Abstract
Objective
To estimate the maternal and fetal risks of smallpox vaccination during pregnancy.
Data Sources
MEDLINE, Web of Science, EMBASE, Global Health, ClinicalTrials.gov, and CINHAL from inception to September 2014.
Methods of Study Selection
We included published articles containing primary data regarding smallpox vaccination during pregnancy that reported maternal or fetal outcomes (spontaneous abortion, congenital defect, stillbirth, preterm birth, or fetal vaccinia).
Tabulations, Integration, and Results
The primary search yielded 887 articles. After hand-searching, 37 articles were included: 18 articles with fetal outcome data and 19 case reports of fetal vaccinia. Outcomes of smallpox vaccination in 12,201 pregnant women were included. Smallpox vaccination was not associated with an increased risk of spontaneous abortion (pooled relative risk [RR] 1.03, confidence interval [CI] 0.76–1.41), stillbirth (pooled RR 1.03, CI 0.75–1.40), or preterm birth (pooled RR 0.84, CI 0.62–1.15). When vaccination in any trimester was considered, smallpox vaccination was not associated with an increased risk of congenital defects (pooled RR 1.25, CI 0.99–1.56); however, first-trimester exposure was associated with an increased risk of congenital defects (2.4% compared with 1.5%, pooled RR 1.34, CI 1.02–1.77). No cases of fetal vaccinia were reported in the studies examining fetal outcomes; 21 cases of fetal vaccinia were identified in the literature, of which three neonates survived.
Conclusion
The overall risk associated with maternal smallpox vaccination appears low. No association between smallpox vaccination and spontaneous abortion, preterm birth, or stillbirth was identified. First-trimester vaccination was associated with a small increase in congenital defects, but the effect size was small and based on limited data. Fetal vaccinia appears to be a rare consequence of maternal smallpox vaccination but is associated with a high rate of fetal loss.
Although the eradication of smallpox is a modern public health triumph, there is ongoing concern that smallpox virus (variola) could be used as a bioterrorist weapon. Given that routine childhood smallpox vaccination was discontinued in 1971, reintroduction of smallpox into the human population could have devastating consequences. Smallpox epidemics are generally associated with case-fatality rates of 30% or higher among unvaccinated populations.1 In the event of a reintroduction, pregnant women are at increased risk of complications from smallpox disease, including a higher rate of hemorrhagic smallpox, with an overall case-fatality rate of 70% for unvaccinated pregnant women.2–4 As part of national preparedness efforts, the U.S. government has stockpiled smallpox vaccine with plans for use in persons exposed to or at high risk for exposure in the event of bioterrorism, including pregnant women.5 Although pregnant women are at increased risk of complications from smallpox disease, past recommendations have varied as to whether pregnant women should receive smallpox vaccine given the concern for fetal infection with vaccinia (the virus that is included in the smallpox vaccine) and reports of an increased risk of pregnancy loss and birth defects with vaccination.6–9 The recommendations of the Advisory Committee on Immunization Practices indicate that pregnant women should not be vaccinated in a preevent setting.10 However, new clinical guidance for smallpox vaccine use in a postevent setting recommends that pregnant women who are exposed to smallpox or at high risk for smallpox infection should be vaccinated.5 To better understand the risks of smallpox vaccination in pregnancy, we conducted a systematic review to address the following questions: 1) What are the risks of adverse maternal outcomes associated with smallpox vaccination in pregnancy? 2) What are the risks of fetal complications (spontaneous abortion, congenital defects, stillbirth, and preterm birth) associated with smallpox vaccination in pregnancy? 3) What is the risk of fetal vaccinia associated with smallpox vaccination in pregnancy?
Sources
Two authors (M.L.B. and D.M.-D.) in conjunction with an expert Centers for Disease Control and Prevention librarian trained in systematic reviews conducted a search of the existing literature. We searched the following databases: MEDLINE, Web of Science, EMBASE, Global Health, ClinicalTrials.gov, and CINHAL from inception to September 2014 to identify all listed publications in the medical literature discussing smallpox vaccine in pregnant women. We searched the databases using standard term indices to cover the concepts “smallpox,” “vaccination,” “pregnant,” “birth defect,” “preterm birth,” “miscarriage,” “maternal health,” and “fetal vaccinia.” We placed no restrictions on the language of publications for this review. After removal of duplicates, two authors (M.L.B. and D.M.-D.) screened the remaining publications for relevance and fulfillment of predefined inclusion and exclusion criteria discrepancies were adjudicated by a third reviewer (D.J.J.). In addition, we hand-searched the bibliographies of all selected articles to identify additional references and communicated with the one of the authors of the most recent smallpox vaccine studies.11,12
Study Selection
In accordance with Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines,13 we conducted a systematic review of the maternal and fetal risks associated with smallpox vaccination during pregnancy. Inclusion criteria for articles were that they must: 1) contain primary data regarding pregnant women who received smallpox vaccination, 2) describe vaccine exposures that occurred at or beyond 2 weeks of gestation, and 3) include a report of maternal or fetal (spontaneous abortion, congenital defect, stillbirth, or preterm birth) outcomes. Unpublished reports, abstracts, policy guidelines, and review articles were excluded; however, bibliographies of these articles were used to identify additional primary references. Given the limited data, no restrictions were placed on the vaccinia virus strain contained in the vaccine, the time period of vaccination, or the country of origin of the report. Non-English articles were professionally translated.
Two reviewers (M.L.B., D.M.-D.) screened all the titles and relevant abstracts and selected articles for full-text review. Abstracts that indicated a primary data source for cases of smallpox vaccination during pregnancy prompted a full-text review of the article. Relevant non-English articles identified by title, abstract, or preliminary translation were evaluated by electronic translation (Google) and if applicable were professionally translated by a medical translationist.
Data elements extracted from the articles included: 1) geographic location, 2) gestational age at time of vaccination, 3) maternal outcomes, and 4) fetal outcomes. The two primary outcomes were spontaneous abortion (defined as pregnancy loss at less than 20 weeks of gestation) and major structural congenital defects (defined as a defect present at birth that has a serious, adverse effect on the neonate's health, development, or functional ability, as described by the Metropolitan Atlanta Congenital Defects Program).14 Primary outcomes were chosen based on previous studies that reported an increased risk of spontaneous abortion, congenital defects, or both.6–8 Secondary outcomes were stillbirth (defined as fetal death at 20 weeks of gestation or greater) and preterm delivery (defined as delivery at less than 37 completed weeks of gestation). In addition, cases of fetal vaccinia were identified and summarized.
Meta-analysis was performed using STATA 11 with the METAN and METAPROP software routines. Pooling of data was considered if there were at least two studies available for a particular outcome. In studies reporting a comparator (no vaccination), categorical data from relevant studies were used to calculate relative risks (RRs) with 95% confidence intervals (CIs). To avoid Simpson's15 paradox, which occurs when the total numerator is simple divided by the total denominator, we estimated the absolute risks by pooling the proportions form the individual studies using a random-effects model. For studies without a comparator, we performed meta-analysis of proportions and their exact binomial CIs using random-effects models. A correction factor of 0.001 was used when data from a study included a value of zero to permit calculation of proportions and 95% CIs. We combined data using DerSimonian-Laird random-effects model even when there was no evidence of statistical heterogeneity.16 This more conservative random-effects model was chosen given the possibility of clinical heterogeneity between studies, regardless of statistical heterogeneity. This approach also provides more conservative estimates of effect size. Heterogeneity between studies was tested using Cochran's Q and Higgins I2 tests.17 We conservatively considered heterogeneity as significant for P <.1 or I2 greater than 30% in recognition of the modest statistical power of these tests for heterogeneity. Publication bias was assessed for the primary outcomes using funnel plots and formally tested using Harbord's test.18 Analyses were stratified by timing of vaccination (first trimester or any trimester) when studies reported that information.
We calculated the rate of spontaneous abortions as the number of documented spontaneous abortions divided by the total number of pregnant women with known final outcome (live birth, spontaneous abortion, or stillbirth) in the study. The rates of preterm birth or congenital defects were calculated using total number of live births reported in each study as the denominator. For congenital defects, if we were unable to classify a defect as major or minor, either because no description was provided or because the description was insufficient, we opted to be more inclusive and counted these as major defects. Given that most congenital defects originate in the first trimester of pregnancy (during embryogenesis), we performed an analysis examining the risk of congenital defects after first-trimester exposure. We also included an analysis of exposure during any trimester because some congenital defects can occur later in pregnancy and a number of studies included in our analysis did not specify trimester of exposure.
Results
The flow diagram of study identification for the systematic review is illustrated in Figure 1. Our search identified a total of 887 nonduplicate articles. A total of 865 English language articles were identified, of which 17 met our inclusion criteria. Reviewing references identified 11 additional English articles for inclusion. A total of 31 non-English articles were professionally translated based on the initial review of the English or translated abstract or from hand-searching, of which nine met inclusion criteria. Overall, 37 articles reported primary data; 18 articles described fetal outcomes in 12,201 pregnant women vaccinated against smallpox (Table 1); none of these included any cases of fetal vaccinia. The other 19 articles describe cases of fetal vaccinia (Table 2). Adverse maternal outcomes were not specifically evaluated in any of the identified articles. No cases of maternal morbidity or mortality with smallpox vaccination were reported. Additionally, maternal morbidity or mortality was also not reported in any of the comparison groups. The results summary for the two primary outcomes, spontaneous abortion and congenital defect, and the secondary outcomes, stillbirth and preterm birth, is displayed in Table 3.
Fig. 1.
Flow diagram of studies included in systematic review and meta-analysis of smallpox vaccination in pregnancy.
Table 1. Characteristics of Included Studies With and Without a Comparison Group Evaluating Risk of Spontaneous Abortion, Congenital Defects, Stillbirth, or Preterm Birth With Smallpox Vaccination in Pregnancy.
| Study | Year | Country | Study Design | Inclusion Criteria | Vaccinated in Pregnancy | Vaccinated in First Trimester | Unexposed (n=33,487) | Level of Evidence* |
|---|---|---|---|---|---|---|---|---|
| Studies Including a Comparison Group | n=10,825 | n=6,040 | ||||||
| Ryan et al12 | 2008 | U.S. | Retrospective cohort | Military women vaccinated during pregnancy | 882 | 672 | Military women with no history of smallpox vaccination, n=23,685 | II |
| Naderi et al8 | 1975 | Iran | Prospective cohort | Registered pregnant women vaccinated during mass vaccination program | 1,522 | 211 | Nonvaccinated pregnant women in same institution during the year after mass vaccination program, n=2,024 | II |
| Ladnyi et al19 | 1974 | Russia | Prospective cohort | Registered pregnant women vaccinated during mass vaccination program | 1,172 | 366 | Pregnant women seen during interepidemic period not vaccinated during pregnancy, n=777 | II |
| Rajhvajn et al20 | 1973 | Croatia | Retrospective survey | Pregnant women conceived before mass vaccination reporting vaccination up to fourth month of pregnancy | 257 | Pregnant women conceived before mass vaccination who were not vaccinated during pregnancy, n=266 | II | |
| Janiszewski et al21 | 1966 | Poland | Retrospective cohort | Pregnant women vaccinated during pregnancy | 205 | 193 | Pregnant women during the same time period who were not vaccinated, n=694 | II |
| Bourke et al22 | 1964 | Ireland | Prospective cohort | Registered pregnant women vaccinated during smallpox outbreak | 122 | 54 | Pregnant women not vaccinated during pregnancy seen for first prenatal visit on same day as exposed (four adjacent charts), n=448 | II |
| Liebeschuetz et al23 | 1964 | England | Prospective cohort | Pregnant women less than 5 mo vaccinated during mass vaccination program | 157 | 126 | Pregnant women not vaccinated during pregnancy, n=1,657 | II |
| Abramowitz et al28 | 1957 | South Africa | Retrospective cohort | Pregnant women less than 20 wk of gestation vaccinated during smallpox outbreak | 1,121 | Pregnant women not vaccinated during pregnancy, n=201 | II | |
| Bieniarz et al7 | 1956 | Poland | Retrospective cohort | Pregnant women vaccinated during mass vaccination | 495 | Pregnant women not vaccinated during pregnancy, n=1,376 | II | |
| Bellows et al24 | 1949 | U.S. | Prospective cohort | Registered pregnant women vaccinated during mass vaccination program | 720 | 246 | Pregnant women not vaccinated during pregnancy, n=173 | II |
| Greenberg et al29 | 1949 | U.S. | Retrospective cohort | Pregnant women with viable neonate who were vaccinated at less than 3 mo during mass vaccination program | 4,172 | 4,172 | Pregnant women in same clinic or hospital who were not vaccinated, n=2,186 | II |
| Studies Without a Comparison Group | n=1,376 | n=331 | ||||||
| Ryan et al11 | 2008 | U.S. | Prospective registry | Military women vaccinated during pregnancy | 208 | 203 | NA | II |
| Topciu et al30 | 1976 | Romania | Prospective cohort | Pregnant women vaccinated during pregnancy | 608 (46)† | 7† | NA | III |
| Krstajic et al25 | 1973 | Croatia | Prospective cohort | Pregnant women vaccinated during pregnancy | 26 (11)† | 11† | NA | III |
| Engstrom et al26 | 1966 | Sweden | Prospective cohort | Pregnant women (nurses, professionals, hospital patients, those traveling abroad) vaccinated during pregnancy | 170 | 19 | NA | III |
| Wentworth et al31 | 1966 | Wales | Retrospective cohort | Pregnant women vaccinated during smallpox outbreak at the time a continuous series of placentas were being collected | 65 | 56 | NA | III |
| MacArthur et al6 | 1952 | Scotland | Retrospective survey | Pregnant women reporting vaccination during pregnancy at time of outbreak | 170 | 34 | NA | III |
| Urner et al27 | 1925 | U.S. | Prospective cohort | Registered pregnant women vaccinated during smallpox outbreak | 129 | 1 | NA | III |
NA, not applicable.
Level of evidence: I, a randomized, controlled trial; II, a cohort or case-controlled study that includes a comparison group; III, an uncontrolled descriptive study including case series.
Excludes women who underwent abortion or had an early pregnancy loss.
Table 2. Cases of Fetal Vaccinia Identified in 19 Articles Reporting a Total of 21 Cases.
| Author | Year | Country | Gestational Age at Vaccination | Gestational Age or Weight at Delivery | Outcome | Pathologic or Clinical Summary | Virus Isolated |
|---|---|---|---|---|---|---|---|
| Dos Santos et al44 | 1985 | Brazil | 12 wk | 20 wk | Live birth: neonatal death | Skin with large number of typical vaccinia lesions | Yes |
| CDC49 | 1979 | Australia | 8 wk | 24 wk | Live birth: neonatal death | Multiple skin lesions and liver lesion | Yes |
| Harley and Gillespie34 | 1972 | England | 3–4 mo | 2,800 g | Live birth: survived | “Very red” scars on body at time of delivery, scarring of eyelid, macula destruction and hypoplastic bone changes | Not tested |
| Aitkens et al43 | 1968 | Australia | 15 wk | 23–24 wk | IUFD | Skin with numerous ring or target-shaped lesions, white necrotic lesions in liver, heart, and lungs | Yes |
| Lane9 | 1970 | U.S. | 1–2 mo | 32 wk | Live birth: survived | Pock-like scarring entire body, well developed normally | Not stated |
| Green et al—case 133 | 1966 | Scotland | 24 wk | 30 wk | IUFD | Skin lesions concentric, target-like, foci of necrosis kidney, liver, lungs | Not tested |
| Green et al—case 233 | 1966 | Scotland | 14 wk | 18 wk | Spontaneous abortion | Scattered target-like lesions on skin, foci of necrosis in lungs and liver | Yes |
| Entwistle et al32 | 1962 | Wales | 19 wk | 25 wk | IUFD | Skin with large, raised, congested umbilicated lesions, lesions in kidney, liver, and lungs | Yes |
| Killpack35 | 1963 | England | 12 wk | 22 wk | IUFD | Numerous hemorrhagic spots on skin | Not tested |
| Lycke et al46 | 1963 | Sweden | 23 wk | 28 wk | Live birth: neonatal death | Generalized skin lesions and liver necrosis | Yes |
| Naidoo and Hirsch37 | 1963 | London | 22–24 wk | 30–32 wk | Live birth: neonatal death | Discrete red circular ulcers covering skin, lesions in lungs, liver, and kidneys | Yes |
| Toendury and Foukas —case 147 | 1964 | Greece | 4 wk | 18 wk | IUFD | Typical smallpox exanthem on body | Not tested |
| Toendury and Foukas—case 247 | 1964 | Greece | 3 wk | 12 wk | Voluntary interruption of pregnancy | Necrosis foci in villi, subepidermal necrosis | Not tested |
| Tucker and Sibson38 | 1962 | England | 2.5 mo | 22 wk | Live birth: neonatal death×2 | Discrete vaccinial lesions over body | Yes |
| Waddington et al39 | 1964 | Wales | 6 mo | 32 wk | Live birth: survived | Widespread vesicles resembling progressive vaccinia | No |
| Kropholler and Voorhoeve-Den Hartog45 | 1962 | Holland | 15 wk | 22 wk | IUFD | Generalized skin lesions which resembled vaccinia eruptions | Not tested |
| Hood41 | 1963 | U.S. | 1.5 mo | 4.5–5 mo | Spontaneous abortion | Numerous circular lesions with slightly depressed central area, necrotic lesions of lung | No |
| Wiersum48 | 1955 | Netherlands | 6 wk before | Premature | IUFD | Numerous spots on skin strongly resembling smallpox pustules | Not tested |
| MacDonald and MacArthur36 | 1953 | Scotland | 3 mo | 6 mo | Live birth: neonatal death | Multiple discrete circular, umbilicated skin lesions | Not tested |
| Lynch42 | 1932 | U.S. | 5 mo | 6 mo | Live birth: neonatal death | Generalized eruptions consisting of raised circular plaques with central depression, lesions on liver | Not tested |
| Jenner40 | 1809 | England | 8 mo | 9 mo | Live birth: neonatal death | Many skin eruptions, bearing much the appearance of smallpox | Yes |
CDC, Centers for Disease Control and Prevention; IUFD, intrauterine fetal death.
Table 3. Results Summary for Spontaneous Abortion, Congenital Defects, Stillbirth, and Preterm Birth With Smallpox Vaccination in Pregnancy.
| Outcome | No. of Studies | Control (n/N) | Control (%)* | Vaccinated (n/N) | Vaccinated (%)* | Measure of Effect* | Effect Size (95% CI) | Heterogeneity (%) |
|---|---|---|---|---|---|---|---|---|
| Primary | ||||||||
| Spontaneous abortion | ||||||||
| First trimester | 3 | 136/3,495 | 5.5 | 44/770 | 5.7 | Pooled RR (random) | 1.62 (0.43–6.19) | I2 = 92.9 |
| All trimesters | 8 | 457/7,560 | 6.7 | 246/4,640 | 7.7 | Pooled RR (random) | 1.03 (0.76–1.41) | I2=62.5 |
| Congenital defect | ||||||||
| First trimester | 5 | 917/28,879 | 1.5 | 80/5,274 | 2.4 | Pooled RR (random) | 1.34 (1.02–1.77) | I2=0.0 |
| All trimesters | 8 | 946/30,804 | 1.6 | 153/8,781 | 2.1 | Pooled RR (random) | 1.25 (0.99–1.56) | I2=0.0 |
| Secondary | ||||||||
| Stillbirth | ||||||||
| All trimesters | 7 | 121/5,974 | 2.4 | 126/5,009 | 2.4 | Pooled RR (random) | 1.03 (0.75–1.4) | I2=10.6 |
| Preterm birth | ||||||||
| All trimesters | 6 | 2,205/29,370 | 10.0 | 638/7,953 | 8.0 | Pooled RR (random) | 0.84 (0.62–1.15) | I2=85.6 |
CI, confidence interval; RR, relative risk.
The percentages are pooled from the individual studies using random-effects models.15
Thirteen of the included studies reported data on spontaneous abortion, a primary outcome of this review.6–8,11,19–27 Figure 2 shows the effect of smallpox vaccination on spontaneous abortion in studies with a comparison group including a subanalysis of first-trimester exposure. Overall, smallpox vaccination was not associated with a significantly increased risk of spontaneous abortion (pooled RR 1.03, 95% CI 0.76–1.41) (Table 3). When the three studies restricted to first-trimester exposure were analyzed, there was also no significantly increased risk of spontaneous abortion (pooled RR 1.62, 95% CI 0.43–6.19). The rate of spontaneous abortion after smallpox vaccination at any time during pregnancy ranged from 0% to 24.1% and after first-trimester vaccination, it ranged from 0% to 29.4%. This was comparable with the range of 1–22.2% in unexposed pregnant women. There was significant statistical heterogeneity between studies (I2=78.6%, P≤.001). The funnel plot and the Harbord test suggest no evidence of publication bias for this outcome (Harbord P=.99; Fig. 2). Additionally, the proportional meta-analysis of five studies without a comparison group found an overall rate of spontaneous abortion in all trimesters of 4% (95% CI 1–8%) and of three studies with first-trimester exposure, the rate of spontaneous abortion was found to be 14% (95% CI 3–26%).6,11,25–27
Fig. 2.
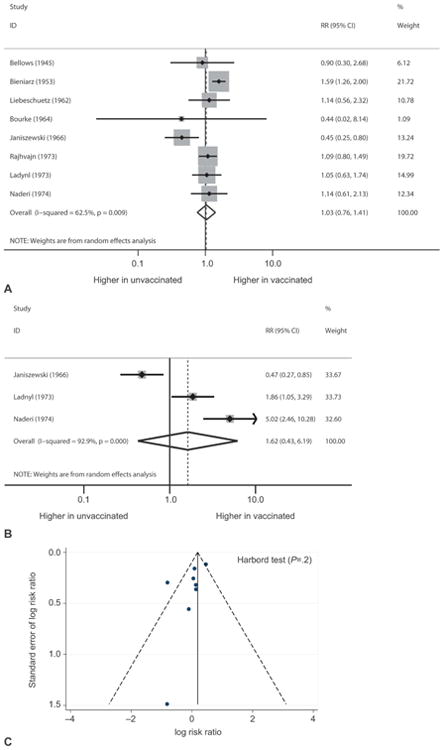
Forest plots showing the effect of smallpox vaccination on risk of spontaneous abortion in all trimesters (A) and in the first trimester (B). Funnel plot with pseudo 95% confidence limits showing the effect of smallpox vaccination on spontaneous abortion (C). RR, risk ratio; CI, confidence interval.
Fifteen studies reported data on congenital defects with smallpox vaccination, another primary outcome of this review.6,8,11,12,20–26,28–31 Figure 3 shows the effect of smallpox vaccination on congenital defects in studies with a comparison group including a subanalysis of first-trimester exposure. Overall, smallpox vaccination was not associated with a significantly increased risk of congenital defects (pooled RR 1.25, 95% CI 0.99–1.56) (Table 3). When the five studies restricted to first-trimester smallpox vaccine exposure were analyzed, an association with congenital defects was seen (2.4% compared with 1.5%, pooled RR 1.34, 95% CI 1.02–1.77).8,12,21,22,29 Overall, the rate of congenital defects ranged from 0.0% to 4.5% among women vaccinated at any time during pregnancy, 0.6–4.5% among women vaccinated in the first trimester, and 0.8–3.7% among unexposed women. In the one study in which first-trimester vaccination was associated with a statistically increased risk of congenital defects (RR 2.60, 95% CI 1.07–6.32), three of the six neonates with congenital defects had clubfoot.8 No specific pattern of multiple defects was observed with smallpox vaccination. The other four studies evaluating first-trimester exposure to smallpox vaccination in pregnancy were not associated with a statistically increased risk of congenital defects.12,21,22,29 No statistical heterogeneity was noted between studies (I2=0.0%, P=.9). The funnel plot and the Harbord test suggest publication bias for this outcome (Harbord P=.045; Fig. 3). Additionally, the proportional meta-analysis of seven studies without a comparison group found a 1% (95% CI 0–1%) overall rate of congenital defects for exposure in all trimesters of pregnancy. For the four studies with first-trimester exposure, the pooled rate of congenital defects was 0% (95% CI 0.0–0.0%).6,11,20,25,26,30,31
Fig. 3.
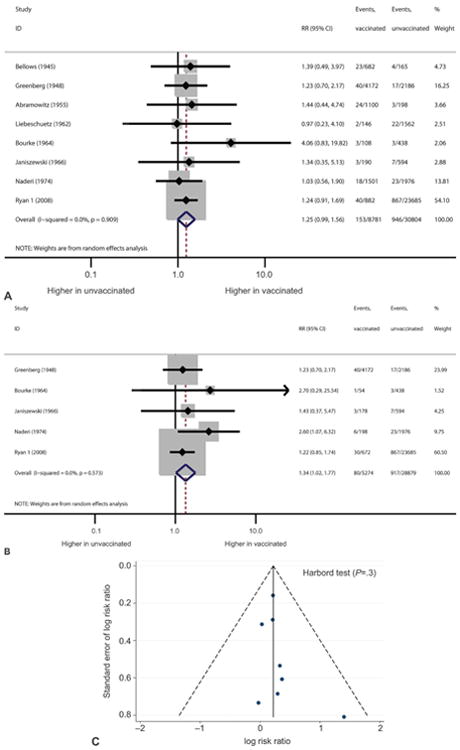
Forest plots showing the effect of smallpox vaccination on risk of congenital defects in all trimesters (A) and in the first trimester (B). Funnel plot with pseudo 95% confidence limits showing the effect of smallpox vaccination on risk of congenital defects (C). RR, risk ratio; CI, confidence interval.
Eleven studies reported data on stillbirth as an outcome.6,8,19,21–24,26,28,30,31 Among eight studies that included a comparison group, there was no significantly increased risk of stillbirth with smallpox vaccination (pooled RR 1.03, 95% CI 0.75–1.40) (Table 3). Stillbirth rates ranged from 1.5% to 4.2% among women vaccinated at any time during pregnancy, 0.9–14.7% among those vaccinated in the first trimester, and 1.2–3.9% among the unexposed cohort. The three largest studies, which each included greater than 1,000 vaccinated pregnant women, found a stillbirth incidence of 1.5%, 1.9%, and 4.2%, none of which were significantly different than the rate among unexposed women of 0.9%, 1.5%, and 3.9%, respectively.8,19,28 The proportional meta-analysis of four studies without a comparison group found an overall rate of stillbirth of 4% (95% CI 2–6%).6,26,30,31
Eleven studies included data on preterm birth.6,8,11,12,19–21,25,29–31 Among six studies that included a comparison group, no increased risk of preterm birth with smallpox vaccination (pooled RR 0.84, 95% CI 0.62–1.15) was observed (Table 3). The preterm birth rate among women vaccinated at any time during pregnancy in these studies ranged from 0.0% to 11.1% compared with an average rate of 7.0–38.8% in unvaccinated women. The proportional meta-analysis of five studies without a comparison group found an overall rate of preterm birth of 3% (95% CI 1–5%).6,11,25,30,31
Twenty-one cases of reported fetal vaccinia were identified from 19 articles (Table 2). The cases were reported from 1809 to 1985; the United Kingdom had the most reported cases (n=10)32–40 and three cases were from the United States.9,41,42 In these reported cases of fetal vaccinia, the stage of pregnancy at which the mother was vaccinated ranged from 3 weeks to 8 months. The interval between vaccination and delivery and pregnancy loss was 4 weeks to 24 weeks of gestation. Fetal vaccinia was associated with a high rate of fetal or neonatal loss; among the 21 reported cases, only three neonates survived.9,34,39 There were two cases of spontaneous abortion, one elective termination of pregnancy, seven stillbirths, and eight live births followed by death immediately or within 8 days of life (including one twin gestation at 22 weeks of gestation, of which both died).9,32–49 In 9 of the 18 cases of fetal and neonatal loss, vaccinia virus was isolated from fetal tissue or placenta.32,33,37,38,40,43,44,46,49 In the three cases in which neonates with reported fetal vaccinia survived, vaccinia virus was not reported to have been isolated.9,34,39 Fetal and neonatal losses from fetal vaccinia occurred after vaccination in all three trimesters.
Discussion
Smallpox vaccination among pregnant women was not associated with an increased risk of spontaneous abortion, preterm birth, or stillbirth, but first-trimester smallpox vaccination was associated with a small increase in the risk of congenital defects. No cases of fetal vaccinia were described in the 12,201 vaccinated pregnant women, and only 21 case reports of fetal vaccinia were identified in the literature. Risk of adverse maternal outcomes with smallpox vaccination could not be evaluated from these data.
Although we found an association between first-trimester exposure and congenital defects, the effect size was small and based on five studies. The pooled RR for congenital defects with first-trimester vaccination was 1.34. When compared with a background risk of congenital defects of approximately 3%, the absolute risk increase is approximately 1% for congenital defects among vaccinated women.50 On review of specific defects, one study reported an increased risk of clubfoot; however, this was an isolated finding.8 Clubfoot is a structural defect involving malposition of the foot and ankle, believed to occur early in pregnancy. Its pathogenesis is not well understood, but vascular disruption, neurologic disorder, and abnormal connective tissue development have all been proposed as possible mechanisms.51,52 In the largest study to date, Ryan et al12 found no increased risk of congenital defects, including clubfoot. Additionally, we found evidence of publication bias, which may exaggerate the association between first-trimester vaccination and smallpox vaccination. With the exception of the study by Ryan et al, the papers included in this analysis were published before 1976 and limited data were available on ascertainment of the defects. Furthermore, in the meta-analysis of proportions, the risk of congenital defects with first-trimester exposure in studies without a comparison group was essentially null (0.0 [95% CI 0.0–0.0]).
Two studies identified did not meet inclusion criteria because they focused on specific birth outcomes. In one study, smallpox vaccination was not associated with ocular abnormalities.53 In the second study, a wide range of neurologic abnormalities including seizures and poor movement were demonstrated more frequently in the vaccinated group.54 No other study reported an association between vaccination and neurologic abnormalities.
Our meta-analysis found that smallpox vaccination during pregnancy was not associated with an increased risk of spontaneous abortion. MacArthur et al6 first examined this question, reporting a 29.4% risk of miscarriage with first-trimester vaccination. Estimating rates of spontaneous abortions is challenging. Spontaneous abortion is common with a baseline risk of approximately 12–15%.55 The range of spontaneous abortion we report is 1.1–29.4%, a broad estimate that lacks precision.
Our meta-analysis also found no association between smallpox vaccination and stillbirth.8,19,21–24,28 Although MacArthur et al demonstrated an increased rate of stillbirth in association with first-trimester vaccination exposure (14.7%), the small sample size (n=34) and lack of a control group limit applicability of these findings.6 Additionally, this meta-analysis found no association between smallpox vaccination and preterm birth. Preterm birth is multifactorial with a number of known risk factors none of which were controlled for in these studies.56
Although smallpox vaccination appears to pose a risk of fetal vaccinia, the risk can be presumed to be very low based on the small number of cases identified (n=21) from our search spanning 1,809 to the present. Fetal vaccinia appears to be associated with a high rate of pregnancy loss. The diagnosis of fetal vaccinia in many reports was presumptive based on fetal and neonatal features. However, vaccinia virus was isolated from the fetus or placenta in some cases, demonstrating that transplacental transmission can occur.
This systematic review provides a comprehensive summary of the literature regarding smallpox vaccination in pregnancy. A strength of this review was our extensive search of the literature, including non-English articles. Given that smallpox is now eradicated and additional data are unlikely to become available, this review is useful to guide clinical recommendations regarding smallpox vaccine use in pregnancy during an emergency bioterrorist response. Our study also has several limitations. There is paucity of evidence regarding safety of smallpox vaccination in pregnancy and the available evidence is generally of poor quality. No randomized clinical trial data were available. Additionally, the studies differed in timeframe, geographic location, smallpox vaccine used, study designs, and outcomes evaluated.
In conclusion, we did not find an association between spontaneous abortion, preterm birth, or stillbirth and smallpox vaccination; however, first-trimester smallpox vaccination was associated with a small increase in congenital defects. The effect size observed was small and no specific pattern of defects was observed. These findings must be viewed in the context of the high morbidity and mortality of smallpox disease in pregnancy, both for the mother and fetus. Fetal vaccinia appears to be an extremely rare consequence of maternal smallpox vaccination. Despite the limits of these data, the overall risk of using smallpox vaccine during pregnancy appears low and supports the recent Centers for Disease Control and Prevention recommendation that pregnant women exposed to smallpox or at high risk for smallpox infection should be vaccinated.5
Acknowledgments
The authors thank Joanna Taliano for assistance with literature searches and the Health Communication Science Office in the National Center for Emerging and Zoonotic Infectious Diseases for translational support.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Fenner F. A successful eradication campaign. Global eradication of smallpox. Rev Infect Dis. 1982;4:916–30. doi: 10.1093/clinids/4.5.916. [DOI] [PubMed] [Google Scholar]
- 2.Rao AR, Prahlad I, Swaminathan M, Lakshmi A. Pregnancy and smallpox. J Indian Med Assoc. 1963;40:353–63. [PubMed] [Google Scholar]
- 3.Lane JM. Remaining questions about clinical variola major. Emerg Infect Dis. 2011;17:676–80. doi: 10.3201/eid1704.101960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishiura H. Smallpox during pregnancy and maternal outcomes. Emerg Infect Dis. 2006;12:1119–21. doi: 10.3201/eid1207.051531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson B, Damon I, Pertowski C, Meaney-Delman D. Clinical guidance for smallpox vaccine use in a post-event vaccination program: CDC recommendations. MMWR Recomm Rep. 2015;64:1–26. [PubMed] [Google Scholar]
- 6.MacArthur P. Congenital vaccinia and vaccinia gravidarum. Lancet. 1952;2:1104–6. doi: 10.1016/s0140-6736(52)90940-9. [DOI] [PubMed] [Google Scholar]
- 7.Bieniarz J, Dabrowski Z. Effect of smallpox vaccination in pregnancy [in Polish] Pol Tyg Lek (Wars) 1956;11:2183–8. [PubMed] [Google Scholar]
- 8.Naderi S. Smallpox vaccination during pregnancy. Obstet Gynecol. 1975;46:223–6. [PubMed] [Google Scholar]
- 9.Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968: results of ten statewide surveys. J Infect Dis. 1970;122:303–9. doi: 10.1093/infdis/122.4.303. [DOI] [PubMed] [Google Scholar]
- 10.Wharton M, Strikas RA, Harpaz R, Rotz LD, Schwartz B, Casey CG, et al. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 2003;52:1–16. [PubMed] [Google Scholar]
- 11.Ryan MA, Seward JF Smallpox Vaccine in Pregnancy Registry Team. Pregnancy, birth, and infant health outcomes from the national smallpox vaccine in pregnancy Registry, 2003–2006. Clin Infect Dis. 2008;46(suppl 3):S221–6. doi: 10.1086/524744. [DOI] [PubMed] [Google Scholar]
- 12.Ryan MA, Gumbs GR, Conlin AM, Sevick CJ, Jacobson IG, Snell KJ, et al. Evaluation of preterm births and birth defects in liveborn infants of US military women who received smallpox vaccine. Birth Defects Res A Clin Mol Teratol. 2008;82:533–9. doi: 10.1002/bdra.20470. [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA National Birth Defects Prevention Study. Guidelines for case classification for the national birth defects prevention study. Birth Defects Res A Clin Mol Teratol. 2003;67:193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- 15.Simpson HE. The interpretation of interaction in contingency tables. J R Stat Soc. 1951;12:238–41. [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 19.Ladnyi ID. Smallpox vaccination during pregnancy [in Russian] Zh Mikrobiol Epidemiol Immunobiol. 1974:121–5. [PubMed] [Google Scholar]
- 20.Rajhvajn B, Krznar B, Stoiljković C, Orescanin M, Smerdel S. Vaccination against smallpox in early pregnancy. Acta Med Iugosl. 1973;27:351–7. [PubMed] [Google Scholar]
- 21.Janiszewski B. Smallpox vaccination in pregnancy [in Polish] Ginekol Pol. 1966:41–5. [Google Scholar]
- 22.Bourke GJ, Whitty RJ. Smallpox vaccination in pregnancy: a prospective study. Br MedJ. 1964;1:1544–6. doi: 10.1136/bmj.1.5397.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liebeschuetz HJ. The effects of vaccination in pregnancy on the foetus. J Obstet Gynaecol Br Commonw. 1964;71:132–4. doi: 10.1111/j.1471-0528.1964.tb04256.x. [DOI] [PubMed] [Google Scholar]
- 24.Bellows MT, Hyman ME, Merritt KK. Effect of smallpox vaccination on the outcome of pregnancy. Public Health Rep. 1949;64:319–23. [PubMed] [Google Scholar]
- 25.Krstajić V, Malbaski S, Tokin S. Smallpox vaccination and pregnancy [in Serbian] Med Pregl. 1973;26:467–9. [PubMed] [Google Scholar]
- 26.Engström L. Smallpox vaccination during pregnancy. Acta Med Scand Suppl. 1966;464:139–46. doi: 10.1111/j.0954-6820.1966.tb05081.x. [DOI] [PubMed] [Google Scholar]
- 27.Urner JA. Some observations on the vaccination of pregnant women and newborn infants. Am J Obstet Gynecol. 1926;1:70–76. [Google Scholar]
- 28.Abramowitz LJ. Vaccination and virus diseases during pregnancy. S Afr Med J. 1957;31:1–3. [PubMed] [Google Scholar]
- 29.Greenberg M, Yankauer A, Jr, Krugman S, Osborn JJ, Ward RS, Dancis J. The effect of smallpox vaccination during pregnancy on the incidence of congenital malformations. Pediatrics. 1949;3:456–67. [PubMed] [Google Scholar]
- 30.Topciu V, Braga V, Plavoşin L, Schiopu S, Moldovan E, Lazăr E. The action of the vaccinia virus upon placenta and fetus in revaccinated pregnants (author's transl) [in German] Zentralbl Bakteriol Orig B. 1976;161:551–6. [PubMed] [Google Scholar]
- 31.Wentworth P. Studies on placentae and infants from women vaccinated for smallpox during pregnancy. J Clin Pathol. 1966;19:328–30. doi: 10.1136/jcp.19.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Entwistle DM, Bray PT, Laurence KM. Prenatal infection with vaccinia virus: report of a case. Br Med J. 1962;2:238–9. doi: 10.1136/bmj.2.5299.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green DM, Reid SM, Rhaney K. Generalised vaccinia in the human foetus. Lancet. 1966;1:1296–8. doi: 10.1016/s0140-6736(66)91202-5. [DOI] [PubMed] [Google Scholar]
- 34.Harley JD, Gillespie AM. A complicated case of congenital vaccinia. Pediatrics. 1972;50:150–3. [PubMed] [Google Scholar]
- 35.Killpack WS. Prenatal vaccinia. Lancet. 1963;1:388. doi: 10.1016/s0140-6736(63)91416-8. [DOI] [PubMed] [Google Scholar]
- 36.Macdonald AM, Macarthur P. Foetal vaccinia. Arch Dis Child. 1953;28:311–5. doi: 10.1136/adc.28.140.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naidoo P, Hirsch H. Prenatal vaccinia. Lancet. 1963;1:196–7. doi: 10.1016/s0140-6736(63)91213-3. [DOI] [PubMed] [Google Scholar]
- 38.Tucker SM, Sibson DE. Foetal complication of vaccination in pregnancy. Br Med J. 1962;2:237–8. doi: 10.1136/bmj.2.5299.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waddington E, Bray PT, Evans AD, Richards ID. Cutaneous complications of mass vaccination against smallpox in South Wales 1962. Trans St Johns Hosp Dermatol Soc. 1964;50:22–42. [PubMed] [Google Scholar]
- 40.Jenner E. Two cases of Small-Pox Infection, communicated to the Foetus in Utero under peculiar circumstances, with additional remarks. Med Chir Trans. 1809;1:271–7. [PMC free article] [PubMed] [Google Scholar]
- 41.Hood CK, McKinnon GE. Prenatal vaccinia. Am J Obstet Gynecol. 1963;85:238–40. doi: 10.1016/s0002-9378(16)35396-0. [DOI] [PubMed] [Google Scholar]
- 42.Lynch FW. Dermatologic conditions of the foetus: case believed to be fetal pemphigoid vaccinia. Arch Derm Syph. 1932;26:997. [Google Scholar]
- 43.Aitkens GH, Bowman R, Hansman D. A case of foetal vaccinia. Med J Aust. 1968;2:173–4. doi: 10.5694/j.1326-5377.1968.tb29384.x. [DOI] [PubMed] [Google Scholar]
- 44.dos Santos AU, Cury CG, Pimenta de Campos E, Sakuma ME, Curti SP, de Barros AC. Clinical, anatomopathological and virological study of a case of fetal vaccinia [in Portuguese] Rev Paul Med. 1985;103:211–4. [PubMed] [Google Scholar]
- 45.Kropholler RW, Voorhoeve-Den Hartog DJ. Fetal death due to vaccinia generalisata of the fetus [in Dutch] Ned Tijdschr Geneeskd. 1962;106:2276–7. [PubMed] [Google Scholar]
- 46.Lycke E, Ahren C, Stenborg R, Bernler G, Spetz S. A case of intrauterine vaccinia. Acta Pathol Microbiol Scand. 1963;57:287–94. doi: 10.1111/j.1699-0463.1963.tb05096.x. [DOI] [PubMed] [Google Scholar]
- 47.Toendury G, Foukas M. The hazard to the human fetus caused by smallpox vaccination during pregnancy [in German] Pathol Microbiol (Basel) 1964;27:602–23. [PubMed] [Google Scholar]
- 48.Wiersum Varioloid in a fetus [in Dutch] Ned Tijdschr Verloskd Gynaecol. 1955;55:417–25. [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. Adverse reaction to smallpox vaccination-1978. MMWR Recomm Res. 1979;28:265–7. [Google Scholar]
- 50.Centers for Disease Control and Prevention (CDC) Update on overall prevalence of major birth defects—Atlanta, Georgia, 1978-2005. MMWR Recomm Res. 2008;57:1–5. [PubMed] [Google Scholar]
- 51.Werler MM, Yazdy MM, Kasser JR, Mahan ST, Meyer RE, Anderka M, et al. Medication use in pregnancy in relation to the risk of isolated clubfoot in offspring. Am J Epidemiol. 2014;180:86–93. doi: 10.1093/aje/kwu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roye BD, Hyman J, Roye DP., Jr Congenital idiopathic talipes equinovarus. Pediatr Rev. 2004;25:124–30. doi: 10.1542/pir.25-4-124. [DOI] [PubMed] [Google Scholar]
- 53.Snyder SS. Effect of maternal smallpox vaccination during pregnancy on the eyes of the infants. Am J Ophthalmol. 1951;34:1713–5. doi: 10.1016/0002-9394(51)90038-4. [DOI] [PubMed] [Google Scholar]
- 54.Roedenbeck SD. Congenital brain disorders after smallpox vaccination [in Spanish] Rev Neuropsiquiatr. 1966;29:354–72. [PubMed] [Google Scholar]
- 55.Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 56.Spong CY. Prediction and prevention of recurrent spontaneous preterm birth. Obstet Gynecol. 2007;110:405–15. doi: 10.1097/01.AOG.0000275287.08520.4a. [DOI] [PubMed] [Google Scholar]