Abstract
There is a need for minimally invasive biomarkers that can accurately and quickly quantify radiation exposure. Radiation-responsive proteins have applications in clinical medicine and for mass population screenings after a nuclear or radiological incident where the level of radiation exposure and exposure pattern complicate medical triage for first responders. In this study, we evaluated the efficacy of the acute phase protein serum amyloid A (SAA) as a biomarker for radiation exposure using plasma from irradiated mice. Ten-week-old female C57BL6 mice received a 1–8 Gy single whole-body or partial-body dose from a Pantak X-ray source at a dose rate of 2.28 Gy/min. Plasma was collected by mandibular or cardiac puncture at 6, 24, 48 and 72 h or 1–3 weeks postirradiation. SAA levels were determined using a commercially available ELISA assay. Data was pooled to generate SAA μg/ml threshold values correlating plasma SAA levels with radiation dose. SAA levels were statistically significant over control at all exposures between 2 and 8 Gy at 24 h postirradiation but not at 6, 48 and 72 h or 1–3 weeks postirradiation. SAA levels at 1 Gy were not significantly elevated over control at all time points. Total-body-irradiated (TBI) SAA levels at 24 h were used to generate a dose prediction model that successfully differentiated TBI mice into dose received cohorts of control/1 Gy and ≥2 Gy groups with a high degree of accuracy in a blind study. Dose prediction of partial-body exposures based on the TBI model correlated increasing predictive accuracy with percentage of body exposure to radiation. Our findings indicate that plasma SAA levels might be a useful biomarker for radiation exposure in a variety of total- and partial-body irradiation settings.
INTRODUCTION
Radiation exposure is a continuing threat both from potential “dirty bomb” terrorist events and industrial accidents involving nuclear power and misplaced radioactive sources. In the case of a radiological event, mass screenings of large sections of the relevant population will be required to triage exposed from nonexposed individuals and to determine the severity of the received dose in exposed individuals (1). The identification of potential biomarker proteins for use as a radiation biodosimeter is critical for effective medical treatment of such occurrences (2). Inflammation is a classically held pathophysiological response to the damaging effects of ionizing radiation exposure and increased serum amyloid A (SAA) expression after exposure has recently been found in both nonhuman primates and mice (3–5).
The apolipoprotein serum amyloid A is a major acute phase reactant protein and plays a central role in the inflammatory response. Present in a wide variety of vertebrate species, SAA is expressed primarily in the liver, although it is also found in extrahepatic sources such as adipocytes and macrophages (6, 7). SAA is involved in cholesterol sequestering and lipid metabolism and has been shown to induce extracellular-matrix-degrading enzymes, proinflammatory cytokines and to recruit immune cells to sites of inflammation by chemotaxis (8, 9). When induced, SAA has a wide dynamic range of expression, increasing up to 1,000-fold over basal expression values and exceeding plasma concentration values of 1 mg/ml (10).
Given the current scarcity of noninvasive markers of radiation response, and expanding on the seminal biodosimetry work of Kim and Ossetrova, the utility of SAA as a potential radiation biodosimeter was further examined (5, 11). Using plasma SAA levels in mice exposed to varying doses of radiation, a predictive model to estimate radiation dose was constructed. This model was then examined for its ability to quantify unknown radiation dose based on SAA expression. Partial-body radiation exposures were also examined to determine the dynamics of SAA response. Our data indicate that plasma SAA in mice is a promising biomarker for radiation exposure.
MATERIALS AND METHODS
Human Cell Lines
Cell lines of human origin were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C, 5% CO2. Total protein was extracted for Western or ELISA assays. Cell lines from various tissues were included in this study: U251, U87, LN18, LN229, MIA PaCa-2, MCF-7, MDA-MB-231, HELA, MRC9, CCD-19Lu and HUVEC. HUVEC were obtained from Lonza (Basel, Switzerland), U251 from the DCTD Tumor Repository (National Cancer Institute, Frederick, MD) and all other cell lines from ATCC® (Gaithersburg, MD).
Human Ex Vivo Model
Human peripheral blood lymphocytes (PBLs) were collected by venipuncture and cultured on Poly-D Lysine/Laminin coated plates. Either PBLs or whole blood were irradiated ex vivo and incubated at 37°C, 5% CO2. PBLs were then harvested and total protein extracted for Western or ELISA assays. Whole blood was spun at 4°C for 10 min at 2,200 RCF and serum or PBLs collected.
Human ELISA and Western Blot
Potential biomarker proteins were screened using either ELISA or Western blot techniques. Commercially available ELISAs were used for transferrin and albumin (Assaypro LLC, St. Charles, MO), sTfR and α-amylase (BioAssay Systems, Hayward, CA), fibrinogen (Innovative™ Research Inc., Novi, MI) and a custom direct ELISA method for γ-H2AX (12). Antibodies for Western blots were obtained from: CUGBP1 (Upstate®, Billerica, MA), CUGBP2 (Zymed Laboratories Inc., San Francisco, CA), ADAM9 (Abcam®, Cambridge, MA), ENO1 (Abcam), MUC1 (Santa Cruz Biotechnology® Inc., Dallas, TX), FMRP (Chemicon International Inc., Billerica, MA) and ST3GAL1 (Novus Biologicals LLC, Littleton, CO).
Mouse ELISA
Potential biomarker proteins were screened using commercially available ELISAs: transferrin (R&D Systems™, Minneapolis, MN), fibrinogen (GenWay Biotech Inc., San Diego, CA), amylase (Bioassay Systems), albumin (GenWay Biotech) and a custom direct ELISA method for γ-H2AX (12).
Dosimetry
Human cell lines, human peripheral blood lymphocytes or whole blood and all in vivo models utilized a Pantak X-ray source at a dose rate of 2.28 Gy/min. Dose rate was calibrated based on the procedures described in the American Association of Physicists in Medicine (AAPM) Task Group Report 61 (TG-61) with regard to the following conditions: X-ray tube potential is 300 kV, half value layer is 0.8 mm copper and source to surface distance is 50 cm. Dose rate was measured at 2 cm depth in a solid water phantom using an ion chamber (model no. N23342; PTW, Freiburg, Germany) and electrometer (model no. 35040; Inovision®, Chesterfield, MI).
Animal Model
For the dose-predictive model, ten-week-old female C57BL6 mice were either exposed to a single dose of 1–8 Gy total-body irradiation (TBI) or sham irraiation. All mice receiving TBI were confined using a standard pie jig preventing movement. Partial-body irradiations were conducted on anesthetized [120 mg/kg ketamine, 16 mg/kg xylazine intraperitoneal (IP)] and fully restrained mice using lead shielding to expose only the desired body fractions.
At various time points after irradiation (6, 24, 48 and 72 h as well as 1–3 weeks), plasma was collected by mandibular or cardiac puncture in lithium heparin blood collection tubes (BD Biosciences, San Jose, CA). Mice received 2.5–5.5% isoflurane anesthesia during cardiac puncture for blood collection. Blood samples were spun at 10,000 RCF for 10 min at room temperature and stored at −80°C.
To test potential processing and storage variables on SAA expression, plasma was isolated from mice exposed to 4 Gy TBI 24 h after irradiation. Age variables were examined by comparing SAA data from C57BL6 mice aged 6–20 weeks at time of TBI with 1, 2, 4 and 8 Gy with plasma collections at 24, 48 h and 72 h postirradiation. Male vs. female SAA expression was examined in nude mice receiving 2, 4 and 8 Gy exposures at 24 h postirradiation. The effect of physical trauma on SAA response was examined in C57BL6 mice that received multiple mandibular venipuncture lacerations at 24 h post-trauma. Psychological stress effects on SAA expression were evaluated in C57BL6 mice receiving a sham exposure of 8 Gy TBI with plasma collected 24 h postirradiation. The effect of anesthesia administered for partial-body exposures was examined in C57BL6 mice receiving a 120 mg/kg ketamine, 16 mg/kg xylazine IP injection with plasma collected 24 h post injection by cardiac puncture with 2.5–5.5% inhaled isoflurane.
Ethics Statement
Samples of human origin were obtained under clinical study no. 02-C-0064 and were approved by the Institutional Review Board of the National Cancer Institute. Informed written consent was obtained from all participants under current IRB guidelines. All animal studies were conducted in accordance with the principles and procedures outlined in the NIH Guide for the Care and Use of Animals and procedures were approved by the NIH Laboratory Animal Safety Program under an approved protocol.
Mouse SAA Measurement
Serum amyloid A levels were determined in mouse plasma using a commercially available ELISA kit (Invitrogen™, Carlsbad, CA) sensitive to all isoforms of SAA according to the manufacturer’s instructions.
Statistical Analysis
In vivo experiments were analyzed independent of each other and unless otherwise indicated the data presented are specific to each individual animal. Statistical analysis of SAA values was done using a Student’s t test and a probability level of P < 0.05 was considered significant. Pooled data is presented as mean ± SEM. For statistical analysis of percentage of SAA response and analysis of clinical variables, a Mann-Whitney test was performed using assigned binary scores for SAA responders ≥150 μg/ml versus nonresponders.
Data was first evaluated for outliers that cause substantial influence on the model. Using studentized residuals and Cook’s distance, 2 points, 1,000 (2 Gy) and 169 (8 Gy) were estimated to be outside the ±2 range at 95% confidence level and were removed. A multiple regression dose prediction threshold for control/1 Gy and ≥2 Gy at 24 h postirradiation was generated using pooled SAA values from multiple animal experiments. SAA μg/ml thresholds were generated using receiver operator curves (ROCs) to establish specificity and sensitivity (Supplementary Figs. S1 and S2; http://dx.doi.org/10.1667/RR13927.1.S1) Using the predicted cutoff value of 168 μg/ml identified from a (control/1 Gy vs. 2–8 Gy) ROC curve, a threshold of 150 μg/ml was selected to ensure high stringency for the lowest false negative rate in the blind study. Blind study SAA values were subsequently evaluated using the predetermined threshold of 150 μg/ml at 24 h postirradiation. Mice were grouped into either control/1 Gy or ≥2 Gy groups. For this study, we required a dose prediction method to accurately predict >70% of the samples to be deemed successful. Contingency table analysis for sensitivity and specificity was performed using a Fisher’s exact test for the TBI blinded study samples.
RESULTS
Prospective Biomarker Screen
Potential biomarker proteins were selected based on literature suggesting increased expression after radiation exposure. To determine if there was a radiation dose-response relationship, prospective protein markers of radiation response were screened using plasma from mice receiving TBI (TBI plasma), human cell lines grown in culture (cell lines), ex vivo irradiated peripheral blood lymphocytes (PBL ex vivo) or ex vivo irradiated whole blood (blood ex vivo). Of the 21 candidate proteins investigated in the cell lines and the two ex vivo sample sets, only γ-H2AX proved responsive to radiation exposure (Table 1). However, in the TBI mouse plasma samples, fibrinogen, MMP9 and SAA showed elevated expression after radiation exposure (Table 1). Fibrinogen had significantly elevated plasma levels (P < 0.05) over control at 1, 4 and 8 Gy 24 h postirradiation (Fig. 1A). Statistically significant (P < 0.001) elevation of plasma MMP9 was seen at 24 h postirradiation at doses of 1, 4 and 8 Gy (Fig. 1B). At 24 h postirradiation, SAA was significantly (P < 0.001) elevated in plasma from TBI mice at doses of 4 and 8 Gy (Fig. 1C). Although fibrinogen, MMP9 and SAA all demonstrated radiation-responsive increases in expression at 24 h postirradiation there were significant differences in their range of expression, with fibrinogen showing a 2.5-fold increase, MMP9 a twofold increase and an over 400-fold increase for SAA. Since SAA had the largest dynamic range of plasma expression it was selected for further study as a potential candidate for radiation response.
TABLE 1.
Prospective Biomarker Proteins
| Candidate | Sample type
|
Species | Method of detection | Radiation dose (collection time point postirradiation) | |||
|---|---|---|---|---|---|---|---|
| TBI plasmaa | Cell lineb | PBL ex vivoc | Blood ex vivod | ||||
| ADAM9 | ≈ | − | − | ≈ | Human | Western blot | PBL (7 Gy, 1 h) |
| Cell (0.5–4 Gy, 6–48 h) | |||||||
| ALBUMIN | − | ≈ | ≈ | − | Human/mouse | ELISA | Blood (2–4 Gy, 2–6 h) |
| TBI plasma (1–8 Gy, 6–48 h) | |||||||
| α-AMYLASE | − | − | ≈ | − | Mouse | ELISA | Cell (8 Gy, 24 h) |
| Blood (8 Gy, 5 h) | |||||||
| TBI plasma (1–8 Gy, 6–72 h) | |||||||
| CUGBP1 | ≈ | − | ≈ | ≈ | Human | Western blot | Cell (2–7 Gy, 6–24 h) |
| CUGBP2 | ≈ | − | ≈ | ≈ | Human | Western blot | Cell (2–7 Gy, 6 h) |
| ENO1 | ≈ | ≈ | − | ≈ | Human | Western blot | PBL (7 Gy, 1 h) |
| Cell (0.5–4 Gy, 1–6 h) | |||||||
| FIBRINOGEN | + | ≈ | ≈ | − | Human/mouse | ELISA | Blood (8 Gy, 5 h) |
| TBI plasma (1–8 Gy, 24 h–1 week) | |||||||
| FMRP | ≈ | ≈ | − | ≈ | Human | Western blot | PBL (7 Gy, 1 h) |
| γH2AX | ≈ | + | + | ≈ | Human/mouse | ELISA | PBL (2–8 Gy, 1–48 h) |
| Cell (4 Gy, 1–24 h) | |||||||
| IL1β | − | ≈ | ≈ | ≈ | Mouse | ELISA | e Partial-body irradiation (8 Gy, 24 h–1 week) |
| MMP2 | − | ≈ | ≈ | ≈ | Mouse | ELISA | TBI plasma (1–8 Gy, 24–72 h) |
| MMP3 | − | ≈ | ≈ | ≈ | Mouse | ELISA | TBI plasma (8 Gy, 24–72 h) |
| MMP9 | + | ≈ | ≈ | ≈ | Mouse | ELISA | TBI plasma (1–8 Gy, 24–72 h) |
| MUC1 | ≈ | − | − | ≈ | Human | Western blot | PBL (7 Gy, 1 h) |
| Cell (2–12 Gy, 2–24 h) | |||||||
| NSE | − | ≈ | ≈ | ≈ | Mouse | ELISA | TBI plasma (1–8 Gy, 24–72 h) |
| S100B | − | ≈ | ≈ | ≈ | Mouse | ELISA | TBI plasma (8 Gy, 24–72 h) |
| SAA | + | ≈ | ≈ | ≈ | Mouse | ELISA | TBI plasma (1–8 Gy, 6 h–3 weeks) |
| ST3GAL1 | ≈ | − | − | − | Human | Western blot | PBL (2–8 Gy, 24–48 h) |
| Cell (0.5–8 Gy, 1–48 h) | |||||||
| Blood (2–8 Gy, 1–48 h) | |||||||
| STFR | ≈ | ≈ | ≈ | − | Human | ELISA | Blood (2–4 Gy, 2–24 h) |
| TRANSFERRIN | − | ≈ | ≈ | − | Human/mouse | ELISA | Blood (2–4 Gy, 2–24 h) |
| TBI plasma (1–4 Gy, 24–72 h) | |||||||
| VEGF | − | ≈ | ≈ | ≈ | Mouse | ELISA | TBI plasma (1–8 Gy, 24–72 h) |
Note. + = Antigen increase with irradiation; − = no response to irradiation; ≈ = not determined.
Plasma was harvested from TBI C57BL6 mice.
Cell lines of human origin were cultured and irradiated in vitro.
Human PBL were harvested, cultured and irradiated ex vivo.
Human blood was collected and irradiated ex vivo.
For this target C57BL6 mice were irradiated in a partial-body model to either head or trunk only.
FIG. 1.
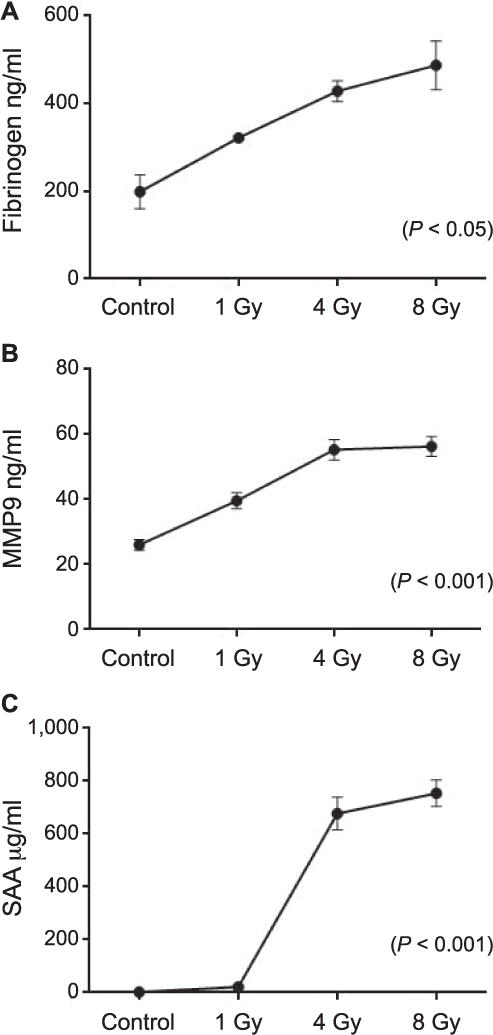
Radiation-responsive plasma proteins. Elevated antigen expression at 24 h after total-body irradiation. Panel A: Significantly elevated plasma fibrinogen levels after 1, 4 and 8 Gy (P < 0.05) total-body irradiation. Panel B: Elevated plasma MMP9 levels after 1, 4 and 8 Gy (P < 0.001) total-body irradiation. Panel C: Elevated levels of plasma SAA after 1, 4 and 8 Gy total-body irradiation in C57BL6 mice (P < 0.001). These values are representative of pooled animal experiments from 19 mice (fibrinogen), 51 mice (MMP9) and 185 mice (SAA). Values reflect the mean ± SEM.
Elevated Plasma SAA Levels in TBI C57BL6 Mice
To more fully characterize the dynamics of SAA response at 24 h, mice received TBI between 2 and 8 Gy. SAA levels from mice exposed to 2 Gy were significantly higher (P < 0.001) than from the unirradiated control mice at 24 h postirradiation (Fig. 2). Irradiated mice in all dose groups between 3 and 8 Gy had SAA values similarly significant over control (P < 0.001) (Fig. 2). To evaluate the early rise and duration of SAA elevation after irradiation we measured plasma SAA at additional time points of 6, 48 and 72 h and 1–3 weeks postirradiation (Fig. 3). At 48 h postirradiation, some mice showed modestly elevated SAA values but this trend was not consistent. Elevated SAA levels were not detected in mice at 6 and 72 h and 1–3 weeks postirradiation. Mice receiving 1 Gy TBI did not show significant SAA elevation at any of the time points examined although a few individual mice had elevated SAA at 24 h postirradiation. Overlapping data points for the respective radiation doses at 24 h are clarified in Fig. 3 (see inset)
FIG. 2.
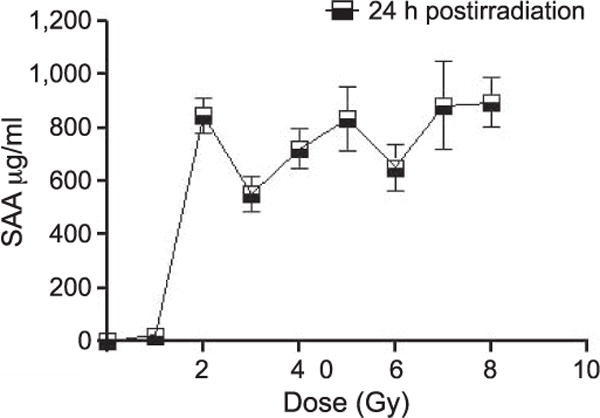
SAA levels after TBI. Plasma SAA levels in C57BL6 mice 24 h postirradiation, between 1–8 Gy. These values are representative of pooled animal experiments from 225 mice (SAA). Values reflect the mean ± SEM.
FIG. 3.
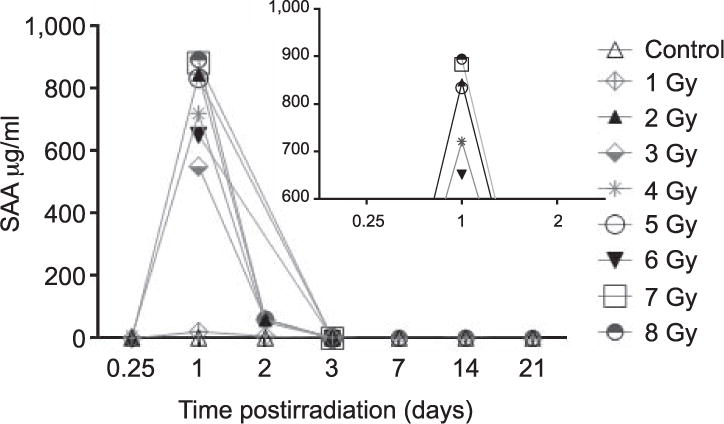
Time course of SAA expression after TBI. SAA expression in C57BL6 mice at 6, 24, 48 and 72 h and 1–3 weeks after TBI. These values are representative of pooled animal experiments from 506 mice (SAA).
Elevated Plasma SAA Levels in Partial-Body-Irradiated C57BL6 Mice
To examine the effects of partial irradiation on SAA response, several partial-body irradiation patterns were examined by irradiating either a gross percentage of 25 or 50% body mass in the upper or lower quadrants, or by isolating distinct regions such as the head, limbs, trunk or trunk and limbs together (Fig. 4A). Partial-body SAA response was evaluated at doses of 2–8 Gy at 24 h postirradiation. Mean SAA (μg/ml) values for each of the partial-body exposure profiles are shown in Fig. 4B and C. For mice receiving a 25% body mass exposure, mean SAA increased with dose and was significantly elevated over control for 4 and 8 Gy (with P < 0.01 and P < 0.001, respectively). For mice receiving a 50% body mass exposure, mean SAA increased with dose of exposure at 2 and 4 Gy, yet 4 and 8 Gy yielded equivalent SAA elevation. SAA increase in mice receiving a 50% exposure was also significant over control at both 4 and 8 Gy (P < 0.001) (Fig. 4B). For mice receiving exposures to only the head, limbs, trunk or trunk and limbs together, an increase in mean SAA was seen with increasing radiation dose for each of the exposure profiles, but was significantly elevated over control for only the 8 Gy trunk and trunk and limbs together exposures (P < 0.001) (Fig. 4C).
FIG. 4.
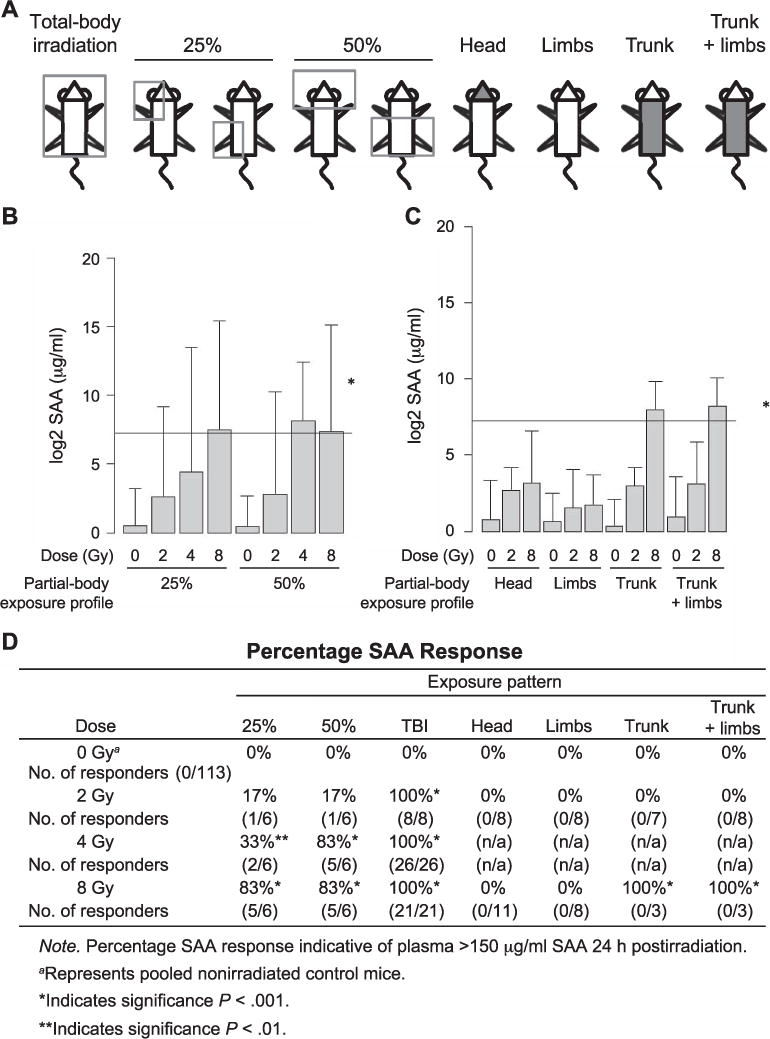
Partial-body irradiation patterns and SAA response. Plasma SAA expression after heterogeneous partial-body irradiation patterns in C57BL6 mice. Panel A: Mice were partially shielded from radiation source using the represented partial-body diagrams with 25 and 50% gross exposures of either upper or lower quadrants or of only the head, limbs, trunk or trunk and limbs. Panel B: Plasma SAA values in mice receiving 0, 2, 4 or 8 Gy radiation with a 25 or 50% partial-body exposure profile. Panel C: Plasma SAA values in mice exposed to 0, 2 or 8 Gy radiation of only the head, limbs, trunk or trunk and limbs. Values reflect the mean ±SEM. *The ≥150 μg/ml SAA threshold is marked for reference. Panel D: Values reflect the percentage of animals with a positive SAA response of ≥150 μg/ml to radiation exposure at various partial-body exposure patterns.
Although mean SAA was sensitive to the level of radiation exposure, the irradiated groups were characterized by extremely low versus high SAA expression. To more fully characterize the SAA response to partial-body irradiation profiles, SAA elevation was analyzed using a binary method for responders versus nonresponders (Fig. 4D). A positive SAA response was defined as plasma SAA exceeding 150 μg/ml. Of the mice receiving a 25% body mass exposure of 2 Gy, 17% responded with elevated SAA at 24 h. This rate of response increased to 33% at 4 Gy and 83% at 8 Gy for the 25% body mass irradiation cohort. A similar trend was seen in mice receiving 2 Gy to 50% of their body mass with 17% of the exposed group responding with elevated SAA at 24 h and 83% of the exposed mice responding at 4 and 8 Gy, respectively. No significant differences in SAA response were observed between the upper and lower quadrant 25 and 50% exposures. At 2, 4 and 8 Gy, 100% of the TBI mice had elevated SAA expression. Exposure of 2 Gy to only the head, limbs, trunk or trunk and limbs together elicited no significant SAA response at 24 h. At 8 Gy, 100% of the mice receiving radiation to the trunk or trunk and limbs together had elevated SAA ≥150 μg/ml. No SAA response was seen in 8 Gy irradiated head or limb profiles at 24 h postirradiation (Fig. 4D).
Plasma SAA Levels Used as Predictor of Dose in a Blind Study of TBI C57BL6 Mice
To determine if SAA levels could be used to separate exposed from unexposed “victims”, a blinded study was performed. C57BL6 mice were total-body irradiated with 1, 2, 4 or 8 Gy and blood was collected at 24 h postirradiation. Samples were run blinded and predictions of received dose were made based on SAA expression. Animals with plasma SAA levels ≥150 μg/ml were predicted to have received a TBI exposure of ≥2 Gy. Mice with plasma SAA levels lower than 150 μg/ml were predicted either to have received 1 Gy TBI or were in the nonirradiated control group. Analysis of SAA using the 150 μg/ml threshold correctly predicted 23/28 mice from the control/1 Gy group and 27/27 mice in the ≥2 Gy group, which included mice receiving TBI doses of 2, 4 and 8 Gy (Table 2). The 5 mice incorrectly grouped as ≥2 Gy actually received 1 Gy TBI. At 24 h postirradiation this SAA prediction method led to correct predictions between control/1 Gy and ≥2 Gy exposures with 91% accuracy and had a high degree of sensitivity (P < 0.0001) and specificity for both positive and negative predictive values (Table 2).
TABLE 2.
Contingency Table: Blinded Total-Body Irradiations
| Treatment group | Percentage correct | Number correcta | Collection time point |
|---|---|---|---|
| Control/1 Gy | 82% | 23/28 | 24 h |
| ≥2 Gy | 100% | 27/27 | 24 h |
| Unexposed | ≥2 Gy | Total | |
| Unexposed | 23% | 5 | 28 |
| ≥2 Gy | 0% | 27 | 27 |
| Total | 23% | 32 | 55 |
Note. Fisher’s exact test P < 0.0001; PPV 0.8214 CI (0.6311–0.9394); NPV 1.000 CI (0.8723–1.000).
Represents the number of correct predictions of ≥2 Gy exposure over the total number of animals in that group.
Potential Confounders Using SAA as a Biomarker of Radiation-Induced Inflammation
To further characterize the utility of SAA as a possible biomarker of radiation exposure, potential biological and sample handling variables were examined for their effects on plasma SAA expression. Physiological factors such as age, sex, exposure to anesthesia and psychological stress did not influence plasma levels of SAA in mice. No significant variation in SAA expression was detected between C57BL6 mice of 6–20 weeks of age. Elevated SAA was also not seen in sham-irradiated mice subjected to the stress of restraint or exposed to anesthesia. Physical trauma due to lacerations caused by repeated mandibular venipuncture attempts to the same area did result in increased SAA expression in 3 of 11 mice examined. None of the processing variables relating to storage conditions or time to processing influenced SAA values (Table 3).
TABLE 3.
Potential Variables in Clinical Screening
| SAA | Dose (Gy) | Time point postirradiation | P value | Notes | |
|---|---|---|---|---|---|
| Biologic variable | |||||
| Agea | − | 0, 1, 2, 4, 8 | 24, 48, 72 h | P > 0.4 | Age range of 6–20 weeks. |
| Sexb | − | 2, 4, 8 | 24 h | P > 0.3 | |
| Physical traumac | + | 0 | n/a | P > 0.2 | Physical trauma from multiple mandibular venipuncture lacerations collected 24 h post trauma. |
| Psychological stressd | − | Sham, 8 | 24 h | P > 0.9 | Psychological stress of physical restraint. |
| Anesthesiae | − | 0 | 24 h | P > 0.9 | Anesthesia 120 mg/kg ketamine, 16 mg/kg xylazine, IP. |
| Processing variablef | |||||
| Type of storage tube | |||||
| PPE 1.7 ml tube | − | 0, 4 | 24 h | *P > 0.9 | All tube types were stored at −80°C |
| Nunc 2 ml Cryovial | − | 0, 4 | 24 h | *P > 0.9 | |
| Corning 2 ml Cryovial | − | 0, 4 | 24 h | *P > 0.9 | |
| Subzero storage | |||||
| −80°C/−20°C | − | 0, 4 | 24 h | *P > 0.9 | SAA values were evaluated at 1 week of subzero storage. |
| Time at 22°C before processing | |||||
| 0.5–4 h | − | 0, 4 | 24 h | *P > 0.9 | Time of whole blood at 22°C before centrifugation and subzero storage. |
Note. + = SAA increase; − = no SAA response.
The age variable reflects similarity of SAA expression between mice aged 6 weeks (n = 33), 7 weeks (n = 19), 9 weeks (n = 102), 10 weeks (n = 95) and 20 weeks (n = 5). P > 0.4 for all relative comparisons.
The sex variable reflects pooled data from experiments involving 9 female versus 19 male nude mice. P > 0.3 for all groups.
The physical trauma variable reflects pooled data from experiments with mice receiving single or multiple mandibular lacerations (11 mice). Three out of eleven (3/11) mice showed SAA elevation >150 μg/ml.
The psychological stress variable reflects data from 10 mice that received 8 Gy of sham irradiation.
The anesthesia variable reflects data from 3 mice receiving anesthesia alone.
Processing variables reflect pooled data from experiments involving 28 mice.
P > 0.9 for both 0 and 4 Gy groups.
DISCUSSION
Biomarkers of Radiation Response
There are few well characterized biomarkers of radiation response (13). Though numerous proteins have been identified as sensitive to radiation no established biomarker for radiation dose has been developed in humans. Currently the most accepted biomarker for estimating radiation dose to an exposed individual is quantification of chromosomal aberrations, a process that is lengthy and requires highly trained personnel working in offsite laboratories (14). γ-H2AX is a well established marker of radiation-induced DNA double-strand breaks and there have been studies demonstrating dose-dependent γ-H2AX expression in ex vivo irradiated lymphocytes, but the early peak of γH2AX expression at 30 min postirradiation and difficulties adapting the assay to a high throughput platform may prevent further development (15, 16).
In an attempt to identify promising novel radiation-responsive proteins, candidate targets were selected from the literature as outlined by Marchetti (17). Of the targets examined using ex vivo human models, only γ-H2AX showed changes in response to ionizing radiation exposure. Given the lack of success using human cell lines and ex vivo samples, candidate proteins were then screened using a mouse model to better simulate radiation response in a mammalian system. Of the molecules examined using plasma from TBI mice, fibrinogen, MMP9 and SAA demonstrated increased expression with radiation exposure. SAA was selected for further study because of the magnitude of its acute response compared to the marginal increases of MMP9 and fibrinogen after irradiation. Unlike fibrinogen and MMP9, SAA also did not show significant elevation at 1 Gy, which is desirable for field applications. SAA had also been identified in a seminal study by Ossetrova and Blakely characterizing SAA elevation in mice after radiation exposure (18, 19). Expanding on this proof of concept work we further characterized the dynamics of SAA expression after radiation exposure using a large cohort and validation using a blinded study.
Applications as a Radiation Biodosimeter
The acute phase response is a highly complex physiologic mechanism through which the body responds to challenges of injury or infection. Inflammation is a key component of the systemic changes introduced during the acute phase response, which also include cytokine cascade, mobilization and increased production of immune cells, metabolic changes and a significant alteration of the protein synthesis profile of the liver. Inflammation is a classically held pathophysiological response to the damaging effects of ionizing radiation, and increased SAA expression after radiation exposure has recently been found in both nonhuman primates and mice (3, 4).
The acute phase protein SAA is a key mediator of the inflammatory response and there is an emerging body of evidence linking SAA expression with radiation exposure. Several key studies have shown SAA elevation after radiation exposure in both TBI and partial-body models. The data presented here are confirmatory to these previous studies in mice where similar TBI exposures ranging from 0.1–14 Gy were isolated at 4 h–7 days postirradiation, collectively (5, 11, 19). Studies done in primates using the TBI model also highlight SAA elevation after exposures of 1–8.5 Gy over a time course of 6 h–18 days (4, 20). Preliminary murine partial-body irradiation studies have shown elevated SAA at 6 Gy at 1–2 days postirradiation, in comparison to the current study using 2 and 8 Gy, 1 day postirradiation, but these studies utilized different partial-body irradiation profiles and restraint models (21, 22).
The data here show a direct correlation between plasma SAA increase and TBI with ≥2 Gy. This acute elevation of SAA at radiation exposures of ≥2 Gy but not 1 Gy may have applications for use as a radiation biodosimeter. In the event of a radiological or nuclear bioterrorist incident, it is expected that mass population screenings will be needed to triage exposed individuals. Identification of exposed and unexposed individuals and subsequent determination of the level of exposure are crucial initial screenings for mass casualty first responders. Determining the level of radiation exposure is critical for identifying those individuals who best benefit from medical intervention, since increased survival is possible with treatment for those exposed to 2–10 Gy TBI, while those exposed to ≤1 Gy do not require treatment to survive and for those exposed to >10 Gy there is no current treatment available to ensure survival (1, 23, 24). A biodosimeter would also be helpful with the infrequent but difficult to quantify accidental exposures that arise from lost radioactive sources in industry (25, 26). In such cases, determining the level of exposure is exceedingly challenging due to variation in irradiation field, heterogeneity of exposure pattern, whether the radiation was TBI, partial body or resulting from inhaled airborne particles or ingested from ground contamination (27, 28). SAA expression dynamics are often compared to another more prominent acute phase reactant, C-reactive protein (CRP). SAA and CRP are both synthesized in the liver in response to injury-related cytokine release and have a large dynamic range of expression (4). SAA as an acute phase protein has been shown to be even more sensitive and responsive than CRP (8). CRP kinetics have been firmly linked with the onset and duration of acute radiation sickness but the relationship between SAA expression and radiation dose has yet to be well defined (14). Although there is limited data available examining SAA as a potential biomarker for radiation exposure, SAA has been shown to be responsive to radiation in both mice and NHP (4, 18).
Our study demonstrates a quantitative relationship between plasma SAA levels and radiation doses between 1 to 2 Gy, and through validation with a blind study demonstrates a working model of SAA as a biodosimeter. Our dose prediction model, based on plasma SAA values from TBI mice, segregated the unirradiated and ≤1 Gy irradiated mice from ≥2 Gy irradiated mice with a high degree of accuracy. Ninety-one percent of the mice investigated were correctly identified as having received a ≥2 Gy exposure or as falling into the ≤1 Gy or control groups. The 5 mice incorrectly grouped as ≥2 Gy actually received 1 Gy TBI. Thus, this dose prediction yielded no false negatives, which is the ideal scenario for mass casualty screening (29). SAA upregulation was seen primarily at 24 h postirradiation with some modest increases in expression seen at 48 h postirradiation. This correlates with the observed half-life of plasma SAA of approximately 24 h and an initial increase of SAA after radiation injury and subsequent rapid clearing from the plasma (10, 30). Unlike the active protein, SAA mRNA has a much longer half-life and has been found to remain upregulated in the bone marrow of irradiated mice up to 28 weeks after exposure, suggesting its potential use for screening past radiation exposures (9, 31). A longer mRNA SAA half-life corresponds with a secondary increase of plasma SAA seen in some studies after an extended latency period postirradiation. An increase in plasma SAA was noted in partial-body lung-irradiated mice 100 days postirradiation and 7 days postirradiation in TBI nonhuman primates (4, 32). Dose prediction of the partial-body-exposed mice using the TBI-generated method was not as accurate in determining the level of exposure, although we found elevated plasma SAA corresponded to both increasing percentage of body mass exposed and increasing radiation dose. We also found that SAA elevation was dependent not only on the percentage of body mass exposure but the specific partial-body exposure profile, since 8 Gy exposures to only the limbs or only the head did not result in significant SAA response. In a previous partial-body irradiation study, Blakely et al. found significant SAA elevation at all partial-body fractions of 6 Gy, but all of these exposure profiles included significant portions of the trunk (21, 22). These exposure profiles would be most closely comparable to our 50% quadrant partial-body exposures where we similarly saw significant SAA elevation at 4 and 8 Gy.
Ultimately it may be more relevant for medical triage to consider not received “dose” but received “damage” from radiation exposure (28, 33). This alleviates many of the issues caused by heterogeneous exposure patterns and variation of individual responses to the same radiation exposure. For example, individuals receiving similar dose exposures between 3–7 Gy may or may not experience hematopoietic toxicity, and one clinical finding from the Chernobyl incident detailed 7 individuals exposed to extremely disparate radiation doses which all fell into the same hematopoietic toxicity category (34). Our TBI dose prediction model might be applied to mass screening using the 150 μg/ml SAA threshold as a marker of “≥2 Gy like” damage where any individual with SAA expression above this value would be triaged for further treatment. Potential confounders not addressed here include variation in SAA expression between C57BL6 mice and humans, as well as differences in human SAA baseline levels, which may vary by race, age, body mass index (BMI) and previous medical history (35). Pre-existing inflammatory states could also elevate SAA irrespective of radiation exposure, given that SAA is a known marker of several inflammatory diseases including rheumatoid arthritis, cardiovascular disease and cancer (36–39).
In this study, the psychological stress of restraint did not affect SAA expression as our TBI exposure model allowed the mice minimal movement and partial-body exposures were performed on anesthetized mice. We also did not see elevated SAA after anesthesia alone. Increased SAA levels have been found in partial-body-irradiated mice subjected to the stress of complete restraint without the use of anesthesia (21). Elevated SAA was evident, in our current study however, after significant physical trauma in some animals, where 3 of 11 mice subjected to repetitive mandibular venipunctures in a short period of time had elevated plasma SAA. Such factors are extremely relevant to a mass-screening situation, as most radiological attacks would present a multicomponent scenario where radiation exposure is but one injury, which may then be complicated by shock, blood loss, thermal burns and physical trauma. SAA elevation after lipopolysaccharide exposure used to mimic infection in such scenarios is also a potential confounder (40). As such, it is acknowledged that the inflammatory response to injury and infection in addition to chronic inflammatory states caused by pre-existing disease conditions may obfuscate SAA increases that are a direct result of radiation exposure.
Psychosomatic-induced symptoms also will complicate triage during mass casualty events particularly where potential radiation exposure is a concern (41, 42). Incorporating SAA into a multiparametric approach with a biomarker panel including other plasma proteins responsive to radiation but not to inflammation would help eliminate these false positives (20, 29, 43). The addition of SAA to such a panel provides an acute response at the critical screening dose of ≥2 Gy but not at 1 Gy and at the earliest time point of potential screening at 24 h post exposure. Since such a diverse biomarker panel of radiation-responsive proteins would ideally include both early and late responders to radiation exposure, the acute early response of SAA may prove useful. Further work with MMP9 and fibrinogen might help identify other valuable biomarkers, as well. Although our TBI data largely correlates with previous studies of SAA elevation after radiation exposure, our study incorporates much larger animal cohorts and examines additional biologic and processing variables relevant to the use of SAA as a biodosimeter. Our partial-body irradiation profiles examine new exposure patterns and add to a growing body of data characterizing SAA expression after radiation exposure.
In conclusion, we have presented here a working plasma SAA biodosimetry model for irradiation in mice. There is an emerging body of data suggesting SAA as a potential biodosimeter in mammalian systems. The dynamics of SAA expression in response to radiation make it a prospective plasma biomarker of radiation dose and radiation damage in both total- and partial-body exposure paradigms. SAA may have many clinical and mass casualty applications as a potential biomarker of radiation exposure.
Supplementary Material
Fig. S1. Receiver operator curve [control vs. treated (1–8 Gy)].
Fig. S2. Receiver operator curve [control (1 Gy) vs. treated (2–8 Gy)].
Acknowledgments
This research was supported in part by funding from the Radiation and Nuclear Countermeasures Program, grant no. Y2-OD-0332-01 NIAID and by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Editor’s note. The online version of this article (DOI: 10.1667/RR13927.1) contains supplementary information that is available to all authorized users.
References
- 1.Ivey RG, Subramanian O, Lorentzen TD, Paulovich AG. Antibody-based screen for ionizing radiation-dependent changes in the mammalian proteome for use in biodosimetry. Radiat Res. 2009;171:549–61. doi: 10.1667/RR1638.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandey BN, Kumar A, Tiwari P, Mishra KP. Radiobiological basis in management of accidental radiation exposure. Int J Radiat Biol. 2010;86:613–35. doi: 10.3109/09553001003746059. [DOI] [PubMed] [Google Scholar]
- 3.Van der Meeren A, Monti P, Lebaron-Jacobs L, Marquette C, Gourmelon P. Characterization of the acute inflammatory response after irradiation in mice and its regulation by interleukin 4 (Il4) Radiat Res. 2001;155:858–65. doi: 10.1667/0033-7587(2001)155[0858:cotair]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Ossetrova NI, Sandgren DJ, Blakely WF. C-reactive protein and serum amyloid A as early-phase and prognostic indicators of acute radiation exposure in nonhuman primate total-body irradiation model. Radiat Measure. 2011;46:1019–24. [Google Scholar]
- 5.Kim D, Marchetti F, Chen Z, Zaric S, Wilson RJ, Hall DA, et al. Nanosensor dosimetry of mouse blood proteins after exposure to ionizing radiation. Sci Reports. 2013;3:2234. doi: 10.1038/srep02234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chait A, Han CY, Oram JF, Heinecke JW. Thematic review series: The immune system and atherogenesis. lipoprotein-associated inflammatory proteins: markers or mediators of cardiovascular disease? J Lipid Res. 2005;46:389–403. doi: 10.1194/jlr.R400017-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Petersen HH, Nielsen JP, Heegaard PM. Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res. 2004;35:163–87. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- 8.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 9.Jensen LE, Whitehead AS. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem J. 1998;334(Pt. 3):489–503. doi: 10.1042/bj3340489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–23. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 11.Ossetrova NI, Condliffe DP, Ney PH, Krasnopolsky K, Hieber KP, Rahman A, et al. Early-response biomarkers for assessment of radiation exposure in a mouse total-body irradiation model. Health Phys. 2014;106:772–86. doi: 10.1097/HP.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 12.Avondoglio D, Scott T, Kil WJ, Sproull M, Tofilon PJ, Camphausen K. High throughput evaluation of gamma-H2AX. Radiat Oncol. 2009;4:31. doi: 10.1186/1748-717X-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okunieff P, Chen Y, Maguire DJ, Huser AK. Molecular markers of radiation-related normal tissue toxicity. Cancer Metastasis Rev. 2008;27:363–74. doi: 10.1007/s10555-008-9138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasanna PG, Blakely WF, Bertho JM, Chute JP, Cohen EP, Goans RE, et al. Synopsis of partial-body radiation diagnostic biomarkers and medical management of radiation injury workshop. Radiat Res. 2010;173:245–53. doi: 10.1667/RR1993.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothkamm K, Horn S. gamma-H2AX as protein biomarker for radiation exposure. Ann Ist Super Sanita. 2009;45:265–71. [PubMed] [Google Scholar]
- 16.Redon CE, Dickey JS, Bonner WM, Sedelnikova OA. gamma-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv Space Res. 2009;43:1171–8. doi: 10.1016/j.asr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchetti F, Coleman MA, Jones IM, Wyrobek AJ. Candidate protein biodosimeters of human exposure to ionizing radiation. Int J Radiat Biol. 2006;82:605–39. doi: 10.1080/09553000600930103. [DOI] [PubMed] [Google Scholar]
- 18.Ossetrova NI, Sandgren DJ, Gallego S, Blakely WF. Combined approach of hematological biomarkers and plasma protein SAA for improvement of radiation dose assessment triage in biodosimetry applications. Health Phys. 2010;98:204–8. doi: 10.1097/HP.0b013e3181abaabf. [DOI] [PubMed] [Google Scholar]
- 19.Ossetrova NI, Blakely WF. Multiple blood-proteins approach for early-response exposure assessment using an in vivo murine radiation model. Int J Radiat Biol. 2009;85:837–50. [PubMed] [Google Scholar]
- 20.Ossetrova NI, Sandgren DJ, Blakely WF. Protein biomarkers for enhancement of radiation dose and injury assessment in nonhuman primate total-body irradiation model. Radiat Prot Dosimetry. 2014;159:61–76. doi: 10.1093/rpd/ncu165. [DOI] [PubMed] [Google Scholar]
- 21.Blakely WF, Sandgren DJ, Nagy V, Kim S-Y, Sigal GB, Ossetrova NI. Further biodosimetry investigations using murine partial-body irradiation model. Radiat Prot Dosimetry. 2014;159:46–51. doi: 10.1093/rpd/ncu127. [DOI] [PubMed] [Google Scholar]
- 22.Blakely W, Sandgren DJ, Nagy V, Kim SY, Ossetrova NI. Murine partial-body radiation exposure model for biodosimetry studies-Premilinary Report. Radiat Measure. 2011;46:898–902. [Google Scholar]
- 23.Bentzen SM, Parliament M, Deasy JO, Dicker A, Curran WJ, Williams JP, et al. Biomarkers and surrogate endpoints for normal-tissue effects of radiation therapy: the importance of dose-volume effects. Int J Radiat Oncol Biol Phys. 2010;76:S145–50. doi: 10.1016/j.ijrobp.2009.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman CN, Adams S, Adrianopoli C, Ansari A, Bader JL, Buddemeier B, et al. Medical planning and response for a nuclear detonation: a practical guide. Biosecur Bioterror. 2012;10:346–71. doi: 10.1089/bsp.2012.1025. [DOI] [PubMed] [Google Scholar]
- 25.Bertho JM, Roy L, Souidi M, Benderitter M, Bey E, Racine R, et al. Initial evaluation and follow-up of acute radiation syndrome in two patients from the Dakar accident. Biomarkers. 2009;14:94–102. doi: 10.1080/13547500902773904. [DOI] [PubMed] [Google Scholar]
- 26.Bertho JM, Roy L, Souidi M, Benderitter M, Gueguen Y, Lataillade JJ, et al. New biological indicators to evaluate and monitor radiation-induced damage: an accident case report. Radiat Res. 2008;169:543–50. doi: 10.1667/RR1259.1. [DOI] [PubMed] [Google Scholar]
- 27.Waller E, Millage K, Blakely WF, Ross JA, Mercier JR, Sandgren DJ, et al. Overview of hazard assessment and emergency planning software of use to RN first responders. Health Phys. 2009;97:145–56. doi: 10.1097/01.HP.0000348464.78396.23. [DOI] [PubMed] [Google Scholar]
- 28.Gourmelon P, Benderitter M, Bertho JM, Huet C, Gorin NC, De Revel P. European consensus on the medical management of acute radiation syndrome and analysis of the radiation accidents in Belgium and Senegal. Health Phys. 2010;98:825–32. doi: 10.1097/HP.0b013e3181ce64d4. [DOI] [PubMed] [Google Scholar]
- 29.Flood AB, Nicolalde RJ, Demidenko E, Williams BB, Shapiro A, Wiley AL, Jr, et al. A framework for comparative evaluation of dosimetric methods to triage a large population following a radiological event. Radiat Measure. 2011;46:916–22. doi: 10.1016/j.radmeas.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takata S, Wada H, Tamura M, Koide T, Higaki M, Mikura SI, et al. Kinetics of c-reactive protein (CRP) and serum amyloid A protein (SAA) in patients with community–acquired pneumonia (CAP), as presented with biologic half-life times. Biomarkers. 2011;16:530–5. doi: 10.3109/1354750X.2011.607189. [DOI] [PubMed] [Google Scholar]
- 31.Goltry KL, Epperly MW, Greenberger JS. Induction of serum amyloid A inflammatory response genes in irradiated bone marrow cells. Radiat Res. 1998;149:570–8. [PubMed] [Google Scholar]
- 32.Ogata T, Yamazaki H, Teshima T, Kihara A, Suzumoto Y, Inoue T, et al. Early administration of IL-6RA does not prevent radiation-induced lung injury in mice. Radiat Oncol. 2010;5:26. doi: 10.1186/1748-717X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertho JM, Griffiths NM, Gourmelon P. The medical diagnosis and treatment of radiation overexposed people (RC-7a). 11th Congress of the International Radiation Protection Association; Madrid, Spain. 2004. ( http://bit.ly/1AgUz4X) [Google Scholar]
- 34.Dainiak N, Berger P, Albanese J. Relevance and feasibility of multi-parameter assessment for management of mass casualties from a radiological event. Exp Hematol. 2007;35:17–23. doi: 10.1016/j.exphem.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Pierce BL, Neuhouser ML, Wener MH, Bernstein L, Baumgartner RN, Ballard-Barbash R, et al. Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Res Treat. 2009;114:155–67. doi: 10.1007/s10549-008-9985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasazuki S, Inoue M, Sawada N, Iwasaki M, Shimazu T, Yamaji T, et al. Plasma levels of C-reactive protein and serum amyloid A and gastric cancer in a nested case-control study: Japan Public Health Center-based prospective study. Carcinogenesis. 2010;31:712–8. doi: 10.1093/carcin/bgq010. [DOI] [PubMed] [Google Scholar]
- 37.Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:726–32. doi: 10.1161/01.CIR.0000115516.54550.B1. [DOI] [PubMed] [Google Scholar]
- 38.Malle E, De Beer FC. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur J Clin Invest. 1996;26:427–35. doi: 10.1046/j.1365-2362.1996.159291.x. [DOI] [PubMed] [Google Scholar]
- 39.Kokubun M, Imafuku Y, Okada M, Ohguchi Y, Ashikawa T, Yamada T, et al. Serum amyloid A (SAA) concentration varies among rheumatoid arthritis patients estimated by SAA/CRP ratio. Clin Chim Acta. 2005;360:97–102. doi: 10.1016/j.cccn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Migita K, Abiru S, Nakamura M, Komori A, Yoshida Y, Yokoyama T, et al. Lipopolysaccharide signaling induces serum amyloid A (SAA) synthesis in human hepatocytes in vitro. FEBS Lett. 2004;569:235–9. doi: 10.1016/j.febslet.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 41.Flynn DF, Goans RE. Nuclear terrorism: triage and medical management of radiation and combined-injury casualties. Surg Clin North Am. 2006;86:601–36. doi: 10.1016/j.suc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Koenig KL, Goans RE, Hatchett RJ, Mettler FA, Jr, Schumacher TA, Noji EK, et al. Medical treatment of radiological casualties: current concepts. Ann Emer Med. 2005;45:643–52. doi: 10.1016/j.annemergmed.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Sproull M, Avondoglio D, Kramp T, Shankavaram U, Camphausen K. Correlation of plasma FL expression with bone marrow irradiation dose. PloS One. 2013;8:e58558. doi: 10.1371/journal.pone.0058558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Receiver operator curve [control vs. treated (1–8 Gy)].
Fig. S2. Receiver operator curve [control (1 Gy) vs. treated (2–8 Gy)].


