Abstract
Prenatal testosterone (T), but not dihydrotestosterone (DHT), excess disrupts ovarian cyclicity and increases follicular recruitment and persistence. We hypothesized that the disruption in the vascular endothelial growth factor (VEGF) system contributes to the enhancement of follicular recruitment and persistence in prenatal T-treated sheep. The impact of T/DHT treatments from Days 30 to 90 of gestation on VEGFA, VEGFB, and their receptor (VEGFR-1 [FLT1], VEGFR-2 [KDR], and VEGFR-3 [FLT4]) protein expression was examined by immunohistochemistry on Fetal Days 90 and 140, 22 wk, 10 mo (postpubertal), and 21 mo (adult) of age. Arterial morphometry was performed in Fetal Day 140 and postpubertal ovaries. VEGFA and VEGFB expression were found in granulosa cells at all stages of follicular development with increased expression in antral follicles. VEGFA was present in theca interna, while VEGFB was present in theca interna/externa and stromal cells. All three receptors were expressed in the granulosa, theca, and stromal cells during all stages of follicular development. VEGFR-3 increased with follicular differentiation with the highest level seen in the granulosa cells of antral follicles. None of the members of the VEGF family or their receptor expression were altered by age or prenatal T/DHT treatments. At Fetal Day 140, area, wall thickness, and wall area of arteries from the ovarian hilum were larger in prenatal T- and DHT-treated females, suggestive of early androgenic programming of arterial differentiation. This may facilitate increased delivery of endocrine factors and thus indirectly contribute to the development of the multifollicular phenotype.
Keywords: ovary, PCOS, sheep, testosterone, VEGF
INTRODUCTION
Initiation and maintenance of follicular growth are dependent on the establishment of an extensive microvascular network that allows preferential delivery of gonadotropins [1]. A reduction in thecal vascularity is one of the early events during follicular atresia, at least in some species [2]. The vascular endothelial growth factor (VEGF) is a major regulator of ovarian angiogenesis [3–5]. Ovarian VEGF production has been demonstrated in many species [6–9], supportive of its paracrine action on endothelial cells of the growing follicle. The VEGF family consists of VEGFA, VEGFB, VEGFC, and VEGFD [10]. VEGFA, also known as VEGF, is a potent angiogenic factor [11], exists in five isoforms [12, 13] and acts through three tyrosine kinase family receptors, namely FLT1 (fms-related tyrosine kinase 1: VEGFR1), KDR (kinase insert domain receptor: VEGFR2/FLK1), and FLT4 (fms-related tyrosine kinase 4: VEGFR3) [14].
Within the ovarian follicle, the angiogenic process is limited to the theca cell layer, where remarkable changes in vascularization occur with newly formed capillaries penetrating the basal lamina and invading the avascular granulosa cell layer and contributing to corpus luteum formation. However, studies have shown dynamic changes in VEGF and its receptors in granulosa cells throughout follicle development [15–17]. The finding of the granulosa cell as a major site of VEGF synthesis within the follicle [15] coupled with increased expression of VEGF and its receptors in the dominant follicle [17, 18] suggest a nonangiogenic role for VEGF and for the VEGF system in mediating and/or enhancing the effects of gonadotropins in granulosa cells [18].
The VEGF system has been implicated in ovarian disorders with disrupted follicular development, such as polycystic ovary syndrome (PCOS) [19–23]. Women with PCOS have increased levels of circulating VEGF that correlate morphologically with increased vascular flow velocities within the ovarian stromal vasculature [24]. Hyperplastic ovarian stroma with excessive collagen accumulation is also a feature of androgenized female-to-male transsexuals [25]. Because androgens can drive ovarian angiogenesis [26] and have a proliferative effect on vascular endothelial and smooth muscle cells [27], changes in ovarian vessel morphology and/or size driven by the hyperandrogenic status of women with PCOS may contribute to the increased vascular flow velocity. Animal models that display ovarian phenotype similar to that of women with PCOS provide a unique resource to help elucidate the initial trigger responsible for morphological changes in vessel development during early ovarian differentiation.
This study tested the hypothesis that disruption in the VEGF system is a contributing factor in enhancing follicular recruitment and persistence in prenatal testosterone (T)-treated sheep. Furthermore, because VEGFA is an angiogenic factor and follicular vascularity plays a role in follicular atresia [3, 6, 10, 15], we also tested if prenatal T excess disrupts ovarian arterial size. Considering that T is an aromatizable androgen, a subtractive approach of comparing the impact of prenatal T with dihydrotestosterone (DHT), a nonaromatizable androgen, was utilized to distinguish if the impact of prenatal T excess was facilitated via androgenic or estrogenic actions of T.
MATERIALS AND METHODS
Breeding and Prenatal Treatment
Institutional Animal Care and Use Committee of the University of Michigan approved all animal procedures used in this study, which are consistent with the National Research Council's Guide for the Care and Use of Laboratory Animals. Details of prenatal treatments to generate prenatal T- and DHT-treated groups have been described previously [28–30]. Diets of breeder sheep and lamb were discussed in detail in previous publications [28–30].
Prenatal T- and DHT-treated sheep were generated by intramuscular administration of 100 mg of 1.2 mg/kg T propionate (Sigma-Aldrich Corp.) or 100 mg of DHT propionate (Steraloids, Inc.) suspended in cottonseed oil to pregnant ewes twice weekly from Days 30 to 90 of gestation. The doses of T and DHT were chosen to mimic doses used in all our earlier studies [31, 32] to enable relating findings from this study to earlier outcomes. Control breeders were injected with the vehicle for the same duration. Ovaries were collected at five different ages from all three experimental groups: control and prenatal T- and DHT-treated, including Fetal Days 90 and 140, 22 wk of age (prepubertal), 10 mo of age (end of first breeding season), and 21 mo of age (end of second breeding season). Collection of postpubertal ovaries (10- and 21-mo-old) was performed during a presumptive follicular phase after synchronization with prostaglandin F2α, given twice 11 days apart. Sample size of five to eight was used per treatment group and per developmental time point. The limited number of DHT-treated females born precluded inclusion of a 21-mo-old group in the study. Time points used for arterial morphometry were restricted to Fetal Day 140 and 10 mo of age. Details of euthanasia, ovarian collection, and processing have been published previously [33]. Paraffin-embedded sections from one ovary from each animal were used in this study.
Immunohistochemistry
A basic local alignment search tool was used to test the homology (>90% for all antibodies) between the target peptide of each antibody and the corresponding ovine protein. For testing antibody specificity (Table 1), sheep ovarian tissue extracts were separated in SDS-PAGE (15% resolving gel for VEGFA and VEGFB and 10% for FLT1, KDR, and FLT4) as previously described [34, 35]. In brief, proteins were transferred onto nitrocellulose membranes, blocked with 2% nonfat milk in 20 mM Tris and 150 mM NaCl containing 0.05% Tween-20 and incubated overnight at 4°C with the primary antibodies. The membranes were treated with a goat-anti-mouse immunoglobulin G peroxidase antibody for VEGFA and VEGFB or a goat-anti-rabbit immunoglobulin G peroxidase antibody for FLT1, KDR, and FLT4. A chemiluminescent detection kit was used to visualize immunopositive bands (ECL-Plus; GE-Amersham).
TABLE 1.
Antibodies used for detection of VEGFA, VEGFB, FLT1, KDR, and FLT4 by immunohistochemistry (IH) and Western blot (WB).
Streptavidin-biotin immunoperoxidase was the method of choice for immunocytochemistry, as described previously [34, 35]. Briefly, after deparaffinization, antigen retrieval was performed in sodium citrate solution in a pressure cooker (FLT1 and FLT4) or a microwave (VEGFA, VEGFB, and KDR). Endogenous peroxidase was blocked with 3% H2O2 in methanol, and nonspecific binding was blocked with 10% (v/v) normal goat serum. Ovarian sections were incubated with the primary antibody for 18 h at 4°C followed by incubation with a biotinylated secondary antibody. Streptavidin-peroxidase solution was used as the detection system combined with 3,3′-diaminobenzidine. Mayer hematoxylin was used to counterstain the sections. Normalization across series was performed by including serial sections of a nonexperimental set of sheep ovaries with each series.
Image Analysis
For each protein (VEGFA, VEGFB, FLT1, KDR, and FLT4), two sections (the first section selected one-third into the ovary and the second two-thirds into the ovary to avoid reimaging the same follicles) were used. All growing follicles in both sections were analyzed (sample size ranged between 8 and 15 for each follicular class). Follicle classes were distinguished using criteria previously established [36]. Only the first 20 primordial follicles per section that were distinct and showed no overlap with neighboring follicles were studied. Follicles that had any signs of atresia were excluded from the analysis. All the analyzed images covered granulosa cells from antrum to theca. Cortical stromal tissue was analyzed using 10 images of random locations. Details of image analysis have been described earlier [34, 35]. Images were digitized with an Olympus C5060 digital camera mounted on a conventional light microscope (Olympus BH-2; Olympus Co.). The image analysis was performed using the Image Pro-Plus 3.0.1 system (Media Cybernetics) as detailed earlier [34, 35]. The observer was blinded to the treatment groups. Quantitative comparisons across proteins are not possible because immunostaining and image analyses were optimized for each protein.
Ovarian Vessel Morphometry
Arterial ovarian morphometry was carried out in Fetal Day 140 and 10-mo-old ovaries. Sample size for arterial morphometry for C, T, and DHT groups were six, eight, and five, respectively, for Fetal Day 140 ovaries and six, seven, and six, respectively, for 10 mo. Fetal ovaries were fixed in Bouin fixative, embedded in plastic (Technovit 7100; Kulzer & Co GmBH) and were serially sectioned at 20 μm. Fetal Day 140 (plastic sections) and 10-mo-old (paraffin sections) ovarian sections were stained with hematoxylin/eosin. Depending on individual ovarian size, 5–10 ovarian sections, with a minimum of 60 μm apart, were used for morphometric analysis. Within the ovarian hilum, the five largest arteries per section were studied. Morphometric analysis of each arterial ring segment was performed as follows. All sections were captured under bright field illumination (Leica DMR microscope and Diagnostic Instruments Spot RT camera) using the same magnification and camera settings. The arterial wall thickness and area were determined by tracking the inner and outer walls of the media and adventitia layers using a microscope-based micrometer. Over 300 observations were generated per treatment group and age for each of the three measures. All the morphometric measurements were performed by two individuals who were blinded to the treatment groups.
Statistical Analysis
Statistical analysis was run using SPSS software (version 11.0 for Windows; SPSS Inc.) and involved analysis of variance followed by the Duncan multiple range test or Tukey test. To compare ovarian arterial morphology, differences among groups were assessed using a linear mixed model after natural log (base e) transformation. Results are expressed as mean ± SEM. P < 0.05 value was considered significant.
RESULTS
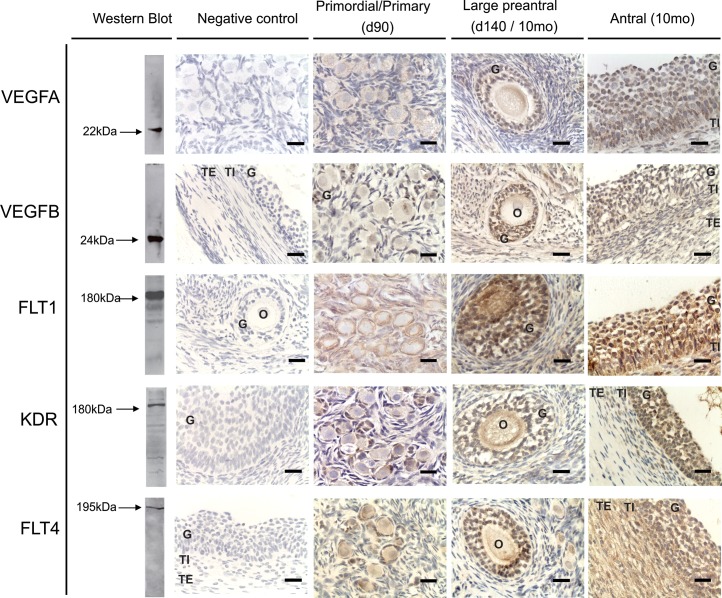
Western blot recognition of VEGFA, VEGFB, and their receptor proteins in ovarian homogenate and their immunohistochemical localization in ovarian sections are shown in Figure 1. Positive bands of appropriate sizes were identified by Western blot analysis for each of the protein studied (Fig. 1, left). The VEGFA, VEGFB, FLT1, KDR, and FLT4 antibodies detected a single band at 22, 24, 180, 180–190, and 195 kDa, respectively. Specific staining was absent in the absence of the primary antibodies (Fig. 1, negative control). Immunostaining of VEGF family members and their receptors in antral follicles of control females are shown in Figure 1 (right panel). All the proteins studied showed nonnuclear localization.
FIG. 1.
Representative images of VEGFA, VEGFB, FLT1, KDR, and FLT4 immunostaining in primordial/primary, preantral, and antral follicles. The first left column represents the results of Western blot analysis conducted in ovarian homogenates (verification of antibody specificity). The next column represents negative controls of immunohistochemistry. VEGFA, vascular endothelial growth factor A; VEGFB, vascular endothelial growth factor B; FLT1, vascular endothelial growth factor receptor 1 (fms-related tyrosine kinase 1); KDR, vascular endothelial growth factor receptor 2 (kinase insert domain receptor); FLT4, vascular endothelial growth factor receptor 3 (fms-related tyrosine kinase 4); G, granulosa cells; TE, theca externa; TI, theca interna; O, oocyte. Bar = 25 μm. Preantral follicles: VEGFA and FLT4: Day 140 (d140); VEGFB, FLT1, and KDR: 10-mo-old (10mo).
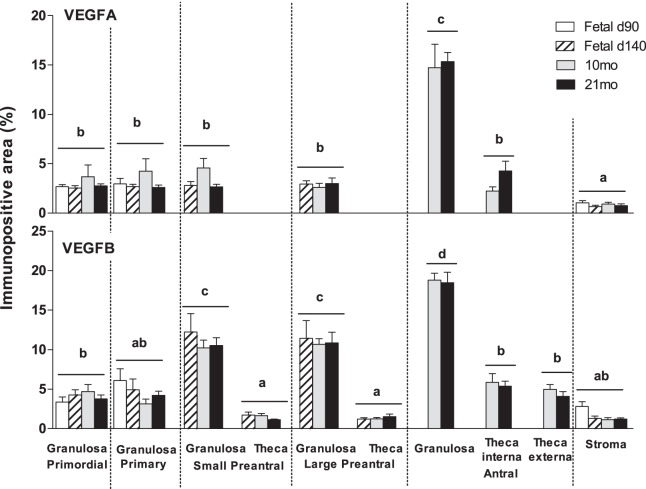
VEGFA expression was found in granulosa cells during all stages of follicular development, as well as in theca interna of antral follicles. A weak expression of VEGFA was found in the stroma. VEGFA levels were similar from primordial through preantral stage of follicular development, increasing in granulosa cells of antral follicles (P < 0.05). In preantral and antral follicles, granulosa cell VEGFB expression was higher compared to expression in the thecal cells (P < 0.05) (Fig. 2). Age-dependent changes in VEGFA expression were not evident.
FIG. 2.
Relative expression of immunopositive area (%) of VEGFA and VEGFB in fetal ovaries on Days 90 (d90) and 140 (d140) as well as in the ovaries of 10- (10mo) and 21-mo-old (21mo) sheep born from control females. Differences among groups were assessed using analysis of variance followed by the Duncan multiple range test. Different letters denote significant differences in the immunopositive area among different follicle types (P < 0.05); no significant differences were found among different days within a follicle type. VEGFA, vascular endothelial growth factor A; VEGFB, vascular endothelial growth factor B.
VEGFB was found in granulosa, theca interna and externa, and stromal cells during all stages of follicular development at all ages. VEGFB expression in the granulosa cells increased progressively from primary to antral follicles (P < 0.05) (Fig. 2). Unlike VEGFA, which was found only in theca interna, VEGFB was expressed in both theca interna and externa. Granulosa cell VEGFB expression was higher relative to expression in the thecal cells (P < 0.05) in preantral and antral follicles (Fig. 2). No age-dependent changes in VEGFB expression was evident in any of the compartments studied.
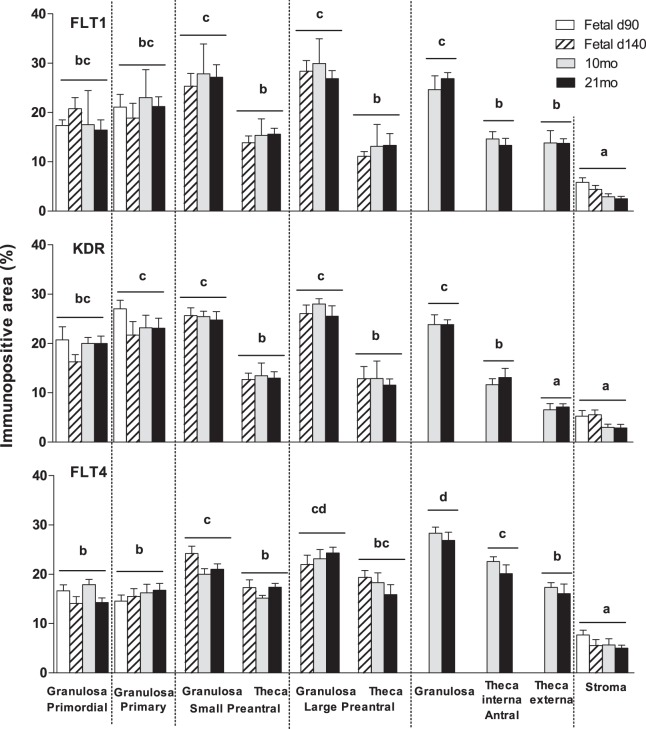
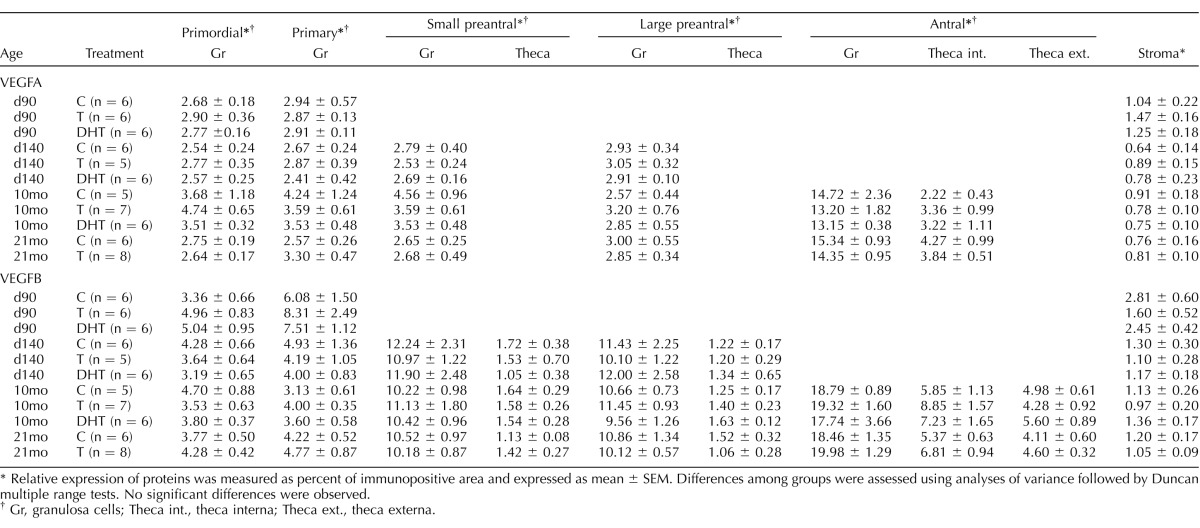
The VEGF receptors (FLT1, KDR, and FLT4) were expressed in the granulosa, theca, and stromal cells during all stages of follicular development. No differences in the levels of FLT1 and KDR expression in granulosa cells were evident across the follicular stages. In contrast, FLT4 increased with follicular differentiation with the highest level seen in the granulosa cells of antral follicles (P < 0.05) (Fig.3). The expression of FLT1 was higher in granulosa compared to thecal cells (P < 0.05) in preantral and antral follicles (Fig. 3). In antral follicles, KDR and FLT4 levels were higher in granulosa cells relative to theca interna, which in turn was higher than levels seen in the theca externa (P < 0.05) (Fig. 3). No differences in level of expression were evident across different ages in any of these proteins. None of the members of VEGF family or their receptors were altered by prenatal T or DHT treatments in granulosa, theca, or stromal cells at any age studied (Tables 2 and 3).
FIG. 3.
Relative expression of immunopositive area (%) of FLT1, KDR, and FLT4 in fetal ovaries on Days 90 (d90) and 140 (d140) as well as in the ovaries of 10- (10mo) and 21-mo-old (21mo) sheep born from control females. Differences among groups were assessed using analysis of variance followed by the Duncan multiple range test. Different letters denote significant differences in the immunopositive area among different follicle types (P < 0.05); no significant differences were found among different days within a follicle type. FLT1, vascular endothelial growth factor receptor 1 (fms-related tyrosine kinase 1); KDR, vascular endothelial growth factor receptor 2 (kinase insert domain receptor); FLT4, vascular endothelial growth factor receptor 3 (fms-related tyrosine kinase 4).
TABLE 2.
Relative expression of VEGFA and VEGFB proteins determined in fetal ovaries on Days 90 (d90) and 140 (d140) as well as in ovaries of 10- (10mo) and 21-mo-old (21mo) sheep born from control (C), testosterone (T)-, and dihydrotestosterone (DHT)-treated mothers.
Relative expression of proteins was measured as percent of immunopositive area and expressed as mean ± SEM. Differences among groups were assessed using analyses of variance followed by Duncan multiple range tests. No significant differences were observed.
Gr, granulosa cells; Theca int., theca interna; Theca ext., theca externa.
TABLE 3.
Relative expression of FLT1, KDR, and FLT4 proteins determined in fetal ovaries on Days 90 (d90) and 140 (d140) as well as in ovaries of 10- (10mo) and 21-mo-old (21mo) sheep born from control (C), testosterone (T)-, and dihydrotestosterone (DHT)-treated mothers.
Relative expression of proteins was measured as percent of immunopositive area and expressed as mean ± SEM. Differences among groups were assessed using analyses of variance followed by Duncan multiple range tests. No significant differences were observed.
Gr, granulosa cells; Theca int., theca interna; Theca ext., theca externa.
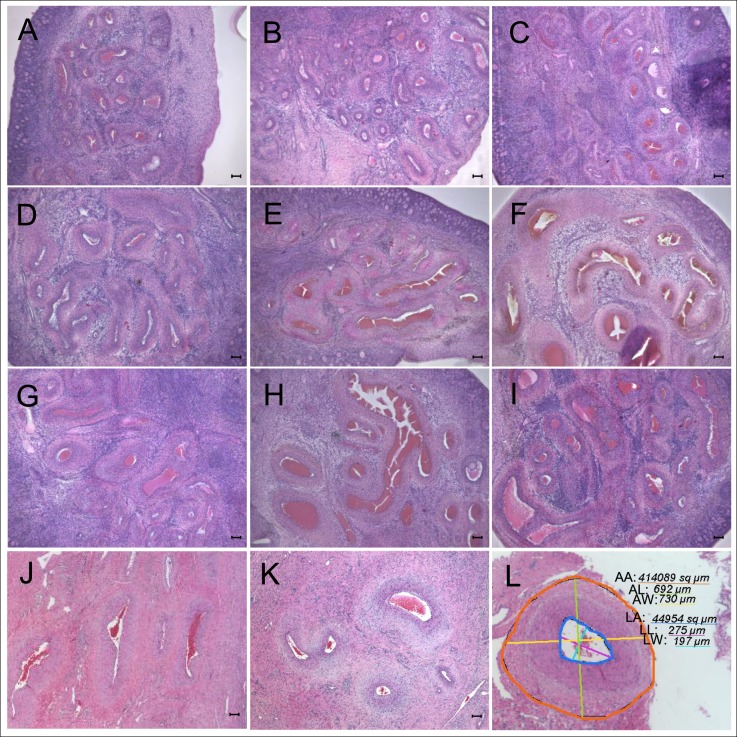
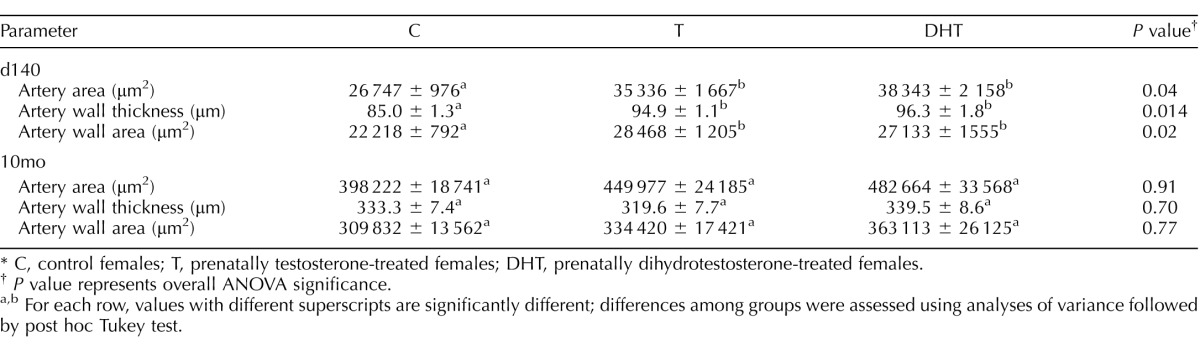
Images with arterial tracing and arterial ovarian morphometry results at Fetal Day 140 and 10-mo-old are summarized in Figure 4 and Table 4. At Day 140, ANOVA revealed significant differences in artery area (P = 0.04), wall thickness (P = 0.014), and wall area (P = 0.02) between groups. All three variables were significantly larger in T- and DHT-treated females compared to controls (Table 4; representative images are shown in Fig. 4, and cumulative area is shown in Fig. 5, left panel). At 10 mo of age, arterial area and wall area were numerically higher in both T- and DHT-treated groups, but were not statistically different from controls (Table 4 and Figs. 4 and 5, right panel).
FIG. 4.
Representative images of the ovarian arteries within the hilum in Fetal Day 140 (d140) and 10-mo-old animals. A–C) Fetal ovaries from untreated mothers (controls). D–F) Fetal ovaries from T-treated mothers. G–I) Fetal ovaries from DHT-treated mothers. Ovarian hilum in control ovaries shows cross sections of ovarian arteries filled with red blood cells (A–C). Ovarian hilum in T- (D–F) and DHT-treated (G–I) groups shows that ovarian arteries in the hilum are larger than in the control group. J–K) The 10-mo-old ovaries from sheep born from control females. L) Arterial tracing of one vessel. Parameters assessed included measurements of arterial area (AA, orange), length (AL, green), and width (AW, yellow), lumen area (LA, dark blue), lumen length (LL, purple), and lumen width (LW. light blue). Numbers in the figure indicate measurement values in μm for diameter or μm2 for area. Each number is underlined by a color line corresponding to the color in the image. Difference between arterial and lumen area provided wall area. Bar = 100 μm. T, testosterone; DHT, dihydrotestosterone.
TABLE 4.
Arterial stromal morphology parameters (mean ± SEM) in fetal ovaries on Day 140 (d140) and in ovaries of 10-mo-old (10mo) sheep.*
C, control females; T, prenatally testosterone-treated females; DHT, prenatally dihydrotestosterone-treated females.
P value represents overall ANOVA significance.
For each row, values with different superscripts are significantly different; differences among groups were assessed using analyses of variance followed by post hoc Tukey test.
FIG. 5.
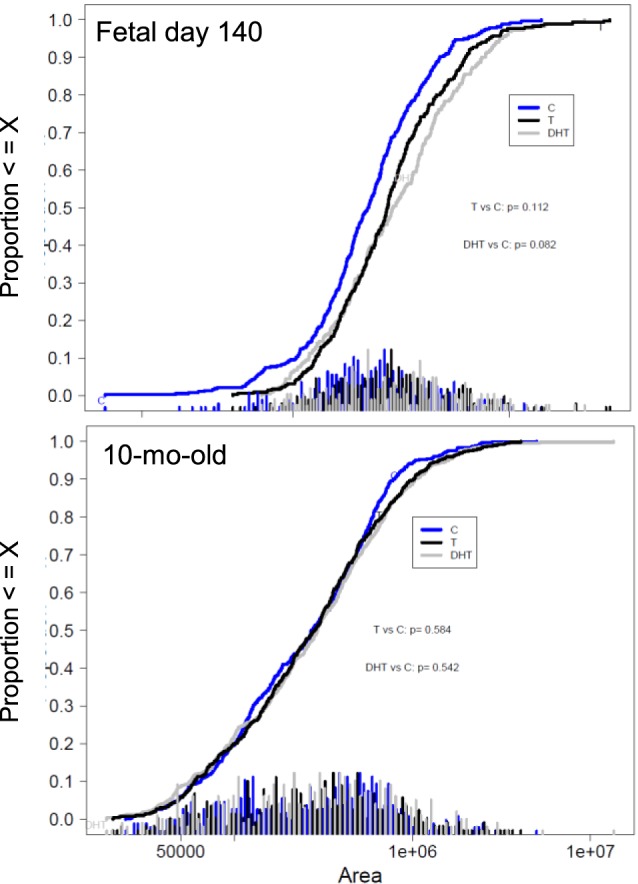
Cumulative area distributions (μm2) of arteries in the hilum of fetal ovaries on Day 140 (left panel) and in the ovaries of 10-mo-old (right panel) sheep born from control (blue), T-treated (black) and DHT-treated (gray) females. T, testosterone; DHT, dihydrotestosterone. Differences among groups were assessed using a linear mixed model after natural log (base e) transformation.
DISCUSSION
The present study demonstrates that 1) VEGFA, VEGFB, and their receptors are expressed in ovine follicles across the reproductive life span, 2) there are distinct differences in the expression and localization patterns of VEGF system across follicular stages and layers, 3) prenatal T or DHT treatment had no effect on the expression of any of the proteins in any of the ovarian components, and 4) prenatal T- and DHT-treatment increased the size of the arteries localized in the medullary region of fetal ovaries but not adult ovaries.
The angiogenic system is involved in the functional changes that occur during follicular development [37]. The finding that VEGFA protein expression was higher in granulosa cells of antral follicles is supportive of granulosa cells being the major site of VEGFA production within the follicle [15]. Findings from this study are consistent with earlier findings of weak VEGFA mRNA expression during early follicular development and enhanced during dominant follicle development in other domestic species [7, 17] and rodents [38]. In primates, VEGFA mRNA expression was found to be absent in primordial, primary, and early secondary follicles, becoming first detectable in theca and granulosa layers of secondary follicles [39], when the follicle starts to develop its own vasculature. In contrast with our findings, Redmer et al. [40] found that VEGFA protein was localized exclusively in the theca layer of ovine preovulatory follicles and that the granulosa layer was devoid of VEGF protein expression. Differences in the expression patterns between studies could be related to sensitivity of the method utilized or specificity of the antibodies toward the various VEGF isoforms. The antibody used in the present study likely recognizes the most common isoform of VEGFA in the ovary, VEGFA165 [41]. Similar to other growth factors [18, 42], VEGFA and VEGFB expression increased in parallel with follicular development. Differential increases in granulosa cell VEGF/VEGF receptor expression during follicle selection [43, 44] may therefore act in combination with FSH to enhance cell proliferation, thus promoting the continuous growth of the dominant follicle.
In contrast to the increasing number of reports suggesting proangiogenic activity of VEGFA in vascular development during folliculogenesis, little is known about the functional role of VEGFB in the ovary. VEGFB expression in granulosa cells increased progressively across follicular stages from primary to antral, with the highest level seen in antral follicles, similar to findings in mares [45]. VEGFB mRNA in human granulosa cells appears to be regulated in an isotype-specific manner, suggesting that VEGFB may play different roles during the vascularization of the human ovarian follicle and corpus luteum [46].
Changes in VEGF receptors were found to be not as dynamic as changes in the ligand, suggesting that regulation for the most part occurs at the ligand rather than the receptor level. Barring FLT4, which increased with follicular differentiation with highest level seen in the granulosa of antral follicles, FLT1 and KDR remained stable across follicular stages in the granulosa, theca, and stromal cell compartments, although expression was higher in the granulosa than theca cell layer of preantral and antral follicles. Functionally, the relative binding capability of VEGFA or VEGFB to FLT4 is unclear, although heterodimerization between FLT1 and KDR, as well as between KDR and FLT4, has been reported and might modulate the activation process [47]. Recent studies in humans indicated that soluble variants of these receptors exist in circulation and are able to bind various VEGF isoforms [48–51]. These variants may bind circulating VEGF and limit the amount of free biological active VEGF available to act at the membrane receptor level [47].
Failure of prenatal T and DHT treatment to alter ovarian VEGFA, VEGFB, and VEGF receptor expression suggests that the VEGF system may not contribute toward the development of the disrupted ovarian phenotype of the prenatal T-treated females or that VEGFA and VEGFB may have redundant functions with other isoforms of VEGF [52]. Alternatively, other angiogenic factors may have contributed to the changes in ovarian stromal vasculature of prenatal T-treated females [53–57].
The increased size of ovarian arteries of the prenatal T-treated females, suggestive of a steroid-mediated programming of angiogenic growth, may facilitate increased delivery of endocrine factors to the differentiating ovary, thus contributing indirectly to the development of multifollicular phenotype. The androgenic mediation of this effect was confirmed by a similar increase in prenatal DHT-treated ovarian arteries. Although very little is known about the angiogenic process and its regulation in fetal ovaries and specifically in sheep fetal ovaries [58], androgens are known to induce proliferation of endothelial cells [27]. While these findings support androgenic mediation of angiogenesis in the differentiating ovary [59], this effect does not appear to be mediated via stimulation of the VEGF system. It needs to be recognized that although DHT was used as a subtractive approach to assess androgenic mediation, the possibility that DHT acts via estrogen receptor 2 upon metabolization into 3-β-diol [60] cannot be eliminated. Similarly, whether the effects of T and DHT are direct or involve other mediators remains to be ascertained. One possibility to consider is that the androgenic effects of T may be secondary to placental compromise [61] and consequent intrauterine growth-retardation [28]. Placental studies carried out with this model show that both prenatal T and DHT induce placental compromise.
From a translational perspective, the increase in size of ovarian vessels of gestational T-treated sheep fetuses points to an ovarian vasculature defect as a potential early compromise contributing to the PCOS-like phenotype seen in these females. In women with PCOS, defects in ovarian stromal vessels are evident, including increased medullar stromal hypervascularity [62], increased vascular flow velocities [24], and increased vascularization index [63]. Paradoxically, significant changes in ovarian stromal vasculature were not evident in 10-mo-old postpubertal sheep ovaries. Whether the vascular defects seen in fetal life will emerge later as animals mature and reproductively age is unclear. Furthermore, whether the increased vascularization observed in prenatal T-treated sheep fetuses play a role in the development of the polycystic follicular trait remains to be elucidated. Yet, our findings of early changes in ovarian stromal vasculature of prenatal T-treated fetal sheep ovaries indicate a potential role for steroids (androgen or estrogen) in early ovarian programming.
ACKNOWLEDGMENT
We are grateful to Mr. Douglas Doop for his help with the generation of the experimental lambs, expert animal care, and facility management; Drs. Mohan Manikkam and Teresa Steckler, Ms. Olga Astapova, Ms. Carol Herkimer, and Mr. James Lee for their assistance with prenatal steroid treatment and help during collection of ovaries; and the staff members of the Laboratory of Cellular Biology (FCV-UNL) for their technical support during the immunohistochemistry.
Footnotes
Current address: Department of Animal Sciences, Michigan State University, East Lansing, MI 48824.
This study was supported by National Institutes of Health grant P01-HD44232 to VP.
REFERENCES
- Zeleznik AJ, Schuler HM, Reichert LE., Jr. Gonadotropin-binding sites in the rhesus monkey ovary: role of the vasculature in the selective distribution of human chorionic gonadotropin to the preovulatory follicle. Endocrinology. 1981;109:356–362. doi: 10.1210/endo-109-2-356. [DOI] [PubMed] [Google Scholar]
- Greenwald GS. Temporal and topographic changes in DNA synthesis after induced follicular atresia. Biol Reprod. 1989;41:175–181. doi: 10.1095/biolreprod41.1.175. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Chen H, Davis-Smyth T, Gerber H-P, Nguyen T-N, Peers D, Chisholm V, Hillan KJ, Schwall RH. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med. 1998;4:336–340. doi: 10.1038/nm0398-336. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Dickson SE, Lunn SF, Wulff C, Morris KD, Carroll VA, Bicknell R. Suppression of luteal angiogenesis in the primate after neutralization of vascular endothelial growth factor. Endocrinology. 2000;141:995–1000. doi: 10.1210/endo.141.3.7369. [DOI] [PubMed] [Google Scholar]
- Wulff C, Wiegand SJ, Saunders PT, Scobie GA, Fraser HM. Angiogenesis during follicular development in the primate and its inhibition by treatment with truncated Flt-1-Fc (vascular endothelial growth factor Trap(A40)) Endocrinology. 2001;142:3244–3254. doi: 10.1210/endo.142.7.8258. [DOI] [PubMed] [Google Scholar]
- Yan Z, Neulen J, Raczek S, Weich HA, Keck C, Grunwald K, Breckwoldt M. Vascular endothelial growth factor (VEGF)/vascular permeability factor (VPF) production by luteinized human granulosa cells in vitro; a paracrine signal in corpus luteum formation. Gynecol Endocrinol. 1998;12:149–153. doi: 10.3109/09513599809015537. [DOI] [PubMed] [Google Scholar]
- Barboni B, Turriani M, Galeati G, Spinaci M, Bacci ML, Forni M, Mattioli M. Vascular endothelial growth factor production in growing pig antral follicles. Biol Reprod. 2000;63:858–864. doi: 10.1095/biolreprod63.3.858. [DOI] [PubMed] [Google Scholar]
- Basini G, Grasselli F, Ponderato N, Bussolati S, Tamanini C. Lipid hydroperoxide and cGMP are not involved in nitric oxide inhibition of steroidogenesis in bovine granulosa cells. Reprod Fertil Dev. 2000;12:289–295. doi: 10.1071/rd00089. [DOI] [PubMed] [Google Scholar]
- Berisha B, Schams D, Kosmann M, Amselgruber W, Einspanier R. Expression and tissue concentration of vascular endothelial growth factor, its receptors, and localization in the bovine corpus luteum during estrous cycle and pregnancy. Biol Reprod. 2000;63:1106–1114. doi: 10.1095/biolreprod63.4.1106. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Endothelial growth factor: a key regulator of physiologic angiogenesis. In: HG Augustin, Iruela-Arispe ML, PAW Rogers, Smithe SK., editors. Vascular Morphogenesis in the Female Reproductive System 1st ed. Boston: Birkhauser; 2001. pp. 149–165. In. (eds) [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Charnock-Jones DS, Sharkey AM, Rajput-Williams J, Burch D, Schofield JP, Fountain SA, Boocock CA, Smith SK. Identification and localization of alternately spliced mRNAs for vascular endothelial growth factor in human uterus and estrogen regulation in endometrial carcinoma cell lines. Biol Reprod. 1993;48:1120–1128. doi: 10.1095/biolreprod48.5.1120. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Wulff C. Angiogenesis in the primate ovary. Reprod Fertil Dev. 2001;13:557–566. doi: 10.1071/rd01055. [DOI] [PubMed] [Google Scholar]
- Grasselli F, Basini G, Bussolati S, Tamanini C. Effects of VEGF and bFGF on proliferation and production of steroids and nitric oxide in porcine granulosa cells. Reprod Domest Anim. 2002;37:362–368. doi: 10.1046/j.1439-0531.2002.00386.x. [DOI] [PubMed] [Google Scholar]
- Greenaway J, Connor K, Pedersen HG, Coomber BL, Lamarre J, Petrik J. Vascular endothelial growth factor and its receptor, Flk-1/KDR, are cytoprotective in the extravascular compartment of the ovarian follicle. Endocrinology. 2004;145:2896–2905. doi: 10.1210/en.2003-1620. [DOI] [PubMed] [Google Scholar]
- Doyle LK, Walker CA, Donadeu FX. VEGF modulates the effects of gonadotropins in granulosa cells. Domest Anim Endocrinol. 2010;38:127–137. doi: 10.1016/j.domaniend.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Kamat BR, Brown LF, Manseau EJ, Senger DR, Dvorak HF. Expression of vascular permeability factor/vascular endothelial growth factor by human granulosa and theca lutein cells. Role in corpus luteum development. Am J Pathol. 1995;146:157–165. [PMC free article] [PubMed] [Google Scholar]
- Stanek MB, Borman SM, Molskness TA, Larson JM, Stouffer RL, Patton PE. Insulin and insulin-like growth factor stimulation of vascular endothelial growth factor production by luteinized granulosa cells: comparison between polycystic ovarian syndrome (PCOS) and non-PCOS women. J Clin Endocrinol Metab. 2007;92:2726–2733. doi: 10.1210/jc.2006-2846. [DOI] [PubMed] [Google Scholar]
- Artini PG, Ruggiero M. Parisen Toldin MR, Monteleone P, Monti M, Cela V, Genazzani AR. Vascular endothelial growth factor and its soluble receptor in patients with polycystic ovary syndrome undergoing IVF. Hum Fertil (Camb) 2009;12:40–44. doi: 10.1080/14647270802621358. [DOI] [PubMed] [Google Scholar]
- Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, Raveendran M, Storey A. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:881–887. doi: 10.1210/jc.2007-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks S. Polycystic ovary syndrome in adolescents. Int J Obesity. 2008;32:1035–1041. doi: 10.1038/ijo.2008.61. [DOI] [PubMed] [Google Scholar]
- Abd El Aal DEM, Mohamed SA, Amine AF, Meki ARMA. Vascular endothelial growth factor and insulin-like growth factor-1 in polycystic ovary syndrome and their relation to ovarian blood flow. Eur J Obstet Gynecol Reprod Biol. 2005;118:219–224. doi: 10.1016/j.ejogrb.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Baba T, Noguchi H, Nagasawa K, Endo T, Kiya T, Saito T. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Hum Reprod. 2013;28:453–461. doi: 10.1093/humrep/des385. [DOI] [PubMed] [Google Scholar]
- Liu D, Iruthayanathan M, Homan LL, Wang Y, Yang L, Wang Y, Dillon JS. Dehydroepiandrosterone stimulates endothelial proliferation and angiogenesis through extracellular signal-regulated kinase 1/2-mediated mechanisms. Endocrinology. 2008;149:889–898. doi: 10.1210/en.2007-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nheu L, Nazareth L, Xu GY, Xiao FY, Luo RZ, Komesaroff P, Ling S. Physiological effects of androgens on human vascular endothelial and smooth muscle cells in culture. Steroids. 2011;76:1590–1596. doi: 10.1016/j.steroids.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007;148:3532–3540. doi: 10.1210/en.2007-0339. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145(2):790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147:1997–2007. doi: 10.1210/en.2005-1338. [DOI] [PubMed] [Google Scholar]
- Wood RI, Kim SJ, Foster DL. Prenatal androgens defeminize activation of GnRH neurons in response to estradiol stimulation. J Neuroendocrinol. 1996;8:617–625. [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Developmental origin of reproductive and metabolic dysfunctions: androgenic versus estrogenic reprogramming. Semin Reprod Med. 2011;29:173–816. doi: 10.1055/s-0031-1275519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep. Biol Reprod. 2009;80:726–736. doi: 10.1095/biolreprod.108.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction. 2009;137:865–877. doi: 10.1530/REP-08-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega HH, Rey F, Velázquez MM, Padmanabhan V. Developmental programming: effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins in sheep. Biol Reprod. 2010;82:1065–1075. doi: 10.1095/biolreprod.109.082719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNatty KP, Juengel JL, Wilson T, Galloway SM, Davis GH, Hudson NL, Moeller CL, Cranfield M, Reader KL, Laitinen MP, Groome NP, Sawyer HR, et al. Oocyte-derived growth factors and ovulation rate in sheep. Reprod Suppl. 2003;61:339–351. [PubMed] [Google Scholar]
- Abramovich D, Rodriguez CA, Hernandez F, Tesone M, Parborell F. Spatiotemporal analysis of the protein expression of angiogenic factors and their related receptors during folliculogenesis in rats with and without hormonal treatment. Reproduction. 2009;137:309–320. doi: 10.1530/REP-08-0130. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Taylor PD, Hillier SG, Fraser HM. Effects of GnRH antagonist treatment on follicular development and angiogenesis in the primate ovary. J Endocrinol. 2004;183:1–17. doi: 10.1677/joe.1.05685. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Doraiswamy V, Bortnem BJ, Fisher K, Jablonka-Shariff A, Grazul-Bilska AT, Reynolds LP. Evidence for a role of capillary pericytes in vascular growth of the developing ovine corpus luteum. Biol Reprod. 2001;65:879–889. doi: 10.1095/biolreprod65.3.879. [DOI] [PubMed] [Google Scholar]
- Tesone M, Stouffer RL, Borman SM, Hennebold JD, Molskness TA. Vascular endothelial growth factor (VEGF) production by the monkey corpus luteum during the menstrual cycle: isoform-selective messenger RNA expression in vivo and hypoxia-regulated protein secretion in vitro. Biol Reprod. 2005;73:927–934. doi: 10.1095/biolreprod.105.039875. [DOI] [PubMed] [Google Scholar]
- Beg MA, Ginther OJ. Follicle selection in cattle and horses: role of intrafollicular factors. Reproduction. 2006;132(3):365–377. doi: 10.1530/rep.1.01233. [DOI] [PubMed] [Google Scholar]
- Yang H, Foxcroft GR, Pettigrew JE, Johnston LJ, Shurson GC, Costa AN, Zak LJ. Impact of dietary lysine intake during lactation on follicular development and oocyte maturation after weaning in promiparous sows. J Anim Sci. 2000;78:993–1000. doi: 10.2527/2000.784993x. [DOI] [PubMed] [Google Scholar]
- Ginther OJ, Gastal EL, Gastal MO, Checura CM, Beg MA. Dose-response study of intrafollicular injection of insulin-like growth factor-I on follicular fluid factors and follicle dominance in mares. Biol Reprod. 2004;70:1063–1069. doi: 10.1095/biolreprod.103.024844. [DOI] [PubMed] [Google Scholar]
- Müller K, Ellenberger C, Schoon H-A. Histomorphological and immunohistochemical study of angiogenesis and angiogenic factors in the ovary of the mare. Res Vet Sci. 2009;87:421–431. doi: 10.1016/j.rvsc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Laitinen M, Ristimäki A, Honkasalo M, Narko K, Paavonen K, Ritvos O. Differential hormonal regulation of vascular endothelial growth factors VEGF, VEGF-B, and VEGF-C messenger ribonucleic acid levels in cultured human granulosa-luteal cells. Endocrinology. 1997;138:4748–4756. doi: 10.1210/endo.138.11.5500. [DOI] [PubMed] [Google Scholar]
- Pietrowski D, Szabo L, Sator M, Just A, Egarter C. Ovarian hyperstimulation syndrome is correlated with a reduction of soluble VEGF receptor protein level and a higher amount of VEGF-A. Hum Reprod. 2012;27:196–199. doi: 10.1093/humrep/der349. [DOI] [PubMed] [Google Scholar]
- Jaroszewicz J, Januszkiewicz M, Flisiak R, Rogalska M, Kalinowska A, Wierzbicka I. Circulating vascular endothelial growth factor and its soluble receptors in patients with liver cirrhosis: possible association with hepatic function impairment. Cytokine. 2008;44:14–17. doi: 10.1016/j.cyto.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Kuemmel S, Thomas A, Landt S, Fuger A, Schmid P, Kriner M, Blohmer JU, Sehouli J, Schaller G, Lichtenegger W, Köninger A, Fuchs I. Circulating vascular endothelial growth factors and their soluble receptors in pre-invasive, invasive and recurrent cervical cancer. Anticancer Res. 2009;29:641–645. [PubMed] [Google Scholar]
- Mouawad R, Spano JP, Comperat E, Capron F, Khayat D. Tumoural expression and circulating level of VEGFR-3 (Flt-4) in metastatic melanoma patients: correlation with clinical parameters and outcome. Eur J Cancer. 2009;45:1407–1414. doi: 10.1016/j.ejca.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Ni XF, Wu CP, Jiang JT. Serum VEGFR-3 and survival of advanced gastric cancer patients treated with FOLFOX. World J Gastroenterol. 2010;16:2163–2169. doi: 10.3748/wjg.v16.i17.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullah SE, Perez-Soler R. Mechanisms of resistance to vascular endothelial growth factor blockade. Cancer. 2012;118:3455–3467. doi: 10.1002/cncr.26540. [DOI] [PubMed] [Google Scholar]
- Robinson RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE. Angiogenesis and vascular function in the ovary. Reproduction. 2009;138:869–881. doi: 10.1530/REP-09-0283. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Hoshino Y, Miyazaki H, Sato E. Angiogenesis and microvasculature in the female reproductive organs: physiological and pathological implications. Curr Pharm Des. 2012;18:303–309. doi: 10.2174/138161212799040367. [DOI] [PubMed] [Google Scholar]
- Berisha B, Sinowatz F, Schams D. Expression and localization of fibroblast growth factor (FGF) family members during the final growth of bovine ovarian follicles. Mol Reprod Dev. 2004;67:162–171. doi: 10.1002/mrd.10386. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Myoken Y, Yabumoto M, Osaki T, Fujita Y, Whitney RG, Kan M, Crabb JW, Sato GH, Kato Y, Takada K, Sato JD. Androgen-dependent expression of fibroblast growth factor-1 in submaxillary gland of mouse. Biochem Biophys Res Commun. 1996;221:795–802. doi: 10.1006/bbrc.1996.0676. [DOI] [PubMed] [Google Scholar]
- Sarić T, Shain SA. Androgen regulation of prostate cancer cell FGF-1, FGF-2, and FGF-8: preferential down-regulation of FGF-2 transcripts. Growth Factors. 1998;16:69–87. doi: 10.3109/08977199809017492. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Caton JS, Arndt W, Burchill K, Thorson C, Borowczyk E, Bilski JJ, Redmer DA, Reynolds LP, Vonnahme KA. Cellular proliferation and vascularization in ovine fetal ovaries: effects of undernutrition and selenium in maternal diet. Reproduction. 2009;137:699–707. doi: 10.1530/REP-08-0375. [DOI] [PubMed] [Google Scholar]
- Lebbe M, Woodruff TK. Involvement of androgens in ovarian health and disease. Mol Hum Reprod. 2013;19:828–837. doi: 10.1093/molehr/gat065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett EM, Astapova O, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programing: impact of testosterone on placental differentiation. Reproduction. 2014;148:199–209. doi: 10.1530/REP-14-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughesdon PE. The deduction of tumor histogenesis, with special reference to teratomas and ovarian tumors. Hum Pathol. 1982;13:1020–1027. doi: 10.1016/s0046-8177(82)80094-4. [DOI] [PubMed] [Google Scholar]
- Alcázar JL, Kudla MJ. Ovarian stromal vessels assessed by spatiotemporal image correlation-high definition flow in women with polycystic ovary syndrome: a case-control study. Ultrasound Obstet Gynecol. 2012;40:470–475. doi: 10.1002/uog.11187. [DOI] [PubMed] [Google Scholar]