Abstract
CTLA-4 is a key immune checkpoint in maintaining self-tolerance, which can be co-opted by cancer to evade immune attack. In Science, Kuehn et al. (2014) describe clinical manifestations from inherited heterozygous CTLA4 mutations, and some are reminiscent of immune-related consequences from anti-CTLA-4 cancer therapy.
Cytotoxic T lymphocyte antigen 4 (CTLA-4) is an essential negative regulator of peripheral T cell function. CTLA-4 and the T cell costimulatory receptor CD28 share the ligands CD80 and CD86, but CTLA-4 is their high-affinity receptor. CTLA-4 is expressed inducibly on CD4+ Foxp3− conventional T (Tconv) cells after activation and constitutively on CD4+ Foxp3+ regulatory T (Treg) cells. The critical inhibitory function of CTLA-4 has been revealed by the rapidly fatal inflammatory phenotype of CTLA-4-null (Ctla4−/−) mice, which spontaneously develop massive T cell expansion with multiorgan lymphocytic infiltration and tissue destruction (Tivol et al., 1995). The similarity of this phenotype to systemic autoimmune disease has catalyzed studies investigating the role of CTLA-4 in T cell tolerance and autoimmunity. CTLA-4 can act on Tconv and Treg cells and is a mediator of Treg cell suppressive function.
Although the crucial roles for CTLA-4 in inhibiting T cell responses and mediating peripheral T cell tolerance are firmly established, there remain many questions about the mechanisms by which CTLA-4 exerts its critical immunoregulatory functions. Data support both cell-intrinsic and cell-extrinsic mechanisms. Antibody-mediated ligation of CTLA-4 (which triggers CTLA-4 crosslinking) inhibits CD3-and CD28-mediated T cell stimulation, supporting cell-intrinsic inhibitory effects. The CTLA-4 cytoplasmic tail has a potentially inhibitory motif, which can negate signals from the T cell receptor (TCR) and from CD28 by recruiting phosphatases. Recent work has shown that kinase PKC-η constitutively binds to the CTLA-4 cytoplasmic domain in Treg cells and that this interaction is an important mediator of CTLA-4 function in these cells (Kong et al., 2014). However, other data support cell-extrinsic mechanisms of CTLA-4 function. CTLA-4 could exert cell-extrinsic effects by attenuating CD80 and/or CD86 expression on antigen-presenting cells (APCs), either indirectly by causing their downregulation or directly by removing them from APCs by transendocytosis, thereby reducing their availability for CD28 engagement and costimulation. In addition, CTLA-4 could reverse signal into APCs through CD80 and/or CD86 and induce the immunosuppressive enzyme indoleamine 2,3 dioxygenase.
The seminal demonstration that CTLA-4 blockade could reverse immunosuppression in the tumor microenvironment and mediate the regression of established cancers in murine models (Leach et al., 1996) led to the clinical development of antibodies blocking CTLA-4 in patients with advanced malignancies. In 2011, the CTLA-4 monoclonal antibody ipilimumab became a standard therapy for patients with advanced metastatic melanoma on the basis of phase 3 clinical trial results showing improved overall survival (Hodi et al., 2010). This established a new treatment paradigm for cancer, namely, blocking immune checkpoints to ‘‘release the brakes’’ on antitumor immunity. However, anti-CTLA-4 therapy was accompanied by autoimmune manifestations that were not predicted by preclinical models and that occurred in patients without a prior medical history of autoimmunity.
A recent report describes severe immune dysregulation in individuals harboring heterozygous CTLA4 germline mutations associated with decreased CTLA-4 expression in immune cells (Kuehn et al., 2014). Seven CTLA4 heterozygotes from four unrelated families were studied intensively. Although one 77-year-old individual remained asymptomatic, six others developed severe immune dysregulation at ages ranging from 2 to 40 years. Clinical manifestations—including pulmonary infiltrates, gut inflammation, cytopenias, and hypogammaglobulinemia (six of six patients); autoantibodies (five of six); and focal brain inflammation (three of six)—were cumulative and progressive. Reminiscent of biopsies of inflamed organs in patients receiving anti-CTLA-4, biopsies of affected organs demonstrated mixed lymphoid infiltrates. CTLA-4 mRNA and protein levels in effector and Treg cells were reduced. Wide-ranging effects on circulating lymphocytes, including effector T cells (hyperproliferative CD4+ and CD8+ cells; see Figure 1), Treg cells (decreased Foxp3 and CD25 expression and decreased suppression of CD4+ T cell proliferation), and B cells (decreased mature CD20+ cells and increased ‘‘exhausted’’ CD21lo cells), were reported. Another report of heterozygous CTLA4 mutations in six different families describes 14 affected individuals with similar clinical findings and eight asymptomatic carriers who nevertheless harbored T cell phenotypic and functional abnormalities (Schubert et al., 2014). Similar to mouse data, these studies of individuals with heterozygous CTLA4 germline mutations support both cell-intrinsic and cell-extrinsic functions for CTLA-4. However, although heterozygous CTLA-4 loss in mice can lead to increased Treg cell frequencies, it does not produce the severe inflammatory manifestations seen in Ctla4−/− mice or in humans with heterozygous CTLA4 mutations.
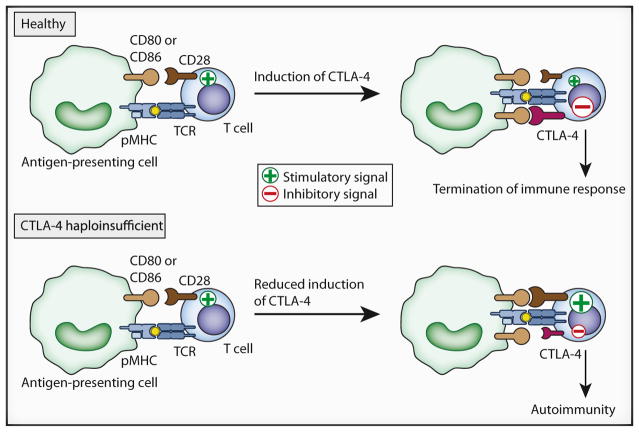
Figure 1. Development of Autoimmunity in Patients with CTLA-4 Haploinsufficiency.
In naive T lymphocytes from healthy CTLA4+/+ individuals, TCR signaling in response to cognate antigen is accompanied by CD28 costimulation, resulting in the rapid induction of inhibitory CTLA-4 coreceptors on the cell surface. CTLA-4 has a higher affinity for the ligands CD80 and CD86 than for CD28. Therefore, a switch to negative signaling occurs to inhibit T cell activation and appropriately conclude the immune response. In contrast, CTLA-4-haploinsufficient patients express reduced levels of CTLA-4 on activated T effector cells. This alters the balance between stimulatory CD28 and inhibitory CTLA-4 signals, sustaining the activation and proliferation of antigen-specific T cells and leading to autoimmunity. pMHC, peptide major histocompatibility complex.
The report by Kuehn et al. describing the consequences of decreased CTLA-4 expression bears notable similarities to, as well as distinctions from, reports of inflammatory disorders associated with anti-CTLA-4 cancer therapy. Among 540 melanoma patients receiving intermittent CTLA-4 blockade with ipilimumab, approximately 60% experienced immune-related adverse events, and 11% had severe symptoms, the most common of which were dermatologic (rash and vitiligo), gastrointestinal (enterocolitis), and endocrine (hypothyroidism and hypophysitis) (Hodi et al., 2010). Less common inflammatory events included hepatitis, uveitis, neurologic disorders, and pneumonitis (Attia et al., 2005). Although most immune-related toxicities were readily managed with immunosuppressive drugs, some were fatal. Biopsies of inflamed organs demonstrated mixed CD4+ and CD8+ T cell infiltrates. Increased serum titers of autoantibodies observed in some patients were directed against thyroid tissue, acetylcholine receptor, pituitary gland, and other targets. A significant correlation between severe immune-related toxicities and major tumor regressions was described (Attia et al., 2005), suggesting common biological mechanisms and highlighting the precarious balance between self-tolerance and autoimmunity in malignant and normal tissues.
Descriptions of asymptomatic adults with heterozygous CTLA4 deficiencies (Kuehn et al., 2014, Schubert et al., 2014), and widely varying ages of onset among symptomatic individuals, imply that additional interacting factors are required for surmounting an autoimmune threshold. These factors might include other genetic or epigenetic events and environmental influences (microbial or other). The variable penetrance of genetic CTLA4 disorders mirrors clinical experience with CTLA-4 blockade. Immune-related toxicities can occur after the first drug dose or much later during treatment, can be mild or severe, and can respond promptly to corticosteroids or require additional immunosuppressants. Exposure of some frequently affected organs (skin and gut) to the microbiome has suggested that this environmental factor might contribute to generating auto-immunity in patients receiving ipilimumab. Interestingly, Ctla4−/− mice die within 2 weeks of age even when they are rederived into germ-free environments, suggesting the importance of self-antigens in driving the inflammatory phenotype.
It is currently unknown how the degree, timing, and cell-type specificity of CTLA-4 deficiency might influence autoimmune consequences. Foxp3-specific CTLA-4 deficiency in mice results in a fatal immunopathology that is delayed in onset and more restricted than that in Ctla4−/− mice (Wing et al., 2008). The rapidly lethal phenotype of Ctla4−/− mice contrasts sharply with murine heterozygous models and cancer-treatment models using short-lived anti-murine CTLA-4 antibodies in wild-type animals. Recent reports suggest that some of the therapeutic effects of anti-CTLA-4 are achieved through depletion of tumor-infiltrating Treg cells (Selby et al., 2013). These findings might explain differences observed between genetic and antibody-mediated CTLA-4 deficiency. Furthermore, severe immune dysfunction in human heterozygotes with partial but chronically reduced CTLA-4 expression can be contrasted to the more limited, organ-specific immune reactivity observed in some cancer patients receiving anti-CTLA-4 intermittently. Collectively, these findings imply that exploring different dosing schedules of anti-CTLA-4 might effectively uncouple antitumor immunity from autoimmunity in patients with cancer.
Kuehn et al. found CTLA-4 expression on B cells and an increased frequency of CD21lo B cells in patients with heterozygous CTLA4 mutations. CTLA-4 can be expressed on B cells, but whether it exerts direct effects on B cells is unclear. Ctla4−/− mice have increased antibody levels, which might reflect CTLA-4 function in T or B cells. Foxp3-specific CTLA-4 deficiency in mice leads to markedly increased autoantibody production, pointing to an essential role for CTLA-4 on Treg cells in regulating B cell responses. Aberrant anti-self immunoglobulin responses, particularly responses against neuronal tissues, are a hallmark of paraneoplastic syndromes and are thought to represent cross-reactivities against determinants shared by tumor and normal cells (Steinman 2014). Interestingly, endocrine gland abnormalities characteristic of anti-CTLA-4 therapy might represent the emergence of previously subclinical paraneoplastic phenomena targeting neural-crest-derived hormone-producing cells.
As Kuehn et al. have shown, a delicate balance exists between self-tolerance and autoimmunity that is governed, at least in part, by quantitative variations in CTLA-4 expression. As predicted by pre-clinical models, the complex interplay between genetics and the environment might determine the evolution of distinct phenotypes associated with CTLA-4 deficits, or conversely, the maintenance of an asymptomatic state. A deeper understanding of these interacting factors will be needed for the optimal development of drugs blocking CTLA-4 and other immune checkpoints (such drugs are showing promise in the clinic). Tumors masquerading as self might then be revealed as foreign to the immune system, resulting in tumor elimination without incurring anti-self consequences. Thus, as revealed by both genetic and drug-induced CTLA-4 imbalances, the immune system appears to have been fine-tuned to live life on the edge.
Acknowledgments
S.L.T. is a recipient of research support from the Bristol Myers Squibb Corporation, which manufactures ipilumab, an anti-CTLA-4 monoclonal antibody. This work was supported in part by NIH P01 AI39671 to A.H.S.
References
- Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, et al. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KF, Fu G, Zhang Y, Yokosuka T, Casas J, Canonigo-Balancio AJ, Becart S, Kim G, Yates JR, 3rd, Kronenberg M, et al. Nat Immunol. 2014;15:465–472. doi: 10.1038/ni.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, Schickel JN, Tran DQ, Stoddard J, Zhang Y, et al. Science. 2014;345:1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, Allison JP. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, Bulashevska A, Petersen BS, Schäffer AA, Grüning BA, et al. Nat Med. 2014 doi: 10.1038/nm.3746. http://dx.doi.org/10.1038/nm.3746. [DOI] [PMC free article] [PubMed]
- Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- Steinman L. Eur J Immunol. 2014 http://dx.doi.org/10.1002/eji.201445191.
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]