Abstract
Objectives
Polymyxins are a last-line therapy to treat MDR Gram-negative bacterial infections. Nephrotoxicity is the dose-limiting factor for polymyxins and recent studies demonstrated significant accumulation of polymyxins in renal tubular cells. However, little is known about the mechanism of polymyxin uptake into these cells. Oligopeptide transporter 2 (PEPT2) is a solute carrier transporter (SLC) expressed at the apical membrane of renal proximal tubular cells and facilitates drug reabsorption in the kidney. In this study, we examined the role of PEPT2 in polymyxin uptake into renal tubular cells.
Methods
We investigated the inhibitory effects of colistin and polymyxin B on the substrate uptake mediated through 15 essential SLCs in overexpressing HEK293 cells. The inhibitory potency of both polymyxins on PEPT2-mediated substrate uptake was measured. Fluorescence imaging was employed to investigate PEPT2-mediated uptake of the polymyxin fluorescent probe MIPS-9541 and a transport assay was conducted with MIPS-9541 and [3H]polymyxin B1.
Results
Colistin and polymyxin B potently inhibited PEPT2-mediated [3H]glycyl-sarcosine uptake (IC50 11.4 ± 3.1 and 18.3 ± 4.2 μM, respectively). In contrast, they had no or only mild inhibitory effects on the transport activity of the other 14 SLCs evaluated. MIPS-9541 potently inhibited PEPT2-mediated [3H]glycyl-sarcosine uptake (IC50 15.9 μM) and is also a substrate of PEPT2 (Km 74.9 μM). [3H]polymyxin B1 was also significantly taken up by PEPT2-expressing cells (Km 87.3 μM).
Conclusions
Our study provides the first evidence of PEPT2-mediated uptake of polymyxins and contributes to a better understanding of the accumulation of polymyxins in renal tubular cells.
Introduction
Seventy years after the first antibiotic, penicillin, was introduced into the clinic, we are now facing a post-antibiotic era.1 Over the last two decades, there have been a very limited number of new antibiotics discovered. Increasing antibiotic resistance has become a leading healthcare problem worldwide.2,3 In particular, there are significant threats from MDR Gram-negative ‘superbugs’ such as Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae that are resistant to almost all currently available antibiotics.4 Due to the dry development pipeline of novel antibiotics,5 polymyxins have been used clinically as last-line therapy for treating the aforementioned ‘superbugs’.6–10
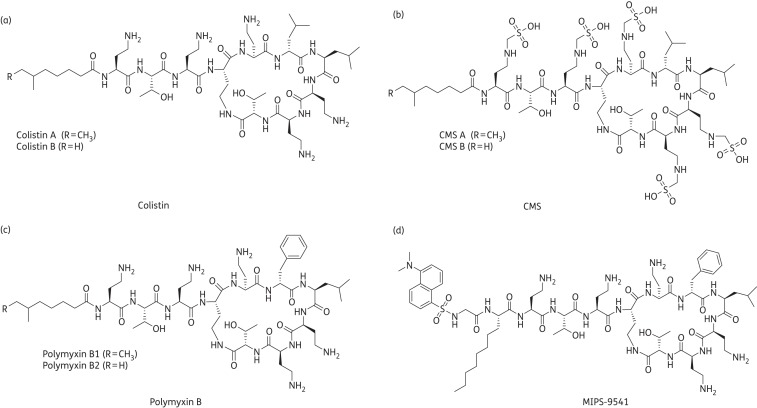
Polymyxins are polycationic cyclic lipopeptides produced by Paenibacillus polymyxa and were discovered in the 1940s.11,12 However, their clinical applications have been largely restricted since the 1970s primarily due to their nephrotoxicity.13 Polymyxin B and colistin (also known as polymyxin E) are the two polymyxins used clinically and they differ by a single amino acid at position 6 (Figure 1).13,14 Commercial products of polymyxin B and colistin contain a variety of components with polymyxin B1 and B2 and colistin A and B as the major components, respectively.14 Polymyxin B and colistin initially bind to the anionic LPS of Gram-negative bacteria, displace divalent Ca2+ and Mg2+ between LPS molecules and destabilize the outer membrane.6 In addition, polymyxins have an antiendotoxin property by neutralizing LPS.15 In practice, colistin is parenterally administered to patients in the form of an inactive prodrug, colistimethate sodium (CMS). In contrast, polymyxin B is used directly in its sulphate form for parenteral administration in North America, South America and South-East Asia.6
Figure 1.
Structures of (a) colistin, (b) CMS, (c) polymyxin B and (d) MIPS-9541.
Polymyxin-induced nephrotoxicity may result from its extensive reabsorption by renal tubular cells.16–20 Therefore, elucidating the mechanism of uptake of polymyxins by kidney cells is fundamental to understand their renal toxicity. It has been reported that megalin facilitates the movement of polymyxins across the membrane via endocytosis in kidney tubular cells.21,22 However, in megalin-knockout rats, renal accumulation of polymyxins was only partially decreased,21 which suggests that other mechanisms also play key roles in the reabsorption of polymyxin by renal tubular cells. The current study demonstrated a novel transporter-facilitated route of polymyxin uptake into renal cells.
Solute carrier transporters (SLCs) are membrane proteins responsible for cellular uptake of a wide range of substances including hormones, steroids, toxins and many clinically important drugs.23 Organic anion-transporting polypeptides (OATPs), organic anion/cation transporters (OATs/OCTs) and oligopeptide transporters (PEPTs) represent the most important SLC subfamilies involved in drug uptake.23–25 These proteins are widely expressed in key human organs including the kidney and liver.26,27 They are responsible for cellular uptake of drug molecules in these tissues, which impacts on drug pharmacokinetics and toxicities. Various antibiotics have been previously found to interact with SLC transporters. For example, PEPTs were shown to mediate the renal uptake of β-lactam antibiotics.28–31
In the current study, we characterized, for the first time, human PEPT2-mediated uptake of polymyxins in overexpressing human embryonic kidney (HEK) 293 cells and evaluated the role of PEPT2 in polymyxin-induced nephrotoxicity.
Materials and methods
Materials
[3H]oestrone sulphate (ES; 57.3 Ci/mmol), [3H]cholecystokinin octapeptide (CCK-8; 97.5 Ci/mmol) and [3H]methyl-4-phenylpyridinium acetate (MPP+; 82.1 Ci/mmol) were purchased from PerkinElmer (Melbourne, VIC, Australia). [3H]4-aminohippuric acid (PAH; 60 Ci/mmol), [3H]l-ergothioneine (1.7 Ci/mmol), [3H]glycyl-sarcosine (Gly-Sar; 2 Ci/μmol) and [14C]l-carnitine (56 mCi/mmol) were purchased from BioScientific (Gymea, NSW, Australia). [3H]polymyxin B1 (120.4 Ci/mol) was synthesized by Quotient Bioresearch (Cardiff, South Glamorgan, UK). Culture media were obtained from Life Technologies (Mount Waverley, VIC, Australia). Gly-Sar, CMS, colistin and polymyxin B were purchased from Sapphire Biosciences (Redfern, NSW, Australia). MIPS-9541 was synthesized in-house as described previously.32 Unless otherwise stated, all other reagents were purchased from Sigma–Aldrich (Castle Hill, NSW, Australia).
Plasmids containing full-length human OCTN1 and OCTN2 cDNAs were obtained from Gene-Ethics (Singapore). The plasmids containing the coding regions of human OAT1, OAT2, OAT3, OCT1, OCT2, OCT3 and PEPT2 were purchased from Australian Biosearch (Balcatta, WA, Australia). The mammalian expressing plasmids of human OATP1A2, OATP1B1, OATP1B3 and OATP2B1 were obtained from United BioResearch (Dural, NSW, Australia).33–36 The plasmid containing the human OAT4 coding region was cloned in-house.37 The plasmid of PEPT1 was kindly provided by Professor Peter J. Meier-Abt (University of Basel, Basel, Switzerland).
Expression of SLC transporters in HEK293 cells
HEK293 cells were maintained at 37°C and 5% CO2 in DMEM supplemented with 10% FCS. Cells were transfected with plasmid DNAs using Lipofectamine 2000 Reagent (Invitrogen, Mount Waverley, VIC, Australia) following the manufacturer's instructions. Transport activities were measured at 24 h after transfection.
Transport studies
To measure the influx of transporter prototypical substrates, influx into control cells (vector transfected) was subtracted from influx measured as the accumulation of radiolabelled compounds in cells overexpressing transporter constructs. Uptake of a mix of radiolabelled and unlabelled typical substrates for each SLC transporter was initiated at 37°C in PBS (pH 7.0 or 5.0) containing 5 mM glucose. Total substrate concentrations and timepoints employed in the study were as described previously: 300 nM [3H]ES for OAT3, OAT4, OATP1A2, OATP1B1 and OATP2B1;38–44 500 nM [3H]ES for OAT2 (pH 5.5);45 1 μM [3H]PAH for OAT1;46 2 nM [3H]CCK-8 for OATP1B3;47 100 nM [3H]MPP+ for OCT1, OCT2 and OCT3;48 1 μM [3H]l-ergothioneine for OCTN1;49 5 μM [14C]l-carnitine for OCTN2;50 and 2.5 μM [3H]Gly-Sar for PEPT1 and PEPT2 (pH 5.5).51 Our preliminary experiments indicated that initial rates of transporter-mediated substrate uptake in HEK293 cells were linear over ≥8 min (data not shown); hence, 8 min was selected for subsequent experiments. The uptake was terminated at 8 min intervals by rapidly washing cells in ice-cold PBS. Cells were then solubilized in 0.2 M NaOH, neutralized with 0.2 M HCl and aliquotted for liquid scintillation counting. The uptake count was standardized to the amount of protein in each well. Background counts of vector-transfected cells were subtracted from all uptake data. The stock solutions of colistin, polymyxin B and MIPS-9541 were all freshly prepared (<30 min before the experiments started). Exposure to up to 500 μM colistin, polymyxin B and MIPS-9541 for 8 min did not significantly influence the background counts observed in the vector-transfected cells, as compared with that of parental cells (data not shown); our preliminary data indicated that the background uptake of vector-transfected cells in the presence of inhibitors represented appropriate values for background correction. Data are presented as the mean ± SEM. The experiments were conducted independently three times with three replicates for each condition.
The inhibitory potency of colistin, polymyxin B and MIPS-9541 was evaluated by IC50 values (the concentration required to inhibit 50% of transporter function). Uptake measurement was performed with varying concentrations of the compound (ranging from 10 nM to 500 μM) added to the uptake buffer containing 2.5 μM [3H]Gly-Sar. The IC50 of each compound was calculated by non-linear regression using GraphPad Prism 6.0 (GraphPad, La Jolla, CA, USA). Each experiment was conducted independently three times with three replicates for each condition.
Uptake measurement of MIPS-9541
Uptake of MIPS-9541 was initiated at 37°C in PBS (pH 5.5) containing 5 mM glucose. Fluorescence accumulation in cells was measured with a Tecan Safire II microplate reader (Life Technologies) with an excitation wavelength of 350 nm and an emission wavelength of 518 nm. Uptake of MIPS-9541 was standardized to the fluorescence counts of 100 μg of protein in each well. Background counts of vector-transfected cells were subtracted from all uptake data. Data are presented as the mean ± SEM. The experiments were conducted three times with three replicates for each data group. Kinetic studies were performed with varying concentrations of MIPS-9541 (0–500 μM) through a 4 min interval incubation. Apparent Km and Vmax values for transport activity were then calculated using GraphPad Prism 6.0.
Uptake of [3H]polymyxin B1 was initiated at 37°C in PBS (pH 5.5) containing 5 mM glucose. Radioactivity in cells was measured with a Hidex S300 liquid scintillation counter (Skudtek Scientific). Uptake of [3H]polymyxin B1 was standardized to the cpm of 100 μg of protein in each well. Background counts of vector-transfected cells were subtracted from all uptake data. Data are presented as the mean ± SEM. The experiments were conducted three times with three replicates for each data group. Kinetic studies were performed with varying concentrations of [3H]polymyxin B1 (0–300 μM) through a 3 min interval incubation. Apparent Km and Vmax values for transport activity were calculated using GraphPad Prism 6.0.
Fluorescence imaging of MIPS-9541 uptake in cells
After the medium was removed from cells transfected with PEPT2 or the vector, cells were incubated with 10 μM MIPS-9541 for 5 min at 37°C. After washing three times in ice-cold PBS, cells were mounted in SlowFade® Gold Antifade Mountant with DAPI (Invitrogen). Samples were visualized with a Leica DMI3000 B epifluorescence microscope (Leica Microsystems, North Ryde, NSW, Australia).
Statistics
Student's t-test was employed to examine the difference between two sets of normally distributed data. Differences in the transport function of PEPT2 with or without treatments were detected by one-way analysis of variance and Dunnett's test. Data are expressed as the mean ± SEM with a P value of <0.05 considered as significant.
Results
Inhibitory effects of polymyxin B and colistin on the specific substrate uptake mediated by essential SLC transporters
We assessed the inhibitory effects of colistin and polymyxin B on the substrate uptake mediated by 15 essential human SLC transporters including OAT1, OAT2, OAT3, OAT4, OATP1A2, OATP1B1, OATP1B3, OATP2B1, OCT1, OCT2, OCT3, OCTN1, OCTN2, PEPT1 and PEPT2. These transporters are involved in drug transport in key human tissues such as the liver and kidney.23–25 The HEK293 cell line was chosen in this study because it is a well-established in vitro renal cell model with favourable transfection efficacy and is widely used in transporter studies.33,34,43,52
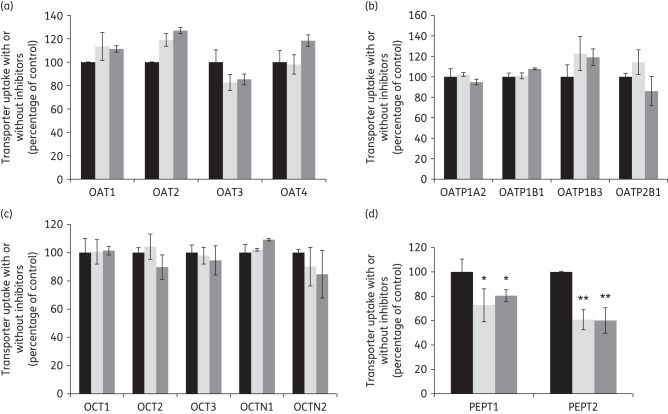
As shown in Figure 2, colistin and polymyxin B at 10 μM had no effect on the substrate uptake mediated through any of the SLC transporters evaluated, except for the PEPTs (Figure 2d). Both polymyxins mildly reduced the influx of Gly-Sar through PEPT1 (∼20%–25% inhibition; P < 0.05). Of note, polymyxin B and colistin significantly inhibited the uptake of Gly-Sar mediated by PEPT2 (∼40% inhibition; P < 0.01), which suggests colistin and polymyxin B are potent inhibitors of PEPT2. PEPT2 is more abundantly expressed at the urine-facing apical membrane of renal proximal tubular cells,53 while PEPT1 is largely distributed in renal distal tubular cells. Considering the majority of the current literature on polymyxin accumulation in the kidney is on proximal tubular cells,17,54 our following experiments focused on PEPT2.
Figure 2.
Inhibitory effects of colistin and polymyxin B on the substrate uptake mediated through 15 essential SLC transporters that were overexpressed by HEK293 cells. Uptake of each radiolabelled substrate was measured in the absence (black bars) and presence of 10 µM colistin (light grey bars) or polymyxin B (dark grey bars) in cells overexpressing human (a) OAT1, OAT2, OAT3 and OAT4; (b) OATP1A2, OATP1B1, OATP1B3 and OATP2B1; (c) OCT1, OCT2, OCT3, OCTN1 and OCTN2; and (d) PEPT1 and PEPT2. Background counts of vector-transfected cells were subtracted from all uptake data and the standardized result is presented as a percentage of the control (i.e. no inhibitors). In all experiments, experiments were conducted independently three times with three replicates in each experiment and values are expressed as the mean ± SEM. Significant difference from the control: *P < 0.05 and **P < 0.01.
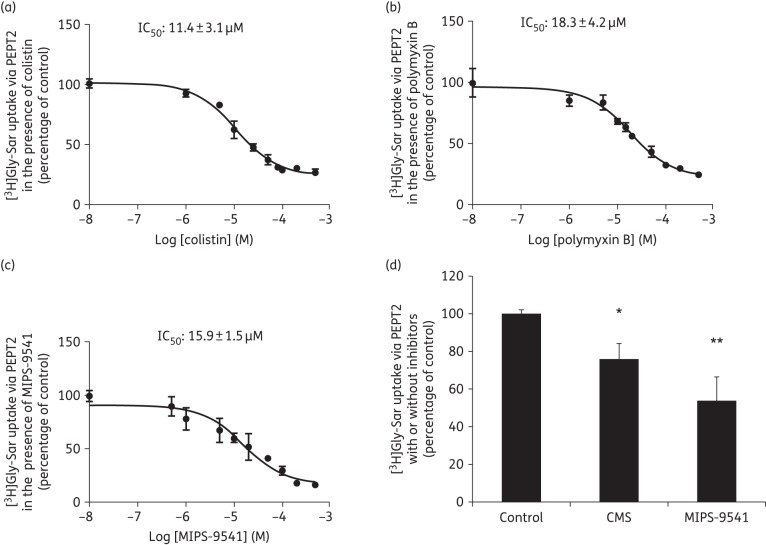
We evaluated the inhibitory potency of colistin and polymyxin B on PEPT2 transport activity (Figure 3a and b) with PEPT2-mediated Gly-Sar uptake measured in the absence or presence of either agent (ranging from 10 nM to 500 μM). It was also observed that uptake by vector-transfected control cells in the presence of both agents at 500 μM was not different from that of parental cells, which indicated that the physiology of the cells was not compromised under our experimental conditions (data not shown). The IC50 value of colistin (11.4 ± 3.1 μM) for inhibiting PEPT2 transport activity was slightly lower than that of polymyxin B (18.3 ± 4.2 μM).
Figure 3.
Inhibitory effect of polymyxins on PEPT2-mediated uptake of Gly-Sar. Cellular uptake of [3H]Gly-Sar was measured in the absence or presence of (a) colistin, (b) polymyxin B or (c) MIPS-9541, ranging from 10 nM to 500 µM in HEK293 cells overexpressing PEPT2 or transfected with the vector alone. (d) The inhibitory effect of CMS and MIPS-9541 on PEPT2-mediated Gly-Sar uptake. [3H]Gly-Sar uptake was measured in the absence or presence of 10 µM CMS or MIPS-9541 in cells overexpressing human PEPT2 or transfected with vector alone. Background counts of vector-transfected cells were subtracted from all uptake data and the standardized result is presented as a percentage of the control (i.e. no inhibitors). In all experiments, experiments were independently conducted three times with three replicates in each experiment and values are expressed as the mean ± SEM. Significant difference from the control: *P < 0.05 and **P < 0.01.
PEPT2-mediated cellular uptake of polymyxins
As an inhibitor may not be a substrate of a transporter, direct uptake is required to determine whether polymyxins are substrates of PEPT2. In a previous study,32 we demonstrated MIPS-9541 is a novel fluorescent probe that favourably maintains the antibacterial and apoptotic effects of polymyxins. It was generated through regioselective modification of the core scaffold of polymyxin B with a dansyl fluorophore and is a preferred representation of the chemical and pharmacological properties of polymyxins.55 In the present study, it was evident that MIPS-9541 also potently inhibited PEPT2-mediated substrate uptake with an IC50 of 15.9 ± 1.5 μM (Figure 3c and d) comparable to that of colistin and polymyxin B; in contrast, CMS, the inactive prodrug of colistin associated with less nephrotoxicity,56,57 only moderately interacts with this transporter (Figure 3d).
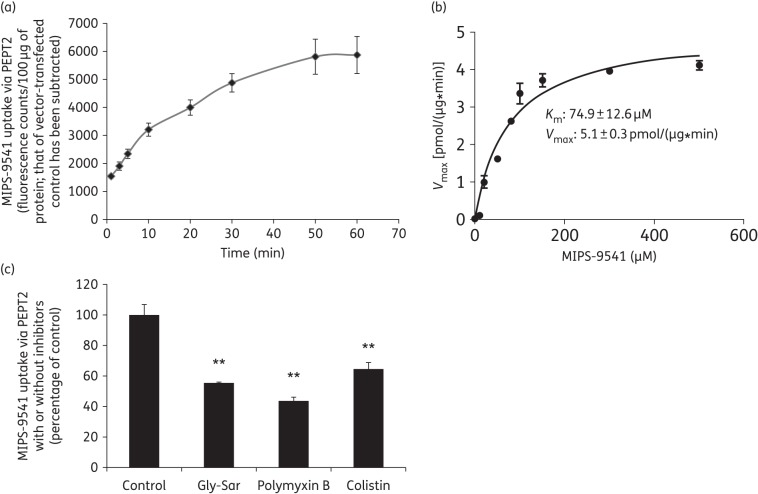
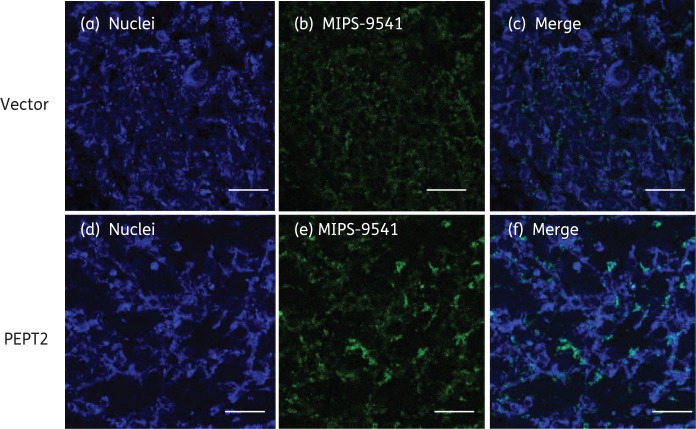
Taking advantage of this fluorescent probe, we then evaluated the involvement of PEPT2 in the cellular uptake of polymyxins. As shown in Figure 4(a and b), the uptake of MIPS-9541 through PEPT2 is time dependent and saturable. In the confirmatory fluorescence imaging analysis (Figure 5), a dramatically increased accumulation of MIPS-9541 in PEPT2-expressing cells was observed compared with that by the vector-transfected control cells. Our kinetic analysis revealed that the Km value of MIPS-9541 to PEPT2 was 74.9 μM, which suggested a favourable binding of MIPS-9541 to PEPT2, comparable to that of the classic PEPT2 substrate Gly-Sar (Km = 70 μM).58 In addition, the PEPT2-mediated MIPS-9541 uptake was inhibited by Gly-Sar, colistin and polymyxin B (Figure 4c), which demonstrated the transporter-specific interactions among these substrates.
Figure 4.
Uptake of MIPS-9541 in HEK293 cells transfected with PEPT2 or the vector alone. (a) Accumulation of MIPS-9541 in HEK293 cells overexpressing PEPT2. The vector- and transporter-expressing cells were incubated with 10 μM MIPS-9541 (in PBS, pH 5.5). Fluorescent signals accumulated in cells were measured using a Tecan Safire II microplate reader over 60 min. Background counts of vector-transfected cells were subtracted from all uptake data and the result was standardized to fluorescence counts/100 μg of protein. (b) Kinetic parameters of MIPS-9541 uptake via PEPT2. The kinetic parameters of MIPS-9541 uptake were derived in HEK293 cells transiently transfected with PEPT2 or the vector. Uptake was assessed with various concentrations of MIPS-9541 (ranging from 0 to 500 µM), subtracting the background of vector-transfected control cells. Km and Vmax values of MIPS-9541 uptake were derived by GraphPad Prism 6.0 software. (c) Inhibition by Gly-Sar, colistin or polymyxin B of PEPT2-mediated MIPS-9541 uptake. MIPS-9541 uptake was measured in the absence or presence of 10 µM Gly-Sar, colistin or polymyxin B in cells overexpressing human PEPT2 or transfected with the vector alone. Background counts of vector-transfected cells were subtracted from all uptake data and the standardized result is presented as a percentage of the control (i.e. no inhibitors). All experiments were conducted independently three times with three replicates in each experiment and values are expressed as the mean ± SEM. Significant difference from the control: **P < 0.01.
Figure 5.
Fluorescence imaging of MIPS-9541 accumulation in HEK293 cells transfected with PEPT2 or the vector alone. MIPS-9541 (10 µM) was applied to HEK293 cells overexpressing PEPT2 or the vector for 5 min at 37°C. Cells were washed with cold PBS (pH 7.4) three times and mounted in SlowFade® Gold Antifade Mountant with DAPI. Panels (a) and (d) show nuclei staining (blue), panels (b) and (e) show MIPS-9541 accumulated in cells (green), panel (c) shows the merged images of panels (a) and (b) and panel (f) shows the merged images of panels (d) and (e). Scale bars = 50 µm.
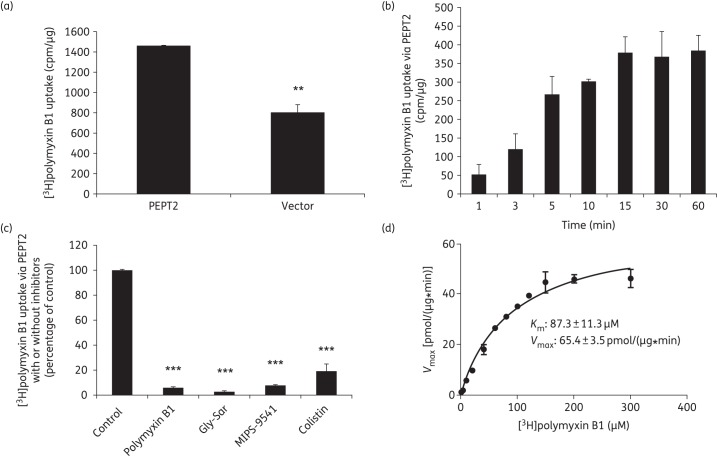
Furthermore, we employed tritium-labelled polymyxin B1 to directly assess polymyxin uptake through PEPT2. As shown in Figure 6(a), the cellular uptake of [3H]polymyxin B1 was significantly higher in cells overexpressing PEPT2 than in vector-transfected cells. Such transporter-mediated uptake was not saturated up to 15 min (Figure 6b) and was inhibited by unlabelled polymyxins including colistin, MIPS-9541, polymyxin B1 as well as the typical substrate of PEPT2 (Figure 6c). Our kinetic analysis revealed that PEPT2 transported [3H]polymyxin B1 into HEK293 cells with a Km value of 87.3 μM (Figure 6d), which is consistent with that of the fluorescent polymyxin probe MIPS-9541 obtained above.
Figure 6.
Molecular characterization of PEPT2-mediated uptake of [3H]polymyxin B1. (a) Uptake of [3H]polymyxin B1 in HEK293 cells transfected with PEPT2 or the vector alone. The vector- and transporter-expressing cells were incubated with 10 μM [3H]polymyxin B1 (in PBS, pH 5.5). Data are expressed as cpm/μg of protein. (b) Time dependence of the PEPT2-mediated [3H]polymyxin B1 uptake. Vector- and transporter-expressing cells were incubated with 10 μM [3H]polymyxin B1 (in PBS, pH 5.5). Data are expressed as cpm/μg of protein. Background counts of vector-transfected cells were subtracted from all uptake data. (c) Inhibition by Gly-Sar, colistin, polymyxin B1 or MIPS-9541 of PEPT2-mediated [3H]polymyxin B1 uptake. [3H]polymyxin B1 uptake was measured in the absence or presence of 20 µM Gly-Sar, colistin, polymyxin B or MIPS-9541 in cells overexpressing human PEPT2 or transfected with the vector alone. Background counts of vector-transfected cells were subtracted from all uptake data and the standardized result is presented as a percentage of the control (i.e. no inhibitors). (d) Kinetic parameters of [3H]polymyxin B1 uptake via PEPT2. Kinetic parameters of [3H]polymyxin B1 uptake were derived in HEK293 cells transiently transfected with PEPT2 or the vector. Uptake was assessed with various concentrations of [3H]polymyxin B1 (ranging from 0 to 300 µM), subtracting the background of vector-transfected control cells. Km and Vmax values of [3H]polymyxin B1 uptake were derived by GraphPad Prism 6.0 software. All experiments were conducted independently three times with three replicates in each experiment and values are expressed as the mean ± SEM. Significant difference from the control: **P < 0.01 and ***P < 0.001.
Discussion
Dose-limiting nephrotoxicity remains a major problem for optimizing the clinical use of polymyxins.16,59 Over the last decade, the pharmacokinetics of colistin and polymyxin B has been studied in animals and human subjects;16,18,20,59–66 however, there are very limited experimental data examining the involvement of transporters in the disposition of polymyxins. It has been shown that colistin is generated after parenteral administration of its inactive prodrug CMS; the latter is eliminated mainly by the kidney, while colistin and polymyxin B are significantly reabsorbed by renal tubular cells.16,18,20,66 Therefore, the significant renal reabsorption of colistin and polymyxin B may contribute greatly to the induced molecular events leading to cell death.59,67
The molecular mechanism underpinning the intracellular accumulation of polymyxins remains unclear. Previous literature reported the involvement of the endocytotic megalin receptor in the renal accumulation of polymyxins.21,22 However, in megalin-knockout rats the renal accumulation of colistin was only reduced to one-third of that observed in the control,21 indicating that megalin-facilitated endocytosis is not the sole mechanism by which polymyxins enter cells. A perfused rat kidney study suggested the role of SLC transporters, in particular OCTs and PEPTs in the tubular reabsorption of polymyxins.19 Unfortunately, there has been no direct evidence to confirm such a role for these transporters in the cellular uptake of polymyxins in animals and humans.
SLCs, in particular OATs, OATPs, OCTs, OCTNs and PEPTs, are drug-related influx transporters widely expressed in key human organs including the kidney.23–27 They are responsible for cellular uptake of clinically important drugs (e.g. antibiotics) and their functions largely determine the disposition and elimination of drugs in the body.28–31 In this study, both colistin and polymyxin B potently inhibited substrate uptake mediated through human PEPT2 (Figure 2d and Figure 3a and b) and also moderately impacted on Gly-Sar uptake through PEPT1 (Figure 2d). However, colistin and polymyxin B have minimal effect on substrate uptake mediated through the classic isomembers of OATs, OATPs and OCTs, which are the SLC subfamilies mainly responsible for the cellular uptake of small molecules (molecular weight <1000 Da).68 Unlike that suggested in the isolated perfused rat kidney study,19 we did not observe any possible contribution of OCTs to the cellular influx of colistin in the present study using human cells.
PEPT1 and PEPT2 are the two important isomembers of PEPTs present in renal tubular cells. PEPT1 is a low-affinity, high-capacity transporter, whereas PEPT2 is a high-affinity, low-capacity transporter.69 It has been recognized widely that PEPT2 binds to its substrates with a 10–15-fold higher affinity than PEPT1.25,70,71 Further, PEPT1 is mainly found in the S1 segment of proximal tubular cells, which is the primary location for renal exchange of potassium, calcium, sodium and protons.72,73 PEPT2 is more abundantly expressed at the urine-facing apical membrane of the S2 and S3 segment of the proximal tubular cells, which are the part of the nephrons responsible for the excretion and/or reabsorption of various molecules, e.g. drugs and their metabolites.53,69 Considering the different tissue localization of PEPT1 and PEPT2, as well as the more potent inhibitory effects of both polymyxins on PEPT2 (Figure 2d), in this study we focused on examining the PEPT2-mediated transport of polymyxins.
In order to assess the direct uptake of polymyxins through PEPT2, we established a method to evaluate the fluorescence accumulation of MIPS-9541 in PEPT2-overexpressing cells. MIPS-9541 is a novel fluorescent probe that retains the pharmacophore of the polymyxins and also maintains their pharmacological effects.32,55 Our results showed consistently that MIPS-9541 can potently inhibit the substrate uptake mediated via PEPT2 (Figure 3). Our kinetic analysis estimated the Km value (reflecting the substrate–transporter binding affinity) of MIPS-9541 to PEPT2 is 74.9 ± 12.6 μM (Figure 4b). This suggested that MIPS-9541 is a potent substrate of PEPT2, since the Km value of the PEPT2 prototype substrate Gly-Sar was ∼70 μM.58 Our further inhibition studies confirmed that Gly-Sar, colistin and polymyxin B significantly impaired PEPT2-mediated MIPS-9541 uptake (Figure 4c; P < 0.01). Compared with the control, our fluorescence imaging results also demonstrate the accumulation of MIPS-9541 in PEPT2-expressing cells (Figure 5). Furthermore, we employed tritium-labelled polymyxin B1 to directly assess the uptake of polymyxins through PEPT2-expressed cells. The result (Figure 6) is consistent with that obtained using MIPS-9541, which confirmed that polymyxins are novel substrates of PEPT2. Collectively, our data elucidate the basic characteristics of PEPT2-facilitated uptake of polymyxins.
PEPT2 transports a number of dipeptides, tripeptides, β-lactam antibiotics and other peptide-like drugs;25 however, it does not require a peptide bond in the substrate.74 Computational modelling with linear di- and tripeptide sequences and β-lactams containing positively charged amino acid side chains suggest that the presence of positive charge and hydrophobicity are important for substrate recognition by PEPT2.70,71 For the polymyxins, it is likely that positively charged l-α-γ-diaminobutyric acid (Dab) residues play a significant role in the recognition of the polymyxins by PEPT2. Results from our inhibition study (Figure 3d) show that CMS (in which the positive charge of the Dab residues is neutralized by their modification with methanesulfonate groups) has a substantially less pronounced interaction with PEPT2. Furthermore, it has been reported that removal of the Dab residues at positions 1 and 3 from the polymyxin core structure leads to a significant increase in the urinary recovery in rats (∼7%–20%75 versus <0.5% for colistin18). As PEPT2 may facilitate the renal tubular reabsorption of polymyxins from tubular fluid, it is postulated that concomitant administration of transporter inhibitors in patients may decrease PEPT2-mediated polymyxin uptake by tubular cells, thereby attenuating polymyxin-induced nephrotoxicity. Importantly, the design of novel polymyxins with decreased binding to PEPT2 may reduce the accumulation and subsequent toxicity in nephrons and widen the therapeutic window. Animal studies are currently underway in our group to examine these hypotheses.
In conclusion, our study is the first to provide evidence of human PEPT2-mediated cellular uptake of polymyxins. Elucidating the mechanism of PEPT2-mediated uptake in renal tubular cells is crucial for discovery of novel, safer polymyxin-like lipopeptides for the treatment of life-threatening Gram-negative ‘superbugs’.
Funding
This study was supported by internal funding from the Faculty of Pharmacy, The University of Sydney. Q. T. Z. is an Australian National Health and Medical Research Council (NHMRC) Early Career Fellow (APP1053528). J. L. is an NHMRC Senior Research Fellow and is funded by grants from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health [R01 AI098771 (J. L. and K. D. R.) and AI111965].
Transparency declarations
None to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Acknowledgements
The generous gift of human PepT1 cDNA from Professor Peter J. Meier-Abt (University of Basel, Basel, Switzerland) is gratefully acknowledged.
References
- 1.Falagas ME, Bliziotis IA. Pandrug-resistant Gram-negative bacteria: the dawn of the post-antibiotic era? Int J Antimicrob Agents 2007; 29: 630–6. [DOI] [PubMed] [Google Scholar]
- 2.Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res 2005; 36: 697–705. [DOI] [PubMed] [Google Scholar]
- 3.Ritchie DJ, Alexander BT, Finnegan PM. New antimicrobial agents for use in the intensive care unit. Infect Dis Clin North Am 2009; 23: 665–81. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Antibiotic Resistance Threats in US. 2013. http://www.cdc.gov/drugresistance/threat-report-2013/.
- 5.Payne DJ, Gwynn MN, Holmes DJ et al. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 2007; 6: 29–40. [DOI] [PubMed] [Google Scholar]
- 6.Velkov T, Roberts KD, Nation RL et al. Pharmacology of polymyxins: new insights into an ‘old’ class of antibiotics. Future Microbiol 2013; 8: 711–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nation RL, Li J, Cars O et al. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 2015; 15: 225–34. [DOI] [PubMed] [Google Scholar]
- 8.Evans ME, Feola DJ, Rapp RP. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant Gram-negative bacteria. Ann Pharmacother 1999; 33: 960–7. [DOI] [PubMed] [Google Scholar]
- 9.Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin Infect Dis 2005; 40: 1333–41. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Nation RL, Milne RW et al. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents 2005; 25: 11–25. [DOI] [PubMed] [Google Scholar]
- 11.Ainsworth GC, Brown AM, Brownlee G. Aerosporin, an antibiotic produced by Bacillus aerosporus Greer. Nature 1947; 159: 263. [DOI] [PubMed] [Google Scholar]
- 12.Benedict RG, Langlykke AF. Antibiotic activity of Bacillus polymyxa. J Bacteriol 1947; 54: 24. [PubMed] [Google Scholar]
- 13.Landman D, Georgescu C, Martin DA et al. Polymyxins revisited. Clin Microbiol Rev 2008; 21: 449–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Nation RL, Turnidge JD et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 2006; 6: 589–601. [DOI] [PubMed] [Google Scholar]
- 15.Rifkind D. Prevention by polymyxin B of endotoxin lethality in mice. J Bacteriol 1967; 93: 1463–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandri AM, Landersdorfer CB, Jacob J et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 2013; 57: 524–31. [DOI] [PubMed] [Google Scholar]
- 17.Azad MA, Roberts KD, Yu HH et al. Significant accumulation of polymyxin in single renal tubular cells: a medicinal chemistry and triple correlative microscopy approach. Anal Chem 2015; 87: 1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Milne RW, Nation RL et al. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother 2003; 47: 1766–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Z, Wang J, Nation RL et al. Renal disposition of colistin in the isolated perfused rat kidney. Antimicrob Agents Chemother 2009; 53: 2857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zavascki AP, Goldani LZ, Cao G et al. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis 2008; 47: 1298–304. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Yamaguchi H, Ogura J et al. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob Agents Chemother 2013; 57: 6319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdelraouf K, Chang KT, Yin T et al. Uptake of polymyxin B into renal cells. Antimicrob Agents Chemother 2014; 58: 4200–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol 2012; 165: 1260–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F, You G. Molecular insights into the structure–function relationship of organic anion transporters OATs. Pharm Res 2007; 24: 28–36. [DOI] [PubMed] [Google Scholar]
- 25.Smith DE, Clemencon B, Hediger MA. Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications. Mol Aspects Med 2013; 34: 323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet 2005; 20: 452–77. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug Metab Pharmacokinet 2008; 23: 22–44. [DOI] [PubMed] [Google Scholar]
- 28.Bretschneider B, Brandsch M, Neubert R. Intestinal transport of β-lactam antibiotics: analysis of the affinity at the H+/peptide symporter (PEPT1), the uptake into Caco-2 cell monolayers and the transepithelial flux. Pharm Res 1999; 16: 55–61. [DOI] [PubMed] [Google Scholar]
- 29.Luckner P, Brandsch M. Interaction of 31 β-lactam antibiotics with the H+/peptide symporter PEPT2: analysis of affinity constants and comparison with PEPT1. Eur J Pharm Biopharm 2005; 59: 17–24. [DOI] [PubMed] [Google Scholar]
- 30.Wenzel U, Gebert I, Weintraut H et al. Transport characteristics of differently charged cephalosporin antibiotics in oocytes expressing the cloned intestinal peptide transporter PepT1 and in human intestinal Caco-2 cells. J Pharmacol Exp Ther 1996; 277: 831–9. [PubMed] [Google Scholar]
- 31.Ganapathy ME, Brandsch M, Prasad PD et al. Differential recognition of β-lactam antibiotics by intestinal and renal peptide transporters, PEPT 1 and PEPT 2. J Biol Chem 1995; 270: 25672–7. [DOI] [PubMed] [Google Scholar]
- 32.Deris ZZ, Swarbrick JD, Roberts KD et al. Probing the penetration of antimicrobial polymyxin lipopeptides into Gram-negative bacteria. Bioconjug Chem 2014; 25: 750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Wang K, Zheng J et al. Interactions of the active components of Punica granatum (pomegranate) with the essential renal and hepatic human solute carrier transporters. Pharm Biol 2014; 52: 1510–7. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Cheung FS, Zheng J et al. Interaction of the bioactive flavonol, icariin, with the essential human solute carrier transporters. J Biochem Mol Toxicol 2014; 28: 91–7. [DOI] [PubMed] [Google Scholar]
- 35.Johnston RA, Rawling T, Chan T et al. Selective inhibition of human solute carrier transporters by multikinase inhibitors. Drug Metab Dispos 2014; 42: 1851–7. [DOI] [PubMed] [Google Scholar]
- 36.Xu F, Li Z, Zheng J et al. The inhibitory effects of the bioactive components isolated from Scutellaria baicalensis on the cellular uptake mediated by the essential solute carrier transporters. J Pharm Sci 2013; 102: 4205–11. [DOI] [PubMed] [Google Scholar]
- 37.Zhou F, Zhu L, Cui PH et al. Functional characterization of nonsynonymous single nucleotide polymorphisms in the human organic anion transporter 4 (hOAT4). Br J Pharmacol 2010; 159: 419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng J, Chan T, Cheung FS et al. PDZK1 and NHERF1 regulate the function of human organic anion transporting polypeptide 1A2 (OATP1A2) by modulating its subcellular trafficking and stability. PLoS One 2014; 9: e94712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou F, Hong M, You G. Regulation of human organic anion transporter 4 by progesterone and protein kinase C in human placental BeWo cells. Am J Physiol Endocrinol Metab 2007; 293: E57–61. [DOI] [PubMed] [Google Scholar]
- 40.Zhou F, Illsley NP, You G. Functional characterization of a human organic anion transporter hOAT4 in placental BeWo cells. Eur J Pharm Sci 2006; 27: 518–23. [DOI] [PubMed] [Google Scholar]
- 41.Zhou F, Lee AC, Krafczyk K et al. Protein kinase C regulates the internalization and function of the human organic anion transporting polypeptide 1A2. Br J Pharmacol 2011; 162: 1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou F, Xu W, Tanaka K et al. Comparison of the interaction of human organic anion transporter hOAT4 with PDZ proteins between kidney cells and placental cells. Pharm Res 2008; 25: 475–80. [DOI] [PubMed] [Google Scholar]
- 43.Zhou F, Zheng J, Zhu L et al. Functional analysis of novel polymorphisms in the human SLCO1A2 gene that encodes the transporter OATP1A2. AAPS J 2013; 15: 1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan T, Zheng J, Zhu L et al. Putative transmembrane domain 6 of the human organic anion transporting polypeptide 1A2 (OATP1A2) influences transporter substrate binding, protein trafficking, and quality control. Mol Pharm 2015; 12: 111–9. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi Y, Ohshiro N, Sakai R et al. Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol 2005; 57: 573–8. [DOI] [PubMed] [Google Scholar]
- 46.Hong M, Zhou F, You G. Critical amino acid residues in transmembrane domain 1 of the human organic anion transporter hOAT1. J Biol Chem 2004; 279: 31478–82. [DOI] [PubMed] [Google Scholar]
- 47.Hirano M, Maeda K, Shitara Y et al. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J Pharmacol Exp Ther 2004; 311: 139–46. [DOI] [PubMed] [Google Scholar]
- 48.Amphoux A, Millan MJ, Cordi A et al. Inhibitory and facilitory actions of isocyanine derivatives at human and rat organic cation transporters 1, 2 and 3: a comparison to human α1- and α2-adrenoceptor subtypes. Eur J Pharmacol 2010; 634: 1–9. [DOI] [PubMed] [Google Scholar]
- 49.Toh DS, Cheung FS, Murray M et al. Functional analysis of novel variants in the organic cation/ergothioneine transporter 1 identified in Singapore populations. Mol Pharm 2013; 10: 2509–16. [DOI] [PubMed] [Google Scholar]
- 50.Toh DS, Murray M, Pern Tan K et al. Functional analysis of pharmacogenetic variants of human organic cation/carnitine transporter 2 (hOCTN2) identified in Singaporean populations. Biochem Pharmacol 2011; 82: 1692–9. [DOI] [PubMed] [Google Scholar]
- 51.Guo X, Meng Q, Liu Q et al. Construction, identification and application of HeLa cells stably transfected with human PEPT1 and PEPT2. Peptides 2012; 34: 395–403. [DOI] [PubMed] [Google Scholar]
- 52.Noshiro R, Anzai N, Sakata T et al. The PDZ domain protein PDZK1 interacts with human peptide transporter PEPT2 and enhances its transport activity. Kidney Int 2006; 70: 275–82. [DOI] [PubMed] [Google Scholar]
- 53.Ocheltree SM, Shen H, Hu Y et al. Role and relevance of peptide transporter 2 (PEPT2) in the kidney and choroid plexus: in vivo studies with glycylsarcosine in wild-type and PEPT2 knockout mice. J Pharmacol Exp Ther 2005; 315: 240–7. [DOI] [PubMed] [Google Scholar]
- 54.Azad MA, Akter J, Rogers KL et al. Major pathways of polymyxin-induced apoptosis in rat kidney proximal tubular cells. Antimicrob Agents Chemother 2015; 59: 2136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azad MA, Yun B, Roberts KD et al. Measuring polymyxin uptake by renal tubular cells: is BODIPY-polymyxin B an appropriate probe? Antimicrob Agents Chemother 2014; 58: 6337–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spapen H, Jacobs R, Van Gorp V et al. Renal and neurological side effects of colistin in critically ill patients. Ann Intensive Care 2011; 1: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergen PJ, Li J, Rayner CR et al. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2006; 50: 1953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ocheltree SM, Shen H, Hu Y et al. Role of PEPT2 in the choroid plexus uptake of glycylsarcosine and 5-aminolevulinic acid: studies in wild-type and null mice. Pharm Res 2004; 21: 1680–5. [DOI] [PubMed] [Google Scholar]
- 59.Garonzik SM, Li J, Thamlikitkul V et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55: 3284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plachouras D, Karvanen M, Friberg LE et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by Gram-negative bacteria. Antimicrob Agents Chemother 2009; 53: 3430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abdelraouf K, He J, Ledesma KR et al. Pharmacokinetics and renal disposition of polymyxin B in an animal model. Antimicrob Agents Chemother 2012; 56: 5724–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He H, Li JC, Nation RL et al. Pharmacokinetics of four different brands of colistimethate and formed colistin in rats. J Antimicrob Chemother 2013; 68: 2311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He J, Abdelraouf K, Ledesma KR et al. Pharmacokinetics and efficacy of liposomal polymyxin B in a murine pneumonia model. Int J Antimicrob Agents 2013; 42: 559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koomanachai P, Landersdorfer CB, Chen G et al. Pharmacokinetics of colistin methanesulfonate and formed colistin in end-stage renal disease patients receiving continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother 2014; 58: 440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J, Coulthard K, Milne R et al. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J Antimicrob Chemother 2003; 52: 987–92. [DOI] [PubMed] [Google Scholar]
- 66.Li J, Milne RW, Nation RL et al. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother 2004; 53: 837–40. [DOI] [PubMed] [Google Scholar]
- 67.Dai C, Li J, Tang S et al. Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob Agents Chemother 2014; 58: 4075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.You G, Morris M. Drug transporters—molecular characterisation and role in drug disposition. 2007: 619–63. [Google Scholar]
- 69.Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch 2004; 447: 610–8. [DOI] [PubMed] [Google Scholar]
- 70.Biegel A, Knutter I, Hartrodt B et al. The renal type H+/peptide symporter PEPT2: structure–affinity relationships. Amino Acids 2006; 31: 137–56. [DOI] [PubMed] [Google Scholar]
- 71.Biegel A, Gebauer S, Brandsch M et al. Structural requirements for the substrates of the H+/peptide cotransporter PEPT2 determined by three-dimensional quantitative structure–activity relationship analysis. J Med Chem 2006; 49: 4286–96. [DOI] [PubMed] [Google Scholar]
- 72.Shen H, Smith DE, Yang T et al. Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am J Physiol 1999; 276: F658–65. [DOI] [PubMed] [Google Scholar]
- 73.Smith DE, Pavlova A, Berger UV et al. Tubular localization and tissue distribution of peptide transporters in rat kidney. Pharm Res 1998; 15: 1244–9. [DOI] [PubMed] [Google Scholar]
- 74.Doring F, Wiͼll J, Amasheh S et al. Minimal molecular determinants of substrates for recognition by the intestinal peptide transporter. J Biol Chem 1998; 273: 23211–8. [DOI] [PubMed] [Google Scholar]
- 75.Ali FE, Cao G, Poudyal A et al. Pharmacokinetics of novel antimicrobial cationic peptides NAB 7061 and NAB 739 in rats following intravenous administration. J Antimicrob Chemother 2009; 64: 1067–70. [DOI] [PubMed] [Google Scholar]