Abstract
Previous studies have demonstrated that perinatal nicotine exposure increased blood pressure (BP) in adult offspring. However, the underlying mechanisms were unclear. The present study tested the hypothesis that perinatal nicotine-induced programming of hypertensive response is mediated by enhanced reactive oxygen species (ROS) in the vasculature. Nicotine was administered to pregnant rats via subcutaneous osmotic mini-pumps from Day 4 of gestation to Day 10 after birth, in the absence or presence of the ROS inhibitor N-acetyl-cysteine (NAC) in the drinking water. Experiments were conducted in 8-mo-old male offspring. Perinatal nicotine treatment resulted in a significant increase in arterial ROS production in offspring, which was abrogated by NAC. Angiotensin II (Ang II)-induced BP responses were significantly higher in nicotine-treated group than in saline-treated control group, and NAC treatment blocked the nicotine-induced increase in BP response. Consistent with that, the nicotine treatment significantly increased both Ang II-induced and phorbol [12, 13]-dibutyrate (PDBu, a Prkc activator)-induced arterial contractions in adult offspring, which were blocked by NAC treatment. In addition, perinatal nicotine treatment significantly attenuated acetylcholine-induced arterial relaxation in offspring, which was also inhibited by NAC treatment. Results demonstrate that inhibition of ROS blocks the nicotine-induced increase in arterial reactivity and BP response to vasoconstrictors in adult offspring, suggesting a key role for increased oxidative stress in nicotine-induced developmental programming of hypertensive phenotype in male offspring.
Keywords: antioxidant, blood pressure, perinatal nicotine, programming, vascular dysfunction
INTRODUCTION
Cardiovascular disease is a leading cause of death in developed countries. Cigarette smoking is one of the major risk factors for development of cardiovascular disease. Recently, epidemiological studies have shown that maternal smoking is associated with increased risk of cardiovascular disease and hypertension in offspring later in life [1–3]. As one of the major active components in cigarette smoking, nicotine readily crosses the placenta and produces higher nicotine concentrations in the fetal circulation than those in the mother [4]. The increased nicotine concentrations in the fetus may contribute to maternal cigarette smoking-induced developmental programming of cardiovascular dysfunction in offspring. Indeed, nicotine treatment during pregnancy caused cardiovascular disorders and hypertension in offspring in several different animal models [5–7]. Our recent studies demonstrated that perinatal nicotine exposure reprogrammed cardiovascular function and caused an exaggerated vascular reactivity and increased blood pressure (BP) response in adult offspring [8–13]. However, the mechanisms remain unknown.
Reactive oxygen species (ROS) are well-recognized key signaling molecules that may mediate diverse biological responses [14–16]. In the cardiovascular system, ROS play a significant pathophysiological role in vascular remodeling and dysfunction associated with hypertension [14]. There is increasing evidence that fetal programming of oxidative stress may be critically important in the causes of cardiovascular disorders in adulthood [17, 18]. Maternal smoking is associated with increased levels of ROS in offspring [19]. Furthermore, exposure to nicotine during gestation results in an increase in ROS in fetal, neonatal, and adult tissues [20, 21]. Our recent studies have demonstrated that perinatal nicotine exposure causes hypertensive reactivity in adult offspring [8–11]. Changes in vascular function are associated with increased ROS production in vasculatures in adult offspring [10]. However, whether oxidative stress is an initiating trigger for perinatal nicotine-induced programming of cardiovascular dysfunction remains unknown. In addition, it is unclear about the potentially profound impact of antenatal antioxidant exposure to counter oxidative stress on the pregnancy outcomes and fetal programming of cardiovascular disorders. Although recent clinical trials suggest that supplementation during pregnancy with a medical food containing l-arginine and antioxidants reduces the incidence of pre-eclampsia in a population at high risk for the condition [22], insufficient evidence existed to demonstrate the use of antioxidants for prevention or treatment of hypertension. There is still a debate whether oxidative stress is a cause or a result of cardiovascular dysfunction. Thus, the present study tested the hypothesis that increased ROS and oxidative stress play a causative role in perinatal nicotine-mediated programming of vascular dysfunction and increased BP response in offspring.
MATERIALS AND METHODS
Experimental Animals
All procedures and protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Time-dated pregnant Sprague-Dawley rats were randomly divided into four groups, as follows: 1) saline control (n = 5), 2) nicotine (n = 5), 3) saline plus the ROS inhibitor N-acetyl-cysteine (NAC [500 mg/kg/day]) (n = 5), and 4) nicotine plus NAC (n = 6). Nicotine was administered to pregnant rats through an osmotic mini-pump at 4 μg/kg/min from Day 4 of pregnancy to Day 10 after birth, as previously described [8–13]. The dose of nicotine resulted in the blood levels closely resembling those occurring in moderate human smokers [1]. Control rats received saline from the osmotic mini-pump as the vehicle control. For the antioxidant treatment, NAC was placed in the drinking water, which was concurrently started at the same time as nicotine infusion at Day 4 of pregnancy and ended at the same time as nicotine treatment ended. Concentrations of NAC placed in the drinking water have been shown to be effective at reducing oxidative stress [23]. Our previous studies [8, 9, 12] and current studies did not show any significant differences in litter size following nicotine exposure. Therefore, the litter size was intact in each dam, and all newborn pups were kept with their mothers until weaning. At weaning (3 wk of age), the male and female offspring were separated. Because our previous studies demonstrated that fetal nicotine exposure causes a hypertensive response in male but not female offspring [9], male offspring were kept and used for present studies at the age of 8 mo old.
Measurement of Arterial Blood Pressure
The adult male rats were implanted with a catheter in the femoral arteries for recording arterial BP and were subcutaneously implanted with another catheter for drug injection, as described previously [9, 11]. The catheters were secured on the backs of rats. Two days after recovery from surgery, BP was measured continuously in conscious animals. After baseline BP was recorded for 60 min, animals received an acute bolus injection of angiotensin II (Ang II) (10 μg/kg) with subcutaneous injection via a subcutaneously implanted catheter, and BP was recorded for 60 min, as described previously [9, 11, 24]. Arterial BP responses at this dose of Ang II reached a submaximal level, as determined in our previous studies [9]. Arterial systolic BP (SBP) and diastolic BP (DBP) and mean arterial BP (MAP) data were recorded continuously throughout each study with data acquisition software (Powerlab 16/SP and Chart version 4; ADInstruments).
Contraction and Relaxation Studies
Aortas were isolated from adult offspring, cut into 4-mm rings, and mounted in 10-ml tissue baths containing modified Krebs solution equilibrated with a mixture of 95% O2 and 5% CO2. Isometric tensions were measured at 37°C, as described previously [9]. Ang II and protein kinase C (Prkc) activator, phorbol [12, 13]-dibutyrate (PDBu)-induced concentration-dependent contractions were obtained by cumulative additions of the agonist in approximately one-half log increments. For relaxation studies, the tissues were precontracted with a submaximal concentration (1 μM) of norepinephrine, followed by acetylcholine (Ach) stimulation, added in a cumulative manner.
Measurement of Vascular ROS
Total ROS in aortic segments was measured with Oxiselect in vitro ROS/RNS assay kit (Cell Biolabs), following the manufacturer's instructions and described previously [25]. Briefly, tissues were homogenized in cold phosphate-buffered saline solution and centrifuged at 10 000 × g for 5 min. The supernatant was collected for assay. Fifty microliters of unknown samples or standard were added to a 96-well plate and mixed with 50 μl of catalyst and 100 μl of 2′7′-dichlorodihydrofluorescein diacetate (DCF). After incubation at room temperature for 30 min, the fluorescence (excitation at 480 nm/emission at 530 nm) was measured using a Synergy HT multi-mode microplate reader (Bio-Tek Instruments).
Statistical Analysis
Concentration-response curves were analyzed by computer-assisted nonlinear regression to fit the data using Prism software (GraphPad) to obtain the maximum response (Emax). Results are given as means ± SEM, and differences were evaluated for statistical significance (P < 0.05) by ANOVA or by t-test, where appropriate.
RESULTS
Antioxidant Inhibited Nicotine-Mediated Changes in Body Weight
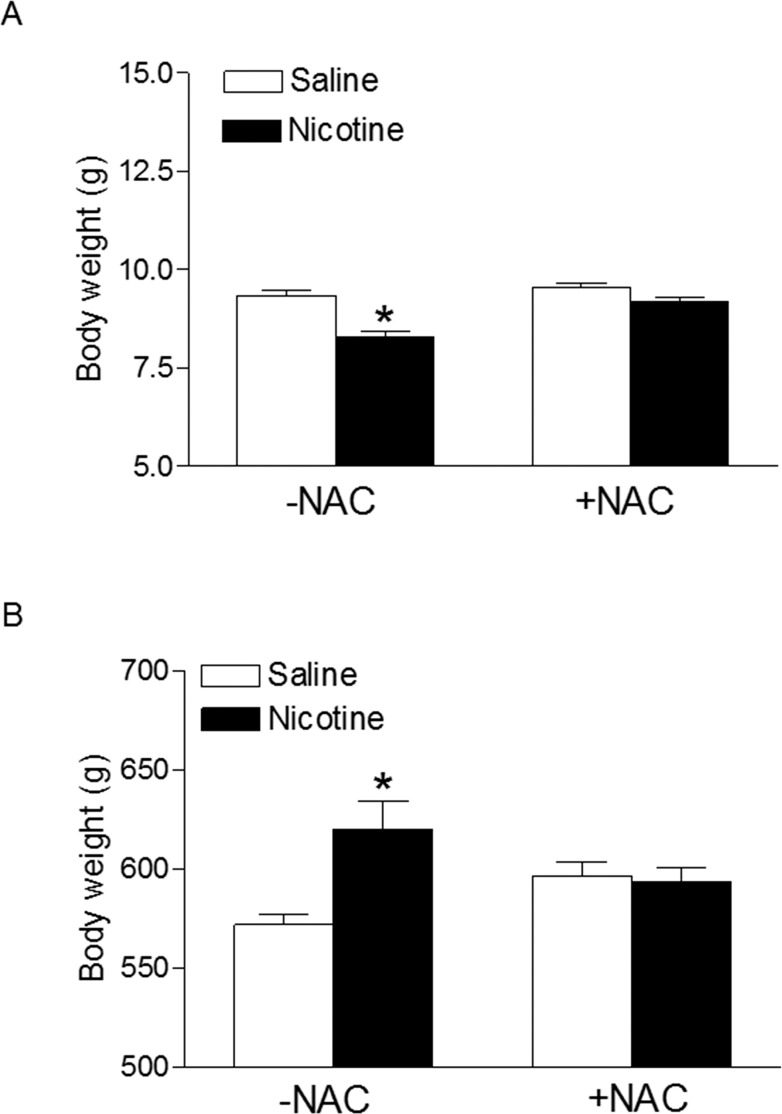
Figure 1 shows the effect of perinatal nicotine treatment on neonatal and adult body weight in the absence or presence of NAC. In 2-day-old pups, the nicotine treatment significantly decreased body weight compared with that in the saline-treated control (8.3 ± 0.1 vs. 9.4 ± 0.1 g, respectively, P < 0.05) (Fig. 1A). However, in 8-mo-old adults, body weight was significantly greater in nicotine-treated animals than in controls (620.3 ± 13.9 vs. 571.9 ± 5.0 g, respectively, P < 0.05) (Fig. 1B). NAC did not affect body weight in the control offspring but blocked the nicotine-induced effects (Fig. 1).
FIG. 1.
Effects of antenatal antioxidant on nicotine-mediated changes in body weight of offspring. Body weights in 2-day-old pups (A) and 8-mo-old adult males (B) were determined in both saline control and nicotine-treated groups with (+) and without (−) treatment of antioxidant (NAC). Data are means ± SEM of animals (n = 4 to 5 litters) from each group. Data were analyzed by Student t-test. *P < 0.05 vs. control.
Antioxidant Blocked Nicotine-Mediated Increase in ROS Production
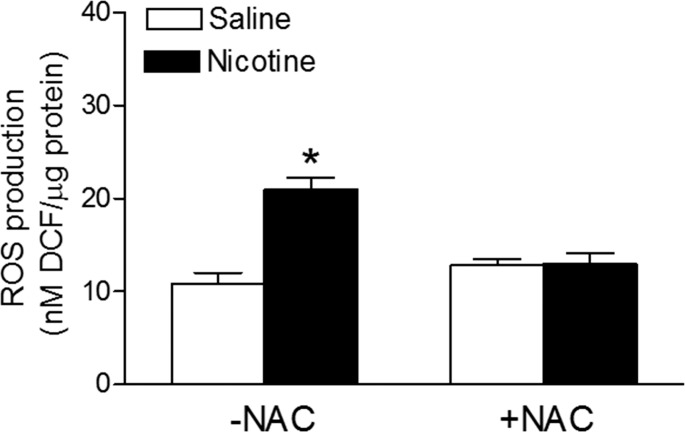
In the absence of NAC, the perinatal nicotine treatment resulted in a significant increase in arterial ROS productions in the adult offspring, compared with that in the saline control animals (Fig. 2). In the presence of NAC, there were no significant differences in ROS production between the two groups (Fig. 2). In addition, NAC treatment did not significantly affect ROS production in control offspring.
FIG. 2.
Effects of antenatal antioxidant on production of ROS in aortic segments. Total ROS production was measured in aortic segments isolated from adult male offspring that had been exposed in utero to saline control or nicotine without (−) or with (+) NAC treatment. Data are means ± SEM from 5 animals per group. *P < 0.05, nicotine vs. control. DCF = 2′,7′-dichlorodihydrofluorescein.
Antioxidant Abrogated Nicotine-Mediated Changes BP Response
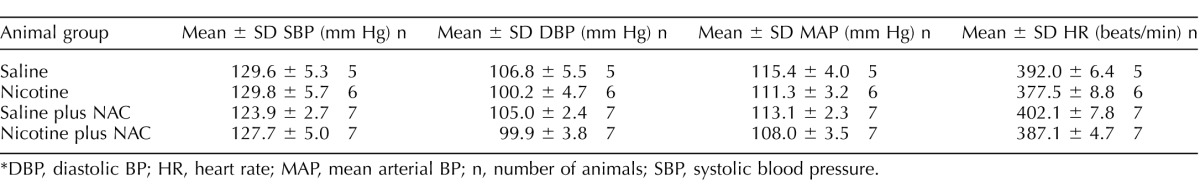
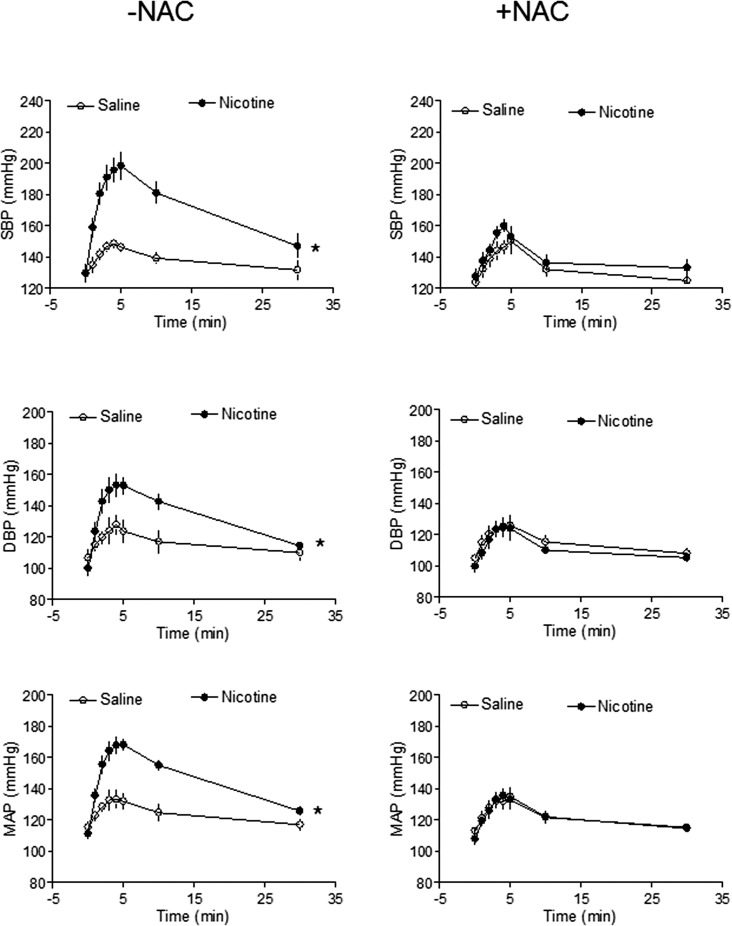
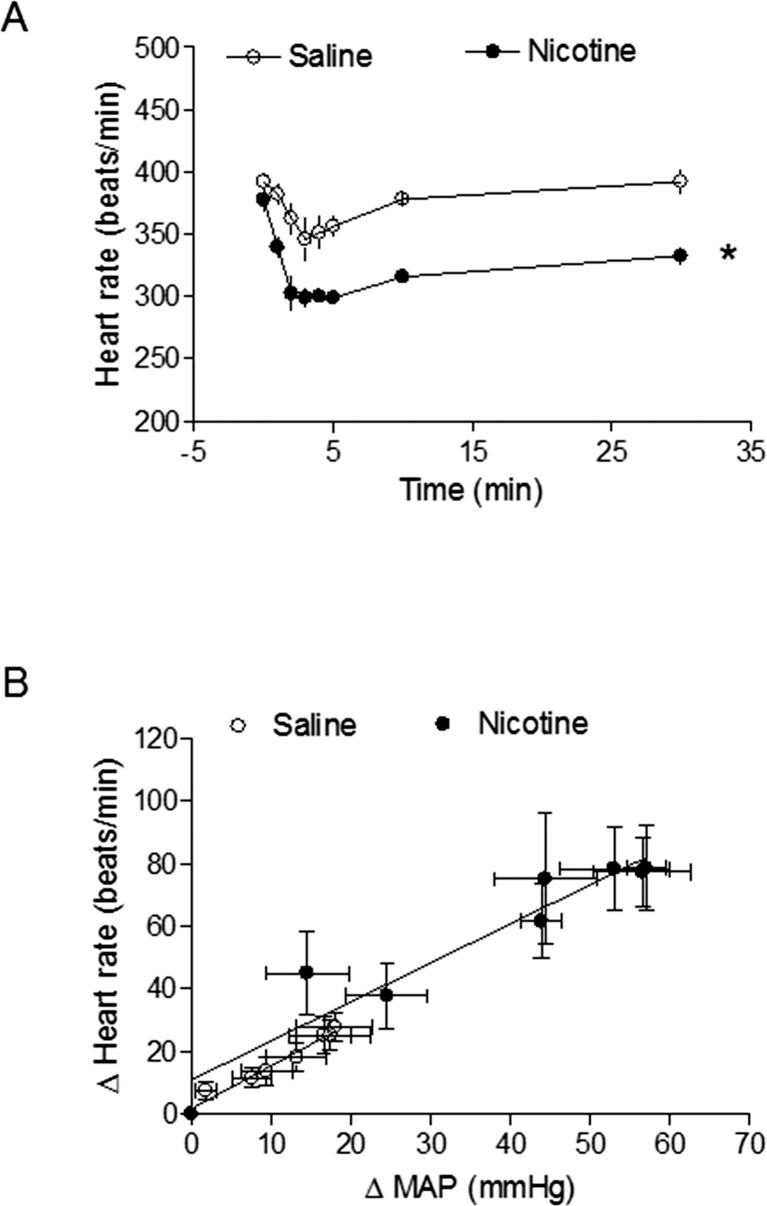
As shown in Table 1, basal arterial BP and heart rate in 8-mo-old male offspring were not significantly different from those in saline-treated controls and nicotine-treated animals, regardless of perinatal NAC supplementation. Figure 3 shows Ang II-induced increases in arterial BP response. In the absence of perinatal NAC supplementation, Ang II-induced SBP, DBP, and MAP responses were significantly increased in the nicotine-treated group compared with those in the saline control group. However, in the presence of perinatal NAC supplementation, there were no differences in Ang II-induced BP responses between the two groups (Fig. 3). As shown in Figure 4A, Ang II resulted in decreases in heart rate (HR) in response to increases in BP. Consistent with the increased BP response (Fig. 3), the decrease in HR was significantly greater in the nicotine-treated group than in the saline control group (Fig. 4A). The baroreflex sensitivity was calculated as slope of ΔHR/ΔMAP and was not significantly different between the saline control and nicotine-treated groups (Fig. 4B).
TABLE 1.
Effect of antenatal antioxidant on basal BP in 8-mo-old male offspring.*
DBP, diastolic BP; HR, heart rate; MAP, mean arterial BP; n, number of animals; SBP, systolic blood pressure.
FIG. 3.
Effects of antenatal antioxidant on Ang II-induced BP response in adult male offspring. Systolic BP (SBP), diastolic BP (DBP), and mean arterial BP (MAP) responses to Ang II (10 μg/kg) were measured in adult male offspring that had been exposed in utero to saline control or nicotine without (left panel) or with (right panel) NAC treatment. Data are means ± SEM and were analyzed by 2-way ANOVA. *P < 0.05, nicotine vs. control; n = 5 to 7.
FIG. 4.
Effects of nicotine on Ang II-induced HR and baroreflex sensitivity in adult male offspring. HR responses to Ang II (10 μg/kg) were measured in adult male offspring that had been exposed in utero to saline control or nicotine (A). (B) Baroreflex sensitivity was calculated as the slope of ΔHR/ΔMAP (beats per minute per mm Hg). Data are means ± SEM; n = 5 to 7. *P < 0.05, nicotine vs. control.
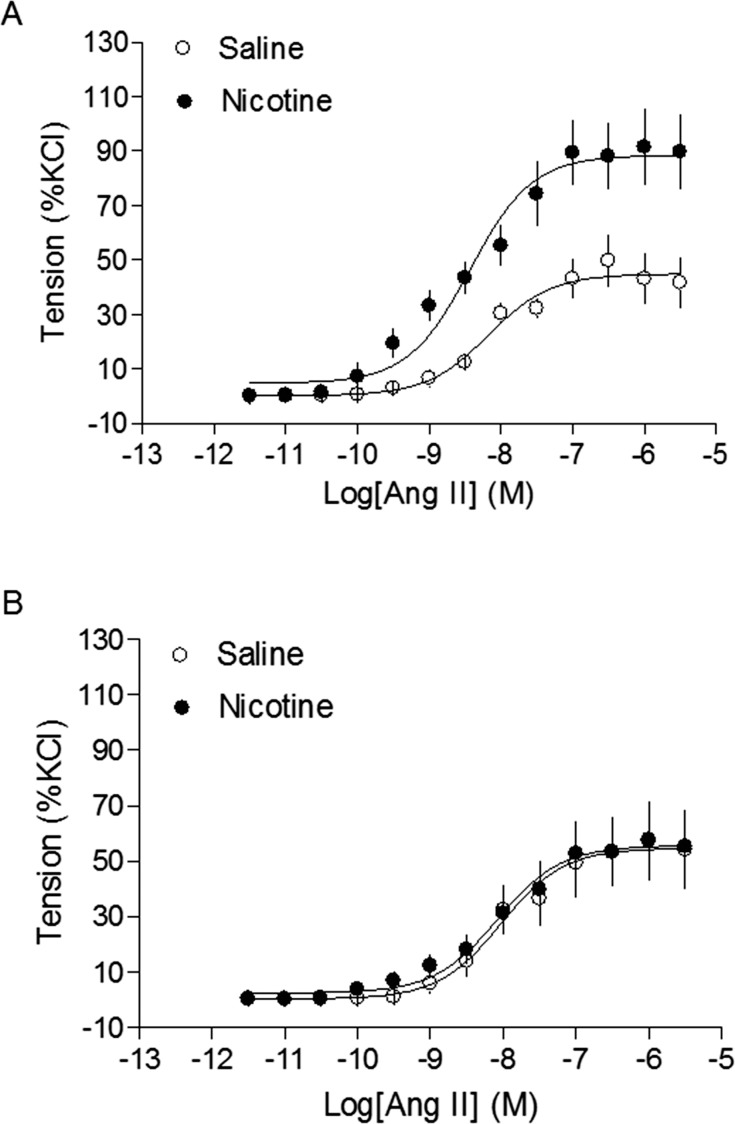
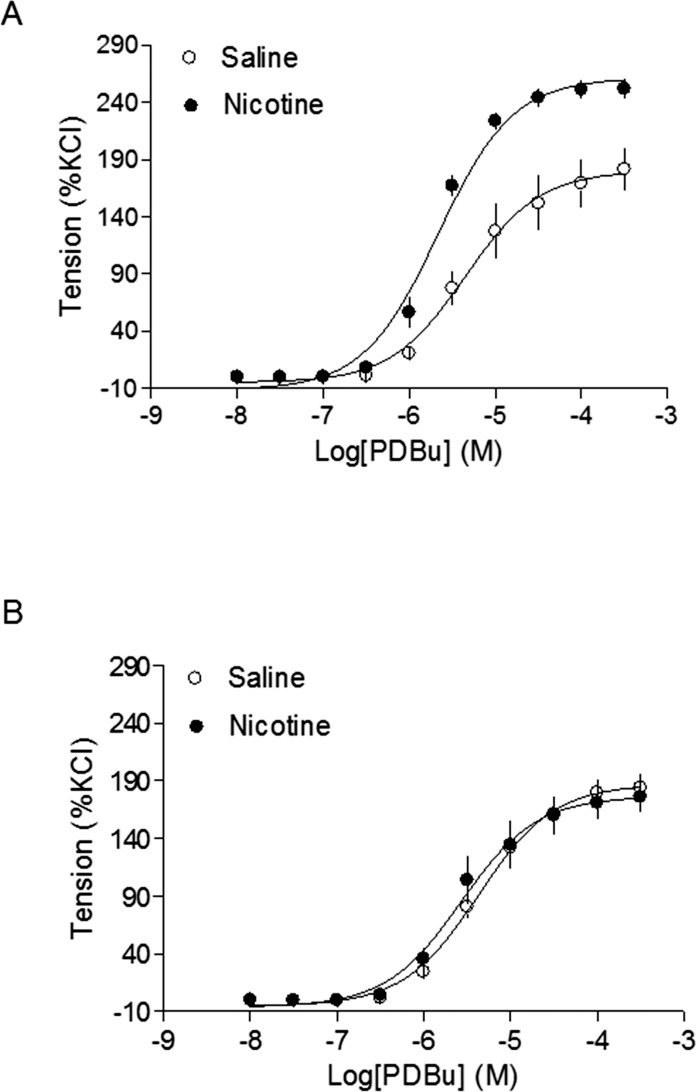
Antioxidant Inhibited Nicotine-Mediated Increase in Vascular Reactivity
As shown in Figure 5, the maximal response of Ang II-induced arterial contractions was significantly increased in the nicotine-treated group compared with that in the saline control group (88.4 ± 4.1% vs. 44.9 ± 2.6%, respectively, P < 0.05) (Fig. 5A), which was inhibited by perinatal NAC supplement (Fig. 5B). Similarly, the nicotine treatment resulted in a significant increase in the maximal response of a Prkc activator, PDBu-induced contractions (261.3 ± 4.9% vs. 180.0 ± 9.6%, respectively, P < 0.05) (Fig. 6A), and this was blocked by perinatal NAC supplementation (Fig. 6B).
FIG. 5.
Effects of antenatal antioxidant on Ang II-induced contractions of aortas in adult male offspring. Ang II-induced contractions were determined in aortas from adult male offspring that had been exposed in utero to saline control or nicotine without (A) or with (B) NAC treatment. Data are means ± SEM of tissues from 5 to 6 animals. Maximal contractile response values are presented in the Results.
FIG. 6.
Effects of antenatal antioxidant on phorbol 12-13-dibutyrate (PDBu)-induced contractions of aortas in adult male offspring. A Prkc activator, PDBu-induced contractions were determined in aortas from adult male offspring that had been exposed in utero to saline control or nicotine without (A) or with (B) NAC treatment. Data are means ± SEM of tissues from 6 to 8 animals. Maximal contractile response values are presented in the Results.
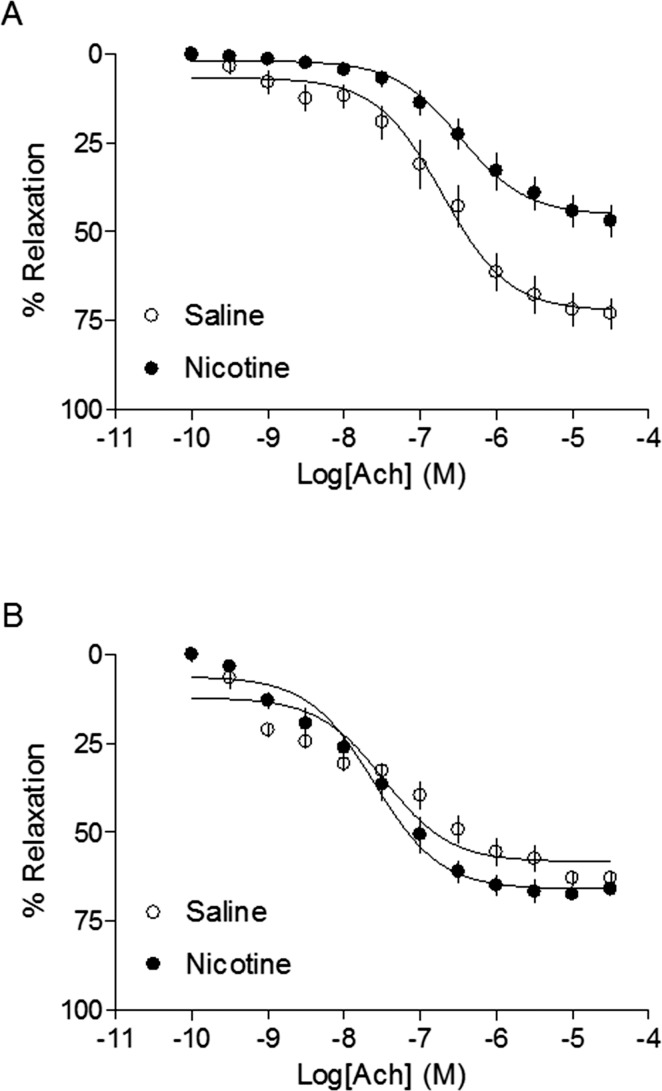
Antioxidant Reversed Nicotine-Mediated Decrease in Ach-Induced Relaxations
Figure 7 shows Ach-induced arterial relaxations in adult offspring. In the absence of perinatal NAC supplementation, the maximal response of Ach-induced relaxation was significantly decreased in the nicotine-treated animals compared with that in the saline control (45.5 ± 2.1% vs. 72.5 ± 2.7%, respectively, P < 0.05) (Fig. 7A). In the presence of perinatal NAC supplementation, there were no significant differences in Ach-induced relaxation between the nicotine-treated and saline control animals (66.1 ± 1.6% vs. 58.5 ± 1.9%, respectively, P > 0.05) (Fig. 7B).
FIG. 7.
Effects of antenatal antioxidant on acetylcholine-induced relaxation of aortas in adult male offspring. Ach-induced relaxation of aortic rings was determined in adult male offspring that had been exposed in utero to saline control or nicotine without (A) or with (B) NAC treatment. Data are means ± SEM of tissues from 5 to 12 animals. Maximal relaxation response values are presented in the Results.
DISCUSSION
Our previous studies demonstrated that prenatal nicotine exposure caused a sex-dependent increase in vascular contractility and BP response in adult male rat offspring, which was associated with an increase in ROS productions in vasculature [9, 10, 25]. The present study provides new direct evidence that increased ROS play a causal role in nicotine-mediated developmental programming of hypertensive phenotype in adult male offspring. The major findings in present study are as follows: 1) perinatal nicotine treatment caused intrauterine fetal/neonatal growth restriction (IUGR) and a rebound catch-up overgrowth in adult offspring, which was inhibited by antenatal antioxidant; (2) antenatal antioxidant blocked perinatal nicotine-induced programming of increased blood pressure response; (3) antenatal antioxidant reversed perinatal nicotine-mediated increase in arterial contractility in offspring; (4) antioxidant abrogated nicotine-mediated decrease in arterial relaxations; and (5) antenatal antioxidant blocked nicotine-induced increase in ROS productions in the vasculature.
Consistent with previous studies [8, 9, 12], the present finding that perinatal nicotine treatment decreased the body weights of 2-day-old pups suggests that, in this animal model, gestational nicotine exposure causes IUGR. In addition, previous studies have demonstrated that offspring with IUGR may develop a catch-up growth at 1 to 5 mo of age in a similar animal model [8, 9, 12]. However, to our surprise, we found that the body weight in 8-mo-old male offspring was higher in nicotine-treated animals than that in the saline-treated control group. These results suggest that the effect of gestational nicotine exposure on the growth of offspring is age-dependent and that it may cause development of obesity in older offspring. Of importance, the finding that antenatal antioxidant eliminated the difference of body weight in offspring between the nicotine-treated and saline-treated control groups, suggests that increased oxidative stress may play an important role in the regulation of nicotine-mediated changes in postnatal growth.
We and other investigators have previously demonstrated that perinatal exposure to nicotine results in increased oxidative damage and ROS productions in offspring [10, 20, 26]. Similarly, the present study showed an increase in arterial ROS productions in offspring that had been prenatally exposed to nicotine. Although the mechanisms underlying heightened vascular ROS production are not completely clear, our previous studies have demonstrated that perinatal nicotine-mediated increased ROS production is associated with an increase in NADPH oxidase 2 (NOX2) expression [10], which suggests that epigenetic up-regulation of the NOX2 gene may be one of the key molecular mechanisms in nicotine-mediated heightened ROS production. The finding that maternal NAC treatment significantly decreased nicotine-induced ROS productions and eliminated the difference of vascular ROS productions between nicotine-treated and saline-treated control groups suggests that NAC is capable of decreasing nicotine-induced oxidative stress. Observation of the anti-oxidative effect of NAC is in accordance with previous reports that have shown that NAC was a potent free radical scavenger with antioxidant properties used in the clinical setting and animal models [27–29].
The finding that perinatal nicotine exposure increased susceptibility of elevated blood pressure in adult offspring is consistent with previous studies showing a direct link between adverse intrauterine environmental exposure and epigenetically increased risk of hypertension and ischemic heart disease in offspring [30–33]. The molecular mechanisms underlying perinatal nicotine-induced heightened blood pressure in offspring are not fully understood. Many studies have shown that intrauterine adverse environmental exposure induces an increase in fetal oxidative stress, resulting in cardiovascular dysfunction and elevation of blood pressure in different animal models [17, 18, 34, 35]. A novel finding of the present study is that maternal antioxidant treatment eliminates nicotine-mediated increased blood pressure responses in adult offspring, which provides a direct evidence that fetal nicotine exposure-induced programming of adult hypertensive response may be mediated by oxidative stress. Similar studies have reported that antioxidant intervention in nicotine-exposed dams prevents pancreas β-cell loss and apoptosis observed in nicotine-exposed male offspring [26]. In addition, a study has demonstrated that antenatal antioxidant prevents adult hypertension and vascular dysfunction associated with in utero exposure to a low-protein diet [17]. Taken together, these findings support the concept that perinatal nicotine exposure leads to fetal oxidative stress, resulting in permanent alterations in the cardiovascular development and increased risk of hypertension in adulthood.
Previously, we found that nicotine-mediated increase in BP response to Ang II was not associated with changes in baroreflex (heart rate response to BP) but was associated with a decrease in the baroreflex sensitivity in 5-mo-old male offspring [9]. In the present study, we found that nicotine-mediated increase in BP response to Ang II was associated with a decrease in heart rate but without changes in the baroreflex sensitivity in 8-mo-old male offspring. These findings suggest that perinatal nicotine-induced changes in baroreflex in offspring is age-dependent. Modulation of vascular contractility by antioxidant is likely to be an important mechanism in the prevention of hypertensive response in adult offspring. Indeed, previous studies demonstrated that maternal antioxidant treatment prevented the enhanced vasomotor response, resulting in reversal of BP elevation in adult offspring in low protein-fed dams and maternal glucocorticoid-treated animals [17, 35]. In agreement with these findings, our present studies also indicated that antenatal antioxidant rescued both Ang II and Prkc-induced vasoconstrictions that were increased by the nicotine treatment. Ang II is an important regulator of vascular contractility that acts by binding to Ang II receptors (Agtr) and activating its down-stream signaling pathway, such as IP3 and Prkc. An increasing body of evidence shows that Ang II/Agtr1 directly regulates ROS signaling pathway, leading to enhanced vasoconstriction under pathophysiological conditions [36–38]. Our previous studies demonstrated that perinatal nicotine exposure epigenetically up-regulated Ang II/Agtr1 signaling [9, 10, 39]. These findings suggest that nicotine-mediated upregulation of Ang II/Agtr1 system may play an important role in enhanced vascular ROS production in adult offspring. In addition, the present finding that antenatal antioxidant rescued nicotine-mediated decrease in Ach-induced arterial relaxations in offspring suggests that ROS may modulate nicotine-mediated, NO-dependent signaling. Similar findings have been reported in different animal models in which the heightened ROS productions results in impairment of NO-mediated vasorelaxation in offspring [17, 34, 35].
Large epidemiological and animal studies indicate a link between in utero adverse stimuli during pregnancy and an increased risk of hypertension and cardiovascular disease later in life. The present study provides direct and translational evidence that antenatal antioxidant treatment during pregnancy reduces fetal oxidative stress, leading to improvement of vascular function and prevention of elevated blood pressure response in adult offspring of nicotine-exposed dam. Although the findings of the present study support the concept that fetal oxidative stress-induced by nicotine exposure can lead to permanent alterations in the cardiovascular system and increase the risk of hypertension late life, further studies are needed to determine how ROS signaling is involved in the epigenetic regulation of contractile protein expression patterns in the developing vasculature. Better understanding of the role of ROS will provide more insights into molecular mechanisms in maternal smoking-related cardiovascular disease and may suggest new directions of therapeutic strategies in the treatment of cardiovascular dysfunction induced by perinatal oxidative insult. In addition, electronic cigarette (e-cigarette), an electronic nicotine delivery system, has been introduced in the global market during the past 5 yr by the industry [40, 41]. Although recent studies have shown that e-cigarettes can alter heart function and affect blood hematology [42], little direct research has been done on the health effects of e-cigarette products on prenatal development or adult health. Therefore, study of the long-term consequences of fetal nicotine exposure either from maternal tobacco smoking or new e-cigarette smoking has significant public health impact and clinic implication. Growing evidences in animal studies have shown a protective and beneficial effect of antioxidant supplementation for cardiovascular dysfunction, however insufficient evidences in humans to demonstrate this protective effect. It is a huge challenge for us to translate the animal study into human clinic.
Footnotes
Supported by National Institutes of Health grants HL118861 to L.Z. and DA032510 to D.X. and by Regents of the University of California Tobacco Related Disease Research Program grant 22XT-0022 to D.X.
REFERENCES
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- Beratis NG, Panagoulias D, Varvarigou A. Increased blood pressure in neonates and infants whose mothers smoked during pregnancy. J Pediatr. 1996;128:806–812. doi: 10.1016/s0022-3476(96)70333-5. [DOI] [PubMed] [Google Scholar]
- Blake KV, Gurrin LC, Rvans SF, Beilin LJ, Landau LI, Stanley FJ, Newnham JP. Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early Hum Dev. 2000;57:137–147. doi: 10.1016/s0378-3782(99)00064-x. [DOI] [PubMed] [Google Scholar]
- Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20:115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- Pausova Z, Paus T, Sedova L, Berube J. Prenatal exposure to nicotine modifies kidney weight and blood pressure in genetically susceptible rats: a case of gene-environment interaction. Kidney Int. 2003;64:829–835. doi: 10.1046/j.1523-1755.2003.00172.x. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Holloway AC, Su LY, Takemori K, Lu C, Lee RM. Effects of fetal and neonatal exposure to nicotine on blood pressure and perivascular adipose tissue function in adult life. Eur J Pharmacol. 2008;590:264–268. doi: 10.1016/j.ejphar.2008.05.044. [DOI] [PubMed] [Google Scholar]
- Tao H, Rui C, Zheng J, Tang J, Wu L, Shi A, Chen N, He R, Wu C, Li J, Yin X, Zhang P, et al. Angiotensin II-mediated vascular changes in aged offspring rats exposed to perinatal nicotine. Peptides. 2013;44:111–119. doi: 10.1016/j.peptides.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Xiao D, Huang X, Lawrence J, Yang S, Fetal Zhang L. and neonatal nicotine exposure differentially regulates vascular contractility in adult male and female offspring. J Pharmacol Exp Ther. 2007;320:654–661. doi: 10.1124/jpet.106.113332. [DOI] [PubMed] [Google Scholar]
- Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension. 2008;51:1239–1247. doi: 10.1161/HYPERTENSIONAHA.107.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Huang X, Yang S, Zhang L. Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. Br J Pharmacol. 2011;164:1400–1409. doi: 10.1111/j.1476-5381.2011.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Huang X, Yang S, Zhang L. Estrogen normalizes perinatal nicotine-induced hypertensive responses in adult female rat offspring. Hypertension. 2013;61:1246–1254. doi: 10.1161/HYPERTENSIONAHA.113.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J, Xaio D, Xue Q, Rejali M, Yang S, Zhang L. Prenatal Nicotine Exposure Increases Heart Susceptibility to Ischemia/Reperfusion Injury in Adult Offspring. J Pharmacol Exp Ther. 2008;324:331–341. doi: 10.1124/jpet.107.132175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J, Chen M, Xiong F, Xaio D, Zhang H, Buchholz JN, Zhang L. Foetal nicotine exposure causes PKCε gene repression by promoter methylation in rat hearts. Cardiovasc Res. 2011;89:89–97. doi: 10.1093/cvr/cvq270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res. 2011;34:5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- Franco R, Schoneveld O, Georgakilas AG. Panayiotidis. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Lassegue B, Griendling Kathy K. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambonie G, Comte B, Yzydorczyk C, Ntimbane T, Germain N, Le NL, Pladys P, Gauthier C, Lahaie I, Abran D, Lavoie JC, Nuyt AM. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am J Physiol. 2007;292:R1236–45. doi: 10.1152/ajpregu.00227.2006. [DOI] [PubMed] [Google Scholar]
- Franco Mdo C, Ponzio BF, Gomes GN, Gil FZ, Tostes R, Carvalho MH, Fortes ZB. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009;85:327–33. doi: 10.1016/j.lfs.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Noakes PS, Thomas R, Lane C, Mori TA, Barden AE, Devadason SG, Prescott SL. Association of maternal smoking with increased infant oxidative stress at 3 months age. Thorax. 2007;62:714–7. doi: 10.1136/thx.2006.061630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin JE, Petre MA, Lehman MA, Raha S, Gerstein HC, Morrison KM. Maternal nicotine exposure increases oxidative in the offspring. Free Radic Biol Med. 2008;44:1919–1925. doi: 10.1016/j.freeradbiomed.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Sharpe GM. Chronic exposure to nicotine alters endothelium-dependent arteriolar dilation: effect of superoxide dismutase. J Appl Physiol. 1999;86:1126–1134. doi: 10.1152/jappl.1999.86.4.1126. [DOI] [PubMed] [Google Scholar]
- Vadillo-ortega F, Perichart-Perera O, Espino S, Avila-Vergara MA, Ibarra I, Ahued R, Godines M, Parry S, Macones G, Strauss JF. BMJ; 2011. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomized controlled trial. 342:d2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AJ, Xiao D, Xiong F, Dixon B, Zhang L. Hypoxia-derived oxidative stress mediates epigenetic repression of PKCε gene in foetal rat hearts. Cardiovasc Res. 2012;93:302–10. doi: 10.1093/cvr/cvr322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman RC, Langley-Evans SC. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin Sci (Lond) 1998;94:373–381. doi: 10.1042/cs0940373. [DOI] [PubMed] [Google Scholar]
- Xiao D, Dasgupta C, Chen M, Zhang K, Buchholz J, Xu Z, Zhang L. Inhibition of DNA methylation reverses norepinephrine-induced cardiac hypertrophy in rats. Cardiovasc Res. 2014;101:373–82. doi: 10.1093/cvr/cvt264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin JE, Woynillowicz AK, Hettinga BP, Tarnopolsky MA, Morrison KM, Gerstein HC, Holloway AC. Maternal antioxidants prevent β-cell apoptosis and promote formation of dual hormone-expressing endocrine cells in male offspring following fetal and neonatal nicotine exposure. J Diabetes. 2012;4:297–306. doi: 10.1111/j.1753-0407.2012.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetlycysteine. Biochim Biophys Acta. 2013;1830:4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Haleagrahara N, Julian V, Chakravarthi S. N-acetylcysteine offers cardioprotection by decreasing cardiac lipid hydroperoxides and 8-isoprostane level in isoproterenol-induced cardiotoxicity in rats. Cardiovasc Toxicol. 2011;11:373–381. doi: 10.1007/s12012-011-9132-0. [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Dluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Xiao D, Yang S, Zhang L. Prenatal cocaine exposure causes sex-dependent impairment in the myigenic reacrivity of coronary arteries in adult offspring. Hypertension. 2009;54:1123–1128. doi: 10.1161/HYPERTENSIONAHA.109.138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadoke PW, Lindsay RS, Seckl JR, Walker BR, Kenyon CJ. Altered vascular contractility in adult female rats with hypertension programmed by prenatal glucocorticoid exposure. J Endocrinol. 2006;188:435–442. doi: 10.1677/joe.1.06506. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Nishina H, Hansan MA, Poston T. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;503:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco Mdo C, Akamine EH, Di Marco GS, Casarini DE, Fortes ZB, Tostes RC, Carvalho MH, Nigro D. NADPH oxidase and enhanced superoxide generation in intrauterine undernourished rats: involvement of the renin-angiotensin system. Cardiovasc Res. 2003;59:767–775. doi: 10.1016/s0008-6363(03)00461-9. [DOI] [PubMed] [Google Scholar]
- Roghair RD, Wemmie JA, Volk KA, Scholz TD, Lamb FS, Segar JL. Maternal antioxidant blocks programmed cardiovascular and behavioural stress responses in adult mice. Clin Sci (Lond) 2011;121:427–436. doi: 10.1042/CS20110153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yzydorczyk C, Gobeil F, Jr, , Camonie G, Lahaie I, Le NL, Samarani S, Ahmad A, Lavoie JC, Oligny LL, Pladys P, Hardy P, Nuyt AM. Exaggerated vasomotor response to Ang II in rats with fetal programming of hypertension associated with exposure to a low-protein diet during gestation. Am J Physiol Regul Comp Physiol. 2006;291:R1060–R1068. doi: 10.1152/ajpregu.00798.2005. [DOI] [PubMed] [Google Scholar]
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2011;164:1400–1409. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- Xiao D, Dasgupta C, Li Y, Huang X, Zhang L. Perinatal nicotine exposure increases angiotensin II receptor-mediated vascular contractility in adult offspring. PLoS One. 2014;9:e108161. doi: 10.1371/journal.pone.0108161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers JW, Ribisl KM, Brownstein JS. Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. Am J Prev Med. 2011;40:448–53. doi: 10.1016/j.amepre.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Noel JK, Rees VW, Connolly GN. Electronic cigarettes: a new “tobacco” industry? Tob Control. 2011;20:81. doi: 10.1136/tc.2010.038562. [DOI] [PubMed] [Google Scholar]
- Flouris AD, Poulianiti KP, Chorti MS, Jamurtas AZ, Kouretas D, Owolabi EO, Tzatzarakis MN, Tsatsakis AM, Koutedakis Y. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem Toxicol. 2012;50:3600–3603. doi: 10.1016/j.fct.2012.07.025. [DOI] [PubMed] [Google Scholar]