Abstract
Oocyte maturation and cumulus cell expansion depend on luteinizing hormone (LH)-mediated upregulation of membrane-bound epidermal growth factor (EGF)-like ligands, including amphiregulin, epiregulin, and betacellulin. These ligands then transactivate the EGF receptor (EGFR) after release by matrix metalloproteinases (MMPs). However, direct measurement of released EGF-like ligands or MMPs from granulosa cells has not been formally evaluated, nor has direct identification of responsible MMPs. Here we address these issues by analyzing LH-induced steroidogenesis, which is also MMP and EGFR dependent, in freshly isolated mouse primary granulosa cells. We demonstrate a correlation between amphiregulin and epiregulin mRNA induction and steroid production in LH-treated granulosa cells as well as in ovaries of human chorionic gonadotropin-treated mice. In contrast, LH does not alter Mmp1, Mmp2, Mmp3, Mmp8, Mmp9, or Adam17 mRNA expression. We demonstrate that, in primary mouse granulosa cells, LH triggers release of soluble amphiregulin that correlates with steroid production, both of which are blocked by MMP2/9 inhibition, confirming that MMP2/9 likely regulates LH-induced amphiregulin release and downstream processes. Notably, LH does not alter secretion of MMP2/9 from primary granulosa cells, nor does it modulate MMP activity. These findings indicate that, in the ovary, LH dictates EGFR-mediated processes not by regulating MMPs, but instead by increasing EGF-like ligand availability. In contrast, LH stimulation of primary mouse Leydig cells does not induce EGF-like ligand expression or require MMP2/9 for steroidogenesis, confirming marked differences in LH receptor-induced processes in the testes. Our results suggest that MMP inhibition may be a means of attenuating excess ovarian steroid production in diseases like polycystic ovary syndrome.
Keywords: amphiregulin, granulosa cells, matrix metalloproteinase, ovary, steroid hormones/steroid hormone receptors, steroidogenesis, testis
INTRODUCTION
At the time of the luteinizing hormone (LH) surge, multiple processes are initiated to prepare for the eventual release of the oocyte (ovulation). These processes include cumulus cell expansion, oocyte maturation (meiotic reentry), and steroidogenesis, all of which coincide and involve the cumulus granulosa cells. However, the finding that the LH receptor is undetectable in cumulus cells of the ovarian follicle at the time of ovulation means that the observed effects of LH on these cells are indirect. It is now known that the paracrine mediators of the ovulatory LH signal are amphiregulin (AREG), epiregulin (EREG), and betacellulin (BTC), which are rapidly and transiently induced by LH in mural granulosa and possibly theca cells [1–3]. All three are epidermal growth factor (EGF)-like ligands that, similar to EGF, signal through the EGF receptor (EGFR). Unlike EGF, however, these EGFR ligands are synthesized as integral membrane precursors. Therefore, in order for these ligands to communicate with the EGFR on the cumulus cells they must be processed by proteases to allow their release from the membrane. In fact, several studies have shown that inhibitors for the matrix metalloproteinase (MMP) class of enzymes effectively dampen LH-mediated events [3–7], with some evidence suggesting that the specific enzyme ADAM17/TACE might be the dominant regulator, at least for LH-induced cumulus cell expansion and subsequent oocyte maturation [8, 9]. Moreover, studies using granulosa cell-specific Erk-depleted mice [10] as well as Areg−/−/Egfrwa2/wa2 double-mutant mice [11] demonstrate that the EGFR is an essential signaling component in ovulation. Finally, the use of Areg−/− and Ereg−/− mice [11] in addition to primate studies [12, 13] has provided great insight into the role and significance of the EGF-like ligands, specifically during cumulus cell expansion and oocyte maturation.
Though steroid production is also initiated by the LH surge, the involvement of these specific EGF-like ligands in LH-induced steroidogenesis has yet to be examined. Importantly, however, several observations implicate the EGF-like ligands as a potential liaison between mural and cumulus granulosa cells during LH-induced steroidogenesis. These observations include that 1) EGF is capable of stimulating steroid production in the LHR negative cumulus cells [5, 14], 2) LH transactivates the EGFR [4], and 3) broad-spectrum MMP inhibitors as well as partial MMP2/MMP9-specific inhibitors interfere with steroid production following an LH stimulus [4, 5]. Interestingly, LH-induced steroidogenesis in the male gonad also involves EGFR transactivation, with some evidence to suggest that LH-mediated EGFR transactivation may similarly require the release of EGF-like ligands [15]. In contrast, other studies show that LH-induced steroidogenesis in Leydig cell lines, as well as in primary Leydig cells, is not affected by global MMP inhibition, suggesting that EGF-like ligands are therefore not required in this tissue [4, 16]. To date, analysis of LH-induced EGF-like ligands in primary Leydig cells has not been examined.
Considering these findings, in combination with the established involvement of the EGFR and EGF-like ligands in cumulus cell expansion and oocyte maturation, it is possible that amphiregulin, epiregulin, and betacellulin may also mediate steroidogenesis that is initiated by LH in the ovary, but not in the testes. Therefore, we wanted to determine whether similar players were regulating LH-induced steroid production in these tissues. Here we analyzed LH-induced ovarian steroidogenesis in a simple primary granulosa cell culture system that allows us to examine EGF-like ligand expression and release, as well as MMP expression, release, and activity. Studies in these primary cells, and in follicle cultures and in vivo studies, show that LH induces amphiregulin and epiregulin expression, as well as amphiregulin release, in ovarian granulosa cells but not testicular Leydig cells, thus confirming a fundamental difference in LH signaling in these two cell types. Although LH does not alter the expression, activity, or release of MMPs in granulosa cells, specific blockade of MMP2 and MMP9 using a fifth-generation MMP2/9 inhibitor attenuates the release of amphiregulin and subsequent LH-induced steroid production. Together, these data suggest that LH-induced production of amphiregulin and epiregulin, followed by their activation by a stable cadre of MMP2 and MMP9, is the critical regulator of steroidogenesis in primary mouse granulosa cells, but not Leydig cells.
MATERIALS AND METHODS
Ethics Statement
Mouse studies were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the University Committee on Animal Resources at the University of Rochester.
Steroid Production in Preovulatory Follicles
Follicle isolation from equine chorionic gonadotropin (eCG; Sigma)-treated mice was carried out as previously described [4]. Follicles were then cultured in M16 medium (Millipore) in the presence of 0.05 μg/ml of LH (Sigma) for 2 h. Follicles were pretreated with either 20 μM AG1478 (Calbiochem) or 250 μM doxycycline (Sigma) for 30 min prior to and during the 2-h LH stimulation. Media was collected for radioimmunoassay (RIA) analysis as described below.
Steroid Production in Primary Mouse Granulosa Cells
Eight- to-nine-week-old female C57BL/6 mice were primed with 5 U eCG and ovaries were collected 40–44 h later. Mice of this age were chosen because they still respond nicely to eCG and provide us with increased numbers of granulosa cells relative to prepubertal female mice. A fine needle was used to puncture the ovaries to release granulosa cells into serum-free Dulbecco modified Eagle medium: nutrient mixture F-12 (DMEM-F12; Gibco), which was then filtered through a 40-micron mesh filter to remove any large pieces of tissue or oocytes before plating. An average of 1.5–2 ovaries (approximately 750 000–1 000 000 cells) per well of a 12-well plate was used. Cells were plated in serum-free DMEM-F12 and immediately stimulated with 0.05 μg/ml LH for 4 h. The inhibitors AG1478 (5 μM; Calbiochem), MMP2/9 V Inhibitor (20 μM; Calbiochem), and doxycycline (75 μM; Sigma) were added 30 min prior to LH stimulation. At the end of the experiment, contents of each well were spun down, media was collected off for RIA, and pelleted cells were lysed for RNA (E.Z.N.A. kit; Omega) or protein (RIPA; Santa Cruz).
Steroid Production in Primary Mouse Leydig Cells
Leydig cell isolation was carried out as previously described with some modifications [16]. Briefly, testes were removed from male mice and decapsulated before being placed in a 0.25 mg/ml collagenase (Sigma)/DMEM-F12 solution that was warmed to 37°C. Testes were incubated at room temperature for 20 min with constant, gentle shaking. Following incubation, the solution was filtered twice through a 70-micron mesh strainer. The Leydig cells were then collected by centrifugation at 200 × g for 5 min. Immunostaining for 3beta-hydroxysteroid dehydrogenase (using an antibody from Mario Ascoli, University of Iowa) confirmed that Leydig cells were about 60% pure. Cells were plated in serum-free DMEM-F12 and immediately stimulated with 0.05 μg/ml LH for 30 min. The inhibitors doxycycline (75 μM) or MMP2/9 V (20 μM) were added 30 min prior to LH stimulation for 30 min. At the end of the experiment, contents of each well were spun down, medium was collected off for RIA, and pelleted cells were lysed for RNA (E.Z.N.A. kit).
Steroid Production In Vivo
Four-week-old C57BL/6 female mice were primed with 1 U eCG. Approximately 40 h later, mice were treated with PBS (mock) or 1 U human chorionic gonadotropin (hCG) and serum and ovaries were collected 2 h later. For animals that received doxycycline treatment, the drug was administered at a dose of 50 mg/kg at the time of eCG, the day after, at the time of hCG treatment, and 4 h into hCG treatment. This dose was chosen based on previous investigation of dose response and doxycycline serum levels in mice [17] and timing of injections was based on the doxycycline metabolism in mice [18]. Serum and ovaries were collected 8 h following hCG. Serum was analyzed by RIA to determine steroid content, and ovaries were processed for RNA (E.Z.N.A. kit) or protein (RIPA).
Real-Time PCR
RNA was isolated from primary mouse granulosa cells (E.Z.N.A. kit) according to the provided instruction manual. Levels of amphiregulin (Areg), epiregulin (Ereg), betacellulin (Btc), heparin-bound EGF (Hb-egf), Mmp1, Mmp2, Mmp3, Mmp8, Mmp9, Adam17, Timp1, Timp2, and Gapdh mRNA expression were analyzed by the ΔΔCt method using inventoried Taqman gene expression assay primers for mouse (Mm00437583_m1, Mm00514794_m1, Mm00432137_m1, Mm00439306_m1, Mm00473485_m1, mm00439498_m1, Mm00440295_m1, Mm00439509_m1, mm00442991_m1, Mm00456428_m1, mm00441818_m1, mm00441825_m1, and Mm03302249_m1) on an ABI StepOne Plus real-time PCR machine. Normalization was to Gapdh.
Gelatin Zymography
Lysates from granulosa cells or whole ovary were quantified using BCA assay (Pierce) and prepared under nonreducing, nondenaturing conditions. Protein from lysates or concentrated medium was separated on a 10% gel containing 1 mg/ml gelatin (Sigma). Purified human MMP-2 (Millipore) and MMP-9 (Millipore) were used as positive controls. After running, the gel was incubated for 1 h on a shaker at room temperature in 2.5% Triton X-100 renaturing buffer. Next, the gel was equilibrated in 1× developing buffer (500 mM Tris-HCL pH 7.8, 2 M NaCl, 50 mM CaCl2, 0.2% Brij 35, 10×) for 30 min on a shaker at room temperature. Then, fresh developing buffer was added before the gel was placed at 37°C overnight to develop. The next day, the gel was washed three times, 5 min each, in doubly distilled water, then stained with brilliant blue R250 for 30 min. Finally, if necessary, the gel was destained until clear bands were visible. Gel was preserved using gel-drying frames (Sigma).
ELISA
Mouse amphiregulin ELISA kit was purchased from Sigma. Culture medium from primary mouse granulosa cell experiments was concentrated using 3K Amicon centrifugal filters (Millipore) before use. CHAPS detergent (Acros) was added to the media at a final concentration of 0.25% 10 min prior to media collection to prevent aggregation and to stabilize the ligand in solution. The addition of CHAPS for these reasons is well known with respect to WNT ligands [19, 20]. Assay was performed according to the protocol provided with the kit.
Steroid Assays
Progesterone concentrations in culture medium or serum and testosterone concentrations in culture medium were measured using an RIA kit (MP Biomedicals). Steroid concentration values were determined as described in the RIA kit manual.
Fluorogenic Enzyme Activity Assays
Medium from granulosa cells treated with or without LH was collected and concentrated while the cells were lysed in 25 mM Tris buffer (pH 7.4) with 1% Triton X plus 1 μM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, 3 μg/ml leupeptin, and 3 μM aprotinin. The lysate or concentrated medium was then used to determine ADAM17 activity using the fluorogenic peptide substrate III (ES003; R&D Systems) or MMP2/9 activity using fluorogenic peptide substrate IX (ES010; R&D Systems). The sample and peptide were incubated in 25 mM Tris buffer (pH 8) in a black-walled 96-well plate for 1 h at 37°C. To determine if MMP2/9 V inhibitor blocks ADAM17 activity, the lysate and inhibitor were preincubated in 25 mM Tris buffer (pH 8) for 1 h at 37°C before addition of peptide III substrate and incubation for an additional hour. To demonstrate MMP2 and MMP9 inhibition by MMP2/9 V inhibitor, 10 ng of purified MMP2 or MMP9 (Millipore) was preincubated with the MMP2/9V inhibitor in 25 mM Tris buffer (pH 8) for 1 h at 37°C before addition of peptide IX substrate and incubation for an additional hour. Following incubation, the plate was read with a spectrofluorophotometer using an excitation wavelength of 320 nm and an emission wavelength of 405 nm to determine fluorescence intensity, which corresponds to enzymatic activity.
RESULTS
Amphiregulin and Epiregulin mRNAs Are Upregulated Following LH Treatment in Primary Mouse Granulosa Cells
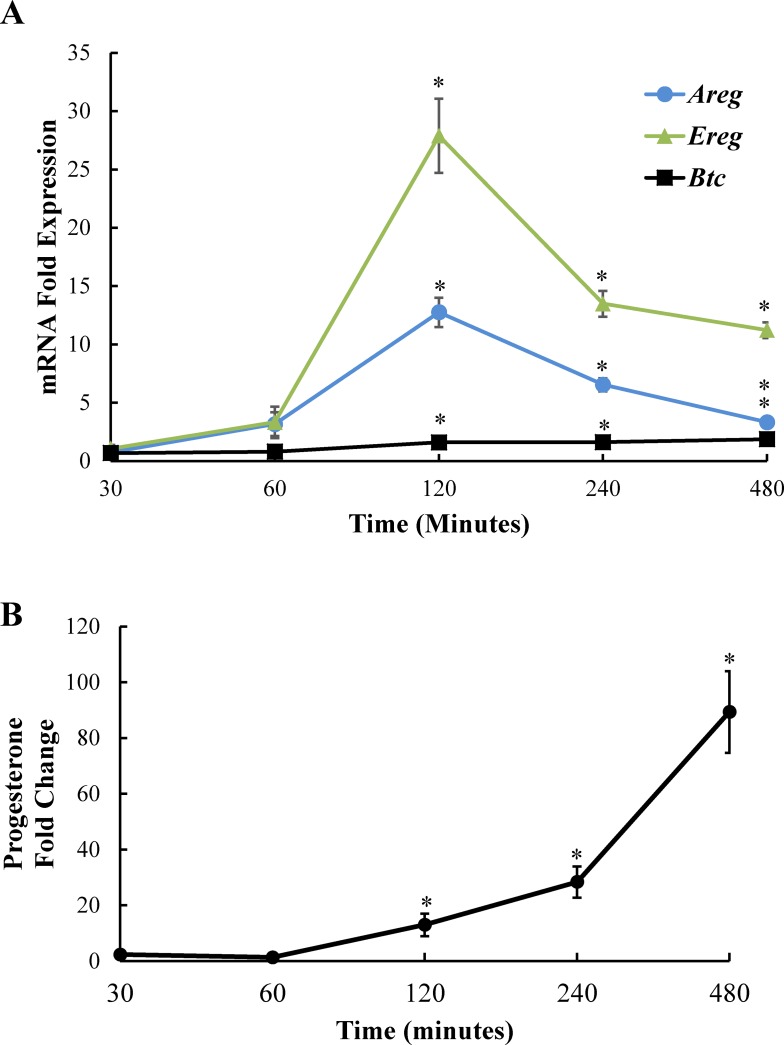
EGF-like ligands such as amphiregulin, epiregulin, and betacellulin are important mediators of events initiated by the LH surge, particularly cumulus cell expansion and oocyte maturation. We wanted to determine if these ligands were also relevant to LH-induced steroidogenesis, which coincides with cumulus cell expansion and oocyte maturation. We also wanted to take advantage of our endpoint of steroidogenesis, which does not require the tertiary structure of cumulus-oocyte complexes or whole follicles, to develop a simple primary mural/cumulus granulosa cell culture system for our studies. Using freshly isolated primary mouse granulosa cells derived from eCG-treated female mice, we examined amphiregulin (Areg), epiregulin (Ereg), and betacellulin (Btc) mRNA expression at various time points following LH exposure with quantitative RT-PCR. All three EGF-like ligands were expressed at low (almost undetectable) levels in the absence of LH. However, we observed a small rise in amphiregulin and epiregulin mRNA levels after 1 h of LH stimulation, with a robust increase in amphiregulin and epiregulin mRNA expression after 2 h of LH stimulation (Fig. 1A). Amphiregulin and epiregulin mRNA expression began to decline at 4 h of LH stimulation, but remained significantly upregulated. Betacellulin mRNA was also induced by LH at 2–4 h but to a much lesser extent; therefore, subsequent experiments focused only on amphiregulin and epiregulin. Notably, the progesterone production by the same freshly isolated primary granulosa cells increased after 2 h with LH, just when the EGF-like ligand mRNAs were also first significantly detectable, and continued to increase over 8 h (Fig. 1B).
FIG. 1.
LH induces EGF-like ligand expression and progesterone production in primary mouse granulosa cells. Primary mouse granulosa cells were cultured with or without LH for 30, 60, 120, 240, or 480 min. Cells were collected for RNA to determine mRNA levels of amphiregulin, epiregulin, and betacellulin by real-time PCR (A) and media was collected to determine progesterone levels (B) at each time point. Results represented as fold induction over unstimulated control at each time point. Each bar represents the average ± SEM (n = 3). All studies were performed at least twice with nearly identical results. *P < 0.05 relative to unstimulated control using Student two-tailed t-test.
EGFR Signaling Is Required During LH-Induced Steroidogenesis
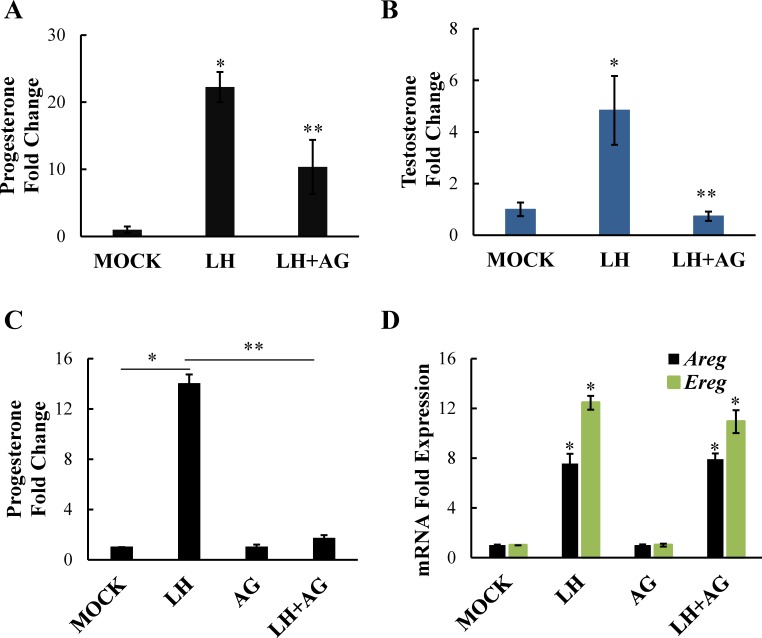
Because amphiregulin and epiregulin signal through the EGFR, we next wanted to confirm the involvement of this receptor. Using the EGFR inhibitor AG1478, we investigated the role of the EGFR signaling using two different systems. First, we used freshly isolated preovulatory follicles from eCG-treated mice and found that AG1478 diminished both LH-induced progesterone (Fig. 2A) and testosterone (Fig. 2B) production, confirming that EGFR signaling is necessary for LH-induced steroidogenesis in both granulosa (progesterone-producing) and theca (androgen-producing) cells. Similarly, the EGFR inhibitor effectively blocked LH-induced progesterone production in freshly isolated primary mural/cumulus granulosa cell cultures (Fig. 2C). As expected, LH minimally promoted testosterone production in the primary granulosa cell cultures because of the lack of significant CYP17-expressing theca cells (not shown). Finally, despite blocking LH-induced progesterone production, AG1478 had no effect on the LH-induced upregulation of amphiregulin and epiregulin mRNA in primary granulosa cells (Fig. 2D), confirming that the EGFR is acting downstream of amphiregulin and epiregulin production. Importantly, previous studies have demonstrated that, following their removal from the oocyte, cumulus granulosa cells show a loss of EGFR expression that is evident by mRNA expression at 2 h and becomes significant by mRNA and protein expression at 20 h [21]. In consideration of this observation, for this and subsequent studies, granulosa cells were stimulated immediately following isolation and were harvested at 2–4 h after isolation, when EGFR expression would not yet be significantly reduced in the absence of oocytes.
FIG. 2.
EGFR signaling is necessary for LH-induced steroid production in the ovary. Whole follicles isolated from mice were stimulated with LH or LH plus AG1478 (20 μM). Progesterone (A) as well as testosterone (B) levels in the medium were measured by RIA. Primary mouse granulosa cells were also treated with LH or LH plus AG1478 (5 μM) and progesterone (C) levels in the medium were measured by RIA and amphiregulin (Areg), or epiregulin (Ereg) mRNA levels (D) were measured by real-time PCR. Data are represented as fold induction over MOCK or inhibitor alone (AG). Each bar represents the average ± SEM (n = 3). Identical results were seen in two experiments. *P < 0.05 relative to MOCK or **P < 0.05 relative to LH using Student two-tailed (B–D) or one-tailed (A) t-test.
Activation of the LH Receptor Does Not Alter MMP Expression
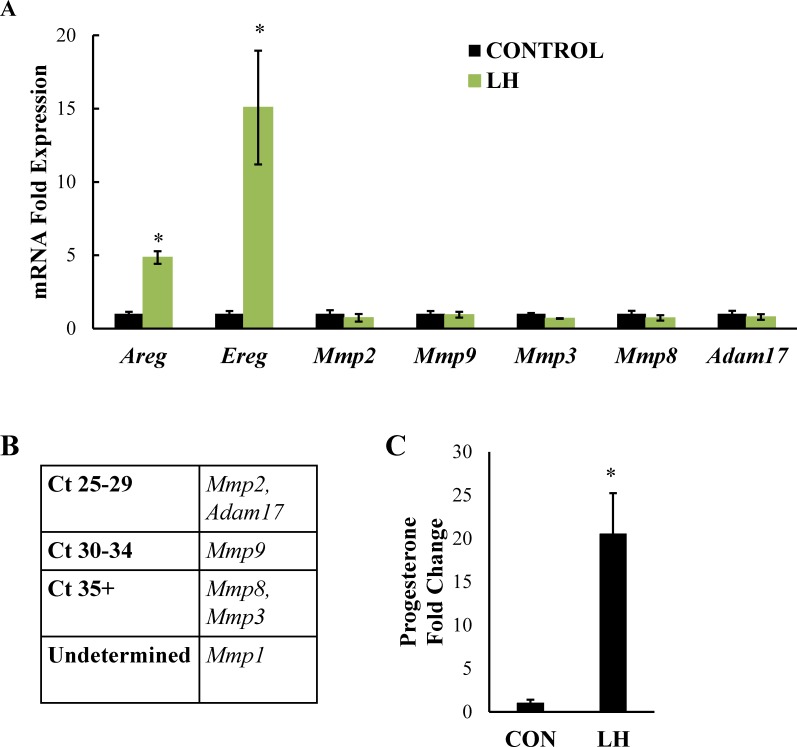
Having established that expression of amphiregulin and epiregulin, but not betacellulin, are significantly upregulated by LH in our system of freshly isolated mouse granulosa cells, and that the EGFR is required for LH-initiated steroid production, we shifted our focus to the MMPs. As mentioned, these enzymes are presumed to cleave amphiregulin and epiregulin at the cell surface to allow subsequent activation of the EGFR. In fact, previous work using ovarian follicles demonstrated that the broad-spectrum MMP inhibitor galardin blocks LH-induced steroidogenesis [4], as well as cumulus cell expansion and oocyte maturation [5, 22]. These observations implicate MMPs as important mediators of LH-induced ovarian processes. MMPs are largely regulated at the transcriptional level, and their expression can be influenced by a wide range of factors including various stimuli, cis-regulatory elements, and changes in cell shape [23]. To determine which MMPs are upregulated during LH-induced steroidogenesis in vitro, we treated freshly isolated mouse granulosa cells from eCG-stimulated mice with LH for 4 h, a time point at which steroidogenesis is abundant and expression of the EGFR ligands are significantly elevated, and looked at mRNA expression of potential galardin targets Mmp1, Mmp2, Mmp3, Mmp8, Mmp9, and Adam17. All values were compared to one another and to Gapdh mRNA expression in the same sample. In primary granulosa cells, Mmp1 mRNA expression was too low to be determined (undetermined), Mmp8 and Mmp3 mRNAs were nearly undetectable (Ct values in the mid-30s), and Mmp9 mRNA expression was detectable but extremely low (Ct values in the low 30s). In contrast, Mmp2 and Adam17 mRNA expression were easily detectable in both control and LH-treated conditions (Fig. 3, A and B). Importantly, LH had no effect on the expression of any of these Mmp mRNAs. As a control, amphiregulin and epiregulin mRNA levels were measured (Fig. 3A), as was progesterone secretion in the media (Fig. 3C). All rose in response to LH, demonstrating that the primary granulosa cells used for these studies were responding appropriately to the LH stimulus.
FIG. 3.
LH treatment in primary mouse granulosa cells does not alter Mmp mRNA expression. Primary mouse granulosa cells were treated with LH for 4 h and mRNA levels of amphiregulin, epiregulin, Mmp1, Mmp2, Mmp3, Mmp8, Mmp9, and Adam17 were measured by real-time PCR (A). Absolute Ct values are also reported (B). Progesterone levels in the medium were measured by RIA (C). Data are represented as fold induction over unstimulated control (CON). Each bar represents the average ± SEM (n = 3). Average of three separate experiments. *P < 0.05 relative to control using Student two-tailed t-test.
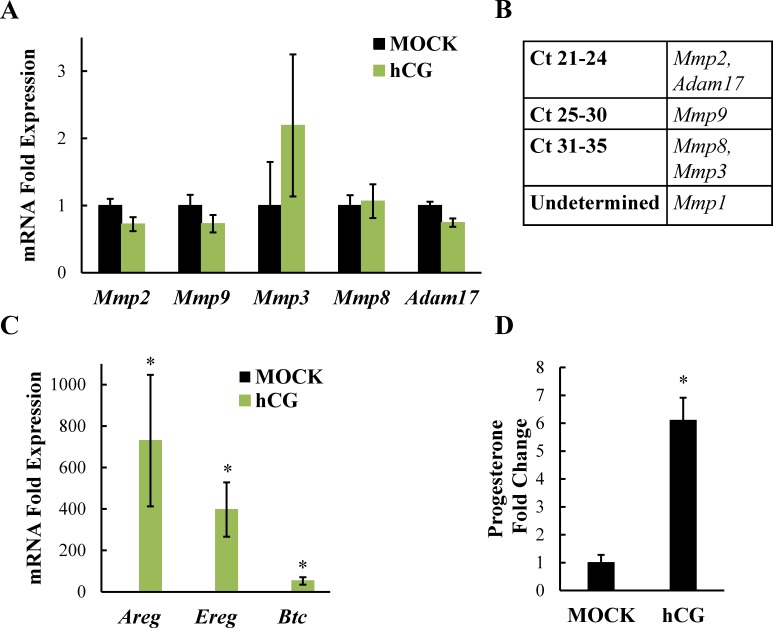
Additionally, we looked at the mRNA expression of these same MMPs in whole ovaries collected from female mice treated with hCG in vivo. The expression pattern of the MMPs was identical to that in granulosa cells (Fig. 4, A and B). Furthermore, similar to LH stimulation in vitro, hCG had no significant effect on Mmp mRNA expression in vivo. As before, amphiregulin and epiregulin mRNA levels in the ovaries (Fig. 4C), as well as progesterone levels in the serum (Fig. 4D), were measured to confirm that the hCG was appropriately stimulating the ovaries in vivo. Together, these findings suggest that, although MMPs are important for LH-induced steroidogenesis in granulosa cells, unlike amphiregulin and epiregulin, LH is not promoting increases in Mmp mRNA expression.
FIG. 4.
In vivo hCG treatment does not alter Mmp mRNA expression. eCG-primed female mice were stimulated intraperitoneally with PBS (MOCK) or hCG for 2 h and mRNA levels of Mmp1, Mmp2, Mmp3, Mmp8, Mmp9, and Adam17 (A) or amphiregulin and epiregulin (C) were measured by real-time PCR analysis and normalized to Gapdh prior to fold induction calculations. Absolute Ct values are also reported (B). Serum progesterone levels were measured by RIA (D). Data are represented as fold induction over MOCK. For (C) MOCK bars are present and normalized to 1. Each bar represents the average ± SEM (n = 9 per treatment). *P < 0.05 relative to MOCK using Student two-tailed t-test.
Activation of the LH Receptor Does Not Alter Mmp2, Mmp9, or Adam17 Expression, Secretion, or Activity
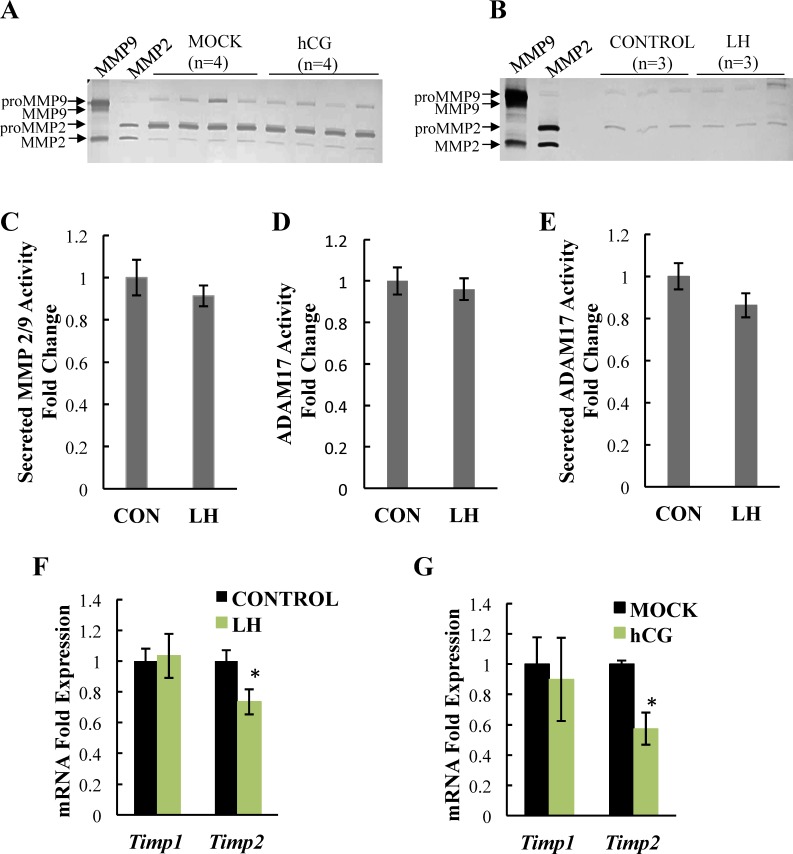
Although LH had no influence on MMPs at the RNA level, MMPs can also be regulated at the protein level through several means, including cleavage of the MMP proenzyme, release of the MMP from the cell, and suppression of endogenous tissue inhibitors of metalloproteinases (TIMPs). We therefore examined the expression and activity of MMPs, focusing on the most abundant enzymes, MMP2, MMP9, and ADAM17. First, using gelatin zymography, we assessed the expression, activity, and secretion of MMP2 and MMP9 using whole ovaries and primary granulosa cells from mice. We found that expression of the pro- and active forms of MMP2 were similar in ovaries from both mock and hCG-treated mice (Fig. 5A). MMP9 expression was lower than that of MMP2, consistent with our mRNA data, but likewise was similar in both mock and hCG-treated animals. In fact, no active MMP9 isoforms were detectable by zymography, consistent with the low overall MMP9 levels. In addition, no differences were found in the levels of secreted MMP9 and MMP2 in medium from primary mouse granulosa cells treated with LH compared to granulosa cells cultured without LH (control, Fig. 5B), indicating that MMP secretion by primary granulosa cells is not altered by LH stimulation. To further address the activity of secreted MMP2 and MMP9, we used fluorogenic peptide IX substrate, as zymography was not sensitive enough to detect active isoforms in the medium. This protease-sensitive optical probe has a target sequence for a variety of MMPs, including MMP2/9, and produces a fluorescent signal when cleaved by the active enzyme. Using this technique, there was 10 times more activity in the medium from untreated primary mouse granulosa cells relative to background. However, there was no difference in MMP activity when comparing media from untreated (control) to LH-treated primary mouse granulosa cells (Fig. 5C). Because gelatin zymography cannot assess ADAM17 activity, we alternatively used fluorogenic peptide substrate III, which uses the same principle as fluorogenic peptide substrate IX but instead has a specific target sequence for ADAM17. Relative to background, there was about 1.5- and 4-fold more ADAM17 activity in untreated granulosa cell lysates (Fig. 5D) and medium (Fig. 5E), respectively; however, we saw no differences in activity between untreated (control) and LH-treated primary mouse granulosa cells. This result again confirms that overall MMP activity, although present, is not increased by LH stimulation.
FIG. 5.
LH does not alter MMP2, MMP9, and ADAM17 expression, activity, or secretion. Levels of proMMP9 (first band), MMP9 (second band), proMMP 2 (third band), and MMP2 (fourth band) were compared in ovaries from hCG- and MOCK (PBS)-treated mice in vivo (A) or in medium collected from LH treated and untreated (CONTROL) primary mouse granulosa cells (B) using gelatin zymography. MMP activity in the concentrated medium from LH-treated and untreated control (CON) granulosa cells was measured using fluorogenic peptide substrate IX (C) and ADAM17 activity was measured in granulosa cell lysates (D) or concentrated medium (E) using fluorogenic peptide substrate III. Timp1 and Timp2 mRNA levels were measured in LH-stimulated primary mouse granulosa cells (F, n = 9) and in ovaries from hCG-stimulated mice in vivo (G) using real-time PCR analysis. Data are represented as fold induction over control (CON) or MOCK. Each bar represents the average ± SEM. Zymography was performed at least twice with identical results. Quantification of zymograms (not shown) showed no significant differences between CON versus LH or MOCK versus hCG. *P < 0.05 relative to CON or MOCK using Student two-tailed t-test.
Finally, we assessed the expression of TIMP1 and TIMP2, which are the endogenous inhibitors of MMP9 and MMP2, respectively. In both primary granulosa cells (Fig. 5F) and ovaries (Fig. 5G) from mice we observed no change in the mRNA level of Timp1 following LH or hCG but noted a modest though statistically significant decrease in Timp2 mRNA levels.
Inhibition of MMP2 and MMP9 Activity Prevents the Processing of Amphiregulin and Blocks LH-Induced Steroidogenesis
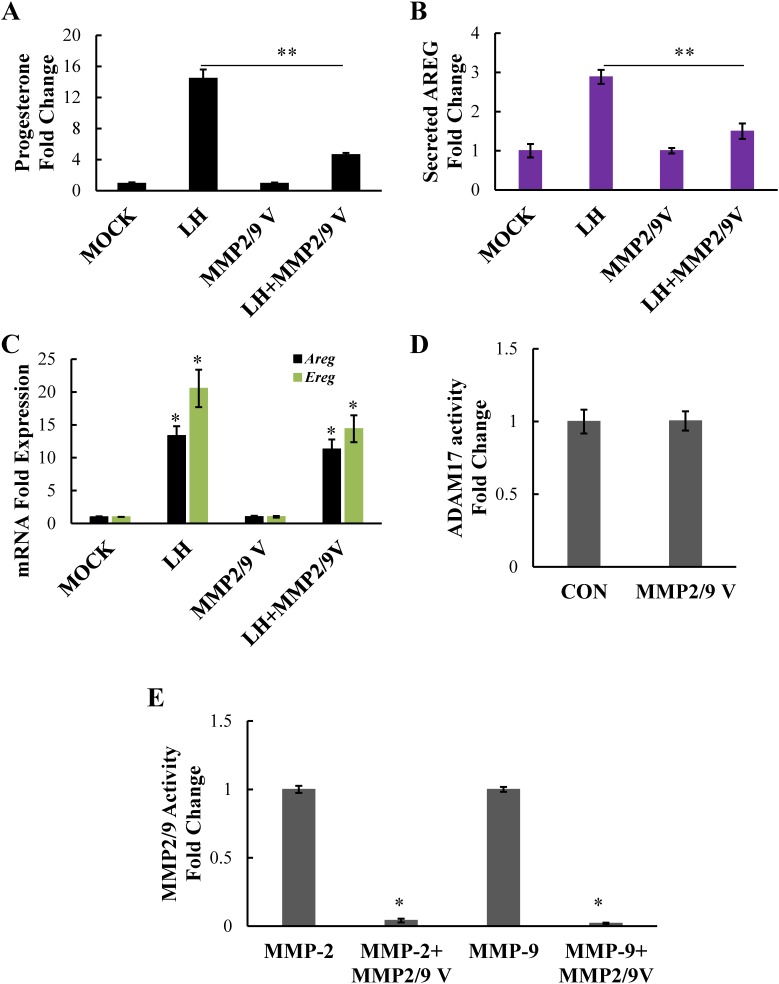
Because MMP expression and activity appear to be unaffected by LH stimulation, but amphiregulin and epiregulin mRNA expression is markedly upregulated by LH stimulation, we postulated that substrate availability may be the primary promoter of EGFR transactivation and subsequent steroidogenesis in response to LH. Therefore, we next focused on determining which MMPs are regulating cleavage of these substrates. Although earlier studies from our laboratory showed that an MMP2/9 inhibitor could block steroid production as effectively as the broad-spectrum MMP inhibitor galardin, the specificity of the MMP2/9 inhibitor used in this study was not clear; thus, the potential off-target effects of the inhibitor on ADAM17 were not ruled out [4]. Therefore, here we used a fifth-generation inhibitor specific for MMP2 and MMP9, MMP2/9 V inhibitor, which offers significantly better specificity than the first-generation inhibitor (MMP2/9 I) used in earlier studies. Notably, this inhibitor effectively blunted steroid production in LH-treated primary mouse granulosa cells (Fig. 6A), suggesting that MMPs 2 and 9 are the critical regulators of LH-induced steroidogenesis. To address whether or not blocking MMP2 and MMP9 activity results in a loss of soluble amphiregulin, we performed an ELISA assay using supernatant from granulosa cells treated with or without LH in the presence of the inhibitor. Indeed, LH induced amphiregulin release from primary granulosa cells, whereas the MMP2/9 V inhibitor suppressed this LH-induced amphiregulin release (Fig. 6B). Of note, the inhibitor did not affect the LH-induced upregulation of amphiregulin or epiregulin mRNA (Fig. 6C).
FIG. 6.
The specific MMP2/9 V inhibitor prevents steroid production in primary mouse granulosa cells. Primary mouse granulosa cells were treated with LH or LH plus MMP2/9V inhibitor (20 μM). Progesterone levels (A) in the medium were measured by RIA and amphiregulin levels in concentrated medium were measured by ELISA (B). The mRNA levels of amphiregulin or epiregulin were measured by real-time PCR (C). Data are represented as fold induction over MOCK or inhibitor alone (MMP2/9V). Fluorogenic peptide substrate III was used to determine the effect of the MMP2/9V inhibitor on ADAM17 activity in granulosa cell lysates (D) and fluorogenic peptide substrate IX was used to determine the effect of the same inhibitor on MMP activity using purified MMP2 and MMP9 (E). Data are represented as fold induction over untreated control (CON) (D) or fold induction over MMP2 and MMP9 (E). All experiments were performed at least twice with identical results. Each bar represents the average ± SEM. *P < 0.05 relative to MOCK or MMP2/9V alone; **P < 0.05 relative to LH using Student two-tailed t-test.
To confirm that the MMP2/9 V inhibitor was specific and did not also block ADAM17 activity, we used the ADAM17-sensitive optical probe (fluorogenic peptide substrate III) with granulosa cell samples as described earlier, but in the presence of the MMP2/9 V inhibitor. Although ADAM17 activity was approximately 2-fold over background, we found no decrease in this ADAM17 protease activity when the MMP2/9 V inhibitor was present (Fig. 6D). On the other hand, using the MMP2/9-sensitive optical probe (fluorogenic peptide substrate IX) we saw robust inhibition of purified MMP2 and purified MMP9 protease activity (Fig. 6E), confirming that MMP2 and MMP9 rather than ADAM17 are the primary mediators of LH-induced amphiregulin release, EGFR transactivation, and subsequent steroidogenesis, in primary mouse granulosa cell cultures.
Doxycycline Prevents the Processing of Amphiregulin and Blocks LH-Induced Steroidogenesis
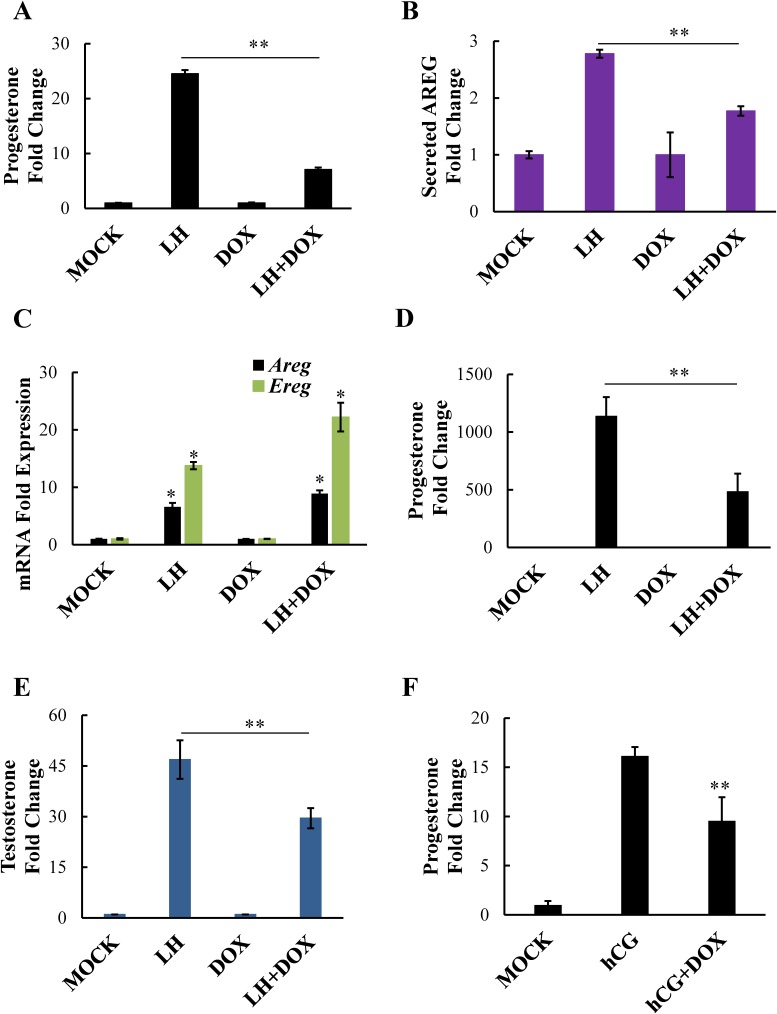
We have previously shown that doxycycline effectively blocks LH-induced progesterone production in isolated mouse follicles [4]. Not only is this drug a known inhibitor of MMP2/9, but it is also FDA approved for human use, giving this drug great translational value. Therefore, we wanted to determine whether doxycycline similarly blocks LH-induced steroid production in our primary granulosa cell model. Further, we wanted to determine whether doxycycline blocks LH-induced steroidogenesis by interfering with amphiregulin processing and release, as seen with the MMP2/9 V inhibitor. In fact, doxycycline markedly reduced LH-induced progesterone production in primary granulosa cells (Fig. 7A). Importantly, although it had no effect on LH-induced amphiregulin or epiregulin mRNA expression (Fig. 7C), doxycycline significantly inhibited LH-induced amphiregulin release in primary granulosa cells (Fig. 7B), indicating that, similar to the MMP2/9 V inhibitor, doxycycline is likely blocking LH-induced steroidogenesis by preventing the MMP-mediated release of EGF-like ligands. Notably, we found that doxycycline significantly reduced both LH-induced progesterone (Fig. 7D) and testosterone (Fig. 7E) production in freshly isolated mouse ovarian follicles, indicating that MMP2 and MMP9 are likely regulating EGFR transactivation and steroidogenesis in both granulosa (where progesterone production would occur) and theca cells (because testosterone is presumably derived from the theca cells). Finally, hCG-stimulated mice treated with doxycycline showed almost a 50% reduction in serum progesterone levels (Fig. 7F), confirming that doxycycline can reduce LH/hCG-induced steroid production in vivo.
FIG. 7.
Doxycycline prevents steroid production through the inhibition of amphiregulin processing. Primary mouse granulosa cells were treated with LH or LH plus doxycycline (75 μM). Progesterone levels (A) and amphiregulin levels (B) in the medium were measured by RIA and ELISA, respectively. Levels of amphiregulin and epiregulin mRNA (C) were measured in primary mouse granulosa cells using real-time PCR. Whole follicles isolated from mouse ovaries were stimulated with LH or LH plus doxycycline (250 μM) and progesterone (D) and testosterone (E) levels in the medium were measured by RIA. Female mice were treated with doxycycline in vivo and serum progesterone levels (F, n = 11 for each group) were determined by RIA. Data are represented as fold induction over MOCK or inhibitor alone (DOX). Each bar represents the average ± SEM (n = 3). Identical results were seen in two experiments. *P < 0.05 relative to MOCK or DOX alone; **P < 0.05 relative to LH/hCG using Student two-tailed t-test.
LH-Induced Steroidogenesis in the Testes Does Not Require the Same EGF-Like Ligands and MMPs as Needed in the Ovary
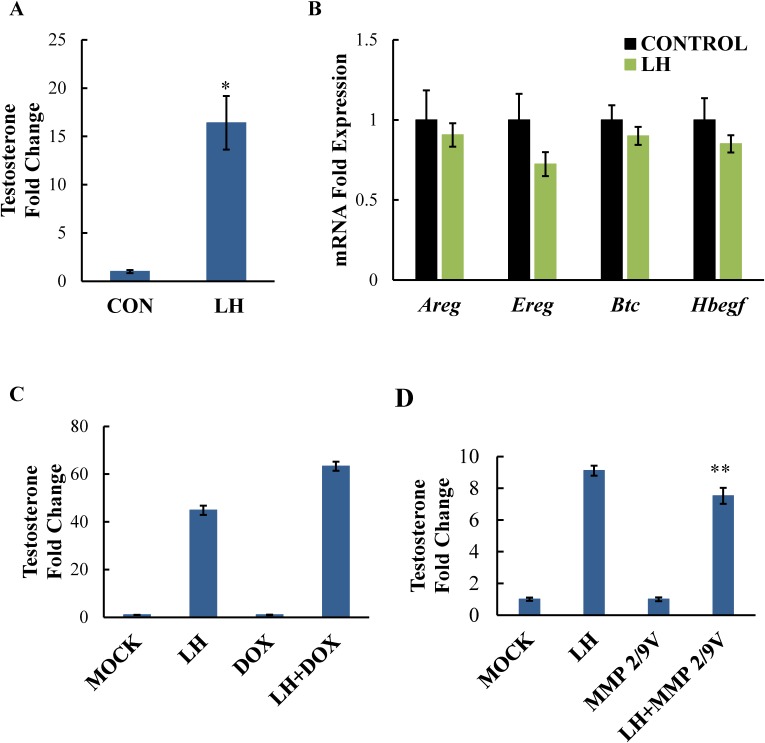
Although several studies have implicated EGFR transactivation in LH-induced steroid production in Leydig cells of the testes, some controversy exists regarding the need for EGF-like ligand release in this process. Therefore, we examined the effects of LH stimulation on the mRNA expression of amphiregulin, epiregulin, and betacellulin as well as heparin-bound EGF to see how they behaved during LH-induced testicular steroidogenesis. Using primary mouse Leydig cells, we looked at the expression of the EGF-like ligands following a 30-min LH treatment, a time point when steroid production is elevated in an EGFR-dependent fashion, as determined by previous studies from our laboratory [16]. We confirmed that, after a 30-min stimulation with LH, significant amounts of testosterone were produced by primary Leydig cells (Fig. 8A). However, no induction of amphiregulin, epiregulin, betacellulin, or heparin-bound EGF mRNA was seen (Fig. 8B), and their expression was extremely low to start. In addition, MMP inhibition using doxycycline (Fig. 8C) or the MMP2/9V inhibitor (Fig. 8D) did not block LH-induced steroidogenesis in primary Leydig cells, in contrast to what was seen in the ovary. Although we observed a subtle decrease in testosterone with the MMP2/9V inhibitor, this drop was not physiologically relevant, especially when compared to the dramatic effects of MMP inhibition of steroid production in ovarian cells. Together, these results indicate that LH signals quite differently in the testes to promote steroidogenesis, with MMP2/9-mediated cleavage of amphiregulin, epiregulin, betacellulin, or heparin-bound EGF being unnecessary.
FIG. 8.
LH induces testosterone production but not EGF-like ligand expression in primary mouse Leydig cells. Primary mouse Leydig cells were stimulated with or without LH for 30 min then medium was collected to measure testosterone levels by RIA (A) and cells were collected to determine mRNA levels of amphiregulin, epiregulin, betacellulin, and heparin bound EGF by real-time PCR (B). Leydig cells were also stimulated with LH in the presence of doxycycline (75 μM, C) or MMP2/9 V inhibitor 9 (20 μM, D) and testosterone levels in the medium were measured as before. Data are represented as fold induction over unstimulated control (CON) in A and B, and fold induction over MOCK or inhibitor alone (DOX, MMP2/9) in C and D. Ct values were in the 30s (relatively low) for amphiregulin, epiregulin, betacellulin, and heparin-bound EGF before and after 30-min stimulation with LH. Each bar represents the average ± SEM. Identical results were seen in two experiments. *P < 0.05 relative to unstimulated control using Student two-tailed t-test. **P < 0.05 relative to LH stimulation using Student two-tailed t-test.
DISCUSSION
The involvement of EGFR transactivation in ovarian LH-mediated events, including cumulus cell expansion, oocyte maturation, and steroidogenesis, has been established; however, a role for the EGF-like ligands amphiregulin, epiregulin, and betacellulin has been confirmed only in cumulus cell expansion and oocyte maturation. Here, using freshly isolated primary granulosa cell cultures, as well as intact mouse ovarian follicles, we demonstrate that these same EGF-like ligands are likely mediating LH-induced steroidogenesis. We find that steroid production following LH stimulation parallels the expression of amphiregulin and epiregulin over time (Fig. 1). Though the upregulation of these ligands in response to LH is well documented in the ovary, the correlation of their expression to steroid production is not. Importantly, the expression pattern we observed is in agreement with previous reports that also observe maximum levels between 1 and 3 h, with amphiregulin being more transient than epiregulin [1, 3, 24, 25]. The finding that betacellulin upregulation in vivo at 2 h is small in relation to amphiregulin and epiregulin conforms with earlier findings that this ligand has a delayed expression [1]. We did not see the more robust upregulation of betacellulin in vitro that has been described elsewhere; however, our studies used primary mouse granulosa cells and most of these studies used whole follicles. Interestingly, one study that also used granulosa cells did not report betacellulin as a rapidly LH-induced gene [24], suggesting that there may be differences related to the type of cell/tissue used. Nonetheless, it is clear that amphiregulin and epiregulin expression is consistent across experimental systems. Notably, it is not surprising that steroid production continues to increase even when EGF-like ligand expression starts to decrease around 4 h, because non-EGFR-dependent mechanisms likely become dominant for long-term LH-induced steroidogenesis (i.e., increased StAR expression) and luteinization.
Importantly, by taking advantage of our primary cell culture conditions (as opposed to working with follicles), we, for the first time, could clearly detect LH-induced release of amphiregulin from mouse granulosa cells (Fig. 6B). Furthermore, we show that MMP inhibition prevented this release. These results demonstrate that LH directly promotes amphiregulin release in an MMP-dependent fashion. Notably, inhibition of MMP-mediated amphiregulin release directly corresponds with a significant decrease in steroidogenesis (Fig. 6A), further supporting the concept that amphiregulin, and likely epiregulin, expression and subsequent release are mediating steroid production in granulosa cells.
In addition to promoting EGFR ligand release, LH was also thought to regulate MMP expression or activation. MMP expression has been investigated during the ovarian reproductive cycle, which has been extensively reviewed elsewhere [26]. As part of these studies, several proteases, namely ADAMTS and cathepsin L, have been implicated in ovulation [27, 28]. However, their expression is regulated by progesterone and peaks well after the ovulatory signal, whereas here we investigated proteases that contribute to the initiation of progesterone synthesis. In fact, studies that examine the regulation of MMP expression specifically at a time when LH is inducing EGF-like ligand release and subsequent steroidogenesis are lacking. Furthermore, no direct examination of LH-induced MMP activation in or release from granulosa cells has been performed. Therefore, we looked at MMP expression, release, and activity at 4 h post-LH in vitro, when amphiregulin and epiregulin mRNA expression is highly induced. We also examined ovarian MMP expression and activity in vivo 2 h after hCG treatment. In both systems, there was no change in MMP expression, and most of the MMPs were nearly undetectable except for ADAM17, MMP2, and MMP9 (Figs. 3–5). Furthermore, using both zymography and fluorogenic peptides, we found that LH does not increase the baseline activities of ADAM17, MMP2, or MMP9. We did observe a slight decrease in Timp2 mRNA expression (the endogenous inhibitor of MMP2) after LH stimulation (Fig. 5, F and G), which could indicate an LH-induced increase in MMP2 activity in vivo that would be missed by zymography. However, we did not measure TIMP2 protein levels. Furthermore, a complex relationship exists between TIMP2 and MMP2, as TIMP2 is also known to be involved in the activation of the enzyme [29, 30]. Thus, it is unclear in which direction a decrease in TIMP2 would influence MMP2 activity. Therefore, although we did not observe changes in overall MMP activity using the fluorogenic peptide substrate IX (Fig. 5C), additional studies are needed to clarify whether this small decrease in Timp2 mRNA expression truly influences MMP2 activity. Finally, we found that, in our primary granulosa cell cultures, LH does not induce secretion of MMPs from granulosa cells. Given the lack of effect on MMP expression, activation, or release in response to LH, we propose that substrate availability (primarily amphiregulin and epiregulin) directs the actions of MMPs that are already present in the cell during LH-induced steroidogenesis.
Currently, ADAM17 prevails as the potential sheddase for amphiregulin and epiregulin during LH-mediated events [9]. However, we have previously reported that neither TAPI-0 nor TAPI-1, ADAM17-specific inhibitors, affects LH-induced steroidogenesis in the ovary [4]. Moreover, most of the evidence for ADAM17 as the enzyme cleaving these EGF-like ligands in the context of ovulation used a porcine model and looked specifically at cumulus granulosa cells [8, 9, 31]. Although small studies in rat granulosa cells appeared to show increased ADAM17 activity in response to gonadotropin, we did not see this trend in mouse granulosa cells, and there are no other studies in mouse that demonstrate ADAM17 activity is elevated in response to LH. Also, though previous studies examining specific MMP2/9 inhibition in rat follicle-enclosed oocytes did not appear to affect meiotic reentry, there could be timing or species differences involved [3]. Moreover, very high concentrations of MMP antagonist were selected for this study, bringing into question the potency and efficacy of this inhibitor.
Here we utilized a potent and specific fifth-generation MMP2/9 inhibitor called MMP2/9V and demonstrated that it effectively suppresses LH-induced steroidogenesis in primary granulosa cell cultures (Fig. 6A). Furthermore, as mentioned, this inhibitor significantly attenuates LH-induced release of amphiregulin from cells into the media (Fig. 6B). Using the ADAM17/TACE-specific optical probe (fluorogenic peptide substrate III), we confirmed that MMP2/9 V does not alter overall ADAM17/TACE activity (Fig. 6D). Thus, in primary mouse granulosa cells, and likely in ovarian follicles as well, MMP2/9 rather than ADAM17/TACE appears to be the primary regulator of LH-induced release of amphiregulin, and likely epiregulin. Attempts were made to detect release of epiregulin; however, the sensitivity of the current ELISA kits is not sufficient to detect epiregulin release, even after concentrating the media.
Accordingly, although ADAM17 is known to cleave membrane-bound EGFR ligands, including TNF alpha, amphiregulin, epiregulin, and heparin-bound EGF [32–37], there is also abundant evidence documenting the involvement of MMPs 2 and 9 in ectodomain shedding [38–42]. Furthermore, MMP2/9 has been shown to be involved in gonadotropin-releasing hormone-mediated EGFR transactivation in pituitary gonadotroph cells [43].
Finally, our studies address the specificity of these EGF-like ligands to LH-induced steroidogenesis in the ovary (Fig. 8). The finding that LH-induced steroidogenesis is significantly increased in the absence of amphiregulin, epiregulin, betacellulin, or heparin-bound EGF upregulation in primary mouse Leydig cells indicates that these EGF-like ligands are not mediating this process in the testes. Moreover, both the specific MMP2/9 V inhibitor and doxycycline had little or no effect on testosterone levels following LH in these cells, further arguing against a role for EGF-like ligands, at least for steroid production. Previous studies have suggested that LH-mediated EGFR transactivation in Leydig cells requires the release of EGF-like ligands. Although our findings suggest that the involvement of MMP2/9, as well as the aforementioned EGFR ligands, is unlikely in Leydig cells, they do not completely eliminate their possible involvement at the level of cleavage, or that of other MMPs and EGFR ligands. Nonetheless, these findings support the notion that LH receptor-mediated signaling, including regulation of EGF-like ligands, EGFR transactivation, and the need for MMP2 and MMP9, is markedly different in the ovary and testes.
To summarize, the studies presented here support the involvement of amphiregulin, epiregulin, and MMPs2/9 in LH-induced ovarian steroidogenesis, and, in combination with previous studies, suggest the following sequence of signaling events in this process: 1) LH signaling upregulates the expression of amphiregulin and epiregulin in mural granulosa cells, and possibly the theca cells; 2) MMP2 and MMP9, which are already expressed and active, process the release of these ligands that are then able to bind to the EGFR; and 3) binding of amphiregulin and epiregulin to the EGFR on mural granulosa cells, cumulus cells [5], and likely the theca cells promotes steroidogenesis (Fig. 9). The finding that inhibition of EGFR signaling (e.g., using AG1478 or doxycycline) decreases testosterone production in intact follicles suggests that EGFR transactivation is occurring in mouse theca cells. That said, additional studies should be pursued to specifically confirm the involvement of theca cells in this process, as we did not directly examine EGFR expression in mouse theca cells. Importantly, however, there is sufficient evidence to suggest that theca cells express EGFR and respond to EGFR ligands in other animal systems, including chicken, hamster, pig, and human [44–48].
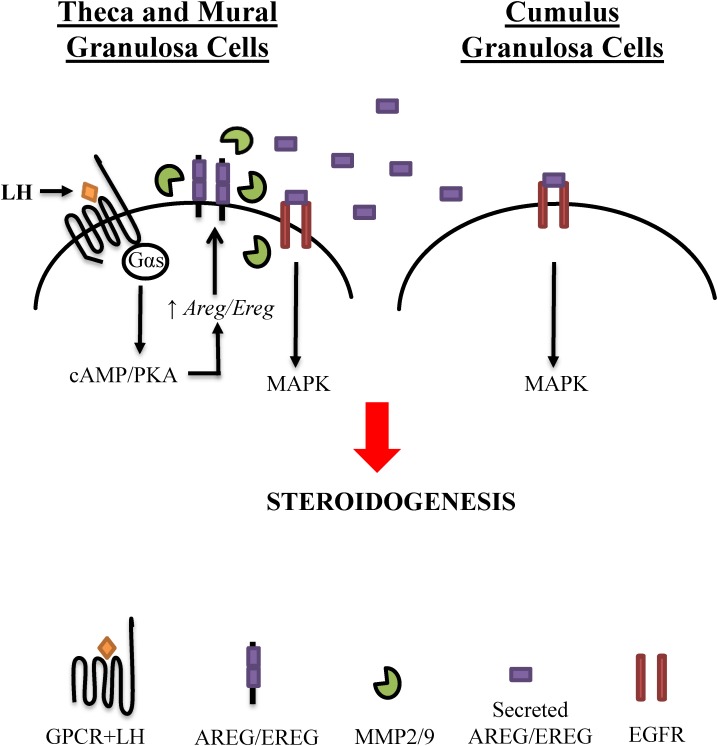
FIG. 9.
Model for EGFR transactivation in LH-induced steroidogenesis. LH binds to its receptor (Gαs-coupled G protein-coupled receptor [GPCR]) on the theca and mural granulosa cells to initiate cAMP and protein kinase A (PKA) signaling. This induces the upregulation of EGF-like ligands, specifically amphiregulin and epiregulin, which are not expressed prior to the LH signal. Production of the secreted form of amphiregulin and epiregulin occurs though MMP-mediated cleavage, via MMP2 and MMP9, which are expressed and active prior to and during the LH stimulus. Upon release, these EGF-like ligands bind to theca, mural, and cumulus granulosa cells to enhance steroid production.
Notably, our studies may have important clinical implications, as they suggest that MMP inhibition could be a possible treatment option for women with polycystic ovary syndrome (PCOS), characterized in part by excess ovarian androgen production. We report here that LH-induced testosterone production in freshly isolated ovarian follicles requires EGFR transactivation, and that doxycycline partially but significantly decreased this LH-induced testosterone production (Fig. 7E). These data indicate that, as in mural granulosa cells, MMP-mediated release of EGFR ligands, followed by autocrine, paracrine, or even juxtacrine signaling in theca cells, may be playing a critical role in androgen production. Doxycycline is a common antibiotic with known MMP2/9 inhibition properties. In fact, this compound has been used in vitro and in vivo to decrease MMP2/9 activity in several diseases where MMP activity contributes to their severity [49–52], and there are numerous active clinical trials exploring this feature of this drug. Furthermore, there is evidence that MMP2 and MMP9 activities are elevated in women with PCOS [53–57]. Therefore, the use of doxycycline or other MMP2/9 inhibitors in the setting of PCOS or other disease of excess ovarian steroidogenesis could prove useful for normalizing elevated androgen levels.
Footnotes
Supported by the National Institutes of Health, R01 GM101709, Division of Endocrinology, University of Rochester School of Medicine and Dentistry. Presented in part at the Society for the Study of Reproduction 45th annual meeting, August 12–15, 2012, State College, Pennsylvania, and the Endocrine Society's 16th International Congress of Endocrinology and the Endocrine Society's 96th Annual Meeting, June 21–24, 2014, Chicago, Illinois.
REFERENCES
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Sekiguchi T, Mizutani T, Yamada K, Kajitani T, Yazawa T, Yoshino M, Miyamoto K. Expression of epiregulin and amphiregulin in the rat ovary. J Mol Endocrinol. 2004;33:281–291. doi: 10.1677/jme.0.0330281. [DOI] [PubMed] [Google Scholar]
- Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84. doi: 10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- Carbajal L, Biswas A, Niswander LM, Prizant H, Hammes SR. GPCR/EGFR cross talk is conserved in gonadal and adrenal steroidogenesis but is uniquely regulated by matrix metalloproteinases 2 and 9 in the ovary. Mol Endocrinol. 2011;25:1055–1065. doi: 10.1210/me.2010-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamnongjit M, Gill A, Hammes SR. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci U S A. 2005;102:16257–16262. doi: 10.1073/pnas.0508521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andric N, Ascoli M. The luteinizing hormone receptor-activated extracellularly regulated kinase-1/2 cascade stimulates epiregulin release from granulosa cells. Endocrinology. 2008;149:5549–5556. doi: 10.1210/en.2008-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigone S, Hsieh M, Fu M, Persani L, Conti M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol. 2008;22:924–936. doi: 10.1210/me.2007-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Kawashima I, Yanai Y, Nishibori M, Richards JS, Shimada M. Hormone-induced expression of tumor necrosis factor alpha-converting enzyme/A disintegrin and metalloprotease-17 impacts porcine cumulus cell oocyte complex expansion and meiotic maturation via ligand activation of the epidermal growth factor receptor. Endocrinology. 2007;148:6164–6175. doi: 10.1210/en.2007-0195. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Shimada M. The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process. J Reprod Dev. 2012;58:510–514. doi: 10.1262/jrd.2012-056. [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo MC, Ting AY, Zamah AM, Conti M, Stouffer RL, Zelinski MB, Hennebold JD. Amphiregulin promotes the maturation of oocytes isolated from the small antral follicles of the rhesus macaque. Hum Reprod. 2012;27:2430–2437. doi: 10.1093/humrep/des158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamah AM, Hsieh M, Chen J, Vigne JL, Rosen MP, Cedars MI, Conti M. Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin. Hum Reprod. 2010;25:2569–2578. doi: 10.1093/humrep/deq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ježová M, Scsuková S, Nagyová E, Vranová J, Procházka R, Kolena J. Effect of intraovarian factors on porcine follicular cells: cumulus expansion, granulosa and cumulus cell progesterone production. Anim Reprod Sci. 2001;65:115–126. doi: 10.1016/s0378-4320(00)00219-0. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Ascoli M. A co-culture system reveals the involvement of intercellular pathways as mediators of the lutropin receptor (LHR)-stimulated ERK1/2 phosphorylation in Leydig cells. Exp Cell Res. 2008;314:25–37. doi: 10.1016/j.yexcr.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evaul K, Hammes SR. Cross-talk between G protein-coupled and epidermal growth factor receptors regulates gonadotropin-mediated steroidogenesis in Leydig cells. J Biol Chem. 2008;283:27525–27533. doi: 10.1074/jbc.M803867200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prall AK, Longo GM, Mayhan WG, Waltke EA, Fleckten B, Thompson RW, Baxter BT. Doxycycline in patients with abdominal aortic aneurysms and in mice: comparison of serum levels and effect on aneurysm growth in mice. J Vasc Surg. 2002;35:923–929. doi: 10.1067/mva.2002.123757. [DOI] [PubMed] [Google Scholar]
- Bocker R, Estler CJ, Maywald M, Weber D. Comparison of distribution of doxycycline in mice after oral and intravenous application measured by a high-performance liquid chromatographic method. Arzneimittelforschung. 1981;31:2116–2117. [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Fahnert B, Veijola J, Roël G, Kärkkäinen MK, Railo A, Destrée O, Vainio S, Neubauer P. Murine Wnt-1 with an internal c-myc tag recombinantly produced in Escherichia coli can induce intracellular signaling of the canonical Wnt pathway in eukaryotic cells. J Biol Chem. 2004;279:47520–47527. doi: 10.1074/jbc.M403207200. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ. Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol Endocrinol. 2010;24:1230–1239. doi: 10.1210/me.2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Park JY, Su YQ. Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol. 2006;20:715–723. doi: 10.1210/me.2005-0185. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti MZ, Christenson LK. Rapid effects of LH on gene expression in the mural granulosa cells of mouse periovulatory follicles. Reproduction. 2009;137:843–855. doi: 10.1530/REP-08-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- Curry TE, Osteen KG. The Matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24:428–465. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci U S A. 2000;97:4689–4694. doi: 10.1073/pnas.080073497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, van Westrum SS, Crabbe T, Clements J, d'Ortho M-P, Murphy G. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A: a kinetic study. J Biol Chem. 1998;273:871–880. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- English WR, Holtz B, Vogt G, Knäuper V, Murphy G. Characterization of the Role of the “MT-loop”: an eight-amino acid insertion specific to progelatinase A (MMP2) activating membrane-type matrix metalloproteinases. J Biol Chem. 2001;276:42018–42026. doi: 10.1074/jbc.M107783200. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Okamoto M, Ikeda M, Okamoto A, Sakai M, Gunji Y, Nishimura R, Hishinuma M, Shimada M. Protein kinase C (PKC) increases TACE/ADAM17 enzyme activity in porcine ovarian somatic cells, which is essential for granulosa cell luteinization and oocyte maturation. Endocrinology. 2014;155:1080–1090. doi: 10.1210/en.2013-1655. [DOI] [PubMed] [Google Scholar]
- Sahin U, Weskamp G, Kelly K, Zhou H-M, Higashiyama S, Peschon J, Hartmann D, Saftig P, Blobel CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwind A, Prenzel N, Ullrich A. Lysophosphatidic acid-induced squamous cell carcinoma cell proliferation and motility involves epidermal growth factor receptor signal transactivation. Cancer Res. 2002;62:6329–6336. [PubMed] [Google Scholar]
- Schafer B, Gschwind A, Ullrich A. Multiple G-protein-coupled receptor signals converge on the epidermal growth factor receptor to promote migration and invasion. Oncogene. 2003;23:991–999. doi: 10.1038/sj.onc.1207278. [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-[alpha] from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Moss ML, Jin SLC, Milla ME, Burkhart W, Carter HL, Chen W-J, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-[alpha] Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi PA, Sabri A, Belmadani S, Matrougui K. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation. 2004;110:3587–3593. doi: 10.1161/01.CIR.0000148780.36121.47. [DOI] [PubMed] [Google Scholar]
- Cheng C-Y, Tseng H-C, Yang C-M. Bradykinin-mediated cell proliferation depends on transactivation of EGF receptor in corneal fibroblasts. J Cell Physiol. 2012;227:1367–1381. doi: 10.1002/jcp.22849. [DOI] [PubMed] [Google Scholar]
- Song RXD, Zhang Z, Chen Y, Bao Y, Santen RJ. Estrogen signaling via a linear pathway involving insulin-like growth factor I receptor, matrix metalloproteinases, and epidermal growth factor receptor to activate mitogen-activated protein kinase in MCF-7 breast cancer cells. Endocrinology. 2007;148:4091–4101. doi: 10.1210/en.2007-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K-T, Sloniowski S, Ethell DW, Ethell IM. Ephrin-B2-induced cleavage of EphB2 receptor is mediated by matrix metalloproteinases to trigger cell repulsion. J Biol Chem. 2008;283:28969–28979. doi: 10.1074/jbc.M804401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Roelle S, Grosse R, Aigner A, Krell HW, Czubayko F, Gudermann T. Matrix metalloproteinases 2 and 9 mediate epidermal growth factor receptor transactivation by gonadotropin-releasing hormone. J Biol Chem. 2003;278:47307–47318. doi: 10.1074/jbc.M304377200. [DOI] [PubMed] [Google Scholar]
- Garnett K, Wang J, Roy SK. Spatiotemporal expression of epidermal growth factor receptor messenger RNA and protein in the hamster ovary: follicle stage-specific differential modulation by follicle-stimulating hormone, luteinizing hormone, estradiol, and progesterone. Biol Reprod. 2002;67:1593–1604. doi: 10.1095/biolreprod.102.005470. [DOI] [PubMed] [Google Scholar]
- Maruo T, Ladines-Llave CA, Samoto T, Matsuo H, Manalo AS, Ito H, Mochizuki M. Expression of epidermal growth factor and its receptor in the human ovary during follicular growth and regression. Endocrinology. 1993;132:924–931. doi: 10.1210/endo.132.2.8425504. [DOI] [PubMed] [Google Scholar]
- Onagbesan OM, Gullick W, Woolveridge I, Peddie MJ. Immunohistochemical localization of epidermal growth factor receptors, epidermal-growth-factor-like and transforming-growth-factor-alpha-like peptides in chicken ovarian follicles. J Reprod Fertil. 1994;102:147–153. doi: 10.1530/jrf.0.1020147. [DOI] [PubMed] [Google Scholar]
- Yao HH, Bahr JM. Chicken granulosa cells show differential expression of epidermal growth factor (EGF) and luteinizing hormone (LH) receptor messenger RNA and differential responsiveness to EGF and LH dependent upon location of granulosa cells to the germinal disc. Biol Reprod. 2001;64:1790–1796. doi: 10.1095/biolreprod64.6.1790. [DOI] [PubMed] [Google Scholar]
- Singh B, Rutledge JM, Armstrong DT. Epidermal growth factor and its receptor gene expression and peptide localization in porcine ovarian follicles. Mol Reprod Dev. 1995;40:391–399. doi: 10.1002/mrd.1080400402. [DOI] [PubMed] [Google Scholar]
- Lee CZ, Xu B, Hashimoto T, McCulloch CE, Yang G-Y, Young WL. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke. 2004;35:1715–1719. doi: 10.1161/01.STR.0000129334.05181.b6. [DOI] [PubMed] [Google Scholar]
- Chang WYC, Clements D, Johnson SR. Effect of doxycycline on proliferation, MMP production, and adhesion in LAM-related cells. Am J Physiol Lung Cell Mol Physiol. 2010;299:L393–L400. doi: 10.1152/ajplung.00437.2009. [DOI] [PubMed] [Google Scholar]
- Chung AW, Yang HH, Radomski MW, van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in Marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res. 2008;102:e73–e85. doi: 10.1161/CIRCRESAHA.108.174367. [DOI] [PubMed] [Google Scholar]
- Sochor M, Richter S, Schmidt A, Hempel S, Hopt UT, Keck T. Inhibition of matrix metalloproteinase-9 with doxycycline reduces pancreatitis-associated lung injury. Digestion. 2009;80:65–73. doi: 10.1159/000212080. [DOI] [PubMed] [Google Scholar]
- Gomes VA, Vieira CS, Jacob-Ferreira AL, Belo VA, Soares GM, Fernandes JB, Ferriani RA, Tanus-Santos JE. Imbalanced circulating matrix metalloproteinases in polycystic ovary syndrome. Mol Cell Biochem. 2011;353:251–257. doi: 10.1007/s11010-011-0793-6. [DOI] [PubMed] [Google Scholar]
- Liu B, Cai LY, Lv HM, Xia L, Zhang YJ, Zhang HX, Guan YM. Raised serum levels of matrix metalloproteinase-9 in women with polycystic ovary syndrome and its association with insulin-like growth factor binding protein-1. Gynecol Endocrinol. 2008;24:285–288. doi: 10.1080/09513590802056995. [DOI] [PubMed] [Google Scholar]
- Shalev E, Goldman S, Ben-Shlomo I. The balance between MMP-9 and MMP-2 and their tissue inhibitor (TIMP)-1 in luteinized granulosa cells: comparison between women with PCOS and normal ovulatory women. Mol Hum Reprod. 2001;7:325–331. doi: 10.1093/molehr/7.4.325. [DOI] [PubMed] [Google Scholar]
- Baka S, Zourla K, Kouskouni E, Makrakis E, Demeridou S, Tzanakaki D, Hassiakos D, Creatsas G. Matrix metalloproteinases 2 and 9 and their tissue inhibitors in the follicular fluid of patients with polycystic ovaries undergoing in vitro fertilisation. In Vivo. 2010;24:293–296. [PubMed] [Google Scholar]
- Lewandowski KC, Komorowski J, O'Callaghan CJ, Tan BK, Chen J, Prelevic GM, Randeva HS. Increased circulating levels of matrix metalloproteinase-2 and -9 in women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1173–1177. doi: 10.1210/jc.2005-0648. [DOI] [PubMed] [Google Scholar]