Abstract
Objectives
The use of the NNRTI rilpivirine in low- and middle-income countries (LMICs) is under debate. The main objective of this study was to provide further clinical insights and biochemical evidence on the usefulness of rilpivirine in LMICs.
Patients and methods
Rilpivirine resistance was assessed in 5340 therapy-naive and 13 750 first-generation NNRTI-failed patients from Europe and therapy-naive HIV-1 subtype C (HIV-1C)-infected individuals from India (n = 617) and Ethiopia (n = 127). Rilpivirine inhibition and binding affinity assays were performed using patient-derived HIV-1C reverse transcriptases (RTs).
Results
Primary rilpivirine resistance was rare, but the proportion of patients with >100 000 HIV-1 RNA copies/mL pre-ART was high in patients from India and Ethiopia, limiting the usefulness of rilpivirine as a first-line drug in LMICs. In patients failing first-line NNRTI treatments, cross-resistance patterns suggested that 73% of the patients could benefit from switching to rilpivirine-based therapy. In vitro inhibition assays showed ∼2-fold higher rilpivirine IC50 for HIV-1C RT than HIV-1B RT. Pre-steady-state determination of rilpivirine-binding affinities revealed 3.7-fold lower rilpivirine binding to HIV-1C than HIV-1B RT. Structural analysis indicated that naturally occurring polymorphisms close to the NNRTI-binding pocket may reduce rilpivirine binding, leading to lower susceptibility of HIV-1C to rilpivirine.
Conclusions
Our clinical and biochemical findings indicate that the usefulness of rilpivirine has limitations in HIV-1C-dominated epidemics in LMICs, but the drug could still be beneficial in patients failing first-line therapy if genotypic resistance testing is performed.
Introduction
HIV-1 subtype C (HIV-1C) dominates the HIV epidemic with >50% of all infections worldwide and is highly predominant in low- and middle-income countries (LMICs), such as South Africa, India and Ethiopia.1 In LMICs, the first-generation NNRTIs efavirenz and nevirapine have been drugs of choice in first-line combination ART (cART), although side effects and a low genetic barrier to resistance are the main drawbacks. A second-generation NNRTI, rilpivirine, has a favourable safety and tolerability profile compared with efavirenz and nevirapine, but a higher virological failure rate in patients with an HIV-1 RNA load [viral load (VL)] of >100 000 copies/mL.2
A recent study from South Africa reported that rilpivirine might be efficacious in patients failing efavirenz- or nevirapine-based therapy.3 However, the feasibility of rilpivirine-based therapy in LMICs is still under debate, mainly due to frequently late HIV diagnosis and the potential of cross-resistance in patients failing efavirenz or nevirapine. Moreover, HIV-1C is more prone to maintain high viraemia (VL >100 000 copies/mL) in untreated subjects.4 In LMICs, the lack of VL testing and drug resistance monitoring, as well as increased trends of NNRTI resistance,5 can potentially affect the therapeutic response to rilpivirine.
In this study, we analysed subtype-tailored primary- and cross-resistance patterns to rilpivirine in a large patient dataset. More detailed patient treatment data from Sweden were also included and compared with the primary-resistance and pre-treatment VL profiles from India and Ethiopia, to assess rilpivirine feasibility in LMICs. The efficacy of rilpivirine on patient-derived HIV-1C versus HIV-1B reverse transcriptases (RTs) was determined by in vitro inhibition and binding assays and in silico molecular modelling analysis. Our results provide important insights into the potential usefulness of rilpivirine in HIV-1C-dominated LMICs in cART.
Patients and methods
Study population
The study population was divided into two groups. The first group included treatment-naive individuals from Sweden (n = 4596)6 and two LMICs, India (n = 623; HIV-1C: 617)7 and Ethiopia (n = 127),8 who were initiating cART. The second group consisted of rilpivirine-naive patients, who had failed first-generation NNRTI therapy (n = 13 750), derived from European HIV clinics and nested in the EuResist database (http://engine.euresist.org/database/). Ethics approval was obtained from all institutions in accordance with national requirements and the principles of the Declaration of Helsinki. Informed consent was obtained from all participants. Patient information was anonymized and delinked prior to analysis.
Genotypic resistance testing and subtyping
Genotypic resistance testing was performed either by the ViroSeq™ HIV-1 Genotyping System (Abbott, USA) or in-house methods. Subtyping was performed using three online HIV-1 tools as described recently (see Figure S1, available as Supplementary data at JAC Online).6 Rilpivirine cross-resistance was projected using the Stanford HIVDB version 7.0.1 tool (http://hivdb.stanford.edu/; accessed in October 2014).
Rilpivirine inhibition and binding affinity assays and molecular modelling
HIV-1C strains were selected for functional studies based on the near full-length HIV-1 genome (∼8.5 kb) sequence as recently described.9 The rilpivirine inhibition and binding kinetics assay was performed as described previously.10 The crystal structure of HIV-1B in complex with rilpivirine (Protein Databank entry 2ZD1)11 was used as a template to generate the homology-derived molecular models of consensus HIV-1B and HIV-1C RTs. Flexible docking of rilpivirine was carried out by the ‘induced-fit docking’ workflow of Schrödinger Suite (Schrödinger, NY, USA). The detailed methodology is available as Supplementary data at JAC Online.
Statistical analysis
Demographic and clinical parameters were assessed by the Mann–Whitney test for continuous variables and χ2 test for categorical variables. Statistical analysis was performed using Stata version 12.1 SE (StataCorp, USA).
Results
Rilpivirine drug-resistant mutations (DRMs) and VL in therapy-naive individuals
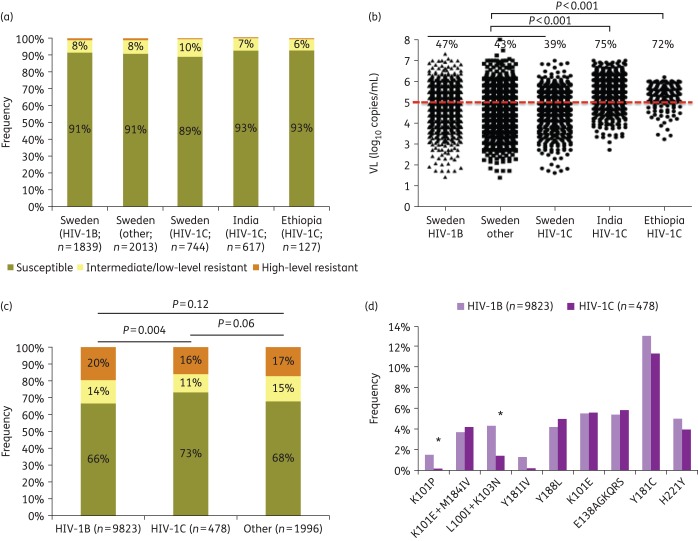
A low prevalence of rilpivirine DRMs was identified in treatment-naive patients, independent of subtype and geographical origin (Figure 1a). There was a significant difference in pre-treatment VL when Swedish patients were compared with patients from India and Ethiopia. A higher proportion of patients from the LMICs had VL >100 000 copies/mL (Figure 1b) compared with the Swedish patients (P < 0.001).
Figure 1.
Primary resistance and cross-resistance to rilpivirine and pre-therapy HIV-1 RNA load. (a) Primary resistance to rilpivirine using Stanford HIVDB version 7.0.1. (b) Subtype-tailored HIV-1 plasma RNA load (log10 copies/mL) at initiation of ART measured using Cobas Amplicor HIV-1 monitor v1.5, Cobas TaqMan HIV-1 v1.0 or Cobas TaqMan HIV-1 v2.0 (Roche Molecular Systems, Basel, Switzerland) for the Swedish cohort or the Abbott m2000rt real-time PCR system (Abbott, Germany) for the Indian and Ethiopian cohorts. The percentages of treatment-naive patients with an HIV-1 RNA load of >100 000 copies/mL at initiation of ART are indicated. (c) Cross-resistance to rilpivirine in the EuResist database using Stanford HIVDB version 7.0.1. Among the 13 750 sequences obtained, 12 297 (89%) passed quality control as per the Stanford database and were thus included in the analysis. (d) Rilpivirine DRM profiles. *Significant difference (P < 0.05). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
DRMs in NNRTI-failing patients naive to rilpivirine
Data were obtained from the EuResist database for 13 750 patients who had failed first-line efavirenz or nevirapine. After excluding the sequences that did not pass quality control (n = 1453), cross-resistance to rilpivirine (sum of high level and intermediate/low level) was predicted more frequently in HIV-1B than HIV-1C patients (34% versus 27%; P < 0.001) (Figure 1c). The K101P (1.6% versus 0.3%; P = 0.03) and L100I + K103N (4.3% versus 1.5%; P = 0.04) mutations were found more frequently in HIV-1B than HIV-1C patients (Figure 1d).
In vitro drug resistance and structural differences between HIV-1C and HIV-1B
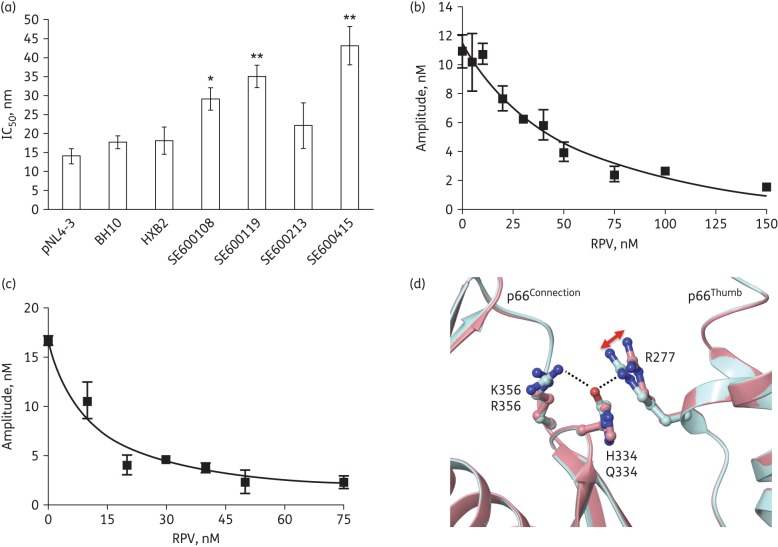
The in vitro inhibition assay showed higher IC50 values (∼2-fold) for WT HIV-1C RT clones obtained from four therapy-naive individuals as compared with the HIV-1B RT clones (Figure 2a). The rilpivirine-binding affinity (Kd.RPV) determined by plotting the amplitude of the burst phase of the biphasic nucleotide incorporation reaction (using pre-steady-state kinetics) in the presence of rilpivirine showed that HIV-1C RT (Figure 2b) binds rilpivirine with 3.7-fold lower affinity than HIV-1B RT (Figure 2c) (Kd.RPV = 67 nM versus 18 nM). Superposition of the rilpivirine-bound X-ray crystal structure of HIV-1B RT (Protein Databank entry 2ZD1) onto the modelled HIV-1C RT/rilpivirine complex showed similar overall folding of the two RTs. However, there are some subtle differences in the interactions between the thumb and connection subdomains of these structures. Specifically, the polar interactions (hydrogen bonds and salt bridges) between residues 277, 334 and 356 at the interface of these subdomains are expected to be slightly different, as HIV-1B RT has R277, Q334 and R356 at these positions whereas HIV-1C has R277, H334 and K356 (Figure 2d). Similarly, a cluster of residues in the vicinity of residues 277 and 334, including residues 245, 359, 360, 376 and 377 (not shown), are also highly polymorphic in various subtypes and expected to affect differently the thumb–connection subdomain interactions, leading to differences in rilpivirine susceptibility.
Figure 2.
(a) Inhibition of RT activity. The HIV-1B clones were pNL4-3, BH10 and HXB2 and the selected HIV-1C sequences were based on WGS of HIV-1C isolated from four patients. None of the sequences had documented rilpivirine mutations. **P < 0.05 and *borderline significance (P = 0.05) compared with pNL4-3. (b and c) Determination of Kd.RPV of WT HIV-1C and HIV-1B RTs. Single nucleotide (dATP) incorporation assays were performed under pre-steady-state conditions to determine Kd.RPV. (d) Structural comparison of rilpivirine binding and residues at the interface between thumb and connection subdomains in HIV-1B and HIV-1C RTs. This figure shows an example of differences in amino acid residues at the interface of thumb and connection subdomains of HIV-1B (turquoise) and HIV-1C (pink) RTs. The change in the conformation of R277 between HIV-1B and HIV-1C RTs is marked with a red arrow. The dotted lines show the hydrogen bond interactions in HIV-1B RT. RPV, rilpivirine. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
We report here clinical, biochemical and structural data that should help rationalize differences in the outcome of rilpivirine-containing therapies in various subtypes. Although primary rilpivirine resistance was rare, a substantial number of patients from LMICs had a pre-ART VL >100 000 copies/mL, limiting the use of rilpivirine in first-line therapy. Additionally, higher rilpivirine IC50 and decreased Kd.RPV were found for HIV-1C compared with HIV-1B RTs. In contrast, analysis of cross-resistance patterns suggested that at least two-thirds of the patients might benefit from rilpivirine if used after failure of efavirenz- or nevirapine-containing regimens. Thus, our findings show a complex pattern of several clinical and biochemical factors, which may influence the outcome of rilpivirine therapy in LMICs.
Several studies have identified primary rilpivirine DRMs among therapy-naive patients, ranging from ≤3% to ∼5%,12–14 as was also found in our study. However, these did not consider the pre-ART VL when the usefulness of rilpivirine was discussed. In the present study, we found that the VL was higher in treatment-naive patients from Ethiopia and India than in therapy-naive individuals in Sweden, which is in line with reports of high viraemia as a feature of HIV-1C infection.4,15 Since treatment initiation in most LMICs is still based upon CD4+ T cell counts and not VL, the high proportion of therapy-naive HIV-1C-infected individuals with VL >100 000 copies/mL limits the use of rilpivirine in these settings.
Several studies have reported on rilpivirine cross-resistance in patients failing first-generation NNRTI-based therapies. A study from Spain identified an average of 20% cross-resistance to rilpivirine in patients failing nevirapine (25%) or efavirenz (14.5%).16 A study from Kenya, primarily in non-B subtypes, identified 14% of the patients as having cross-resistance to rilpivirine,17 while a French study reported as much as ∼59% cross-resistance and also observed a higher frequency of rilpivirine DRMs in non-B subtypes compared with HIV-1B.18 Unlike the French cohort, we observed that HIV-1B had significantly higher rilpivirine cross-resistance than HIV-1C. However, low rilpivirine cross-resistance among HIV-1C infected individuals should be interpreted with caution that HIV-1C-infected patients may fail virologically because of lower adherence or less exposure to ART. Therefore, in agreement with earlier studies,19 the use of rilpivirine in patients who failed nevirapine- or efavirenz-containing therapies should not be initiated without support of genotypic resistance testing, regardless of the subtype and geographical area.
The flexibility of the RT mainly in the NNRTI-binding pockets due to mutations has been recognized as an important factor for the efficacy of NNRTIs including rilpivirine.11 Moreover, the connection subdomain mutations that are known to impact NNRTI susceptibility are either part of, or close to, these peptides (such as residues 334, 335, 348, 359, 371, 376 and 509) (reviewed in Singh et al.20). Hence, polymorphic changes at these regions may explain our higher IC50 values even in WT (without any rilpivirine DRMs) HIV-1C RTs. Such differences could be associated with flexibility changes that impact NNRTI binding or nucleic acid alignment at the active sites of HIV-1 RT, leading to decreased susceptibility to these drugs. Furthermore, the decreased Kd.RPV of HIV-1C RT compared with HIV-1B RT signifies a decreased rilpivirine IC50 for HIV-1C.
In conclusion, the clinical and biochemical data presented here indicate that the usefulness of rilpivirine as a first-line drug in HIV-1C-dominated epidemics is likely to have limitations. However, if genotypic resistance testing is performed, it is possible to use rilpivirine as part of a second-line regimen in patients who have failed a first-generation NNRTI-based treatment.
Funding
This work was partly supported by a Young Investigator Grant from Karolinska Institutet Research Foundation Grants (2014fobi41250) to U. N., Swedish International Developing Agency, the Swedish Civil Contingencies Agency (SWE-2009-151), and the Swedish Research Council (521-2012-3476 and 2007-7092) (to A. S.). S. G. S. acknowledges funding by Mizzou Advantage and from NIH grant P50-GM103368. The biochemical assays for rilpivirine binding and susceptibility were conducted with support from P50-GM103368, and the molecular modelling was supported by Mizzou Advantage funds.
Transparency declarations
None to declare.
Author contributions
Conceived and designed the experiments: U. N. and A. S. Performed the experiments: A. H., U. N., K. S., L. C. R. and S. D. R. Analysed the data: U. N. and K. S. Contributed patient materials and clinical data: W. A. and A. S. Provided database management and query framework: E. S. and A. H. Reviewed the biochemical and structural portion of the study: S. G. S. and E. A. Reviewed the study design and clinical implications of the study: M. Z., A. H. and A. S. Wrote the paper: U. N., reviewed and revised by K. S., A. S., M. Z., E. A. and S. G. S. All authors approved the final the version of the paper.
Supplementary data
Acknowledgements
We thank the HIVIND consortium (ISRCTN79261738) and EuResist Network study group (www.euresist.org). We thank Dr Soham Gupta and Dr Irene Bontell for assistance in the partial analysis of the data and lab work. Rilpivirine, BH10 and HXB2 were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH.
References
- 1.Hemelaar J, Gouws E, Ghys PD et al. . Global trends in molecular epidemiology of HIV-1 during 2000–2007. AIDS 2011; 25: 679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molina JM, Clumeck N, Redant K et al. . Rilpivirine vs. efavirenz in HIV-1 patients with baseline viral load 100,000 copies/ml or less: week 48 phase III analysis. AIDS 2013; 27: 889–97. [DOI] [PubMed] [Google Scholar]
- 3.Basson AE, Rhee SY, Parry CM et al. . Impact of drug resistance-associated amino acid changes in HIV-1 subtype C on susceptibility to newer nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother 2015; 59: 960–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novitsky V, Ndung'u T, Wang R et al. . Extended high viremics: a substantial fraction of individuals maintain high plasma viral RNA levels after acute HIV-1 subtype C infection. AIDS 2011; 25: 1515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta RK, Jordan MR, Sultan BJ et al. . Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet 2012; 380: 1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neogi U, Haggblom A, Santacatterina M et al. . Temporal trends in the Swedish HIV-1 epidemic: increase in non-B subtypes and recombinant forms over three decades. PLoS One 2014; 9: e99390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shet A, De Costa A, Kumarasamy N et al. . Effect of mobile telephone reminders on treatment outcome in HIV: evidence from a randomised controlled trial in India. BMJ 2014; 349: g5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdurahman S, Barqasho B, Nowak P et al. . Pattern of microbial translocation in patients living with HIV-1 from Vietnam, Ethiopia and Sweden. J Int AIDS Soc 2014; 17: 18841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossmann S, Nowak P, Neogi U. Subtype-independent near full-length HIV-1 genome sequencing and assembly to be used in large molecular epidemiological studies and clinical management. J Int AIDS Soc 2015; 18: 20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh K, Marchand B, Rai DK et al. . Biochemical mechanism of HIV-1 resistance to rilpivirine. J Biol Chem 2012; 287: 38110–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das K, Bauman JD, Clark AD Jr et al. . High-resolution structures of HIV-1 reverse transcriptase/TMC278 complexes: strategic flexibility explains potency against resistance mutations. Proc Natl Acad Sci USA 2008; 105: 1466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert-Niclot S, Charpentier C, Storto A et al. . Prevalence of pre-existing resistance-associated mutations to rilpivirine, emtricitabine and tenofovir in antiretroviral-naive patients infected with B and non-B subtype HIV-1 viruses. J Antimicrob Chemother 2013; 68: 1237–42. [DOI] [PubMed] [Google Scholar]
- 13.Picchio GR, Rimsky LT, Van Eygen V et al. . Prevalence in the USA of rilpivirine resistance-associated mutations in clinical samples and effects on phenotypic susceptibility to rilpivirine and etravirine. Antivir Ther 2014; 19: 819–23. [DOI] [PubMed] [Google Scholar]
- 14.Sungkanuparph S, Jiamsakul A, Kiertiburanakul S et al. . Rilpivirine resistance-associated mutations among antiretroviral-naive patients infected with HIV-1 in Asia. J Acquir Immune Defic Syndr 2013; 62: e98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neogi U, Rao SD, Bontell I et al. . Novel tetra-peptide insertion in Gag-p6 ALIX-binding motif in HIV-1 subtype C associated with protease inhibitor failure in Indian patients. AIDS 2014; 28: 2319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anta L, Llibre JM, Poveda E et al. . Rilpivirine resistance mutations in HIV patients failing non-nucleoside reverse transcriptase inhibitor-based therapies. AIDS 2013; 27: 81–5. [DOI] [PubMed] [Google Scholar]
- 17.Crawford KW, Njeru D, Maswai J et al. . Occurrence of etravirine/rilpivirine-specific resistance mutations selected by efavirenz and nevirapine in Kenyan patients with non-B HIV-1 subtypes failing antiretroviral therapy. AIDS 2014; 28: 442–5. [DOI] [PubMed] [Google Scholar]
- 18.Lambert-Niclot S, Charpentier C, Storto A et al. . Rilpivirine, emtricitabine and tenofovir resistance in HIV-1-infected rilpivirine-naive patients failing antiretroviral therapy. J Antimicrob Chemother 2014; 69: 1086–9. [DOI] [PubMed] [Google Scholar]
- 19.Rossotti R, Fonte L, Meini G et al. . Rilpivirine resistance and the dangerous liaisons with substitutions at position 184 among patients infected with HIV-1: analysis from a national drug-resistance database (ARCA). J Med Virol 2014; 86: 1459–66. [DOI] [PubMed] [Google Scholar]
- 20.Singh K, Flores JA, Kirby KA et al. . Drug resistance in non-B subtype HIV-1: impact of HIV-1 reverse transcriptase inhibitors. Viruses 2014; 6: 3535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.