Abstract
Background
Tim4 is a transmembrane protein known as T cell immunoglobulin and mucin domain containing protein-4. We speculated that Tim4 might be associated with glioma. This study aimed to investigate the expression level of Tim4 in gliomas and the regulatory role of Tim4 on the growth and apoptosis of LN-18 glioma cells.
Material/Methods
Tumor tissues and adjacent normal tissues were collected from patients with glioma. The expression level of Tim4 mRNA and protein was determined by RT-PCR and Western blot analyses, respectively to evaluate their association with glioma. Tim4 was overexpressed or silenced by siRNA interference in cultured human glioma cells LN-18. The growth and apoptosis of LN-18 cells was detected by MTT assay and flow cytometry. The colony-forming ability of LN-18 cells was assessed by the colony formation assay. The collection of human tissues was approved by the Research Ethics Committee at the Harbin Medical University Cancer Hospital and performed in strict accordance with international standards. All patients were required to sign the informed consent.
Results
The expression level of Tim4 mRNA and protein in tumor tissues was significantly higher compared with adjacent normal tissues. Antisense miRNA targeting Tim4 inhibited the growth of LN-18 cells, induced their apoptosis, and reduced their clonogenic capacity. In contrast, overexpression of Tim4 promoted the growth of LN-18 cells, inhibited their apoptosis, and enhanced their clonogenic potential.
Conclusions
The expression level of Tim-4 is closely associated with glioma. Decreased expression of Tim4 inhibited the growth and colony-forming ability of LN-18 cells and induced their apoptosis, whereas increased expression of Tim4 stimulated the growth and clonogenic potential of LN-18 cells and suppressed their apoptosis.
MeSH Keywords: Adenovirus Infections, Human; Apoptosis Inducing Factor; MicroRNAs
Background
Glioma is a type of primary tumor of the central nervous system, accounting for approximately 50% of intracranial tumors. The disease has been a serious threat to the life and health of patients [1]. Traditional treatment for glioma such as surgery, chemotherapy, and radiation therapy often causes a variety of side effects and clinical complications [2,3]. Therefore, there is currently a great need for more clinically effective, novel gene therapy [4]. The selection and identification of efficient molecular targets has become a priority.
Studies based on genetics and molecular biology have shown that the occurrence and development of malignant glioma is associated with mutations and abnormal expression of multiple genes as well as the disorder of several signal transduction pathways [5,6]. These genes and molecules in the signaling pathways directly or indirectly regulate the progression of cell cycles including the growth, proliferation and even the apoptosis of cells [7]. Theoretically, these regulatory molecules may inhibit or stimulate the growth of cells [8]. With the development of molecular biology and genetic therapeutic techniques, novel gene therapy targeting these molecules might become novel strategies for the treatment of glioma. Therefore, the screening for genes, proteins and other molecules related to the occurrence, development and prognosis of glioma is crucial for the development of molecular targeting therapy against glioma and has become a research hotspot [9,10].
Currently, very few glioma-associated molecular markers have been identified. The search for novel markers of glioma is important for molecular targeted treatment and intervention of the tumor [10]. Tim4 is a transmembrane protein known as T cell immunoglobulin and mucin domain containing protein-4 [11]. Although the role of Tim4 in human gliomas is not yet clarified, we speculate that the expression of Tim4 might be involved in the development of glioma by regulating the growth and apoptosis of glioma cells.
In the present study, tumor tissues and adjacent normal tissues were collected from patients with glioma (The collection of human tissues was approved by the Research Ethics Committee at the Harbin Medical University Cancer Hospital and performed in strict accordance with international standards. All patients were required to sign the informed consent.). The expression level of Tim4 mRNA and protein was determined by RT-PCR and Western blot analyses, respectively to evaluate their association with glioma. Tim4 was overexpressed or silenced by siRNA interference in cultured human glioma cells LN-18. The growth and apoptosis of LN-18 cells was detected by MTT assay and flow cytometry. The colony-forming ability of LN-18 cells was assessed by the colony formation assay. This study will provide a theoretical basis for the identification of novel therapeutic targets of glioma gene therapy.
Material and Methods
Cells and reagents
Human glioma cells LN-18 were purchased from American Type Culture Collection (ATCC). MTT kit was purchased from Shanghai Bioleaf Technology Co. (Shanghai, China). Apoptosis detection kits including caspase-3 activity assay kit and FITC-Annexin-V apoptosis detection kit for phosphatidylserine (PS) externalization were purchased from Beijing Dingguo Biotechnology Co. (Beijing, China). Anti-Tim4 and anti-actin antibodies were purchased from Beyotime Biotechnology (Shanghai, China). DMEM culture media and fetal bovine serum (FBS) were purchased from Sigma (St. Louis, MO, USA). Antisense miRNA targeting Tim4 and control miRNA were designed and synthesized by Fengshou Biotechnology Co. (Shanghai, China). Lipofectamine 2000 transfection reagent was purchased from Shanghai Bioleaf Technology Co. The collection of human tissues was approved by the Research Ethics Committee at Qilu Hospital and performed in strict accordance with international standards. All patients were required to sign the informed consent.
Culture of LN-18 glioma cells
LN-18 human glioma cells were cultured in DMEM medium containing 10% FBS at 37°C in an incubator with 5% CO2 [12].
Transfection of LN-18 glioma cells
LN-18 cells were transfected with antisense miRNA targeting Tim4 and control miRNA, and 1 μg of Tim4 plasmid using the conventional method [13]. LN-18 cells were subjected to subsequent experiments at 24 h after transfection.
MTT assay
The growth and proliferation of LN-18 cells in each group was detected by MTT assay as previously described [14].
Analysis of apoptosis by flow cytometry
The apoptosis of LN-18 cells in each group was assessed by flow cytometry as previously described [15]. Briefly, LN-18 cells in each group were collected by centrifugation at 150 g for 10 min, and resuspended in PBS solution. FITC-Annexin V and reaction solution were added into the cell suspension (volume ratio: 1:250:50). The mixture was incubated in the dark for 30 min. All samples were detected by flow cytometry.
Western blot analyses of Tim4 level in LN-18 cells
The level of Tim4 in LN-18 cells in each group was determined by Western blot analyses using anti-Tim4 and anti-actin antibodies as previously described [16].
RT-PCR analyses of Tim4 mRNA level in LN-18 cells
The level of Tim4 mRNA in LN-18 cells in each group was determined by RT-PCR analyses as previously described [17].
Caspase-3 activity assay
The activity of caspase-3 in LN-18 cells in each group was determined using caspase-3 activity assay kit as previously described [18].
Colony formation assay
LN-18 cells transfected with antisense miRNA targeting Tim4 or over-expressing Tim4 were collected. Colony formation assay was performed using the conventional method to detect the clonogenic capacity of these cells [19].
Statistical analyses
All data were expressed as mean ± standard. Statistical analyses were performed using SPSS 18.0. Differences among groups were compared by one-way analysis of variance (ANOVA). P<0.05 is considered statistically significant.
Results
Increased expression of Tim4 in human glioma cells LN-18
As shown in Figure 1, the expression level of Tim4 mRNA in glioma tissues was significantly higher compared with normal control tissues (P=0.001). As shown in Figure 2, the level of Tim4 protein in glioma tissues was also significantly higher than that in normal control tissues (P=0.0021). These results suggested that Tim4 might be associated with the occurrence and progression of glioma.
Figure 1.
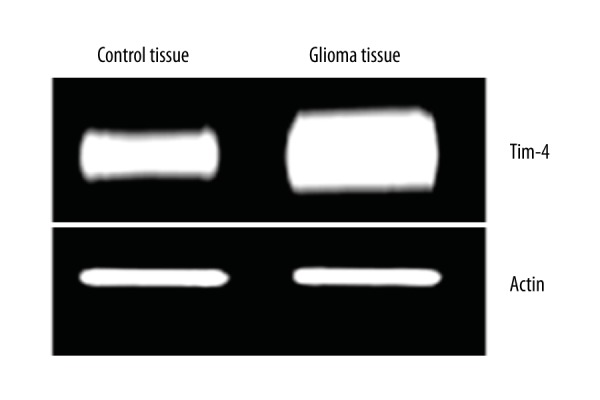
RT-PCR analyses showing increased Tim4 mRNA level in glioma tissues.
Figure 2.
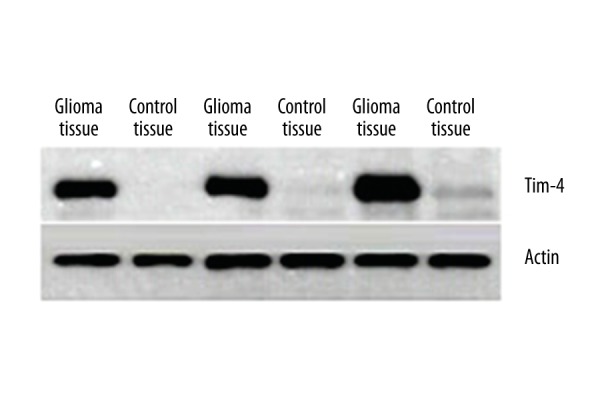
Western blot analyses showing increased Tim4 protein level in glioma tissues.
Inhibitory effect of antisense miRNA targeting Tim4 on the growth of LN-18 cells
As shown in Figure 3, the percentage of viable cells in the group transfected with antisense miRNA targeting Tim4 was significantly lower compared with the control miRNA group (P=0.028).
Figure 3.
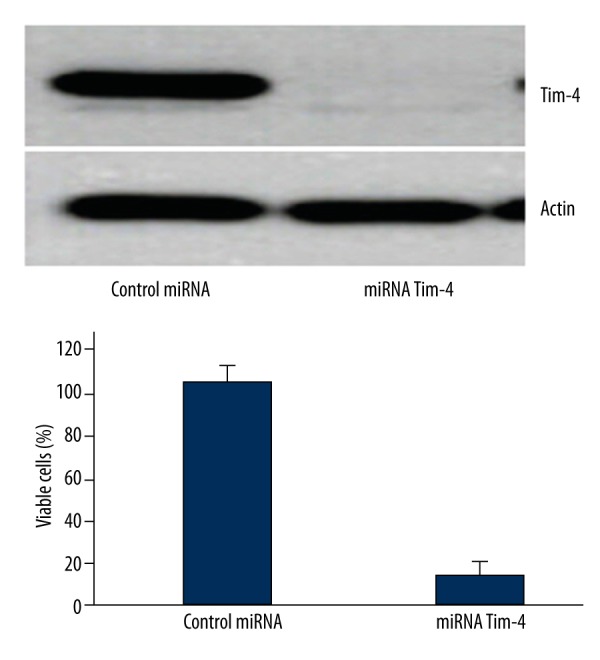
Antisense miRNA targeting Tim4 inhibited the growth of LN-18 cells.
Antisense miRNA targeting Tim4-induced apoptosis in LN-18 cells
As shown in Figures 4 and 5, both the extent of PS externalization and caspase-3 activity in LN-18 cells transfected with antisense miRNA targeting Tim4 was significantly higher compared with the control miRNA group, suggesting that antisense miRNA targeting Tim4 induced the apoptosis of LN-18 cells.
Figure 4.

Antisense miRNA targeting Tim4 induced PS externalization in LN-18 cells.
Figure 5.
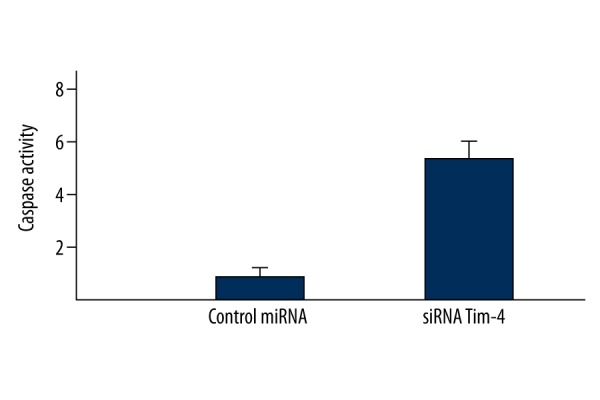
Antisense miRNA targeting Tim4 induced higher caspase-3 activity in LN-18 cells.
Inhibitory effect of antisense miRNA targeting Tim4 on the clonogenic capacity of LN-18 cells
As shown in Figure 6, the clonogenic potential of LN-18 cells transfected with antisense miRNA targeting Tim4 was significantly lower than that in the control miRNA group, which indicating the inhibitory effect of antisense miRNA targeting Tim4 on the colony formation of LN-18 cells.
Figure 6.
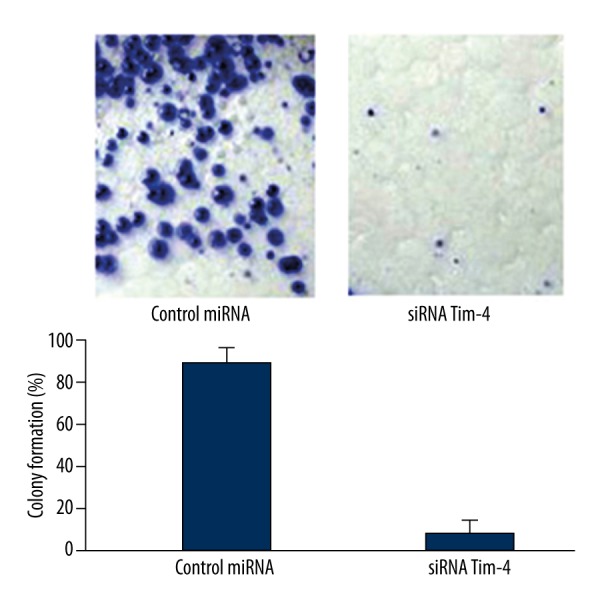
Antisense miRNA targeting Tim4 inhibited the clonogenic capacity of LN-18 cells.
Stimulation of Tim4 overexpression on the growth of LN-18 cells
As shown in Figure 7, the percentage of viable cells in the Tim4 overexpression group was significantly higher compared with the control group transfected with empty plasmid, suggesting that the overexpression of Tim4 did not promote apoptosis, but stimulated the growth of LN-18 cells.
Figure 7.
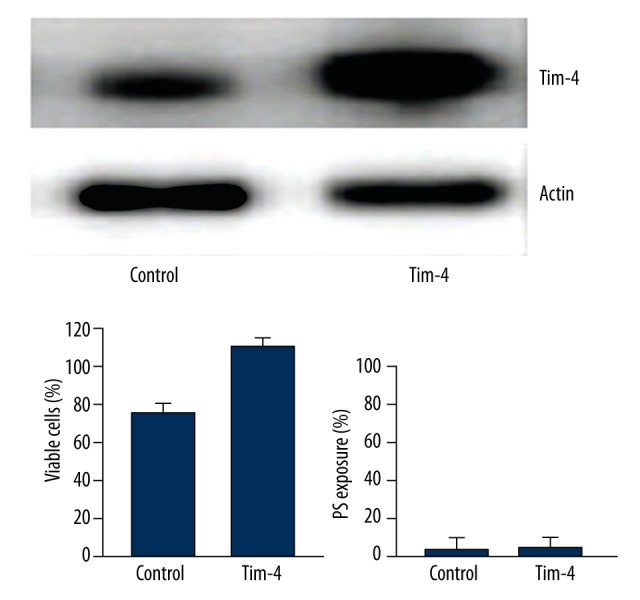
The overexpression of Tim4 stimulated the growth of LN-18 cells.
Discussion
In this study, we investigated the expression level of Tim4 in glioma and its regulatory role on glioma cells LN-18. Our work is unique in that: 1) it was shown that the expression level of Tim4 mRNA and protein in glioma tissues was significantly higher compared with adjacent normal tissues, suggesting that Tim4 might be associated with the occurrence and development of glioma. 2) Antisense miRNA targeting Tim4 inhibited the growth of LN-18 cells, induced their apoptosis, and reduced their clonogenic capacity, whereas the overexpression of Tim4 led to completely opposite effects in LN-18 cells. These results suggested that Tim4 modulated the growth and apoptosis of LN-18 cells at the cellular level, and thereby might be a potential anti-apoptotic protein, which is consistent with the inhibition of anti-apoptotic proteins on the apoptosis and colony formation of tumor cells in previous studies [20,21].
Apoptosis is primarily mediated by the mitochondrial intrinsic pathway and death receptor extrinsic pathway [22]. In this study, the expression of intracellular Tim4 was silenced by short interfering RNA (siRNA), PS externalization was clearly observed in LN-18 cells, which revealed that significant apoptosis of these cells had occurred. The Tim4-induced apoptosis signaling pathway in LN-18 cells treated by RNA interference was further investigated. Results of caspase activity assay showed that the activity of caspase-3 was significantly increased in LN-18 cells with decreased Tim4 expression, whereas the marker for the death receptor extrinsic pathway, caspase-8, was not detected (data not shown), which suggested that the Tim4-induced apoptosis signaling pathway in LN-18 cells was mediated by mitochondria. The result was inconsistent with previous findings that the apoptosis of tumor cells was regulated by caspase-8 [23]. Such a discrepancy might be associated with different sensitivity of different tumor cells to external stimuli [24]. Additionally, we speculate that Tim4 might direct or indirectly activate the mitochondria-mediated signaling pathway, and thus initiate the process of apoptosis [25]. However, further studies are needed to verify the speculation.
The present study has the following limitations: First, it did not investigate the association between Tim4 expression and the extent of glioma such as the clinical staging and grading of the tumor mostly because it failed to predict the possible correlation between Tim4 expression and the extent of glioma development during sample collection. Moreover, it was difficult in such a short time to collect glioma tissues at different staging and grading due to the limited availability of precious clinical specimens. The association between Tim4 expression and the extent of glioma shall be investigated further in future studies. The elucidation of such association will shed light on the diagnosis, treatment and prognosis of glioma. Secondly, no rat glioma model was constructed to verify the association between Tim4 and the occurrence and development of gliomas in this study. In addition, no animal study was performed to evaluate the potential of Tim4 intervention by siRNA in the treatment of gliomas. Third, this study included only a limited number of glioma cases, and therefore the conclusions need to be further verified. Finally, since the post-treatment expression of Tim4 in glioma tissues was not measured, the association between Tim4 level and prognosis of glioma could not be assessed. Nevertheless, despite all the limitations mentioned above, the current study on the Tim4 overexpression and siRNA-mediated Tim4 knockdown at the cellular level (LN-18 cells) has provided a theoretical basis for the application of molecular targeting therapy in the treatment of glioma. However, the behavior of LN-18 glioma cells was not compared to the known clinical subtypes and grading in this study due to the unavailability of glioma tissues at different staging and the limited experimental conditions, which shall be included in future studies.
Conclusions
The expression level of Tim-4 is closely associated with glioma. Decreased expression of Tim4 inhibited the growth and colony-forming ability of LN-18 cells and induced their apoptosis, whereas increased expression of Tim4 stimulated the growth and clonogenic potential of LN-18 cells and suppressed their apoptosis. The present study will provide a theoretical basis for the identification of novel therapeutic targets of glioma gene therapy.
Footnotes
Source of support: Promotive research fund for excellent young and middle-aged scientists of Shandong Province (BS2011YY010)
References
- 1.Chen W, Wang D, Du X, et al. Glioma cells escaped from cytotoxicity of temozolomide and vincristine by communicating with human astrocytes. Med Oncol. 2015;32(3):43. doi: 10.1007/s12032-015-0487-0. [DOI] [PubMed] [Google Scholar]
- 2.Zhang B, Wu T, Wang Z, et al. p38MAPK activation mediates tumor necrosis factor-α-induced apoptosis in glioma cells. Mol Med Rep. 2015;11(4):3101–7. doi: 10.3892/mmr.2014.3002. [DOI] [PubMed] [Google Scholar]
- 3.Li X, He X, Tian W, Wang J. Short hairpin RNA targeting Notch2 inhibits U87 human glioma cell proliferation by inducing cell cycle arrest and apoptosis in vitro and in vivo. Mol Med Rep. 2014;10(6):2843–50. doi: 10.3892/mmr.2014.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao D, Song Y, Che Z, Liu Q. Calcium overload and in vitro apoptosis of the C6 glioma cells mediated by sonodynamic therapy (hematoporphyrin monomethyl ether and ultrasound) Cell Biochem Biophys. 2014;70(2):1445–52. doi: 10.1007/s12013-014-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koneru M, O’Cearbhaill R, Pendharkar S, et al. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto )directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med. 2015;13(1):102. doi: 10.1186/s12967-015-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder KA, Hughes MR, Hedberg B, et al. Podocalyxin enhances breast tumor growth and metastasis and is a target for monoclonal antibody therapy. Breast Cancer Res. 2015;17(1):46. doi: 10.1186/s13058-015-0562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosch A, Li Z, Bergamaschi A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med. 2015;7(283):283ra51. doi: 10.1126/scitranslmed.aaa4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heller G, Rommer A, Steinleitner K, et al. EVI1 promotes tumor growth via transcriptional repression of MS4A3. J Hematol Oncol. 2015;8(1):28. doi: 10.1186/s13045-015-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang JT, Li ZL, Wu JY, et al. An oxidative stress mechanism of shikonin in human glioma cells. PLoS One. 2014;9(4):e94180. doi: 10.1371/journal.pone.0094180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JB, Dong DF, Gao K, Wang MD. Mechanisms underlying the biological changes induced by isocitrate dehydrogenase-1 mutation in glioma cells. Oncol Lett. 2014;7(3):651–57. doi: 10.3892/ol.2014.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang XJ, Lu JT, Tu HJ, et al. TRAIL-engineered bone marrow-derived mesenchymal stem cells: TRAIL expression and cytotoxic effects on C6 glioma cells. Anticancer Res. 2014;34(2):729–34. [PubMed] [Google Scholar]
- 12.Kus G, Oztopcu-Vatan P, Uyar R, Kabadere S. Cytotoxic and apoptotic functions of licofelone on rat glioma cells. Acta Biol Hung. 2013;64(4):438–52. doi: 10.1556/ABiol.64.2013.4.4. [DOI] [PubMed] [Google Scholar]
- 13.Hagemann C, Fuchs S, Monoranu CM, et al. Impact of MACC1 on human malignant glioma progression and patients’ unfavorable prognosis. Neuro Oncol. 2013;15(12):1696–709. doi: 10.1093/neuonc/not136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awde AR, Boisgard R, Thézé B, et al. The translocator protein radioligand 18F-DPA-714 monitors antitumor effect of erufosine in a rat 9L intracranial glioma model. J Nucl Med. 2013;54(12):2125–31. doi: 10.2967/jnumed.112.118794. [DOI] [PubMed] [Google Scholar]
- 15.García-Álvarez I, Garrido L, Romero-Ramírez L, et al. The effect of antitumor glycosides on glioma cells and tissues as studied by proton HR-MAS NMR spectroscopy. PLoS One. 2013;8(10):e78391. doi: 10.1371/journal.pone.0078391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bota DA, Alexandru D, Keir ST, et al. Proteasome inhibition with bortezomib induces cell death in GBM stem-like cells and temozolomide-resistant glioma cell lines, but stimulates GBM stem-like cells’ VEGF production and angiogenesis. J Neurosurg. 2013;119(6):1415–23. doi: 10.3171/2013.7.JNS1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SM, Yoon SM, Yim MS, et al. Targeted delivery of a phosphopeptide prodrug inhibits the proliferation of a human glioma cell line. Amino Acids. 2013;45(5):1149–56. doi: 10.1007/s00726-013-1570-5. [DOI] [PubMed] [Google Scholar]
- 18.Kopatz J, Beutner C, Welle K, et al. Siglec-h on activated microglia for recognition and engulfment of glioma cells. Glia. 2013;61(7):1122–33. doi: 10.1002/glia.22501. [DOI] [PubMed] [Google Scholar]
- 19.Priester M, Copanaki E, Vafaizadeh V, et al. STAT3 silencing inhibits glioma single cell infiltration and tumor growth. Neuro Oncol. 2013;15(7):840–52. doi: 10.1093/neuonc/not025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Chen Y, Wan J, et al. ABT-263 enhances sorafenib-induced apoptosis associated with Akt activity and the expression of Bax and p21((CIP1/WAF1)) in human cancer cells. Br J Pharmacol. 2014;171(13):3182–95. doi: 10.1111/bph.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsebatlela T, Gallicchio V, Becker R. Lithium modulates cancer cell growth, apoptosis, gene expression and cytokine production in HL-60 promyelocytic leukaemia cells and their drug-resistant sub-clones. Biol Trace Elem Res. 2012;149(3):323–30. doi: 10.1007/s12011-012-9438-1. [DOI] [PubMed] [Google Scholar]
- 22.Ashkenazi A. Targeting the extrinsic apoptotic pathway in cancer: lessons learned and future directions. J Clin Invest. 2015;125(2):487–89. doi: 10.1172/JCI80420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akl H, Vervloessem T, Kiviluoto S, et al. A dual role for the anti-apoptotic Bcl-2 protein in cancer: mitochondria versus endoplasmic reticulum. Biochim Biophys Acta. 2014;1843(10):2240–52. doi: 10.1016/j.bbamcr.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Gajate C, Mollinedo F. Lipid rafts, endoplasmic reticulum and mitochondria in the antitumor action of the alkylphospholipid analog edelfosine. Anticancer Agents Med Chem. 2014;14(4):509–27. doi: 10.2174/1871520614666140309222259. [DOI] [PubMed] [Google Scholar]
- 25.Koehler BC, Jäger D, Schulze-Bergkamen H. Targeting cell death signaling in colorectal cancer: current strategies and future perspectives. World J Gastroenterol. 2014;20(8):1923–34. doi: 10.3748/wjg.v20.i8.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]


