Introduction
Progressive encephalomyelitis with rigidity and myoclonus (PERM) is a severe syndrome that presents with autonomic features, hyperekplexia (brainstem myoclonus or excessive startle), painful spasms and breathing problems [Carvajal-Gonzalez et al. 2014]. Symptoms can be explained by the disruption of the inhibitory glycinergic synaptic transmission, which is prominent in the spinal cord and brainstem [Carvajal-Gonzalez et al. 2014]. The documented presence in the serum and cerebrospinal fluid (CSF) of antibodies against glycine receptors suggests an antibody-mediated pathogenesis with possible good response to immunotherapies [Dalakas et al. 2001; Kosmidis and Dalakas, 2010]. The precise immunopathogenetic mechanism and the triggering factors of PERM have not yet been clarified.
Brucella spp. is a pathogen able to invade the central nervous system (CNS) and cause an inflammatory response with activation of microglia and macrophages, apoptosis and increased expression of antigen-presenting molecules. Neurobrucellosis often manifests with meningoencephalitis and seems that T-cell mediated mechanisms in concert with microglia activation play a key role [Seidel et al. 2003].
We present a case of PERM preceded by a meningoencephalitis caused by Brucella spp. infection and highlight response to immunotherapy, especially rituximab, many months after disease onset. The possibility that neurobrucellosis may share some common immunopathogenic mechanisms with PERM is discussed.
Case report
A 47 year-old man was admitted to the intensive care unit (ICU) of our tertiary hospital because of progressive confusional mental state followed by tonic–clonic movements interpreted as seizures. The patient required intubation and mechanical ventilation. Some 3 weeks earlier, he had complained for sudden onset of diplopia. No chest pain, cough, diarrhea, nausea, fever, rash or arthralgias were reported. His past medical history was notable only for arterial hypertension.
Neurological examination showed restricted upward, downward and vertical gaze movements, bilateral horizontal gaze-evoked nystagmus, and diffuse spontaneous myoclonic spasms (supplementary video). Cranial and spinal magnetic resonance (MRI) scans were normal. Electroencephalography showed diffuse dysrhythmia without epileptiform discharges. CSF analysis revealed mild lymphocytic pleocytosis (Table 1). CSF polymerase chain reaction (PCR) studies for herpes simplex virus 1 (HSV1), HSV2, cytomegalovirus (CMV), Epstein–Barr virus (EBV), varicella zoster virus (VZV), Listeria, human immunodeficiency virus (HIV), Mycobacterium tuberculosis and West Nile virus were negative. Tests for paraneoplastic and autoimmune encephalitis antibodies were also negative. Thyroid function was normal.
Table 1.
CSF and serum profile of the patient on admission, after starting administration of antineurobrucellosis (ANB) treatment and after receiving rituximab.
| ICU admission | First course γ-globulin, methylprednizolone ANB therapy 10th day ICU |
Second course γ-globulin, diazepam ANB therapy 63th day ICU |
Third course γ-globulin, diazepam, baclofen ANB therapy 75th day ICU |
First course of 2 gr rituximab ANB therapy 5 months ICU |
Second course of 2 gr rituximab ANB therapy 8 months ICU |
Third course of 2 gr rituximab ANB therapy 12 months ICU |
|
|---|---|---|---|---|---|---|---|
| CSF cells/mm3 (lymphocytes) | 39 | 184 | 21 | – | 1 | 2 | N/A |
| CSF proteins (mg/dl) | 65 | 45 | 43 | – | 50 | 34 | N/A |
| CSF GlyR anti- | N/A | +++ (1:20) | ++ (1:20) | + (1:20) | NEG (1:20) | NEG (1:20) | N/A |
| Serum GlyR anti- | N/A | ++ (1:40) | ++ (1:40) | ++ (1:40) | + (1:40) | + (1:40) | N/A |
| Othalmoplegia | Yes | Yes | No | No | No | No | No |
| Hyperidrosis | Yes | Severe | Severe | Severe | Severe | Less | None |
| Myoclonus | Severe | Severe | Severe | Less | Less | None | None |
| Diplopia | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Startle –panic attacks | No | No | Yes | Yes | Yes | Less | No |
| Respiratory spasms bradycardia | No | No | Yes | Yes | No | No | No |
The fourth column represents the status of the patient just before the introduction of rituximab and while on ABN treatment. After the 3rd course of rituximab, muscle and respiratory spasms, hyperidrosis, panic attacks and episodes of bradycardia completely disappeared. Stiffness slightly improved.
CSF, cerebrospinal fluid; GlyR, glycine receptors; ICU, intensive care unit; N/A, not available; NEG, negative; POS, positive.
The patient was treated sequentially with anticonvulsants, acyclovir and ampicillin followed by high dose intravenous (IV) methylprednisolone (1 mg/kg) and IV immunoglobulin (0.4 per kg per day for 5 days).
The patient did not improve but continued to worsen with more myoclonic jerks, necessitating deep suppression with midazolame and temporarily with penthothal. Repeat computerized tomography (CT) brain scans showed a linear hemorrhagic focal lesion in the left thalamus. Because the patient had travelled to Turkey in the preceding 2 months, where he consumed fresh (unpasteurized) cheese, the CSF was tested for Brucella spp. by PCR and yielded positive results. The test was repeated and confirmed. Serologic tests for brucellosis were also performed on admission and 2 months later but were negative in both serum and CSF. Rifambicin 600 mg × 1, doxycycline 100 mg × 2 and trimethoptrime-sulphomethoxazole (160/800 SMX/TMP × 2) were then initiated for possible neurobrucellosis. On day 20, tracheostomy was performed.
In spite of treatment for meningoencephalitis-associated neurobrucellosis, the patient’s myoclonic jerks continued while symptoms of rigidity, hyperexcitability aggravated by noises, fear and dysautonomia with profound perspirations and episodes of bradycardia, dominated the clinical picture. Treatment with a combination of diazepam and baclofen failed.
The myoclonic spasms and rigidity were so severe that they resulted in hip fractures facilitated by osteoporosis due to prolonged immobility. Gradually muscle stiffness, particularly of axial and lower proximal limb muscles became prominent. Periodically, the patient required mechanical respiratory support due to sudden episodes of breath holding and consequent oxygen desaturation. PERM was suspected and testing for antiglutamic acid decarboxylase (GAD) and antiglycine receptors (GlyR) antibodies was performed in serum and CSF [Alexopoulos et al. 2013]. GAD testing was performed with a commercially available ELISA kit (Euroimmun). For anti-GlyR testing we used a cell-based assay where we transfected HEK293T cells with the glycine receptor α1 cDNA. Live cells were incubated with serum (1:40 dilution) or CSF (1:20 dilution) for 1 hour, then fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) and incubated with an antihuman secondary antibody (goat anti-human AlexaFluor® 568, Invitrogen).
Both CSF and serum were positive for anti-GlyR antibodies, corroborating PERM. Rituximab (1 g per 15 days) was then administrated; 6 courses of plasma exchange were also performed 2 months later without obvious benefit. The patient’s rigidity and mobility slowly started to improve. Assuming that his response was probably related to rituximab, he received a further 2 g in the following 7 month period. A slow but steady improvement became clear. Neurobrucellosis treatment (rifambicin and doxycycline) was maintained for 1 year. Despite a long, 12-month ICU stay, the patient no longer required ventilatory assistance, the sweating had improved, the myoclonic spasms resolved and he had started moving all his extremities. He was eventually discharged to a rehabilitation centre in perfect mental state. Now, 24 months after disease onset, he is unable to sit mainly due to the painful hip fractures for which replacement surgery is scheduled, he walks with a walker and is relapse free.
Repeated testing of CSF samples (including the original sample) showed that the anti-GlyR antibodies titers were falling as the patient was improving (Figure 1). After the second dose of rituximab, the antibodies were undetectable in the CSF but were still present (weakly positive) in the serum (Table 1).
Figure 1.
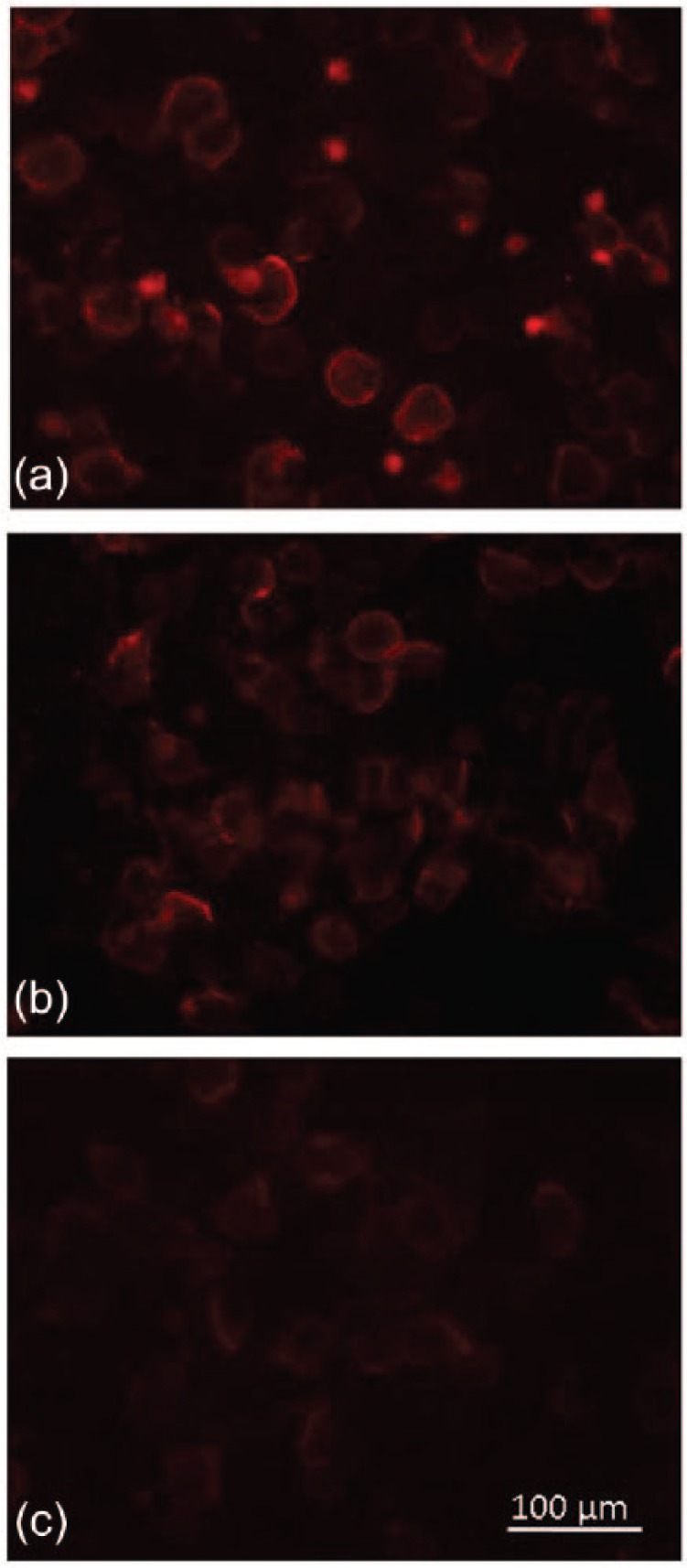
Screening for antiglycine receptor (GlyR) antibodies was performed using a cell-based-assay. Patient cerebrospinal fluid (CSF) (1:20 dilution) was applied on live human embryonic kidney 293T cells, transiently transfected with full-length GlyR enhanced green fluorescent protein (EGFP), followed by a goat-anti-human secondary antibody (AlexaFluor-568). (a) CSF obtained at 10 days in the intensive care unit (ICU). (b) CSF obtained at 63 days in the ICU (c) CSF obtained at 75 days in the ICU. Falling titers of anti-GlyR antibodies can be observed.
Scale bar 100μm.
Discussion
We report a patient with progressive encephalomyelitis with rigidity and myoclonus (PERM) associated with anti-GlyR antibodies in the CSF and serum possibly triggered by Brucella spp. who improved after 12 months in an ICU following intense immunotherapy. Before antibody testing for anti-GlyR antibodies, the patient was considered as having neurobrucellosis based on the positive PCR finding in the CSF and symptoms of myoclonus diplopia and meningoencephalitis. In retrospect, it is difficult to ascertain whether these symptoms were due to neurobrucellosis or constituted early symptoms of PERM. It is tempting to conclude that the introduction of antineurobrucellosis (ABN) therapy might have resulted in the early improvement of meningoencephalitis including his mental status, opthalmoplegia and disappearance of the Brucella spp. from the CSF, as was retested by PCR in the same laboratory. The brain hemorrhagic focal lesion also disappeared. It is very likely that rituximab was the most beneficial immunotherapy because improvement of PERM’s symptomatology was temporally connected to the initiation of rituximab therapy and was associated with reduction of GlyR titers. The persistence of low titers antibodies in the serum even after disease resolution is not, however, unexpected as often antineuronal antibodies may persist in the serum [Alexopoulos et al. 2011]. Whether this constitutes a sign for a possible relapse is unclear.
It may seem unusual that the patient’s serologic tests for brucellosis were negative both in blood and CSF, but this is not unprecedented as it has been described in neurobrucellosis when immunocompetent patients with localized disease fail to stimulate the host immune response [Celik et al. 2010]. A retrospective analysis of 1028 patients with focal and generalized brucellosis revealed that the standard tube agglutination (STA) and Coombs test were negative in 12 cases (1.1%) [Buzgan et al. 2010]. Overall, four different types of PCR assays, which ampify genomic DNA from Brucella melitensis, have excellent sensitivity for the detection of acute and relapsing brucellosis reaching 98–99% compared with conventional methods [Mitka et al. 2007]. Furthermore, various nonstandardized serological methods may lead to false negative or false positive results [Zerva et al. 2001].
Continuous muscular activity and partial stiff person syndrome (SPS) had been reported in 3 patients with positive Borrellia burgdorferi serology in the bloodstream, as well as in the CSF [Martin et al. 1990; Requena et al. 1995]. It is possible therefore that neurobrucellosis may have acted as a trigger for the anti-GlyR antibodies and PERM manifestations. Molecular mimicry cannot be excluded as has been documented for SPS following an acute West Nile virus infection [Hassin-Baer et al. 2004]. We tested for amino acid sequence similarity between the bacterial proteins and glycine receptors but none was found. However, this does not rule out mimicry as structural epitopes may be involved in cross-antigenic recognition.
It is also a possibility that the association between neurobrucellosis and PERM may be due to a common immunopathogenic basis via a T-cell mediated immune response and microglial activation. In this case, antibody generation in the CSF may be a secondary event. Post-mortem brain examination of a PERM patient has shown brain areas with CD3+ T-cell infiltrates in close apposition to neurons, along with invasion of hippocampal and pyramidal cells by CD8+ T cells and CD68+ microglial cell activation. These findings have been confirmed in more than one case [Whiteley et al. 1976]. This raises the possibility that, in PERM, autoantibody production in meningeal or perivascular spaces may be a secondary event following an initial sensitization in the periphery [Whiteley et al. 1976] by a pathogen, such as Brucella spp. A similar mechanism has also been implied in N-methyl-d-aspartate receptor (NMDAR) encephalitis following HSV infection [Prüss et al. 2012]. However, the possibility that a coincidental infection might have triggered a pre-existing, but not yet manifested, autoimmune encephalitic condition, as reported for HSV in NMDAR encephalitis, cannot be excluded.
Supplementary Material
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not for-profit sectors
Conflict of Interest Statement: The authors declare no conflict of interest in preparing this article.
Contributor Information
Eleni E. Magira, First Department of Critical Care, Evangelismos Hospital, School of Medicine, University of Athens, Athens, Greece
Harry Alexopoulos, Neuroimmunology Unit, Department of Pathophysiology, School of Medicine, University of Athens, Greece.
Evangelos Charitatos, First Department of Critical Care, Evangelismos Hospital, School of Medicine, University of Athens, Athens, Greece.
Dimitris Michas, Department of Neurology, Evangelismos Hospital, Athens, Greece.
Marinos C. Dalakas, Neuroimmunology Unit, Department of Pathophysiology, Faculty of Medicine, National and Kapodistrian University of Athens, 75 Mikras Asias Street, Athens, 115 27, Greece.
References
- Alexopoulos H., Akrivou S., Dalakas M. (2013) Glycine receptor antibodies in stiff-person syndrome and other GAD-positive CNS disorders. Neurology 81: 1962–1964. [DOI] [PubMed] [Google Scholar]
- Alexopoulos H., Kosmidis M., Dalmau J., Dalakas M. (2011) Paraneoplastic anti-NMDAR encephalitis: long-term follow-up reveals persistent serum antibodies. J Neurol 258: 1568–1570. [DOI] [PubMed] [Google Scholar]
- Buzgan T., Kasim Karahocagil M., Irmak H., Baran A., Karsen H., Evirgen O. (2010) Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int J Infect Dis 14: e469–e478. [DOI] [PubMed] [Google Scholar]
- Carvajal-González A., Leite M., Waters P., Woodhall M., Coutinho E., Balint B., et al. (2014) Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain 137: 2178–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik A., Yulugkural Z., Kilincer C., Hamamcioglu M., Kuloglu F., Akata F. (2010) Negative serology: could exclude the diagnosis of brucellosis? Rheumatol Int 32: 2547–2549. [DOI] [PubMed] [Google Scholar]
- Dalakas M., Fujii M., Li M., Lutfi B., Kyhos J., McElroy B. (2001) High-dose intravenous immune globulin for stiff-person syndrome. New Engl J Med 34: 1870–1876. [DOI] [PubMed] [Google Scholar]
- Hassin-Baer S., Kirson E., Shulman L., Buchman A., Bin H., Hindiyeh M., et al. (2004) Stiff-person syndrome following West Nile fever. Arch Neurol 61: 938–941. [DOI] [PubMed] [Google Scholar]
- Kosmidis M., Dalakas M. (2010) Practical considerations on the use of rituximab in autoimmune neurological disorders. Ther Adv Neurol Disord.3: 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Meinck H., Schulte-Mattler W., Ricker K., Mertens H. (1990) Borrelia burgdorferi myelitis presenting as a partial stiff man syndrome. J Neurol 237: 51–54. [DOI] [PubMed] [Google Scholar]
- Mitka S., Anetakis C., Souliou E, Diza E., Kansouzidou A. (2007) Evaluation of different PCR assays for early detection of acute and relapsing brucellosis in humans in comparison with conventional methods. J Clin Microbiol. 45:1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüss H., Finke C., Höltje M., Hofmann J., Klingbeil C., Probst C., et al. (2012) N-Methyl-d-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 72: 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena I., Arias M., Pardo J., Portela M., Alvarez J. (1995) Syndromes of continuous muscular activity: report of a central case (stiff-man) and a peripheral case (neuromyotonia) associated with neuroborreliosis. Rev Neurol 23: 129–133. [PubMed] [Google Scholar]
- Seidel G., Pardo C., Newman-Toker D., Olivi A., Eberhart C. (2003) Neurobrucellosis presenting as leukoencephalopathy: the role of cytotoxic T lymphocytes. Arch Pathol Lab Med 127: e374–e377. [DOI] [PubMed] [Google Scholar]
- Whiteley A., Swash M., Urich H. (1976) Progressive encephalomyelitis with rigidity. Brain 99: 27–42. [DOI] [PubMed] [Google Scholar]
- Zerva L., Bourantas K., Mitka S., Kansouzidou A., Legakis N. (2001) Serum is the preferred clinical specimen for diagnosis of human brucellosis by PCR. J Clin Microbiol 39: 1661–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


