Abstract
In Parkinson's disease, the efficacy of L-Dopa treatment changes over time, as dyskinesias emerge with previously beneficial doses. Using MitoPark mice, that models mitochondrial failure in dopamine (DA) neurons and mimics the progressive loss of dopamine observed in Parkinson’s disease, we found that the severity of DA denervation and associated adaptations in striatal neurotransmission at the time of initiation of L-Dopa treatment determines development of L-Dopa induced dyskinesias. We treated 20-week, and 28-week old MitoPark mice with L-Dopa (10 mg/kg i.p. twice a day) and found locomotor responses to be significantly different. While all MitoPark mice developed sensitization to L-Dopa treatment over time, 28-week old MitoPark mice with extensive striatal DA denervation developed abnormal involuntary movements rapidly and severely after starting L-Dopa treatment, as compared to a more gradual escalation of movements in 20-week old animals that started treatment at earlier stages of degeneration. Our data support that it is the extent of loss of DA innervation that determines how soon motor complications develop with L-Dopa treatment. Gene array studies of striatal neurotransmitter receptors revealed changes in mRNA expression levels for DA, serotonin, glutamate and GABA receptors in striatum of 28-week old MitoPark mice. Our results support that delaying L-Dopa treatment until Parkinson’s disease symptoms become more severe does not delay the development of L-Dopa-induced dyskinesias. MitoPark mice model genetic alterations known to impair mitochondrial function in a subgroup of Parkinson patients and provide a platform in which to study treatments to minimize the development of dyskinesia.
Keywords: basal ganglia, nigrostriatal function, MitoPark mouse, behavior, sensitization, motor complications
1. Introduction
Development of treatment-induced motor complications constitutes a major problem in the long-term management of patients suffering from Parkinson's disease. Particularly, L-Dopa-induced dyskinesias (LIDs), also known as abnormal involuntary movements, pose a significant challenge. Most Parkinson’s disease patients develop dyskinesia within a decade from onset of treatment (Tambasco et al, 2012). Despite the clinical importance of this side effect, the mechanisms underlying the generation of LIDs are not fully understood, although there are data to suggest it is the degree of dopamine loss, and the dose of L-Dopa, rather than the length of L-Dopa treatment, that correlates to the development and severity of dyskinesia (Cilia et al 2014). Animal models using selective neurotoxins to unilaterally mimic the selective loss of substantia nigra neurons typical of Parkinson’s disease, and have been used to assess LIDs (Lundblad et al, 2004; Francardo et al, 2011; Winkler et al, 2002) and the efficacy of standard and novel therapeutic treatments. Several models based on genes implicated in the pathogenesis of familial Parkinson’s disease (e.g., α-synuclein and parkin) have been developed over the last decade (Le et al, 2014). Although these animal models display important characteristics of Parkinson’s disease, including dystrophic neurites, neuronal atrophy, and intracellular inclusions, degeneration of the nigrostriatal DA system has been variable (Le et al, 2014). There is thus a need for additional genetic models of Parkinson’s disease that carry hallmark features in the basal ganglia, such as slow progressive, robust bilateral degeneration of the nigrostriatal DA system, accompanied by a gradual motor impairment, responsiveness to Parkinson’s disease medications (e.g. DA agonists and L-Dopa), and associated development of dyskinesias.
MitoPark mice mimic the progression of Parkinson’s disease in humans, with adult onset and slowly progressing neurological symptoms (Ekstrand et al, 2007). The MitoPark mouse has a homozygous disruption of mitochondrial transcription factor A (TFAM) selectively in midbrain DA neurons, leading to mitochondrial dysfunction and gradual degeneration of DA neurons. While the MitoPark mouse may not be directly suitable to study the etiology of Parkinson’s disease, as there are almost no indications of TFAM polymorphisms being linked to Parkinson’s disease, the MitoPark mouse does model mitochondrial failure, strongly implicated as one common genetic denominator in Parkinson’s disease pathology (Ekstrand et al, 2007; Galter et al, 2010). The MitoPark phenotype results in uniform and progressive loss of DA afferents in areas of the basal ganglia, starting in early adulthood, in which nigrostriatal dopaminergic projections innervating the dorsal striatum are strongly affected, while mesolimbic dopaminergic projections innervating the ventral striatum are relatively spared (Ekstrand et al, 2007; Galter et al, 2010). This pattern of progressive DA neuron loss recapitulates the gradual degeneration of DA projections and associated motor symptoms typical of Parkinson’s disease in humans. The slow bilateral time course of nigrostriatal degeneration in MitoPark animals, over weeks to months, compared with the days to weeks time of neurodegeneration in most toxin models also allows study of the major risk factor for idiopathic Parkinson’s disease – age. Our genetic model also provides greater uniformity of animals with respect to degree of DA degeneration, compared to toxin models. Additionally, MitoPark mice are responsive to L-Dopa therapy with a differential response depending on disease stage (Galter et al, 2010; Gellhaar et al, 2015). Here we show behavioral evidence of striatal neuroplasticity in response to denervation of dopaminergic afferents, as well as in response to chronic L-Dopa treatment. As severity of Parkinson’s disease has been recognized as a risk factor for LIDs in humans (Schrag et al 2003; Schrag and Quinn, 2000; Kostic et al, 1991; Kumar et al, 2005), we used the mouse model to test whether involuntary movements in response to chronic L-Dopa treatment was dependent on length of treatment, or degree of degeneration. Our results suggest that the MitoPark mouse may provide a preclinical model to study circuit-related questions, and to probe therapeutic treatments in relation to the formation of LIDs. Our results support the current view that delaying the onset of L-Dopa treatment does not delay development of LIDs.
A recent paper (Gellhaar, et al, 2015) came to similar conclusions about the relationship of development of hyperactivity and dyskinesia to the age of MitoPark mice and therefore degree of DA loss, rather than duration of L-Dopa treatment. There are some differences between this paper and the current study. The MitoPark mice used here had an internal ribosome entry sequence to drive cre recombinase from the 3' untranslated region of the endogenous DAT gene, minimizing any effects on DAT mRNA formation. In addition, we used measurement of DA and metabolites by HPLC, rather than tyrosine hydroxylase immunocytochemistry, to document the age related DA loss. Finally, we also used array analysis to investigate striatal mRNA encoding relevant neurotransmittor receptors to monitor changes which accompany the dyskinetic/hyperactivity behavior in these mice.
2. Results
Assessment of DA depletion in the striatum of MitoPark mice
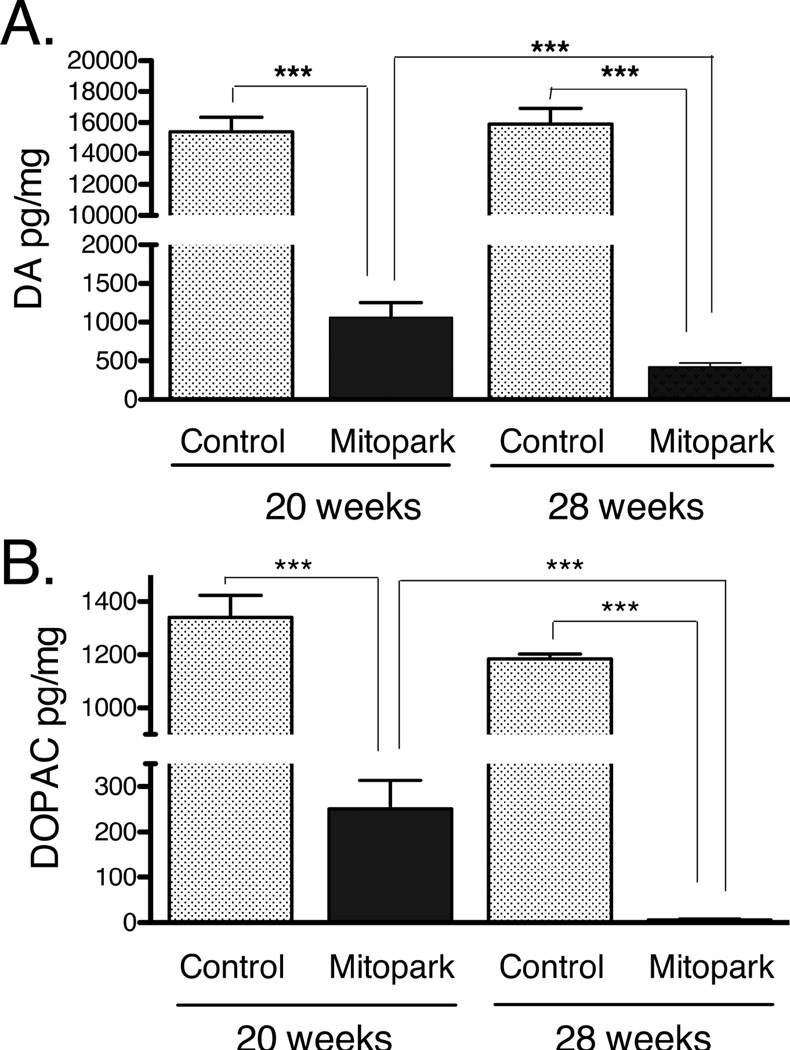
Neurochemical studies in dorsal striatum from MitoPark and WT mice at 20- and 28 weeks of age points confirmed here the progressive DA neuron loss previously observed in substantia nigra and the striatal DA depletion in MitoPark mice (Ekstrand et al, 2007; Ekstrand and Galter, 2009), and showed significant loss of DA and DOPAC in the dorsal striatum compared to WT animals (Fig. 1). Previous studies have shown a progressive loss of striatal dopamine in MitoPark mice starting at 12 weeks of age, and continuing until 40 weeks of age (Ekstrand et al, 2007; Ekstrand and Galter, 2009). In this study, a further significant decline in DOPAC levels was observed in 28-week old MitoPark mice, compared to 20-week old MitoPark mice. DA levels were also significantly decreased in 28-week old, compared to 20-week old, MitoPark mice (Fig. 1). This gradual degeneration over time is also characteristic of Parkinson’s disease in humans. Dopamine turnover, as HVA+DOPAC/DA, was measured in MitoPark mice vs wild type at both 20 and 28 weeks of age. The values were 0.217± 0.08 for 20 week wt, 0.177±0.09 for 28 week wt, 0.883±0.08 for 20 week MitoPark and 0.706±0.09 for 28 week old MitoPark. Turnover rates between wt and MitoPark were significantly different (p< 0.001, ANOVA).
Figure 1.
Concentrations of DA (A) and DOPAC (B) levels in striatum of control and MitoPark mice at 20 and 28 weeks of age. Bars represent mean dopamine (A) and DOPAC (B) levels from 5 animals per group and error bars indicate SEM. DA and DOPAC levels were significantly lower in both 20 and 28 week MitoPark mice compared to control animals. A significant DA reduction was observed as well in 28 week old MitoPark mice as compared to 20-week old MitoPark mice. (***p<0.001 ANOVA followed by Fisher’s protected least significant difference, PLSD). DOPAC levels were significantly reduced in 28-week old MitoPark mice, as compared to 20-week old MitoPark mice (B; ***p<0.001, ANOVA followed by Fisher’s protected least significant difference, PLSD).
L-Dopa induced locomotor behavior
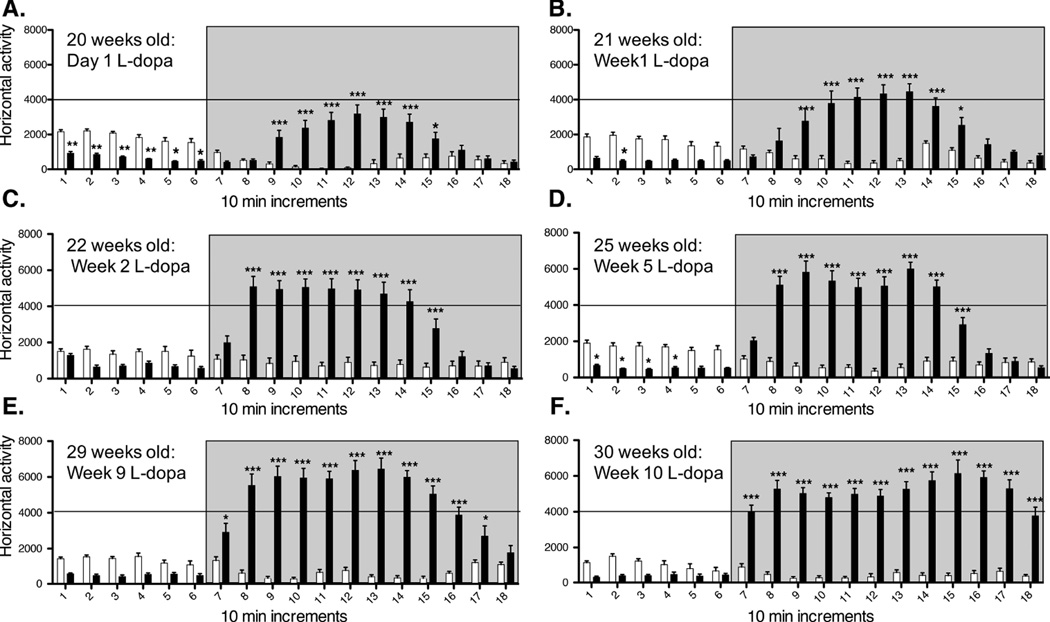
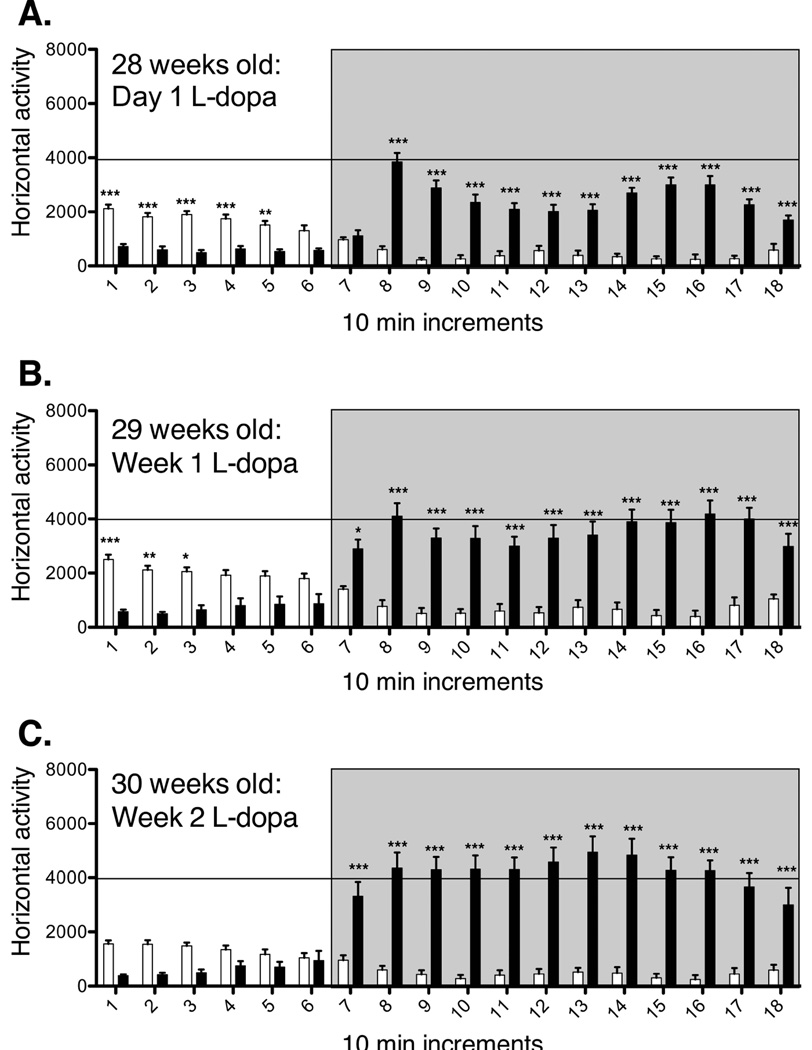
Oral intake of the DA precursor L-Dopa elicits therapeutic relief in Parkinson’s disease patients by restoring extracellular DA levels within the dorsal striatum. However, unlike physiological DA transmission, this therapeutic regimen is thought to lead to abnormal peaks of non-synaptic DA, which over time trigger behavioral sensitization, eventually manifested as abnormal involuntary movements (Iravani et al, 2012). In this study we followed the pattern of horizontal locomotor activity in MitoPark mice and age-matched controls through chronic L-Dopa treatment. There was little overall movement and no LIDs in 20 week old MitoPark mice prior to L-Dopa administration. MitoPark mice in both age groups (20- and 28- week old) developed sensitization to L-Dopa treatment (Figs. 2 and 3). Interestingly, the initial and subsequent response to L-Dopa differed between the two age groups. Twenty eight week old parkinsonian mice showed an increased sensitivity to initial L-Dopa administration (Fig. 3), as the latency to reach L-Dopa peak activity decreased in this age group, and total duration of the response to L-Dopa increased when compared to 20-week old MitoPark mice (Fig. 2). This trend continued throughout treatment, suggesting that the continuous degeneration of DA terminals plays an important role in modeling adaptations in the brain that ultimately result in very different behavioral responses depending on the stage of the disease at initiation of L-Dopa treatment.
Figure 2.
Acute (A) and chronic (B–F) L-Dopa induced locomotor behavior in 20-week old MitoPark (dark bars) and control (white bars) mice. Horizontal locomotor activity is plotted in 10 min increments. L-Dopa injections were given after 60 min of baseline recordings, and recorded for 2 additional hours (grey box). MitoPark and control mice received the initial L-Dopa injection at 20 weeks of age (A), and continued to receive daily L-Dopa injections until 30-weeks of age (B-F). Control mice did not develop sensitization to L-Dopa treatment (white bars). 20-week old MitoPark mice developed a progressive sensitization to L-Dopa treatment with chronic treatment. After 10 weeks of L-Dopa treatment (F, 30 weeks old), MitoPark mice showed a faster onset and a longer response to L-Dopa injections, when compared to the same MitoPark cohort after the first weeks of treatment.
Figure 3.
Acute (A) and chronic (B and C) L-Dopa induced locomotor behavior in 28-week old MitoPark and control mice. Horizontal locomotor activity is plotted in 10 min increments. L-Dopa injections were given after 60 min of baseline recordings, and recordings continued for 2 additional hours (grey box). MitoPark and control mice received the initial L-Dopa injection at 28 weeks of age (A), and continued to receive daily L-Dopa injections for 2 weeks until 30-weeks of age (C). Control mice did not develop sensitization to L-Dopa treatment (white bars). Twenty eight-week old MitoPark mice developed a rapid sensitization to L-Dopa treatment (dark bars). After 2 weeks of chronic L-Dopa treatment (C, 30 weeks old), MitoPark mice showed a significantly faster onset to L-Dopa treatment, as compared to the same cohort of mice after the first day of treatment (A). In addition, expression of sensitization as related to chronicity of L-Dopa treatment was more rapid and pronounced (both in onset and duration) in MitoPark mice starting L-Dopa treatment at 28 weeks of age as compared to MitoPark mice starting treatment at 20 weeks of age (Fig. 2).
Abnormal Involuntary movements
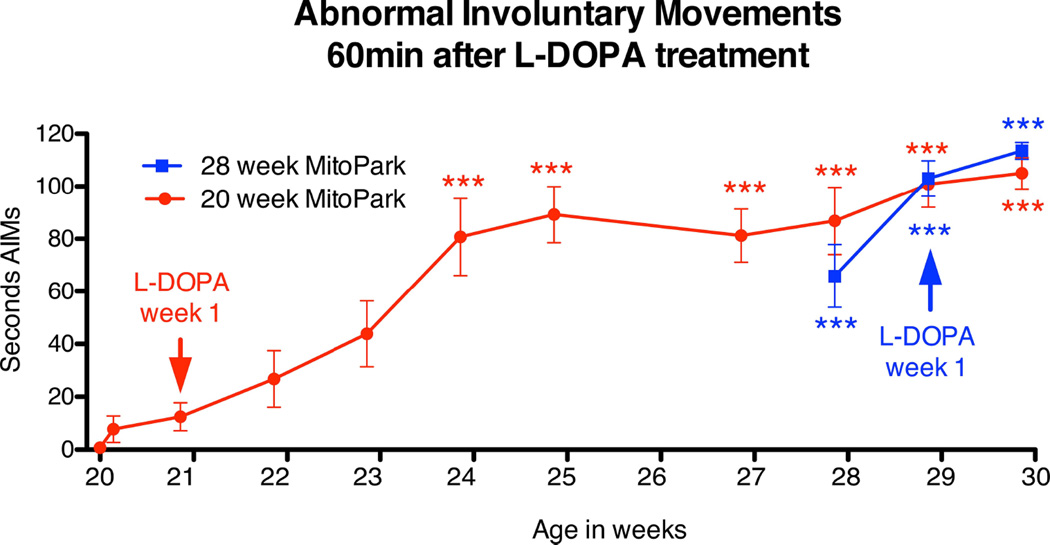
Development of L-Dopa induced dyskinesias correlated well with locomotor activity data. Twenty-week old MitoPark mice showed a very low incidence of LIDs at the initial stages of L-Dopa treatment (Figure 4), and did not develop more severe degrees of LIDs until after 4–5 weeks of chronic L-Dopa treatment (Figure 4). Thus LIDs in 20-week old MitoPark mice developed progressively with L-Dopa treatment. In contrast, 28-week MitoPark mice manifested severe LIDs immediately after the first L-Dopa injection (Figure 4). The development of LIDs in 28-week old MitoPark mice was thus abrupt and marked even after the 1st L-Dopa injection. Control animals treated with L-Dopa did not develop LIDs and therefore are not represented in Figure 4. These data suggest that the extent of loss of DA innervation and subsequent adaptations in the striatum may determine how rapidly motor complications develop after initiation of L-Dopa treatment.
Figure 4.
Development of L-Dopa induced abnormal involuntary movements over time in 20 and 28-week old MitoPark mice after treatment with chronic daily L-Dopa injections (10 mg/kg). All 28-week old MitoPark mice showed abrupt and severe dyskinesias after the first L-Dopa injection. Twenty week old MitoPark mice did not develop such severe dyskinetic movements until after 4–5 weeks of chronic L-Dopa treatment. These data suggest that priming for L-Dopa induced dyskinesia is significantly accelerated in MitoPark mice with severe DA depletion.
Striatal neurotransmitter receptor mRNA expression in 28-week old Mitopark mice
Behavioral results from 20- and 28-week old MitoPark mice treated with L-Dopa, shown above, suggest that changes in the basal ganglia as a result of the progressive loss of DA neurons may be related to susceptibility to L-Dopa induced dyskinesias in MitoPark mice. As 28-week old MitoPark mice developed severe LIDs after acute treatment with L-Dopa, real-time quantitative PCR analyses were performed in striatal tissue to determine mRNA expression levels for various neurotransmitter receptor families, as compared to aged-matched WT controls. Progressive dopamine neuron degeneration resulted in significant alterations in the expression of mRNA species encoding neurotransmitter receptors in striatum in 28-week old naïve MitoPark mice, as compared to controls. Such alterations included dopaminergic, glutamatergic, gabaergic, cholinergic and serotonergic neurotransmitter receptors (Table 1). As 28-week Mitopark mice are particularly susceptible to developing L-Dopa induced dyskinesias, dysregulation of these neurotransmitter systems may contribute to the increased susceptibility to LIDs.
Table 1.
Dysregulation of neurotransmitter receptor genesin striatum of 28-week old MitoPark mice as compared to age-matched controls. Marked alterations are shown in bold.
| GeneBank | Symbol | Description | Fold change |
p value |
|---|---|---|---|---|
| NM_013461 | Adra1a | Adrenergic receptor, alpha 1a | 1.2713 | 0.01034 |
| NM_013460 | Adra1d | Adrenergic receptor, alpha 1d | 1.6758 | 0.334303 |
| NM_007417 | Adra2a | Adrenergic receptor, alpha 2a | 1.6216 | 0.033766 |
| NM_007420 | Adrb2 | Adrenergic receptor, beta 2 | 1.3255 | 0.00113 |
| NM_013462 | Adrb3 | Adrenergic receptor, beta 3 | 1.3003 | 0.256787 |
| NM_016847 | Avpr1a | Arginine vasopressin receptor 1A | 2.1497 | 0.009146 |
| NM_011924 | Avpr1b | Arginine vasopressin receptor 1B | 1.0206 | 0.670543 |
| NM_009766 | Brs3 | Bombesin-like receptor 3 | 1.6186 | 0.125584 |
| NM_007627 | Cckbr | Cholecystokinin B receptor | 1.1834 | 0.208665 |
| NM_007698 | Chrm1 | Cholinergic receptor, muscarinic 1, CNS | 0.8805 | 0.101805 |
| NM_007699 | Chrm4 | Cholinergic receptor, muscarinic 4 | 0.7734 | 0.003584 |
| NM_205783 | Chrm5 | Cholinergic receptor, muscarinic 5 | 1.685 | 0.040201 |
| NM_145129 | Chrna3 | Cholinergic receptor, nicotinic, alpha polypeptide 3 | 0.8013 | 0.124339 |
| NM_015730 | Chrna4 | Cholinergic receptor, nicotinic, alpha polypeptide 4 | 1.3541 | 0.220213 |
| NM_176844 | Chrna5 | Cholinergic receptor, nicotinic, alpha polypeptide 5 | 0.9552 | 0.783436 |
| NM_021369 | Chrna6 | Cholinergic receptor, nicotinic, alpha polypeptide 6 | 1.0048 | 0.943585 |
| NM_007390 | Chrna7 | Cholinergic receptor, nicotinic, alpha polypeptide 7 | 0.8026 | 0.011896 |
| NM_009603 | Chrne | Cholinergic receptor, nicotinic, epsilon polypeptide | 1.0776 | 0.186884 |
| NM_007726 | Cnr1 | Cannabinoid receptor 1 (brain) | 0.6518 | 0.007608 |
| NM_010076 | Drd1a | Dopamine receptor D1A | 0.7846 | 0.017427 |
| NM_010077 | Drd2 | Dopamine receptor D2 | 0.9356 | 0.497982 |
| NM_013503 | Drd5 | Dopamine receptor D5 | 1.4165 | 0.00071 |
| NM_019439 | Gabbr1 | (GABA) B receptor, 1 | 0.9429 | 0.135509 |
| NM_001081141 | Gabbr2 | (GABA) B receptor, 2 | 1.163 | 0.145911 |
| NM_010250 | Gabra1 | (GABA) A receptor, subunit alpha 1 | 1.2985 | 0.07144 |
| NM_008066 | Gabra2 | (GABA) A receptor, subunit alpha 2 | 1.3524 | 0.071727 |
| NM_010251 | Gabra4 | (GABA) A receptor, subunit alpha 4 | 0.8071 | 0.003188 |
| NM_176942 | Gabra5 | (GABA) A receptor, subunit alpha 5 | 1.2922 | 0.011022 |
| NM_008068 | Gabra6 | (GABA) A receptor, subunit alpha 6 | 1.0883 | 0.586606 |
| NM_008069 | Gabrb1 | (GABA) A receptor, subunit beta 1 | 1.0397 | 0.400714 |
| NM_008071 | Gabrb3 | (GABA) A receptor, subunit beta 3 | 0.8539 | 0.051473 |
| NM_008072 | Gabrd | (GABA) A receptor, subunit delta | 0.7827 | 0.007242 |
| NM_017369 | Gabre | (GABA) A receptor, subunit epsilon | 1.385 | 0.135854 |
| NM_010252 | Gabrg1 | (GABA) A receptor, subunit gamma 1 | 1.2759 | 0.03047 |
| NM_008073 | Gabrg2 | (GABA) A receptor, subunit gamma 2 | 1.0238 | 0.710742 |
| NM_008074 | Gabrg3 | (GABA) A receptor, subunit gamma 3 | 0.8601 | 0.144219 |
| NM_020488 | Gabrq | (GABA) A receptor, subunit theta | 1.2317 | 0.281796 |
| NM_008075 | Gabrr1 | (GABA) C receptor, subunit rho 1 | 1.3599 | 0.021585 |
| NM_008076 | Gabrr2 | (GABA) C receptor, subunit rho 2 | 1.9866 | 0.001767 |
| NM_008101 | Gcgr | Glucagon receptor | 1.0847 | 0.603755 |
| NM_008165 | Gria1 | Glutamate receptor, ionotropic, AMPA1 | 1.1595 | 0.001987 |
| NM_013540 | Gria2 | Glutamate receptor, ionotropic, AMPA2 | 0.8209 | 0.014424 |
| NM_016886 | Gria3 | Glutamate receptor, ionotropic, AMPA3 | 0.9255 | 0.330373 |
| NM_146072 | Grik1 | Glutamate receptor, ionotropic, kainate 1 | 1.6701 | 0.047624 |
| NM_010349 | Grik2 | Glutamate receptor, ionotropic, kainate 2 | 0.8522 | 0.005992 |
| NM_175481 | Grik4 | Glutamate receptor, ionotropic, kainate 4 | 1.5347 | 0.000566 |
| NM_008168 | Grik5 | Glutamate receptor, ionotropic, kainate 5 | 0.7479 | 0.01214 |
| NM_008169 | Grin1 | Glutamate receptor, ionotropic, NMDA1 | 0.8635 | 0.017452 |
| NM_008170 | Grin2a | Glutamate receptor, ionotropic, NMDA2A | 0.8861 | 0.074721 |
| NM_008171 | Grin2b | Glutamate receptor, ionotropic, NMDA2B | 0.8552 | 0.014799 |
| NM_010350 | Grin2c | Glutamate receptor, ionotropic, NMDA2C | 0.907 | 0.258151 |
| NM_016976 | x | Glutamate receptor, metabotropic 1 | 0.9209 | 0.022333 |
| NM_181850 | Grm3 | Glutamate receptor, metabotropic 3 | 0.8678 | 0.020762 |
| NM_001013385 | Grm4 | Glutamate receptor, metabotropic 4 | 0.882 | 0.109941 |
| NM_001081414 | Grm5 | Glutamate receptor, metabotropic 5 | 0.7745 | 0.004797 |
| NM_173372 | Grm6 | Glutamate receptor, metabotropic 6 | 1.0388 | 0.980826 |
| NM_177328 | Grm7 | Glutamate receptor, metabotropic 7 | 1.0267 | 0.690845 |
| NM_008174 | Grm8 | Glutamate receptor, metabotropic 8 | 1.0772 | 0.371807 |
| NM_008177 | Grpr | Gastrin releasing peptide receptor | 1.3413 | 0.198779 |
| NM_198962 | Hcrtr2 | Hypocretin (orexin) receptor 2 | 1.5415 | 0.018631 |
| NM_008285 | Hrh1 | Histamine receptor H1 | 1.219 | 0.193255 |
| NM_153087 | Hrh4 | Histamine receptor H4 | 1.1032 | 0.369374 |
| NM_008308 | Htr1a | Sertonin receptor 1A | 1.6091 | 0.007967 |
| NM_010482 | Htr1b | Serotonin receptor 1B | 1.0809 | 0.385587 |
| NM_008309 | Htr1d | Serotonin receptor 1D | 0.9676 | 0.670621 |
| NM_008310 | Htr1f | Serotonin receptor 1F | 0.9772 | 0.654426 |
| NM_172812 | Htr2a | Serotonin receptor 2A | 1.3284 | 0.032927 |
| NM_008311 | Htr2b | Serotonin receptor 2B | 1.1198 | 0.001279 |
| NM_008312 | Htr2c | Serotonin receptor 2C | 1.3043 | 0.05377 |
| NM_013561 | Htr3a | Serotonin receptor 3A | 1.2509 | 0.044708 |
| NM_008313 | Htr4 | Serotonin receptor 4 | 1.1655 | 0.003944 |
| NM_008315 | Htr7 | Serotonin receptor 7 | 1.7551 | 0.048707 |
| NM_008731 | Npy2r | Neuropeptide Y receptor Y2 | 1.561 | 0.003761 |
| NM_016708 | Npy5r | Neuropeptide Y receptor Y5 | 0.9597 | 0.502927 |
| NM_008747 | Ntsr2 | Neurotensin receptor 2 | 1.2378 | 0.02537 |
| NM_001081147 | Oxtr | Oxytocin receptor | 1.5892 | 0.068579 |
| NM_144944 | Prokr2 | Prokineticin receptor 2 | 1.0258 | 0.854078 |
| NM_009216 | Sstr1 | Somatostatin receptor 1 | 1.1627 | 0.354588 |
| NM_009217 | Sstr2 | Somatostatin receptor 2 | 1.4627 | 0.00043 |
| NM_009219 | Sstr4 | Somatostatin receptor 4 | 1.123 | 0.088049 |
| NM_009313 | Tacr1 | Tachykinin receptor 1 | 1.0869 | 0.235536 |
| NM_009314 | Tacr2 | Tachykinin receptor 2 | 0.7827 | 0.125587 |
| NM_021382 | Tacr3 | Tachykinin receptor 3 | 1.0642 | 0.435184 |
| NM_009775 | Tspo | Translocator protein | 1.4052 | 0.031808 |
3. Discussion
A prerequisite for the development of L-Dopa induced dyskinesias appears to be the loss of dopamine nerve cells in substantia nigra and of DA nerve terminals in striatum. The rapid emergence of dyskinesia in Parkinson’s disease patients with late diagnosis or a young onset, where denervation is extensive at diagnosis, and the absence of dyskinesia in normal humans chronically treated with L-Dopa (i.e., mistaken diagnosis) support this conjecture (Jankovic et al, 2000; Kumar et al, 2005; Schrag and Quinn, 2000). Chronic stimulation of the denervated striatum via the dopamine precursor, L-Dopa, induces a process of sensitization that modifies the response to subsequent dopaminergic treatments (Nadjar et al, 2009). This process, called priming, is associated with changes in receptors for DA and other neurotransmitters and may play a role in the development of neuronal maladaptations and motor complications in humans. As data from Parkinson’s disease patients suggest a close relationship between priming, the development of LIDs in response to treatment, and the level of DA loss in striatum (Nadjar et al, 2009), it is of importance to develop a preclinical animal model that recapitulates this aspect of the disease. Using a parkinsonian mouse model, the MitoPark mouse, we show that the interaction of L-Dopa with the denervated basal ganglia is quite different depending on disease progression at the time of treatment. These findings support both previous experimental work (Francardo et al, 2011; Lundblad et al, 2004; Winkler et al, 2002) and clinical observations (Cilia et al, 2014).
To establish the MitoPark mouse as a preclinical animal model to study the mechanisms and neuronal adaptations occurring in the basal ganglia in response to DA replacement therapy, and that eventually lead to the formation of LIDs, it is important to first demonstrate that this model recapitulates some of the events associated with dyskinesias in humans. The MitoPark genotype results in progressive loss of DA afferents that spans several months. Starting at 12 weeks of age MitoPark mice show decreased locomotion in the open field. By 20 weeks of age, they have lost 60–70% of their DA neurons in the SN and the decline in locomotor activity is substantial (Ekstrand et al, 2007; Galter et al, 2010; Good et al, 2011). As in humans suffering from Parkinson’s disease, this motoric decline can be reversed by treatment with the DA precursor L-Dopa (Ekstrand et al, 2007; Ekstrand and Galter, 2009; Galter et al, 2010). From this point on, motor deficits progress, along with loss of fine motor skills. This eventually prevents MitoPark mice from eating and grooming properly, and leads to a decline in their quality of life reminiscent of the situation for Parkinson’s disease patients before L-Dopa became a treatment option (Ekstrand et al, 2007). By 33 weeks of age MitoPark mice have lost 70–80% of the nigral DA neurons, and present advanced parkinsonian symptoms (Ekstrand et al, 2007; Ekstrand and Galter, 2009; Galter et al, 2010). Also, as shown here by PCR arrays, the state of neurotransmitter dynamics in the striatum of 28 week old MitoPark mice has fundamentally changed. Severe loss of DA afferents in striatum of 28-week old MitoPark mice causes significant changes in the mRNA expression pattern for DA, GABA, glutamatergic, serotonergic and cholinergic receptors. We therefore hypothesized that L-Dopa induced dyskinesia would be significantly accelerated in MitoPark animals with advanced DA degeneration (28-week old), as compared to animals with less advanced DA loss (20-week old). Interestingly, the duration and peak response to acute L-Dopa treatment was significantly enhanced in MitoPark mice with severe DA depletion, suggesting that older MitoPark mice with more advanced Parkinson’s disease symptoms sensitize more rapidly to DA replacement therapy. These data correlated well with the development of LIDs. While all 28-week MitoPark mice showed severe dyskinesias after the first L-Dopa injection, 20-week old MitoPark mice did not develop such severe dyskinetic movements until 4–5 weeks after chronic L-Dopa treatment.
Recent literature underscores the relevance of the PCR array data, reported here, that DA degeneration modified the expression of a multitude of neurotransmitter receptors in 28-week old MitoPark mice, as compared to controls. For example, 28-week old MitoPark mice showed a significant dysregulation of many glutamatergic receptor units (of 19 investigated levels of glutamate receptor mRNA species, 8 were significantly decreased, while 3 were significantly increased). Recent studies have shown the value of NMDA receptor blockade in humans to treat the development of LIDs. A study in advanced Parkinson’s disease patients showed that amantadine reduced dyskinesia severity and improved motor fluctuations in L-Dopa treated individuals (Verhagen et al, 1998). Furthermore, a Phase IIb study showed that patients with moderate to severe Parkinson’s disease showed significant improvement in dyskinesia severity after treatment with AFQ056, a potent selective mGluR5 inhibitor (Stocchi et al, 2013). We also found significant changes in mRNA expression for cholinergic receptors in striatum of 28-week old MitoPark mice. A study using Pitx-3 deficient aphakia mice has shown that chronic L-Dopa induces ERK activation in striatal cholinergic interneurons, and that this neuronal maladaptation plays a role in the expression of LIDs (Ding et al 2011). As cholinergic tone contributes to DA and glutamate release locally via presynaptic nicotinic receptors on DA and glutamatergic terminals, our data suggest changes in cholinergic neurotransmission may be involved in the development of motor complications. It will be important to dissect which of the neurotransmitter changes observed in this study contribute to the antiparkinsonian effects associated with L-Dopa treatment, and/or to the development of dyskinesias.
We used HPLC measurement of DA and DOPAC to document the age related loss of dopaminergic projection in these mice. We did not carry out a more extensive characterization because, with respect to the nigrostriatal DA system, of previously published immunohistochemical studies, biochemical studies, cell counts, pharmacological studies and electrophysiological studies documenting the neurodegenerative events in MitoPark mice (Ekstrand et al 2007; Galter et al 2010; Gellhaar et al, 2015; Good et al 2011). The genetic nature of the model, leading to identical progressive loss of the DA system with time in all MitoPark mice, shows a correlation between the response to L-Dopa and the degree of degeneration, based on the age-related changes in the MitoPark DA system which are extensively documented in the studies cited above.
To conclude, we show that MitoPark mice develop a differential type of response to L-Dopa treatment depending on the extent of DA neurodegeneration. The MitoPark mouse model offers an opportunity to study plastic rearrangements occurring in the basal ganglia during slowly progressive DA loss, and the potential impact of therapeutic interventions when animals are suffering from mild, moderate or severe Parkinson’s disease symptoms. This may help progress towards the goal of improving the effectiveness of L-Dopa replacement therapies, while avoiding or inhibiting the development of L-Dopa induced dyskinesias. Moreover, our data strongly supports the view that delaying the initiation of L-Dopa treatment does not delay the development of LIDs.
4. Experimental Procedure
Animals
All animal studies were conducted under National Institutes Health (NIH) Guidelines using the NIH handbook Animals in Research and were approved by the Institutional Animal Care and Use Committee (National Institute on Drug Abuse, Intramural Research Program, Baltimore, MD). The TfamloxP (Ekstrand et al, 2007) line was mixed with the Slc6a3Cre (Backman et al, 2006) to obtain regional knockout (Slc6a3Cre/wtTfamloxP/loxP, or MitoPark) and control mice (Slc6a3Cre/wt Tfamwt/wt, or WT). Animals were genotyped using TfamloxP and Slc6a3Cre primers as described elsewhere (Backman et al, 2006; Ekstrand et al, 2007). Animals received pelleted food and water ad libitum. From 20 weeks of age, ground mouse chow was also supplied. Mice were sacrificed using CO2 inhalation at the end of the experimental procedures and tissue samples were collected after death was verified.
HPLC analyses of DA and metabolites
Striatal tissue from 20 and 28 week old WT and MitoPark mice (n=5) for each genotype and age group) were dissected, weighed and stored at −80°C until extraction. Tissue samples were homogenized in 0.1M perchloric acid and centrifuged at 25,000 g for 12 min. DOPAC, DA, and HVA in supernatants were measured by HPLC with electrochemical detection (Waters 717 Plus automated injection system; Waters 1525 Binary pump; ESA Coulochem III detector). The column (Waters SunFire C18 5 µm; 4.6 × 150.0 mm) received a mobile phase (0.01 M sodium dihydrogenphosphate, 0.01 M citric acid, 1.2 mM sodium EDTA, 1.2 mM sodium 1-heptane sulfonic acid, 10% methanol, pH 3.5) at flow rate 1.0 ml/min and a column temperature of 34°C. Concentrations of DOPAC, DA and HVA were calculated as pg/mg of tissue weight. Statistical analysis was performed using analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD) (StatView 4.02,SAS Institute, Cary, NC). Criteria for significance were set at p < 0.05 (* p<0.05, ** p<0.01, ***p<0.001).
RT2 profiler neurotransmitter receptors arrays
To determine how DA denervation affected the pattern of mRNA expression for various neurotransmitter receptors in striatum, PCR microarrays for 84 mRNA species involved in neurotransmission were used. Striatal tissue was dissected from 28-week old naïve WT (n=6) and Mitopark mice (n=6) and was immediately processed for total RNA isolation and DNAse I treatment (RNAqueous-Micro Ambion kit, Applied Biosystems Ambion, Austin, TX), following the manufacturer’s instructions. Total RNA from individual animals were reverse-transcribed into cDNA (RT2 First strand Kit, SABiosciences, mixed with a qPCR mastermix containing SYBR Green, SA Biosciences), and aliquoted in equal volumes to each well of the PCR microarrays (Mouse synaptic plasticity RT2 Profiler™PAMM-060Z, SA Biosciences). A separate array was used for each animal. After real-time PCR (HT7900 Cycler, Applied Biosystems), data were normalized using appropriate software (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php).
Treatment regimen
L-Dopa, and benserazide hydrochloride (Sigma-Aldrich, St. Louis, MO) were dissolved in 0.9% saline. L-Dopa (10 mg/kg) was injected i.p. together with benserazide (5 mg/kg) to inhibit peripheral dopa decarboxylase. L-Dopa was injected twice a day (morning and late afternoon), except for weekends, when injections were given only in the morning. Daily L-Dopa treatment was initiated in two different cohorts of MitoPark mice and age-matched controls (n=8 in each group) at 20 weeks of age (moderate DA loss), and 28 weeks of age (marked DA loss), respectively. 20-week old mice were treated with L-Dopa for a period of 10 weeks, until they were 30 weeks old. 28-week old mice were treated with L-Dopa for a period of 2 weeks, also until they were 30 weeks old. Behavioral analyses were performed weekly after initiation of L-Dopa treatment.
Locomotor Behavior
Locomotor activity was recorded using activity chambers placed into analyzers, where activity was monitored through a grid of infrared light beams (Versamax, Accuscan instruments). Control and MitoPark mice were habituated (day 0) to the chambers and to the injection protocol prior to L-Dopa treatment. On day 0, control and Mitopark mice were placed in the locomotor activity chambers for a 1-h habituation period. Thereafter, animals received an i.p. saline injection and were placed back into the chamber for two additional hours. Behavioral recordings started at 20 weeks of age (moderate DA degeneration), or 28 weeks of age (severe dopamine degeneration) and continued every week, until the end point of the study at 30 weeks of age. There were 8 animals in each of the four treatment groups. We chose to use 20 weeks as an initial age since there is a robust PD-like phenotype, as oppose to smaller changes in behavior and dopamine dynamics at younger MitoPark ages. Recording sessions lasted 180 minutes. All behavioral tests were done between 9:00 am and 1:00 pm. Data are presented as the mean and standard error of the mean. Two-way analysis of variance with Bonferroni post hoc comparisons for multiple measurements was performed. A p value of <0.05 was considered significant (* p<0.05, ** p<0.01, ***p<0.001).
Quantification of L-Dopa induced dyskinesia
Animals were placed in a small transparent cylinder 60 minutes after treatment initiation with L-Dopa, as it coincided with maximal horizontal activity in 20-week old Mitopark mice, and was continued each week thereafter. Spontaneous activity was videotaped for 3 minutes and the middle 2 minutes were analyzed manually to quantify dyskinesias and L-Dopa induced motor abnormalities. Abnormal paw movements exhibited in the cylinder were scored as previously described (Ding et al, 2007). Front paw dyskinesia was noted when mice stood on both hind paws, with both front paws moving repeatedly up and down along the surface of the cylinder wall. Hind paw touch was noted when mice stood on their hind paws and repeatedly touched the surface of the cylinder wall with one hind paw. Three paw dyskinesia was noted when mice stood on their hind paws close to the wall of the cylinder moving both front paws as noted above and repeatedly lifting the hind paws up and down in an alternating fashion while maintaining their weight on the other hind paw. A few MitoPark mice developed an abnormal rotational response to L-Dopa treatment, which increased over treatment time. This type of L-Dopa-induced uninterrupted rotational behavioral response was considered a motor complication induced by L-Dopa treatment. The sum of hind paw, three paw dyskinesia and abnormal rotational behavior were used to score total abnormal involuntary movements or L-Dopa induced dyskinesias (LIDs). Data are presented as the mean and standard error of the mean. Two-way analysis of variance with Bonferroni post hoc comparison was performed (measured points in both 20- and 28-week old groups, were compared to abnormal involuntary movements in 20-week old Mitopark mice chronically treated with L-Dopa for 1 week; see Fig. 4). A p value of <0.05 was considered significant (* p<0.05, ** p<0.01, ***p<0.001).
Highlights.
L-DOPA dyskinesia related to DA loss.
L-DOPA dyskinesia less related to prior L-DOPA exposure
Changes in striatal mRNA seen with DA loss
Acknowledgments
The study was supported by the Intramural Research Program, NIDA, NIH (LS, OD, YZ, BL, JC, CB), NIH grant NS070825 (BH), ERC Advanced Investigator grant (322744 LO), the Swedish Research Council (K2012-62X-03185-42-4 LO), the Swedish Parkinson Foundation (LO), and Taiwan NSC101-2632-B-038-001-MY3 (YC).
LO is a co-owner of Kampavata AB, which owns commercial rights to the MitoPark mouse. This does not alter our adherence to Brain Research policies on sharing data and materials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
The authors have no other conflicts of interests.
Contributor Information
Yajun Zhang, Email: ychiang@tmu.edu.tw.
Lars Olson, Email: Lars.Olson@ki.se.
Barry J. Hoffer, Email: barry.hoffer@case.edu.
Cristina M. Bäckman, Email: Cristina.Backman@nih.gov.
References
- Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, et al. Characterization of a mouse strain expressing Cre recombinase from the 3' untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- Cilia R, Akpalu A, Sarfo FS, Cham M, Amboni M, et al. The modern pre levodopa era of Parkinson's disease: insights into motor complications from sub-Saharan Africa. Brain. 2014;137:2731–2742. doi: 10.1093/brain/awu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Won L, Britt JP, Lim SA, McGehee DS, et al. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc Natl Acad Sci USA. 2011;108:840–845. doi: 10.1073/pnas.1006511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Restrepo J, Won L, Hwang DY, Kim KS, et al. Chronic 3,4-dihydroxyphenylalanine treatment induces dyskinesia in aphakia mice, a novel genetic model of Parkinson's disease. Neurobiol Dis. 2007;27:11–23. doi: 10.1016/j.nbd.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand MI, Galter D. The MitoPark Mouse - an animal model of Parkinson's disease with impaired respiratory chain function in dopamine neurons. Parkinsonism Relat Disord. 2009;15(Suppl 3):S185–S188. doi: 10.1016/S1353-8020(09)70811-9. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francardo V, Recchia A, Popovic N, Andersson D, Nissbrandt H, et al. Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkinson's disease. Neurobiol Dis. 2011;42:327–340. doi: 10.1016/j.nbd.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Galter D, Pernold K, Yoshitake T, Lindqvist E, Hoffer B, et al. MitoPark mice mirror the slow progression of key symptoms and L-DOPA response in Parkinson's disease. Genes Brain Behav. 2010;9:173–181. doi: 10.1111/j.1601-183X.2009.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellhaar S, Marcellino D, Abrams MB, Galter D. Chronic L-DOPA induces hyperactivity, normalization of gait and dyskinetic behavior in MitoPark mice. Genes Brain Behav. 2015 Mar 9; doi: 10.1111/gbb.12210. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CH, Hoffman AF, Hoffer BJ, Chefer VI, Shippenberg TS, et al. Impaired nigrostriatal function precedes behavioral deficits in a genetic mitochondrial model of Parkinson's disease. FASEB J. 2011;25:1333–1344. doi: 10.1096/fj.10-173625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani MM, McCreary AC, Jenner P. 452 Striatal plasticity in Parkinson's disease and L-Dopa induced dyskinesia. Parkinsonism Relat Disord. 2012;18(Suppl 1):S123–S125. doi: 10.1016/S1353-8020(11)70038-4. [DOI] [PubMed] [Google Scholar]
- Jankovic J, Rajput AH, McDermott MP, Perl DP. The evolution of diagnosis in early Parkinson disease. Parkinson Study Group. Arch Neurol. 2000;57:369–372. doi: 10.1001/archneur.57.3.369. [DOI] [PubMed] [Google Scholar]
- Kostic V, Przedborski S, Flaster E, Sternic N. Early development of levodopa induced dyskinesias and response fluctuations in young-onset Parkinson's disease. Neurology. 1991;41:202–205. doi: 10.1212/wnl.41.2_part_1.202. [DOI] [PubMed] [Google Scholar]
- Kumar N, Van Gerpen JA, Bower JH, Ahlskog JE. Levodopa-dyskinesia incidence by age of Parkinson's disease onset. Mov Disord. 2005;20:342–344. doi: 10.1002/mds.20360. [DOI] [PubMed] [Google Scholar]
- Le W, Sayana P, Jankovic J. Animal models of Parkinson's disease: a gateway to therapeutics? Neurotherapeutics. 2014;11:92–110. doi: 10.1007/s13311-013-0234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004;16:110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Gerfen CR, Bezard E. Priming for L-Dopa-induced dyskinesia in Parkinson's disease: a feature inherent to the treatment or the disease? Prog Neurobiol. 2009;87:1–9. doi: 10.1016/j.pneurobio.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M. Young- versus older onset Parkinson's disease: impact of disease and psychosocial consequences. Mov Disord. 2003;18:1250–1256. doi: 10.1002/mds.10527. [DOI] [PubMed] [Google Scholar]
- Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson's disease. A community-based study. Brain. 2000;123(Pt 11):2297–2305. doi: 10.1093/brain/123.11.2297. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Rascol O, Destee A, Hattori N, Hauser RA, et al. AFQ056 in Parkinson patients with levodopa-induced dyskinesia: 13-week, randomized, dose-finding study. Mov Disord. 2013;28:1838–1846. doi: 10.1002/mds.25561. [DOI] [PubMed] [Google Scholar]
- Tambasco N, Simoni S, Mar 408 sili E, Sacchini E, Murasecco D, et al. Clinical aspects and management of levodopa-induced dyskinesia. Parkinsons Dis. 2012;2012:410, 745947. doi: 10.1155/2012/745947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen Metman L, Del Dotto P, van den Munckhof P, Fang J, Mouradian MM, et al. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson's disease. Neurology. 1998;50:1323–1326. doi: 10.1212/wnl.50.5.1323. [DOI] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Bjorklund A, Cenci MA. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson's disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2002;10:165–186. doi: 10.1006/nbdi.2002.0499. [DOI] [PubMed] [Google Scholar]