Abstract
Spasticity, one of the main symptoms of multiple sclerosis (MS), can affect more than 80% of MS patients during the course of their disease and is often not treated adequately. δ-9-Tetrahydrocannabinol-cannabidiol (THC-CBD) oromucosal spray is a plant-derived, standardized cannabinoid-based oromucosal spray medicine for add-on treatment of moderate to severe, resistant multiple sclerosis-induced spasticity. This article reviews the current evidence for the efficacy and safety, with dizziness and fatigue as the most common treatment-related adverse events, being mostly mild to moderate in severity. Results from both randomized controlled phase III studies involving about,1600 MS patients or 1500 patient-years and recently published studies on everyday clinical practice involving more than 1000 patients or more than,1000 patient-years are presented.
Keywords: cannabinoids, CBD, multiple sclerosis, nabiximols, spasticity, THC
Introduction
Multiple sclerosis (MS) is a progressive, chronic, immune-mediated disease of the central nervous system (CNS) [Zettl et al. 2012; Kutzelnigg and Lassmann, 2014], diagnosed predominantly in young adults with approximately 500,000 patients in Europe and more than 2.3 million people worldwide [Flachenecker and Stuke, 2008; Browne et al. 2014]. It is the most common neurological disease in young and middle-aged adults resulting in marked physical disability, inability to work or early retirement, significantly impaired quality of life (QoL) and a substantial burden on society in terms of associated costs as it evolves [Zettl et al. 2013; Svensson et al. 2014]. MS is characterized by a broad range of signs and symptoms, the most common being restricted mobility, spasticity, fatigue, sensory deficits, palsy, pain, bladder dysfunction, cognitive dysfunction, depression and visual impairment [Rizzo et al. 2004; Goldenberg, 2012].
Spasticity is one of the more common symptoms of MS, as it affects more than 80% of MS patients during the course of the disease [Barnes et al. 2003; Beard et al. 2003; Rizzo et al. 2004]. Spasticity can be defined from the pathophysiological perspective as a ‘disordered sensorimotor control resulting from an upper motor neuron lesion, presenting as intermittent or sustained involuntary activation of muscles’ [Stevenson, 2010]. It is perceived by the patients as continuous muscle stiffness, often associated with exacerbating spasms, and further restricting the already MS compromised mobility, fatigue, bladder dysfunction, pain or impaired sexual activities [Hobart et al. 2006; Crayton and Rossman, 2006; Zwibel et al. 2009; Flachenecker et al. 2014a].
The impact of MS-induced spasticity on QoL was examined by a Swedish retrospective, cross-sectional study involving 105 patients. Health-related QoL measured by generic EuroQol (EQ-5D) and 0-100 Visual Analogue Scale (VAS) significantly decreased with increased severity of spasticity. Mean quality-adjusted life-year (QALY) weight was 0.36 compared with 0.82 in the healthy Swedish population, with 21 patients displaying a value below 0 (worse than death). QALY weight decreased with increased severity. This study emphasizes the negative impact of spasticity on QoL [Svensson et al. 2014].
The association between severity of MS spasticity and QoL was also confirmed by a recent Spanish multicenter, cross-sectional study where the SF-12® questionnaire was used to assess QoL, which was correlated to the modified Ashworth scale and a 0-10 Numerical Rating Scale (NRS) used to assess severity of spasticity [Arroyo et al. 2013]. The results showed an association between severity of spasticity and QoL for both scales (p ⩽ 0.002), where the correlation was stronger with the NRS in patients with MS. A second Spanish cross-sectional study has shown a direct correlation between increasing severity of spasticity and worsening of spasticity-related symptoms, especially day and nighttime spasms (p < 0.001), urinary dysfunction (p < 0.001) and sleep disturbances (p = 0.015). The study involved 2029 MS patients; 65.7% suffered from spasticity, with 40% of them moderate to severe [Oreja-Guevara et al. 2013].
Costs for society increase with severity of spasticity, being 2.4 times higher in MS patients with severe spasticity than to those with mild spasticity [Svensson et al. 2014].
Severity of spasticity is difficult to quantify. Even mild spasticity may cause restrictions of the patient’s physical capacities in comparison with persons with no motor neuron damage [Henze et al. 2006]. First of all, an intensive anamnestic and neurological evaluation is key to identifying spasticity in MS patients. In addition to neurophysiological methods [Voerman et al. 2005] and biomechanical techniques [Wood et al. 2005], objective but often applicable only in experimental settings, reliable, responsive and validated clinical scales can be applied [Platz et al. 2005]. The most widely used scale reflecting the healthcare professional’s perspective is the (modified) Ashworth Scale [Ashworth, 1964; Bohannon and Smith, 1987], but its validity and reliability have been questioned [Fleuren et al. 2010; Sunnerhagen, 2010]. This is because, although an increase in score represents more spasticity, the difference in spasticity between two consecutive scores is not the same across the whole scale (categorical scale, no linearity). Therefore, the differences in scores and changes during time obtained with this scale and possible analyses (for example, central or dispersion tendency measures such as means and standard deviations) are likely to be imprecise.
The 0-10 NRS reflects the severity of spasticity from patient’s perspective [Farrar et al. 2008; Anwar and Barnes, 2009], displayed as mean perception over time. It is both reliable and valid for the measurement of spasticity, and it substantially increases the likelihood of making an accurate diagnosis and assessment of the level of severity. The scale has a moderate to high level of correlation with other clinician rated instruments used to assess spasticity. It allows simplified patient self-rating of spasticity and provides more details on the severity of spasticity-associated symptoms experienced by patients who are also influenced by the presence of spasticity.
Two other important scales to measure spasticity are the Penn Spasms Scale, which is a two component self-report measure of frequency with a five-point scale and of severity with a three-point scale to quantify spasticity severity [Penn et al. 1989] and the Multiple Sclerosis Spasticity Scale (MSSS-88), a validated self-assessment tool, retrieving 88 items [Hobart et al. 2006; Henze et al. 2014]. Due to its extent, use of the MSSS-88 scale in daily practice is limited. However, so far no single standard method or a direct comparison between scales is available.
Management of MS-induced spasticity
The most important treatment goals in patients with MS-induced spasticity are avoidance or elimination of triggers which may initiate or enhance spasticity, pain reduction, improvement or maintenance of functional abilities, QoL and facilitation of nursing. If physiotherapy, as a generally accepted basic treatment option, is not sufficient, drugs should be tried [Henze et al. 2006]. Depending on the severity of spasticity, drug treatment varies widely, reliant on approved drugs which may differ between geographical regions. Commonly used medications like baclofen, tizanidine, gabapentin or dantrolene are administered orally. Their mode of action varies, but all cause muscle relaxation.
There is limited evidence of the effectiveness and efficacy of these four anti-spasticity drugs. According to a number of reviews, they appear to be approximately equally effective at reducing spasticity when assessed clinically, but there is no convincing proof of functional benefit [Beard et al. 2003; Stevenson, 2010]. Clinical studies date back to the 1970s and spasticity measured by the (modified) Ashworth Scale might not have led to valid and reliable results [Fleuren et al. 2010; Sunnerhagen, 2010].
A systematic review by the Cochrane Group concluded that the absolute and comparative efficacy, as well as tolerability of classical anti-spasticity medication, is limited [Shakespeare et al. 2003]. The most commonly used medication, oral baclofen, yielded a moderate effectiveness score of 3.68 (±0.97) in a rating carried out by patients on a 1 to 5 categorical rating scale from (3 = no change and 4 = a little better; Rizzo et al. 2004). Further limitations are mainly due to a high occurrence of side effects such as muscle weakness with risk of falls, sedation, cognitive difficulties, withdrawal syndrome or dizziness at patient required doses.
However, the evaluation of the efficacy of a symptomatic treatment in the context of a chronic di-sease like MS might be afflicted with methodological problems. Spasticity is a very complex and not yet fully understood condition. As the extent of upper motor neuron damage varies from patient to patient, the response to a peripheral or centrally acting antispasticity medication cannot be predicted and can vary. An overlap of various symptoms can influence the detection of the improvement of one specific symptom. Therefore psychometric qualities of the selected instruments need to be reliable and valid. An instrument with poor psychometric properties will be less likely to detect treatment effects.
Invasive medication like intrathecal baclofen pumps for resistant spasticity [Smyth and Peacock, 2000] or intramuscular injections of botulinum toxin A for focal cases [Kabus et al. 2006] are used only for a small number of patients. Few patients are treated with intrathecal triamcinolone-acetonide [Kamin et al. 2014]. Surgical interventions are applied only very rarely [Beard et al. 2003].
There is ample evidence that spasticity is not being managed adequately in everyday clinical practice. Various published cross-sectional European survey-based studies showed that only about half of the patients with MS-related spasticity were receiving antispasticity medication. In Spain, 42.4% of patients with moderate and 52.6% with severe spasticity received spasticity related medication [Oreja-Guevara et al. 2013]. In Germany, 55.1% of MS patients with spasticity were treated with antispasticity medication [Zettl et al. 2013]. The number of prescribed drugs increased with severity of spasticity, indicating insufficient effectiveness [Flachenecker et al. 2014a]. These results are confirmed by a North American study. About one-third of the patients reported their level of spasticity as moderate or worse despite ongoing single or multiple drug use [Rizzo et al. 2004]. Additionally, in the North American Research Committee on Multiple Sclerosis (NARCOMS) study, it was shown that MS patients showed a continuous worsening of spasticity through MS disease evolution despite available treatments [Kister et al., 2013].
Recent burden-of-disease studies [Zettl et al. 2013] confirm those early findings. MOVE 1 (MObility ImproVEment), a cross-sectional and retrospective study from Germany, has shown low satisfaction with the effectiveness of the currently available pharmacotherapy of spasticity in a total of 414 MS patients with mild (27.3%), moderate (44.0%) and severe spasticity (28.7%); 36.1% of the patients under pharmacotherapy and 41.3% of the participating physicians were partially or fully dissatisfied with the effectiveness of the medication used (including baclofen, tol-perisone or tizanidine). In both groups, dissatisfaction increased with increasing severity of spasticity [Flachenecker et al. 2014a]. Similar trends are seen in the MOVE 1 EU study, with 300 participating patients from seven countries: 48.0% physicians and 34.0% patients are at least partly dissatisfied with spasticity relief from current drug treatment [Vermersch, 2014].
Considering these limitations, there was an urgent need to find alternative drugs. Since 2011, δ-9-tetrahydrocannabinol-cannabidiol (THC-CBD) oromucosal spray [US Adopted Name (USAN nabiximols; trade name Sativex®] is available as add-on therapy for patients with moderate to severe treatment-resistant spasticity in a growing number of European countries.
Role of the endocannabinoid system in spasticity
The search for alternative antispasticity drugs reignited the interest in Cannabis sativa, one of the oldest herbal plants in the history of medicine [von Linnaeus, 1753]. Smoked or otherwise processed cannabis has been used for medical purposes for a long time either to achieve or to investigate antispastic, muscle relaxant and analgesic effects [Killestein and Polman, 2004; Russo and Guy, 2006]. However, concerns were raised regarding cannabinoid tolerability, in particular with respect to the contribution to psychiatric or cognitive disorders by repeated use [Johns, 2001; Arseneault et al. 2004; Solowij et al. 2002].
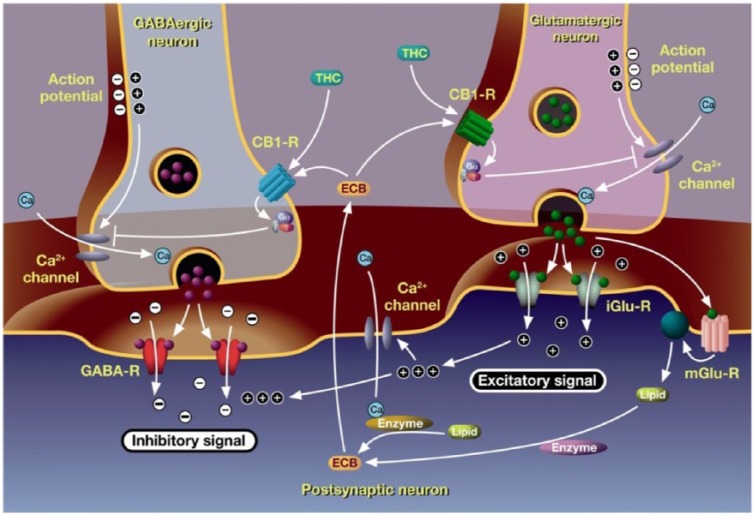
After the discovery of the human endocannabinoid system, the identification of the cannabinoid receptors CB1 and CB2 and of the endogenous cannabinoids including anandamide and 2-arachidonoylglycerol, its role was extensively examined [DiMarzo and Petrosino, 2007]. One important action of endocannabinoids is their interaction with presynaptic cannabinoid receptors, predominantly present in the CNS on both GABAergic and glutamatergic synapses. CB1 receptor-mediated inhibition of ion channels is associated with a reduced release of the excitatory glutamatergic and the inhibitory GABAergic neurotransmitters [Pertwee, 2006]. It has been shown that the CB1 receptors in the aforementioned pathways are the main cannabinoid target to deliver an antispastic effect [Pryce and Baker, 2007]. The stimulation of the endocannabinoid system has an impact on many different effects including a reduction of the severity of both MS-induced spasticity and pain [Pertwee, 2006]. This gave rise to the development of specifically combined cannabinoids for the treatment of MS-induced spasticity [Ware et al. 2005; Pertwee, 2009; Perez and Ribera, 2008].
THC-CBD oromucosal spray for the management of MS-induced spasticity
A THC-CBD oromucosal spray is available as add-on therapy for patients with moderate to severe treatment-resistant spasticity in a growing number of European countries for patients who are not satisfactorily relieved with their current first-line antispastic therapy. Two exogenous endocannabinoid agonists, the phytocannabinoids THC [Gaoni and Mechoulam, 1964] and CBD [Guy and Stott, 2005], derived from cloned Cannabis sativa chemovars, are the main components of this oromucosal spray. They have both inhibitory and excitatory effects on different neurotransmitter systems [Hoffman and Lupica, 2000]:
THC is a partial agonist at both human CB1 and CB2 receptors. Its main pharmacological effects at different doses include analgesia, muscle rela-xation, anti-emesis or appetite stimulation as well as psychotropic effects which limited its clinical use [Pertwee, 2000].
CBD has little activity at the CB1 receptor but greater activity at the CB2 receptor [Showalter et al. 1996]. It also stimulates vanilloid pain receptors (VR1) and inhibits the uptake of anandamide, while also weakly inhibiting its breakdown [Bisogno et al. 2001]. Studies have also shown that it can have anti-inflammatory, neuroprotective, anticonvulsant, muscle relaxant, antioxidant and anti-psychotropic effects at different doses [Russo and Guy, 2006; Perez, 2006].
Figure 1 illustrates the mode of action of cannabinoids. They modulate the human endocannabinoid system and act synergistically to increase the analgesic effects on muscles. Mimicking the negative feedback mechanism of endocannabinoids [Pertwee, 2006], a modulation of the transmission of impulses at synapses in the central and peripheral nervous system is therefore ensured [Di Marzo and Petrosino, 2007]. THC binds to the CB1 receptor which is predominantly present in the CNS at glutamatergic synapses. This reduces the effects of the excitatory glutamate typical for spasticity [Pertwee, 1999; Izzo et al. 2009]. The mode of action of THC has been studied in an in vivo MS mouse model [Baker et al. 2000]. As CBD alleviates the psychotrophic effects of THC, a higher dose of THC can be administered and thus a higher benefit can be achieved [Pertwee, 2000; Russo and Guy, 2006]. The synergistic potential in MS-induced spasticity was proven in an in vivo MS mouse model where THC-CBD dose dependently reduced spasticity to the same extent as baclofen [Hilliard et al. 2012].
Figure 1.
Endocannabinoid system and mode of action of cannabinoids.
Endo- and phytocannabinoids mediate neuronal signaling. The neurotransmitters glutamate and γ-aminobutyric acid (GABA) are released after an action potential has induced calcium ions to flow into the axonal terminal bulb. Glutamate binding to its ionotropic receptor (iGluR) leads to membrane depolarization in the postsynaptic neuron and an excitatory signal. GABA binding to its receptor leads to an inhibitory signal. Synthesis of endocannabinoids (ECBs) is induced by the activation of postsynaptic metabotropic glutamate receptors (mGlu-R) and high cytoplasmic calcium levels. ECBs activate presynaptic G-protein-coupled CB1 receptors, thus inhibiting presynaptic calcium influx and neurotransmitter release. Tetrahydrocannabinol (THC) mimics the action of the ECB anandamide (Perez, J. Combined cannabinoid therapy via an oromucosal spray. Drugs of Today 2006; 42:495-501. Copyright© 2006-2014 Prous Science, S.A.U. or its licensors. All rights reserved. DOI NUMBER: 10.1358/dot.2006.42.8.1021517).
THC-CBD oromucosal spray contains a stan-dardized, fixed 1:1 ratio of THC and CBD and is so far the only cannabinoid approved as add-on treatment for MS induced spasticity. Other orally administered cannabinoid-containing medications such as dronabinol (synthetic THC) or nabilone (THC analog) lack CBD and are not approved for this indication.
A summary of pharmacokinetic data from five phase I studies [Guy and Robson, 2003; Karschner et al. 2011; Stott et al. 2013a, 2013b, 2013c] was published recently in a review [Garcia-Merino et al. 2014]. Both THC and CBD are absorbed rapidly and appear in the plasma within 15 minutes of a single oromucosal administration. A mean plasma concentration Cmax of about 4 ng/ml was reached about 45–120 minutes after a single dose administration of 4 sprays in a row. As cannabinoids are highly lipophilic, they are quickly absorbed and distributed into body fat. From there they are slowly released at subtherapeutic levels back into the blood stream. Then they are metabolized in the liver and excreted via the urine and feces [Karschner et al. 2011]. Food does alter the relative bioavai-lability of the THC-CBD oromucosal spray; however, variations in bioavailability caused by food are less than the intersubject variations observed and therefore the findings are unlikely to be clinically relevant [Stott et al. 2013b]. A comparison between the THC-CBD oromucosal spray and smoked cannabis confirmed that the likelihood of achieving psychoactive effects is minimal due to a much lower Cmax compared with inhaled THC [Stott et al. 2008].
Clinical efficacy of THC-CBD oromucosal spray in pivotal and recent studies
The THC-CBD spray clinical study program included basically three pivotal randomized, double blind, placebo-controlled, multicenter phase III efficacy studies [Collin et al. 2007, 2010; Novotna et al. 2011], one supporting phase II pilot study and its open label extension [Wade et al. 2004, 2006], one long-term extension study [Serpell et al. 2013], one withdrawal study [Notcutt et al. 2012] and one safety phase IV study [Vachova et al. 2014] in MS patients with moderate to severe treatment-resistant spasticity. Inclusion criteria were in line with the Summary of Product Characteristics (SmPC).
THC-CBD spray is approved as to be used in addition to the patient’s current antispastic medication. Usually, the NRS was used to determine spasticity in the studies, only early studies applied the 0–100 mm VAS, which is a horizontal line on which patients mark the point that they feel represents the perception of their current state. Efficacy outcomes have been summarized and discussed in detail in other publications [Sastre-Garriga et al. 2011; Leussink et al. 2011; Garcia-Merino et al. 2014; Syed et al. 2014] and are only briefly discussed here. Table 1 summarizes the main characteristics and key efficacy results.
Table 1.
Main efficacy results from pivotal THC-CBD spray clinical phase III studies.
| Study | Design | Study period | Number of patients (n) | Mean duration since MS diagnosis in years ± SD (min-max) | Mean duration of spasticity in years ± SD (minimum– maximum) | Mean daily sprays in active group ± SD | Key efficacy outcome | Key efficacy results |
|---|---|---|---|---|---|---|---|---|
| Collin et al. [2007] | R, DB, PC, MC | 6 weeks | THC-CBD: n = 124 Placebo: n = 65 |
13.6 ± 8.6 12.2 ± 7.7 |
n.a. | 9.6 ±6.4* | Primary: efficacy (change in mean spasticity NRS score) Secondary: Ashworth Scale, subjective measure of spasm |
Statistically significant reduction of mean spasticity NRS in THC-CBD group (ITT analysis). Treatment difference of 0.52 points (95% CI: -1.029 to -0.004 points; p < 0.0001) in favor of THC-CBD Responder analysis favored THC-CBD group: 40% of patients achieved ⩾30% reduction in mean NRS score versus placebo at study end (p = 0.014). No statistical significance in Ashworth Scale or subjective measure of spasm. |
| Collin et al. [2010] | R, DB, PC, MC | 15 weeks | THC-CBD: n = 167 Placebo: n = 170 (ITT, n = 335) (PP, n = 265) |
15.2 ± 8.41 | 7.73 ± 5.33 | 8.5 (range 1–22)$ | Primary: efficacy (change in mean spasticity NRS score) Secondary: responder analysis 30% improvement in NRS spasticity; timed 10 metre walk, Barthel Index, carer GIC |
Numeric, but no statistically significant reduction of mean spasticity NRS in THC-CBD group (ITT analysis). Treatment difference of 0.23 points (p = 0.219) in favor of THC-CBD. Statistically significant reduction of mean spasticity NRS in THC-CBD group (PP analysis). Treatment difference of 0.46 points (p = 0.035) in favor of THC-CBD. No difference between treatment groups in 30% responder analysis. Significant change in carer GIC (p = 0.013) and timed 10 metre walk (p = 0.042). |
| Novotna et al. [2011] | R, DB, PC, MC, enriched | Phase A: 4 weeks Phase B: 12 weeks |
Phase A: n = 572 Phase B (ITT): n = 241 (THC-CBD n = 124, placebo n = 117) |
12.4 ± 7.66 (0.5–42.4) |
7.5 ± 5.86 (0.2–40.4) |
8.3 ±2.43‡ | Primary: efficacy (change in mean spasticity NRS score in phase B) Secondary: responder analysis >30% improvement in spasticity, subjective measure of spasm, sleep disruption, Barthel Index, physician GIC, patient GIC, carer GIC |
Statistically significant reduction of mean spasticity NRS in THC-CBD group (ITT analysis). Treatment difference in phase B: 0.84 points (95% CI: -1.29 to -0.40 points; p = 0.0002) in favor of THC-CBD. Significant differences were found in favor of THC-CBD in the responder analysis (p = 0.0003), spasm frequency (p = 0.005), sleep disruption (p <0.0001), Barthel Index (p =0.0067), physician GIC: (p = 0.005), patient GIC (p = 0.023) and carer GIC (p = 0.005). |
Maximum of 48 sprays per day.
Maximum of 24 sprays per day.
Maximum of 12 sprays per day.
CI, confidence interval; DB, double blind; GIC, Global Impression of Change; ITT, intention-to-treat; MS, multiple sclerosis; MC, multicenter studies; n.a., not available; NRS, Numerical Rating Scale; PC, placebo-controlled; PP, per protocol; R, randomized; SD, standard deviation; THC-CBD, δ-9-tetrahydrocannabinol-cannabidiol.
In a phase II pilot study (n = 160 patients), THC-CBD spray showed a significant improvement in the 100 mm VAS compared with placebo (p = 0.0001) after 10 weeks [Wade et al. 2004]. This effect was maintained in 137 patients in a subsequent extension study for up to 82 weeks without significant changes in mean dosages [Wade et al. 2006].
In the first pivotal study (n = 337 patients), the primary endpoint was the change in the patient-rated, mean spasticity NRS score from baseline after 6 weeks [Collin et al. 2007]. It was statistically significant in favor of THC-CBD spray in the intention-to-treat (ITT) population (difference 0.52 points, p = 0.048). A responder analysis (⩾30% NRS reduction at study end) of the primary variable was presented as a secondary outcome measure. In the THC-CBD group, 40% of patients reported at least a 30% improvement in spasticity compared with 20% in the placebo group (p < 0.01). The mean daily spray number was 9.6 ± 6.4. The secondary endpoint [a composite Ashworth Scale and Motricity Index in muscles affected by spasticity score, mean daily spasm scores and patient Global Impression of Change (GIC)] was in favor of THC-CBD spray, but did not reach statistical significance. In a subsequent noncomparative, open-label extension study to provide safety and tolerability information on the long-term use of THC-CBD, a total of 146 patients from the previous parent study were enrolled [Serpell et al. 2013]. All patients with continuous data up to 52 weeks of treatment were analyzed. Of these patients, 90% (n = 55 patients) reported continued benefit over a mean treatment time of 334 (± 209) days on mean spasticity NRS score, the subjective effect in spasticity and quality of sleep. Only 10% of patients withdrew from the study due to lack of efficacy.
Using results from the study by Collin and colleagues [Collin et al. 2007], Farrar and colleagues proved the validity, reliability and clinical relevance of the spasticity NRS [Farrar et al. 2008]. The minimal clinically important difference was defined by a reduction of 18% in spasticity NRS score and the clinically important difference was defined by a reduction of 29.5%. A ⩾20% spasticity NRS improvement was therefore consi-dered an initial response and a ⩾30% improvement as clinically relevant response in the following studies.
The second pivotal study by Collin and colleagues included 335 patients in the ITT analysis and 265 patients in the per protocol (PP) analysis after 15 weeks [Collin et al. 2010]. The primary endpoint, the change in mean spasticity NRS score from baseline, was in favor of THC-CBD in the PP population (difference -0.46 points, p = 0.035), but not statistically significant in the ITT population (difference -0.23 points; p = 0.219). Responder analysis at the 30% spasticity reduction level showed nonsignificant treatment diffe-rences in the ITT population (p = 0.23); in the PP analysis, however, 36% of patients reported at least a 30% improvement in spasticity compared with 21% in the placebo group (p = 0.04). The difference between ITT and PP outcome was thought to be attributed to a group of 72 patients in the verum group who terminated treatment early and had a poor treatment response. This group potentially masked the response in the ITT population, while it was excluded in the PP analysis. It was shown that a 20% reduction in spasti-city NRS score during the first 4 weeks (initial responder) was predictive of the clinically relevant response of a ⩾30% reduction in spasticity NRS score (clinically relevant responder). The mean daily spray number was 8.5 (range 1–22). QoL, measured by the generic QoL questionnaire EQ-5D and the disease-specific QoL questionnaire MSQoL-54, showed a positive trend in favor of THC-CBD spray.
A meta-analysis of the studies by Collin and colleagues [Collin et al. 2007, 2010] and Wade and colleagues [Wade et al. 2004] proved that treatment with THC-CBD spray (n = 356) yielded a statistically significant greater proportion of clinically relevant responders versus placebo (n = 296) (37% versus 26%, respectively; p = 0.0073) at study end with an odds ratio (OR) in favor of THC-CBD spray [OR = 1.62; 95% confidence interval (CI): 1.15–2.28] [Wade et al. 2010].
Based on the findings from these three studies [Collin et al. 2007, 2010; Wade et al. 2010], an enriched study design with two phases (single blind trial period followed by randomized phase of the trial period responders) was developed. This design was accepted by the European Medicines Agency (EMA) for the conduction of the third pivotal study [Novotna et al. 2011]. This enriched study design is a well-accepted concept often used in analgesic studies [Hewitt et al. 2011].
In a single blind, 4 week trial period, all patients (n = 522) received THC-CBD. Only those patients achieving an improvement of ⩾20% in their spasticity NRS score were selected and continued into a 12 week double blind randomized phase. Of the patients who completed the week phase, 47% (n = 271) were initial responders. In this trial period, the mean spasticity NRS of the early responder MS patients with moderate to severe spasticity improved by 3.01 points [standard deviation (SD) ± 1.38] from 6.91 ± 1.25 to 3.90 ± 1.51 points. The mean daily spray number was 6.9 (±1.78). A total of 241 patients were randomized. The primary endpoint, the change in mean spasticity NRS score from beginning of randomization phase, was statistically significant in favor of THC-CBD (difference of 0.85, p = 0.0002). Responder analysis (⩾30% NRS reduction at study end compared with screening) was presented as a secondary outcome measure. In the THC-CBD group, 74% of patients reported at least a 30% improvement in spasticity compared with 51% in the placebo group (p = 0.0003). The mean daily spray number was 8.3 (±2.43) in the active group compared with 8.9 (±2.31) in the placebo group.
In addition, secondary endpoints were statistically significant in favor of THC-CBD versus placebo, including spasm frequency (p = 0.0005), sleep disturbance (p < 0.0001), Barthel Index of Daily Living (p = 0.0067), physician GIC (p = 0.005), subject GIC (p = 0.023) and carer GIC in function (p = 0.005). The secondary endpoints modified Ashworth Scale (p = 0.094) and timed 10 metre walk (p = 0.069) were in favor of THC-CBD spray but did not reach statistical significance, while QoL measured by the generic questionnaires EQ-5D and SF-36 showed 19–37% improvement in initial responders.
In a randomized, double blind, placebo-controlled, long-term multicenter phase IV study with 121 patients, long-term tolerability (cognition and mood) and efficacy was investigated over 50 weeks [Vachova et al. 2014]. Efficacy of spasticity was measured using the GIC determined by patients, physicians and carers, and in all analysis the THC-CBD arm was significantly improved compared with placebo (p = 0.002; p = 0.0001 and p = 0.014, respectively). From the first to the last 3 months of the study, the mean daily spray number decreased from 7.6±3.1 to 6.4±3.1 sprays per day in the THC-CBD group.
The phase III studies and the phase IV study provide conclusive evidence of the efficacy of THC-CBD spray in MS-induced, moderate to severe spasticity that cannot be fully relieved with first-line therapies. Initial responders can be identified in a 4 week trial period. About 40% of previously unsuccessful treated MS patients reached a clinically relevant mean spasticity NRS score improvement of at least 30% with a mean daily spray number of around eight.
There are some relevant aspects to be considered in the interpretation of the results. Not all these previously resistant patients profited from a treatment with THC-CBD spray. THC-CBD did not show significant benefit in the four points observer rated spasticity modified Ashworth Scale secondary endpoint, although a number of other spasticity related end points such as the patient-rated NRS, spasm frequency or the patient, carer and clinician GICs improved significantly. Regarding the mode of action, it is potentially related to inhibitory signals in the GABAergic system, but patient’s perception and an analgesic effect of THC-CBD cannot be excluded completely. Moreover, the therapeutic gain (active arm effect minus placebo effect) seems limited if the trial period gains are not considered.
Clinical effectiveness of THC-CBD spray: results from observational studies
Evidence-based medicine relies on randomized controlled clinical studies to evaluate efficacy, safety and tolerability of new drugs. Nevertheless, well-designed observational post approval studies to monitor use in everyday clinical practice are important to confirm results obtained under experimental conditions. All observational stu-dies described here included only patients with moderate to severe spasticity not responding to first-line oral antispastic treatment, in alignment with the THC-CBD approved label. The main characteristics and key effectiveness results with effectiveness as ‘efficacy in real-life or under conditions of an observational study’ are described and are summarized in Table 2.
Table 2.
Effectiveness results from the main recent THC-CBD spray studies.
| Study | Design | Study period | Number of patients (n) | Mean duration since MS diagnosis in years ± SD (min-max) | Mean duration of spasticity in years ± SD (minimum–maximum) | Mean daily sprays ± SD | Key effectiveness outcomes | Key effectiveness results |
|---|---|---|---|---|---|---|---|---|
|
Flachenecker et al. [2014b] (MOVE 2) |
Observational, prospective, multicenter, noninterventional | 3 months | n = 276 | 15.4 ± 9.0 (1–53) |
8.7 ± 6.6 (0–36) |
Month 1: 6.9 ± 2.8 Month 3: 6.7 ± 2.9 |
Effectiveness (change in mean spasticity NRS score) | Statistically significant reduction of spasticity after 3 months: Total group (n = 75): from 6.3 to 4.7 points (p < 0.0001) IR group* (n = 39): from 6.4 to 4.0 points (p < 0.0001) CR group$ (n = 25): from 6.5 to 3.4 points (p < 0.0001) Statistically significant improvement of sleep disturbances after 1 month (mean sleep NRS score): Total group (n = 212): from 4.1 ± 3.0 to 3.3 ± 2.5 points (p < 0.0001) IR group* (n = 85): from 3.9 ± 3.1 to 2.6 ± 2.1 points (p < 0.0001) CR group$ (n = 52): from 3.5 ± 3.0 to 2.0 ± 1.7 points (p = 0.0008) |
|
Flachenecker et al. [2014c] (MOVE 2 Long-term) |
Observational, prospective, multicenter, noninterventional | 12 months | n = 52 | 14.1 ± 8.0 (2 - 30) |
7.9 ± 5.3 (1 - 26) |
Month 12: 6.2 ±2.6 | Long-term effectiveness (change in mean spasticity NRS score) | Statistically significant reduction of spasticity after 12 months: Total group (n = 40): from 6.2 to 4.6 points (p < 0.0001) IR group* (n = 24): from 6.3 to 4.3 points (p = 0.0004) CR group$ (n = 13): from 6.6 to 4.2 points (p = 0.0038) Statistically significant improvement of sleep disturbances after 12 months (mean sleep NRS score): Total group (n = 50): from 5.1 ± 2.9 to 3.2 ± 2.5 (p < 0.0001) IR group* (n = 26): from 5.4 ± 3.1 to 2.4 ± 1.9 (p < 0.0001) CR group$ (n = 20): from 5.3 ± 3.2 to 1.9 ± 1.6 (p < 0.0001) |
| Koehler et al. [2014] | Observational, medical charts’ data collection study, single center | Mean follow-up 9 months | n = 166 | n.a. | n.a. | Month 9: 4 (range 1-12) | Effectiveness (change in mean spasticity NRS score; response rate) | Reduction of spasticity from 7.0 (range 4–10) to 3.0 (range 0–6) within 10 days (57% reduction); response rate of 72% |
| Russo et al. [2015] | Observational, single group, interventional, single center | 1 month | n = 30 | n.a. | n.a. | n.a. | Effectiveness (change in mean spasticity NRS score) neurophysiological assessment |
Significant reduction of mean spasticity NRS treatment difference of 2.8 points (p = 0.035) versus baseline Increase of short intracortical inhibition (p = 0.0002) Reduction of intracortical facilitation (p = 0.01) Mild but significant Hmax/Mmax ratio reduction (p = 0.05) |
IR, initial responder (NRS ⩾20% versus baseline).
CR, clinically relevant responder (NRS ⩾30% versus baseline).
n.a. not available; NRS, Numerical Rating Scale; SD = standard deviation; THC-CBD, δ-9-tetrahydrocannabinol-cannabidiol.
MOVE 2 German observational study
MOVE 2 was an observational, prospective, multicenter, noninterventional study. It included 276 patients for a follow-up period of 3–4 months [Flachenecker et al. 2014b]. The main objective of this study was to provide longitudinal data on the effectiveness and tolerability of the THC-CBD oromucosal spray from everyday clinical practice. The initial response rate (NRS ⩾20% versus baseline after 4 weeks trial period) was 41.7%. Analysis for all endpoints was done for the total sample, the 20% NRS responders (MCID) and the 30% NRS responders (CID). After 1 month, the mean spasticity NRS score decreased significantly in the total sample group (p < 0.0001), in the 20% responder group (p < 0.0001) and the 30% responder group (p < 0.0001) compared with study start. Similarly, the mean spasticity NRS constantly decreased in all groups after 3 months in the total sample group (p < 0.0001), in the 20% responder group (p < 0.0001) and in the 30% responder group (p = 0.008). The mean daily number of sprays was 6.9 (±2.8) after 1 month and 6.7 (±2.9) at the end of the study (3 months). Spasticity is known to disturb sleep [Beard et al. 2003], often due to cramps or spasms that occur at night. The THC-CBD spray was found to improve sleep disturbances. The mean sleep NRS score decreased significantly in all groups after 1 month (Table 2), with effects maintained to the end of the study.
After 3 months, the generic QoL EQ-5D-3L index remained stable compared with baseline, while the disease-specific MSQoL-54 improved statistically significantly in both physical health (p = 0.0003) and mental health composite scores (p = 0.0012).
Patients’ satisfaction with the effectiveness of the antispastic drug treatment increased from 44.8% at baseline to 79.5% after 3 months. Similarly, physicians rated that 74.6% of their patients showed improvement of their spasticity after 1 month and the effect was maintained up to 3 months.
MOVE 2 long-term observational study
The MOVE 2 long-term study was an observational extension of the MOVE 2 study [Flachenecker et al. 2014c] to investigate effectiveness and safety. At the end of the 3 months’ treatment period, 100 MS resistant spasticity patients were tracked up to 12 months. A total of 62 patients were still on treatment at the end of the follow-up period, reaching a mean duration of exposure of 379.1 (±32.6) days. At the end of the study, 12 months after start of initial MOVE 2 study, 52.9% (n = 27) of the remaining patients had a spasticity reduction of at least 20% in their NRS and 41.2% (n = 21) a reduction of at least 30% in their NRS compared with the baseline value. The mean spasticity overall NRS score also reduced significantly in the total sample group remaining patients (n = 40) from 6.2 to 4.6 points (p < 0.0001((Table 2). The degree of spasticity mainly decreased within the first month of treatment and remained stable over the following 11 months. At the end of the study, the mean daily spray number was 6.2 (±2.6).
Sleep disturbances also improved statistically significantly after 12 months (Table 2). The generic QoL EQ-5D-3L index was in favor of THC-CBD spray versus baseline values of MOVE 2, but did not reach statistical significance. The disease-specific MSQoL-54 scores improved in all patients and were statistically significant in both the 20% NRS responder and the 30% NRS responder group for physical health (p = 0.0121 and p = 0.0127, respectively) and mental health compo-site scores (p = 0.0203 and p = 0.0019, respectively).
Satisfaction or complete satisfaction with the effectiveness of the antispastic drug treatment increased from 17.1% at baseline to 70.2% after 12 months (n = 47) in the remaining patients. From the physicians’ perspective, the most disturbing symptoms (muscle stiffness and pain) showed statistically significant improvements after 12 months (p = 0.00023 and p = 0.0076, respectively).
German single center data collection study
This observational, single center medical charts’ retrospective data collection study included 166 patients over a period of 15 months [Koehler et al. 2014]. The objective was to provide data on the effectiveness of THC-CBD spray from everyday clinical practice; 72% of the patients were considered initial responders by physicians and remained on the medication for a mean of 9 months. The higher initial responder rate can partially be explained by the fact that the definition of responders was not based on the ⩽20% NRS improvement; instead responders were patients who continued therapy due to an overall reduction of spastic-associated symptoms judged by the physician. The response usually showed within the first weeks, similar to what had been seen elsewhere. The mean spasticity NRS score of responders decreased by 57% within the first 10 days of treatment, whereby first clinical effects were already visible after 1 week. For 60 patients treated with THC-CBD spray, data were present for more than 60 days at the time of analysis and response was found to be maintained over this period. The mean daily number of sprays used by the patients was 4, being lower than other stu-dies. Although THC-CBD spray is approved as an add-on therapeutic, some patients who had tried other anti-spasticity drugs before but were unable to tolerate their side effects received THC-CBD spray as monotherapy.
Role of THC-CBD in the corticospinal modulation study
A study by Russo and colleagues (2015) exa-mined the role of corticospinal modulation of THC-CBD in the management of 30 MS patients with spasticity [Russo et al. 2015]. Beside analysis of spasticity parameters like NRS, modified Ashworth Scale, Penn Spasm Scale, bladder control scale and mobility, patients underwent a neurophysiological assessment of sensory motor circuits at baseline and 1 month after continuous treatment with THC-CBD. Significant improvements in spasticity (mean NRS, treatment diffe-rences = -2.8; p < 0.01 and modified Ashworth Scale treatment difference = -1.0; p < 0.05), mobility (Ambulation Index treatment difference = -1.3; p < 0.01 and timed 10 metre walk treatment difference = -29 sec; p < 0.01) and spasticity associated symptoms (Penn Spasms Scale treatment difference = -0.6; p < 0.05, bladder control scale treatment difference = -4.0; p < 0.05) were found after 1 month of treatment compared with baseline.
Interestingly, THC-CBD was found to modulate both cortical excitability, shown by an increase of short intracortical inhibition (p = 0.0002) and a reduction of intracortical facilitation (p = 0.01) and spinal excitability, shown by a mild but significant Hmax/Mmax ratio reduction (p = 0.05). The Hmax/Mmax amplitude ratio can serve as an index for a quantitative evaluation of the monosynaptic reflex excitability of the motor neuron pool. These results led to the hypothesis that THC-CBD may have an impact on the function of remote spinal circuits. As the study has an observational character and a placebo group is missing, assessments should be repeated in a clinical trial setting to confirm these results.
Despite being limited by their observational character, it can be concluded that effectiveness of the THC-CBD spray was shown in everyday clinical practice studies, with similar results to those seen in the clinical studies. Somehow higher responder rates and lower mean dosages, with a broad range of spray per day in some of these studies, emphasize the importance of individual dose titration. As not all patients benefit from the treatment, the initial 4 week trial period is important to select THC-CBD responders.
Long-term studies confirmed the stable and sustained effect over 1 year in line with the findings of the open-label clinical extension study by Serpell and colleagues [Serpell et al. 2013] and patient registries in the UK, Germany and Spain (reviewed below) with long-term benefit being maintained for up to 2 years [Garcia-Merino et al. 2013].
Beyond the findings from the clinical trials, observational studies report a positive impact on a number of QoL scales during long-term use. However, QoL measurements by generic instruments like SF-36 have not found differences and might not be sensitive enough to detect differences of symptomatic nature. Future studies should be set up to examine the influence on chronic spasticity in the long run.
Overall, clinical experience with THC-CBD spray involves approximately 1600 MS patients or 1500 patient-years in randomized controlled phase III studies and over 1000 patients or more than 3000 patient-years in studies on everyday clinical practice. More than 800 patients have been treated continuously for 6 months or more in controlled studies [Garcia-Merino et al. 2013].
The economic assessment of new therapies becomes increasingly important but is not part of this review. So far, cost-effectiveness for the THC-CBD spray has been shown for Spain, Italy and Germany [Slof and Gras, 2012; Slof et al. 2015; Flachenecker, 2013].
Safety and tolerability of THC-CBD spray
Clinical studies
The two phase III pivotal studies by Collin and colleagues [Collin et al. 2007, 2010] and the study by Wade and colleagues [Wade et al. 2004] were combined, as described above, in a meta-analysis [Wade et al. 2010]. Treatment-related adverse events (AEs) occurred in 79.3% of the patients treated with THC-CBD spray and 55.8% of the patients treated with placebo. They were mostly mild to moderate in severity (84.6% versus placebo 93.4%), with dizziness being the most common adverse reaction in the active group (32.0% versus 11.0% in the placebo group). Withdrawal/abandonment because of tolerability reasons was observed in 11.0% of the patients treated with THC-CBD spray mainly due to nausea, dizziness or vertigo compared with a rate of 3.6% in the placebo group. Serious adverse events (SAEs) were observed in 5.8% of the patients treated with THC-CBD spray versus 4.3% in the placebo group. All treatment-related SAE resolved.
In the study by Novotna and colleagues, the AE rate was lower than in the previous phase III pi-votal studies, with 46.9% of the patients in the trial phase reporting AEs [Novotna et al. 2011]. In the randomized phase, the rate was comparable between THC-CBD spray (53.0%) and placebo (49.0%) (OR 1.20; 95% CI 0.72–1.99; p = 0.484) (Table 3). These results are potentially due to the slower uptitration regimen that was implemented for the first time in this study. OR for treatment-emergent AEs was 2.03 (95% CI 0.65–6.36; p = 0.222) and OR for SAEs was 5.90 (95% CI 0.70–49.76; p = 0.103) (Table 3). Two treatment-related SAEs were reported and resolved. Study discontinuation due to AEs was low in both phases (Peto-OR 7.47; 95% CI 1.98–38.22; p = 0.003) (Table 3).
Table 3.
Main safety results from pivotal THC-CBD spray clinical phase III study [Novotna et al. 2011] and phase IV study [Vachova et al. 2014].
| Study Group | N | n (%) | OR (95% CI) | Peto-OR (95% CI) |
|---|---|---|---|---|
| Novotna et al. [2011], ITT population | ||||
| Patients with adverse events | ||||
| Therapy start | ||||
| THC-CBD spray | 572 | 268 (46.9) | ||
| Randomization | 1.20 (0.72–1.99)p = 0.484 | 1.20 (0.72–1.98) p = 0.485 | ||
| THC-CBD spray | 124 | 66 (53.2) | ||
| Placebo | 117 | 57 (48.7) | ||
| Patients with treatment-related adverse events | ||||
|
Therapy start
THC-CBD spray |
572 | 226 (39.5) | ||
| Randomization | 1.99 (1.05–3.79)p = 0.035 | 1.95 (1.05–3.62) p = 0.033 | ||
| THC-CBD spray | 124 | 33 (26.6) | ||
| Placebo | 117 | 18 (15.4) | ||
| Patients with severe adverse events | ||||
| Therapy start | ||||
| THC-CBD spray | 572 | 8 (1.4) | ||
| Randomization | 5.90 (0.70–49.76) p = 0.103 | 4.08 (0.91–18.32) p = 0.066 | ||
| THC-CBD spray | 124 | 6 (4.8) | ||
| Placebo | 117 | 1 (0.9) | ||
| Study discontinuation due to adverse events | ||||
| Therapy start | ||||
| THC-CBD spray | 572 | 35 (6.1) | ||
| Randomization | 7.47 (1.98–28.22) p = 0.003 | |||
| THC-CBD spray | 124 | 9 (7.3) | – | |
| Placebo | 117 | 0 (0.0) | ||
| Vachova et al. [2014] | ||||
| Patients with adverse events | ||||
| THC-CBD spray | 62 | 39 (62.9) | 3.57 (1.68–7.56)p< 0.001 | 3.39 (1.66–6.89)p< 0.001 |
| Placebo | 59 | 19 (32.2) | ||
| Patients with treatment-related adverse events | ||||
| THC-CBD spray | 62 | 25 (40.3) | 7.30 (2.56–20.80)p < 0.001 | 5.44 (2.39–12.38)p < 0.001 |
| Placebo | 59 | 5 (8.5) | ||
| Patients with severe adverse events | ||||
| THC-CBD spray | 62 | 5 (8.1) | – | 7.53 (1.27–44.81) p = 0.027 |
| Placebo | 59 | 0 (0.0) | ||
| Study discontinuation due to adverse events | ||||
| THC-CBD spray | 62 | 9 (14.5) | 4.84 (1.00–23.43) p = 0.050 | 3.80 (1.11–13.07) p = 0.034 |
| Placebo | 59 | 2 (3.4) | ||
CI, confidence interval; ITT, intention-to-treat; N, number of patients included in study; n, number of patients included in study group; THC-CBD, δ-9-tetrahydrocannabinol-cannabidiol.
Observational studies
In the MOVE 2 Germany study, the majority of patients (84%) did not report a treatment-related AE [Flachenecker et al. 2014b]. The most common of the 115 all-casuality AEs (⩾1%) reported in 54 patients were dizziness (4.0%), fatigue (2.5%), drowsiness (1.9%), nausea (1.9) and dry mouth (1.2%). Of these, 113 AEs in 51 patients were deemed treatment-related, nearly all events were mild; 8 were serious but patients recovered fully once medication was discontinued. These results were confirmed by the extension study [Flachenecker et al. 2014c], with 84% of patients not reporting a treatment-related AE. The 22 recorded AEs in the other patients were considered treatment-related; 21 were nonserious and one was serious (fall with fracture) from which the patient recovered.
All other previously described observational studies showed similar AE rates with dizziness and fatigue being the most common treatment-related AEs. AEs were mild to moderate, all patients recovered fully from their AEs and withdrawal rates were low.
Overall, the AE rate seen in the observational studies was lower than in the randomized clinical trial studies, although that is often due to diffe-rent patient perception and a less controlled environment. Possible methodological difficulties like data collection gaps can further reduce the rate of AEs in observational studies and should be considered when interpreting the lower event rates. Finally, AEs that have occurred only in a small number of patients may still be of relevance to patients and should be discussed with the respective patient before starting the therapy.
Adverse events of special interest
Several studies and a review [Robson, 2011] have addressed the theoretical question of psychiatric symptoms, withdrawal syndrome, dependence or abuse prompted by the THC component of the THC-CBD spray.
There is no evidence for psychopathological or cognitive effects at dosages within the therapeutic setting as proven in an 8 week randomized, double blind, placebo-controlled crossover study with cannabis-naïve MS-patients [Aragona et al. 2009].
Investigation of the influence of THC-CBD on cognition and mood in a 1 year randomized double blind study with 121 patients was performed with the Paced Auditory Serial Addition test (PASAT) screening cognitive decline and with the Beck-Depressions-Inventory screening depression. As no gold standard exists, both tests are used extensively worldwide despite possible limitations. Patients showed no association for THC-CBD with cognitive decline, nor with depression or changes in mood. The most common treatment-related AEs identified in this study were vertigo, dizziness and fatigue, and no relevant psychiatric safety signals were identified (OR 3.57; 95% CI 1.68–7.56; p < 0.001; Table 3). The OR reported for any AE was 3.57 (95% CI 1.68–7.56; p < 0.001) and for study discontinuation due to AEs was 4.84 (95% CI 1.00–23.43; p = 0.050) (Table 3) [Vachova et al. 2014].
Similarly, no evidence for a withdrawal syndrome using THC-CBD spray has been identified. Results from an open-label long-term study with 137 patients, followed over an average of 434 days, showed no consistent withdrawal syndrome once patients stopped using THC-CBD abruptly [Wade et al. 2006]. There were 46% of patients (11 out of 25) who reported symptoms such as fatigue or vivid dreams for 2 weeks after discontinuation, which might have been associated with the withdrawal. In a randomized, 4 week placebo-controlled study by Notcutt and colleagues [Notcutt et al. 2012] THC-CBD spray was stopped for 4 weeks abruptly in 36 patients to identify whether withdrawal syndromes are present. Similarly, no clear withdrawal symptoms were seen in these patients.
If patients experience dependence or abuse from THC-CBD, one would expect that dosage intake would increase steadily over the study periods. However, stable dosages and low levels of intoxication were seen after long-term application of THC-CBD spray and in one study dosages even reduced further over a 50 week study period compared with study start [Serpell et al. 2013; Vachova et al. 2014]. Additionally, a randomized, double blind, placebo controlled, crossover study performed to evaluate the subjective abuse potential and cognitive effects of THC-CBD oromucosal spray in subjects with a history of recreational cannabis use showed that the THC-CBD oromucosal spray in otherwise healthy marijuana smo-kers was not different from placebo for change in the Addiction Research Centre Inventory Morphine Benzedrine Group (ARCI-MBG) scale, Drug Liking Visual Analogue Scale (DL-VAS) and Subjective Drug Value (SDV) scale at dose administration of four sprays in a row, while the usual administration does not allow two sprays in a row [Schoedel et al. 2011].
Driving is regarded as an essential activity of daily living for a large percentage of subjects. So far, only limited, inconsistent evidence from clinical studies is available on driving ability of MS patients [Marcotte et al. 2008; Devos et al. 2013]. Since centrally acting drugs may interfere with the ability to drive safely, the question arose as to whether or not patients starting THC-CBD spray therapy show an additional influence on driving ability.
In a prospective, multicenter, noninterventional German pilot study that included 33 MS patients with moderate to severe spasticity [Freidel et al. 2015], influence on driving ability after starting THC-CBD spray was investigated. Using a validated standardized computer-based driving test, also used in the official German driving ability assessments [Schuhfried GmbH, 2012], patients were tested before and after 4–6 weeks of THC-CBD treatment. The test included five categories: visual pursuit; reaction time; adaptive tachistoscopic capability; traffic perception; and determination. A total of 31 patients completed the driving test at baseline and final visit. No overall difference was detected between the test before and after study completion in these patients. Interestingly, one of these categories testing the reactive stress tolerance level (determination) even showed a significant difference in favor of THC-CBD (p = 0.0255). Overall, all patients responded to the treatment with THC-CBD showing a significant improvement of the mean spasticity NRS score from 6.0 to 3.6 (p < 0.0001). The mean daily number of sprays at the end of the study was 5.1.
Although this study showed that treatment initiation with THC-CBD spray did not affect patients’ ability to drive, this needs to be determined individually for every patient by their physician. It is particularly important to take into account an influence on driving ability due to the underlying disease MS and also possible CNS AEs (somnolence, dizziness) which might appear in the first weeks of treatment, especially if the titration is done too fast. Therefore neurological deficits should be judged carefully and the respective national framework of traffic regulations needs to be considered.
The influence of possible short- and long-term AEs with a particular interest in addiction, abuse, misuse, memory impairment or loss of driving ability were also examined in two registry studies, one collecting data in UK and Germany, and one in Spain, which were implemented following the medicine approval process by the EMA to fulfill the requirements of the Risk Management Plan in 2010.
The first interim results from 687 completed case report forms from the UK/German registry (613 in the UK, 74 in Germany) showed that the rate of AEs was low, with 10.5% being treatment-related. There was no evidence of addiction, abuse, misuse, memory impairment or loss of driving ability reported in these treated patients [Eltayb et al. 2013; Fernández, 2014]. Similarly, in the Spanish registry, no new safety findings were reported in a 6 and 12 month interim analysis [Fernández, 2014; Oreja-Guevara et al. 2014]. After 6 months THC-CBD treatment, there were a total of five psychiatric/psychotic incidences but no incidences of suicidal thoughts, attempts, abuse, misuse or indication of driving impairment.
Overall, all compiled safety data show a very low risk for patients to suffer one of the discussed AEs. This indicates that treatment with THC-CBD is generally well tolerated. However, patients should be controlled by their treating physician, especially in the initial trial period, to identify and resolve possible occurring AEs. If AEs cannot be fully resolved, discontinuation of THC-CBD needs to be considered.
Aspects in clinical practice
Therapy should start following a relatively simple titration schema to achieve maximum effect with increasing doses limited by the appearance of mild AEs. Patients gradually titrate their number of sprays per day during the first 2 weeks of treatment until reaching their ideal individualized dose. Each patient will require a different dose depending on their own features and sensitivity to cannabinoids. The patient’s response should be reviewed after 4 weeks (trial period). If a clinically significant improvement in spasticity or spasticity-related symptoms is not seen by the physician at that stage, treatment should be stopped. In case of mild to moderate AEs, it is recommended to continue treatment with a constant spray number or to reduce it by 1 or 2 sprays until AEs disappear. After this, the spray number can be slowly increased further within the recommended range to the best possible relief of spasticity.
The mean daily number of sprays in randomized clinical trial studies was around 8 sprays, but varied on an individual basis from 4.0 to 9.6 (Tables 1 and 2). In the previously reviewed observational studies, the mean doses have been between 5 and 7 sprays per day, similarly showing wide ranges of individual variability and clearly highlighting the importance of an individual dose titration for each patient. Doses greater than 12 sprays per day are not recommended. During maintenance therapy, patients may spread the doses throughout the day for their own convenience to reduce spasticity according to their individual symptoms. Keeping a diary can strengthen the responsibility and adherence of the patient and can additionally be used by the physician to select THC-CBD treatment responders.
Future therapeutic potential of THC-CBD spray
Chronic pain in patients with late stage cancer
The high prevalence and incidence of global chronic pain has made pain a global public health priority. It is estimated that 1 in 5 adults suffers from pain and another 1 in 10 adults are diagnosed with chronic pain globally each year [International Association for the Study of Pain, 2004]. In cancer patients, up to 33% continue to have pain after curative therapy [Pachman et al. 2012]. Consequently, a number of clinical investigations with cannabinoids are currently focusing on the potential anti-analgesic effect of both THC and CBD.
So far, two randomized, double blind, placebo-controlled studies have been conducted with THC-CBD spray in chronic cancer pain. In a 2- week randomized double blind clinical study of 177 chronic cancer pain patients with resistant opioid-refractory pain, THC-CBD spray was given as an add-on therapy to step III opioids. The mean number of THC-CBD sprays by day were 8.75 (SD 5.14) by the end of the titrations phase (days 1–7) [Johnson et al. 2010]. The primary endpoint ‘change in mean pain NRS score from baseline’ was significantly reduced in patients using THC-CBD compared with placebo (p = 0.014). Most treatment-related AEs were mild or moderate. The results were similar in a long-term extension of the study [Johnson et al. 2013].
The second 5-week multicenter, randomized, double blind, placebo-controlled, parallel group, graded-dose design study by Portenoy and colleagues included 360 patients with advanced cancer and opioid-refractory pain [Portenoy et al. 2012]. They received either THC-CBD in three different doses (low dose: 1–4 sprays/day; medium dose: 6–10 sprays/day; high dose: 11–16 sprays/day) or placebo while doses of opioid medication were kept stable. Although the primary efficacy endpoint of 30% pain NRS responder rate did not reach statistical significance, the secondary endpoint continuous responder rate (0–100%) was statistically significant in favor of THC-CBD spray, especially in the low and medium dose groups (p = 0.008 and p = 0.038, respectively).
A phase III study program has been set up to test THC-CBD spray as add-on analgesic for adult patients with advanced cancer and opioid-refractory cancer pain who experience inadequate pain relief from optimized chronic opioid therapy; results are expected by the end of 2015 or early 2016. In Canada, an approval [Notice of Compliance with conditions (NOC/c)] for this indication was granted in 2007.
Spasticity in children with cerebral palsy or traumatic CNS injury
Cerebral palsy is a lifelong disorder and encompasses a large group of childhood movement and posture disorders. Prevalence has remained stable in the past 40 years at 2–3.5 cases per 1000 live births despite changes in antenatal and perinatal care [Colver et al. 2014] and spasticity is often a resistant concomitant symptom. Traumatic CNS injury in children is frequent and may have lifelong consequences in survivors, including spasticity [Pinto et al. 2012]. Based on the pediatric regulation plan of the European Union [Regulation (EC) No 1901/2006], a 12 week randomized, double blind, placebo-controlled study, followed by a 24 week open-label extension phase, has been set up for children aged between 8 and 18 years with spasticity related to cerebral palsy or traumatic CNS injury. The primary objective is to assess the efficacy of THC-CBD spray treatment. The final data collection date for primary outcome measure is December 2015.
Conclusion
This review has summarized the evidence for the efficacy and effectiveness of THC-CBD oromucosal spray in symptom management for patients with spasticity due to MS. About 80% of patients suffer from MS-associated spasticity or spasticity associated symptoms during their MS disease course. Of these, a substantial proportion is dissatisfied with the limited efficacy of classical oral antispasticity medications at tolerable doses or limited access to physiotherapy. For these patients with resistant moderate to severe MS-induced spasticity, THC-CBD spray can be a treatment option. Through its action via a different pathway (the endocannabinoid pathway) it might help patients who have not responded adequately to different first-line medication and might even lead to a reduction of the underlying medication and associated adverse events.
Efficacy of THC-CBD oromucosal spray has been proven in randomized, controlled clinical studies and its effectiveness confirmed in observational studies. It shows alleviation of symptoms together with improvement of daily activities and improvement in QoL for patients: approximately every second patient can benefit to achieve a re-levant reduction of spasticity. Responders can be identified in an initial 4 week titration period. This allows an early discontinuation of the drug in patients who do not perceive initial improvement and the maintenance of the therapy in those who are likely to gain a good long-term response. However, a patient’s perception as a confounder for the efficacy and effectiveness of the drug, due to the usage of a subjective measure (NRS scale), cannot be excluded completely, and so improvement of spasticity associated symptoms (spasms, urinary dysfunction, pain) and/or activities of daily living/QoL should be checked.
In addition, THC-CBD spray has been shown to have a good safety profile and is generally well tolerated, especially in comparison with other cannabis products that have been suspected to promote psychiatric AEs as well as abuse or addiction. This has not been reported for THC-CBD oromucosal spray. The more common AEs that are typical for THC-CBD treatment are dizziness, tiredness, fatigue and dryness of mouth, which are in most patients mild to moderate in intensity and often most pronounced in the initial phase. They may be prevented by a slow dose titration following the titration schema. However, the treating physician should pay attention to the patients during the first weeks of therapy to detect potential AEs and manage them adequately. In case of mild to moderate AEs, it is recommended to continue treatment with a constant spray number or to reduce it by 1 or 2 sprays until the AEs disappear. If the AEs cannot be fully resolved, discontinuation of THC-CBD needs to be considered.
Finally, THC-CBD oromucosal spray is able to respond to individual patient’s needs through a self-adaptable dosage form. This allows patients to optimize the doses to their personal needs and helps them to have self-influence on relief of their symptoms, AEs and factors that influence QoL.
As spasticity is a chronic disease, other studies over the next few years should examine the long-term influence of THC-CBD spray in daily practice to further confirm the findings of clinical trials regarding its effectiveness, especially in relation to first-line therapies, and to provide directions for use and methodological improvements.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest Statement: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: U.Z. has received honoraria for lecturing, travel expenses for attending meetings and support for research from Almirall, Bayer, Biogen Idec, Merck Serono, Novartis, Sanofi Aventis and TEVA. P. H. provided editorial support with funding from Almirall Hermal GmbH, Reinbek, Germany. The content was written entirely independently of Almirall and suggestions from Almirall were not considered mandatory. All conclusions are expressions of the author’s scientific viewpoint. The other authors declare no conflicts of interest in preparing this article.
Contributor Information
Uwe K. Zettl, Department of Neurology, University of Rostock, Gehlsheimer Straße 20, D-18147 Rostock, Germany.
Paulus Rommer, Department of Neurology, University of Rostock, Germany; Department of Neurology, Medical University of Vienna, Austria.
Petra Hipp, Saproma, Roetgen, Germany.
Robert Patejdl, Department of Neurology, University of Rostock, Germany Oscar-Langendorff-Institute of Physiology, University of Rostock, Germany.
References
- Anwar K., Barnes M. (2009) A pilot study of a comparison between a patient scored numeric rating scale and clinician scored measures of spasticity in multiple sclerosis. NeuroRehabilitation 24: 333–340. [DOI] [PubMed] [Google Scholar]
- Aragona M., Onesti E., Tomassini V., Conte A., Gupta S., Gilio F., et al. (2009) Psychopathological and cognitive effects of therapeutic cannabinoids in multiple sclerosis: a double-blind, placebo controlled, crossover study. Clin Neuropharmacol 32: 41–47. [DOI] [PubMed] [Google Scholar]
- Arroyo R., Vila C., Dechant K. (2013) Impact of Sativex® on quality of life and activities of daily living in patients with multiple sclerosis spasticity. J Comp Eff Res 3: 435–444. [DOI] [PubMed] [Google Scholar]
- Arseneault L., Cannon M., Witton J., Murray R. (2004) Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry 184: 110–117. [DOI] [PubMed] [Google Scholar]
- Ashworth B. (1964) Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 192: 540–542. [PubMed] [Google Scholar]
- Baker D., Pryce G., Croxford J., Brown P., Pertwee R., Makriyannis A., et al. (2000) Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J 15: 300–302. [DOI] [PubMed] [Google Scholar]
- Barnes M., Kent R., Semlyen J., McMullen K. (2003) Spasticity in multiple sclerosis. Neurorehabil Neural Repair 17: 66–70. [DOI] [PubMed] [Google Scholar]
- Beard S., Hunn A., Wight J. (2003) Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technology Assessment 7: 1–111. [DOI] [PubMed] [Google Scholar]
- Bisogno T., Hanus L., De Petrocellis L., Tchilibon S., Ponde D.E., Brandi I., et al. (2001) Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Brit J Pharmacol 134: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon R., Smith M. (1987) Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 67: 206–207. [DOI] [PubMed] [Google Scholar]
- Browne P., Chandraratna D., Angood C., Tremlett H., Baker C., Taylor B., et al. (2014) Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology 83: 1022–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin C., Davies P., Mutiboko I., Ratcliffe S. (2007) Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol 14: 290–296. [DOI] [PubMed] [Google Scholar]
- Collin C., Ehler E., Waberzinek G., Alsindi Z., Davies P., Powell K., et al. (2010) A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res 32: 451–459. [DOI] [PubMed] [Google Scholar]
- Colver A., Fairhurst C., Pharoah P. (2014) Cerebral palsy. Lancet 383: 1240–1249. [DOI] [PubMed] [Google Scholar]
- Crayton H., Rossman H. (2006) Managing the symptoms of multiple sclerosis: a multimodal approach. Clin Ther 28: 445–460. [DOI] [PubMed] [Google Scholar]
- Devos H., Brijs T., Alders G., Wets G., Feys P. (2013) Driving performance in persons with mild to moderate symptoms of multiple sclerosis. Disabil Rehabil 35: 1387–1393. [DOI] [PubMed] [Google Scholar]
- Di Marzo V., Petrosino S. (2007) Endocannabinoids and the regulation of their levels in health and disease. Curr Opin Lipidol 18: 129–140. [DOI] [PubMed] [Google Scholar]
- Eltayb A., Etges T., Wright S. (2013) An observational post-approval registry study of patients prescribed Sativex®. Results from clinical practice. Mult Scler 19(Suppl.): P1041 [Google Scholar]
- Farrar J., Troxel A., Stott C., Duncombe P., Jensen M. (2008) Validity, reliability, and clinical importance of change in a 0–10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther 30: 974–985. [DOI] [PubMed] [Google Scholar]
- Fernández O. (2014) Advances in the management of MS spasticity: recent observational studies. Eur Neurol 72 (Suppl. 1): 12–14. [DOI] [PubMed] [Google Scholar]
- Flachenecker P. (2013) A new multiple sclerosis spasticity treatment option: effect in everyday clinical practice and cost-effectiveness in Germany. Expert Rev Neurother 13(Suppl. 1): 15–19. [DOI] [PubMed] [Google Scholar]
- Flachenecker P., Stuke K. (2008) National MS registries. J Neurol 255: 102–108. [DOI] [PubMed] [Google Scholar]
- Flachenecker P., Henze T., Zettl U. (2014a) Spasticity in patients with multiple sclerosis-clinical characteristics, treatment and quality of life. Acta Neurol Scand 129: 154–162. [DOI] [PubMed] [Google Scholar]
- Flachenecker P., Henze T., Zettl U. (2014b) Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice - results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur Neurol 71: 271–279. [DOI] [PubMed] [Google Scholar]
- Flachenecker P., Henze T., Zettl U. (2014c) Long-term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannabidiol oromucosal spray) in clinical practice. Eur Neurol 72: 95–102. [DOI] [PubMed] [Google Scholar]
- Fleuren J., Voerman G., Erren-Wolters C., Snoek G., Rietman J., Hermens H., et al. (2010) Stop using the Ashworth Scale for the assessment of spasticity, J Neurol Neurosurg Psychiatry 81: 46–52. [DOI] [PubMed] [Google Scholar]
- Freidel M., Tiel-Wilck K., Schreiber H., Prechtl A., Essner U., Lang M. (2015) Drug-resistant MS spasticity treatment with Sativex® add-on and driving ability. Acta Neurol Scand 131: 9–16. [DOI] [PubMed] [Google Scholar]
- Gaoni Y., Mechoulam R. (1964) Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc 86: 1646–1647. [Google Scholar]
- García-Merino A. (2013) Endocannabinoid system modulator use in everyday clinical practice in the UK and Spain. Expert Rev Neurother 13: 9–13. [DOI] [PubMed] [Google Scholar]
- Goldenberg M. (2012) Multiple sclerosis review. P T 37: 175–184. [PMC free article] [PubMed] [Google Scholar]
- Guy G., Robson P. (2003) A Phase I, double blind, three-way crossover study to assess the pharmacokinetic profile of cannabis based medicine extract (CBME) administered sublingually in variant cannabinoid ratios in normal healthy male volunteers (GWPK02125). J Cannabis Ther 3: 121–152. [Google Scholar]
- Guy G., Stott C. (2005) The development of Sativex® – a natural cannabis-based medicine. In: Mechoulam R. (ed.), Cannabinoids as Therapeutics. Basel: Birkhäuser Verlag, pp. 231–263. [Google Scholar]
- Henze T., Mackensen S., von Lehrieder G., Zettl U., Pfiffner C., Flachenecker P. (2014) Linguistic and psychometric validation of the MSSS-88 questionnaire for patients with multiple sclerosis and spasticity in Germany. Health Qual Life Outcomes 12: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze T., Rieckmann P., Toyka K. (2006) Symptomatic treatment of multiple sclerosis. Multiple Sclerosis Therapy Consensus Group (MSTCG) of the German Multiple Sclerosis Society. Eur Neurol 56: 78–105. [DOI] [PubMed] [Google Scholar]
- Hewitt D., Ho T., Galer B., Backonja M., Markovitz P., Gammaitoni A., et al. (2011) Impact of responder definition on the enriched enrollment randomized withdrawal trial design for establishing proof of concept in neuropathic pain. Pain 152: 514–521. [DOI] [PubMed] [Google Scholar]
- Hilliard A., Stott C., Wright S., Guy G., Pryce G. Al-Izki, S.et al. (2012) Evaluation of the Effects of Sativex (THC BDS: CBD BDS) on inhibition of spasticity in a chronic relapsing experimental allergic autoimmune encephalomyelitis: a model of multiple sclerosis. ISRN Neurol, 2012: article ID 802649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobart J., Riazi A., Thompson A., Styles I., Ingram W., Vickery P., et al. (2006) Getting the measure of spasticity in multiple sclerosis: the Multiple Sclerosis Spasticity Scale (MSSS-88). Brain 129: 224–234. [DOI] [PubMed] [Google Scholar]
- Hoffman A., Lupica C. (2000) Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci 20: 2470–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Association for the Study of Pain (2004) Unrelieved pain is a major global healthcare problem. Available at: http://www.iasp-pain.org/files/Content/ContentFolders/GlobalYearAgainstPain2/20042005RighttoPainRelief/factsheet.pdf (accessed 7 October 2015).
- Izzo A., Borrelli F., Capasso R., Di Marzo V., Mechoulam R. (2009) Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 30: 515–527. [DOI] [PubMed] [Google Scholar]
- Johns A. (2001) Psychiatric effects of cannabis. Br J Psychiatry 178: 116–122. [DOI] [PubMed] [Google Scholar]
- Johnson J., Burnell-Nugent M., Lossignol D., Ganae-Motan E., Potts R., Fallon M. (2010) Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 39: 167–179. [DOI] [PubMed] [Google Scholar]
- Johnson J., Lossignol D., Burnell-Nugent M., Fallon M. (2013) An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage 46: 207–218. [DOI] [PubMed] [Google Scholar]
- Kabus C., Hecht M., Japp G., Jost W., Pohlau D., Stuckrad-Barre S., et al. (2006) Botulinum toxin in patients with multiple sclerosis. J Neurol 25 (Suppl. 1): I26–I8. [DOI] [PubMed] [Google Scholar]
- Kamin F., Rommer P., Abu-Mugheisib M., Koehler W., Hoffmann F., Winkelmann A., et al. (2014) Effects of intrathecal triamincinolone-acetonide treatment in MS patients with therapy-resistant spasticity. Spinal Cord. DOI: 10.1038/sc.2014.155. [DOI] [PubMed] [Google Scholar]
- Karschner E., Darwin W., Goodwin R., Wright S., Huestis M. (2011) Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem 57: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killestein J., Polman C. (2004) The therapeutic value of cannabinoids in MS: real or imaginary? Mult Scler 10: 339–340. [DOI] [PubMed] [Google Scholar]
- Kister I., Bacon T., Chamot E., Salter A., Cutter G., Kalina J., Herbert J. (2013) Natural history of multiple sclerosis symptoms. Int J MS Care 15: 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler J., Feneberg W., Meier M., Pollmann W. (2014) Clinical experience with THC:CBD oromucosal spray in patients with multiple sclerosis-related spasticity. Int J Neurosci 124: 652–656. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A., Lassmann H. (2014) Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Handb Clin Neurol 122: 15–58. [DOI] [PubMed] [Google Scholar]
- Leussink V., Husseini L., Warnke C., Broussalis E., Hartung H., Kieseier B. (2012) Symptomatic therapy in multiple sclerosis: the role of cannabinoids in treating spasticity. Ther Adv Neurol Disord 5: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte T., Rosenthal T., Roberts E., Lampinen S., Scott J., Allen R., et al. (2008) The contribution of cognition and spasticity to driving performance in multiple sclerosis’. Arch Phys Med Rehabil 89: 1753–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno Torres I., Sanchez A.J., Garcia-Merino A. (2014) Evaluation of the tolerability and efficacy of Sativex in multiple sclerosis. Expert Rev Neurother 14: 1243–1250. [DOI] [PubMed] [Google Scholar]
- Notcutt W., Langford R., Davies P., Ratcliffe S., Potts R. (2012) A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex(R) (nabiximols). Mult Scler 18: 219–228. [DOI] [PubMed] [Google Scholar]
- Novotna A., Mares J., Ratcliffe S., Novakova I., Vachova M., Zapletalova O., et al. (2011) A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex®) as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol 18: 1122–1131. [DOI] [PubMed] [Google Scholar]
- Oreja-Guevara C., Blasco R., Casanova B., Ordás C., Vila Silván C., Asensio D., et al. (2015) Observational safety study of THC/CBD oromucosal spray (Sativex) in multiple sclerosis patients with spasticity. Clin Exp Pharmacol 5: 184. doi: 10.4172/2161-1459.1000184. [DOI] [Google Scholar]
- Oreja-Guevara C., Gonzalez-Segra D., Vila C. (2013) Spasticity in multiple sclerosis: results of a patient survey. Int J Neurosci 123: 400–408.4 [DOI] [PubMed] [Google Scholar]
- Pachman D., Barton D., Swetz K., Loprinzi C. (2012) Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol 30: 3687–3696. [DOI] [PubMed] [Google Scholar]
- Penn R., Savoy S., Corcos D., Latash M., Gottlieb G., Parke B., et al. (1989) Intrathecal baclofen for severe spinal spasticity. New Engl J Med 320: 517–1521. [DOI] [PubMed] [Google Scholar]
- Perez J. (2006) Combined cannabinoid therapy via an oromucosal spray. Drugs Today 42: 495–503. [DOI] [PubMed] [Google Scholar]
- Perez J., Ribera M. (2008) Managing neuropathic pain with Sativex: a review of its pros and cons. Expert Opin Pharmacother 9: 1189–1195. [DOI] [PubMed] [Google Scholar]
- Pertwee R. (1999) Pharmacology of cannabinoid receptor ligands. Curr Med Chem 6: 635–664. [PubMed] [Google Scholar]
- Pertwee R. (2000) Neuropharmacology and therapeutic potential of cannabinoids. Addict Biol 5: 37–46. [DOI] [PubMed] [Google Scholar]
- Pertwee R. (2006) Cannabinoid pharmacology: the first 66 years. Br J Pharmacol 147(Suppl. 1): S163–S171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R. (2009) Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol 156: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto P., Poretti A., Meoded A., Tekes A., Huisman T. (2012) The unique features of traumatic brain injury in children. Review of the characteristics of the pediatric skull and brain, mechanisms of trauma, patterns of injury, complications and their imaging findings – part 1. J Neuroimaging 22: e1–e17. [DOI] [PubMed] [Google Scholar]
- Platz T., Eickhof C., Nuyens G., Vuadens P. (2005) Clinical scales for the assessment of spasticity, associated phenomena, and function: a systematic review of the literature. Disabil Rehabil 27: 7–18. [DOI] [PubMed] [Google Scholar]
- Portenoy R., Ganae-Motan E., Allende S., Yanagihara R., Shaiova L., Weinstein S., et al. (2012) Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain 13: 438–449. [DOI] [PubMed] [Google Scholar]
- Pryce G., Baker D. (2007) Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br J Pharmacol 150: 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo M., Hadjimichael O., Preiningerova J., Vollmer T. (2004) Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler 10: 589–595. [DOI] [PubMed] [Google Scholar]
- Robson P. (2011) Abuse potential and psychoactive effects of delta-9-tetrahydrocannabinol and cannabidiol oromucosal spray (Sativex), a new cannabinoid medicine. Expert Opin Drug Saf 10: 675–685. [DOI] [PubMed] [Google Scholar]
- Russo E., Guy G. (2006) A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses 66: 234–246. [DOI] [PubMed] [Google Scholar]
- Russo M., Calabro R., Naro A., Sessa E., Rifici C. D’Aleo, G.et al. (2015) Sativex in the management of multiple sclerosis-related spasticity: role of the corticospinal modulation. Neural Plast 2015: article ID 656582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre-Garriga J., Vila C., Clissold S., Montalban X. (2011) THC and CBD oromucosal spray (Sativex®) in the management of spasticity associated with multiple sclerosis. Expert Rev Neurother 11: 627–637. [DOI] [PubMed] [Google Scholar]
- Schoedel K., Chen N., Hilliard A., White L., Stott C., Russo E., et al. (2011) A randomized, double-blind, placebo-controlled, crossover study to evaluate the subjective abuse potential and cognitive effects of nabiximols oromucosal spray in subjects with a history of recreational cannabis use. Hum Psychopharmacol 26: 224–236. [DOI] [PubMed] [Google Scholar]
- Schuhfried GmbH (2012) Wiener test system, Manual FEV. Anlage 5 Nr. 2. Mödling, Austria: Schuhfried GmbH. [Google Scholar]
- Serpell M., Notcutt W., Collin C. (2013) Sativex long-term use: an open-label trial in patients with spasticity due to multiple sclerosis. J Neurol 260: 285–295. [DOI] [PubMed] [Google Scholar]
- Serpell M., Ratcliffe S., Hovorka J., Schofield M., Taylor L., Lauder H., et al. (2014) A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 18: 999–1012. [DOI] [PubMed] [Google Scholar]
- Shakespeare D., Boggild M., Young C. (2003) Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst Rev 4: CD001332. [DOI] [PubMed] [Google Scholar]
- Showalter V.M., Compton D.R., Martin B.R., Abood M.E. (1996) Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther 278: 989–999. [PubMed] [Google Scholar]
- Slof J., Gras A. (2012) Sativex® in multiple sclerosis spasticity: a cost-effectiveness model. Expert Rev Pharmacoecon Outcomes Res 12: 439–441. [DOI] [PubMed] [Google Scholar]
- Slof J., Ruiz L., Vila C. (2015) Cost-effectiveness of Sativex in multiple sclerosis spasticity: new data and application to Italy. Expert Rev Pharmacoecon Outcomes Res 16: 1–13. [DOI] [PubMed] [Google Scholar]
- Smyth M., Peacock W. (2000) The surgical treatment of spasticity. Muscle Nerve 23: 153–163. [DOI] [PubMed] [Google Scholar]
- Solowij N., Stephens R., Roffman R., Babor T., Kadden R., Miller M., et al. (2002) Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 287: 1123–1131. [DOI] [PubMed] [Google Scholar]
- Stevenson V. (2010) Rehabilitation in practice: spasticity management. Clin Rehabil 24: 293–304. [DOI] [PubMed] [Google Scholar]
- Stott C., White L., Wright S., Wilbraham D., Guy G. (2013a) A phase I study to assess the single and multiple dose pharmacokinetics of THC/CBD oromucosal spray. Eur J Clin Pharmacol 69: 1135–1147. [DOI] [PubMed] [Google Scholar]
- Stott C., White L., Wright S., Wilbraham D., Guy G. (2013b) A phase I study to assess the effect of food on the single dose bioavailability of the THC/CBD oromucosal spray. Eur J Clin Pharmacol 69: 825–834. [DOI] [PubMed] [Google Scholar]
- Stott C., White L., Wright S., Wilbraham D., Guy G. (2013c) A Phase I, open-label, randomized, crossover study in three parallel groups to evaluate the effect of Rifampicin, Ketoconazole, and Omeprazole on the pharmacokinetics of THC/CBD oromucosal spray in healthy volunteers. Springerplus 2: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott C., Wright S., Guy G. (2008) Comparison of pharmacokinetic profiles of inhaled delta-9-tetra-hydro-cannabinol (THC) from smoked cannabis with Sativex oromucosal spray in humans, implications for possible symptomatic treatment in multiple sclerosis. Eur J Neurol 15(Suppl. 3): 365. [Google Scholar]
- Sunnerhagen K. (2010) Stop using the Ashworth scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry 81: 2. [DOI] [PubMed] [Google Scholar]
- Svensson J., Borg S., Nilsson P. (2014) Costs and quality of life in multiple sclerosis patients with spasticity. Acta Neurol Scand 129: 13–20. [DOI] [PubMed] [Google Scholar]
- Syed Y., McKeage K., Scott L. (2014) Delta-9-tetrahydrocannabinol/ cannabidiol (Sativex®): a review of its use in patients with moderate to severe spasticity due to multiple sclerosis. Drugs 74: 563–578. [DOI] [PubMed] [Google Scholar]
- Vachová M., Novotná A., Mares J., Taláb R., Fiedler J., et al. (2014) A multicentre, double-blind, randomised, parallel-group, placebo-controlled study of effect of long-term Sativex® treatment on cognition and mood of patients with spasticity due to multiple sclerosis. J Mult Scler 1: 122. doi: 10.4172/jmso.1000122 [DOI] [Google Scholar]
- Vermersch P. (2014) MObility ImproVEment with spasticity in multiple sclerosis in Europe: the MOVE 1 EU study. Neurodegener Dis Manag 4: 407–415. [DOI] [PubMed] [Google Scholar]
- Voerman G., Gregoric M., Hermens H. (2005) Neurophysiological methods for the assessment of spasticity: the Hoffmann reflex, the tendon reflex, and the stretch reflex. Disabil Rehabil 27: 33–68. [DOI] [PubMed] [Google Scholar]
- Von Linnaeus C. (1753) Species plantarum, Species plantarum 2. Stockholm: L. Salvius. [Google Scholar]
- Wade D., Collin C., Stott C., Duncombe P. (2010) Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult Scler 16: 707–714. [DOI] [PubMed] [Google Scholar]
- Wade D., Makela P., House H., Bateman C., Robson P. (2006) Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler 12: 639–645. [DOI] [PubMed] [Google Scholar]
- Wade D., Makela P., Robson P., House H., Bateman C. (2004) Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients, Mult Scler 10: 434–441. [DOI] [PubMed] [Google Scholar]
- Ware M., Adams H., Guy G. (2005) The medicinal use of cannabis in the UK: results of a nationwide survey. Int J Clin Pract 59: 291–295. [DOI] [PubMed] [Google Scholar]
- Wood D., Burridge J., van Wijck F., McFadden C., Hitchcock R., Pandyan A., et al. (2005) Biomechanical approaches applied to the lower and upper limb for the measurement of spasticity: a systematic review of the literature. Disabil Rehabil 27: 19–32. [DOI] [PubMed] [Google Scholar]
- Zettl U., Henze T., Essner U., Flachenecker P. (2013) Burden of disease in multiple sclerosis patients with spasticity in Germany: mobility improvement study (MOVE 1). Eur J Health Econ 15: 953–966. [DOI] [PubMed] [Google Scholar]
- Zettl U., Stüve O., Patejdl R. (2012) Immune-mediated CNS diseases: a review on nosological classification and clinical features. Autoimmun Rev 11: 167–173. [DOI] [PubMed] [Google Scholar]
- Zwibel H. (2009) Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv Ther 26: 1043–1057. [DOI] [PubMed] [Google Scholar]