Abstract
Objectives
Sterile α motif and histidine–aspartate domain-containing protein 1 (SAMHD1) has been shown to restrict retroviruses and DNA viruses by decreasing the pool of intracellular deoxynucleotides. In turn, SAMHD1 is controlled by cyclin-dependent kinases (CDK) that regulate the cell cycle and cell proliferation. Here, we explore the effect of CDK6 inhibitors on the replication of herpes simplex virus type 1 (HSV-1) in primary monocyte-derived macrophages (MDM).
Methods
MDM were treated with palbociclib, a selective CDK4/6 inhibitor, and then infected with a GFP-expressing HSV-1. Intracellular deoxynucleotide triphosphate (dNTP) content was determined using a polymerase-based method.
Results
CDK6 inhibitor palbociclib blocked SAMHD1 phosphorylation, intracellular dNTP levels and HSV-1 replication in MDM at subtoxic concentrations. Treatment of MDM with palbociclib reduced CDK2 activation, measured as the phosphorylation of the T-loop at Thr160. The antiviral activity of palbociclib was lost when SAMHD1 was degraded by viral protein X. Similarly, palbociclib did not block HSV-1 replication in SAMHD1-negative Vero cells at subtoxic concentrations, providing further evidence for a role of SAMHD1 in mediating the antiviral effect.
Conclusions
SAMHD1-mediated HSV-1 restriction is controlled by CDK and points to a preferential role for CDK6 and CDK2 as mediators of SAMHD1 activation. Similarly, the restricting activity of SAMHD1 against DNA viruses suggests that control of dNTP availability is the major determinant of its antiviral activity. This is the first study describing the anti-HSV-1 activity of palbociclib.
Introduction
Sterile α motif and histidine–aspartate domain-containing protein 1 (SAMHD1) is a deoxynucleotide triphosphate (dNTP) triphosphohydrolase found in myeloid cells that has been described as a restriction factor for the HIV-1 in resting T cells and non-cycling myeloid cells (reviewed in Ballana and Esté1). The dNTPase function of SAMHD1 has been suggested to be responsible for the blocking of HIV-1 reverse transcription through regulation of the intracellular dNTP pool.2 The dNTPase activity of SAMHD1 is widely accepted,3 but other possible functions such as DNA and RNA endonuclease activity have been proposed as alternative mechanisms of virus restriction, but are currently under scrutiny.1,4 Samhd1 knockout mice have shown that SAMHD1 is able to reduce dNTP levels, and mouse SAMHD1 is able to restrict retrovirus infections.5,6 Rhesus macaques infected with a WT or a truncated form of viral protein X (Vpx) that abrogates its capacity to induce SAMHD1 degradation exhibited a differential virus replication capacity and plasma viraemia.7 Moreover, CD4+ T cell subsets that are more prone to HIV-1 infection showed reduced SAMHD1 levels.8 A subset of HIV elite controllers had lower SAMHD1 mRNA expression than patients with viraemic HIV or seronegative healthy donors,9 but the true role of SAMHD1 in vivo remains unclear.10
Regulation of SAMHD1 activity is associated with cyclin-dependent kinases (CDK), which lead to SAMHD1 inactivation through phosphorylation.11–15 CDK family members are responsible for leading the cell through to different stages of the cell cycle. CDK4/6 and their regulatory D-type cyclins, upstream of CDK2 and CDK1, have been proposed to regulate cell cycle entry (G0 to G1).13,16 We have previously shown that CDK6 upstream of CDK2 may induce the phosphorylation of SAMHD1 in proliferating CD4+ T cells and macrophages and block HIV-1 infection.12 CDK2,12,13,17 CDK118 or both14 are thought responsible for SAMHD1 phosphorylation and are thus pivotal in SAMHD1 regulation.
CDK4/6 expression also leads to phosphorylation of the retinoblastoma protein (Rb), which triggers E2F-dependent transcription19,20 and S-phase entry. Hyperphosphorylation of Rb is strongly associated with aberrant cell proliferation and cancer. We have recently shown that palbociclib, a selective CDK4/6 inhibitor, recently approved for treating a specific advanced breast cancer,21,22 inhibits SAMHD1 phosphorylation and HIV-1 infection in monocyte-derived macrophages (MDM).11 Indeed, the antiviral activity of palbociclib against HIV-1 is dependent of SAMHD1, since degradation of SAMHD1 through Vpx induces a complete loss of palbociclib activity.11
Moreover, it has also been shown that herpes simplex virus (HSV)-1, a double-stranded DNA virus belonging to the Herpesviridae family is susceptible to SAMHD1 restriction,23 a result that confirmed the role of SAMHD1 in restricting DNA viruses in non-dividing myeloid cells.23,24 CDK are required for the replication of multiple viruses in dividing and non-dividing cells, including HSV-1 and -2. CDK play multiple roles required for viruses to complete their replication cycle25,26 and CDK inhibitors have long been investigated for their potential use as antivirals. It has already been shown that CDK inhibitors block the replication of HSV by targeting cellular proteins.27 Here we show that palbociclib blocked HSV-1 replication in MDM by impeding inactivation of SAMHD1 by phosphorylation. SAMHD1 function decreased the intracellular dNTP pool, thus we suggest that intracellular pool limitation is responsible for inhibition of viral replication in both RNA and DNA viruses, reinforcing the hypothesis of the dNTPase activity of SAMHD1 as the mechanism restricting viral infections. This is the first study describing the anti-HSV-1 activity of palbociclib.
Materials and methods
Cells
PBMCs were isolated by Ficoll-Paque density gradient centrifugation from human healthy donors and used for fresh purification of monocytes using a negative selection immunomagnetic cocktail (Stem Cell Technologies). Monocytes were resuspended in RPMI 1640 medium supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin (all from Life Technologies). MDM were obtained after culturing monocytes for 4 days in the presence of 100 ng/mL monocyte colony-stimulating factor or granulocyte macrophage colony-stimulating factor (M-CSF or GM-CSF, respectively; Peprotech). Buffy coats were purchased anonymously from the Catalan Banc De Sang i Teixits (http://www.bancsang.net/en/index.html). The buffy coats received were totally anonymous and untraceable and the only information given was whether they have been tested for disease.
African green monkey kidney Vero cells were kindly provided by Dr Ana Angulo (IDIBAPS, University of Barcelona) and were maintained in DMEM (Life Technologies) supplemented with 10% heat-inactivated FBS (Gibco, Life Technologies), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, Life Technologies). HEK293T (AIDS Reagent Program, NIH, Bethesda, MD, USA) and THP-1 (ATCC) cells were maintained in complete DMEM and RPMI 1640 medium, respectively.
Compounds
Zidovudine and aciclovir were purchased from Sigma-Aldrich. PD-0332991 (palbociclib)21 was purchased from Selleckhem. All compounds were resuspended in DMSO and stored at −20°C until use.
Virus production and infections
HSV-1 is a laboratory-adapted 17syn+ strain containing a cytomegalovirus (CMV)-GFP cassette in the US5 region.23 HSV-1 was grown in Vero cells and stored at −80°C. Envelope-deficient HIV-1 NL4-3 clone encoding IRES-GFP (NL4-3-GFP+) was pseudotyped with VSV-G by cotransfection of HEK293T cells using polyethyleneimine (Polysciences, Eppelheim, Germany) as previously described.28 Viral-like particles carrying Vpx (VLPVpx) were produced as previously described.29
MDM and Vero cells were plated in 24- or 96-well plates, depending on the experiment read-out. Cells were pretreated with serial dilutions of palbociclib for 16 h in the case of MDM or 6 h for Vero cells. Afterwards and when appropriate, VLPVpx were added 4 h prior to VSV-HIV-1 NL4-3-GFP+ or HSV-1-GFP+ infection at an moi of 0.1. No drug, 1 μg/mL aciclovir or 1 μg/mL zidovudine were used as a controls. After 48 h of incubation, infections were measured as the percentage of GFP+ cells by flow cytometry (LSRII; BD Biosciences). Data were analysed using FlowJo software (Single Cell Analysis Software).
Flow cytometry and viability assays
For intracellular Ki67 staining, cells were fixed for 3 min with fixation buffer (FIX & PERM; Life Technologies) before adding precooled 50% methanol for 10 min at 4°C. Cells were washed in PBS with 5% FBS and incubated for 30 min with the Ki67 FITC (1 : 10; clone B56; BD Biosciences) diluted in permeabilization buffer. For cell cycle analysis, cells were suspended in 0.03% saponin (7AAD; Sigma-Aldrich) in PBS and then incubated in 20 mM 7-aminoactinomycin D (Sigma-Aldrich) for 30 min at room temperature in the dark. Cells were kept on ice for 5 min before addition of pyronin Y (Sigma-Aldrich) to a final concentration of 5 μM. After incubation for 10 min on ice, cells were directly analysed by flow cytometry using a LSRII (BD Biosciences). The data were analysed using FlowJo software.
Cell viability was measured by relative quantification of live cells by flow cytometry (LSRII; BD Biosciences) according to forward scatter and side scatter parameters and analysed using FlowJo software (Single Cell Analysis Software).
Western blot
Vero cells and MDM were rinsed in ice-cold PBS and extracts prepared in lysis buffer (50 mM Tris-HCl pH 7.5, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 10 mM sodium β-glycerophosphate, 50 mM NaF, 5 mM sodium pyrophosphate, 270 mM sucrose and 1% Triton X-100) supplemented with protease inhibitor cocktail (Roche, Madrid, Spain) and 1 mM phenyl-methylsulfonyl fluoride. Denatured lysates were subjected to SDS-PAGE and transferred to a polyvinylidene fluoride membrane (ImmunolonP, Thermo). The following antibodies were used for immunoblotting: Hsp90 (BD Bioscience), SAMHD1 (Abcam), pThr592SAMHD1 (kindly provided by O. Keppler, University of Frankfurt), phospho-CDK2 (Thr160;2561), CDK2, CDK6, Rb and phospho-Rb (Ser807/811) (all at 1:1000 and, if not otherwise stated, purchased from Cell Signaling Technology). The anti-HSV-1 thymidine kinase (TK) mouse monoclonal antibody 4C8 (1:1000) was obtained from W. Summers (Yale University, New Haven, CT, USA) and used as described previously.30 At the moi used, HSV-TK from the incoming virus was not detectable (data not shown). Thus, HSV-TK is a reflection of newly expressed protein and virus replication.
Intracellular dNTP levels
dNTP content was determined using a polymerase-based method as previously described.12 Briefly, MDM were rinsed and lysed with trichloroacetic acid (0.5 mol/L). Samples were centrifuged to recover the supernatant free of cellular proteins. Supernatant was neutralized with 0.5 mol/L tri-n-octylamine in 1,1,2-trichlorotrifluoroethane and then dried in a SpeedVac. Pellets were resuspended in Tris-HCl buffer (40 mmol/L, pH 7.4) and dNTP concentration was determined using a polymerase-based method as previously described.11 Briefly, 20 μL of reaction mixture contained 5 μL of dNTP extract in 40 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 5 mM DTT, 0.25 μM oligoprimer, 0.75 μM [8-3H]deoxyadenosine triphosphate (dATP) 12–21 Ci/mmol, or [methyl-3H]deoxythymidine triphosphate (dTTP) for the dATP assay, and 0.5 U of Sequencing DNA Polymerase (Bioron). Reaction mixtures with aqueous dNTP standards were processed in parallel. After incubation at 48°C for 1 h, 18 μL of the mixture was spotted on a Whatman DE81 paper and left to dry. The filters were washed three times for 10 min with 5% Na2HPO4, once with water and once with absolute ethanol and left to dry again. The retained radioactivity was determined by scintillation counting and dNTP amounts were calculated from interpolation on the calibration curves. To ensure the reliability of the results, triplicates of both dilutions obtained from each sample (not diluted or diluted 1:3 in water) were processed in each independent experiment.
Statistical methods
Data are presented as mean ± SD and significance was calculated using a Student's t-test with the GraphPad PRISM software (GraphPad Software, San Diego, CA, USA).
Results
The selective CDK4/6 inhibitor, palbociclib, inhibits HSV-1 and HIV-1 infection in MDM
Palbociclib antiviral activity against HSV-1 and HIV-1 was evaluated in MDM from healthy donors. Differentiated MDM were pretreated with serial dilutions of palbociclib for 16 h prior to infection. Palbociclib showed antiviral activity against HIV-1 and HSV-1 in MDM in a dose–response manner at subtoxic concentrations (Figure 1a–c). The calculated 50% effective drug concentration (EC50) for HIV-1 infection was 0.016 μM (Figure 1a and Table 1), similar to that previously reported for HIV.11 For HSV-1 infection, viral replication was reduced (up to 84.3%; P = 0.0001) in MDM, showing an EC50 value similar to that reported for HIV-1 (EC50 = 0.020 μM; Figure 1b and Table 1). Maximum inhibition achieved with palbociclib treatment was comparable to that observed for control drugs: zidovudine in the case of HIV-1 and aciclovir in the case of HSV-1.
Figure 1.
Evaluation of selective CDK4/6 inhibitor palbociclib on HSV-1 and HIV-1 infection in MDM. MDM were cultured with increasing concentrations of palbociclib (μM) for 12 h, followed by 4 h with or without VLPVpx and then infected with (a) pseudotyped VSV-HIV-1 NL4-3-GFP+ or (b) HSV-1-GFP+. Infection was measured by flow cytometry as the percentage of GFP+-positive cells 48 h post-infection. (c) Cell viability of macrophages treated for 2 days with palbociclib, zidovudine and aciclovir was measured by flow cytometry and normalized to the no-drug control. (d) HSV-1 entry results in inhibition of SAMHD1 phosphorylation as measured by western blot of lysates from MDM infected with HSV-1, treated or not with VLPVpx and coincubated with aciclovir (1 μg/mL) or palbociclib (4 μM) respectively. HSV-TK was determined to assess viral infection. ACV, aciclovir; ZDV, zidovudine; UN, uninfected; INF, infected; ND, no drug.
Table 1.
Antiviral activity of palbociclib in MDM
| Virus | Zidovudine |
Aciclovir |
Palbociclib |
||||
|---|---|---|---|---|---|---|---|
| EC50 (μM) | CC50 (μM) | EC50 (μM) | CC50 (μM) | EC50 (μM) | EC50 (Vpx) (μM) | CC50 (μM) | |
| HIV-1 | 0.0003 | >0.3 | not tested | >4.4 | 0.016 | >4 | >4 |
| HSV-1 | not tested | >0.3 | 0.002 | >4.4 | 0.020 | 1.68 | >4 |
EC50, measured by inhibition of the percentage of GFP-positive cells infected with the corresponding virus measured by flow cytometry.
CC50, in uninfected MDM as measured by flow cytometry.
Data represent the mean of three independent experiments.
The anti-HSV-1 activity of palbociclib was confirmed at the protein level, as treatment with palbociclib inhibited the expression of viral protein HSV-TK by western-blot analysis, similarly to the aciclovir control (Figure 1d).
Next, antiviral activity of palbociclib was assessed after degradation of SAMHD1 with VLPVPX. As expected expression of HIV-2 Vpx led to SAMHD1 degradation (Figure 1d) and an increase in both HIV-1 and HSV-1 infectivity measured either by the increase of GFP+ cells (Figure 1a and b, black bars) or expression of HSV-TK protein by western blot (Figure 1d). Interestingly, SAMHD1 degradation by VLPVpx revealed a differential loss of palbociclib antiviral activity in HIV-1 and HSV-1 infections in MDM (Figure 1a and b). In the case of HIV-1, palbociclib antiviral activity was completely lost in MDM treated with VLPVpx, even at the highest concentration tested. However, antiviral activity of palbociclib was only partially lost in HSV-1-infected MDM treated with VLPVpx, and the antiviral activity is completely lost at 0.0064 μM. These results may suggest an alternative mechanism of action for palbociclib, in addition to that dependent on SAMHD1 previously reported in HIV-1 infections.11 Notably, HSV-1 infection led to inhibition of SAMHD1 phosphorylation in all cases (Figure 1d) even in the presence of aciclovir (Figure 1d), suggesting that HSV-1 immediate early or early gene expression may account for this effect. These results emphasize the role of SAMHD1 as a restriction factor for both RNA and DNA viruses.
Palbociclib treatment inhibits SAMHD1 phosphorylation mediated through CDK2 in MDM
To delineate further the cellular and molecular determinants leading to palbociclib antiviral activity, SAMHD1 expression and phosphorylation was measured by western blot. As previously shown,11 palbociclib blocked SAMHD1 phosphorylation in a dose–response manner, whereas SAMHD1 protein expression was not affected (Figure 2). In concordance with the hypothesis of CDK2-mediated phosphorylation of SAMHD1, palbociclib inhibited CDK2 activation (measured by phosphorylated forms of CDK2; pCDK2T160), but not CDK6 expression, confirming that CDK2 is controlled upstream by CDK6. In addition, we observed a concomitant dephosphorylation of Rb, the natural substrate of CDK6, which demonstrates the specificity of palbociclib activity (Figure 2).
Figure 2.

Palbociclib activity leads to inhibition of SAMHD1 phosphorylation mediated through CDK2 in MDM. Samples were blotted using α-phospho-Rb [pRB (Ser807/811)], α-Rb, α-phospho-CDK2 [pCDK2 (Thr160)], α-CDK2, α-CDK6, α-SAMHD1, α-phospho-SAMHD1 [pSAMHD1 (Thr592)] and α-Hsp90. ND, no drug.
The antiviral effect of palbociclib is dependent on SAMHD1 expression
To investigate further the correlation between palbociclib antiviral effect on HSV-1 infection and SAMHD1 function, a series of experiments were performed in Vero cell lines, which are easily infected with HSV-1. First, SAMHD1 expression was evaluated in Vero cells either by RNA or protein expression, finding in both cases a reduced expression of SAMHD1 compared with the monocytic THP-1 cell line (Figure 3a and b). Similar to previous reports in stable cell lines with low-expressing levels of SAMHD1,31 neither HIV-1 nor HSV-1 infection increased after SAMHD1 degradation by VLPVpx (Figure 3c). In concordance, palbociclib treatment did not show antiviral activity against HIV-1 or HSV-1 (Figure 3d), further demonstrating that palbociclib antiviral activity is dependent on SAMHD1 expression. In contrast, palbociclib treatment was able to inhibit phosphorylation of both Rb and CDK2 (Figure 3e), indicating that palbociclib is inhibiting CDK6 function, but is not able to affect SAMHD1-mediated viral restriction in SAMHD1 low-expressing cells.
Figure 3.
Selective CDK4/6 inhibitor palbociclib has no effect on HSV-1 and HIV-1 infection in SAMHD1-negative Vero cells. (a) Characterization of gene expression (mRNA) of SAMHD1 in Vero cells compared with THP-1 cells as a positive control of SAMHD1-expressing cells. (b) Lysates of Vero cells and THP-1 cells were subjected to SDS-PAGE and blotted with α-Rb, α-SAMHD1, α-pSAMHD1 (Thr592), α-CDK6 and α-Hsp90 antibodies. (c) Incubation with VLPVpx does not increase HSV-1 or HIV-1 infection in Vero cells. (d) Palbociclib does not inhibit HSV-1 or HIV-1 infection in Vero cells. Antiviral drugs aciclovir (1 μg/mL) and zidovudine (1 μg/mL) were used as controls for HSV-1 and HIV-1 infections, respectively. (e) Palbociclib inhibits phosphorylation of Rb and CDK2 in Vero cells after 24 h of exposure to palbociclib (4 μM). Mean ± SD values of three independent experiments are shown while representative blots of one of three independent experiments are shown for western-blot analyses. UN, uninfected; INF, infected; ND, no drug; AD, antiviral drugs.
Effect of palbociclib on the levels of intracellular dNTPs in the presence of Vpx
We have previously shown that palbociclib treatment decreased intracellular dNTP levels in M-CSF MDM. Evaluation of cell proliferation and cell cycle staining showed that palbociclib treatment decreased the percentage of cycling cells as measured by Ki67 staining (Figure 4a) and arrested cells in G1 (Figure 4b). MDM differentiated with M-CSF have up to 2-fold increased dNTP levels as compared with non-cycling GM-CSF MDM, but considerably lower than cycling, immortalized, stable cell lines such as MT-4 (Figure 4c) or Vero cells.32 Treatment with VLPVpx lead to SAMHD1 degradation (Figure 5a) and increased dNTP intracellular levels compared with the untreated control (no drug), having the greatest effect on dATP (P < 0.0001) (Figure 5c). These results are in concordance with the reported dNTPase activity of SAMHD1 and support the hypothesis that SAMHD1-mediated restriction is due to its dNTPase function. However, degradation of SAMHD1 by VLPVpx partially recovered the levels of dNTPs in palbociclib-treated MDM (P = 0.0005), i.e. although an increase is observed, degradation of SAMHD1 did not lead to a complete restoration of dNTP levels observed in the no-drug condition (Figure 5b). These results would suggest an alternative mechanism of action for palbociclib in down-regulating dNTP intracellular nucleotide levels, which may not be mediated by SAMHD1.
Figure 4.
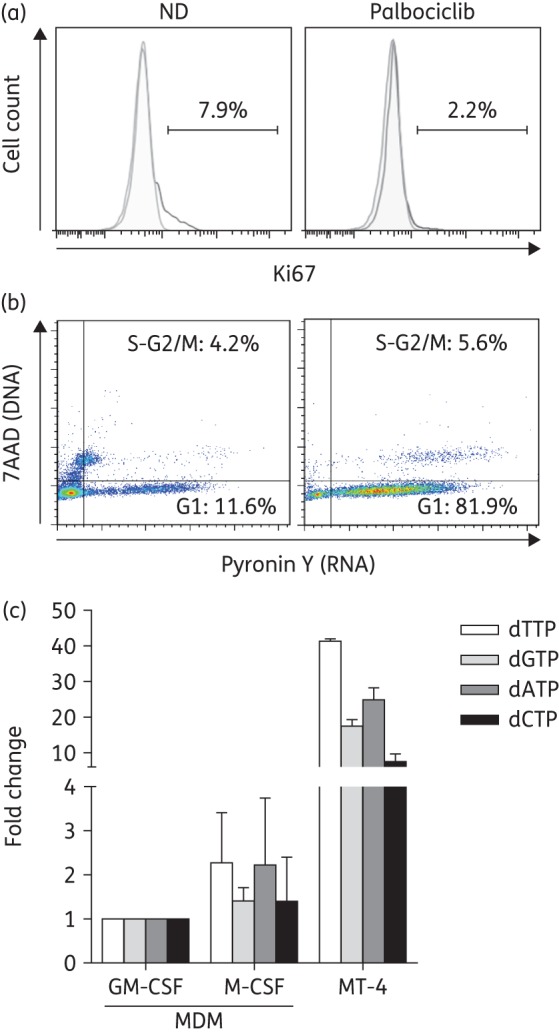
Palbociclib induced cell cycle arrest at G1 and reduced dNTP levels in MDM. (a) Percentage of Ki67+ cells in M-CSF-stimulated MDM treated with palbociclib (4 μM) for 16 h compared with untreated (no drug) controls. (b) Cell cycle profile of MDM treated or not with palbociclib. Representative histograms of two independent experiments are shown. (c) Intracellular dNTPs were extracted from primary MDM differentiated with GM-CSF or M-CSF or the lymphoid cell line MT-4 and dNTP content was determined using a polymerase-based method. Fold change compared with GM-CSF MDM is shown. Mean ± SD values of two independent experiments are shown. ND, no drug. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 5.

Effect of palbociclib on the levels of intracellular dNTPs in the presence of Vpx. (a) Lysates of MDM treated or not with palbociclib (4 μM) in the presence or absence of VLPVpx were monitored by western blot. (b) Intracellular dNTP content was determined using a polymerase-based protocol. Mean ± SD values of three independent experiments are shown while representative blots of one of three independent experiments are shown for western-blot analyses. ND, no drug.
Discussion
The dNTPase activity of SAMHD1 regulates the intracellular dNTP availability as part of a regulatory mechanism of the cell cycle.33 dNTP availability may become a roadblock for cell proliferation, viral replication,2 triggering of an immune response and susceptibility to dNTP analogues with antiproliferative or antiviral activities.31,34 Sufficient evidence begins to indicate that the antiviral mechanism of SAMHD1 may solely depend on its control of intracellular dNTP levels and that structural/conformational changes induced by its phosphorylation state may strongly affect the hydrolase activity of SAMHD1.4
The observation that inhibition of CDK function leading to disruption of SAMHD1 phosphorylation results in a substantial reduction of the intracellular level of dNTPs,11,14,15 supports the hypothesis that phosphorylation impairs the dNTPase activity of SAMHD1, strongly affecting the replication of HIV-1, but also of DNA viruses such as HSV-1. An RNAse activity of SAMHD1 has been proposed as the responsible function for the inhibition of HIV infection in myelocytic cells.35,36 This conclusion is difficult to reconcile with the effect of SAMHD1 function in the replication of a DNA virus, such as HSV-1. Moreover, SAMHD1 has been shown to be a single-stranded nucleic acid binding protein with no active site-associated nuclease activity.4 Nevertheless, additional mechanisms by which SAMHD1 affects viral replication are not totally discarded.
The degradation of SAMHD1 by Vpx abrogated the antiviral activity of palbociclib, suggesting that its antiviral activity against DNA and RNA viruses converges on a common mechanism mediated through SAMHD1, most likely by controlling the intracellular dNTP pool. However, palbociclib retained partial antiviral activity against HSV-1 in spite of SAMHD1 degradation. The observation that Vpx only partially restored dNTP levels in the presence of palbociclib may suggest an alternative mechanism, dependent on dNTP levels. CDK2 is responsible for controlling dNTP synthesis by the ribonucleotide reductase.37 Inhibition of CDK6-dependent CDK2 activation may not only control dNTP hydrolase, but dNTP synthesis, as dNTP requirements may be different for the HIV-1 reverse transcriptase and the HSV-1 DNA polymerase.38,39 Additionally, the ribonucleotide reductases encoded by HSV-1 additionally affect the overall dNTP pool (for review see Lembo and Brune40), making them less dependent on the enzymatic machinery of the host and adding a confounding element in evaluating the role of SAMHD1 in DNA virus replication. SAMHD1 degradation may be only sufficient to allow for HIV-1 replication, but not HSV-1.
Lack of activity of palbociclib in SAMHD1 low-expressing Vero cells may be explained by the simple reason that immortalized cells present a highly deregulated cell cycle and, in that case, the metabolic pathway in charge of synthesizing and degrading nucleotides may be dysregulated. In that case, where high amounts of dNTPs are present41 alteration of the dNTP pool induced by palbociclib may not be sufficient to reduce dNTP levels below the threshold required by HSV-1 DNA polymerase.42
Independently from the effect induced by palbociclib, we observed that HSV-1 infection of MDM induced the inhibition of SAMHD1 phosphorylation, even in the presence of aciclovir. HSV-1 infection has been shown to cause inhibition of CDK4/6 and CDK2-specific phosphorylation of Rb (pRb),43 leading to cell cycle arrest at G1, a similar effect as that observed by palbociclib treatment (Figure 2). Further research should elucidate how these events affect HIV-1 and HSV-1 coinfection of macrophages, as previously reported.44
In summary, our findings support the dNTPase activity of SAMHD1 as a primary mechanism for the restriction of HIV-1 and HSV-1 infections. Alternatively, we have indicated a novel antiviral mechanism of palbociclib, probably mediated by regulation of the pRB-E2F1-RNR2 pathway, blocking dNTP synthesis and impeding HSV-1 DNA replication. We have used primary MDM as a relevant model in which to test and compare the effect of SAMHD1 on HSV-1 and HIV-1 replication. Our findings should be re-evaluated in other models and cells types relevant for the replication and pathogenesis of HSV-1 infection.
Funding
This work was supported in part by the Spanish Ministerio de Economía y Competitividad (MINECO) project BFU2012-31569 and Fondo de Investigación Sanitaria (FIS) projects PI13/01083 and CP14/00016. R. B. and E. B. are research fellows from FIS.
Transparency declarations
None to declare.
Acknowledgements
We thank Dr Matthew D. Weitzman and Dr Ana Angulo for providing reagents and advice, and the National Institutes of Health (AIDS Research and Reference Reagent Program) and the EU Programme EVA Centralised Facility for AIDS Reagents, NIBSC, UK for reagents.
References
- 1.Ballana E, Esté JA. SAMHD1: at the crossroads of cell proliferation, immune responses, and virus restriction. Trends Microbiol 2015; doi:10.1016/j.tim.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Lahouassa H, Daddacha W, Hofmann H et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol 2012; 13: 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pauls E, Ballana E, Esté JA. Nucleotide embargo by SAMHD1: a strategy to block retroviral infection. Antiviral Res 2013; 97: 180–2. [DOI] [PubMed] [Google Scholar]
- 4.Seamon KJ, Sun Z, Shlyakhtenko LS et al. SAMHD1 is a single-stranded nucleic acid binding protein with no active site-associated nuclease activity. Nucleic Acids Res 2015; 43: 6486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rehwinkel J, Maelfait J, Bridgeman A et al. SAMHD1-dependent retroviral control and escape in mice. EMBO J 2013; 32: 2454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rehwinkel J. Mouse knockout models for HIV-1 restriction factors. Cell Mol Life Sci 2014; 71: 3749–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shingai M, Welbourn S, Brenchley JM et al. The expression of functional Vpx during pathogenic SIVmac infections of rhesus macaques suppresses SAMHD1 in CD4+ memory T cells. PLoS Pathog 2015; 11: e1004928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruffin N, Brezar V, Ayinde D et al. Low SAMHD1 expression following T-cell activation and proliferation renders CD4+ T cells susceptible to HIV-1. AIDS 2015; 29: 519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riveira-Munoz E, Ruiz A, Pauls E et al. Increased expression of SAMHD1 in a subset of HIV-1 elite controllers. J Antimicrob Chemother 2014; 69: 3057–60. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan EL, McAlexander MA, Witwer KW. SAMHD1 expression in blood cells of HIV-1 elite suppressors and viraemic progressors. J Antimicrob Chemother 2015; 70: 954–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pauls E, Badia R, Torres-Torronteras J et al. Palbociclib, a selective inhibitor of cyclin-dependent kinase4/6, blocks HIV-1 reverse transcription through the control of sterile α motif and HD domain-containing protein-1 (SAMHD1) activity. AIDS 2014; 28: 2213–22. [DOI] [PubMed] [Google Scholar]
- 12.Pauls E, Ruiz A, Badia R et al. Cell cycle control and HIV-1 susceptibility are linked by CDK6-dependent CDK2 phosphorylation of SAMHD1 in myeloid and lymphoid cells. J Immunol 2014; 193: 1988–97. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz A, Pauls E, Badia R et al. Cyclin D3-dependent control of the dNTP pool and HIV-1 replication in human macrophages. Cell Cycle 2015; 14: 1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan J, Hao C, DeLucia M et al. CyclinA2-cyclin-dependent kinase regulates SAMHD1 protein phosphohydrolase domain. J Biol Chem 2015; 290: 13279–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pauls E, Ruiz A, Riveira-Muñoz E et al. p21 regulates the HIV-1 restriction factor SAMHD1. Proc Natl Acad Sci USA 2014; 111: E1322–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 2009; 9: 153–66. [DOI] [PubMed] [Google Scholar]
- 17.St Gelais C, de Silva S, Hach JC et al. Identification of cellular proteins interacting with the retroviral restriction factor SAMHD1. J Virol 2014; 88: 5834–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cribier A, Descours B, Valadao AL et al. Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep 2013; 3: 1036–43. [DOI] [PubMed] [Google Scholar]
- 19.Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer 2002; 2: 910–7. [DOI] [PubMed] [Google Scholar]
- 20.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev 2004; 18: 2699–711. [DOI] [PubMed] [Google Scholar]
- 21.Leonard JP, LaCasce AS, Smith MR et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood 2012; 119: 4597–607. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health and Human Services. FDA Information on Drugs. Approved Drugs. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm432886.htm. [Google Scholar]
- 23.Kim ET, White TE, Brandariz-Nunez A et al. SAMHD1 restricts herpes simplex virus 1 in macrophages by limiting DNA replication. J Virol 2013; 87: 12949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollenbaugh JA, Gee P, Baker J et al. Host factor SAMHD1 restricts DNA viruses in non-dividing myeloid cells. PLoS Pathog 2013; 9: e1003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schang LM. Cyclin-dependent kinases as cellular targets for antiviral drugs. J Antimicrob Chemother 2002; 50: 779–92. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M, Onogi H, Kii I et al. CDK9 inhibitor FIT-039 prevents replication of multiple DNA viruses. J Clin Invest 2014; 124: 3479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schang LM, Bantly A, Knockaert M et al. Pharmacological cyclin-dependent kinase inhibitors inhibit replication of wild-type and drug-resistant strains of herpes simplex virus and human immunodeficiency virus type 1 by targeting cellular, not viral, proteins. J Virol 2002; 76: 7874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badia R, Riveira-Muñoz E, Clotet B et al. Gene editing using a zinc-finger nuclease mimicking the CCR5Δ32 mutation induces resistance to CCR5-using HIV-1. J Antimicrob Chemother 2014; 69: 1755–99. [DOI] [PubMed] [Google Scholar]
- 29.Badia R, Grau J, Riveira-Muñoz E et al. The thioacetate-ω(γ-lactam carboxamide) HDAC inhibitor ST7612AA1 as HIV-1 latency reactivation agent. Antiviral Res 2015; 123: 62–9. [DOI] [PubMed] [Google Scholar]
- 30.Neyts J, Reymen D, Letourneur D et al. Differential antiviral activity of derivatized dextrans. Biochem Pharmacol 1995; 50: 743–51. [DOI] [PubMed] [Google Scholar]
- 31.Ballana E, Badia R, Terradas G et al. SAMHD1 specifically affects the antiviral potency of thymidine analog HIV reverse transcriptase inhibitors. Antimicrob Agents Chemother 2014; 58: 4804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aduma PJ, Gupta SV, Stuart AL et al. Deoxyribonucleoside triphosphate pools of herpes simplex virus infected cells: the influence of selective antiherpes agents and the role of the deaminase pathway. Biochem Cell Biol 1991; 69: 409–14. [DOI] [PubMed] [Google Scholar]
- 33.Franzolin E, Pontarin G, Rampazzo C et al. The deoxynucleotide triphosphohydrolase SAMHD1 is a major regulator of DNA precursor pools in mammalian cells. Proc Natl Acad Sci USA 2013; 110: 14272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clifford R, Louis T, Robbe P et al. SAMHD1 is mutated recurrently in chronic lymphocytic leukemia and is involved in response to DNA damage. Blood 2014; 123: 1021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi J, Ryoo J, Oh C et al. SAMHD1 specifically restricts retroviruses through its RNase activity. Retrovirology 2015; 12: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryoo J, Choi J, Oh C et al. The ribonuclease activity of SAMHD1 is required for HIV-1 restriction. Nat Med 2014; 20: 936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allouch A, David A, Amie SM et al. p21-mediated RNR2 repression restricts HIV-1 replication in macrophages by inhibiting dNTP biosynthesis pathway. Proc Natl Acad Sci USA 2013; 110: E3997–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amie SM, Noble E, Kim B. Intracellular nucleotide levels and the control of retroviral infections. Virology 2013; 436: 247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Souza TM, De Souza MC, Ferreira VF et al. Inhibition of HSV-1 replication and HSV DNA polymerase by the chloroxoquinolinic ribonucleoside 6-chloro-1,4-dihydro-4-oxo-1-(β-d-ribofuranosyl) quinoline-3-carboxylic acid and its aglycone. Antiviral Res 2008; 77: 20–7. [DOI] [PubMed] [Google Scholar]
- 40.Lembo D, Brune W. Tinkering with a viral ribonucleotide reductase. Trends Biochem Sci 2008; 34: 25–32. [DOI] [PubMed] [Google Scholar]
- 41.Gandhi VV, Samuels DC. A review comparing deoxyribonucleoside triphosphate (dNTP) concentrations in the mitochondrial and cytoplasmic compartments of normal and transformed cells. Nucleosides Nucleotides Nucleic Acids 2011; 30: 317–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lund TJ, Cavanaugh NA, Joubert N et al. B family DNA polymerases asymmetrically recognize pyrimidines and purines. Biochemistry 2011; 50: 7243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ehmann GL, McLean TI, Bachenheimer SL. Herpes simplex virus type 1 infection imposes a G1/S block in asynchronously growing cells and prevents G1 entry in quiescent cells. Virology 2000; 267: 335–49. [DOI] [PubMed] [Google Scholar]
- 44.Heng MCY, Heng SY, Allen SG. Co-infection and synergy of human immunodeficiency virus-1 and herpes simplex virus-1. Lancet 1994; 343: 255–8. [DOI] [PubMed] [Google Scholar]




