mrfmmm.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Simbulan-Rosenthal CM, Haddad BR, Rosenthal DS, Weaver Z, Coleman A, Luo R, et al. Chromosomal aberrations in PARP(−/𢈒) mice: genome stabilization in immortalized cells by reintroduction of poly(ADP-ribose) polymerase cDNA. Proc Natl Acad Sci USA. 1999;96:13191–13196. doi: 10.1073/pnas.96.23.13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong WM, Hande MP, Lansdorp PM, Wang ZQ. DNA strand break-sensing molecule poly(ADP-Ribose) polymerase cooperates with p53 in telomere function, chromosome stability, and tumor suppression. Mol Cell Biol. 2001;21:4046–4054. doi: 10.1128/MCB.21.12.4046-4054.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espejel S, Klatt P, Menissier-de Murcia J, Martin-Caballero J, Flores JM, Taccioli G, et al. Impact of telomerase ablation on organismal viability, aging, and tumor-igenesis in mice lacking the DNA repair proteins PARP-1 Ku86, or DNA-PKcs. J Cell Biol. 2004;167:627–638. doi: 10.1083/jcb.200407178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurley KE, Vo K, Kemp CJ. DNA double-strand breaks, p53, and apoptosis during lymphomagenesis in scid/scid mice. Cancer Res. 1998;58:3111–3115. [PubMed] [Google Scholar]
- 24.Espejel S, Martin M, Klatt P, Martin-Caballero J, Flores JM, Blasco MA. Shorter telomeres, accelerated ageing and increased lymphoma in DNA-PKcs-deficient mice. EMBO Rep. 2004;5:503–509. doi: 10.1038/sj.embor.7400127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piskunova TS, Yurova MN, Ovsyannikov AI, Semenchenko AV, Zabezhinski MA, Popovich IG, et al. Deficiency in poly(ADP-ribose) polymerase-1 (PARP-1) accelerates aging and spontaneous carcinogenesis in mice. Curr Gerontol Geriatr Res. 2008:754190. doi: 10.1155/2008/754190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurley KE, Kemp CJ. p53 induction, cell cycle checkpoints, and apoptosis in DNAPK-deficient scid mice. Carcinogenesis. 1996;17:2537–2542. doi: 10.1093/carcin/17.12.2537. [DOI] [PubMed] [Google Scholar]
- 27.Blunt T, Finnie NJ, Taccioli GE, Smith GC, Demengeot J, Gottlieb TM, et al. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995;80:813–823. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- 28.Kirchgessner CU, Patil CK, Evans JW, Cuomo CA, Fried LM, Carter T, et al. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. 1995;267:1178–1183. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- 29.Morrison C, Smith GC, Stingl L, Jackson SP, Wagner EF, Wang ZQ. Genetic interaction between PARP and DNA-PK in V(D)J recombination and tumorigenesis. Nat Genet. 1997;17:479–482. doi: 10.1038/ng1297-479. [DOI] [PubMed] [Google Scholar]
- 30.Gao Y, Chaudhuri J, Zhu C, Davidson L, Weaver DT, Alt FW. A targeted DNA-PKcs-null mutation reveals DNA-PK-independent functions for KU in V(D)J recombination. Immunity. 1998;9:367–376. doi: 10.1016/s1074-7613(00)80619-6. [DOI] [PubMed] [Google Scholar]
- 31.Mills KD, Ferguson DO, Alt FW. The role of DNA breaks in genomic instability and tumorigenesis. Immunol Rev. 2003;194:77–95. doi: 10.1034/j.1600-065x.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 32.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 33.Bailey SM, Meyne J, Chen DJ, Kurimasa A, Li GC, Lehnert BE, et al. DNA double-strand break repair proteins are required to cap the ends of mammalian chromosomes. Proc Natl Acad Sci USA. 1999;96:14899–14904. doi: 10.1073/pnas.96.26.14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goytisolo FA, Samper E, Edmonson S, Taccioli GE, Blasco MA. The absence of the DNA-dependent protein kinase catalytic subunit in mice results in anaphase bridges and in increased telomeric fusions with normal telomere length and G-strand overhang. Mol Cell Biol. 2001;21:3642–3651. doi: 10.1128/MCB.21.11.3642-3651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey SM, Cornforth MN, Kurimasa A, Chen DJ, Goodwin EH. Strand-specific post-replicative processing of mammalian telomeres. Science. 2001;293:2462–2465. doi: 10.1126/science.1062560. [DOI] [PubMed] [Google Scholar]
- 36.Espejel S, Franco S, Sgura A, Gae D, Bailey SM, Taccioli GE, et al. Functional interaction between DNA-PKcs and telomerase in telomere length maintenance. Embo J. 2002;21:6275–6287. doi: 10.1093/emboj/cdf593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maser RS, Wong KK, Sahin E, Xia H, Naylor M, Hedberg HM, et al. DNA-dependent protein kinase catalytic subunit is not required for dysfunctional telomere fusion and checkpoint response in the telomerase-deficient mouse. Mol Cell Biol. 2007;27:2253–2265. doi: 10.1128/MCB.01354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey SM, Cornforth MN, Ullrich RL, Goodwin EH. Dysfunctional mammalian telomeres join with DNA double-strand breaks. DNA Repair (Amst) 2004;3:349–357. doi: 10.1016/j.dnarep.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Guidos CJ, Williams CJ, Grandal I, Knowles G, Huang MT, Danska JS. V(D)J recombination activates a p53-dependent DNA damage checkpoint in scid lymphocyte precursors. Genes Dev. 1996;10:2038–2054. doi: 10.1101/gad.10.16.2038. [DOI] [PubMed] [Google Scholar]
- 40.Gomez M, Wu J, Schreiber V, Dunlap J, Dantzer F, Wang Y, et al. PARP1 Is a TRF2-associated poly(ADP-ribose)polymerase and protects eroded telomeres. Mol Biol Cell. 2006;17:1686–1696. doi: 10.1091/mbc.E05-07-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.d’Adda di Fagagna F, Hande MP, Tong WM, Lansdorp PM, Wang ZQ, Jackson SP. Functions of poly(ADP-ribose) polymerase in controlling telomere length and chromosomal stability. Nat Genet. 1999;23:76–80. doi: 10.1038/12680. [DOI] [PubMed] [Google Scholar]
- 42.Zhu C, Mills KD, Ferguson DO, Lee C, Manis J, Fleming J, et al. Unrepaired DNA breaks in p53-deficient cells lead to oncogenic gene amplification subsequent to translocations. Cell. 2002;109:811–821. doi: 10.1016/s0092-8674(02)00770-5. [DOI] [PubMed] [Google Scholar]
- 43.Liyanage M, Weaver Z, Barlow C, Coleman A, Pankratz DG, Anderson S, et al. Abnormal rearrangement within the alpha/delta T-cell receptor locus in lymphomas from Atm-deficient mice. Blood. 2000;96:1940–1946. [PubMed] [Google Scholar]
- 44.Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF. Abnormal centro-some amplification in the absence of p53. Science. 1996;271:1744–1747. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- 45.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 46.Jordan JJ, Inga A, Conway K, Edmiston S, Carey LA, Wu L, et al. Altered-function p53 missense mutations identified in breast cancers can have subtle effects on transactivation. Mol Cancer Res. 2010;8:701–716. doi: 10.1158/1541-7786.MCR-09-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 48.Miyazaki M, Miyazaki K, Itoi M, Katoh Y, Guo Y, Kanno R, et al. Thymocyte proliferation induced by pre-T cell receptor signaling is maintained through polycomb gene product Bmi-1-mediated Cdkn2a repression. Immunity. 2008;28:231–245. doi: 10.1016/j.immuni.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Robertson KD, Jones PA. The human ARF cell cycle regulatory gene promoter is a CpG island which can be silenced by DNA methylation and down-regulated by wild-type p53. Mol Cell Biol. 1998;18:6457–6473. doi: 10.1128/mcb.18.11.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stimpson KM, Song IY, Jauch A, Holtgreve-Grez H, Hayden KE, Bridger JM, et al. Telomere disruption results in non-random formation of de novo dicentric chromosomes involving acrocentric human chromosomes. PLoS Genet. 2010;6:e1001061. doi: 10.1371/journal.pgen.1001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi L, Strong MA, Karim BO, Huso DL, Greider CW. Telomere fusion to chromosome breaks reduces oncogenic translocations and tumour formation. Nat Cell Biol. 2005;7:706–711. doi: 10.1038/ncb1276. [DOI] [PubMed] [Google Scholar]
- 52.Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11:319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 53.Veuger SJ, Curtin NJ, Smith GC, Durkacz BW. Effects of novel inhibitors of poly(-ADP-ribose) polymerase-1 and the DNA-dependent protein kinase on enzyme activities and DNA repair. Oncogene. 2004;23:7322–7329. doi: 10.1038/sj.onc.1207984. [DOI] [PubMed] [Google Scholar]
- 54.Veuger SJ, Curtin NJ, Richardson CJ, Smith GC, Durkacz BW. Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res. 2003;63:6008–6015. [PubMed] [Google Scholar]
- 55.Mitchell J, Smith GC, Curtin NJ. Poly(ADP-Ribose) polymerase-1 and DNA-dependent protein kinase have equivalent roles in double strand break repair following ionizing radiation. Int J Radiat Oncol Biol Phys. 2009;75:1520–1527. doi: 10.1016/j.ijrobp.2009.07.1722. [DOI] [PubMed] [Google Scholar]
- 56.Orsburn B, Escudero B, Prakash M, Gesheva S, Liu G, Huso D, et al. Differential requirement for H2AX and 53BP1 in organismal development and genome maintenance in the absence of poly(ADP)ribosyl polymerase 1. Mol Cell Biol. 2010;30:2341–2352. doi: 10.1128/MCB.00091-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franco S, Gostissa M, Zha S, Lombard DB, Murphy MM, Zarrin AA, et al. H2AX prevents DNA breaks from progressing to chromosome breaks and transloca-tions. Mol Cell. 2006;21:201–214. doi: 10.1016/j.molcel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Bassing CH, Suh H, Ferguson DO, Chua KF, Manis J, Eckersdorff M, et al. Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell. 2003;114:359–370. doi: 10.1016/s0092-8674(03)00566-x. [DOI] [PubMed] [Google Scholar]
- 59.Morales JC, Franco S, Murphy MM, bassing CH, Mills KD, Adams MM, et al. 53BP1 and p53 synergize to suppress genomic instability and lymphomagenesis. Proc Natl Acad Sci USA. 2006;103:3310–3315. doi: 10.1073/pnas.0511259103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Celeste A, Difilippantonio S, Difilippantonio MJ, Fernandez-Capetillo O, Pilch DR, Sedelnikova OA, et al. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell. 2003;114:371–383. doi: 10.1016/s0092-8674(03)00567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109(Suppl):S45–S55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 62.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 63.Scaringe WA, Li K, Gu D, Gonzalez KD, Chen Z, Hill KA, et al. Somatic microindels in human cancer: the insertions are highly error-prone and derive from nearby but not adjacent sense and antisense templates. Hum Mol Genet. 2008;17:2910–2918. doi: 10.1093/hmg/ddn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 65.Maser RS, DePinho RA. Connecting chromosomes, crisis, and cancer. Science. 2002;297:565–569. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- 66.Davoli T, Denchi EL, de Lange T. Persistent telomere damage induces bypass of mitosis and tetraploidy. Cell. 2010;141:81–93. doi: 10.1016/j.cell.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bosma MJ, Carroll AM. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- 68.McIlrath J, Bouffler SD, Samper E, Cuthbert A, Wojcik A, Szumiel I, et al. Telomere length abnormalities in mammalian radiosensitive cells. Cancer Res. 2001;61:912–915. [PubMed] [Google Scholar]
- 69.Stracker TH, Couto SS, Cordon-Cardo C, Matos T, Petrini JH. Chk2 suppresses the oncogenic potential of DNA replication-associated DNA damage. Mol Cell. 2008;31:21–32. doi: 10.1016/j.molcel.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.