Abstract
There is an increasing interest in targeting the MDM2 oncogene for cancer therapy. SP-141, a novel designed small molecule MDM2 inhibitor, exerts excellent in vitro and in vivo anticancer activity. To facilitate the preclinical development of this candidate anticancer agent, we have developed an HPLC method for the quantitative analysis of SP-141. The method was validated to be precise, accurate, and specific, with a linear range of 16.2–32,400 ng/mL in plasma, 16.2–6480 ng/mL in homogenates of brain, heart, liver, kidneys, lungs, muscle and tumor, and 32.4–6480 ng/mL in spleen homogenates. The lower limit of quantification was 16.2 ng/mL in plasma and all the tissue homogenates, except for spleen homogenates, where it was 32.4 ng/mL. The intra- and inter-assay precisions (coefficient of variation) were between 0.86 and 13.39%, and accuracies (relative errors) ranged from −8.50 to 13.92%. The relative recoveries were 85.6–113.38%. SP-141 was stable in mouse plasma, modestly plasma bound and metabolized by S9 microsomal enzymes. We performed an initial pharmacokinetic study in tumor-bearing nude mice, demonstrating that SP-141 has a short half-life in plasma and wide tissue distribution. In summary, this HPLC method can be used in future preclinical and clinical investigations of SP-141.
Keywords: SP-141, HPLC, pyrido[b]indole, pharmacokinetics
Introduction
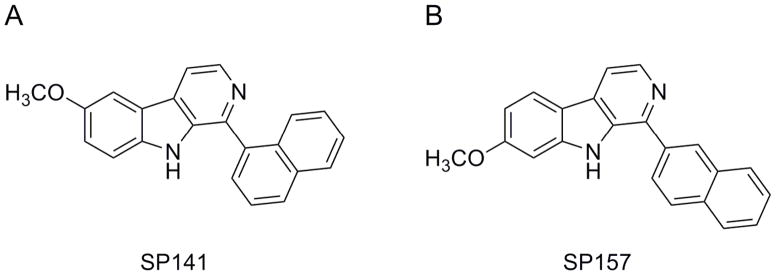
Cancer poses a major public health problem worldwide, and chemotherapy remains one of the most effective approaches to clinical cancer treatment (Seigel et al., 2013). In spite of recent improvements made in early diagnosis and treatment of various cancers, the mortality rates are still high for most advanced cancers such as pancreatic cancer (Cao et al., 2013). There is an urgent need to develop novel agents for these cancer types that respond poorly to currently available therapies (Paulson et al., 2013). We have recently generated a number of novel synthetic analogs of the pyrido[b]indole class and have identified several candidate anticancer compounds. Among them is SP-141 {6-methoxy-1-(naphthalen-1-yl)-9H-pyrido[3,4-b]indole} (Fig. 1A), a specific inhibitor of the MDM2 oncogene that has high potency against several cancers, including pancreatic cancer.
Figure 1.
Chemical structures of (A) SP-141 and (B) SP-157.
We and others have suggested that MDM2 is a valid molecular target for cancer chemotherapy and several MDM2 inhibitors are under preclinical and clinical development (Chen et al., 1998; Zhang and Wang, 2000; Zhang et al., 2003, 2004, 2005; Wang et al., 2008, 2009). One of the unique aspects of SP-141 as a novel class of MDM2 inhibitor is its mechanism of action: it binds directly to MDM2 protein and induces its degradation. This mechanism differs remarkably from other MDM2 inhibitors under investigation that primarily block MDM2-p53 binding (Rayburn et al., 2005; Qin et al., 2012; Nag et al., 2013). Therefore, there is strong interest in developing SP-141 as a lead candidate for novel cancer chemotherapy.
Based on its impressive in vitro and in vivo activities, we have decided to conduct further preclinical development of SP-141 as a novel anticancer agent. As this compound represents a new class of anticancer drugs, the development of an analytical method is essential for its preclinical and clinical development. Without a sensitive and reliable analytical method, it will not be possible to determine its key characteristics, including in vitro stability, pharmacokinetics, tissue distribution and toxicological properties (Singh, 2006; Gad, 2008). Herein, we report the development and validation of a high-performance liquid chromatographic (HPLC) method with UV detection for the quantitation of SP-141 in biological matrices such as plasma and tissue samples. We also carried out an initial pharmacokinetic study in tumor-bearing nude mice following intraperitoneal administration, a dosing route used in our preclinical efficacy study. It is hoped that the developed and validated method will facilitate the future investigations of this compound in both preclinical models and clinical settings.
Experimental
Chemicals and reagents
The structure of the test compound SP-141 and the internal control SP-157 (Fig. 1B) was confirmed by UV, IR, MS and NMR spectroscopy. The purity of the compounds was determined to be >99%. HPLC-grade methanol was obtained from Fisher Scientific (Fair Lawn, NJ, USA). HPLC-grade water was obtained from the Elga Purelab Ultra® water purifier system. Formic acid and triethanolamine (TEA) were purchased from Sigma (St Louis, MO, USA). Blank mouse plasma (heparinized, non-Swiss albino) was obtained from Lampire Biological Laboratories (Pipersville, PA, USA). Other chemicals and reagents were of highest grade available.
HPLC Instrumentation
The liquid chromatography experiments were conducted on an Agilent 1200 series® liquid chromatography system equipped with a G1379B Micro Vacuum Degasser, a G1312B SL binary pump, a G1367C high-performance autosampler (Hip-ALS SL), a G1330B FC/ALS Therm, a G1316B TCC SL and a G1315C DAD SL (all from Agilent Technologies, Inc., CA, USA). Chromatographic separation was performed on a Zorbax SB C18 column (4.6 × 150 mm, 5 μm, Agilent) with a guard-column cartridge (Agilent, SB C18). All data were acquired and analyzed using the Agilent Chemstation software (Agilent Technologies, Inc., CA, USA).
Chromatographic conditions
The mobile phase was composed of (A) water containing 0.05% formic acid (v/v) and 0.05% TEA (v/v), and (B) methanol containing 0.1% formic acid (v/v) and 0.05% TEA (v/v). Prior to application, both water and methanol were filtered using a Millipore glass filter system with nylon membrane (0.2 μm) and degassed for 10–12 min. Analytical separations were conducted at 35°C. The column elute was monitored by DAD at 295 nm, preceded by an RRLC in-line filter, 4.6 mm, 0.2 μm. The gradient elution was applied at a flow rate of 1.0 mL/min, starting at 45% A and 55% B from 0 min linearly programmed to 5% A and 95% B in 6 min; then the gradient was changed to 45% A and 55% B and maintained for 2 min. Total running time was 8 min.
Preparation of standards and quality control samples
A 10 mM stock solution of SP-141 was prepared in methanol and stored at −80°C. The working solutions for calibration standards and quality control samples were prepared from the stock solution. Calibration curves were prepared at concentrations of 16.2–32,400 ng/mL in mouse plasma, 16.2–6480 ng/mL in mouse tissue homogenates of brain, heart, kidneys, liver, lungs and tumor, and 32.4–6480 ng/mL in mouse spleen homogenates, which were prepared by adding the working solution to the plasma or homogenates, followed by immediate extraction. All extraction procedures were carried out in an ice bath to avoid possible degradation of the analyte. The quality control samples of low (32.4 ng/mL; 162 ng/mL for spleen), medium (324 ng/mL; 1620 ng/mL for spleen) and high (3240 ng/mL) concentrations were prepared in the same way to assess intra-day and inter-day precision and accuracy and recovery. The stability studies in mouse plasma and the various tissue matrices were performed at 324 and 3240 ng/mL.
Sample preparation
All samples were processed on ice at 4°C. Blood samples were collected into heparinized tubes via a retro-orbital bleeding and plasma was separated by centrifugation (14,000 rpm, 15 min). Various tissue samples (brain, heart, liver, kidneys, lungs, spleen and skeletal muscle) and the subcutaneous tumors were collected at necropsy, immediately trimmed of extraneous fat and connective tissues, blotted on filter paper, and weighed. The tissues were homogenized in two volumes (v/w) of PBS with a Polytron PT-10-35-GT homogenizer (Thomas Scientific, Swedesboro, NJ, USA) in an ice bath. The blank tissue homogenates were prepared by pooling three portions of tissue from different mice. If a sample concentration in initial assay was not covered in the range of a given standard curve, proper dilution and concentration procedures were used in repeated testing.
SP-141 was extracted from the plasma and homogenized tissue samples by precipitating proteins with methanol (containing 0.04% formic acid). Briefly, ice-cold methanol (600 μL) was added to 200 μL plasma or tissue homogenates; the samples were vortexed for 30 s and then centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was transferred to a glass tube and evaporated to dryness at 35°C under a nitrogen stream, using a TurboVap® LV Concentration Workstation (Caliper Lifesciences, Hopkinton, MA, USA). Finally, the dried samples were reconstituted in 200 μL of mobile phase, vortex mixed well for 1 min, and then centrifuged at 14,000 rpm for 10 min at 4°C. After syringe filtered, 10 μL of the reconstituted samples was injected onto the HPLC.
Method validation
Selectivity
The specificity of the method was assessed by analyzing blank plasma and tissue samples spiked with SP-141 to observe the possible endogenous interference from plasma and tissue samples with the analyte. A structurally related compound (SP-157, 7-methoxy-1-(naphthalen-2-yl)-9H-pyrido[3,4-b]indole; Fig. 1B) was chosen as the internal standard due to its similarity to SP-141 in terms of extractability and chromatographic behavior.
Linearity
The linearity of the relationship between the detector response and SP-141 concentrations was confirmed within the concentration range of 16.2–32,400 ng/mL for mouse plasma, 16.2–6480 ng/mL for mouse brain, heart, kidney, liver, lung and tumor tissue homogenates, and 32.4–6480 ng/mL for mouse spleen homogenates. Calibration curves of the slope, intercept and determination coefficients were calculated by plotting the peak area ratios (y) for SP-141 vs the nominal concentrations (x) in standard plasma or tissue by the 1/x2 weighted least-square linear regression.
Precision and accuracy
To evaluate the precision and accuracy of the method, we analyzed quality control samples at three concentration levels (low, 162 ng/mL; medium, 1620 ng/mL; and high, 3240 ng/mL for spleen homogenates; low, 32.4 ng/mL; medium, 324 ng/mL; and high, 3240 ng/mL for plasma and other tissues homogenates) in five replicates on four separate days. Mean, standard deviation (SD) and the ratio of the standard deviation to the mean (coefficient of variation, CV) were calculated and used to evaluate the precision. The accuracy of the assay was assessed by comparing the calculated mean concentrations to the actual concentrations of serial dilutions. Accuracy was required to be within ±15%, and the intra- and interday precisions (represented by CV) were not to exceed 15%.
Lower limits of detection and quantification
The lower limit of detection (LLOD) was defined as the peak signal of the compound equal to three times the average noise level. The lower limit of quantification (LLOQ) was defined as the lowest concentration of compound giving a signal-noise ratio of 5:1 with a precision and accuracy of 100 ± 20%.
Sample extraction recovery
The percentage recoveries of SP-141 at three quality control levels (n = 3) from mouse plasma and eight tissues were determined by comparing the mean peak area of the quality controls with those of pure compound prepared in the mobile phase.
Stability studies
The stability of SP-141 was assessed by comparing the initial concentration with the final concentration following incubation of the compound at the noted conditions, and expressing the final results as a percentage of the initial concentration.
Freeze–thaw stability
The stabilities of SP-141 samples of two concentrations (324 and 3240 ng/mL) were analyzed in triplicate, after undergoing three freeze–thaw cycles. Briefly, the samples were stored at −20°C for 24 h. They were then removed from the freezer and allowed to thaw unassisted at room temperature. When completely thawed, the samples were refrozen for 24 h under the same conditions. This freeze–thaw cycle was repeated twice more, for a total of three cycles. Then the samples were extracted using the above methods before HPLC analysis.
Short- and long-term stability
The stability of SP-141 in mouse plasma and various tissue homogenates was evaluated by analyzing duplicates of the samples that were exposed to different conditions (time and temperature) at concentrations of 324 and 3240 ng/mL. The short-term stability in plasma was assessed by analyzing samples kept at 37°C for 2, 4, 6, 8, 12 and 24 h and samples kept at 4°C for 4, 8, 12 and 24 h. The long-term stability was determined by assaying samples after storage at −80°C for 2, 4, 6 and 8 weeks. Then the samples were extracted using the above methods before HPLC analysis. These final results were compared with those obtained for freshly prepared samples.
Bench-top stability
To assess the bench-top stability of SP-141 in a particular biological matrix at room temperature, SP-141 (324 and 3240 ng/mL) was incubated in plasma or tissue homogenates and kept at ambient temperature (25°C) for 4, 8, 12 and 24 h, which far exceeded the routine sample preparation time.
Homogenization stability
To assess the effects of the homogenization and extraction procedures on the stability of the analyte, SP-141 at 324 and 3240 ng/mL was injected into equal weights of blank control tissues (brain, heart, liver, kidneys, lungs, spleen, tumor and muscle) in the collection tube. Care was taken to ensure the drug solution was contained inside the tissue/organs. The tissue samples were homogenized as above, and extracted and immediately analyzed.
Stock solution stability
Fresh and stored at room temperature (25°C) stock solutions of comparison standards (SP-141 and SP-157) with low and high quality control concentrations (324 and 3240 ng/mL) were tested at 6, 12 and 24 h after sample preparation. The extraction and analytical methods were identical to the above sample preparation.
Nonspecific binding
The nonspecific binding was determined by using a sequential transfer test as described previously (Ji et al. 2010). The test was performed by spiking plasma and tissue homogenates with the low (324.0 ng/mL) or high (3240.0 ng/mL) quality control concentrations of SP-141. The samples were collected in four clean and dry test tubes with same composition and size. A portion of the solution was transferred into the first tube, and then from there to the second tube, then to the remaining tubes. All transfers were done without the use of a pipette. Between transfers, the solution in the donor tube was left at room temperature (5–20 min) for analyte adsorption. From each tube, an appropriate amount (100 μL) was pipetted out for analyte detection using the same HPLC method.
Protein binding
The extent to which SP-141 was bound by mouse plasma proteins was assessed by a micro-ultrafiltration system as described previously (Agarwal et al., 1998). Mouse plasma samples spiked with SP-141 at concentrations of 324 and 3240 ng/mL were maintained at 37°C for 1 h. The control samples were prepared using PBS instead of plasma. From each of these preparations, a portion was aliquoted and placed in a sample reservoir of an Amicon Centrifree® ultrafiltration system (Millipore Co., Bedford, MA). The filter systems were centrifuged at 2000g until the reservoirs were dry. From each sample, triplicate portions were taken for analysis by HPLC. The amounts present in the filtrates were designated as ‘free drug’ (F). The concentrations of the unfiltered solutions were also determined by triplicate analyses. This amount represented the ‘total drug’ concentration (T). The amount bound nonspecifically to the filter (X) also was determined. The percentage of SP-141 bound to plasma proteins was calculated by the following formula:
S9 Metabolism in vitro
We used hepatic microsomal S9 fractions from CD2F1 mice (In Vitro Technologies, Boston, MA, USA) to determine the in vitro metabolism of SP-141. The reaction mixture consisted of 20 μL of 3240 ng/mL SP-141 (in DMSO) and 1280 μL of cold 100 mM Tris buffer (pH 7.4). The reactions were initiated by adding 200 μL of the appropriate S9 fractions and were maintained at 37°C. The negative controls did not contain the hepatic S9 fractions. Metabolic reactions were initiated by adding phase I (NADPH regenerating systems) or phase II (UDPGA and PAPS) reagents to the reaction mixtures, respectively (Ezell et al., 2010; Rayburn et al., 2012; Wang et al., 2012; Zhang et al., 2012). At designated time points (0, 15, 30, 45 and 60 min), duplicate 100 μL portions of the incubation mixture were removed and subjected to HPLC analysis by the same procedure described for plasma samples above. The stability of the drug was determined by analysis of intact SP-141, in comparison with negative control (without S9 fractions).
Pharmacokinetics of SP-141 in tumor-bearing nude mice
To demonstrate the utility of the HPLC method, the pharmacokinetics of the compound in mouse plasma and the compound’s tissue distribution were determined in pancreatic cancer cell (MIA PaCa-2) xenograft tumors implanted in female nu/nu mice (4–6 weeks) following intraperitoneal administration. The animal study protocol was approved by the Institutional Animal Care and Use Committee of Texas Tech University Health Sciences Center. To establish the MIAPaCa-2 human pancreatic cancer xenograft tumors, cultured MIAPaCa-2 (1 × 107) cells were harvested from monolayer cultures, washed twice with serum-free medium, re-suspended, and injected subcutaneously (total volume 0.2 mL) into the left inguinal area of the mice. All animals were monitored for activity, physical condition, body weight and tumor growth. Tumor size was determined every three days by caliper measurement of two perpendicular diameters of the implant. Tumor mass (in grams) was calculated by the formula, where a is the long diameter and b is the short diameter (in centimeters) (Wang et al., 2009, 2012; Hao et al., 2011; Zhang et al., 2012). When the tumor mass reached 100 mg, the animals were randomly divided into groups of three each, and 40 mg/kg of SP-141 formulated in PEG400–EtOH–saline (57.1:14.3:28.6, v/v/v) was administered. Plasma and tissue samples were collected at 0 (pre-dose), 5, 10, 30 and 60 min, and 2, 4, 6, 8, and 24 h after drug administration. Plasma was obtained from the retro-orbital plexus of the anesthetized mice. Various tissues (liver, heart, lungs, kidneys, spleen and brain) and the subcutaneous tumors were collected at necropsy, immediately trimmed of extraneous fat and connective tissue, blotted on filter paper and weighed. Urine and feces were collected on ice after 24 h from mice housed in metabolic cages (n = 3/study). The collection containers and cages were washed twice with PBS solution and each urine collection and each wash was analyzed separately. Values for each mouse were then added together to determine the total SP-141 present in urine during the collection period. Tissues were stored at −80°C until processing. For SP-141 extraction, tissue samples were homogenized in PBS. The samples were then processed as described above, and concentration–time curves were obtained for plasma and each tissue.
Results
Method validation
Chromatographic separation and quantitation of SP-141
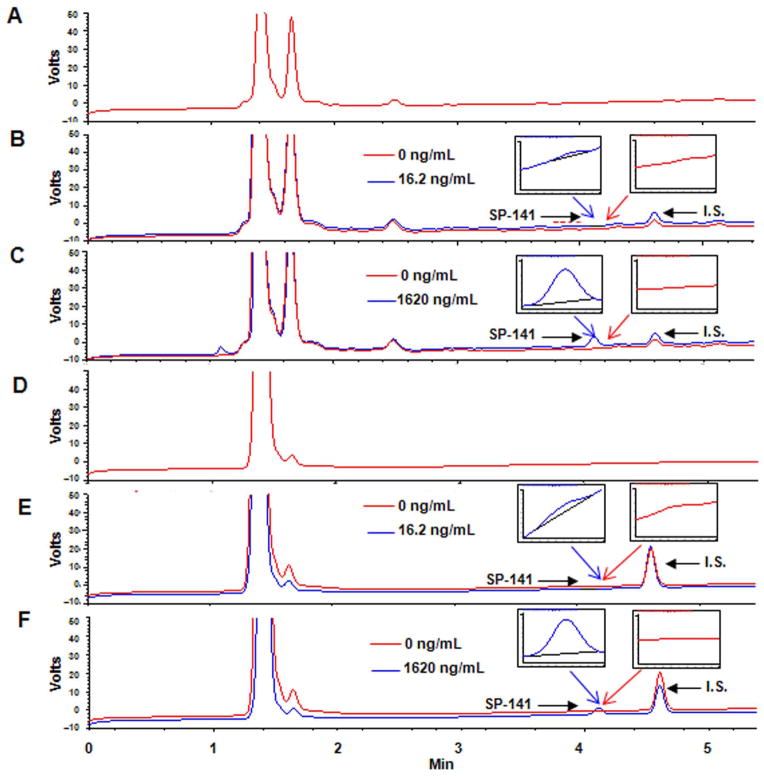
The specificity of the assay was examined using the described chromatographic conditions. Figure 2 depicts the representative chromatograms of the samples of blank mouse plasma (Fig. 2A), control (drug-free) mouse plasma spiked to contain 16.2 ng/mL (LLOQ, Fig. 2B) and 1620 ng/mL (Fig. 2C) SP-141, blank tumor tissue (Fig. 2D) and control (drug-free) tumor tissue spiked to contain 16.2 ng/mL (LLOQ, Fig. 2E) and 1620 ng/mL (Fig. 2F) SP-141, respectively. No endogenous interference at the retention time of the peak of interest (~4.1 min) was detected in plasma and tissue homogenates. The theoretical plate number of the column was 11,975 and the asymmetry factor was 1.04.
Figure 2.
Representative chromatograms of (A) blank mouse plasma; control (drug-free) mouse plasma spiked to contain (B) 16.2 ng/mL (LLOQ); (C) 1620 ng/mL SP-141; (D) blank tumor tissue; and control (drug-free) tumor tissue spiked to contain (E) 16.2 ng/mL (LLOQ); and (F) 1620 ng/mL SP-141. The internal standard (SP-157) elutes at 4.6 min while SP-141 elutes at 4.1 min.
Linearity of the calibration curve, lower limit of detection, and lower limit of quantitation
The correlation coefficients (R) of the standard curves for SP-141 in plasma and various tissue homogenates were all >0.9888. All the linearity and range parameters and their related validation data are shown in Table 1. The LLOD and LLOQ for SP-141 in plasma using the optimized conditions were 3.24 ng/mL (LLOD) and 16.2 ng/mL (LLOQ). The present method afforded an LLOD of 3.24 ng/mL and an LLOQ of 16.2 ng/mL for SP-141 in seven mouse tissues, and an LLOD of 6.48 ng/mL and an LLOQ of 32. 4 ng/mL for SP-141 in mouse spleen.
Table 1.
Calibration data resulting from linear least squares regression analysis for the determination of SP-141 in plasma and various tissue samples
| LLOD (ng/mL) | LLOQ (ng/mL) | Linear Fit | Slope | Intercept | Correlation Coefficient (R) | Linear range (ng/mL) | |
|---|---|---|---|---|---|---|---|
| Plasma | 3.24 | 16.2 | y = 7.5391x − 0.0006 | 7.5391 ± 0.0894 | 0.0006 ± 0.0000 | 0.9999 | 16.2–32,400 |
| Brain | 3.24 | 16.2 | y = 5.2704x + 0.0162 | 5.2704 ± 0.0409 | 0.0162 ± 0.0002 | 0.9993 | 16.2–6480 |
| Heart | 3.24 | 16.2 | y = 8.8606x − 2.1321 | 8.8606 ± 0.3775 | −2.1321 ± 0.1192 | 0.9909 | 16.2–6480 |
| Liver | 3.24 | 16.2 | y = 6.6509x + 0.5497 | 6.6509 ± 0.0301 | 0.5497 ± 0.0015 | 0.9999 | 16.2–6480 |
| Kidneys | 3.24 | 16.2 | y = 6.3212x − 0.6021 | 6.3212 ± 0.1409 | −0.6021 ± 0.1480 | 0.9994 | 16.2–6480 |
| Lungs | 3.24 | 16.2 | y = 4.9182x + 0.1313 | 4.9182 ± 0.1422 | 0.1313 ± 0.0326 | 0.9986 | 16.2–6480 |
| Spleen | 6.48 | 32.4 | y = 7.3217x − 3.0542 | 7.3217 ± 0.2544 | −3.0542 ± 0.0432 | 0.9888 | 32.4–6480 |
| Tumor | 3.24 | 16.2 | y = 4.8694x − 1.1571 | 4.8694 ± 0.2745 | −1.1571 ± 0.0309 | 0.9963 | 16.2–6480 |
| Muscle | 3.24 | 16.2 | y = 5.5120x + 0.1212 | 5.5120 ± 0.2896 | 0.1212 ± 0.0197 | 0.9985 | 16.2–6480 |
Precision and accuracy
The intra- and interday precision and accuracy for detection of SP-141 in mouse plasma and tissue samples are presented in Table 2. Precision was defined as the variation between replicate samples. Accuracy was defined as the percentage of the observed concentration calculated from peak areas compared with the known concentration of the prepared samples. The CV of the intra- and inter-day precision for SP-141 was 10.61, 13.39 and 10.74 for the low, moderate and high concentrations, respectively. The accuracy of the quantitative analysis of the compound ranged from 91.50 to 113.92% for intra-day and from 91.84 to 108.68% for inter-day analyses. The precision and accuracy values were well within the acceptable range as described by the United States Food and Drug Administration (CDER/US FDA, 2007).
Table 2.
Accuracy and precision of the method for detection of SP-141 in plasma and tissue samples
| Nominal concentration (ng/mL) | Intraday assay (n = 5) | Interday assay (n = 4) | |||||
|---|---|---|---|---|---|---|---|
| Mean measured concentration ± %SD (ng/mL) | CV (%) | Accuracy (%) | Mean measured concentration ± %SD (ng/mL) | CV (%) | Accuracy (%) | ||
| Plasma | 32.4 | 32.4 ± 3.2 | 5.78 | 2.67 | 35.6 ± 0.0 | 3.18 | 5.64 |
| 324 | 311.0 ± 22.7 | 6.89 | −4.01 | 314.3 ± 16.2 | 5.14 | −3.17 | |
| 3240 | 3142.8 ± 110.2 | 3.48 | −2.95 | 3045.6 ± 217.1 | 7.07 | −5.99 | |
| Brain | 32.4 | 35.6 ± 0.0 | 2.95 | 13.92 | 32.4 ± 0.0 | 3.65 | 0.35 |
| 324 | 369.4 ± 48.6 | 13.39 | 13.62 | 307.8 ± 22.7 | 6.97 | −5.19 | |
| 3240 | 3262.7 ± 239.8 | 7.29 | 0.72 | 3262.7 ± 304.6 | 9.29 | 0.68 | |
| Heart | 32.4 | 35.6 ± 3.2 | 4.41 | 5.21 | 32.4 ± 3.2 | 5.59 | 3.33 |
| 324 | 298.1 ± 19.4 | 6.74 | −7.52 | 298.1 ± 6.5 | 2.19 | −8.16 | |
| 3240 | 3133.1 ± 110.2 | 3.52 | −3.37 | 3216.6 ± 126.4 | 4.05 | −3.49 | |
| Liver | 32.4 | 35.6 ± 3.2 | 9.78 | 6.80 | 35.6 ± 3.2 | 8.32 | 8.68 |
| 324 | 343.4 ± 13.0 | 3.99 | 6.19 | 343.4 ± 16.2 | 4.45 | 5.67 | |
| 3240 | 3476.5 ± 200.9 | 5.77 | 7.32 | 3489.5 ± 230.0 | 6.60 | 7.64 | |
| Kidney | 32.4 | 32.4 ± 0.0 | 1.63 | 4.39 | 32.4 ± 0.0 | 1.40 | 3.92 |
| 324 | 298.1 ± 3.2 | 12.13 | −8.50 | 301.3 ± 0.0 | 2.84 | −6.85 | |
| 3240 | 3175.2 ± 103.7 | 3.24 | −2.07 | 3272.4 ± 220.3 | 6.69 | 0.97 | |
| Lungs | 32.4 | 35.6 ± 3.2 | 8.72 | 9.94 | 35.6 ± 0.0 | 3.74 | 5.44 |
| 324 | 330.5 ± 13.0 | 4.31 | 2.37 | 320.8 ± 25.9 | 8.91 | −0.45 | |
| 3240 | 3295.1 ± 220.3 | 6.65 | 1.72 | 3230.3 ± 187.9 | 5.85 | −0.29 | |
| Muscle | 32.4 | 32.4 ± 0.0 | 3.62 | 2.50 | 32.4 ± 3.2 | 9.93 | 1.76 |
| 324 | 362.9 ± 9.7 | 3.11 | 12.55 | 349.9 ± 13.0 | 3.66 | 8.09 | |
| 3240 | 3149.3 ± 64.8 | 2.02 | −2.80 | 3207.6 ± 175.0 | 5.51 | −0.90 | |
| Tumor | 32.4 | 35.6 ± 3.2 | 5.10 | 6.01 | 32.4 ± 3.2 | 9.78 | 3.86 |
| 324 | 343.4 ± 16.2 | 4.82 | 6.94 | 337.0 ± 16.2 | 5.56 | 4.73 | |
| 3240 | 3382.6 ± 337.0 | 10.74 | 4.10 | 3321.0 ± 259.2 | 8.21 | 2.49 | |
| Spleen | 162 | 175.0 ± 3.2 | 0.86 | 7.60 | 171.7 ± 19.4 | 10.61 | 5.45 |
| 1620 | 1837.1 ± 110.2 | 6.64 | 13.41 | 1749.6 ± 132.8 | 8.64 | 7.34 | |
| 3240 | 3133.1 ± 42.1 | 1.36 | −3.32 | 3000.2 ± 77.8 | 2.56 | −7.42 | |
Extraction recovery
Extraction recoveries of the compound at low (32.4 ng/mL), moderate (324 ng/mL) and high (3240 ng/mL) concentrations (in triplicate) in plasma and in all tissue homogenates, including tumor tissue, were >85% (Table 3). The recoveries determined at low (32.4 ng/mL), medium (324 ng/mL) and high (3240 ng/mL) concentrations (in triplicate) in plasma, brain, heart, liver, kidney, lungs and muscle were 86.35 ± 6.76, 86.34 ± 2.87 and 89.78 ± 5.62%, respectively. The recoveries determined at low (162 ng/mL), medium (324 or 1620 ng/mL), and high (3240 ng/mL) concentrations (in triplicate) in spleen and tumor tissue were 90.32 ± 5.58, 85.60 ± 5.13 and 95.60 ± 2.87%, respectively.
Table 3.
Recovery of SP-141 from plasma and tissue homogenates
| Concentration (ng/mL) | Recovery (%) ± SD (%) | CV (%) | |
|---|---|---|---|
| Plasma | 32.4 | 90.03 ± 1.73 | 1.92 |
| 324 | 102.84 ± 6.56 | 6.37 | |
| 3240 | 100.60 ± 2.18 | 2.27 | |
| Brain | 32.4 | 91.33 ± 3.04 | 3.32 |
| 324 | 88.30 ± 6.26 | 7.09 | |
| 3240 | 93.70 ± 7.52 | 8.43 | |
| Heart | 32.4 | 88.33 ± 2.64 | 2.99 |
| 324 | 95.40 ± 2.64 | 2.76 | |
| 3240 | 96.17 ± 2.50 | 2.60 | |
| Liver | 32.4 | 109.27 ± 8.29 | 7.59 |
| 324 | 106.57 ± 2.32 | 2.18 | |
| 3240 | 113.38 ± 7.44 | 6.56 | |
| Kidney | 32.4 | 86.35 ± 6.76 | 7.83 |
| 324 | 91.18 ± 5.24 | 5.74 | |
| 3240 | 89.78 ± 5.62 | 6.26 | |
| Lungs | 32.4 | 105.01 ± 1.11 | 0.96 |
| 324 | 102.10 ± 7.50 | 9.00 | |
| 3240 | 101.10 ± 9.19 | 4.86 | |
| Muscle | 32.4 | 89.06 ± 3.88 | 4.36 |
| 324 | 86.34 ± 2.87 | 3.32 | |
| 3240 | 92.16 ± 4.79 | 5.20 | |
| Tumor | 162 | 90.32 ± 5.58 | 6.18 |
| 324 | 92.16 ± 7.85 | 6.95 | |
| 3240 | 95.60 ± 2.87 | 3.00 | |
| Spleen | 162 | 105.30 ± 3.85 | 4.60 |
| 1620 | 85.60 ± 5.13 | 6.00 | |
| 3240 | 96.46 ± 1.62 | 1.37 |
Stability of SP-141
Freeze–thaw stability
SP-141 was found to hace excellent freeze–thaw stability. After three freeze–thaw cycles, the percentages of the remaining concentrations were 98.24 ± 0.69% at 324 ng/mL and 97.44 ± 1.53% at 3240 ng/mL of SP141.
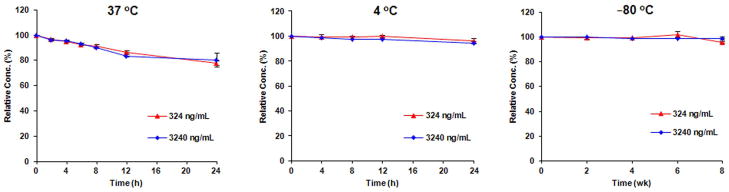
Short- and long-term stability
SP-141 was relatively stable in mouse plasma at 37°C, with more than 77% of the compound remaining after a 24 h incubation period for both the low (324 ng/mL) and high (3240 ng/mL) concentrations (Fig. 3A). We also found that SP-141 can be stored at either 4°C for a short period of time, or −80°C for a longer period of time. After a 24 h storage in mouse plasma at 4°C, >94% of the compound still remained intact (95.9% for the 324 ng/mL and 94.3% for the 3240 ng/mL, respectively; Fig. 3B). When the plasma was stored at −80°C for 8 weeks, 95.7 and 98.7% of the compound remained for the 324 and 3240 ng/mL concentrations, respectively (Fig 3C).
Figure 3.
Stability of SP-141 in mouse plasma at 37°C (A), 4°C (B) and −80°C (C).
Bench-top stability
As shown in Fig. 4, we found that SP-141 was relatively stable in plasma (Fig. 4A) and in most tissue homogenates except for liver (Fig. 4B–I). There was 76% of the compound remaining after a 4 h incubation period for high (3240 ng/mL) concentrations (Fig. 4D).
Figure 4.
Bench-top stability of SP-141 at room temperature (25°C) in mouse plasma and different tissue homogenates.
Homogenization stability
As shown in Table 4, the recovery of SP-141 ranged from 81.79 ± 4.47 to 99.61 ± 0.79 at a concentration of 324 ng/mL, and from 86.58 ± 1.39 to 100.89 ± 1.45 at a concentration of 3240 ng/mL in all tissue homogenates. These results agreed well with the recovery data (Table 3) and indicate that there is no significant loss of test compounds during the homogenization process.
Table 4.
Homogenization stability of SP-141 in various tissues
| Concentration (ng/mL) | Recovery (%) ± SD (%) | CV (%) | |
|---|---|---|---|
| Brain | 324 | 91.35 ± 0.98 | 1.08 |
| 3240 | 100.89 ± 1.45 | 1.44 | |
| Heart | 324 | 81.79 ± 4.47 | 4.47 |
| 3240 | 86.58 ± 1.39 | 1.70 | |
| Liver | 324 | 86.70 ± 1.36 | 1.56 |
| 3240 | 89.22 ± 0.77 | 0.88 | |
| Kidney | 324 | 82.52 ± 2.60 | 2.59 |
| 3240 | 87.82 ± 1.36 | 1.56 | |
| Lungs | 324 | 88.97 ± 1.57 | 1.77 |
| 3240 | 93.31 ± 1.02 | 1.09 | |
| Muscle | 324 | 97.18 ± 0.90 | 0.93 |
| 3240 | 97.45 ± 1.47 | 1.51 | |
| Tumor | 324 | 99.61 ± 0.79 | 0.81 |
| 3240 | 99.12 ± 0.19 | 0.19 | |
| Spleen | 324 | 92.95 ± 5.20 | 5.62 |
| 3240 | 99.71 ± 1.15 | 1.15 |
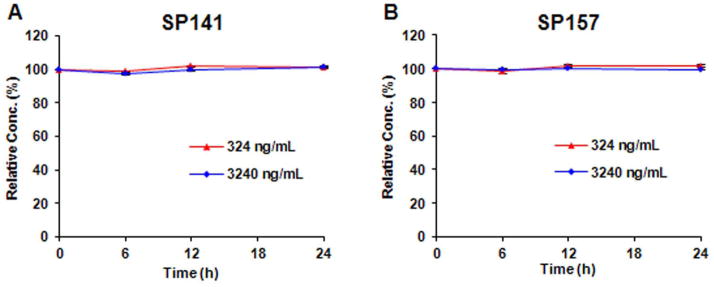
Stock solution stability
As shown in Fig. 5, both low and high levels (324 and 3240 ng/mL) of SP-141 and SP-157 stock solutions were found to be unchanged even after 24 h at room temperature.
Figure 5.
Stock solution stability of (A) SP-141 and (B) SP-157 at room temperature (25°C).
Nonspecific binding
As shown in Table 5, the recoveries of SP-141 ranged from 96.29 ± 2.54 to 104.91 ± 6.65 at low concentration, and from 97.13 ± 1.04 to 100.58 ± 0.81 at high concentration in plasma and all tissue homogenates, including tumor tissues. This sequential transfer test indicates that there is minimal binding of SP-141 to the container surface, and this negligible nonspecific binding will not affect the results of analyses performed.
Table 5.
Nonspecific binding of SP-141 in plasma and various tissues
| Concentration (ng/mL) | Recovery (%) ± SD (%) | CV (%) | |
|---|---|---|---|
| Plasma | 324 | 98.95 ± 1.49 | 1.51 |
| 3240 | 100.49 ± 0.04 | 0.04 | |
| Brain | 324 | 96.29 ± 2.54 | 2.64 |
| 3240 | 100.58 ± 0.81 | 0.81 | |
| Heart | 324 | 100.41 ± 0.27 | 0.27 |
| 3240 | 98.82 ± 0.38 | 0.38 | |
| Liver | 324 | 104.91 ± 6.65 | 6.35 |
| 3240 | 99.69 ± 1.38 | 1.40 | |
| Kidney | 324 | 99.88 ± 3.11 | 3.12 |
| 3240 | 97.13 ± 1.04 | 1.08 | |
| Lungs | 324 | 96.64 ± 2.24 | 2.33 |
| 3240 | 99.99 ± 0.21 | 0.22 | |
| Muscle | 324 | 101.12 ± 2.33 | 2.31 |
| 3240 | 99.19 ± 1.01 | 1.02 | |
| Tumor | 324 | 96.84 ± 2.30 | 2.47 |
| 3240 | 99.20 ± 0.57 | 0.58 | |
| Spleen | 324 | 99.54 ± 2.10 | 2.12 |
| 3240 | 99.99 ± 0.22 | 0.22 |
Protein binding
SP-141 was considerably bound to proteins in mouse plasma, with 57.23% at 324 ng/mL and 62.61% at 3240 ng/mL.
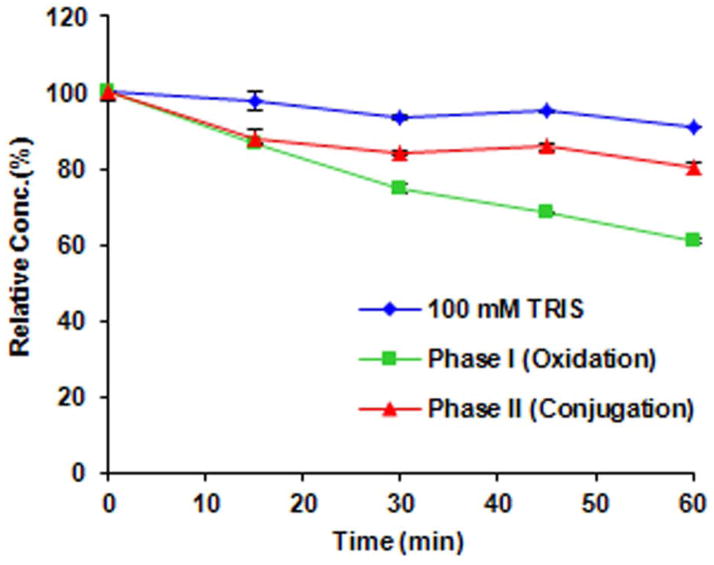
In vitro metabolism of SP-141
Since SP-141 was minimally excreted in the feces and urine (data not shown), exhibited low plasma concentrations, and wide tissue distribution, we hypothesized that SP-141 may underwent extensive metabolism in vivo. As a preliminary study of the metabolism of SP-141, the compound was incubated with S9 fractions containing phase I and phase II metabolic enzymes and their co-factors for 15, 30, 45 and 60 min (Fig. 6). These studies indicate that SP-141 undergoes significant metabolism in the presence of both phase I and phase II enzymatic components, with approximately 38% of the compound being metabolized by phase I and 18.8% of the compound being metabolized by phase II components during the 60 min incubation.
Figure 6.
Degradation of SP-141 by isolated mouse S9 liver fractions.
Pharmacokinetics and tissue distribution of SP-141 in nude mice bearing pancreatic cancer xenografts
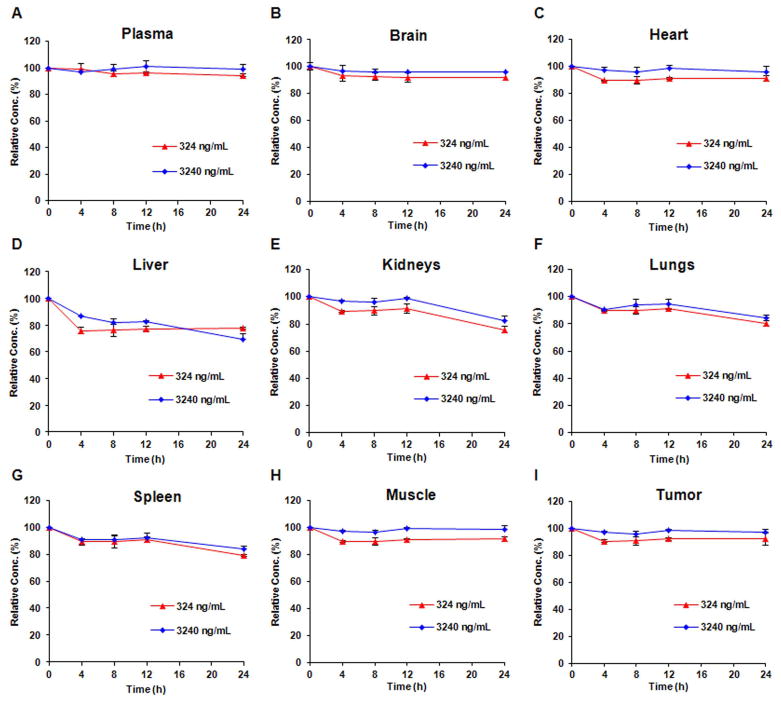
The concentrations of SP-141 in mouse plasma and tissue samples were determined after administration of a single intraperitoneal dose of 40 mg/kg to nude mice (Fig. 7). Following administration, the concentration of the compound in plasma decreased rapidly, from around 3000 ng/mL at 10 min to 200 ng/mL at 4 h, to undetectable levels at 6 h after administration (Fig. 7A). The highest overall concentrations in the tissues examined were in the following order: spleen > lungs > liver > kidney > brain > tumor > muscle > heart (Fig. 7B). SP-141 could be detected in tumor tissue at 24 h after administration, albeit at low concentrations. Although the distribution of the compound in the tumor is lower than in some other tissues, the concentrations are higher than in vitro IC50 values (132.84 ng/mL). We also have observed that the parent compound was minimally excreted in urine and feces (data not shown), suggesting that it is highly metabolized. Of note, no specific metabolite peaks were detected using the current HPLC method.
Figure 7.
(A) Plasma concentration–time curves for SP-141 following i.p. injection of 40 mg/kg in nude (nu/nu) mice implanted with MIAPaca-2 xenografts; (B) time-dependent distribution of SP-141 in various tissues.
Discussion
HPLC presents a popular technique for the analysis of drug molecules, and is widely used for the evaluation of nonvolatile small molecules (Bansal and DeStefano, 2007; Lee, 2003). Since SP-141 presents a λmax at 295 nm where no interferences from DNA and protein impurities present in the biological specimen (which absorb at a lower wavelength range) are expected, UV detection was determined as a suitable method for monitoring the compound (Wolfender, 2009). For any effective HPLC method, sample preparation techniques must be rapid without compromising efficient extraction of the analyte as well as selectivity. In the current study, we used a protein precipitation/extraction technique using acidified methanol with syringe filtering of the final sample. This protocol led to satisfactory purification of the compound, resulting in good selectivity of the chromatographic technique. To evaluate the utility of this method for practical purposes, we applied our newly developed and validated method to analyze drug concentrations in plasma and tissue samples obtained from nude mice implanted with subcutaneous pancreatic cancer cell xenograft tumors (Sinha et al., 2008; Zhou et al., 2002).
The stability determination of a new chemical entity in plasma is crucial, since a rapid degradation of drugs typically indicates poor in vivo efficacy. Plasma instability may also leads to misunderstanding of in vitro results. In addition, the stability of compound may exhibit inter-tissue differences. In the present study, we demonstrated that SP-141 was stable in plasma and various tissues under different temperature and storage conditions and homogenization process employed in tissue sample preparation, indicating that SP-141 can be prepared and stored under routine laboratory conditions, which is very important for future preclinical and clinical studies of this compound.
In general, a candidate compound with extensive binding to plasma can impede its biodistribution and affect their therapeutic performance and possible toxicity profile. Typically, minimally protein-bound drugs are able to reach the desired site of action easily but are excreted quickly. In this circumstance, free drug concentrations are more accurate than total concentrations in drug efficacy evaluation. Our results indicated that SP-141 showed approximately 60% protein binding, which is lower than most anticancer agents. Generally, in drugs exhibiting <85% protein binding, differences in extent of binding do not appear to be clinically important (Scheife, 1989). Therefore, we speculate that the total plasma and tissue SP141 concentrations may represent the active compound very well and therefore can be used in pharmacokinetic and pharmacodynamic studies in the future.
Our initial in vivo pharmacokinetic data (low urinary and fecal excretion, short plasma half-life and wide tissue distribution) suggest that SP141 is metabolized in vivo. In vitro metabolism by S9 enzymes is an important preliminary study to gauge the possible metabolic transformations of the drug in vivo. Our initial data suggest that SP-141 may be metabolized by both phase I (38%) and phase II (18.8%) metabolism, with degradation by phase I enzymes being higher. Of note, the HPLC method was developed and validated for the analyses of intact SP-141 only. This method apparently was not affected by its possible metabolites. To facilitate the metabolic studies of the drug, method development and validation for identification of possible metabolites of the drug is needed in the future.
To facilitate future clinical testing of SP141 for its antitumor activity, there is a necessity to determine its pharmacological properties such as pharmacokinetics and tissue distribution and toxicity in animal model after various routes of administration. Our previous studies demonstrated that SP-141 is effective against pancreatic cancer cells at submicromolar levels in vitro and suppresses the tumor growth with no significant host toxicity at 40 mg/kg intraperitoneal doses in vivo. The current pharmacokinetic study demonstrated that the effective dose of SP141 showed a short half-life and low concentration in plasma (but at or above the in vitro IC50), extensive tissue distribution and significant tissue residence times. Based on the results, we speculate that further optimization of administration route and schedule, change in formulation, development of delivery strategies are needed to improve antitumor activity of the compound.
Conclusions
In summary, a sensitive and reliable HPLC method for the determination of SP-141 in mouse plasma was developed and validated according to USA Food and Drug Administration guidelines. In addition, the analytical method was applied to a preliminary pharmacokinetic study in mice wherein we measured the concentrations of SP-141 in the plasma and tissues of nude mice following intraperitoneal administration. We believe that these results will support the future preclinical and clinical evaluation of SP141 as a novel anticancer therapeutic agent.
Acknowledgments
This work was supported by NIH grant R01 CA186662 (to R.Z.). J.K.B. was supported by NIH grant R15 CA100102. M.H.W. was supported by NIH R01 CA91980. The contents of the paper are solely the responsibility of the authors, and do not necessarily represent the official views of the National Institutes of Health or other funding agencies.
Abbreviations
- SP-141
6-methoxy-1-(naphthalen-1-yl)-9H-pyrido[3,4-b]indole
- SP-157
7-methoxy-1-(naphthalen-2-yl)-9H-pyrido[3,4-b]indole
- PBS
phosphate buffered saline
- TCC
thermostatted column compartment
- DAD
diode array detector
- TEA
triethanolamine
References
- Agrawal S, Zhang X, Cai Q, Kandimalla ER, Manning A, Jiang Z, Marcel T, Zhang R. Effect of aspirin on protein binding and tissue disposition of oligonucleotide phosphorothioate in rats. Journal of Drug Targeting. 1998;5:303–312. doi: 10.3109/10611869808995883. [DOI] [PubMed] [Google Scholar]
- Bansal S, DeStefano A. Key elements of bioanalytical method validation for small molecules. AAPS Journal. 2007;9:E109–114. doi: 10.1208/aapsj0901011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Le D, Yang LX. Current status in chemotherapy for advanced pancreatic adenocarcinoma. Anticancer Research. 2013;33:1785–1791. [PubMed] [Google Scholar]
- CDER/US FDA. [accessed 4 April 2014];Guidance for Industry: Bioanalytical Methods Validation. 2007 Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf.
- Chen L, Agrawal S, Zhou W, Zhang R, Chen J. Synergistic activation of p53 by inhibition of MDM2 expression and DNA damage. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:195–200. doi: 10.1073/pnas.95.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezell SJ, Li H, Xu H, Gurpinar E, Zhang X, Rayburn ER, Sommers CI, Yang X, Velu SE, Wang W, Zhang R. Preclinical pharmacology of BA-TPQ, a novel synthetic iminoquinone anticancer agent. Marine Drugs. 2010;8:2129–2141. doi: 10.3390/md8072129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad SC. Preclinical Development Handbook: ADME and Biopharmaceutical Properties. John Wiley & Sons; Hoboken, NJ: 2008. [Google Scholar]
- Hao M, Wang W, Zhao Y, Zhang R, Wang H. Pharmacokinetics and tissue distribution of 25-hydroxyprotopanaxadiol, an anti-cancer compound isolated from Panax ginseng, in athymic mice bearing xenografts of human pancreatic tumors. European Journal of Drug Metabolism and Pharmacokinetics. 2011;35:109–113. doi: 10.1007/s13318-010-0022-9. [DOI] [PubMed] [Google Scholar]
- Ji AJ, Jiang Z, Livson Y, Davis JA, Chu JX, Weng N. Challenges in urine bioanalytical assays: overcoming nonspecific binding. Bioanalysis. 2010;2:1573–1586. doi: 10.4155/bio.10.114. [DOI] [PubMed] [Google Scholar]
- Lee MS. LC/MS Applications in Drug Development. John Wiley & Sons; Hoboken, NJ: 2003. [Google Scholar]
- Nag S, Qin JJ, Srivenugopal K, Wang MH, Zhang R. MDM2-p53 pathway revisited. Journal of Biomedical Research. 2013;27:254–271. doi: 10.7555/JBR.27.20130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–1326. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- Qin JJ, Nag SN, Voruganti S, Wang W, Zhang R. Natural product MDM2 inhibitors: anticancer activity and mechanisms of action. Current Medicinal Chemistry. 2012;19:5705–5725. doi: 10.2174/092986712803988910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayburn E, Zhang R, He J, Wang H. MDM2 and human malignancies: expression, clinical pathology, prognostic markers, and implications for chemotherapy. Current Cancer Drug Targets. 2005;5:27–42. doi: 10.2174/1568009053332636. [DOI] [PubMed] [Google Scholar]
- Rayburn E, Wang W, Li M, Zhang X, Xu H, Li H, Qin JJ, Jia L, Covey J, Lee M, Zhang R. Preclinical pharmacology of novel indolecarboxamide ML-970, an investigative anti-cancer agent. Cancer Chemotherapy and Pharmacology. 2012;69:1423–1431. doi: 10.1007/s00280-012-1851-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheife RT. Protein binding: what does it mean? DICP. 1989;23:S27–31. doi: 10.1177/106002808902300706. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Singh SS. Preclinical pharmacokinetics: an approach towards safer and efficacious drugs. Current Drug Metabolism. 2006;7:165–182. doi: 10.2174/138920006775541552. [DOI] [PubMed] [Google Scholar]
- Sinha VK, De Buck SS, Fenu LA, Smit JW, Nijsen M, Gilissen RA, Van Peer A, Lavrijsen K, Mackie CE. Predicting oral clearance in humans: how close can we get with allometry? Clinical Pharmacokinetics. 2008;47:35–45. doi: 10.2165/00003088-200847010-00004. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang H, Rayburn E, Zhao Y, Hill D, Zhang R. 20(S)-25-methoxyl-dammarane-3β, 12β, 20-triol, a novel natural product for prostate cancer therapy: activity in vitro and in vivo and mechanisms of action. British Journal of Cancer. 2008;98:792–802. doi: 10.1038/sj.bjc.6604227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Rayburn ER, Zhao Y, Wang H, Zhang R. Novel ginsenosides 25-OH-PPD and 25-OCH3-PPD as experimental therapy for pancreatic cancer: anticancer activity and mechanisms of action. Cancer Letter. 2009a;278:241–248. doi: 10.1016/j.canlet.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Rayburn ER, Velu SE, Nadkarni DH, Murugesan S, Zhang R. In vitro and in vivo anticancer activity of makaluvamine analogues. Clinical Cancer Research. 2009b;15:3511–3518. doi: 10.1158/1078-0432.CCR-08-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Ao L, Rayburn ER, Xu H, Zhang XR, Zhang X, Nag SA, Wu X, Wang MH, Wang H, Van Meir EG, Zhang R. KCN1, a novel synthetic sulfonamide anticancer agent: in vitro and in vivo anti-pancreatic cancer activities and preclinical pharmacology. PLoS ONE. 2012;7:e44883. doi: 10.1371/journal.pone.0044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfender JL. HPLC in natural product analysis: the detection issue. Planta Medica. 2009;75:719–734. doi: 10.1055/s-0028-1088393. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang H. MDM2 oncogene as a novel target for human cancer therapy. Current Pharmaceutical Design. 2000;6:393–416. doi: 10.2174/1381612003400911. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang H, Agrawal S. Novel antisense anti-MDM2 mixed-backbone oligonucleotides: proof of principle, in vitro and in vivo activities, and mechanisms. Current Cancer Drug Targets. 2005;5:43–50. doi: 10.2174/1568009053332663. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xu H, Xu Z, Sukesh V, Srinivasan M, Dwayaja NH, Sadanandan VE, Wang M-H, Wang W, Zhang R. Preclinical evaluation of anticancer efficacy and pharmacological properties of FBA-TPQ, a novel synthetic makaluvamine analog. Marine Drugs. 2012;10:1138–1155. doi: 10.3390/md10051138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li M, Wang H, Agrawal S, Zhang R. Antisense therapy targeting MDM2 oncogene in prostate cancer: Effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11636–11641. doi: 10.1073/pnas.1934692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wang H, Prasad G, Li M, Yu D, Bonner J, Agrawal S, Zhang R. Radiosensitization by antisense anti-MDM2 mixed-backbone oligonucleotide in in vitro and in vivo human cancer models. Clinical Cancer Research. 2004;10:1263–1273. doi: 10.1158/1078-0432.ccr-0245-03. [DOI] [PubMed] [Google Scholar]
- Zhou S, Kestell P, Paxton JW. Predicting pharmacokinetics and drug interactions in patients from in vitro and in vivo models: the experience with 5,6-dimethylxanthenone-4-acetic acid (DMXAA), an anti-cancer drug eliminated mainly by conjugation. Drug Metabolism Reviews. 2002;34:751–790. doi: 10.1081/dmr-120015693. [DOI] [PubMed] [Google Scholar]