Abstract
Concern about use of anthrax as a bioweapon prompted development of novel anthrax antitoxins for treatment. Clinical guidelines for the treatment of anthrax recommend antitoxin therapy in combination with intravenous antimicrobials; however, a large-scale or mass anthrax incident may exceed antitoxin availability and create a need for judicious antitoxin use. We conducted a systematic review of antitoxin treatment of inhalation anthrax in humans and experimental animals to inform antitoxin recommendations during a large-scale or mass anthrax incident. A comprehensive search of 11 databases and the FDA website was conducted to identify relevant animal studies and human reports: 28 animal studies and 3 human cases were identified. Antitoxin monotherapy at or shortly after symptom onset demonstrates increased survival compared to no treatment in animals. With early treatment, survival did not differ between antimicrobial monotherapy and antimicrobial-antitoxin therapy in nonhuman primates and rabbits. With delayed treatment, antitoxin-antimicrobial treatment increased rabbit survival. Among human cases, addition of antitoxin to combination antimicrobial treatment was associated with survival in 2 of the 3 cases treated. Despite the paucity of human data, limited animal data suggest that adjunctive antitoxin therapy may improve survival. Delayed treatment studies suggest improved survival with combined antitoxin-antimicrobial therapy, although a survival difference compared with antimicrobial therapy alone was not demonstrated statistically. In a mass anthrax incident with limited antitoxin supplies, antitoxin treatment of individuals who have not demonstrated a clinical benefit from antimicrobials, or those who present with more severe illness, may be warranted. Additional pathophysiology studies are needed, and a point-of-care assay correlating toxin levels with clinical status may provide important information to guide antitoxin use during a large-scale anthrax incident.
Bacillus anthracis, a Gram-positive and spore-forming bacterium that causes anthrax, is considered a high-priority threat because of its widespread availability, easy dissemination, and ability to cause substantial morbidity and mortality.1–5 In the United States, Bacillus anthracis is a select agent and subject to the select agent regulations (42 CFR Part 73). Inhalation anthrax is one of the most lethal forms of anthrax; without treatment, its fatality rates range from 92% to almost 100%.6,7 In the 2001 US anthrax incident, antimicrobial treatment was associated with a 55% mortality reduction among inhalation anthrax patients,4 but there is ongoing interest in reducing mortality even further with adjunctive treatments.
Although antimicrobials can effectively eliminate bacteremia, anthrax is a toxin-mediated disease, and toxin accumulation is associated with mortality.3,5 In addition to the poly-D-glutamic acid capsule of B. anthracis, lethal toxin (LT) and edema toxin (ET) represent major virulence factors in anthrax pathogenesis. LT inhibits immune function and is primarily responsible for vasomotor instability observed in patients with anthrax; ET causes cellular and tissue edema.8–10 LT and ET are formed when protective antigen (PA) binds to lethal factor (LF) and edema factor (EF), respectively.8–11 Protective antigen is essential to toxin formation, promotes intracellular toxin translocation, and is the main target of vaccines and antitoxins.10
Two novel antibody-based antitoxins, ABthrax (also known as Raxibacumab) and Anthrax Immune Globulin Intravenous (AIGIV, proprietary name Anthrasil) are available in the United States Strategic National Stockpile (SNS), and additional antitoxins are being investigated.6,10,12–15 Raxibacumab is an IgG1γ monoclonal antibody, and Anthrasil is a human IgG polyclonal antibody. Both antitoxins have been approved by the Food and Drug Administration (FDA) under the animal rule.16,17 The Centers for Disease Control and Prevention (CDC) recommend antitoxin in addition to antimicrobials to treat patients with systemic anthrax.18 However, these recommendations apply in situations where antitoxin is readily available, and, other than a diagnosis of systemic anthrax, no additional clinical parameters guide antitoxin use. During a large-scale or mass anthrax incident in which the demand for antitoxin exceeds antitoxin availability, criteria for antitoxin use may change to ensure judicious, efficient, and consistent use of the finite supplies of antitoxin stockpiled in the SNS. In the absence of human randomized controlled trials, decisions on antitoxin use are likely to be based on available data, predominantly from animal studies and human case reports.
We conducted a systematic review of animal studies and human reports involving antitoxin treatment of inhalation anthrax. The goal of the review was to compile evidence to inform antitoxin recommendations during a large-scale or mass anthrax incident in which the demand for antitoxin treatment would be anticipated to exceed the supply. Clinical findings and experimental outcomes associated with the timing of antitoxin administration are reported in this review.
Methods
Data Sources and Search Strategy
This review follows the methods outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).19 A search strategy was developed in conjunction with an expert systematic review librarian. Eleven databases were searched from their inception to April 2015: Commonwealth Agricultural Bureaux (1973-), Cumulative Index to Nursing and Allied Health Literature (1981-), Defense Technical Information Center (1950-), EconLit (1886-), Embase (1988-), Federal Research in Progress (1930-), Global Health (1910-), MEDLINE (1946-), National Technical Information Service (1964-), Web of Science (1980-), World Health Organization (1948-), and WorldCat (1967-). Article types that met a priori inclusion criteria were: randomized controlled trials; case-control, cross-sectional, or observational studies; case reports; and case series involving inhalation anthrax. Consistent search terms were used among databases, and searches were restricted to articles in English. Search terms are shown in Figure 1. We identified additional studies through hand searching of references, communication with subject matter experts, and searches of the FDA website.
Figure 1.
Search Strategy
Study Selection
Relevant articles included both animal studies and human case reports involving antitoxin treatment for inhalation anthrax. For articles to be included, they had to contain information on the type of antitoxin, dose administered, timing of administration and/or clinical triggers, and survival. Additionally, included studies were restricted to those with information on antitoxins available in the SNS or currently under development for clinical use. Animal studies were restricted to nonhuman primates (NHPs) and rabbits, because the pathophysiology of anthrax in these animals is similar to that of anthrax in humans, and these models are accepted for licensure of anthrax medical countermeasures under the FDA Animal Rule.10,20–25 Animal studies of antitoxin use as anthrax prophylaxis (ie, administered prior to spore exposure or shortly after spore exposure) were excluded because these antitoxin uses do not represent treatment. Animal studies were included if they involved antitoxin administration at: (1) detection of significant increase in body temperature (SIBT) or serum protective antigen, which indicate the onset of bacteremia; (2) 39 hours or more postexposure in nonhuman primates; or (3) 30 hours or more postexposure in rabbits. The specific time points were chosen because they represent the average time points for the onset of bacteremia following exposure to B. anthracis spores in animal species.26,27
Titles and abstracts of relevant articles were reviewed independently by 2 reviewers using a priori inclusion criteria. Full-text reviews of articles were then conducted to identify eligible studies for data abstraction.
Data Abstraction and Analysis
An Excel data abstraction tool was developed by 2 systematic reviewers using templates from previous anthrax systematic reviews.7,28 Data extracted for animal studies included study design, exposure, treatment time points or clinical trigger, type of treatment, and survival. For human inhalation anthrax case reports, data extracted included age, sex, exposure, clinical presentation, antimicrobial type and administration timing, antitoxin type and administration timing, supportive care, and survival. Clinical characteristics documented in published case reports were consolidated with unpublished CDC data. The level for statistical significance was set at p ≤ 0.05. Because of heterogeneity in study designs, analyses, treatment groups, and treatment triggers, we did not perform a meta-analysis.
Results
Search Results
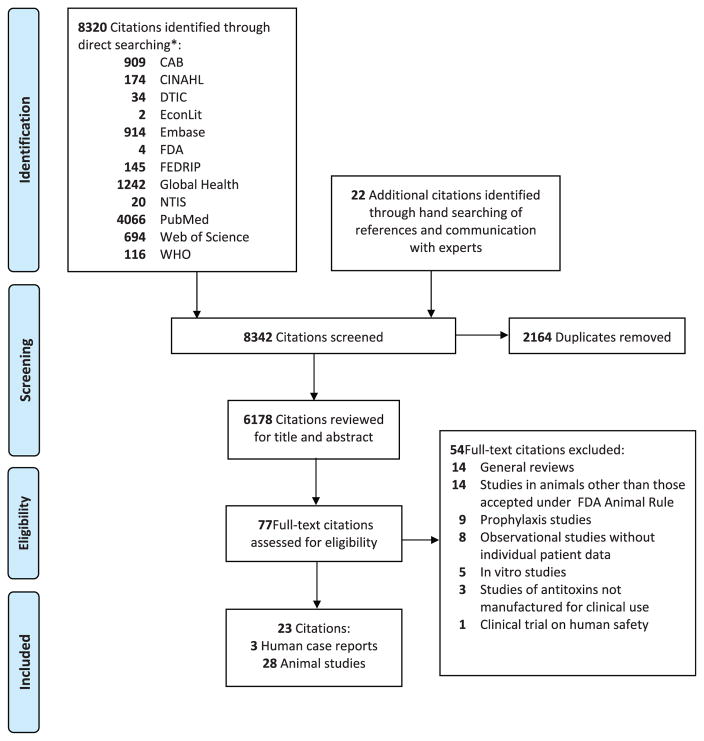
After initial removal of 2,164 duplicate references, 6,178 citations were screened by title and abstract. Twenty-two additional citations were identified for review from hand searching of references and communication with subject-matter experts. Seventy-seven citations were selected for full-text review, and 23 citations met the inclusion criteria. Citations were excluded for the following reasons: general reviews of antitoxins, animal studies using models other than nonhuman primate and rabbit, animal studies of antitoxin prophylaxis, reviews of human anthrax cases that lacked sufficient information for data abstraction, in vitro antitoxin studies, studies or cases involving antitoxins that are not available in the SNS or under development for clinical use, and human clinical safety trials (Figure 2). Among the 23 included citations, 3 human cases of inhalation anthrax treated with antitoxin provided relevant clinical and outcome data, and 28 animal studies that involved antitoxin treatment provided data on animal survival. The case studies represent low-quality evidence, and no widely accepted quality grading schema for extrapolation of animal studies to human data exists.
Figure 2.
Flow Diagram of Search Strategy
Animal Studies
Antitoxin Monotherapy
Data from 20 animal studies of antitoxin monotherapy are summarized in this review. Six antibody-based antitoxins targeting protective antigen were included: Anthrasil, Thravixa, Raxibacumab, Anthrivig, Anthim, and Valortim (Table 1). Five nonhuman primate studies report the therapeutic effect of antitoxin at various doses and administration time points. Studies in which nonhuman primates were treated with antitoxin suggested a higher likelihood of survival than those that were untreated or that received a placebo. Among nonhuman primates treated at detection of serum PA (31–49 hours postexposure), the following were associated with an increase in survival compared to that of the controls: Anthrasil at doses 15 U/kg and 30 U/kg; Thravixa at doses of 1 mg/kg, 5 mg/kg, and 20 mg/kg; and Raxibacumab. Anthrivig survival rates were 2/6 (33.3%), 1/6 (16.7%), and 2/6 (33.3%) at doses of 7.1, 14.2, and 21.3 mg/kg, respectively, whereas survival for controls was 0/6 (0.0%); this difference was not statistically significant.26,29–33 Among nonhuman primates treated with Anthim 48 hours postexposure, 4/14 (28.6%) survived, while 1/10 (10%) survived among the controls.34
Table 1.
Animal Studies: Antitoxin Monotherapy
| Study | Study Design | Antitoxin | Trigger for Treatment | Treatment | Outcome (No. Survived/ No. Treated) | P valuea |
|---|---|---|---|---|---|---|
| Nonhuman Primates: treatment at detection of serum protective antigen (PA) | ||||||
| 32, 33 | Cynomolgus macaques, randomized, placebo-controlled | Anthrasil | Detection of serum PA | IVIG | 1/16 (6%) | – |
| 7.5 U/kg | 4/15 (27%) | 0.1462 | ||||
| 15 U/kg | 7/16 (44%) | 0.0373 | ||||
| 30 U/kg | 10/14 (71%) | 0.0009 | ||||
|
| ||||||
| 30 | Cynomolgus macaques, placebo-controlled | Thravixa | Detection of serum PA (31–49 h postexposure) | Controls | 0/8 (0.0%) | – |
| 1 mg/kg | 6/10 (60.0%) | 0.0385 | ||||
| 5 mg/kg | 6/9 (66.7%) | 0.0385 | ||||
| 10 mg/kg | 4/10 (40.0%) | n.s. | ||||
| 20 mg/kg | 6/10 (60.0%) | 0.0385 | ||||
|
| ||||||
| 26, 29 | Cynomolgus macaques, randomized, blinded, placebo-controlled | Raxibacumab | Detection of serum PA (avg. 39 h postexposure) | Controls | 0/12 (0.0%) | – |
| 20 mg/kg | 7/14 (50.0%) | 0.0064 | ||||
| 40 mg/kg | 9/14 (64.3%) | <0.0007 | ||||
|
| ||||||
| 31 | Cynomolgus macaques, blinded, randomized, placebo-controlled | Anthrivig | Detection of serum PA (avg. 48 h postexposure) | Gamunex | 0/6 (0.0%) | – |
| 7.1 mg/kg | 2/6 (33.3%) | n.s. | ||||
| 14.2 mg/kg | 1/6 (16.7%) | n.s. | ||||
| 21.3 mg/kg | 2/6 (33.3%) | n.s. | ||||
|
| ||||||
| Nonhuman Primates: treatment at preset time points | ||||||
| 34 | Cynomolgus macaques, randomized, placebo- controlled | Anthim | 48 h postexposure | Controls | 1/10 (10.0%) | – |
| 16 mg/kg | 4/14 (28.6%) | – | ||||
|
| ||||||
| Rabbits: treatment at detection of significant increase in body temperature (SIBT) or serum protective antigen (PA) | ||||||
| 30 | New Zealand white rabbits, placebo-controlled | Thravixa | Detection of SIBT or serum PA (geometric mean: 24–48 h postexposure) | Controls | 0/6 (0.0%) | – |
| 1 mg/kg | 3/9 (33.3%) | n.s. | ||||
| 5 mg/kg | 12/13 (92.3%) | <0.05 | ||||
| 10 mg/kg | 10/11 (90.9%) | <0.05 | ||||
| 20 mg/kg | 6/ 9 (66.7%) | <0.05 | ||||
|
| ||||||
| 23 | New Zealand white rabbits, untreated controls | Thravixa | Detection of SIBT (avg. 27 h postexposure) | Controls | 0/9 (0.0%) | – |
| 8 mg/kg | 9/10 (90.0%) | <0.001 | ||||
|
| ||||||
| 23 | New Zealand white rabbits, untreated controls | Thravixa | Detection of SIBT (avg. 27 h postexposure), 6 h or 12 h post-SIBT | Controls | 0/6 (0.0%) | – |
| 10 mg/kg at SIBT | 5/6 (83.3%) | <0.05 | ||||
| 10 mg/kg 6 h post-SIBT | 6/6 (100.0%) | <0.05 | ||||
| 10 mg/kg 12 h post-SIBT | 4/5 (80.0.%) | <0.05 | ||||
|
| ||||||
| 31 | New Zealand white rabbits, open-label, randomized, placebo-controlled | Anthrivig | Detection of SIBT (avg. 28 h postexposure) | Gamunex | 0/8 (0.0%) | – |
| 7.1 mg/kg | 0/8 (0.0%) | – | ||||
| 14.2 mg/kg | 6/8 (75.0%) | <0.05 | ||||
| 21.3 mg/kg | 2/8 (25.0%) | <0.05 | ||||
|
| ||||||
| 26, 29 | New Zealand white rabbits, randomized, open-label, placebo-controlled | Raxibacumab | Detection of SIBT or serum PA (avg. 30 h postexposure) | Controls | 0/17 (0.0%) | – |
| 20 mg/kg | 5/18 (27.8%) | 0.0455 | ||||
| 40 mg/kg | 8/18 (44.4%) | 0.0029 | ||||
|
| ||||||
| 29 | New Zealand white rabbits, randomized, double-blind, placebo-controlled | Raxibacumab | Detection of SIBT or serum PA (avg. 30 h postexposure) | Controls | 0/24 (0.0%) | – |
| 40 mg/kg | 11/24 (45.8%) | <0.0001 | ||||
|
| ||||||
| 32, 33 | New Zealand white rabbits, Placebo-controlled | Anthrasil | Detection of serum PA (average 32.4 h postexposure) | IVIG | 1/48 (2%) | – |
| 15 U/kg | 13/50 (26%) | 0.0009 | ||||
|
| ||||||
| Rabbits: treatment at preset time points | ||||||
| 32, 33 | New Zealand white rabbits, placebo-controlled | Anthrasil | 30 h postexposure | IVIG | 0/10 (0%) | – |
| 7.5 U/kg | 4/14 (29%) | 0.0489 | ||||
| 15 U/kg | 6/14 (43%) | 0.0239 | ||||
| 30 U/kg | 5/14 (36%) | 0.0407 | ||||
|
| ||||||
| 35, 36 | New Zealand white rabbits, placebo-controlled | Raxibacumab | 36 h postexposure | Controls | 1/12 (8.3%) | – |
| 40 mg/kg | 5/12 (41.7%) | 0.1550 | ||||
|
| ||||||
| 35, 36 | New Zealand white rabbits, placebo-controlled | Raxibacumab | 36 h postexposure | Controls | 0/12 (0.0%) | – |
| 20 mg/kg | 0/12 (0.0%) | 1.0000 | ||||
|
| ||||||
| 38 | Dutch Belted rabbits, untreated controls | Thravixa | 36 h postexposure | Controls | 0/6 (0.0%) | – |
| 2 mg/kg | 2/6 (33.0%) | n.s. | ||||
|
| ||||||
| 38 | Dutch Belted rabbits, untreated controls | Thravixa | 36 h postexposure | Controls | 0/6 (0.0%) | – |
| 0.5 mg/kg | 0/6 (0.0%) | n.s. | ||||
|
| ||||||
| 37 | New Zealand white rabbits, randomized, placebo-controlled | Anthim | 36 h or 48 h postexposure | Controls | 0/10 (0.0%) | – |
| 10 mg at 36 h | 5/10 (50.0%) | 0.041 | ||||
| 10 mg at 48 h | 3/7 (42.9%) | 0.424 | ||||
|
| ||||||
| 39 | Dutch Belted rabbits, placebo-controlled | Thravixa | 48 h postexposure | Controls | 0/5 (0.0%) | – |
| 10 mg/kg | 3/5 (60.0%) | n.s. | ||||
|
| ||||||
| 40 | New Zealand white rabbits, placebo-controlled | Valortim | 48 h postexposure | Controls | 0/10 (0.0%) | – |
| 10 mg/kg | 3/7 (42.9%) | – | ||||
p-value Fisher’s Exact Test: compared the treatment group with the control group; n.s. = not significant.
Antitoxin treatment of rabbits at or after the onset of symptoms (detection of either significant increase in body temperature or serum PA, whichever occurred first) enhanced survival (Table 1). Among rabbits treated with Thravixa, 5/6 (83.3%) survived at the detection of significant increase in body temperature, 6/6 (100%) survived at 6 hours post-SIBT, and 4/5 (80.0%) survived at 12 hours post-SIBT, while none survived in the control group; all of these differences were statistically significant.23 Dose-response studies of Raxibacumab and Anthrivig demonstrated that treatment at detection of significant increase in body temperature or serum PA resulted in 44.4% (8 of 18 treated with 40mg/kg Raxibacumab) to 75.0% (6 of 8 treated with 14.2 mg/kg Anthrivig) survival, respectively, while none survived among the controls; both of these differences were statistically significant.26,29,31 Treatment with Anthrasil demonstrated similar results: 26% survival among animals treated compared to 0% survival in the nontreated group, with a statistically significant difference between the 2 groups.32,33
Additionally, 8 rabbit studies reported the survival rates after administration of antitoxin at 36 or 48 hours post-exposure. Survival following administration at 36 hours or 48 hours postexposure ranged from 0.0% (0 of 12 treated with 20mg/kg Raxibacumab at 36 h) to 60.0% (3 of 5 treated with 10 mg/kg Thravixa at 48h). In these 8 studies, only Anthrasil (7.5, 15, and 30 U/kg) at 30 h and 10 mg Anthim at 36 h were found to show statistically significant differences ( p < 0.05) (Table 1).32,33,35–40
Antitoxin-Antimicrobial Therapy
Eight animal studies compared antimicrobial-antitoxin therapy to antimicrobial monotherapy. Two studies were conducted in nonhuman primates (Table 2). In the Raxibacumab study, treatment was initiated at the detection of serum PA, which averaged 42 hours postexposure. Survival proportions for treatment groups were 14/14 (100%) in the ciprofloxacin group and 12/14 (85.7%) in the ciprofloxacin-Raxibacumab group.29 In the Anthrasil study, treatment was initiated at 64 hours postexposure. Survival proportions for treatment groups were 75% in the ciprofloxacin group and 79% to 83% in ciprofloxacin-Anthrasil groups.32,33
Table 2.
Animal Studies: Antimicrobial-Antitoxin Therapy
| Study | Study Design | Antitoxin | Trigger for Treatment | Treatment | Outcome (No. Survived/ No. Treated) | Survival Rate (95% Confidence Interval) | P valuea |
|---|---|---|---|---|---|---|---|
| Nonhuman Primates: treatment at detection of serum protective antigen (PA) | |||||||
| 29 | Cynomolgus macaques, randomized, double-blind, placebo-controlled | Raxibacumab | Detection of serum PA (avg. 42 h postexposure) | Controls | 0/12 (0.0%) | – | – |
| 75 mg cipro bid × 3 d | 14/14 (100.0%) | – | <0.0001 | ||||
| 75 mg cipro bid × 3 d + 40 mg/kg Raxibacumab | 12/14 (85.7%) | – | <0.0001 | ||||
|
| |||||||
| Nonhuman Primates: treatment at preset time point | |||||||
| 32, 33 | Cynomolgus macaques, randomized, double-blind, placebo-controlled | Anthrasil | 64 h postexposure | Controls | 1/12 (8%) | – | |
| Cipro + IVIG | 9/12 (75%) | – | |||||
| Cipro +15 U/kg Anthrasil | 10/12 (83%) | n.s. | |||||
| Cipro +30 U/kg Anthrasil | 11/14 (79%) | n.s. | |||||
|
| |||||||
| Rabbits: treatment at detection of significant increase in body temperature (SIBT) or serum protective antigen (PA) | |||||||
| 29 | New Zealand white rabbits, randomized, double blind, placebo-controlled | Raxibacumab | Detection of SIBT or serum PA (avg. 28 h postexposure) | Controls | 0/12 (0.0%) | – | – |
| 50 mg/kg levo od × 3 d | 19/20 (95.0%) | – | <0.0001 | ||||
| 50 mg/kg levo od × 3 d + 40 mg/kg Raxibacumab | 19/20 (95.0%) | – | <0.0001 | ||||
|
| |||||||
| Rabbits: treatment at preset time points | |||||||
| 27, 32, 33b | New Zealand white rabbits, randomized, placebo-controlled | Anthrasil | 30, 36, 48, or 60 h postexposure | Controls | 0/8 (0.0%) | 0.00 (0.00, 0.37) | – |
| 50 mg/kg levo od × 3 d at 30 h+ IVIG | 8/8 (100.0%) | 1.00 (0.63, 1.00) | 1.0000 | ||||
| 50 mg/kg levo od × 3 d + 15 U/kg Anthrasil at 30 h | 7/8 (87.5%) | 0.88 (0.47, 1.00) | – | ||||
| 50 mg/kg levo od × 3 d at 36 h+ IVIG | 8/8 (100.0%) | 1.00 (0.63, 1.00) | NA | ||||
| 50 mg/kg levo od × 3 d + 15 U/kg Anthrasil at 36 h | 7/7 (100.0%) | 1.00 (0.59, 1.00) | – | ||||
| 50 mg/kg levo od × 3 d at 48 h+ IVIG | 8/8 (100.0%) | 1.00 (0.63, 1.00) | NA | ||||
| 50 mg/kg levo od × 3 d + 15 U/kg Anthrasil at 48 h | 8/8 (100.0%) | 1.00 (0.63, 1.00) | – | ||||
| 50 mg/kg levo od × 3 d at 60 h + IVIG | 7/8 (87.5%) | 0.88 (0.47, 1.00) | 1.0000 | ||||
| 50 mg/kg levo od × 3 d + 15 U/kg Anthrasil at 60 h | 6/8 (75.0%) | 0.75 (0.35, 0.97) | – | ||||
|
| |||||||
| 27, 32, 33c | New Zealand white rabbits, randomized, placebo-controlled | Anthrasil | 60, 72, 84, or 96 h postexposure | Controls | 0/18 (0.0%) | 0.00 (0.00, 0.19) | – |
| 50 mg/kg levo od × 3 d at 60 h + IVIG | 9/10 (90.0%) | 0.90 (0.55, 1.00) | 1.0000 | ||||
| 50 mg/kg levo od × 3 d + 15 U/kg Anthrasil at 60 h | 8/8 (100.0%) | 1.00 (0.63, 1.00) | – | ||||
| 50 mg/kg levo od × 3 d at 72 h + IVIG | 11/20 (55.0%) | 0.55 (0.32, 0.77) | 0.5450 | ||||
| 50 mg/kg levo od × 3 d + 15 U/kg Anthrasil at 72 h | 15/23 (65.2%) | 0.65 (0.43, 0.84) | – | ||||
| 50 mg/kg levo od × 3 d at 84 h+ IVIG | 3/9 (33.3%) | 0.33 (0.07, 0.70) | 1.0000 | ||||
| 50 mg/kg levo od × 3 d + 15 U/kg Anthrasil at 84 h | 4/10 (40.0%) | 0.40 (0.12, 0.74) | – | ||||
| 50 mg/kg levo od × 3 d at 96 h+ IVIG | 2/8 (25.0%) | 0.25 (0.03, 0.65) | 0.1319 | ||||
| 50 mg/kg levo od × 3 d + 15 U/kg Anthrasil at 96 h | 5/7 (71.4%) | 0.71 (0.29, 0.96) | – | ||||
| 29, 41 | New Zealand white rabbits, randomized, placebo-controlled | Raxibacumab | 84 h postexposure | 50 mg/kg levo od × 3 d | 24/37 (64.9%) | – | – |
| 50 mg/kg levo od × 3 d + 40 mg/kg Raxibacumab | 32/39 (82.1%) | – | 0.0874 | ||||
| 32, 33 | New Zealand white rabbits, placebo-controlled | Anthrasil | 96 h postexposure | 50 mg/kg levo od × 3 d + IVIG | 13/33 (39%) | – | – |
| 50 mg/kg levo +15 U/kg Anthrasil | 18/31 (58%) | – | 0.1353 | ||||
| 41 | New Zealand white rabbits, placebo-controlled | Anthim | Detection of PA | Saline | 0/4 (0%) | ||
| 2 mg/kg Doxycycline bid × 3 days | 5/10 (50%) | 0.8446 | |||||
| 2 mg/kg Doxycycline + ETI-204 bid × 3 | 9/10 (90%) | 0.0051 | |||||
p-value Fisher’s Exact Test: compared the treatment group with the control group.
p-value Pairwise Log-rank test: time-to-death and overall survival rates between groups by pairwise Log-rank test. NA- Log-rank test was not possible due to no deaths occurred in either group.
p-value Fisher’s Exact Test: compared overall-survival between the groups.
n.s. = not significant; cipro = ciprofloxacin; levo = levofloxacin; bid = twice a day; od = once.
In rabbit studies, survival rates (95%) did not vary between levofloxacin and levofloxacin-Raxibacumab groups when treatment was initiated at the onset of symptoms (either significant increase in body temperature or detection of serum PA, whichever occurred first; onset of symptoms occurred on average 28 hours postexposure).29 In addition, at less than 60 hours, no significant difference in survival rates was observed between levofloxacin-IVIG and levofloxacin-Anthrasil treatment groups.32 In the most recent rabbit study, treatment with doxycycline-Anthim at detection of PA resulted in survival of 9/10 (90%) rabbits compared to 5/10 (50%) in the doxycycline alone group ( p = 0.1031).41
Given the similar survival rates of early antimicrobial and antimicrobial-antitoxin treatment, additional studies assessed whether adding antitoxin improved survival when treatment was delayed. Among 76 (42%) surviving rabbits treated at 84 hours postexposure with levofloxacin or levofloxacin-Raxibacumab, 24/37 (65%) survived in the levofloxacin group, while 32/39 (82%) survived in the levofloxacin-Raxibacumab group ( p = 0.0874).26,42 Additional studies were conducted with levofloxacin-Anthrasil at later time points. In one study, 18/31 (58%) rabbits treated with levofloxacin-Anthrasil at 96 hours postexpo-sure survived, compared to a 13/33 (39%) survival rate in animals treated with levofloxacin-IVIG ( p = 0.1353). A similar trend was observed in rabbits treated at 72 or 84 hours postexposure, but no significant difference in survival was observed between the treatment and control groups (Table 2).27,32,33
Human Case Reports
Limited information was published regarding the 3 inhalation anthrax human cases who received Anthrasil treatment (Table 3). B. anthracis was culture-confirmed in all 3 cases. Infected individuals were 34 to 61 years old, and all were male. Exposure was known in 2 patients—animal hide drums—while in 1 case the exposure was not conclusively established.43–48 Of the 3 cases, 2 patients survived and 1 died.
Table 3.
Antitoxin Treatment in Human Inhalation Anthrax
| Case | Age | Gender | Exposure | Clinical Presentation | Antimicrobial Initiation Post Symptom Onset (Days) | Antimicrobial Treatment | Additional Supportive Care | Anthrasil Administration Post Symptom Onset (Days) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 44, 47 | 44 | M | Animal hide drums | Dyspnea, cough, syncope, chest pain, bilateral pleural effusions, mediastinal fluid, progressive respiratory failure, elevated liver enzymes, elevated creatinine | 2–3 | Ceftriaxone, azithromycin, moxifloxacin, clindamycin, piperacillin/tazobactam | Mechanical ventilation, pleural fluid drainage, vasopressors | 9 | Lived |
| 45, 46 | 61 | M | Unknown, presumed environmental | Fever, productive cough, exertional dyspnea, progressive respiratory failure, elevated creatinine | 2 | Ceftriaxone, azithromycin, ciprofloxacin, meropenem, vancomycin, rifampin, clindamycin | Mechanical ventilation, pleural fluid drainage | 7 | Lived |
| 42, 43 | 34 | M | Animal hide drums | Fever, night sweats, rigors, rapid respiratory failure, pleural effusions, widened mediastinum, multi-organ failure | 2 | Amoxicillin, clarithromycin, piperacillin/tazobactam, rifampicin, ciprofloxacin, clindamycin | Multi-organ support: mechanical ventilation, pleural fluid drainage, vasopressors, steroids | 8 | Died |
All patients demonstrated clinical evidence of sepsis, and all received multidrug intravenous antimicrobials at the time of hospital admission.43–48 All patients required mechanical ventilation and underwent pleural fluid drainage. One survivor and the nonsurvivor also received vasopressors. For all patients, Anthrasil was administered as a single 420-unit dose under the CDC-sponsored Investigational New Drug protocol.49
A comparison of the clinical course of survivors and nonsurvivors is presented below. Among the 2 survivors, antimicrobials were started 2 to 3 days after symptom onset, and Anthrasil was administered 7 or 9 days after symptom onset.45–48 The first survivor initially presented with respiratory distress, which progressively worsened despite his receiving intravenous antimicrobials. Prior to receiving Anthrasil, he had bilateral pulmonary effusions and large amounts of mediastinal fluid requiring drainage by thoracentesis and thoracotomy, and he required mechanical ventilation. He also had evidence of multiorgan dysfunction as evidenced by progression of pulmonary infiltrates and elevated liver enzymes and serum creatinine. He received Anthrasil on day 9 post–symptom onset; however, he experienced a reaccumulation of right pleural effusions requiring drainage and then subsequently developed acute respiratory distress syndrome. His condition gradually improved starting 2 days after Anthrasil administration, with reports of less prominent infiltrates on chest radiographs and improved WBC counts over the next several days. He was discharged approximately 5 weeks after initial presentation.45,46
The second survivor initially presented with fever, cough, and dyspnea on admission. Prior to receiving Anthrasil, he had progressive respiratory failure requiring endotracheal intubation, mechanical ventilation, and pleural fluid drainage. The patient received Anthrasil 7 days post–symptom onset (4 days post–antimicrobial initiation). Two days after Anthrasil administration, his condition improved: renal dysfunction, hyponatremia, and thrombocytopenia gradually resolved. He was extubated 6 days after Anthrasil administration and then discharged approximately 4 weeks after his initial presentation.47,48
Limited information is available on the nonsurvivor. He initially presented to the hospital with a 2-day history of fever and rigors, and within 2 days of initial presentation to the hospital, he required mechanical ventilation. Antimicrobial therapy was initiated at admission. Prior to receiving Anthrasil, he had multisystem organ failure requiring mechanical ventilation, vasopressor support, and bilateral pleural fluid drainage (CDC unpublished data). Anthrasil was administered 8 days post–symptom onset. Despite administration of Anthrasil, the patient remained in critical condition and died 6 days after Anthrasil administration.43,44
Lethal factor levels were measured in all patients, and similar patterns were observed. Serum and pleural fluid lethal factor levels declined with antimicrobial use, followed by a faster decline after Anthrasil administration. For the first survivor, the lethal factor levels decreased steadily from 294.30 ng/mL (3 days post–symptom onset) to 16.0 ng/mL (9 days post–symptom onset) with 6 days of antimicrobial treatment prior to Anthrasil administration, and decreased further to 0.85 ng/mL 1 hour post-Anthrasil administration.46 For the second survivor, the lethal factor levels decreased from 58.0 ng/mL (2 days post–symptom onset) to 1.5 ng/mL (7 days post–symptom onset) with 5 days of antimicrobial treatment prior to Anthrasil administration, and decreased further to 0.02 ng/mL within a day post–Anthrasil administration.48 For the nonsurvivor, the lethal factor levels declined more slowly 12 hours post–Anthrasil administration compared to those of the survivors.50 Of note, the absolute values of initial serum lethal factor and pleural fluid lethal factor levels were much lower in the nonsurvivor, and his first sample was obtained 6 days post–antimicrobial initiation, whereas the first samples of the survivors were obtained prior to antimicrobial initiation.51 Based on measurements of anti-PA, the 2 survivors developed a quantifiable antibody response prior to AIGIV administration, and the first survivor was fully seroconverted (>4-fold change in anti-PA IgG concentration).46,48
Discussion
Animal studies that evaluate the treatment of inhalation anthrax had small numbers but suggest that both antimicrobial monotherapy and antimicrobial-antitoxin therapy at or shortly after symptom onset are associated with survival. However, when the treatment is delayed 60 hours or more, improved survival with addition of antitoxin to antimicrobial therapy was suggested, but was not demonstrated statistically. This may be due to the small sample size; many animals did not survive long enough to receive delayed treatment.27,42 While antimicrobials prevent bacterial replication, they do not prevent the uptake of toxin and the subsequent formation of toxin complexes.18,46,48,52 There is a biologically plausible conceptual basis to support antitoxin use in addition to antimicrobial therapy—to prevent intracellular uptake of circulating toxin. Antitoxin appears safe and well tolerated,18,32,52 and thus the benefits appear to outweigh the risks.
In animal studies in which any therapy was delayed, data suggest combined antimicrobial-antitoxin therapy improves survival compared to antimicrobial therapy alone. However, specific clinical predictors that define the greatest likelihood of survival are not known in animals or in humans. All 3 human cases of inhalation anthrax received antitoxin treatment after clinical signs of sepsis and the initiation of mechanical ventilation. Two of these patients survived; the nonsurvivor’s clinical findings may provide important insights. Despite a similar timeframe for receipt of antitoxin (ie, from day 6 to day 9 post–symptom onset), the nonsurvivor developed respiratory distress earlier in the course of illness. He remained in critical condition, required multiorgan support after antitoxin administration, and ultimately died 2 weeks post–symptom onset.43,44 Many factors may have contributed to this poor outcome: Extrinsic factors such as large inoculum of inhaled spores or intrinsic factors such as individual immunologic and physiologic differences may have resulted in more severe disease. There may also be a time point in disease progression after which intracellular damage is irrevocable. The low serum lethal factor measurement obtained 6 days post–antimicrobial initiation in the nonsurvivor may support this notion. The low value may indicate an accumulation of toxin intracellularly, and thus further blockade of toxin translocation may not have enhanced survival. Intracellular toxin is not affected by the administration of antitoxin; therefore, there might be a yet-to-be determined point in illness where addition of antitoxin may not improve survival.
For the 2 survivors who received antitoxin treatment, we are unable to disentangle whether antitoxin, pleural fluid drainage, intensive supportive care, combination or high-dose antimicrobial treatment, or, more likely, a combination of these therapies, was associated with survival. Additionally, the impact of endogenous antibody formation prior to antitoxin administration is unknown.46,48 In animal studies of severe anthrax disease, antitoxin-vasopressor combinations were found to improve survival, suggesting an additive effect of antitoxin is possible even in patients with vasomotor instability.53
There is limited evidence to guide the judicious use of antitoxin in a large-scale or mass anthrax incident. In non–resource limited settings, CDC recommends antitoxin use for all patients with systemic anthrax, which is supported by the findings of this review. However, during a large-scale or mass incident, depending on the scope of the event and the number of individuals infected, antitoxin may not be available to treat all suspected or confirmed anthrax cases. Based on the data compiled in this review, when antitoxin supplies are limited it may be reasonable to reserve antitoxin for patients who have not demonstrated an obvious clinical benefit from antimicrobials, and for patients who present with more severe illness at the onset but who have a reasonable chance of survival. More research is needed to define specific clinical criteria for antitoxin treatment in resource-limited settings, particularly to identify the clinical threshold beyond which antitoxin therapy may not confer a survival benefit. Animal studies designed to target the optimal therapeutic window and real-time capture of clinical data from naturally occurring cases of anthrax may provide additional insight to guide antitoxin use.
Direct assessment of toxin levels may also inform antitoxin use. Measurement of lethal factor, which has been shown to be the earliest indicator of B. anthracis infection, presents particular promise.54–57 Edema factor, edema toxin, and lethal toxin assays and real-time measurements of protective antigen, anti-PA, and capsular antigen may also be useful.58–60 Finally, a point-of-care toxin assay may provide a mechanism to monitor antimicrobial or antimicrobial-antitoxin treatment response.27,34,46,48
Our review has several limitations. There is a dearth of relevant human data, an inherent challenge when studying the treatment of rare and highly fatal diseases. Furthermore, animal studies do not capture clinical indices, which, even if available, are difficult to correlate with those of humans. However, animal pathophysiology studies may provide information to correlate toxin levels with organ dysfunction or failure, which may improve understanding of anthrax pathogenesis and treatment. Since our review is limited to English articles, animal studies in other languages may provide additional indirect evidence for antitoxin adjunctive therapy. It is not known whether different antitoxin products have different levels of efficacy or potency since head-to-head comparison studies were not identified. Lastly, our review is subject to publication and reporting biases, which are intrinsic to all systematic reviews.
Conclusions
Despite the paucity of relevant human data, limited animal data suggest that adjunctive antitoxin therapy may improve survival. While early initiation of antimicrobial therapy has previously been shown to improve survival, adjunctive antitoxin therapy may also have a role in enhancing survival, particularly for patients in whom serum toxin accumulation is likely, or for whom antimicrobial therapy alone does not provide a cure. Additional pathophysiology studies are needed, and a point-of-care assay correlating toxin levels with clinical status may provide important information to guide antitoxin use.
Acknowledgments
The authors thank Onnalee Gomez for conducting the searches for this systematic review, Theresa Turski and Stefan Katharios-Lanwermeyer for reviewing articles, and the members of the CDC Workgroup on Anthrax Clinical Guidelines for providing additional references for our review. This project is funded by the Centers for Disease Control and Prevention, Office of Public Health Preparedness and Response.
References
- 1.Abramova FA, Grinberg LM, Yampolskaya OV, Walker DH. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc Natl Acad Sci U S A. 1993;90(6):2291–2294. doi: 10.1073/pnas.90.6.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. [Accessed November 10, 2015];Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) Strategy and Implementation Plan. 2012 http://www.phe.gov/Preparedness/mcm/phemce/Documents/2014-phemce-sip.pdf.
- 3.Inglesby TV, O’Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, McDade J, Osterholm MT, Parker G, Perl TM, Russell PK, Tonat K Working Group on Civilian Biodefense. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA. 2002;287(17):2236–2252. doi: 10.1001/jama.287.17.2236. [DOI] [PubMed] [Google Scholar]
- 4.Jernigan DB, Raghunathan PL, Bell BP, et al. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis. 2002;8(10):1019–1028. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jernigan JA, Stephens DS, Ashford DA, et al. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis. 2001;7(6):933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grabenstein JD. Vaccines: countering anthrax: vaccines and immunoglobulins. Clin Infect Dis. 2008;46(1):129–136. doi: 10.1086/523578. [DOI] [PubMed] [Google Scholar]
- 7.Holty JE, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med. 2006;144(4):270–280. doi: 10.7326/0003-4819-144-4-200602210-00009. [DOI] [PubMed] [Google Scholar]
- 8.Collier RJ, Young JA. Anthrax toxin. Annu Rev Cell Dev Biol. 2003;19:45–70. doi: 10.1146/annurev.cellbio.19.111301.140655. [DOI] [PubMed] [Google Scholar]
- 9.Mourez M. Anthrax toxins. Rev Physiol Biochem Pharmacol. 2004;152:135–164. doi: 10.1007/s10254-004-0028-2. [DOI] [PubMed] [Google Scholar]
- 10.Kummerfeldt CE. Raxibacumab: potential role in the treatment of inhalational anthrax. Infect Drug Resist. 2014;7:101–109. doi: 10.2147/IDR.S47305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brossier F, Mock M. Toxins of Bacillus anthracis. Toxicon. 2001;39(11):1747–1755. doi: 10.1016/s0041-0101(01)00161-1. [DOI] [PubMed] [Google Scholar]
- 12.Baillie LW. Past, imminent and future human medical countermeasures for anthrax. J Appl Microbiol. 2006;101(3):594–606. doi: 10.1111/j.1365-2672.2006.03112.x. [DOI] [PubMed] [Google Scholar]
- 13.Chitlaru T, Altbourn Z, Reuveny S, Shafferman A. Progress and novel strategies in vaccine development and treatment of anthrax. Immunol Rev. 2011;239(1):221–236. doi: 10.1111/j.1600-065X.2010.00969.x. [DOI] [PubMed] [Google Scholar]
- 14.Rainey GJ, Young JA. Antitoxins: novel strategies to target agents of bioterrorism. Nat Rev Microbiol. 2004;2(9):721–726. doi: 10.1038/nrmicro977. [DOI] [PubMed] [Google Scholar]
- 15.Schneemann A, Manchester M. Anti-toxin antibodies in prophylaxis and treatment of inhalation anthrax. Future Microbiol. 2009;4(1):35–43. doi: 10.2217/17460913.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration; Dec 14, 2012. [Accessed November 10, 2015]. FDA approves raxibacumab to treat inhalational anthrax [news release] http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm332341.htm. [Google Scholar]
- 17.US Food and Drug Administration; Mar 25, 2015. [Accessed November 10, 2015]. FDA approves treatment for inhalation anthrax [news release] http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm439752.htm. [Google Scholar]
- 18.Hendricks KA, Wright ME, Shadomy SV, Bradley JS, Morrow MG, Pavia AT, Rubinstein E, Holty JE, Messonnier NE, Smith TL, Pesik N, Treadwell TA, Bower WA. Workgroup on Anthrax Clinical Guidelines. Centers for Disease Control and Prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis. 2014;20(2) doi: 10.3201/eid2002.130687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed November 10, 2015];Pathway to Licensure for Protective Antigen-based Anthrax Vaccines for a Post-exposure Prophylaxis Indication Using the Animal Rule. 2010 Nov 16; http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM232400.pdf.
- 21.Committee on Animal Models for Assessing Countermeasures to Bioterrorism Agents; National Research Council. Animal Models for Assessing Countermeasures to Bioterrorism Agents. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 22.Phipps AJ, Premanandan C, Barnewall RE, Lairmore MD. Rabbit and nonhuman primate models of toxin-targeting human anthrax vaccines. Microbiol Mol Biol Rev. 2004;68(4):617–629. doi: 10.1128/MMBR.68.4.617-629.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comer JE, Ray BD, Henning LN, Stark GV, Barnewall RE, Mott JM, Meister GT. Characterization of a therapeutic model of inhalational anthrax using an increase in body temperature in New Zealand white rabbits as a trigger for treatment. Clin Vaccine Immunol. 2012;19(9):1517–1525. doi: 10.1128/CVI.00292-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henning LN, Comer JE, Stark GV, et al. Development of an inhalational Bacillus anthracis exposure therapeutic model in cynomolgus macaques. Clin Vaccine Immunol. 2012;19(11):1765–1775. doi: 10.1128/CVI.00288-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Twenhafel NA. Pathology of inhalational anthrax animal models. Vet Pathol. 2010;47(5):819–830. doi: 10.1177/0300985810378112. [DOI] [PubMed] [Google Scholar]
- 26.Migone TS, Subramanian GM, Zhong J, et al. Raxibacumab for the treatment of inhalational anthrax. N Engl J Med. 2009;361(2):135–144. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- 27.Kammanadiminti S, Patnaikuni RK, Comer J, Meister G, Sinclair C, Kodihalli S. Combination therapy with antibiotics and anthrax immune globulin intravenous (AIGIV) is potentially more effective than antibiotics alone in rabbit model of inhalational anthrax. PLoS One. 2014;9(9):e106393. doi: 10.1371/journal.pone.0106393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meaney-Delman D, Zotti ME, Rasmussen SA, et al. Anthrax cases in pregnant and postpartum women: a systematic review. Obstet Gynecol. 2012;120(6):1439–1449. doi: 10.1097/aog.0b013e318270ec08. [DOI] [PubMed] [Google Scholar]
- 29.Human Genome Sciences, Inc. [Accessed November 10, 2015];Anti-Infective Drugs Advisory Committee Meeting: Raxibacumab, Treatment of Inhalational Anthrax, BLA 125349. 2012 Nov 2; http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM326185.pdf.
- 30.Malkevich NV, Hopkins RJ, Bernton E, et al. Efficacy and safety of AVP-21D9, an anthrax monoclonal antibody, in animal models and humans. Antimicrob Agents Chemother. 2014;58(7):3618–3625. doi: 10.1128/AAC.02295-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mytle N, Hopkins RJ, Malkevich NV, et al. Evaluation of intravenous anthrax immune globulin for treatment of inhalation anthrax. Antimicrob Agents Chemother. 2013;57(11):5684–5692. doi: 10.1128/AAC.00458-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. [Accessed November 10, 2015];Clinical Pharmacology BLA Review; Division of Hematology, Office of Blood Review and Research. 2014 Jul 25; http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM443660.pdf.
- 33.ANTHRASIL/Anthrax Immune Globulin Intravenous (Human) Cangene Corporation; Mar 25, 2015. [Accessed November 10, 2015]. Summary Basis for Regulatory Action. http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM443728.pdf. [Google Scholar]
- 34.Elusys Therapeutics, Inc. [Accessed November 10, 2015];Elusys releases new data on anthrax anti-toxin administered via intramuscular injection from three recent animal studies [news release] 2013 Jul 31; http://www.prnewswire.com/news-releases/elusys-releases-new-data-on-anthrax-anti-toxin-administered-via-intramuscular-injection-from-three-recent-animal-studies-217754071.html.
- 35.Beebe LB, Barnewall R, Zhong J, Choi G. Post-exposure therapeutic potential of PAmAb in an inhalation model of anthrax in New Zealand white rabbits. Presented at: American Society of Microbiology Biodefense Meeting; March 2004; Baltimore, MD. [Google Scholar]
- 36.Mazumdar S. Raxibacumab. MAbs. 2009;1(6):531–538. doi: 10.4161/mabs.1.6.10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohamed N, Clagett M, Li J, et al. A high-affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge. Infect Immun. 2005;73(2):795–802. doi: 10.1128/IAI.73.2.795-802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson JW, Comer JE, Baze WB, et al. Human monoclonal antibody AVP-21D9 to protective antigen reduces dissemination of the Bacillus anthracis Ames strain from the lungs in a rabbit model. Infect Immun. 2007;75(7):3414–3424. doi: 10.1128/IAI.00352-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson JW, Comer JE, Noffsinger DM, et al. Human monoclonal anti-protective antigen antibody completely protects rabbits and is synergistic with ciprofloxacin in protecting mice and guinea pigs against inhalation anthrax. Infect Immun. 2006;74(2):1016–1024. doi: 10.1128/IAI.74.2.1016-1024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vitale L, Blanset D, Lowy I, et al. Prophylaxis and therapy of inhalational anthrax by a novel monoclonal antibody to protective antigen that mimics vaccine-induced immunity. Infect Immun. 2006;74(10):5840–5847. doi: 10.1128/IAI.00712-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biron B, Beck K, Dyer D, Mattix M, Twenhafel N, Nalca A. Efficacy of ETI-204 monoclonal antibody as an adjunct therapy in a New Zealand white rabbit partial survival model for inhalational anthrax. Antimicrob Agents Chemother. 2015;59(4):2206–2214. doi: 10.1128/AAC.04593-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corey A, Migone TS, Bolmer S, et al. Bacillus anthracis protective antigen kinetics in inhalation spore-challenged untreated or levofloxacin/raxibacumab-treated New Zealand white rabbits. Toxins (Basel) 2013;5(1):120–138. doi: 10.3390/toxins5010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.A single case of inhalation anthrax in a drum maker in London. [Accessed November 30, 2015];Health Protection Report. 2008 Oct 31;2(44) http://webarchive.nationalarchives.gov.uk/20140714084352/http://www.hpa.org.uk/hpr/archives/2008/news4408.htm. [Google Scholar]
- 44.Anaraki S, Addiman S, Nixon G, et al. Investigations and control measures following a case of inhalation anthrax in East London in a drum maker and drummer, October 2008. Euro Surveill. 2008;13(51):19076. [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. Inhalation anthrax associated with dried animal hides—Pennsylvania and New York City, 2006. MMWR Morb Mortal Wkly Rep. 2006;55(10):280–282. [PubMed] [Google Scholar]
- 46.Walsh JJ, Pesik N, Quinn CP, et al. A case of naturally acquired inhalation anthrax: clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin Infect Dis. 2007;44(7):968–971. doi: 10.1086/512372. [DOI] [PubMed] [Google Scholar]
- 47.Griffith J, Blaney D, Shadomy S, Lehman M, Pesik N, Tostenson S, Delaney L, Tiller R, DeVries A, Gomez T, Sullivan M, Blackmore C, Stanek D, Lynfield R Anthrax Investigation Team. Investigation of inhalation anthrax case, United States. Emerg Infect Dis. 2014;20(2):280–283. doi: 10.3201/eid2002.130021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprenkle MD, Griffith J, Marinelli W, et al. Lethal factor and anti-protective antigen IgG levels associated with inhalation anthrax, Minnesota, USA. Emerg Infect Dis. 2014;20(2):310–314. doi: 10.3201/eid2002.130245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. E-IND protocol: one time emergency use of liquid 5% anthrax immune globulin for treatment of severe anthrax. Centers for Disease Control and Prevention; Feb 22, 2006. [Google Scholar]
- 50.Boyer AE. Clinical Utilization Plan for Anthrax Countermeasures in a Mass Event Setting Workshop. Centers for Disease Control and Prevention; Atlanta, Georgia: 2014. Prognostic indicators, anthrax immune globulin intravenous use and toxin levels in recent systemic anthrax cases. [Google Scholar]
- 51.Subramanian GM, Cronin PW, Poley G, et al. A phase 1 study of PAmAb, a fully human monoclonal antibody against Bacillus anthracis protective antigen, in healthy volunteers. Clin Infect Dis. 2005;41(1):12–20. doi: 10.1086/430708. [DOI] [PubMed] [Google Scholar]
- 52.Barochia AV, Cui X, Sun J, et al. Protective antigen antibody augments hemodynamic support in anthrax lethal toxin shock in canines. J Infect Dis. 2012;205(5):818–829. doi: 10.1093/infdis/jir834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyer AE, Gallegos-Candela M, Lins RC, et al. Quantitative mass spectrometry for bacterial protein toxins—a sensitive, specific, high-throughput tool for detection and diagnosis. Molecules. 2011;16(3):2391–2413. doi: 10.3390/molecules16032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyer AE, Quinn CP, Beesley CA, et al. Lethal factor toxemia and anti-protective antigen antibody activity in naturally acquired cutaneous anthrax. J Infect Dis. 2011;204(9):1321–1327. doi: 10.1093/infdis/jir543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyer AE, Quinn CP, Hoffmaster AR, et al. Kinetics of lethal factor and poly-D-glutamic acid antigenemia during inhalation anthrax in rhesus macaques. Infect Immun. 2009;77(8):3432–3441. doi: 10.1128/IAI.00346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boyer AE, Quinn CP, Woolfitt AR, et al. Detection and quantification of anthrax lethal factor in serum by mass spectrometry. Anal Chem. 2007;79(22):8463–8470. doi: 10.1021/ac701741s. [DOI] [PubMed] [Google Scholar]
- 57.Fish DC, Lincoln RE. In vivo-produced anthrax toxin. J Bacteriol. 1968;95(3):919–924. doi: 10.1128/jb.95.3.919-924.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kobiler D, Gozes Y, Rosenberg H, Marcus D, Reuveny S, Altboum Z. Efficiency of protection of guinea pigs against infection with Bacillus anthracis spores by passive immunization. Infect Immun. 2002;70(2):544–550. doi: 10.1128/IAI.70.2.544-550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rossi CA, Ulrich M, Norris S, Reed DS, Pitt LM, Leffel EK. Identification of a surrogate marker for infection in the African green monkey model of inhalation anthrax. Infect Immun. 2008;76(12):5790–5801. doi: 10.1128/IAI.00520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]