Abstract
Non-native plants are now a pervasive feature of ecosystems across the globe1. One hypothesis for this pattern is that introduced species occupy open niches in recipient communities2,3. If true, then non-native plants should often benefit from low competition for limiting resources that define niches. Many plants have evolved larger size after introduction, consistent with increased access to limiting resources4–9. It has been difficult to test whether larger size reflects adaptation to exploit open resources, however, because vacant niches are generally challenging to identify in plants. Here we take advantage of a situation in which a highly invasive non-native plant, Centaurea solstitialis L. (yellow starthistle, hereafter ‘YST’), occupies a well-described environmental niche, wherein water is a known limiting resource10,11. We use a glasshouse common environment and climatic niche modeling to reveal that invading YST has evolved a higher-fitness life history at the expense of increased dependence on water. Critically, historical declines in resident competitors have made water more available for introduced plants11,12, demonstrating how native biodiversity declines can open niches and create opportunities for introduced species to evolve increased resource use, a potentially widespread basis for introduction success and the evolution of invasive life histories.
In general, we expect invasive species to have a low probability of establishment and spread where there is strong competition among functionally similar resident species for limiting resources13. These ideas have been articulated as the “Biotic Resistance” of a community14 and as “Darwin’s Naturalization Hypothesis”2, wherein Darwin noted that successful introductions appear to be those least like resident species. Both experimental studies and analyses of phylogenetic similarity within communities provide some support for reduced establishment of new species as functional similarity to the resident community increases2,12,14–16. In line this with this thinking, introduced species are increasingly hypothesized to fill novel or recently-vacated functional roles in communities1, perhaps even reinstating benefits to the ecosystem where native biodiversity has declined17.
Critically, niche-filling by introduced species could facilitate their evolution into invasive genotypes. If introductions often succeed where competition for resources is low, then establishing populations may have novel opportunities to exploit available resources to increase their fitness and spread. In plants, invading individuals are often larger than their native conspecifics, consistent with an increase in the resources available for growth4. Experimental studies of these observations frequently find that size increases are genetically-based and potentially adaptive4–8,18. What remains unclear is how often increased size reflects increased uptake of available resources, versus re-allocation of a fixed quantity of resources away from investment in other non-growth functions, such as resistance to enemies19.
We tested for the evolution of increased resource exploitation in YST populations that have invaded grasslands in California, USA. California grasslands experienced a conversion from native perennial to introduced annual grasses during ecosystem change starting in the late 1800’s (cattle grazing, tilling, and drought), which effectively eliminated native species by the mid-1900’sreferences in 16. This is arguably one of the best-studied degraded ecosystems to datee.g.10,12,15,16. When native biodiversity declines in this region, some of the first species lost from the community are late-season annual forbs12. Late-season annual species are a distinct functional group, continuing to grow and reproduce into the summer drought season in the Mediterranean-type climate, when most other species have senesced10. YST is a Eurasian late-season annual that began to invade in the mid 1900’s, after the decline of natives20. Both observational and experimental studies have shown that YST is a poor invader against native late-season species10–12. Competitive exclusion of YST appears to be mediated by water as a limiting resource11. Nevertheless, YST is considered one of the most invasive species in western North America21, and it occurs at densities that far exceed those seen in its native range22,23. Thus current evidence suggests that historical decline of functionally similar native species opened a niche into which YST established and became a highly successful invader10,11.
To test for evolutionary changes in resource use and fitness, we used a glasshouse common environment to quantify trait variation among YST genotypes from 20 invading populations from the west coast of North America and 22 native populations from the native range (Fig 1A,B; Supplementary Table 1). We observed significant divergence in size between regions: invaders were 49.5% larger by 3.5 weeks and maintained a significant size difference throughout growth and reproduction (Fig. 2A; Supplementary Fig. 1; Supplementary Table 2 for model statistics for all analyses). In a smaller previous study, genotypes from the California invasion produced seeds and plants that were significantly larger in size than genotypes from parts of the native range across two generations in multiple common environments18, consistent with evolutionary change in both traits, though persistent trans-generational plasticity remains a potential source of variation in all common-environment studiese.g. 24. Size variation across populations in our study was not associated with differences in germination timing (no effect of plant age; Supplementary Table 2), and was not explained by differences in seed size (Supplementary Table 2).
Figure 1. YST distribution and niche.
Geographic and climatic distribution of YST in its native Eurasian range (a) and invasion into the western Unites States (b). Black dots indicate all geo-referenced localities (185 native and 372 invading), and colored dots indicate 22 native (blue) and 20 invading (red) source populations in our common garden. A principal components analysis using seven CliMond bioclimatic variables (c; where TEMP = temperature, RAD = solar radiation, PPT = precipitation; CliMond codes indicated in parentheses) for all localities was used to define climatic niche space along the first two axes in both ranges (d,e). Grey shading in d and e shows the density of the occurrences of the species by cell, and colored dots the location of our common garden source populations within the niche space. The solid and dashed contour lines illustrate 100% and 50% of the available (background) climate, respectively.
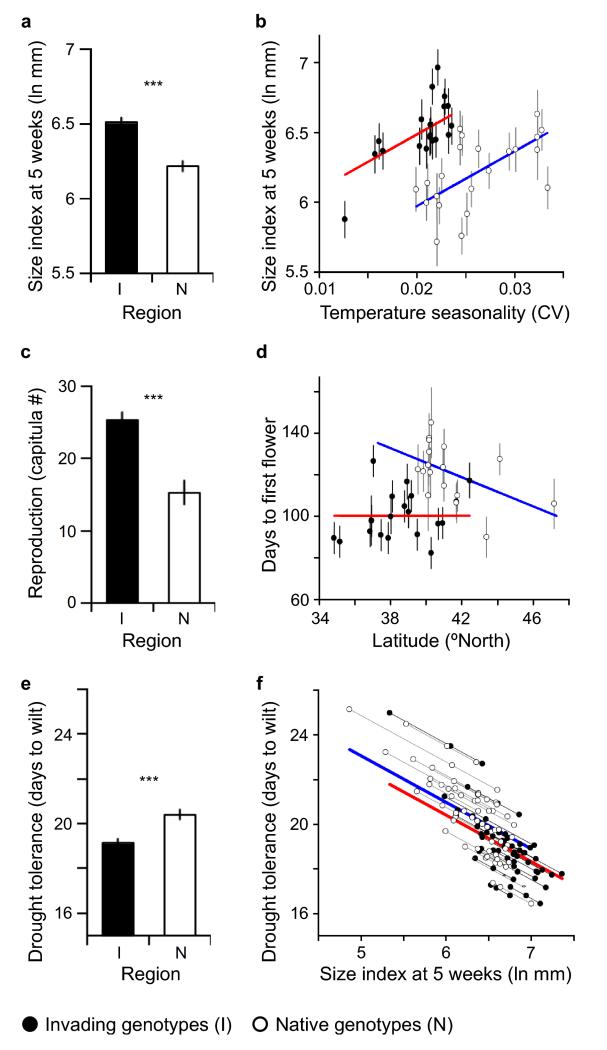
Figure 2. Trait variation across YST populations in a common environment.
Size differences between regions (a) reflect a shift in inter-population variation that is positively correlated with temperature seasonality (CliMond variable b4) within regions (b). Solid lines in b show significant fitted relationships from ANCOVA to native (blue) and invading (red) populations (P < 0.0001). Capitula production is higher among invading populations (c) and correlated with earlier flowering; flowering time is not correlated with latitude for invaders as it is for native populations (d, solid lines show regressions fitted individually for each region, significant only for native populations: P = 0.04). Invaders show reduced drought tolerance (e) that is strongly correlated with size (f, lines show predicted model fits connecting median, 25th percentile, and 75th percentile size values from each source population), with a small significant difference remaining between regions after taking size into account (P = 0.02; heavy red and blue lines show significant model fits for each region). Size data shown in a and b are from 10 maternal lineages (one plant per lineage) from each of 22 native and 20 invading populations grown in a glasshouse. Reproductive plants were followed through flowering as shown in c and d. A drought treatment tested for tolerance in 4-10 lineages/population (mean 8.4) as shown in e and f. Panels a,c,e show REML ANOVA tests for differences between native and invading genotypes (*** P < 0.0001), and b,f show significant REML ANCOVA tests for regional effects on covariation of population variance within each region. Panels a-e show Least Squares Means +/− s.e.m. (Additional statistics in Supplementary Tables 2,3,5).
Size differences between plants from native and invading populations were associated with an apparent increase in fitness in the introduction. Invaders were equally likely to flower as native genotypes, but they produced 60.5% more capitula (Fig. 2C; Supplementary Table 3). The date of first flowering explained the variation in reproductive output when included in the model (Supplementary Table 3), suggesting that increased capitula production in the invaders was the result of an earlier first flowering date that lengthened the flowering season (Fig. 2C,D). Higher reproductive output of larger invaders supports a previous inference of adaptive size evolution in this invasion, based on size differences exceeding expectations from molecular differentiation (QST > FST)25.
Trait divergence between regions can be generated by underlying environmental gradients, such that sampled native and invading populations represent different parts of the same clines in locally adapted traits across regions, rather than evolution of novel traits associated with invasiveness per se26. Within our study there was significant population variation in both plant size and reproduction within regions (Fig. 2B,D; Supplementary Tables 2,3), allowing us to test for the possibility of underlying environmental gradients affecting each trait. Size variation was positively related to temperature seasonality in both regions, such that larger plants are found in more highly seasonal habitats, but this cline was shifted toward larger size in the invasion (Fig. 2B; Supplementary Table 4). Though plants from invading populations occupy less-seasonal habitats on the west coast of North America, they were as large or larger than plants in the most seasonal climates sampled in Europe, particularly those from Spain – a putative major source of the invasions27 (Supplementary Figure 2).
Similarly, mean first flowering date of native populations showed a negative relationship with latitude (linear regression: r2 = 0.24, P = 0.04), typical of temperate plants wherein higher latitude populations flower earlier under a shorter overall growing season, but invading populations did not show this relationship (Fig. 2D). Instead, invaders flowered earlier across populations, irrespective of latitude. The lack of a latitudinal cline in flowering date is surprising, as other introduced species have readily re-evolved similar clines post-introduction, even when introduced from geographically-limited source populationse.g. 6,28. Earlier flowering in these larger invaders may reflect selection for a longer reproductive season without the fitness costs of lost opportunities for growth seen in other species28. In sum, invading genotypes show a shift toward larger size and increased reproduction that is uncharacteristic of similar environments in the native range.
We found that this high fitness life history was gained at the expense of increased demand for the limiting resource of water in the invasion. We simulated drought conditions for half of the plants in our experiment, and quantified drought tolerance by the number of days to wilting. Invading genotypes were significantly less drought tolerant, and drought tolerance was strongly negatively correlated with plant size (Fig. 2E,F; Supplementary Table 5). Larger plants could mitigate these increased water demands by evolving increased water use efficiency (WUE), and we tested for this possibility using carbon isotope estimates of integrated lifetime WUE. Consistent with increased water demand in larger plants, isotopic discrimination estimates indicated that there has been no evolutionary change in WUE (native: mean Δ = 25.4 +/− 0.40 SD; invader: 25.2 +/− 0.55; t-test: P = 0.11). Invaders also wilted significantly earlier than natives after accounting for the correlation with size (Fig. 2F; Supplementary Table 5). A correlation between larger size and reduced drought tolerance observed in several other introduced plants5,7,8 suggests that reduced resource limitation relative to native populations may be a common advantage for invaders.
If invading populations occupy benign or unique climates relative to the native range (e.g. higher water availability than native populations), climatic differences could enhance opportunities for the evolution of increased resource use, and/or complicate our interpretation of relationships between traits and the environment29. We tested for this possibility by comparing the climatic niche occupied by YST established across the western United States to climates occupied throughout its native range. In general, available climate space in the two regions is similar (Fig. 1D,E), and invading populations occupy climatic niche space that is included within the range of YST in Europe (test for rejection of niche similarity: P = 0.30). There has been a significant shift in the distribution of YST across climatic space (test for rejection of niche equivalency: P = 0.02), and the invading populations studied here occupy some of the warmest, most seasonally dry habitats available – a relatively marginal condition for the species in its native range (Fig. 1D,E). Populations invading the inter-mountain western United States (eastern WA, OR, ID not studied here; Fig. 1B) occupy a wetter and more seasonal climate (upper high-density region in Fig. 1E), and may be experiencing different selective pressures than populations invading California. In general, the presence of YST invasions in climates already occupied in its native range suggests that novel/missing biotic interactions are more likely than novel climates to change natural selection on introduced genotypes, consistent with an emerging picture of conservatism in the fundamental niche of many invaders30.
An alternative explanation for the life history evolution that we observe in YST could be an adaptive acceleration of growth and reproduction to avoid summer drought. Though possible, we found that biomass both accumulated faster and remained higher in the invaders throughout life (Fig S1), indicating that either increased resource exploitation (as we argue here) or re-allocation of resources must underlie gains in biomass. The loss of natural enemies during long-distance dispersal has often been hypothesized to facilitate invasiveness by allowing the re-allocation of resources away from defense functions19. YST in California has lost a variety of its seed predators, herbivores, and pathogens from the native range, though seven insect and fungal biocontrol agents have been introduced and have not controlled YST21, and negative microbial interactions persist in the invasions23. Instead, previous demonstrations of the poor invading ability of YST against now-rare functionally similar native species, together with our results, suggest that a vacated niche has relaxed selection imposed by competitors, facilitating the evolution of increased reproduction and invasiveness, potentially at the expense of a decrease in competitive ability. A recent study of competitive interactions in this system suggests that invading YST genotypes have evolved to be less impacted by functionally dissimilar grass species31, consistent with the avoidance of competition and increased exploitation of a vacant niche.
In the face of declines in native biodiversity, the widespread opportunity for non-native species to fill vacated niches has led some biologists to suggest that introductions might be beneficial for recipient communities, as lost functional roles of natives species are replaced by newcomers17. We show for the first time that a plant has evolved to increase its resource use where competition in its niche is low, and by doing so has achieved higher fitness and the ability to spread more aggressively. Our results caution that native biodiversity loss may make ecosystems more vulnerable to the evolution of new invaders with adverse ecosystem and economic impacts, as introduced species evolve in response to altered communities.
Methods
Glasshouse experiment
Seeds were collected in 2008 from 10 maternal plants separated by >1m along a transect at each site. Germination of pappus-bearing seeds on moist potting soil was recorded daily. Two-week-old seedlings were transplanted to 410ml Deepots (Steuwe & Sons, Tangent, OR) and randomly assigned to control or drought treatment ([1 plant × 10 families]/population/treatment).
Size
The length and width of the longest leaf and leaf counts were recorded at 3.5 and 5 weeks. One plant per population was destructively sampled from the control treatment at 5.5 weeks, washed and dried at 60°C for root and shoot biomass. Root and shoot biomass were strongly correlated across all plants (Pearson’s r = 0.94, P < 0.0001), and a linear size index [leaf number * (maximum leaf length*maximum leaf width)1/2] strongly predicted total biomass (ANCOVA on ln transformed variables, with fixed effect of observer: r2adj = 0.82, F(1,38) = 90.3, P < 0.0001). We tested for regional (native vs. invading) and population differences in size using restricted maximum likelihood (REML) analyses of covariance (ANCOVA), with fixed effects of region, population nested within region, observer, individual plant age (days since germination, the continuous variable), and the interaction of region and plant age, as well as a random effect of block. Least Squares Means (LSMs) were extracted for individual populations and correlated with climatic variables for those sites (Supplementary Table 4). Size indices were ln transformed to improve normality. For all analyses, highly non-significant effects (P > 0.1) were removed from the model. Likelihood ratio tests (df = 1) determined significance of random effects by comparing the full model with a reduced model lacking the random effect. Analyses were performed in JMP 11 (SAS Institute, Cary, NC).
Reproduction
Reproductive control plants were maintained through senescence and date of first flower was recorded daily. Post-reproduction, the total number of flowering heads (capitula) were counted and aboveground biomass (including flowers) was dried and weighed. A logistic regression was used to test for regional differences in the tendency to flower. For plants that flowered, REML analyses were used to model the age at first flower, the number of capitula produced, and total biomass, with fixed effects of region, population nested within region, and harvest date, and a random effect of block. LSMs were extracted for individual populations from these analyses and correlated with latitude using an ANCOVA with a fixed effect of region. A fixed effect of age at reproduction was examined as a covariate in the model of number of capitula produced.
Water use
In the drought treatment, watering ceased at 5 weeks, and plants were monitored for wilting daily. A REML analysis of variance (ANOVA) was used to model the number of days to wilt with fixed effects of region and population nested within region, and a random effect of block. A fixed effect of size index at 5 weeks (ln transformed) and its interaction with region were added to the model to test for a relationship between size and drought tolerance.
Water use efficiency was assessed by analyzing carbon isotope composition of leaf tissues at 6 weeks of age. Tissue was collected from one plant per population, dried, and homogenized. Isotope ratios were quantified by the University of Arizona Geosciences Stable Isotope Facility and converted to discrimination values (Δ)32.
Climatic niche
CliMond33 variables at 18.5 × 18.5 km resolution were used to describe the climatic niche, encompassing occurrences in the native region of Eurasia (9.5-49.5°E and 35-64°N) and the western United States (‘WUS’: 114-124.6°W and 32.5-49°N). Occurrence records were compiled during March 2014 from the Global Biodiversity Information Facility (www.gbif.org), Oregon WeedMapper (www.oregon.gov/oda/plant/weeds/weedmapper/), CalFlora (www.calflora.org/), published literature22,23, and unpublished data (M. Cristafaro, E. Coombs, M. Schwarzlaender, O. Sert, and the authors). For niche modeling, we removed occurrence records that were duplicated within grid cells, and thinned records to reduce bias in densely sampled regions34,35. The final dataset included 185 records from Eurasia and 372 from the WUS. Climatic variables were extracted for localities using ArcGIS 10.2 (ESRI, Redlands, CA). Many variables were strongly correlated with one another and were reduced to seven representative variables (Supplementary Table 6).
Climatic niches in Eurasia and the WUS were compared using scripts provided by O. Broennimann29. Principal components analysis summarized environmental gradients across the entire dataset using R/ade4. Climatic space was gridded and a kernel density function used to determine the density of occurrences in each cell. Niche overlap and similarity were quantified using the D metric36. For niche equivalency, a null distribution was obtained by randomly assigning all occurrences to two pools and calculating D, with 100 repetitions. For niche similarity, a null distribution was obtained by randomly shifting the observed density of occurrences in one range and calculating the overlap with the observed niche in the other range (100 repetitions).
Supplementary Material
Acknowledgements
We thank A. Guggisberg and M.S. Barker for assistance with seed collections; B.M. Anderson, S. Lin and K. Nurkowski for help with greenhouse measurements; D. Kaplan for assistance with greenhouse logistics; O. Broennimann for sharing scripts and advice for climate analyses; and B.M. Anderson and J. Braasch for helpful comments on drafts of this manuscript. This work was supported by funding from a Natural Sciences and Engineering Research Council of Canada grant #353026 to L.H.R., from University of Arizona startup funds to K.M.D, and from a Postdoctoral Excellence in Research and Teaching (National Institutes of Health program K12 GM000708) fellowship to B.S.B.
Footnotes
Author contributions
K.M.D. and L.H.R. designed the research. K.A. and S.M.S. contributed seed collections; K.M.D., F.A.C. and B.S.B performed the research. K.M.D. and B.S.B. analyzed the data and created the figures. K.M.D. wrote the paper.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Vellend M, et al. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proc. Natl. Acad. Sci. USA. 2013;110:19456–19459. doi: 10.1073/pnas.1312779110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daehler CC. Darwin’s naturalization hypothesis revisited. Am. Nat. 2001;158:324–330. doi: 10.1086/321316. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy TA, et al. Biodiversity as a barrier to ecological invasion. Nature. 2002;417:636–638. doi: 10.1038/nature00776. [DOI] [PubMed] [Google Scholar]
- 4.Bossdorf O, et al. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia. 2005;144:1–11. doi: 10.1007/s00442-005-0070-z. [DOI] [PubMed] [Google Scholar]
- 5.Mayrose M, Kane NC, Mayrose I, Dlugosch KM, Rieseberg LH. Increased growth in sunflower correlates with reduced defences and altered gene expression in response to biotic and abiotic stress. Mol. Ecol. 2011;20:4683–4694. doi: 10.1111/j.1365-294X.2011.05301.x. [DOI] [PubMed] [Google Scholar]
- 6.Dlugosch KM, Parker IM. Invading populations of an ornamental shrub show rapid life history evolution despite genetic bottlenecks. Ecol. Lett. 2008;11:701–709. doi: 10.1111/j.1461-0248.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- 7.Turner KG, Hufbauer RA, Rieseberg LH. Rapid evolution of an invasive weed. New Phytol. 2014;202:309–321. doi: 10.1111/nph.12634. [DOI] [PubMed] [Google Scholar]
- 8.Hodgins KA, Rieseberg L. Genetic differentiation in life-history traits of introduced and native common ragweed (Ambrosia artemisiifolia) populations. J. Evol. Biol. 2011;24:2731–2749. doi: 10.1111/j.1420-9101.2011.02404.x. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal DM, Hufbauer RA. Increased plant size in exotic populations: a common-garden test with 14 invasive species. Ecology. 2007;88:2758–2765. doi: 10.1890/06-2115.1. [DOI] [PubMed] [Google Scholar]
- 10.Hooper DU, Dukes JS. Functional composition controls invasion success in a California serpentine grassland. J. Ecol. 2010;98:764–777. [Google Scholar]
- 11.Hulvey KB, Zavaleta ES. Abundance declines of a native forb have nonlinear impacts on grassland invasion resistance. Ecology. 2012;93:378–388. doi: 10.1890/11-0091.1. [DOI] [PubMed] [Google Scholar]
- 12.Zavaleta ES, Hulvey KB. Realistic species losses disproportionately reduce grassland resistance to biological invaders. Science. 2004;306:1175–1177. doi: 10.1126/science.1102643. [DOI] [PubMed] [Google Scholar]
- 13.Shea K, Chesson P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002;17:170–176. [Google Scholar]
- 14.Levine JM. Species diversity and biological invasions: relating local process to community pattern. Science. 2000;288:852–854. doi: 10.1126/science.288.5467.852. [DOI] [PubMed] [Google Scholar]
- 15.Strauss SY, Webb CO, Salamin N. Exotic taxa less related to native species are more invasive. Proc. Natl. Acad. Sci. USA. 2006;103:5841–5845. doi: 10.1073/pnas.0508073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seabloom EW, Harpole WS, Reichman OJ, Tilman D. Invasion, competitive dominance, and resource use by exotic and native California grassland species. Proc. Natl. Acad. Sci. USA. 2003;100:13384–13389. doi: 10.1073/pnas.1835728100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis MA, et al. Don’t judge species on their origins. Nature. 2011;474:153–154. doi: 10.1038/474153a. [DOI] [PubMed] [Google Scholar]
- 18.Widmer TL, Guermache F, Dolgovskaia MY, Reznik SY. Enhanced growth and seed properties in introduced vs. native populations of yellow starthistle (Centaurea solstitialis) Weed Sci. 2007;55:465–473. [Google Scholar]
- 19.Keane R, Crawley M. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002;17:164–170. [Google Scholar]
- 20.Gerlach JD. How the West was lost: reconstructing the invasion dynamics of yellow starthistle and other plant invaders of western rangelands and natural areas. Calif. Exot. Pest Plant Counc. Symp. Proc. 1997;3:67–72. [Google Scholar]
- 21.DiTomaso JM, Healy E. Weeds of California and other western states. University of California Department of Agriculture and Natural Resources; 2007. [Google Scholar]
- 22.Uygur S, Smith L, Uygur FN, Cristofaro M, Balciunas J. Population densities of yellow starthistle (Centaurea solstitialis) in Turkey. Weed Sci. 2004;52:746–753. [Google Scholar]
- 23.Andonian K, et al. Range-expanding populations of a globally introduced weed experience negative plant-soil feedbacks. PLoS One. 2011;6:e20117. doi: 10.1371/journal.pone.0020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galloway LF, Etterson JR. Transgenerational plasticity is adaptive in the wild. Science. 2007;318:1134–1136. doi: 10.1126/science.1148766. [DOI] [PubMed] [Google Scholar]
- 25.Eriksen RL, Desronvil T, Hierro JL, Kesseli R. Morphological differentiation in a common garden experiment among native and non-native specimens of the invasive weed yellow starthistle (Centaurea solstitialis) Biol. Invasions. 2012;14:1459–1467. [Google Scholar]
- 26.Colautti RI, Maron JL, Barrett SCH. Common garden comparisons of native and introduced plant populations: latitudinal clines can obscure evolutionary inferences. Evol. Appl. 2009;2:187–199. doi: 10.1111/j.1752-4571.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erikson RL, et al. Dispersal pathways and genetic differentiation among worldwide populations of the invasive weed Centaurea solstitialis L. (Asteraceae) PLoS ONE. 2014;9:e114786. doi: 10.1371/journal.pone.0114786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colautti RI, Barrett SCH. Rapid adaptation to climate facilitates range expansion of an invasive plant. Science. 2013;342:364–366. doi: 10.1126/science.1242121. [DOI] [PubMed] [Google Scholar]
- 29.Broennimann O, et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012;21:481–497. [Google Scholar]
- 30.Petitpierre B, et al. Climatic niche shifts are rare among terrestrial plant invaders. Science. 2012;335:1344–1348. doi: 10.1126/science.1215933. [DOI] [PubMed] [Google Scholar]
- 31.Graebner RC, Callaway RM, Montesinos D. Invasive species grows faster, competes better, and shows greater evolution toward increased seed size and growth than exotic non-invasive congeners. Plant Ecol. 2012;213:545–553. [Google Scholar]
- 32.Farquhar G, Ehleringer J, Hubick K. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989;40:503–537. [Google Scholar]
- 33.Kriticos DJ, et al. CliMond: global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol. Evol. 2012;3:53–64. [Google Scholar]
- 34.Warren DL, Glor RE, Turelli M. ENMTools: a toolbox for comparative studies of environmental niche models. Ecography. 2010;33:607–611. [Google Scholar]
- 35.Verbruggen H, et al. Improving transferability of introduced species’ distribution models: new tools to forecast the spread of a highly invasive seaweed. PLoS One. 2013;8:e68337. doi: 10.1371/journal.pone.0068337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warren DL, Glor RE, Turelli M. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution. 2008;62:2868–2883. doi: 10.1111/j.1558-5646.2008.00482.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.