Abstract
Oocyte aging has a significant impact on reproductive outcomes both quantitatively and qualitatively. However, the molecular mechanisms underlying the age-related decline in reproductive success have not been fully addressed. BRCA is known to be involved in homologous DNA recombination and plays an essential role in double-strand DNA break repair. Given the growing body of laboratory and clinical evidence, we performed a systematic review on the current understanding of the role of DNA repair in human reproduction. We find that BRCA mutations negatively affect ovarian reserve based on convincing evidence from in vitro and in vivo results and prospective studies. Because decline in the function of the intact gene occurs at an earlier age, women with BRCA1 mutations exhibit accelerated ovarian aging, unlike those with BRCA2 mutations. However, because of the still robust function of the intact allele in younger women and because of the masking of most severe cases by prophylactic oophorectomy or cancer, it is less likely one would see an effect of BRCA mutations on fertility until later in reproductive age. The impact of BRCA2 mutations on reproductive function may be less visible because of the delayed decline in the function of normal BRCA2 allele. BRCA1 function and ataxia-telangiectasia-mutated (ATM)-mediated DNA repair may also be important in the pathogenesis of age-induced increase in aneuploidy. BRCA1 is required for meiotic spindle assembly, and cohesion function between sister chromatids is also regulated by ATM family member proteins. Taken together, these findings strongly suggest the implication of BRCA and DNA repair malfunction in ovarian aging.
Keywords: BRCA, BRCA1, BRCA2, fertility, infertility, mechanisms of ovarian aging, mutations, oocyte, oocyte DNA repair, ovarian reserve
INTRODUCTION
Declining fertility is among the earliest phenotypic manifestations of aging in humans. The human genome is subject to continuous DNA damage, amounting up to 1 000 000 molecular lesions per cell per day [1]. Without continuous active DNA repair mechanisms, an organism's life span would be extremely limited. DNA damage can occur in the form of both single-strand DNA breaks and double-strand DNA breaks (DSBs). Because DSBs can affect both copies of a gene, they can result in lethal consequences for cells if left unrepaired, including severe mutagenesis, carcinogenesis, cell senescence, or apoptotic cell death [2].
BRCA1 and BRCA2 genes belong to the family of ataxia-telangiectasia-mutated (ATM)-mediated DNA DSB repair genes that play a critical role in the safeguarding of DNA integrity [3]. The best-known clinical consequence of BRCA mutations is breast and ovarian carcinogenesis, especially that occurring prior to menopause. In addition, several other malignancies, such as pancreatic cancer, melanoma, and prostate cancer, are also associated with such mutations [4]. Increasing evidence shows that mutations in other DNA repair genes are linked to breast and other cancer types [5, 6].
DNA damage is particularly problematic for nondividing or slowly dividing cells, where unrepaired damage will tend to accumulate over time. In contrast, in rapidly dividing cells, unrepaired DNA damage that does not kill the cell by blocking replication will tend to cause replication errors and thus mutation, and nearly all mutations are typically deleterious to cell's survival and hence are not passed on to the next generation of cells. However, rare mutations that create a survival advantage may result in the clonal expansion of the mutant cells, potentially giving rise to cancer. Thus, DNA damage is a common initiator of carcinogenesis in somatic cells, as a result of elevated mutations in frequently dividing cells [7]. In contrast, in resting cells, such as the oocyte of the primordial follicle, DNA damage likely accumulates over time due to the absence of the mechanisms to eliminate the faulty cells during replication, and thus causes aging. In fact, this paradigm would perfectly explain the triangular relationship between BRCA mutations, breast/ovarian malignancies, and oocyte aging.
Unlike in the instance of single-strand DNA breaks, when the repair can take place using the complementing strand, DSB repair requires a more complex process. The two main mechanisms of DNA DSB repair include nonhomologous end joining repair and homologous recombination (HR) repair. Although nonhomologous end joining may play a more predominant role in mitotic cells, HR is the main mode of DSB repair in meiotic cells [8]. Hence, in oocytes HR would be expected to take the dominant role to mend accumulating DSBs.
Recently emerging evidence began to tie DNA DSB repair and BRCA function to ovarian aging. Given that a sufficient body of laboratory and clinical evidence has accumulated, we undertook this systematic review to summarize the current understanding of the role of DNA repair and BRCA mutations in human reproduction.
METHODOLOGY
We searched the published articles in PubMed database, containing key words BRCA, BRCA1, BRCA2, Mutations, Fertility, Ovarian Reserve, Infertility, Mechanisms of Ovarian Aging, Oocyte, and Oocyte DNA repair in the English-language literature. We did not include abstracts or conference proceedings because the data are usually difficult to assess. This yielded 2426 articles, and by cross-referencing a final number of 64 articles were found to be relevant to the topic. Of these articles, 45 were laboratory studies, 17 were clinical studies, and 1 was both [9]. Of those, four were prospective and three of them studied Anti-Müllerian-Hormone (AMH) levels.
OVERVIEW OF ATM-MEDIATED DNA DSB REPAIR
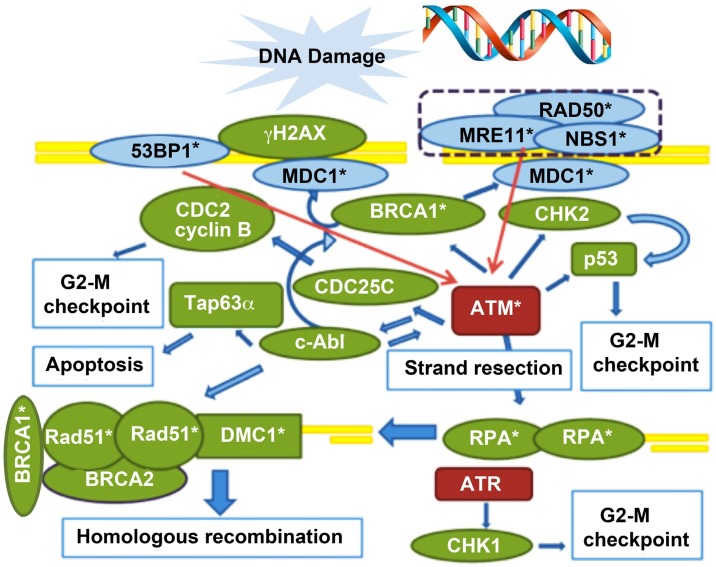
A complex process of recognition and repair mechanisms evolved in the cell (Fig. 1). When DNA DSBs occur, this damage is sensed within seconds by the MRN complex, which consists of MRE11, RAD50, and NBS1. This complex serves as the platform for the other DNA repair proteins to carry on the vital repair. The MRN complex is assisted in DNA damage recognition by P53BP1, which senses the changes in chromatin structure, and they consequently activate ATM. ATM is the orchestrator of the DNA DSB repair via HR, phosphorylating many downstream modulators. The γH2AX is another critical member of the process that, after being phosphorylated (γH2AX), is localized to DSB sites in a one-to-one fashion. Because of this precise match to individual DSB sites, DNA DSBs can be quantified by visualization of γH2AX foci via confocal microscopy.
FIG. 1.
ATM-mediated DNA DSB repair pathway. DNA damage is sensed by the MRN complex (sensor of DSBs, consists of MRE11, RAD50, and NBS1) and 53BP1 (sensor of changes in chromatin structure), which consequently activate ATM (red arrows). MDC1 binds to γH2AX via BRCA1 and is involved in the retention of the MRN complex to chromatin, accumulation of ATM, and mediation of the interaction between ATM and γH2AX. ATM phosphorylates γH2AX and activates downstream pathways leading to either DNA repair (activation of DNA strand resection, which leads to HR), or cell cycle arrest (via CHK2 and inhibition of CDC2, only G2/M highlighted as applicable to oocyte) and/or apoptosis (via c-abl and TAp63α). DNA strand resection is necessary to invade in the homologous DNA strand. The resulting single-strand (ss) DNA is coated with RPA, which in turn activates ATR and leads to cell cycle arrest (via CHK1). In germ cells, RPA is eventually replaced by Rad51and DMC1 (the latter is germ cell specific) through a BRCA2-mediated process, which results in the initiation of HR. Hence the declining function of key DNA repair proteins, such as BRCA1, Rad51, MRE11, and ATM, would lead to impaired DNA repair function, which would then shift the pathways from cell survival mode to cell death for the genomic protection of the species. DSB sensor proteins are in blue, effectors are in green, and transducers are in red. Molecules also involved in meiotic recombination are denoted with an asterisk (*). (Reproduced from Titus et al. [9] with permission from Science Translational Medicine.)
Another protein in this pathway, MDC1, binds to γH2AX via BRCA1 and is involved in the retention of the MRN complex to chromatin, accumulation of ATM, and mediation of the interaction between ATM and γH2AX. ATM phosphorylates γH2AX and activates downstream pathways, leading to cell cycle arrest (via CHK2 and inhibition of CDC2) [10], DNA repair (activation of DNA strand resection, which leads to HR), and/or apoptosis (via c-abl and TAp63α) [11]. DNA strand resection is necessary to invade in the homologous DNA strand. The resulting single-strand DNA is coated with RPA, which in turn activates ATR and leads to cell cycle arrest (via CHK1). In germ cells, RPA is eventually replaced by Rad51 and DMC1 (the latter being germ cell specific) through a BRCA2-mediated process, which results in the initiation of HR [12]. It is through this complex interaction of ATM-mediated DNA DSB repair molecules that the cell, in our case the oocyte, fights to shield itself against the continuous onslaught of genotoxic stressors.
CLINICAL AND EARLY TRANSGENIC MICE DATA IN SUPPORT OF THE ROLE OF DNA DSB REPAIR IN OOCYTE AGING
There are numerous lines of evidence from clinical observations that supported the relationship between DNA repair and reproduction. While performing ovarian stimulation for fertility preservation in women with breast cancer, we observed that those with BRCA1 mutations especially produced fewer oocytes in comparison with those who tested negative for those mutations, as well as an untested low-risk population [13]. Furthermore, a prior study investigating the hormonal status of BRCA1 mutation carriers reported earlier menopause compared with those tested for the same mutations [14].
Also, the disorders of DNA DSB repair mechanisms are associated with gonadal failure. In Fanconi anemia (FA), a genetic disease of DSB repair, females experience early menopause and gonadal failure [15]. Ovaries of Fanconi-gene mutant mice are hypoplastic and exhibit greatly reduced numbers of primordial follicles [16]. Interestingly, FANCD1, one of the genes responsible for FA, is none other than the BRCA2 gene, and the activated version of another gene involved in FA, FANCD2, interacts with BRCA1 [17].
ATM is a major orchestrator of DSB repair pathways, involving BRCA1 and FANCD1/BRCA2. Most strikingly, gonadal failure occurs in both sexes of ATM-knockout mice, as a result of massive germ cell apoptosis at or shortly after the prophase I of the first meiotic division [18]. Collectively, these observations lend strong support to the validity and significance of the hypothesis that the intact DNA repair is critical for successful reproduction, and impaired DNA repair can lead to reproductive aging and premature depletion of ovarian reserve.
RECENT LABORATORY AND TRANSLATIONAL DATA IN SUPPORT OF THE ROLE OF IMPAIRED DNA DSB REPAIR IN OVARIAN AGING
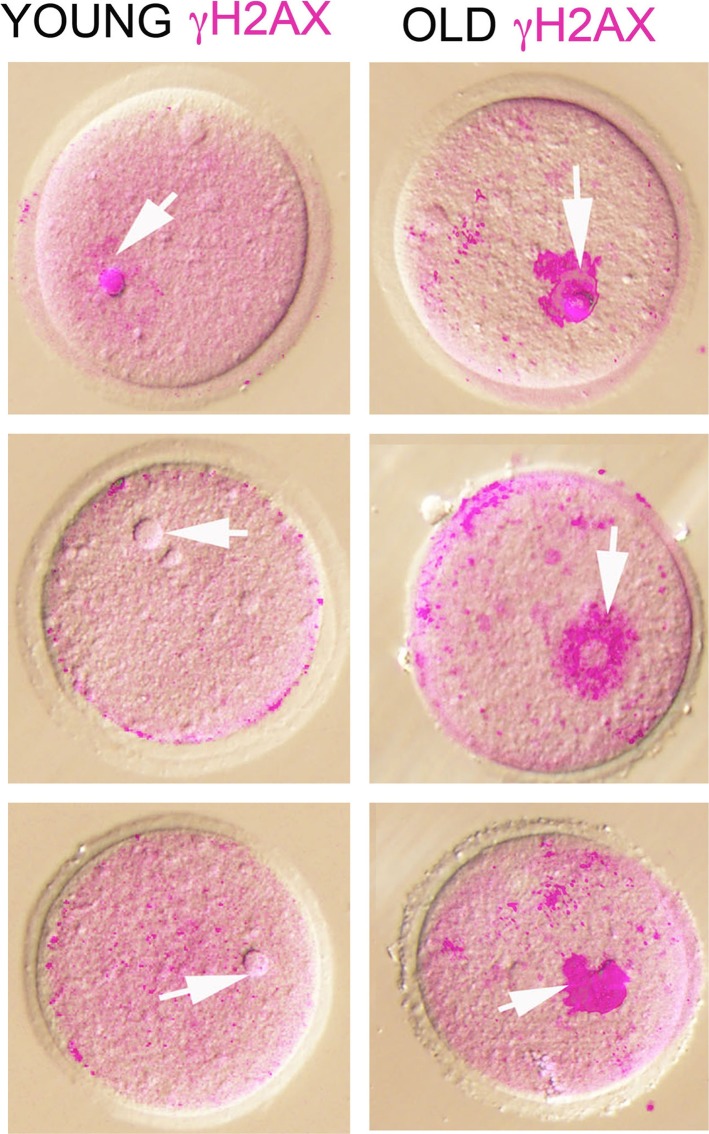
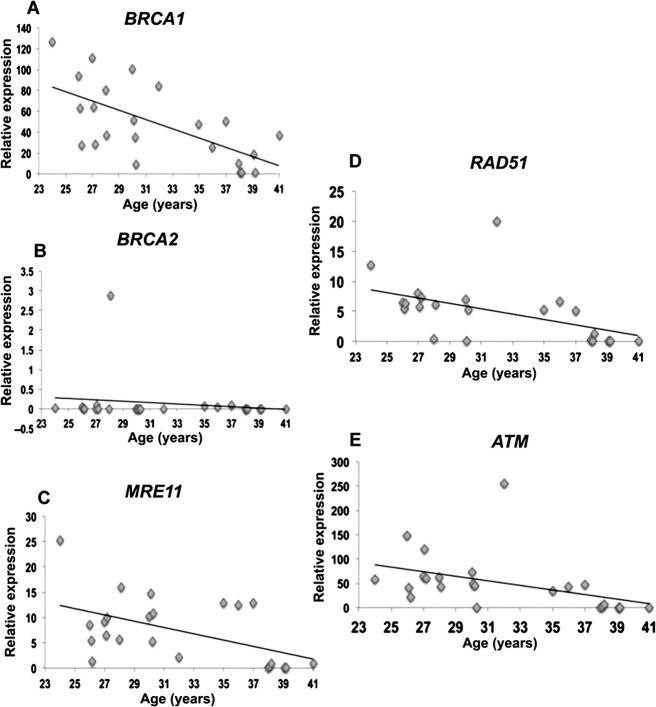
Given this background and to determine the role of DNA DSB repair in oocyte aging, we designed studies in mice and human. We hypothesized that DNA DSB repair efficiency declines with age such that oocytes accumulate DNA DSBs over time, resulting in reduced ovarian reserve and quality, and this may explain age-related decline in fertility. First we observed that with age there was significant accumulation of DNA DSBs in primordial follicles of mice (Fig. 2). We then analyzed human primordial follicle oocytes and found that oocytes from older females were more likely to accumulate DNA DSBs. Next, to test whether DNA DSB repair efficiency declines with age, we collected immature oocytes during in vitro fertilization procedures for women ages 21–43 yr with nonovarian infertility, and quantified expression of key ATM-mediated DNA DSB repair genes, including BRCA1, BRCA2, ATM, MRE11, and Rad51 (Fig. 3). Notably, expression of all genes but BRCA2 declined with age. This decline was more prominent after age 36 yr, consistent with the well-known sharp decline in the indices of human fertility as well as the acceleration of primordial follicle loss seen after the same age [9]. These findings heralded the existence of an important role for DNA DSB repair in human oocyte aging.
FIG. 2.
Superimposed confocal immunofluorescence and differential interference contrast images of young and old germinal vesicle oocytes stained with anti-γH2AX antibody. Nuclear γH2AX (pink), representing DSBs, is consistently and drastically increased in the old oocytes. Arrows point to the nuclear region. Original magnification ×20. (Reproduced from Titus et al. [9] with permission from Science Translational Medicine.)
FIG. 3.
Expression of DNA repair genes in human oocytes. Scatter plots represent the relative expression of DNA repair genes assessed by quantitative RT-PCR in human oocytes from 24 patients ages 24 to 41 yr, some of which show significant age-related declines. Relative expression is defined as the fold change in expression of the DNA repair genes compared with the housekeeping gene hypoxanthine-guanine phosphoribosyl transferase (HPRT). There is a significant decrease in the expression of DNA repair genes: (A) BRCA1 (r = 0.60; P < 0.001, linear regression analysis and Student t-test), (C) MRE11 (r = 0.447; P < 0.05), (D) RAD51 (r = 0.5; P < 0.01,), and (E) ATM (r = 0.4; P < 0.01), when the expression in old is compared with young human oocytes. B) BRCA2 gene expression did not decline significantly within the age range studied (r = 0.1, P = 0.75). (Reproduced from Titus et al. [9] with permission from Science Translational Medicine.).
Further, to test whether acutely active DNA DSB repair was critical in oocyte survival, we downregulated BRCA1, ATM, MRE11, and Rad51 in mouse oocytes and subjected them to genotoxic stress via H202 exposure, and we found that the oocytes became more sensitive to environmental stress. Compared with the controls, these oocytes were more likely to accumulate DNA DSB, become apoptotic, and die in vitro. When BRCA1 was overexpressed, the oocytes tended to be more resistant to genotoxic stress, suggesting that DNA DSB repair is critical in oocyte survival and its resistance to oxidative stress [9].
Recently the relationship between DNA DSB repair and aging in the primordial follicles of female rats has been reported [19]. Consistent with our findings, the investigators also observed a significant decline in mRNA levels of key DNA DSB repair genes, such as BRCA1 and RAD51, as well as H2AX. In addition, a decline in the phosphoprotein levels of BRCA1 was detected in the primordial follicles of aged rats. Besides the DNA DSB repair genes, the same study reported a decline in the expression of ERCC2 (excision repair cross-complementing group 2, also called xeroderma pigmentosum group [D-XPD]), which is an integral member of the basal transcription factor BTF2/TFIIH complex. These results further support the potential importance of DNA repair in oocyte aging.
The above laboratory findings were also confirmed in an in vivo, transgenic mouse model. In that model, a BRCA1-mutant mouse carrying a deletion of 330 bp in intron 10 plus 407 bp in exon 11 of the Brca1 gene, which severely impairs DNA repair functions, was used [20]. It has been reported that in mouse embryonic cells that have a defective exon 11 of BRCA1, the checkpoint at the G2–M phase of the cell cycle is impaired, leading to a phenotype of unequal chromosomal segregation, abnormal nuclear division, and aneuploidy [21]. Homozygous mutation of the BRCA1 gene is lethal; hence, we only studied the heterozygote mouse with this deletion. We also studied BRCA2-mutant mice that carried a deletion in exon 27 of the Brca2 gene. This region codes for domains that interact with RAD51 [22]; the latter interaction is essential for the HR repair function of BRCA2 protein. This mutation does not result in lethality, so we were able to study both the heterozygote and homozygote mouse.
The reproductive performance studies in these transgenic mice showed that the BRCA1- but not the BRCA2-mutant mice produced fewer oocytes in response to ovarian stimulation, and had a smaller litter size and fewer primordial follicles at 5 days of life compared with the wild-type (WT) mice. Moreover, the BRCA1 but not the BRCA2 mice accumulated more DNA DSBs with age compared with WT mice. In addition to BRCA1 deficiency in oocytes, BRCA1 reduction in granulosa cells also is associated with ovarian aging in monkeys [23].
To translate these findings to human, we finally studied women with breast cancer who were tested for BRCA mutations. We measured their serum AMH levels and found that women with BRCA1 but not BRCA2 mutations had a significantly lower levels of AMH compared with BRCA mutation-negative women with breast cancer [9]. Therefore, both in vitro and in vivo studies in mice and human gave us parallel results, firmly supporting an essential role for intact DNA DSB repair in the maintenance of oocyte health and ovarian reserve.
Furthermore, a meta-analysis of 22 genome-wide association studies in 38 968 women of European decent identified several DNA repair genes to be associated with age at natural menopause [24]. One of the five candidate genes that were significantly associated with age at natural menopause was DMC1. DMC1 encodes for a protein that is essential for meiotic HR and is regulated by NOBOX, mutations that cause primary ovarian insufficiency [25].
Collectively, these data pointed to a critical role for DNA DSB repair in human oocyte aging. The seeming absence of an impact of BRCA2 gene dysfunction on ovarian reserve in the mutant mice as well as the women we studied arises, we believe, from the fact that the decline in the function of the BRCA2 gene occurs toward the very end of reproductive life and that it has functions differing from those of the BRCA1 gene. In fact, the prominence and timing of BRCA1 expression decline compared with BRCA2 also fit well with the earlier occurrence of BRCA1 mutation-related ovarian cancers [26].
POTENTIAL ROLE OF DNA DSB REPAIR AND BRCA GENES IN OOCYTE QUALITY
One of the most consequential manifestations of human oocyte aging is the increased incidence of aneuploidy in human MII oocytes. The percentage of aneuploid oocytes has been reported to increase from 20% in females in their twenties to more than 50% in women older than 40 yr [27]. The mechanisms of this age-related increase in oocyte aneuploidy are not fully revealed. The current hypothesis dictates that the oocytes of primordial follicles, which rest in the prophase of first meiotic division (PI), are passively subjected to environmental and other unknown factors affecting oocyte health, and this results in the aging of the oocyte pool [28–30].
Homologous recombination takes place at PI in primary oocytes prior to meiotic arrest. Oocytes use the same DNA repair mechanisms to mend DSBs that naturally occur during HR [31]. Although this process increases genetic diversity, it also may play a role in stabilizing the metaphase plate by creating physical bonds. In fact, crossovers are associated with HR events and can have stabilizing or destabilizing effects on the genome. In meiosis, crossovers are highly regulated such that at least one crossover occurs between each pair of homologous chromosomes to ensure proper chromosome segregation, while excess crossovers are suppressed [32]. In mouse, it has long been observed that chiasmata frequency decreases with age [33], and in some strains of mice an age-related increase in aneuploidy rates in offspring is associated with a decrease in recombination frequency between homologs [30]. Failure to repair DSBs, or misrepair, indeed results in large-scale chromosome changes, including deletions, translocations, and chromosome loss in numerous cell types [34].
Moreover, cohesins regulate sister chromatid cohesion and recent evidence indicates that they migrate to DSB repair sites independently of the normal replication cycle [35]. Additional studies indicate that cohesion function is also regulated by BRCA1 and the member of the ATM-mediated DNA DSB repair family [36, 37].
DNA damage also could occur at telomeres and shortens telomeres, causing aneuploidy and abortion or genetic diseases, such as Down syndrome [38, 39]. Old mouse oocytes show shorter telomeres than those of young oocytes [40]. Short telomeres also can reduce homologous pairing and recombination and cause meiotic abnormality, including aberrant chromosome alignment at metaphase and disruption of spindles, resulting in aneuploidy [41–43]. Notably, BRCA1/2 is involved in telomere maintenance [44–48]. Interestingly, telomere shortening also alters kinetics of the DNA DSB in response to ionization radiation in human cells [49].
Furthermore, it has been shown that BRCA1 is required for meiotic spindle assembly and spindle assembly checkpoint activation in mouse oocytes [50]. Recent laboratory studies also showed that severe DNA DSBs reduced and delayed entry into M phase through activation of DNA damage checkpoint in mouse oocytes. Chromosome spread of MI and MII oocytes with severe DNA DSBs display fragmented chromosomes after germinal vesicle breakdown (GVBD), indicating that intact DNA DSB repair may be vital in maintaining oocyte quality [51]. Interestingly, activated oocytes harboring damaged DNA manifest cytofragmentation, a morphological hallmark of apoptosis, and this represents a unique checkpoint for DNA integrity at cleavage stages of embryonic development [52].
Thus, it is probable that the formation and repair of DSBs are ongoing acute processes throughout reproductive life. During aging in oocytes, diminished DSB repair capacity may drive the accumulation of lethal DSBs, leading to oocyte compromise and death. In tandem, alteration of the ATM-mediated DSB repair/HR mechanism results in meiotic dysfunction. This hypothesis would explain the age-related decline in reproductive performance, and the increase in the incidence of aneuploidy in children born to older women under the same mechanism. However, further laboratory evidence and translational studies are required to prove and substantiate this hypothesis.
RECENT CLINICAL STUDIES INVESTIGATING THE ASSOCIATION OF BRCA MUTATIONS WITH ACCELERATED OVARIAN AGING
Reproduction studies from women with BRCA mutations are important for a number of reasons. First, a substantial percentage of women undergoing fertility preservation for breast cancer carry BRCA mutations, and it is important to understand whether carrying these mutations will affect the success of fertility preservation. Second, the studies of ovarian function in BRCA mutation carriers provide essential clues into the role of DNA repair in reproduction. However, given the heterogeneity of the BRCA mutation population and the end points that were studied, as well as the confounding factors, not all studies can be considered under the same light.
The studies that are investigating the role of BRCA mutations in ovarian aging can be grouped into four:
Those that use menopausal age as the end point;
Those that use serum ovarian reserve marker or primordial follicle count as the end point;
Those that use ovarian stimulation outcomes as the surrogate;
Those that assess fertility.
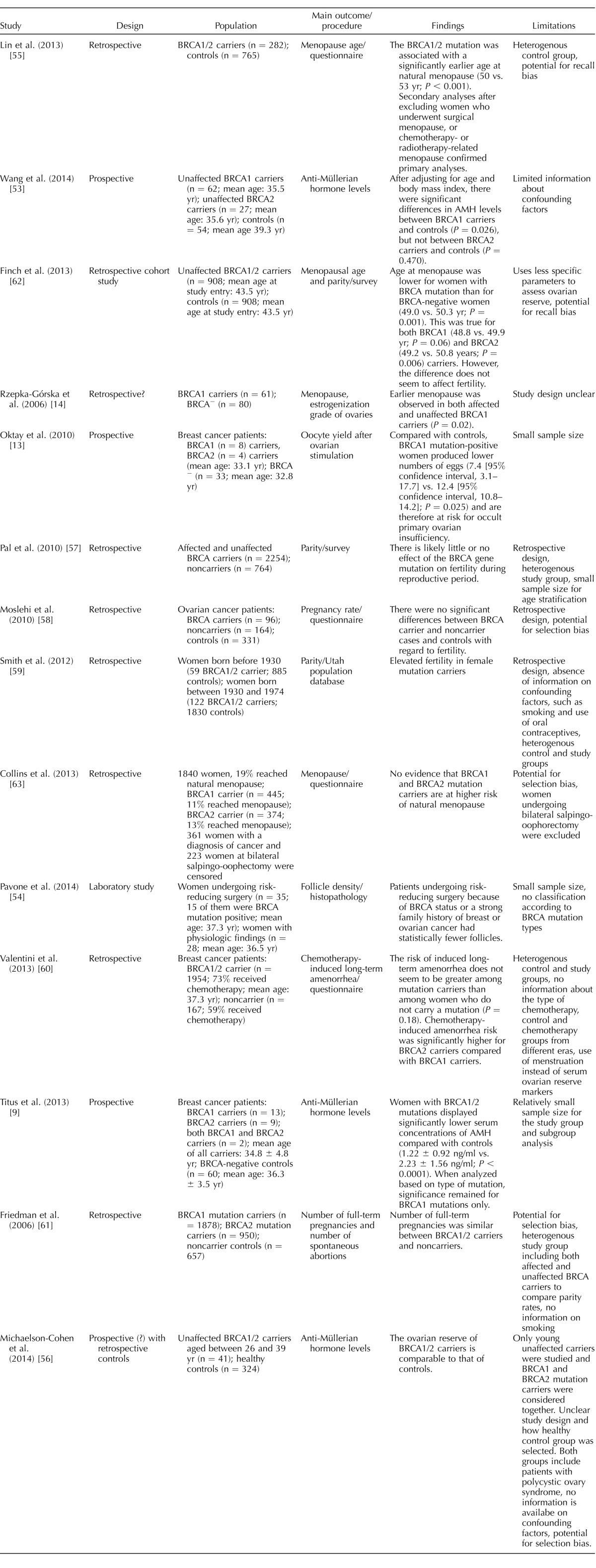
Overall, the best quality evidence comes from those studies that investigate the serum ovarian reserve marker AMH or primordial follicle counts in women with and without known BRCA mutations. These studies are generally prospective and, by virtue of hormone measurements or follicle counts, are quantitative [9, 13, 14, 53, 54]. Those that looked at menopausal age and fertility rates are retrospective/cohort studies [55–63]. Some are also subject to data heterogeneity and lack of appropriate controls [59, 60]. These studies as well as their major findings and weaknesses are summarized in Table 1.
TABLE 1.
Studies investigating the relationship of BRCA mutations with ovarian reserve and fertility.
Overall, the majority of the studies with sufficient sample size and/or which are prospective in nature support the notion that ovarian reserve is diminished in women with BRCA mutations, especially in those who are affected [9, 13, 14, 53, 54]. Other studies that are retrospective in nature and without any stratification for confounders (such as BRCA mutation type; age; region; confounding factors, such as oral contraceptive use and smoking; and chemotherapy type if a patient is an affected carrier) did not detect any differences between BRCA mutation carriers and those who were not carriers [56–61]. The two studies that looked at the parity were questionnaire studies, which also did not have the numbers to address age stratification [57, 58]. Interestingly, one study claimed increased fertility in women with BRCA mutations [59]. However, the study was hampered by retrospective design; absence of information on confounding factors, such as smoking and oral contraceptive use; and heterogeneous control (including untested women) and study (including both affected and unaffected carriers and no differentiation of BRCA1 vs. BRCA2 mutations) groups.
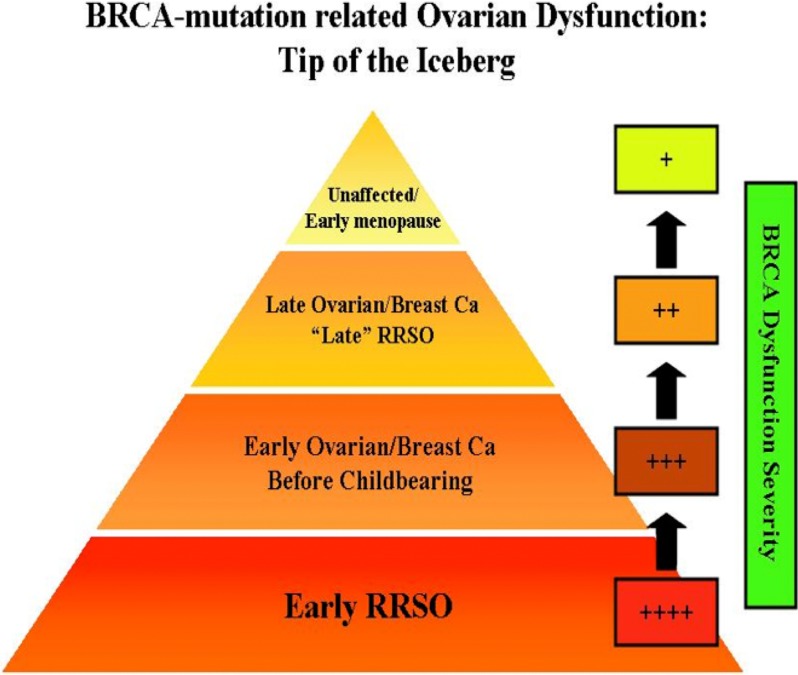
Another important selection bias that may hamper the detection of accelerated ovarian aging in women with BRCA mutations is the removal of the most severely affected individuals from a likely study population because of earlier risk-reducing salpingo-oophorectomy (RRSO), ovarian cancer, premature ovarian failure, and chemotherapy for breast cancer (Fig. 4). This earlier attrition of severe cases of BRCA dysfunction would enrich the unaffected and least affected individuals for analysis in a general BRCA mutation population. Moreover, studies that target unaffected carriers may miss any small effect of BRCA mutations, because the decline in the function of the intact BRCA gene may be less significant in unaffected women. Supporting this possibility, the studies found small but significant differences (1–3 yr) in menopausal age between the unaffected BRCA mutation carriers and the controls. Such small differences in ovarian reserve may be difficult to detect by serum AMH assessments or fertility evaluations.
FIG. 4.
A proposed explanation for the limitations in detecting BRCA mutation-related decline in ovarian reserve and fertility. Those with the most diminished BRCA function due to a severe mutation plus early age-related decline in the function of the normal allele either have a highly significant history of early ovarian cancer and are subjected to RRSO early on, or experience early ovarian cancer and have ovarian resection or compromised ovarian function due to chemotherapy for breast cancer, and are thereby unable to display the signs of early ovarian aging. Those with the least BRCA dysfunction may have breast or ovarian cancer late in reproductive life or may have RRSO relatively late, typically after age 40 yr. Because their BRCA and DNA repair function has not been severely compromised during the majority of their reproductive life, they will not have apparent fertility issues. The majority of women will fall into the latter category. Then there are those who do not develop cancer during their reproductive age. Those people will only have minimal BRCA dysfunction and will show a slight decline in ovarian reserve, which will translate into menopause that is earlier by 1–3 yr. In addition, BRCA2 function declines more slowly with age compared with BRCA1, explaining the more common association of BRCA1 mutations with diminished ovarian reserve. Because of these confounders, it may be difficult to clinically detect the impact of BRCA mutations on human fertility. We may have several windows into the relationship between BRCA function and ovarian aging—among the most severely affected and undergoing ovarian stimulation for fertility preservation or among the less affected and postponing childbearing to later reproductive ages. This pyramid explains why BRCA mutations are not eliminated from the population and why retrospective studies may fail to detect any association between BRCA mutations and ovarian function.
Interestingly, our first observation of low response to ovarian stimulation came from prospectively studying affected women who presented for fertility preservation with a novel protocol using aromatase inhibitors [13]. If these women were not given this new option to undergo ovarian stimulation and preserve their fertility prior to chemotherapy, their diminished ovarian function would have been solely attributed to the impact of gonadotoxic chemotherapy.
On the other hand, had BRCA mutations created an absolute infertility state, the mutations would have become extinct. Furthermore, the function of the intact BRCA allele does not significantly decline until past age 36–37 yr, and hence it is unlikely that BRCA mutations will cause infertility in younger women. However, given the strong evidence of earlier menopausal age and lower AMH levels, infertility may become more likely in women carrying BRCA mutations who are in their later reproductive years [64].
CONCLUSIONS
In conclusion, there seems to be a relationship between BRCA gene function, intact DNA DSB repair function, and maintenance of ovarian reserve. Because of the earlier age-related decline in the function of the intact allele, women with BRCA1 mutations may have lower ovarian reserve in reproductive age compared with those with BRCA2 mutations. In general, because of the robust function of the intact allele in younger women, it is unlikely to see a widespread effect of BRCA mutations on fertility until later in reproductive age. Further prospective studies with homogeneous control and study groups with sufficient sample size are required to confirm the preexisting evidence and whether BRCA mutations result in accelerated reproductive aging and premature infertility. Additional basic science studies studying the relationship between BRCA function, DNA repair mechanisms, and oocyte quality are also needed.
Footnotes
Supported by National Institutes of Health/National Institute of Child Health and Human Development grant R01HD053112.
REFERENCES
- Peterson CL, Cote J. Cellular machineries for chromosomal DNA repair. Genes Dev. 2004;18:602–616. doi: 10.1101/gad.1182704. [DOI] [PubMed] [Google Scholar]
- Panier S, Boulton SJ. Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol. 2014;15:7–18. doi: 10.1038/nrm3719. [DOI] [PubMed] [Google Scholar]
- Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- Mersch J, Jackson MA, Park M, Nebgen D, Peterson SK, Singletary C, Arun BK, Litton JK. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian Cancer 2015. 15; 121 269 275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiiski JI, Pelttari LM, Khan S, Freysteinsdottir ES, Reynisdottir I, Hart SN, Shimelis H, Vilske S, Kallioniemi A, Schleutker J, Leminen A, Bützow R, et al. Exome sequencing identifies FANCM as a susceptibility gene for triple-negative breast cancer. Proc Natl Acad Sci U S A. 2014;111:15172–15177. doi: 10.1073/pnas.1407909111. 21; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longerich S, Li J, Xiong Y, Sung P, Kupfer GM. Stress and DNA repair biology of the Fanconi anemia pathway. Blood. 2014;124:2812–2819. doi: 10.1182/blood-2014-04-526293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best BP. Nuclear DNA damage as a direct cause of aging. Rejuvenation Res. 2009;12:199–208. doi: 10.1089/rej.2009.0847. [DOI] [PubMed] [Google Scholar]
- Sung P, Klein H. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat Rev Mol Cell Biol. 2006;7:739–750. doi: 10.1038/nrm2008. [DOI] [PubMed] [Google Scholar]
- Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, Oktay K. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans Sci Transl Med 2013. 5.172ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science. 2014;343:533–536. doi: 10.1126/science.1247671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Suh E. Defying DNA double-strand break-induced death during prophase I meiosis by temporal TAp63α phosphorylation regulation in developing mouse oocytes. Mol Cell Biol. 2014;34:1460–1473. doi: 10.1128/MCB.01223-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki A, Schoenmakers S, Baarends WM. DNA double strand break repair, chromosome synapsis and transcriptional silencing in meiosis. Epigenetics. 2010;5:255–266. doi: 10.4161/epi.5.4.11518. [DOI] [PubMed] [Google Scholar]
- Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28:240–244. doi: 10.1200/JCO.2009.24.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepka-Górska I, Tarnowski B, Chudecka-Głaz A, Górski B, Zielińska D, Tołoczko-Grabarek A. Premature menopause in patients with BRCA1 gene mutation. Breast Cancer Res Treat. 2006;100:59–63. doi: 10.1007/s10549-006-9220-1. [DOI] [PubMed] [Google Scholar]
- Alter BP, Frissora CL, Halpérin DS, Freedman MH, Chitkara U, Alvarez E, Lynch L, Adler-Brecher B, Auerbach AD. Fanconi's anaemia and pregnancy. Br J Haematol. 1991;77:410–418. doi: 10.1111/j.1365-2141.1991.tb08593.x. [DOI] [PubMed] [Google Scholar]
- Luo Y, Hartford SA, Zeng R, Southard TL, Shima N, Schimenti JC. Hypersensitivity of primordial germ cells to compromised replication-associated DNA repair involves ATM-p53-p21 signaling. PLoS Genet. 2014;10:e1004471. doi: 10.1371/journal.pgen.1004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, Grompe M, D'Andrea AD. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell. 2001;7:249–262. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- Morita Y, Maravei DV, Bergeron L, Wang S, Perez GI, Tsutsumi O, Taketani Y, Asano M, Horai R, Korsmeyer SJ, Iwakura Y, Yuan J, Tilly JL. Caspase-2 deficiency prevents programmed germ cell death resulting from cytokine insufficiency but not meiotic defects caused by loss of ataxia telangiectasia-mutated (Atm) gene function. Cell Death Differ. 2001;8:614–620. doi: 10.1038/sj.cdd.4400845. [DOI] [PubMed] [Google Scholar]
- Govindaraj V. Keralapura Basavaraju R, Rao AJ. Changes in the expression of DNA double strand break repair genes in primordial follicles from immature and aged rats. Reprod Biomed Online. 2015;30:303–310. doi: 10.1016/j.rbmo.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Huber LJ, Yang TW, Sarkisian CJ, Master SR, Deng CX, Chodosh LA. Impaired DNA damage response in cells expressing an exon 11-deleted murine Brca1 variant that localizes to nuclear foci. Mol Cell Biol. 2001;21:4005–4015. doi: 10.1128/MCB.21.12.4005-4015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Weaver Z, Linke SP, Li C, Gotay J, Wang XW, Harris CC, Ried T, Deng CX. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell. 1999;3:389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- Donoho G, Brenneman MA, Cui TX, Donoviel D, Vogel H, Goodwin EH, Chen DJ, Hasty P. Deletion of Brca2 exon 27 causes hypersensitivity to DNA crosslinks, chromosomal instability, and reduced life span in mice. Genes Chromosomes Cancer. 2003;36:317–331. doi: 10.1002/gcc.10148. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang X, Zeng M, Yuan J, Liu M, Yin Y, Wu X, Keefe DL, Liu L. Increased DNA damage and repair deficiency in granulosa cells are associated with ovarian aging in rhesus monkey. J Assist Reprod Genet. 2015;32:1069–1078. doi: 10.1007/s10815-015-0483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk L, Perry JR, Chasman DI, He C, Mangino M, Sulem P, Barbalic M, Broer L, Byrne EM, Ernst F, Esko T, Franceschini N. Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat Genet. 2012;44:260–268. doi: 10.1038/ng.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- Oktay K, Moy F, Titus S, Stobezki R, Turan V, Dickler M, Goswami S. Age-related decline in DNA repair function explains diminished ovarian reserve, earlier menopause, and possible oocyte vulnerability to chemotherapy in women with BRCA mutations. J Clin Oncol. 2014;32:1093–1094. doi: 10.1200/JCO.2013.53.5369. [DOI] [PubMed] [Google Scholar]
- Anaya Y, Tran ND. Delayed childbearing: impacts on fecundity and treatment. Med J Obstet Gynecol. 2013;1:1009. [Google Scholar]
- Vaskivuo TE, Anttonen M, Herva R, Billig H, Dorland M, te Velde ER, Stenbäck F, Heikinheimo M, Tapanainen JS. Survival of human ovarian follicles from fetal to adult life: apoptosis, apoptosis-related proteins, and transcription factor GATA-4. J Clin Endocrinol Metab. 2001;86:3421–3429. doi: 10.1210/jcem.86.7.7679. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- Jones KT. Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Hum Reprod Update. 2008;14:143–158. doi: 10.1093/humupd/dmm043. [DOI] [PubMed] [Google Scholar]
- Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- Champion MD, Hawley RS. Playing for half the deck: the molecular biology of meiosis. Nat Cell Biol. 2002;4(suppl):s50–s56. doi: 10.1038/ncb-nm-fertilityS50. [DOI] [PubMed] [Google Scholar]
- Henderson SA, Edwards RG. Chiasma frequency and maternal age in mammals. Nature. 1968;218:22–28. doi: 10.1038/218022a0. [DOI] [PubMed] [Google Scholar]
- Tronov VA. Loginova MIu, Kramarenko II. Methylnitrosourea as challenge mutagen in assessment of the DNA mismatch repair (MMR) activity: association with some types of cancer [in Russian] Genetika. 2008;44:686–692. doi: 10.1134/s1022795408050128. [DOI] [PubMed] [Google Scholar]
- Watrin E, Peters J. Cohesin and DNA damage repair. Exp Cell Res. 2006;312:2687–2693. doi: 10.1016/j.yexcr.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Skibbens RV. Cell biology of cancer: BRCA1 and sister chromatid pairing reactions? Cell Cycle. 2008;7:449–452. doi: 10.4161/cc.7.4.5435. [DOI] [PubMed] [Google Scholar]
- Kim ST, Xu B, Kastan MB. Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage. Genes Dev. 2002;16:560–570. doi: 10.1101/gad.970602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE. The evolution of ovarian oocyte decline with aging and possible relationships to Down syndrome and Alzheimer disease. Exp Gerontol. 1994;29:299–304. doi: 10.1016/0531-5565(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Feingold E, Chakraborty S, Dey SK. Telomere length is associated with types of chromosome 21 nondisjunction: a new insight into the maternal age effect on Down syndrome birth. Hum Genet. 2010;127:403–409. doi: 10.1007/s00439-009-0785-8. [DOI] [PubMed] [Google Scholar]
- Yamada-Fukunaga T, Yamada M, Hamatani T, Chikazawa N, Ogawa S, Akutsu H, Miura T, Miyado K, Tarín JJ, Kuji N, Umezawa A, Yoshimura Y. Age-associated telomere shortening in mouse oocytes. Reprod Biol Endocrinol. 2013;11:108. doi: 10.1186/1477-7827-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DL, Liu L. Telomeres and reproductive aging. Reprod Fertil Dev. 2009;21:10–14. doi: 10.1071/rd08229. [DOI] [PubMed] [Google Scholar]
- Liu L, Franco S, Spyropoulos B, Moens PB, Blasco MA, Keefe DL. Irregular telomeres impair meiotic synapsis and recombination in mice. Proc Natl Acad Sci U S A. 2004;101:6496–6501. doi: 10.1073/pnas.0400755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treff NR, Su J, Taylor D, Scott RT. Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011;7:e1002161. doi: 10.1371/journal.pgen.1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabuy E, Newton C, Slijepcevic P. BRCA1 knock-down causes telomere dysfunction in mammary epithelial cells. Cytogenet Genome Res. 2009;122:336–342. doi: 10.1159/000167820. [DOI] [PubMed] [Google Scholar]
- Martinez-Delgado B, Yanowsky K, Inglada-Perez L, de la Hoya M, Caldes T, Vega A, Blanco A, Martin T, Gonzalez-Sarmiento R, Blasco M, Robledo M, Urioste M, et al. Shorter telomere length is associated with increased ovarian cancer risk in both familial and sporadic cases. J Med Genet. 2012;49:341–344. doi: 10.1136/jmedgenet-2012-100807. [DOI] [PubMed] [Google Scholar]
- McPherson JP, Hande MP, Poonepalli A, Lemmers B, Zablocki E, Migon E, Shehabeldin A, Porras A, Karaskova J, Vukovic B, Squire J, Hakem R. A role for Brca1 in chromosome end maintenance. Hum Mol Genet. 2006;15:831–838. doi: 10.1093/hmg/ddl002. [DOI] [PubMed] [Google Scholar]
- Rosen EM. BRCA1 in the DNA damage response and at telomeres. Front Genet. 2013;4:85. doi: 10.3389/fgene.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu L, Montagna C, Ried T, Deng CX. Haploinsufficiency of Parp1 accelerates Brca1-associated centrosome amplification, telomere shortening, genetic instability, apoptosis, and embryonic lethality. Cell Death Differ. 2007;14:924–931. doi: 10.1038/sj.cdd.4402105. [DOI] [PubMed] [Google Scholar]
- Drissi R, Wu J, Hu Y, Bockhold CA, Dome JS. Telomere shortening alters the kinetics of the DNA damage response after ionizing radiation in human cells. Cancer Prev Res (Phila) 2011;4:1973–1981. doi: 10.1158/1940-6207.CAPR-11-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong B, Li S, Ai JS, Yin S, Ouyang YC, Sun SC, Chen DY, Sun QY. BRCA1 is required for meiotic spindle assembly and spindle assembly checkpoint activation in mouse oocytes. Biol Reprod. 2008;79:718–726. doi: 10.1095/biolreprod.108.069641. [DOI] [PubMed] [Google Scholar]
- Lin F, Ma XS, Wang ZB, Wang ZW, Luo YB, Huang L, Jiang ZZ, Hu MW, Schatten H, Sun QY. Different fates of oocytes with DNA double-strand breaks in vitro and in vivo. Cell Cycle. 2014;13:2674–2680. doi: 10.4161/15384101.2015.945375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Trimarchi JR, Smith PJS, Keefe DL. Checkpoint for DNA integrity at the first mitosis after oocyte activation. Mol Reprod Dev. 2002;62:277–288. doi: 10.1002/mrd.10094. [DOI] [PubMed] [Google Scholar]
- Wang ET, Pisarska MD, Bresee C, Chen YD, Lester J, Afshar Y, Alexander C, Karlan BY. BRCA1 germline mutations may be associated with reduced ovarian reserve. Fertil Steril. 2014;102:1723–1728. doi: 10.1016/j.fertnstert.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone ME, Hirshfeld-Cytron J, Tingen C, Thomas C, Thomas J, Lowe MP, Schink JC, Woodruff TK. Human ovarian tissue cortex surrounding benign and malignant lesions. Reprod Sci. 2014;21:582–589. doi: 10.1177/1933719113506498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WT, Beattie M, Chen LM, Oktay K, Crawford SL, Gold EB, Cedars M, Rosen M. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California. Cancer. 2013;119:1652–1659. doi: 10.1002/cncr.27952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson-Cohen R, Mor P, Srebnik N, Beller U, Levy-Lahad E, Eldar-Geva T. BRCA mutation carriers do not have compromised ovarian reserve. Int J Gynecol Cancer. 2014;24:233–237. doi: 10.1097/IGC.0000000000000058. [DOI] [PubMed] [Google Scholar]
- Pal T, Keefe D, Sun P, Narod SA. Fertility in women with BRCA mutations: a case-control study. Fertil Steril. 2010;93:1805–1808. doi: 10.1016/j.fertnstert.2008.12.052. [DOI] [PubMed] [Google Scholar]
- Moslehi R, Singh R, Lessner L, Friedman JM. Impact of BRCA mutations on female fertility and offspring sex ratio. Am J Hum Biol. 2010;22:201–205. doi: 10.1002/ajhb.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR., Hanson HA, Mineau GP, Buys SS. Effects of BRCA1 and BRCA2 mutations on female fertility. Proc Biol Sci. 2012;279:1389–1395. doi: 10.1098/rspb.2011.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini A, Finch A, Lubinski J, Byrski T, Ghadirian P, Kim-Sing C, Lynch HT, Ainsworth PJ, Neuhausen SL, Greenblatt E, Singer C, Sun P, et al. Chemotherapy-induced amenorrhea in patients with breast cancer with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2013;31:3914–3919. doi: 10.1200/JCO.2012.47.7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E, Kotsopoulos J, Lubinski J, Lynch HT, Ghadirian P, Neuhausen SL, Isaacs C, Weber B, Foulkes WD, Moller P, Rosen B, Kim-Sing C, et al. Spontaneous and therapeutic abortions and the risk of breast cancer among BRCA mutation carriers. Breast Cancer Res. 2006;8:R15. doi: 10.1186/bcr1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch A, Valentini A, Greenblatt E, Lynch HT, Ghadirian P, Armel S, Neuhausen SL, Kim-Sing C, Tung N, Karlan B, Foulkes WD, Sun P, et al. Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertil Steril. 2013;99:1724–1728. doi: 10.1016/j.fertnstert.2013.01.109. [DOI] [PubMed] [Google Scholar]
- Collins IM, Milne RL, McLachlan SA, Friedlander M, Hickey M, Weideman PC, Birch KE, Hopper JL, Phillips KA. Do BRCA1 and BRCA2 mutation carriers have earlier natural menopause than their noncarrier relatives?: results from the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer. J Clin Oncol. 2013;31:3920–3925. doi: 10.1200/JCO.2013.49.3007. [DOI] [PubMed] [Google Scholar]
- Santoro N. BRCA mutations and fertility: do not push the envelope! Fertil Steril. 2013;99:1560. doi: 10.1016/j.fertnstert.2013.01.091. [DOI] [PubMed] [Google Scholar]