Abstract
During the first trimester of pregnancy, appropriate regulation of estradiol (E2) is essential for normal placental development. Previous studies demonstrate that premature elevation in E2 concentrations can lead to abnormal placentation, but have not fully elaborated the mechanism of this effect in the first-trimester trophoblast. Our aim was to determine whether E2 elicits trophoblast cell death or inhibits proliferation. The first-trimester human cytotrophoblast cell line HTR-8/SVneo was cultured in phenol red-free medium containing charcoal-stripped serum and treated with 17beta-E2 at concentrations between 0 and 100 nM. TUNEL and invasion assays indicated that E2 significantly increased cell death and reduced cell invasion at 10 nM, and nuclear Ki67 expression revealed that it decreased cell proliferation at 1 nM. A similar effect on cell death was observed in first-trimester placental explants. The E2 antagonist fulvestrant blocked all effects of E2. Immunohistochemistry showed that protein expression of proapoptotic caspases 3, 8, and 9 increased at E2 concentrations of 25 nM and greater, whereas expression of antiapoptotic BCL2-alpha decreased at E2 concentrations of 10 nM and greater. Additionally, treatments with estrogen receptor (ER) alpha-specific and ERbeta-specific agonists at concentrations between 0 and 1000 nM indicated that only ERalpha mediates E2's effects, although immunohistochemistry and Western immunoblotting showed that HTR-8/SVneo cells and placental explants express both ERalpha and ERbeta. Taken together, these findings reveal the interplay between elevated serum E2 and apoptosis in the first trimester of pregnancy. These factors could be associated with pregnancy complications including infertility and uteroplacental insufficiency.
Keywords: apoptosis, developmental biology, estradiol, first-trimester placenta, steroid hormone receptors, steroid hormones
INTRODUCTION
The physiology of the placenta during the first trimester of pregnancy impacts its ultimate establishment [1]. Thus, in order for normal placentation to take place, a careful dynamic between a number of agents and processes must take place. For instance, the trophoblast itself, marking the boundary between the placenta and mother, is comprised of layers that carry out distinct functions and are critical to the development of the human embryo: the multinucleate syncytiotrophoblast layer regulates the transport of nutrients and wastes and maintains blood chemistry at the interface [2] and mononuclear cytotrophoblast cells demonstrate invasive behavior following proper differentiation [3, 4]. Further, in the tips of the villi, cytotrophoblasts differentiate into penetrable extravillous trophoblasts (EVTs). These cells invade the uterine maternal tissue, anchor the placenta, and contribute to the uterine and spiral artery remodeling essential for expanding blood supply to the growing embryo [5, 6].
Normal placental development also depends in part on estradiol (E2), which regulates growth, differentiation, and metabolic processes of the placenta [7, 8]. However, maternal serum E2 levels do not increase indefinitely, especially during the first trimester: although E2 serum levels can reach up to 7192 pg/ml (about 26.404 nM) in the second trimester of normal pregnancies [9], in the first trimester, E2 levels remain between 188 and 2497 pg/ml (0.690–9.167 nM). It is not fully understood what influences serum E2 levels, but others have suggested it to be linked to factors including diet [10, 11] and exposure to environmental pollutants [12]. Studies of the baboon model, a good correlate to human spiral artery invasion and transformation [13], show that premature E2 elevation in the first 60 days of pregnancy compromises EVT invasion and uterine blood flow dynamics [14, 15]. Specifically, premature E2 elevation increases uterine artery pressure and decreases uterine blood flow, ultimately disrupting fetal homeostasis and development. Also, in humans undergoing IVF, elevated serum E2 in the first trimester is linked to impaired placentation and pregnancy complications, including preeclampsia and intrauterine growth restriction [16–18]. The aforementioned conditions are also associated with apoptosis [19–21].
Like E2, apoptosis is another important regulator of placentation. Apoptosis is ubiquitous among multicellular organisms, helping to maintain normal cell turnover and ultimately contributing to the homeostasis of a number of physiological systems and processes, including those involved in pregnancy. As delineated by Straszewski-Chavez et al. [22], apoptosis is present in varying capacities throughout the beginning of normal pregnancies. The implantation stage, for example, involves blastocysts eliciting apoptosis in the uterine epithelium [23] and in the decidua. Additionally, as Allaire et al. [24] suggested, the invading trophoblast and maternal immune system require a careful and mutual balance of apoptosis in order to prevent fetal allograft rejection. Proapoptotic proteins are also involved in promoting differentiation and cell turnover in the villous trophoblast, with emphasis in the syncytial compartment [25]. Furthermore, along with proliferation and cyclin and cyclin-dependent kinase expression, apoptosis is involved with EVT invasion and eventual placental development [22, 26]. Specifically, it has been proposed that through factors including the Fas ligand and members of the TNF family, trophoblast apoptosis mediates uterine and spiral artery remodeling and aids in vascular smooth muscle cell death, ultimately contributing to the vascular integrity of the growing placenta [27, 28]. Apoptosis is thus an apparent limiting factor during trophoblast endothelial and interstitial invasion [29].
In normal pregnancy, apoptosis is low in the first trimester but increases throughout the course of pregnancy [30, 31], and E2 level follows a similar progression [32]. Hence, left unimpeded, high levels of both E2 and apoptosis in early pregnancy could be harmful to placentation. The link between E2 and apoptosis and its possible correlations with impaired placentation, nevertheless, are not fully understood.
The present study was designed to explore how abnormally elevated E2 negatively impacts placental trophoblast viability at the beginning of pregnancy. Accordingly, we examined the effects of E2 on cell death, proliferation, and invasion using TUNEL, Ki67 nuclear expression, and invasion assays respectively, and we also studied the immunohistochemistry of caspases associated with apoptosis in the first-trimester human cytotrophoblast cell line, HTR-8/SVneo. This cell line shares similarities with the parental trophoblasts from which it is derived, including invasive abilities and expression of trophoblast marker proteins, and cells also have prolonged lifespans and proliferative capacity [33, 34]. Moreover, our primary experiments were confirmed using a first-trimester villous explant model. Our findings indicate that E2 elevation above the upper range of its circulating concentration in maternal serum in the first trimester is detrimental to trophoblast survival.
MATERIALS AND METHODS
Human Cytotrophoblast Cells and Villous Explant Cultures
A first-trimester immortalized human cytotrophoblast cell line, HTR-8/SVneo (provided by Dr. Charles Graham of Queen's University), cultured in vitro [34], was used in all experiments. Cells were cultured in phenol red-free Dulbecco modified Eagle medium/Ham F12 medium (Life Technologies), supplemented with 10% charcoal-stripped fetal bovine serum (FBS; Sigma Aldrich), in 96-well tissue culture plates (Becton Dickinson).
First-trimester villous explants were also used to determine the effect of E2 on apoptosis. Specimens were isolated from first-trimester terminations at a Michigan family planning facility. Placental tissues were obtained with Wayne State University Institutional Review Board approval and patient informed consent. Fresh tissue was placed on ice in PBS and immediately transported to our lab. Here, the chorionic villi were dissected into individual villi or villous clusters and cultured in 24-well culture plates (Becton Dickinson) to be used in subsequent assays.
Preparation of Ligands
A 10 mM stock solution was prepared with E2 powder and dimethyl sulfoxide (DMSO; Sigma Aldrich) for assays testing the effects of E2. The stock solution was then diluted in culture medium to create 1, 10, 25, and 100 nM solutions. Additionally, because each E2 solution contained DMSO in a 1:1000 ratio, 1 μl DMSO was added to FBS media to create the vehicle control (0 nM E2). For the cell death and cell proliferation assays, 2 μl of a stock solution of 5 mM fulvestrant (ICI 182,780; Sigma Aldrich) was prepared with DMSO and diluted to 25 nM in culture medium. Cells were exposed to E2 for 24 h and first-trimester villous explants were exposed for 18 h after arrival from the clinic. Stock solutions of 10 mM progesterone (P4) were likewise made using powder (Sigma Aldrich) and FBS media.
Similarly, 1 mM stock solutions of estrogen receptor α (ERα)-specific agonist 4,4′,4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (PPT) and the ERβ-specific agonist diarylpropionitrile (2,3-bis(4-hydroxyphenyl)-propionitrile) (DPN; Tocris Bioscience) were prepared with DMSO. The stock solutions were diluted into culture medium, creating 1, 10, 100, and 1000 nM concentrations, and 1 μl DMSO was added to medium for the vehicle control as described above.
Finally, 5 mM stock solutions of pan-caspase inhibitor Z-VAD-FMK, caspase 3 inhibitor Z-D(OMe)E(Ome)VD(OMe)-FMK, caspase 8 inhibitor Z-IE(OMe)TD(OMe)-FMK, caspase 9 inhibitor Z-LEHD-FMK, and the inactive analog Z-FA-FMK (EMD Millipore) were prepared with DMSO (Sigma Aldrich). The aforementioned caspase inhibitors were diluted 1:5000 in 0 and 25 nM E2.
Cell Death Assays
TUNEL was used to assess cell death. After the incubation period, cells were fixed with 4% paraformaldehyde for 30 min and permeabilized with 0.1% Triton-X100 for 10 min. Cells were then washed for 5 min with PBS three times. The same procedure was used for the villous explants, but the tissue was incubated with 4% paraformaldehyde overnight at 4°C, exposed to 0.1% Triton-X100 for 20 min at room temperature, and washed for 10 min with PBS three times. A fluorescein-based kit from Roche Applied Science was used to perform the TUNEL assay, and cells were counterstained with 5 μg/ml 4′,6-diamidino-2-phenylindole HCl (DAPI). A Hamamatsu Orca digital camera with a Leica DM IRB epifluorescence microscope and Simple PCI (Hamamatsu) imaging software were utilized for quantitative analysis. Three regions per well were chosen to obtain images of total cell number (DAPI labeled) and apoptotic cells (TUNEL labeled) and to calculate the percentage of apoptotic nuclei (TUNEL index = [TUNEL labeled/DAPI labeled] * 100).
Immunohistochemistry
Fixed, permeabilized cells were treated with 10 μg/ml of each of the following mouse primary antibodies: cleaved caspase 3, caspase 8, caspase 9, and BCL2-α (Cell Signaling Technology). In addition, 10 μg/ml nonimmune mouse immunoglobulin G (IgG; Jackson Immunoresearch Laboratories, Inc.) was added to control wells. Plates were incubated at 4°C overnight. An Envision System peroxidase anti-mouse kit (DAKO), bright-field microscopy, the digital camera, and the imaging software were used to visually analyze antibody labeling. Images of three different regions of each well were taken with a background light intensity set at a gray level of 255, and mean gray levels were recorded for each image. Values obtained for the control wells (incubated with nonimmune IgG) were averaged and subtracted from values obtained with specific antibody.
In addition, first-trimester villous explants at a gestational age of 8 wk were used to test for the presence of ERα and ERβ receptors. As described above, first-trimester placental samples were obtained with informed consent. They were washed, sectioned, and mounted onto glass slides, and later deparaffinized and rehydrated as delineated by Imudia et al. [35]. The slides were then incubated at 4°C with either 200 μg/ml anti-α (HC-20) (sc-543; Santa Cruz Biotechnology Inc.) or 1 μg/ml anti- ERβ antibody [14C8] (ab288; Abcam) overnight. As a control, another slide was incubated overnight with 10 μg/ml nonimmune mouse IgG. Tissues were then washed three times in 1× PBS, labeled with 10 μg/ml biotin-conjugated goat anti-rabbit or rabbit anti-mouse antibodies (Jackson ImmunoResearch), and stained using Envision System peroxidase kits. After the tissues were dehydrated and coverslipped, they were viewed under light microscopy.
Invasion Assay
Cells (20 000 per well) were cultured for 72 h on Matrigel (Collaborative Research) in 6.5-mm transwell inserts (Corning Inc.), as previously reported [34]. Cells were cultured in 0, 1, 10, 25, and 100 nM E2 and also 25 nM E2 + ICI 182,780 on the Matrigel for 72 h at 37°C. Cells that penetrated the Matrigel and populated the lower chamber were detached using a trypsin-EDTA solution and fixed. They were later visually analyzed using light microscopy and counted.
Cell Proliferation Assays
Proliferation was assessed by immunohistochemistry, using nuclear labeling of Ki67, a marker for cell proliferation. Nuclei expressing Ki67 were labeled with 10 μg/ml affinity-purified mouse antibody (DAKO) and stained using an Envision System peroxidase anti-mouse kit (DAKO). Bright-field microscopy and the digital camera and imaging software were used to count the number of cells that stained positively for Ki67 and the total cell number in three regions of each well in order to ultimately calculate the percentage of Ki67-positive nuclei (Ki67 index = [Ki67 positive cells/total number of cells] * 100).
Western Immunoblotting
Cells were cultured in tissue culture flasks for 72 h in serum-free medium. A rubber policeman and ice-cold PBS were used to dislodge the nonadherent cells, and they were recovered by centrifugation at 1000 × g. Ice-cold SDS sample buffer (3.00 g Tris-acetate, 8.00 g SDS, 40.00 ml glycerol, 100 ml distilled water, and HCl to lower pH to 6.8) containing a mixture of protease inhibitors (1 mM PMSF, 25 KIU/ml aprotinin, 2 M leupeptin, 2 μM antipain, 10 μM benzamidine, 1 μM pepstatin, and 1 μM chymostatin; Sigma Aldrich) was added to lyse the cells. The lysate was centrifuged at 14 000 × g for 5 min, and the protein concentration of the resulting supernatant was determined using a Bio-Rad DC protein assay. An SDS loading buffer containing β-mercaptoethanol was added to the protein extract (35 μg/lane), and the resulting solution was heated at 95°C for 5 min. SDS gel electrophoresis was conducted according to Laemmli [36], using biotinylated protein ladders (Cell Signaling Technology). After electrophoretic transfer of the proteins to a 0.45-μm nitrocellulose membrane (Fisher Scientific), membranes were blocked for 1 h with 5% nonfat dry milk in 20 mM Tris-HCl, pH 7.6, 145 mM NaCl, and 0.1% Tween-20 (TTBS), then incubated at 4°C overnight with 10 ml of the blocking solution containing one of the following: 200 μg/ml anti-ERα (HC-20) or 1 μg/ml anti-ERβ antibody [14C8]. Blots were washed with TTBS three times and then incubated in 10 ml of blocking solution containing either 20 μg/ml anti-rabbit IgG horseradish peroxidase (HRP)-linked antibody (Jackson ImmunoResearch) and anti-biotin (for ERα) or 20 μg/ml anti-mouse IgG HRP-linked antibody (Jackson ImmunoResearch) and anti-biotin (for ERβ) for 1 h at 25°C. Blots were then washed three times with TTBS. ECL solution (Amersham) was added to the blots for 1 min and exposed to x-ray film to visualize labeled protein bands. For the six first-trimester placental samples of gestational ages 8, 8, 9, 12, 12, and 12 wk, the aforementioned method did not allow for us to visualize distinct bands; therefore, for increased sensitivity, the V3 Western Workflow system (Bio-Rad Laboratories) protocol as described by Posch et al. [37] was utilized.
Statistical Analysis
All experiments were repeated at least three times and all assays were run using at least triplicate samples. Comparisons with vehicle controls were made for TUNEL indices, antibody staining intensity gray levels, invading cell numbers, and Ki67 indices by analyzing results using ANOVA and following with Dunnett or Bonferroni t-test for post hoc analysis.
RESULTS
E2 Induces Cell Death
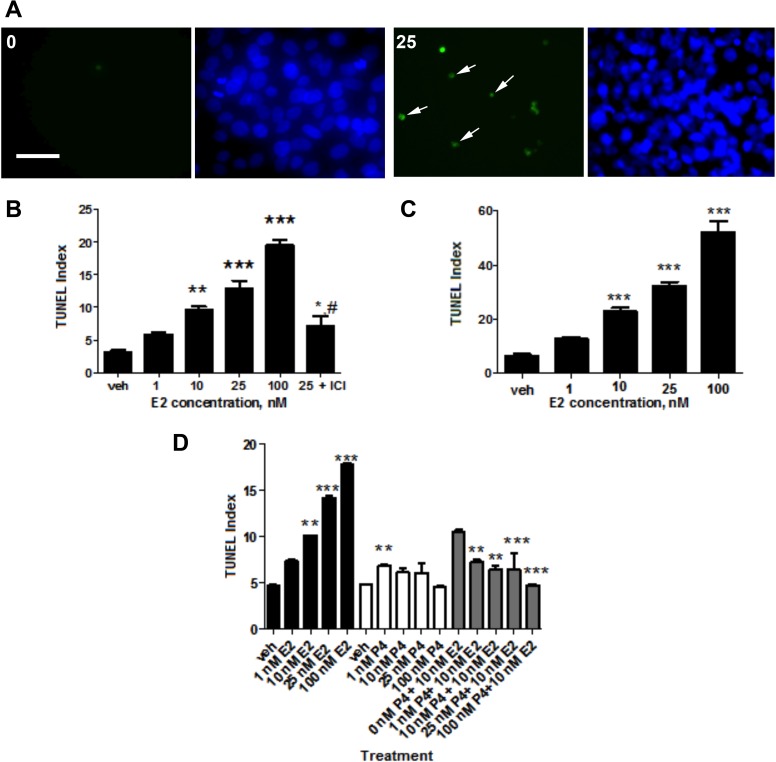
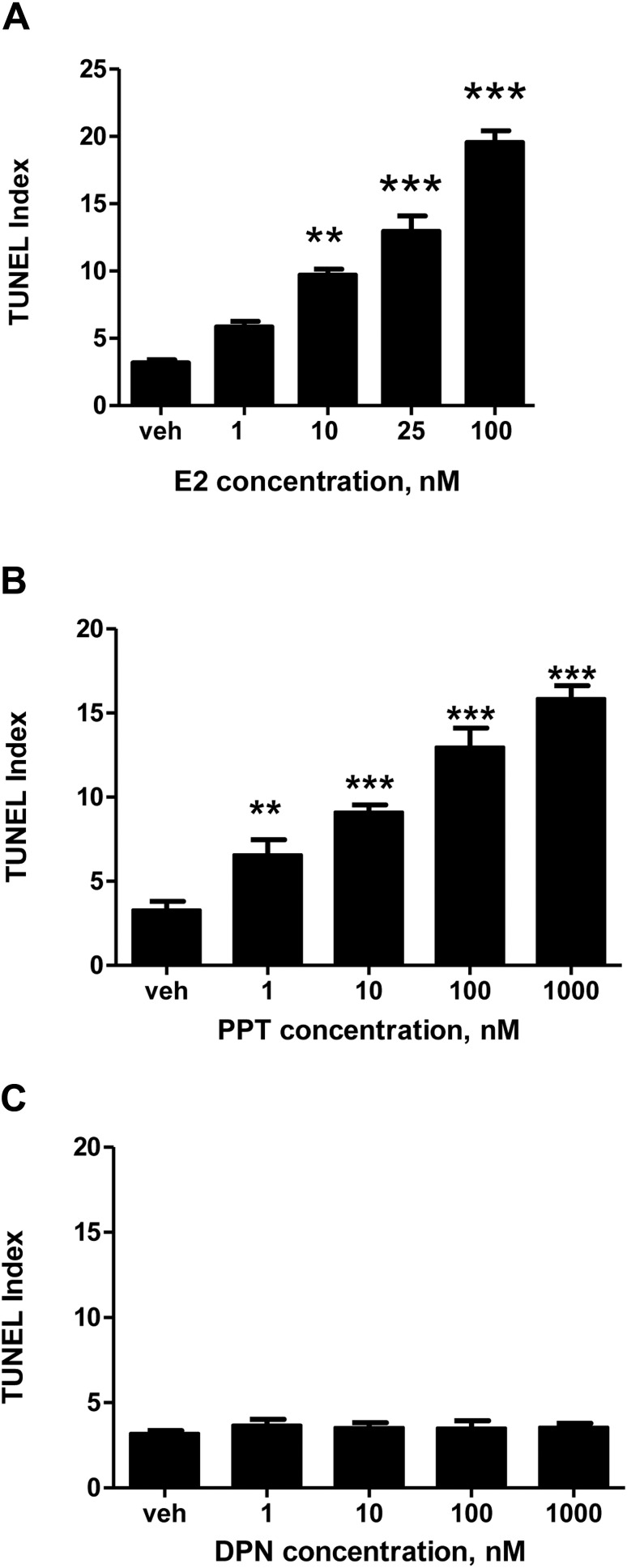
Cell death was first analyzed using the TUNEL assay and quantified as TUNEL indices, as delineated in Materials and Methods. Compiled data show that in relation to the vehicle control, E2 elevated cell death of HTR-8/SVneo cells in a dose-dependent manner (Fig. 1, A and B). Significantly, 10 nM E2 increased cell death by 300% and 25 nM E2 increased cell death by almost 400% (P < 0.01). Additionally, 100 nM solution increased cell death by greater than 600% (P < 0.001). Moreover, addition of the anti-estrogen inhibitor ICI 182,780 to 25 nM E2 led to a decrease in the cell death effect by greater than 170% compared to 25 nM E2 alone (P < 0.05). Cell death also was elevated by E2 in trophoblast cells of first-trimester villous explants (Fig. 1C).
FIG. 1.
The effect of E2 on cell death. A) DNA fragmentation and pyknotic nuclei of HTR-8/SVneo cells were visualized using a fluorescein-based stain and DAPI. The total cell densities are represented by the DAPI-labeled nuclei (right) and shown alongside TUNEL-positive nuclei (left; indicated by arrows) expressed in the same area. Images of cells from the 0 and 25 nM E2 treatments were taken under fluorescence at ×400. Bar = 50 μm. B) The effect of E2 on TUNEL indices of HTR-8/SVneo cells. As denoted by the hash mark, the anti-estrogen ICI 182,780 was combined with 25 nM E2 and compared to the TUNEL index elicited by 25 nM E2 alone (#P < 0.05). C) The effect of E2 on TUNEL indices of villous explants. D) TUNEL indices were measured and compared for HTR-8/SVneo cells treated with the indicated concentrations of E2 (black) and P4 (white). Combination treatments comprised of 10 nM E2 with differing P4 concentrations were also tested (gray). Statistical significance is denoted by asterisks comparing each concentration with the vehicle control for B, C, and D and also for comparing each combination treatment with 10 nM E2 alone for D (*P < 0.05, **P < 0.01, ***P < 0.001). Bars represent mean ± SEM (n = 3).
We observed disparate cell death effects of E2, P4, and the combination of 10 nM E2 and varying P4 concentrations (Fig. 1D). Consistent with our earlier experiments, E2 led to increased cell death in a concentration-dependent manner. However, TUNEL indices remained relatively stable despite higher P4 concentrations, and in the combination solutions, E2's effect on cell death was reduced by the addition of higher P4 concentrations. For example, there were significant differences between the E2 and P4 combination solutions containing 10 nM E2 and 10 nM P4 and higher (P < 0.05 and P < 0.001).
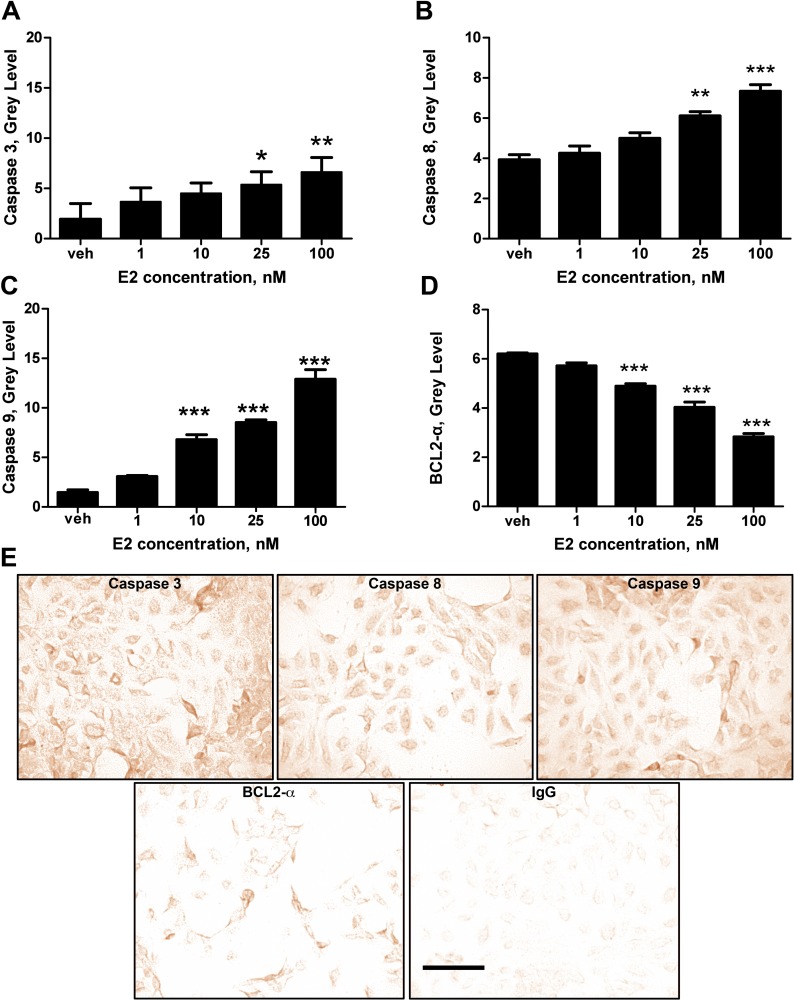
We further tested our hypothesis that E2 induces apoptosis by immunostaining of cleaved caspase 3, a proapoptotic protein (Fig. 2A). Just as TUNEL indices increased with E2 concentrations, protein activity (quantified by gray-level staining intensity) increased in an E2 concentration-dependent manner; and it was significantly elevated at 25 nM E2 (P < 0.05) relative to vehicle. Further, compared to the vehicle, 100 nM of E2 increased caspase 3 gray level by about 320% (P < 0.01). The positive relationship between caspase 3 staining and E2 concentrations was also observed with the initiator proapoptotic proteins, caspase 8 (Fig. 2B) and caspase 9 (Fig. 2C). At 25 nM, E2 led to about a 150% higher gray level of cleaved caspase 8 than the control (P < 0.01), and at 100 nM E2, about a 190% higher gray level in comparison to the vehicle (P < 0.001). Compared to the control, caspase 9 gray levels were increased by about 470%, 590%, and 890% in the 10, 25, and 100 nM E2 solutions, respectively (P < 0.001). In contrast, immunostaining of BCL2-α, an antiapoptotic protein, displayed a negative relationship as E2 concentrations increased (Fig. 2D). Relative to vehicle, gray levels decreased by 120% in the 10 nM E2 solution, 150% in the 25 nM E2 solution, and 210% in the 100 nM solution (P < 0.001).
FIG. 2.
E2 affects proapoptotic and antiapoptotic proteins. Antibody activity was quantified using immunohistochemistry and gray-level image analysis on HTR-8/SVneo cells. A) The effect of E2 on the activity of effector proapoptotic protein caspase 3. B and C) The effect of E2 on the activity of initiator proapoptotic proteins caspase 8 and caspase 9. D) The effect of E2 on the activity of the antiapoptotic protein BCL2-α. Statistical differences relative to the vehicle are indicated by the asterisks (*P < 0.05, **P < 0.01, ***P < 0.001). Bars represent mean ± SEM (n = 3). E) Immunohistochemical labeling shows the expression of caspase 3, caspase 8, caspase 9, BCL2-α, and IgG control at 25 nM E2. Images were taken under light microscopy at ×400. Bar = 50 μm.
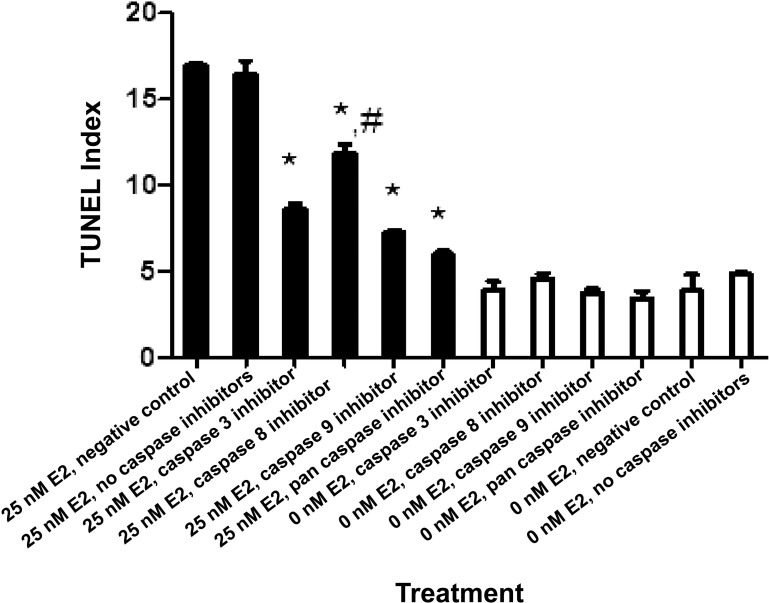
As measured through TUNEL indices, inhibitors of caspase 3, 8, and 9, as well as the pan-caspase inhibitor, appeared to reduce the cell death effect induced by E2 (Fig. 3). In particular, compared to 25 nM E2 alone, the treatment containing 25 nM E2 plus one of the aforementioned caspase inhibitors significantly reduced TUNEL indices (P < 0.05). Also of note, the inhibitor of caspase 8 in the 25 nM E2 solution led to significantly different TUNEL indices than did the caspase 3, caspase 9, and pan-caspase inhibitor (P < 0.05).
FIG. 3.
The effects of E2 and caspase inhibitors on cell death. HTR-8/SVneo cells were incubated with 0 (white) and 25 nM E2 (black) containing the indicated caspase inhibitors. Statistically significant differences between TUNEL indices of 25 nM E2 with and without caspase inhibitors are denoted by the asterisk (*P < 0.05). In addition, the statistical difference between the effect of the caspase 8 inhibitor and the caspase 3, caspase 9, and pan-caspase inhibitors on TUNEL index in the presence of 25 nM E2 is indicated by the hash mark (#P < 0.05). Bars represent mean ± SEM (n = 3).
E2 Negatively Affects Cell Invasion
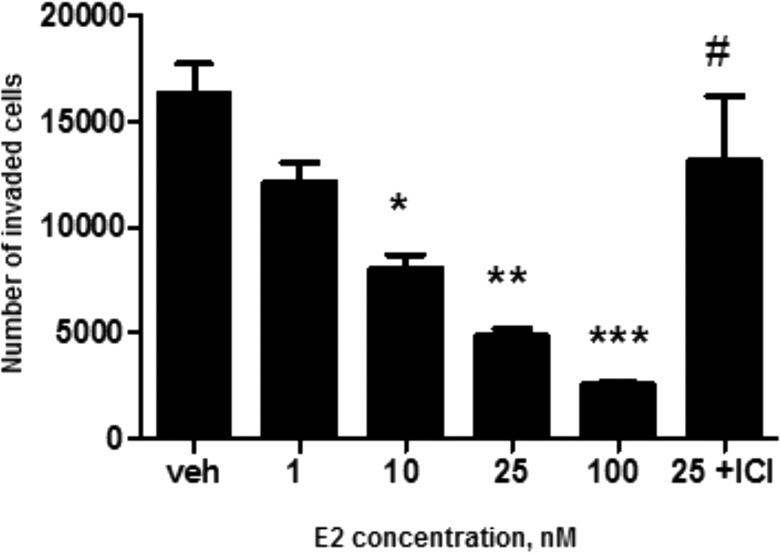
HTR-8/SVneo cells cultured on Matrigel were affected by E2 in a dose-dependent manner (Fig. 4). Significant reduction in cell invasion was observed at and above 10 nM: the number of invading cells was reduced by an average of 210% (P < 0.05), 340% (P < 0.01), and 630% (P < 0.001) in the 10, 25, and 100 nM E2 solutions, respectively, compared to the vehicle control. Also, there was about 220% fewer cells invading the pores in the 25 nM E2 solution compared to solution containing the addition of ICI 182,780 (P < 0.05). As the inserts were not moved to new wells each day during the 72-h incubation, it is not clear whether the final count of the cells was affected by proliferation from the cells that had previously entered the lower chamber.
FIG. 4.
The effect of E2 on cell invasion. The indicated concentrations of E2 were tested to study their effect on invasion of HTR-8/SVneo cells. Asterisks denote statistical difference from the vehicle (*P < 0.05, **P < 0.01, ***P < 0.001), and the hash mark denotes statistical difference between 25 nM E2 with and without the antiestrogen ICI 182,780 (#P < 0.05). Bars represent mean ± SEM (n = 3).
E2 Reduces Cell Proliferation
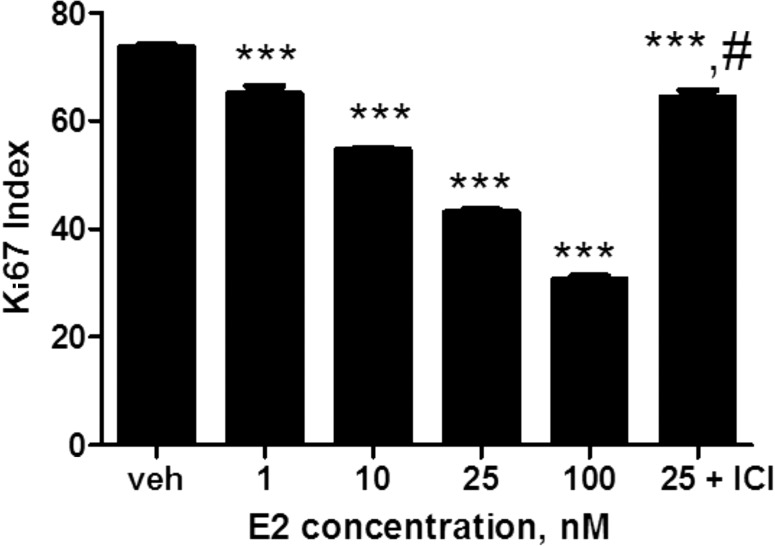
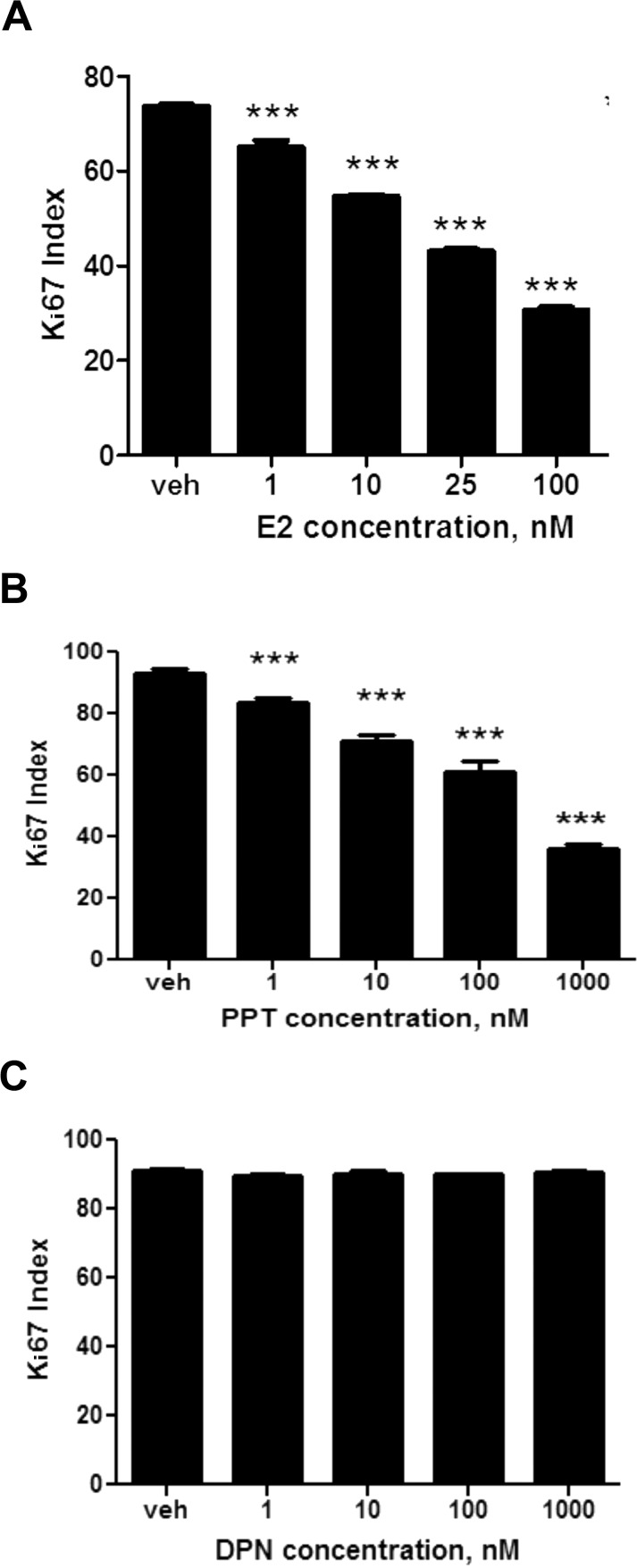
Nuclear Ki67 immunohistochemistry was performed to determine whether E2 affected proliferation of the HTR-8/SVneo cell line. Concentration-dependent effects were observed (Fig. 5), with significant reduction of nuclear Ki67 labeling at E2 concentrations greater than 1 nM, compared to the vehicle (P < 0.001). For example, at 100 nM, E2 decreased proliferation by 240%. Furthermore, relative to 25 nM E2 alone, the addition of ICI 182,780 to 25 nM E2 increased cell proliferation by 150% (P < 0.01). Overall, the finding that E2 led to higher TUNEL indices and promoted cell death was consistent with the trend represented by the Ki67 labeling indices.
FIG. 5.
The effect of E2 on cell proliferation. Proliferation of HTR-8/SVneo cells was quantified using the Ki67 assay. Statistically significant differences are denoted by asterisks comparing each concentration with the vehicle control (***P < 0.001). Additionally, the statistical difference between the 25 nM E2 and antiestrogen ICI 182,780 combination treatment and 25 nM E2 without the inhibitor is indicated by the hash mark (#P < 0.05). Bars represent mean ± SEM (n = 3).
ERα and ERβ
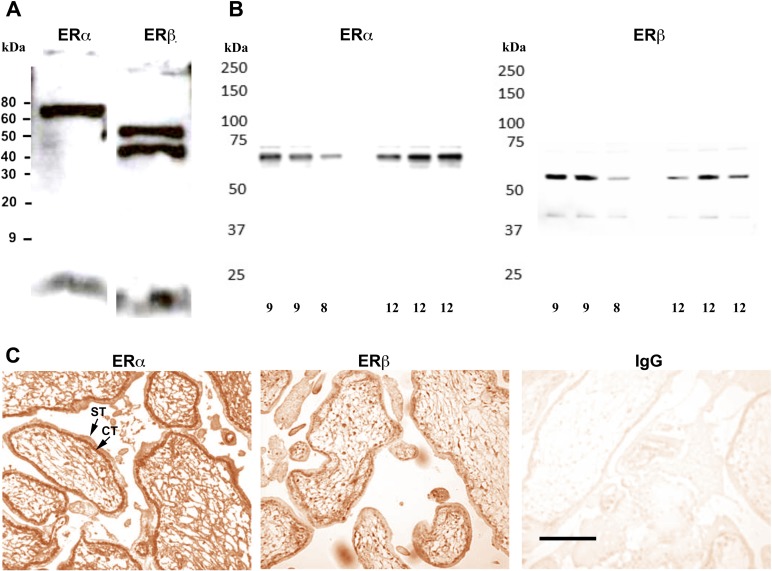
The estrogen receptor has two nuclear receptor subtypes, ERα (ESR1) and ERβ (ESR2), with differing activities [38]. Both subtypes were expressed in the cell line HTR-8/SVneo (Fig. 6A) and in first-trimester trophoblast cells of villous explants (Fig. 6B). Additionally, substantial nuclear staining of ERα was found in first-trimester placentas, and ERβ was also detected (Fig. 6C).
FIG. 6.
Estrogen receptor subtypes in the first trimester. A) Western blot shows that both ERα and ERβ are present in the HTR-8/SVneo cell line. B) Western blot shows that ERα and ERβ are also both present in the first-trimester placenta. The age (in weeks) of each sample is indicated below each lane on both blots. C) Immunohistochemical labeling indicates that ERα and ERβ are expressed in the first-trimester placenta (8 wk). Cytotrophoblasts (CT) and syncytiotrophoblasts (ST) are indicated by the arrows. Another sample was labeled with nonimmune mouse IgG antibody as a control. Images were taken at ×400. Bar = 50 μm.
To determine whether ERα or ERβ mediated the observed E2-induced apoptosis and decreased cell proliferation in human cytotrophoblast cells, the effects of receptor subtype-specific ligands PPT and DPN were studied in the HTR-8/SVneo cell line. The ERα-specific ligand PPT mimicked the effect of E2 in terms of apoptosis (Fig. 7, A and B). Using the TUNEL assay, cell death increased by 280% after treatment with 10 nM PPT, 390% at 100 nM, and 480% at 1000 nM (P < 0.001), compared to the vehicle. In contrast, the ERβ-specific ligand DPN displayed no significant effect on apoptosis (Fig. 7C). These results were consistent with our findings on cell proliferation: we found that the effects of E2 and PPT on Ki67 followed a similar trend (Fig. 8, A and B), where cell proliferation at 1000 nM PPT was decreased 260% compared to the vehicle control (P < 0.001). However, the ERβ-specific ligand DPN again displayed no significant effect on cell proliferation (Fig. 8C). The findings of these experiments indicate that although both ERα and ERβ are present in the HTR-8/SVneo cell line and in the first-trimester trophoblast, E2 elevates cell death and reduces cell proliferation exclusively through the ERα subtype.
FIG. 7.
The effects of E2, ERα agonist PPT, and ERβ agonist DPN on cell death. Receptor-subtype-specific ligands were used to determine which subtype mediated apoptosis in HTR-8/SVneo cells. A) The effect of E2 on TUNEL indices. B) The effect of ERα agonist PPT on TUNEL indices. C) The effect of ERβ agonist DPN on TUNEL indices. Statistical differences compared to the vehicle are denoted by the asterisks (**P < 0.01, ***P < 001). Bars represent mean ± SEM (n = 3).
FIG. 8.
The effects of E2, ERα agonist PPT, and ERβ agonist DPN on cell proliferation. Receptor-subtype-specific ligands were used to determine which subtype mediated cell proliferation in HTR-8/SVneo cells. A) The effect of E2 on Ki67 indices. B) The effect of ERα agonist PPT on Ki67 indices. C) The effect of ERβ agonist DPN on Ki67 indices. Statistical differences compared to the vehicle are denoted by the asterisks (***P < 0.001). Bars represent mean ± SEM (n = 3).
DISCUSSION
From guiding neuronal growth and survival to affecting body weight and metabolism, E2 carries out essential roles in the body, and the placenta is no exception. But although E2 is necessary throughout gestation for trophoblast differentiation and migration [39, 40] and overall success of pregnancies [8], its levels in the first trimester do not increase without consequence. Although some studies have not found high E2 levels to negatively affect placental integrity [41–43] others report that elevated serum E2 can be detrimental to implantation [16–18]. In mice, for example, low E2 levels sustain uterine receptivity for blastocyst implantation, whereas high levels impede it [44, 45]. There is also an increased risk for low birth weight and small-for-gestational-age birth in women with high serum E2 levels in the first trimester of pregnancy [46]. Hence, elevated serum E2 levels in first-trimester trophoblast could contribute to suboptimal perfusion of the developing placenta and reduced uterine artery blood flow, possibly precipitating uteroplacental vascular insufficiency and perinatal disorders [47, 48].
Because E2 concentrations range between 0 and 10 nM at the end of the first trimester [9], by testing E2 concentrations between 0 and 100 nM, we were able to bracket the endogenous concentrations found in maternal blood during the first trimester of normal pregnancies. Based on our findings, it appears that elevation of E2 to concentrations above 10 nM initiates significant apoptotic, antiproliferative effects in first-trimester human cytotrophoblast cells. The induction of cell death and inhibition of cell proliferation displayed by E2, as well as the ERα-specific ligand PPT, are mediated by ERα and exhibit concentration dependence. Further, these effects are blocked by the nuclear ER antagonist ICI 182,780. In our experiments, we exposed the cells to E2 for only 24 h. Therefore, rather than actually counting the dead and living cells, we utilized TUNEL and Ki67 assays. The aforementioned sufficiently sensitive methods allowed us to detect short-term differences in cell death and cell proliferation; but in the future, if we wanted to understand if E2 exposure over a period of days would have an impact on the cells, we could determine actual cell counts.
Our results were contingent on the fact that the immortalized cell line HTR-8/SVneo has many advantages, including its ability to resist aging and to be induced to differentiate to an extravillous phenotype [34]. But although the HTR-8/SVneo cell line is an effective model system for first-trimester cytotrophoblasts [33, 34], there is always concern that a cell line could lack certain properties of the parental cells. However, our results confirmed that a culture of primary trophoblast cells in villous explants also exhibited elevated cell death with increasing E2 concentrations, as measured through TUNEL.
The cell death observed could have been the result of either necrosis or apoptosis, although pyknotic nuclei were observed in the E2-exposed cells during TUNEL assays, which is thought to differentiate between the two forms of cell death [49]. Analysis of cleaved caspases 3, 8, and 9 and BCL2-α activity strongly suggests that cell death was most likely a consequence of apoptosis. Caspase 8 is an initiator of the death receptor extrinsic pathway, whereas caspase 9 is an initiator of the mitochondrial intrinsic pathway, and both can directly or indirectly activate downstream effector caspase 3 [50]. Because both caspase 8 and 9 activities increased with E2 concentrations, and caspase activity does not always trigger apoptosis [51], it is difficult to firmly establish the mechanism of apoptosis. Nonetheless, because BCL2-α inhibits apoptosis by blocking cytochrome c release from the mitochondria [50], and BCL2-α activity decreased with increasing E2, it appears that apoptosis is mediated through the intrinsic pathway. Our data also show that the inhibitor of caspase 8 resulted in significantly higher TUNEL indices in the 25 nM E2 solution than did the inhibitors of the other caspases. This reinforces the possibility that E2 operates through the intrinsic apoptotic pathway. Although our data show that caspase inhibitors 3, 8, and 9 and the pan-caspase inhibitor reduced TUNEL indices compared to controls, additional experiments are needed to determine whether caspase activity is actually required for this cell death.
Moreover, our results revealed that E2 and P4 show differing effects on TUNEL indices in first-trimester cytotrophoblast cells. The contrasting effects of the aforementioned hormones have been highlighted by others, including Sweeney et al. [52], who found that P4 blocked an apoptotic effect induced by E2 administration in MCF-7:5C, a cell line derived from MCF-7 breast cancer cells by long-term estrogen deprivation. In the first trimester of pregnancy, serum P4 levels increase rapidly and reach up to 113 nM, whereas E2 levels rise slowly up to no greater than 10 nM [9, 53]. In contrast to the increased cell death displayed by E2 at concentrations at 10 nM and above, our results show that P4 did not cause a specific effect at concentrations ranging from 0 to 100 nM. Higher P4 concentrations might have revealed different results, as Lui et al. [54] found P4 concentrations at 10 and 100 ng/ml (31.8005 and 318.005 nM) significantly reduced TUNEL indices. However, when combined with 10 nM E2, P4 appeared to reduce the cell death effect induced by E2. There could be various reasons to account for this, including the down-regulation of ER expression by P4 suggested by Chang and Zhang [55]. Future tests on the effect of the combination E2 and P4 treatment should be carried out in order to analyze if P4 reduces E2's effect in other areas, including caspase activity and cell proliferation.
Although E2 is associated with antiapoptotic effects in certain cell populations, including cardiomyocytes [56] and skeletal myoblasts [57], it is also linked with proapoptotic effects, as we have demonstrated in the present study. Based on our experimental design, we were not able to conclude that the apoptosis observed would lead to impaired placental development or to regrowth and repair of damaged tissue in actual pregnancies. However, we speculate that because many studies have found that increased apoptosis in the first trimester is abnormal [30, 31, 58, 59], the heightened apoptosis could be detrimental to placental structure and function. Additionally, our finding that E2 displays dose-dependent effects and elicits statistically significant injurious effects is in agreement with other studies. For example, Wong et al. [60] found that E2 concentrations at 10 nM and above led to a significant gonadotropin-releasing hormone mRNA decrease, halting the proinvasiveness of EVTs required for implantation [61]. Given that E2's effects on invasion are modulated by different growth factors [62], it remains to be determined whether E2 directly affects EVT differentiation, migration, and growth factors involved with invasion.
Overall, our findings regarding E2's effects on cell death, cell proliferation, and cell invasion were notable, but studying how E2 carried out the previously mentioned effects was also important. E2 can instigate its effects through receptor-mediated mechanisms by binding to either ERα or ERβ [63]. Although we found that both isoforms were present in the first-trimester placenta, and the ER antagonist ICI 182,780 reversed E2-induced apoptosis, only the ERα agonist PPT induced apoptosis and inhibited cell proliferation in HTR-8/SVneo cells. The interaction between E2 and ERα is highlighted in related studies. Das and Basak [64] suggested that E2 participates in autoregulation, stimulating the expression of ERα in human first-trimester cytotrophoblast cells, and Ni et al. [65] found that E2 inhibited the gene expression of CRH in a concentration-dependent manner through an ERα mediated mechanism in primary placental cells.
Taken together, this study reveals that abnormally high E2 concentrations during early pregnancy could hamper normal development of the placenta through increased apoptosis of trophoblast cells. This is physiologically important, as errors in the first trimester of pregnancy contribute to pregnancy complications involving many key players, including those implicated with apoptosis. Nevertheless, many of the mechanisms through which E2 acts and the interactions it forms with other regulators of the first-trimester cytotrophoblast have yet to be examined and thus merit further investigation.
ACKNOWLEDGMENT
The authors thank Dr. Charles Graham of Queen's University for providing the HTR-8/SVneo cell line and the Northland Family Planning Centers of Michigan for participating in this research study.
Footnotes
Supported in part by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH grants HD045966 and HD071408, and the Office of the Vice President for Research of Wayne State University. Presented in part at the 96th Annual Meeting of the Endocrine Society, 21–24 June 2014, Chicago, Illinois.
REFERENCES
- Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano T, Itzasa H, Hwang IW, Hirose Y, Morita T, Maeda T, Nakashima E. Conditionally immortalized syncytiotrophoblast cell lines as new tools for study of the blood-placental barrier. Biol Pharm Bull. 2004;27:753–759. doi: 10.1248/bpb.27.753. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Fitzgerald ML, Fisher SJ. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest. 1992;89:210–222. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows TD, King A, Loke YW. Trophoblast migration during human placental implantation. Hum Reprod Update. 1996;2:301–321. doi: 10.1093/humupd/2.4.307. [DOI] [PubMed] [Google Scholar]
- Vićovac L, Aplin JD. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat. 1996;156:202–216. doi: 10.1159/000147847. [DOI] [PubMed] [Google Scholar]
- Villee CA, Hagerman DD. Effects of estradiol on the metabolism of human placenta in vitro. J Biol Chem. 1953;205:873–882. [PubMed] [Google Scholar]
- Albrecht ED, Aberdeen GW, Pepe GI. The role of estrogen in the maintenance of primate pregnancy. Am J Obstet Gynecol. 2000;182:432–438. doi: 10.1016/s0002-9378(00)70235-3. [DOI] [PubMed] [Google Scholar]
- Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009;114(6):1326–1331. doi: 10.1097/AOG.0b013e3181c2bde8. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Onajafe I, Raygada M, Cho E, Clarke R, Lippman M. Breast cancer risk in rats fed a diet high in n-6 polyunsaturated fatty acids during pregnancy. J Natl Cancer Inst. 1996;88(24):1821–1827. doi: 10.1093/jnci/88.24.1821. [DOI] [PubMed] [Google Scholar]
- Rose DP, Goldman M, Connolly JM, Strong LE. High-fiber diet reduces serum estrogen concentrations in premenopausal women. Am J Clin Nutr. 1991;54(3):520–525. doi: 10.1093/ajcn/54.3.520. [DOI] [PubMed] [Google Scholar]
- Janssen PAH, Lambert JGD, Vethaak AD, Goos HJT. Environmental pollution caused elevated concentrations of oestradiol and vitellogenin in the female flounder, Platichthys flesus (L.) Aquat Toxicol. 1997;39(3–4):195–214. [Google Scholar]
- Carter AM. Animal models of human placentation—a review. Placenta. 2007;28:S41–S47. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Bonagura TW, Burleigh DW, Enders AC, Aberdeen GW, Pepe GJ. Suppression of extravillous trophoblast invasion of uterine spiral arteries by estrogen during early baboon pregnancy. Placenta. 2006;27:483–490. doi: 10.1016/j.placenta.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Aberdeen GW, Bonagura TW, Harman CR, Pepe GJ, Albrecht ED. Suppression of trophoblast uterine spiral artery remodeling by estrogen during baboon pregnancy: impact on uterine and fetal blood flow dynamics. Am J Physiol Heart Circ Physiol. 2012;302:H1936. doi: 10.1152/ajpheart.00590.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman R, Fries N, Testart J, Belaisch-Allart J, Hazout A, Frydman R. Evidence for an adverse effect of elevated serum estradiol concentrations on embryo implantation. Fertil Steril. 1988;49:118–122. doi: 10.1016/s0015-0282(16)59661-7. [DOI] [PubMed] [Google Scholar]
- Simón C, Cano F, Valbuena D, Remohí J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. 1995;10:2432–2437. doi: 10.1093/oxfordjournals.humrep.a136313. [DOI] [PubMed] [Google Scholar]
- Farhi J, Haroush AB, Andrawus N, Pinkas H, Sapir O, Fisch B, Ashkenazi J. High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reprod Biomed Online. 2010;21:331–337. doi: 10.1016/j.rbmo.2010.04.022. [DOI] [PubMed] [Google Scholar]
- DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155:293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B, Kadyrov M, Kingdom JC. Apoptosis and its role in the trophoblast. Am J Obstet Gynecol. 2006;195:29–39. doi: 10.1016/j.ajog.2005.07.039. [DOI] [PubMed] [Google Scholar]
- Kadyrov M, Kingdom JC, Huppertz B. Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am J Obstet Gynecol. 2006;194:557–563. doi: 10.1016/j.ajog.2005.07.035. [DOI] [PubMed] [Google Scholar]
- Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev. 2005;26:877–897. doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- Parr EL, Tung HN, Parr MB. Apoptosis as the mode of uterine epithelial cell death during embryo implantation in mice and rats. Biol Reprod. 1987;36(1):211–225. doi: 10.1095/biolreprod36.1.211. [DOI] [PubMed] [Google Scholar]
- Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol. 2000;96(2):271–276. doi: 10.1016/s0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- Kar M, Ghosh P, Sengupta J. Histochemical and morphological examination of proliferation and apoptosis in human first trimester villous trophoblasts. Hum Reprod. 2007;22(11):2814–2823. doi: 10.1093/humrep/dem284. [DOI] [PubMed] [Google Scholar]
- Whitley GS, Cartwright JE. Trophoblast-mediated spiral artery remodeling: a role for apoptosis. J Anat. 2009;215(1):21–26. doi: 10.1111/j.1469-7580.2008.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton SV, Whitley GS, Dash PR, Wareing M, Crocker IP, Baker PN, Cartwright JE. Uterine spiral artery remodeling involves endothelial apoptosis induced by extravillous trophoblasts through Fas/FasL interactions. Arterioscler Thromb Vasc Biol. 2005;25(1):102–108. doi: 10.1161/01.ATV.0000148547.70187.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rango U, Krusche CA, Kertschanska S, Alfer K, Kauffman P, Beier HM. Apoptosis of extravillous trophoblast cells limits the trophoblast invasion in uterine but not in tubal pregnancy during first trimester. Placenta. 2003;24(10):929–940. doi: 10.1016/s0143-4004(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Smith S, Baker PN, Symonds EM. Placental apoptosis in normal human pregnancy. Am J Obstet Gynecol. 1997;177:57–65. doi: 10.1016/s0002-9378(97)70438-1. [DOI] [PubMed] [Google Scholar]
- Smith SC, Leung TN, To KF, Baker PN. Apoptosis is a rare event in first-trimester placental tissue. Am J Obstet Gynecol. 2000;183:697–699. doi: 10.1067/mob.2000.106555. [DOI] [PubMed] [Google Scholar]
- Bukovsky A, Caudle MR, Cekanova M, Fernando RI, Wimalasena J, Foster JS, Elder RF. Placental expression of estrogen receptor beta and its hormone binding variant—comparison with estrogen receptor alpha and a role for estrogen receptors in asymmetric division and differentiation of estrogen-dependent cells. Reprod Biol Endocrinol. 2003;1:36–46. doi: 10.1186/1477-7827-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving JA, Lysiak JJ, Graham CH, Hearn S, Han VKM, Lala PK. Characteristics of trophoblast cells migrating from first trimester chorionic villus explants and propagated in culture. Placenta. 1995;16:413–433. doi: 10.1016/0143-4004(95)90100-0. [DOI] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- Kilburn BA, Wang J, Duniec-Dmuchkowski ZM, Leach RE, Romero R, Armant DR. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol Reprod. 2000;62:739–742. doi: 10.1095/biolreprod62.3.739. [DOI] [PubMed] [Google Scholar]
- Imudia AN, Kilburn BA, Petkova A, Edwin SS, Romero R, Armant DR. Expression of heparin-binding EGF-like growth factor in term chorionic villous explants and its role in trophoblast survival. Placenta. 2008;29:784–789. doi: 10.1016/j.placenta.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Posch A, Kohn J, Oh K, Hammond M, Liu N. V3 stain-free workflow for a practical, convenient, and reliable total protein loading control in western blotting. J Vis Exp. 2013;82:50948. doi: 10.3791/50948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson JA. Estrogen receptor beta—a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163:379–383. doi: 10.1677/joe.0.1630379. [DOI] [PubMed] [Google Scholar]
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- Cronier L, Guibourdenche J, Niger C, Malassine A. Oestradiol stimulates morphological and functional differentiation of human villous cytotrophoblast. Placenta. 1999;20:669–676. doi: 10.1053/plac.1999.0423. [DOI] [PubMed] [Google Scholar]
- Chenette PE, Sauer MV, Paulson RJ. Very high serum estradiol concentrations are not detrimental to clinical outcome of in vitro fertilization. Fertil Steril. 1990;61:858–863. doi: 10.1016/s0015-0282(16)53946-6. [DOI] [PubMed] [Google Scholar]
- Sharara F, McClamrock HD. High estradiol levels and high oocyte yield are not detrimental to in vitro fertilization outcome. Fertil Steril. 1999;72:401–405. [PubMed] [Google Scholar]
- Ng EHY, Yeung WSB, Lau EYL, So WWK, Ho PC. High serum oestradiol concentrations in fresh IVF cycles do not impair implantation and pregnancy rates in subsequent frozen–thawed embryo transfer cycles. Hum Reprod. 2000;15:250–255. doi: 10.1093/humrep/15.2.250. [DOI] [PubMed] [Google Scholar]
- Simón C, Domínguez F, Valbuena D, Pellicer A. The role of estrogen in uterine receptivity and blastocyst implantation. Trends Endocrinol Metab. 2003;14:197–199. doi: 10.1016/s1043-2760(03)00084-5. [DOI] [PubMed] [Google Scholar]
- Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100:2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XL, Feng C, Lin XH, Zhong ZX, Zhu YM, Lv PP, Lv M, Meng Y, Zhang D, Lu XE, Jin F, Sheng JZ, et al. High maternal serum estradiol environment in the first trimester is associated with the increased risk of small-for-gestational-age birth. J Clin Endocrinol Metab. 2014;99:2217–2224. doi: 10.1210/jc.2013-3362. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hossain N, Paidas MJ. Adverse pregnancy outcome, the uteroplacental interface, and preventive strategies. Semin Perinatol. 2007;31:208–212. doi: 10.1053/j.semperi.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Kraupp BG, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995;21:1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham MC, Shaham S. Death without caspases, caspases without death. Trends Cell Biol. 2004;14:184–193. doi: 10.1016/j.tcb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Sweeney EE, Fan P, Jordan VC. Molecular modulation of estrogen-induced apoptosis by synthetic progestins in hormone replacement therapy: an insight into the women's health initiative study. Cancer Res. 2014;74:7060–7068. doi: 10.1158/0008-5472.CAN-14-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutanen EM, Koistinen R, Wahlström T, Sjöberg J, Stenman UH, Seppälä M. Placental protein 12 (PP12) in the human endometrium: tissue concentration in relation to histology and serum levels of PP12, progesterone, and oestradiol. Br J Obstet Gynaecol. 1984;91:377–381. doi: 10.1111/j.1471-0528.1984.tb05927.x. [DOI] [PubMed] [Google Scholar]
- Lui J, Matsuo H, Laog-Fernandex JB, Xu Q, Maruo T. The effects of progesterone on apoptosis in the human trophoblast-derived HTR-8/SV neo cells. Mol Hum Reprod. 2007;13:869–874. doi: 10.1093/molehr/gam078. [DOI] [PubMed] [Google Scholar]
- Chang K, Zhang L. Review article: steroid hormones and uterine vascular adaptation to pregnancy. Reprod Sci. 2008;15:336–348. doi: 10.1177/1933719108317975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzer T, Schumann M, Neumann M, deJager T, Stimpel M, Sefling E, Neyses L. 17beta-estradiol prevents programmed cell death in cardiac myocytes. Biochem Biophys Res Commun. 2000;268:192–200. doi: 10.1006/bbrc.2000.2073. [DOI] [PubMed] [Google Scholar]
- La Colla A, Vasconsuelo A, Boland R. Estradiol exerts antiapoptotic effects in skeletal myoblasts via mitochondrial PTP and MnSoD. J Endocrinol. 2013;216:331–341. doi: 10.1530/JOE-12-0486. [DOI] [PubMed] [Google Scholar]
- Whitley GSJ, Dash PR, Ayling LJ, Prefumo F, Thilaganathan B, Cartwright JE. Increased apoptosis in first trimester extravillous trophoblasts from pregnancies at higher risk of developing preeclampsia. Am J Pathol. 2007;170:1903–1909. doi: 10.2353/ajpath.2007.070006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naicker T, Dorsamy E, Ramsuran D, Burton GJ, Moodley J. The role of apoptosis on trophoblast cell invasion in the placental bed of normotensive and preeclamptic pregnancies. Hypertens Pregnancy. 2013;32:245–256. doi: 10.3109/10641955.2013.796969. [DOI] [PubMed] [Google Scholar]
- Wong BC, Oehninger S, Gibbons WE, Dong KW. Estrogen down-regulates GnRH gene expression in human placental cytotrophoblast cells. Mol Cell Endocrinol. 2004;213:199–210. doi: 10.1016/j.mce.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Lui J, Maccalman CD, Wang YL, Leung PC. Promotion of human trophoblasts invasion by gonadotropin-releasing hormone (GnRH) I and GnRH II via signaling pathways. Mol Endocrinol. 2009;23:1014–1021. doi: 10.1210/me.2008-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Liu H, Fang W, Yang Y, Wang H, Peng J. Insulin-like growth factor binding protein 7 modulates estrogen-induced trophoblast proliferation and invasion in HTR-8 and JEG-3 cells. Cell Biochem Biophys. 2012;63:73–84. doi: 10.1007/s12013-012-9342-5. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Gustafsson JÅ. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- Das C, Basak S. Expression and regulation of integrin receptors in human trophoblast cells: role of estradiol and cytokines. Indian J Exp Biol. 2003;41:748–755. [PubMed] [Google Scholar]
- Ni X, Nicholson RC, King BR, Chan EC, Read MA, Smith R. Estrogen represses whereas the estrogen-antagonist ICI 182780 stimulates placental CRH gene expression. J Clin Endocrinol Metab. 2002;87:3374–3378. doi: 10.1210/jcem.87.8.8745. [DOI] [PubMed] [Google Scholar]