Abstract
Objective:
To evaluate and compare the effects of exercise therapy and electrical stimulation on muscle strength and functional activities in patients with limb-girdle muscular dystrophy (LGMD).
Methods:
This controlled clinical trial included 24 subjects who were diagnosed with LGMD by the Neurology Department of the Hacettepe University Hospital, Ankara, Turkey and were referred to the Physical Therapy Department between May 2013 and December 2014. Subjects were enrolled into an electrical stimulation (11 patients) group, or an exercise therapy (13 patients) group.
Results:
The mean age of patients was 31.62 years in the electrical stimulation group, and 30.14 years in the exercise therapy group. The most important results in this controlled clinical study were that the muscle strength in both groups was significantly decreased and post-treatment evaluation results indicated that muscle strength of the Deltoideus was higher in the electrical stimulation group, and the difference between the groups was maintained in the follow-up period (p<0.05). However, the muscle strength of quadriceps was similar in both groups, according to the post-treatment and follow-up evaluation results (p>0.05). Additionally, the electrical stimulation group presented more obvious overall improvements than the exercise therapy group according to muscle strength, endurance, and timed performance tests.
Conclusions:
Since no definitive treatments currently exist for patients with LGMD, these results provide important information on the role of exercise therapy and electrical stimulation for clinicians working in rehabilitation.
Limb-girdle muscular dystrophy (LGMD) is a group of typically inherited, slowly progressive neuromuscular diseases, primarily involving the pelvis and/or shoulder girdle muscles.1 During the last 20 years, although important steps have been taken toward the etiology and treatment of neuromuscular diseases through developments in genetic science, no definitive treatments exists for a large number of neuromuscular diseases such as LGMD.1,2 The absence of the methods to control the progress of the diseases increases the importance of physiotherapy and rehabilitation in order to support, maintain, and increase quality of life.3 In patients with LGMD, the aim of exercise therapy is to maintain muscle strength, joint mobility, and functional activity level as long as possible.4 As it is well known, resistive exercise training is one of the most efficient methods in improving the functional capacity of the neuromuscular system.5 However, potential benefits and risks of resistive exercise training in neuromuscular diseases are currently controversial in the literature.6-11 It is frequently stated in the literature that effects of exercise therapy vary according to the type of myopathy.3,6 In dystrophy types with rapid progress and with membrane instability (such as Duchenne muscular dystrophy [DMD]), high intensive (50-70% of maximum weight) and excentric exercise programs are not recommended since they are considered to cause mechanical stress on muscle fibers, which may lead to injury and an increase in muscle weakness. Contrarily, other studies in the literature stated that high intensive exercise training was of benefit with appropriate patient selection.3,6 In recent years, studies have shown that mild and moderate intensity (25-40% of maximum weight) strength training had positive effects on muscle strength when applied in the early period, especially in slowly progressive neuromuscular diseases.9-12 However, the contribution of improvement by strength training, to a functional level and daily living of patients has not been discussed extensively in the literature. Electrical stimulation is another method, which has recently been considered to be of benefit in the rehabilitation of patients with neuromuscular diseases, due to its effects in preventing muscle atrophies, maintaining, and improving muscle strength.13,14 In a limited number of studies on DMD and other forms of dystrophies, the effects of low frequency and long duration of electrical stimulation on muscle strength have been studied.13-16 These studies indicated an increase in the number of muscle fibers contributing to contraction and changes in biochemical and contractile elements of the muscle fibers, and thus, improvement in the maximal volunteer contraction strength, without causing any changes in fatigue and time of relaxation, especially when applied in the early period of the disease.13-16 Although the results of these studies are promising, the role of electrical stimulation in the management of patients with muscular dystrophies, optimal current type, method and duration of application, superiority over exercise therapy, and functional acquisition by the treatment are not clear. Subject related studies to date are on the rapid progressing type of neuromuscular group, and it is difficult to interpret these results in the slowly progressing neuromuscular group. High voltage pulsed galvanic stimulation (HVPGS) has positive effects on producing efficient muscle contraction, improving muscle strength and circulation, reducing pain and edema, and acceleration of wound healing.17-22 Recently, HVPGS became the most preferred among the electrical stimulation methods, which are used with the aim of improving muscle strength, since they require lower current intensity and being more tolerable by the patients.17-19 However, there is a lack of studies in the literature, which investigated the effects of HVPGS on muscle strength in neuromuscular diseases. The aim of this study was to investigate and compare the effects of exercise therapy and HVPGS on muscle strength, functional activities, and independence level of activities of daily living (ADL) in patients with LGMD. Therefore providing information to the subject related literature, which is currently deficient in defining the role of exercise therapy and electrical stimulation in rehabilitation of these patients.
Methods
Subjects
The present study was a clinical controlled study. All of the subjects (N=42) who were diagnosed with LGMD by the Neurology Department of the Hacettepe University Hospital, Ankara, Turkey and were referred to the Physical Therapy Department of the Health Sciences Faculty between May 2013 and December 2014 were invited to participate in the study. However, 15 subjects declined to participate as they were living out of town and were not able to attend regular treatment sessions. All of the patients who were accepted to participate to the study (N=27), signed the informed consent forms after explanation of the aims and methods of the study, and after ethical approval was obtained according to the Helsinki Declaration principles. Two patients discontinued treatment, and one patient did not attend post-treatment evaluations. Therefore, this study was completed on 24 patients with LGMD. The study design is summarized in Figure 1.
Figure 1.
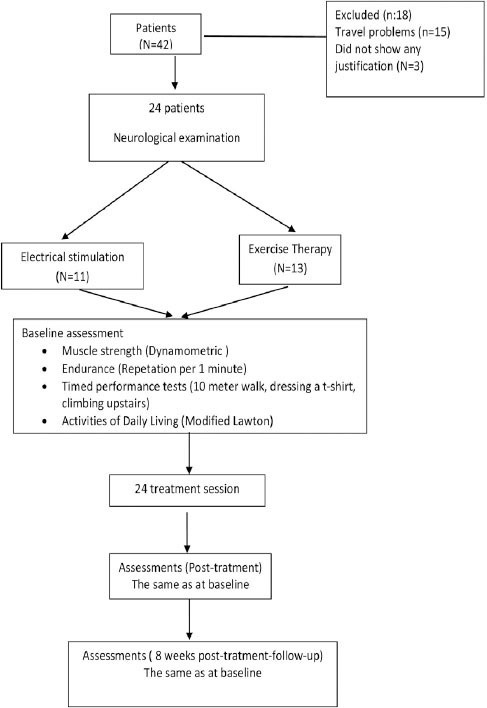
Shows the study design to evaluate and compare the effects of the exercise therapy and electrical stimulation on muscle strength and functional activities in patients with limb-girdle muscular dystrophy
Inclusion criteria
i) Patients who were diagnosed as LGMD by a neurologist were invited to the study. The diagnoses were made based on the history and signs of the patients and the results of muscle biopsy. ii) Ability to ambulate without any assistive devices. iii) A value of 3+ or over (being able to contract against gravity and oppose a little resistance) according to manual muscle testing of the muscles to be tested.
Exclusion criteria
i) Joint limitations in shoulders and knees. ii) Other neurological or cardiovascular problems that could be an obstacle for the exercise program.
Treatment protocol
Patients were enrolled into electrical stimulation (n=11) or exercise therapy (n=13) groups, based on willingness to undergo electrical stimulation. In the exercise therapy group, moderate intensity progressive resistive exercises was applied bilaterally to the muscles that are considered to be most effective in functional activities (deltoideus as shoulder abductors in the upper limbs, and quadriceps femoris as knee extensors in the lower limbs). In the electrical stimulation group, HVPGS was applied to the same muscles. According to their strengths, active or active-assistive strengthening exercises were given to the limb muscles except the ones mentioned above and to the trunk muscles, in both groups. Also, all of the subjects performed stretching exercises and received functional mobility training. The treatment programs of the groups were carried out by the same physiotherapist, with a frequency of 3 days a week for 8 weeks. At the end of the treatment programs, subjects were followed up by a home program, and were invited to come to control visits after 8 weeks.
At the beginning of the moderate intensity of progressive resistive exercise program, 1-maximum repetition (1 MR) values of quadriceps femoris and deltoideus muscles were determined by connecting free-weights to the related extremities. The characteristics of the exercise program are presented in Table 1.
Table 1.
Progressive resistive exercise program among LGMD patients.
| Progressive resistive exercise program | Intensity | Number of repetitions × set / session | Number of treatment per week |
|---|---|---|---|
| 1-2 weeks | 25% of 1MR | 10×2 | 3 |
| 2-4 weeks | 30% of 1MR | 10×3 | 3 |
| 5-6 weeks | 35% of 1MR | 10×3 | 3 |
| 7-8 weeks | 40% of 1MR | 10×3 | 3 |
1 MR - 1 maximum repetition, LGMD - limb-girdle muscular dystrophy
Electrical stimulation application
Electrical stimulation was performed using an HVPG stimulator (Elettronica Pagani, Performer 982, Class Type BF, S/N:181, 2004, Supplier: Libor Medical Products, Ankara/Turkey, Manufacturer: Medical Expo (Paderno, Dugnano, Italy), using monophasic wave type (twin peak pulse) via surface electrodes. The pulse frequency of the device was 2-100 Hz, voltage output was 0-500 V, and pulse duration was 200 µs.
Similarly to previous studies, a pulse frequency of 50 Hz was preferred in this study for optimal contraction. Four electrodes were placed around the muscle and current intensity was increased up to significant contraction. Duty cycle was set at 5 seconds on and 10 seconds off, during 10 minutes of stimulation of each muscle. The findings indicating muscle fatigue and pain intensity were assessed by 0-10 cm visual analogue scales, 24 hours after electrical stimulation application.18
Assessment
At the beginning of the study, age, gender, height, body weight, and dominant side of the subjects, and the duration of the disease were recorded. All subjects were evaluated at the beginning of the study, after 8 weeks (post-treatment) and 2 months after the treatment (follow-up). The evaluated parameters were; 1) Muscle strength - dynamometric muscle strengths of shoulder abductors and knee extensors were evaluated by Power Track II Commander (TECH 801-478-0680, JTECH Medical, Utah, USA), in standard muscle testing positions. Results were recorded in Newton units.23 2) Pain and fatigue - muscle pain and fatigue problems of the patients after exercise and electric stimulation were measured by 10 cm visual analogue scales (0=no pain, 10=intolerable pain for pain intensity, and 0=no fatigue, 10=unbearable fatigue).20 3) Timed performance - the durations of climbing up 8 stairs,24 walking 10 meters,25 and dressing with a t-shirt26 were recorded (seconds). 4) Endurance - number of shoulder abduction and knee extension repetitions per one minute were recorded.27 5) Activities of daily living - Modified Lawton ADL test was used for the assessment. Each of feeding, self-care, dressing, transfer, bed, hands, and walking activities were evaluated on a 1-4 score range (1=completely dependent, 2=needs assistance, 3=needs observation, 4=completely independent). Total score was recorded as the ADL score.28
Statistical analyses
Statistical analysis was performed by the Statistical Package for Social Sciences, version 18.0 (SPSS Inc., Chicago, IL, USA). The quantitative and qualitative data were expressed as arithmetic mean ± standard deviation (X±SD). Wilcoxon signed-rank test was used for intra-group analyses. Inter-group analyses were performed by using Mann-Whitney U test. The limit of statistical significance was set at p<0.05.
Results
In this study, which investigated and compared the effects of exercise therapy and HVPGS on muscle strength and functional activities, 19 male (79.2%) and 5 (20.8%) female subjects with the diagnosis of LGMD were included. Eleven (48.1%) patients accepted to be included in the electrical stimulation group, and 13 subjects were involved in the exercise therapy group. The mean age of patients was 31.62 years in the electrical stimulation group, and 30.14 years in the exercise therapy group. Additionally, duration of illness of patients was 14.92 months in the electrical stimulation group, and 14.71 months in the exercise therapy group. Physical characteristics of the subjects were similar in both groups (p>0.05) (Table 2).
Table 2.
Comparison of the physical characteristics of the LGMD patients in electrical stimulation and exercise therapy groups.
| Demographics of patients | Electrical stimulation group | Exercise therapy group | P-value |
|---|---|---|---|
| Age (year) | 31.62±16.92 | 30.14±11.04 | 0.696 |
| Height (cm) | 174.23±7.07 | 169.21±9.36 | 0.150 |
| Body weight (kg) | 65.92±9.04 | 67.93±13.01 | 0.808 |
| Duration since diagnosis (month) | 14.92±23.81 | 14.71±55.01 | 0.092 |
Mann-Whitney U test was used, the limit of statistical significance was set at p<0.05, LGMD - limb-girdle muscular dystrophy
According to between group analysis pre-treatment muscle strengths of the quadriceps femoris and deltoideus were similar in both groups (right deltoid p=0.32, left deltoid p=0.22; right quadriceps p=0.81, left quadriceps p=0.90). Post-treatment evaluation results indicated that the muscle strength of the deltoideus was higher in the electrical stimulation group (right p=0.00, and left p=0.01), and the difference between the groups was maintained in the follow-up period (p=0.00 for right and left) (Table 3).
Table 3.
Pre-treatment, post-treatment and follow-up results of muscle strength within and between groups of LGMD patients.
| Electrical stimulation group (n=11) | Exercise therapy group (n=13) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | p0 | Follow-up | p1 | Pre | Post | p0 | Follow-up | p1 | pa | pb | pc | |
| X (SD) (95%CI) | X (SD) (95%CI) | ||||||||||||
| Muscle strength (Newton) | |||||||||||||
| Musculus Deltoideus | |||||||||||||
| Right | 85.04(32.73) (66.69-104.05) | 128.31(40.48) (105.55-151.54) | 0.00* | 122.89(38.41) (100.60-144.79) | 0.15 | 69.39(42.35) | 76.78(38.25) | 0.09 | 69.89(28.73) | 0.14 | 0.32 | 0.00* | 0.00* |
| Left | 92.18(29.25) (76.24-109.42) | 128.86(40.38) (106.64-152.59) | 0.00* | 121.23(34.58)(102.27-140.90) | 0.32 | 71.43(49.63) (47.73-98.44) | 82.77(36.07)(66.16-102.92) | 0.11 | 77.00(30.32)(62.62-93.84) | 0.08 | 0.22 | 0.01* | 0.00* |
| Musculus Quadriceps femoris | |||||||||||||
| Right | 147.04(104.00) (91.86-208.05) | 192.35(112.07) (131.95-256.40) | 0.00* | 178.55(108.65) (119.76-239.72) | 0.03* | 137.81(82.63) (95.74-182.76) | 158.42(88.48)(114.53-206.34) | 0.01* | 144.92(78.56) (105.40-187.15) | 0.06 | 0.81 | 0.43 | 0.40 |
| Left | 133.98(84.59) (89.07-183.34) | 179.51(99.08) (127.44-236.53) | 0.00* | 164.84(90.5) (119.04-219.45) | 0.19 | 138.23(72.39) (100.16-176.37) | 156.62(79.74) (117.17-199.54) | 0.05* | 149.46(80.54) (110.46-192.60) | 0.19 | 0.90 | 0.55 | 0.67 |
p0 - p-values of pre and post treatment within groups, p1 - p values of post-treatment and follow-up within groups, pa - p-values of pre-treatment results between groups, pb - p-values of post-treatment results between groups, pc - p-values of follow up results between groups. Mann Whitney U test was used for between group analysis and Wilcoxon signed rank test for within group analysis.
p<0.05
Within group analyses showed that dynamometric strengths of the deltoideus and quadriceps femoris increased bilaterally after treatment in the electrical stimulation group (p=0.00 for all), and this increase was maintained on the deltoids (right p=0.15; left p=0.32) and left quadriceps (p=0.19) muscles during the 2 months after the treatment. In the exercise therapy group, the quadriceps femoris muscle strength increased bilaterally post treatment (right p=0.01; left p=0.05) and this improvement was maintained during follow up (p=0.06 right and p=0.19 left quadriceps). There were no significant increases in the deltoideus muscles strength in the exercise group (p=0.09 for right and p=0.11 for left deltoids). The detailed analysis of muscle strength is summarized in Table 3. Groups were homogeneous for pre-treatment endurance tests results, and no inter-group difference was found in latter measurements (p>0.05). Intra-group analyses revealed that endurance of the upper extremity improved bilaterally after the treatment in the electrical stimulation group (p=0.002 for right, and p=0.03 for left) (Table 4). Timed performance test results improved after treatment in both groups (p<0.05), except for dressing with a t-shirt duration in the exercise therapy group (p=0.14). According to the inter-group analyses, pre-treatment values were similar in both groups, and subjects in the electrical stimulation group were found to perform the performance tests in a shorter time after the treatment and in the follow-up period (p<0.05) (Table 4). Activities of daily living scores did not improve significantly after treatments, and no difference was found between and within the groups (p>0.05) (Table 4). Muscle pain and fatigue symptoms did not increase after exercise therapy and electrical stimulation applications.
Table 4.
Pre-treatment. post-treatment and follow-up values of endurance tests. timed performance tests and activities of daily living test within group and between group among LGMD patients.
| Electrical stimulation group (n=11) | Exercise therapy group (n=13) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-treatment | Post | p0 | Follow-up | p1 | Pre | Post | p0 | Follow-up | p1 | Pa | pb | pc | |
| X (SD) (95%CI) | X (SD) (95%CI) | ||||||||||||
| Endurance test (times/minute) | |||||||||||||
| Upper extremity | |||||||||||||
| Right | 52.55(19.69) (43.09-65.27) | 60.91(22.62) (49.82-76.00) | 0.002* | 59.45(18.83) (50.00-71.81) | 0.44 | 51.62(11.38) (46.38-58.15) | 54.23(14.51) (47.31-62.15) | 0.26 | 53.23(13.42) (47.16-60.54) | 0.40 | 0.89 | 0.41 | 0.37 |
| Left | 51.82(14.37) (44.00-61.09) | 59.00(14.75) (51.00-68.27) | 0.03* | 56.91(13.01) (49.82-65.00) | 0.18 | 49.92(11.41) (44.38-56.38) | 52.69(14.41) (45.92-60.31) | 0.26 | 50.46(11.82) (44.85-56.69) | 0.19 | 0.73 | 0.30 | 0.22 |
| Lower extremity | |||||||||||||
| Right | 57.64(11.19) (51.64-64.27) | 62.64(14.91) (53.91-70.90) | 0.16 | 62.45(13.19) (54.64-69.64) | 0.89 | 54.38(15.30) (46.54-62.69) | 58.77(17.73) (49.47-67.69) | 0.01* | 55.85(15.91) (46.93-64.00) | 0.06 | 0.55 | 0.57 | 0.28 |
| Left | 57.64(14.189 (50.27-66.18) | 62.36(16.41) (53.28-71.91) | 0.21 | 64.64(13.74) (56.91-72.82) | 0.16 | 57.08(23.23) (45.85-70.46) | 59.38(24.46) (46.54-72.53) | 0.19 | 58.46(23.75) (46.46-71.54) | 0.48 | 0.94 | 0.73 | 0.44 |
| Timed performance tests (seconds) | |||||||||||||
| Climbing up stairs | 7.16(3.26) (5.50-9.18) | 5.30(2.83) (3.43-6.65) | 0.00* | 5.94(2.44) (3.72-6.37) | 0.85 | 11.58(9.50) (7.32-16.73) | 9.38(7.489 (6.05-13.62) | 0.01* | 9.97(7.71) (6.46-14.27) | 0.09 | 0.14 | 0.05* | 0.04* |
| Walking 10 m | 7.96(1.13) (7.31-8.60) | 6.08(1.10) (5.51-6.74) | 0.00* | 6.25(3.21) (4.39-8.05) | 0.83 | 10.07(3.55) (8.27-11.94) | 8.45(3.10) (7.00-10.19) | 0.01* | 8.53(2.51) (7.29-9.91) | 0.89 | 0.07 | 0.02* | 0.07 |
| Dressing a t-shirt | 4.05(1.81) (3.97-6.01) | 3.52(1.10) (2.98-4.19) | 0.02* | 3.91(1.03) (3.41-4.53) | 0.03* | 7.22(3.86) (4.56-6.33) | 5.98(3.80) (5.00-8.56) | 0.14 | 6.21(3.00) (4.79-7.75) | 0.47 | 0.58 | 0.01* | 0.02* |
| Activities of daily living test (0-96) | |||||||||||||
| Lawton ADL test score | 93.36(5.80) (89.36-95.99) | 94.00(5.12) (90.55-96.00) | 0.09 | 94.00(5.12) (90.55-96.00) | 1 | 87.69(9.57) (85.31-95.08) | 88.23(9.81) (82.69-93.08) | 0.16 | 87.92(9.62) (82.54-92.77) | 0.37 | 0.41 | 0.08 | 0.07 |
p0 - p-values of pre and post treatment within groups. p1 - p-values of post-treatment and follow-up within groups. pa - p values of pre-treatment results between groups. pb: p-values of post-treatment results between groups. pc- p values of follow up results between groups. Man Whitney U test was used for between group analysis and Wilcoxon signed rank test for within group analysis.
p<0.05
Discussion
The results of this study indicate that electrical stimulation, which was applied 3 times a week, for 8 weeks, was effective in increasing the strengths of the deltoid and quadriceps femoris muscles in adult LGMD patients. Also, mild-moderate intensity progressive resistive exercise therapy, which was applied with the same frequency and duration as the electrical stimulation, was effective in improving quadriceps femoris muscle strength. Electrical stimulation was found more effective than exercise therapy in increasing deltoideus muscle strength. This result may due to the higher type 2 fiber content (approximately 60%) of the quadriceps muscle requiring higher stimulation frequencies. Dirks et al,29 used a 100 Hz frequency to prevent muscle wasting in the quadriceps muscle. Therefore, our frequency of 50 Hz could not improve the muscle performance as in the case with resistive exercise. However, this frequency seems more appropriate to stimulate the deltoid muscle due to its type 1 predominancy (approximately 55%). The improvement in the deltoid muscle after HVPGS can be explained by this frequency coherency to the type distribution. Also, the obtained muscle strength improvements were maintained in the electrical stimulation group, according to the follow-up measurements. Cup et al5 stated in their review that there are no electrical stimulation studies carried out on adult NMD patients, and the present study addresses a major limitation in the literature.
Scott et al13,14 revealed in 2 separate studies that the maximum voluntary contraction of electrically stimulated quadriceps femoris and tibialis anterior muscles increased significantly when compared with the values of a nonstimulated control group of muscles, without causing any adverse effects, in children with DMD. Zupan et al15,16 showed that ankle torque and maximal voluntary contraction of the tibialis anterior muscles of 12 children with progressive muscular dystrophy increased significantly, when compared with a nonstimulated ankle, by daily application of low-intensity electrical stimulation with a duration of 2 hours over 3 months. The findings of our study are parallel to the studies of Scott et al13,14 and Zupan et al,15,16 which presented the positive effects of electrical stimulation on dystrophic muscle strength. However, the difference in this current study is that it was carried on adult patients with a diagnosis of homogeneous dystrophy with relatively slow progress, including upper extremity applications, using the technique of electric stimulation, and included the long-term results.
In standard percutaneous electrical stimulation applications, it is an important problem that the current required for muscle contraction, generally causes pain and discomfort. This problem is a limitation for electrical stimulation applications in neuromuscular diseases. Therefore, use of low-frequency currents was generally recommended in the previous studies.14 In this study, HVPGS was preferred since high voltage output during application, and twin peak wave form has the advantages of enabling the current to penetrate into deeper tissues without causing pain, decreasing the skin resistance, and so, requiring a lower intensity current for muscle contraction. The HVPGS related literature samples present its positive effects on providing efficient muscle contraction, improving muscle strength, and reducing pain and edema.19,30,31 However, we could not source studies that investigated its effects on muscle strength in neuromuscular diseases. The present study indicates that HVPGS can be used in patients with LGMD in order to improve muscle strength, without causing muscular pain or fatigue problems. On literature review, there is a lack of studies that investigate the impact of muscle strengthening on ADL or functional level in neuromuscular diseases. However, assessment of ADL is very important as the main expectation of the patients with LGMD, and so the main goal of management is the maintenance of functional independence.32 The timed performance and independence level of the groups were similar at the beginning of this study. The improvement of timed performance parameters in the electrical stimulation group may indicate that an increase in the strengths of 2 functionally important muscles effects daily living activities. At the same time, although no significant improvement was found in ADL scores of the subjects according to the intra-group analyses, post-treatment and follow-up values were higher in the electrical stimulation group. This result may be due to the higher muscle strength of the deltoideus muscle both in the post-treatment and follow-up measurements in the electrical stimulation group.
Milner-Brown and Miller,11 used low-resistive weight training and electrical stimulation of the quadriceps muscle in combination in 10 children with muscular dystrophy. They stated that when electrical stimulation and weight training were used in combination, they caused a greater increase in muscle strength, depending on the type of neuromuscular disease. In the electrical stimulation group of this study, exercise therapy was also used in combination, and in addition to muscle strength, upper extremity muscular endurance, and timed performance improved significantly after the treatment, and this effect was found to be maintained or continued increasing, in the follow-up period. The same variables presented also positive but more limited improvements in the exercise therapy group. These findings indicate that a combination of electrical stimulation and exercise therapy may increase the effectiveness of the treatment.
The most important limitation of this study is the low number of subjects. However, involving a homogenous group of patients with the diagnosis of LGMD enabled us to eliminate the methodological limitations of other subject related studies in the literature, which investigated LGMD in combination with other neuromuscular diseases while commenting on the results of treatment programs. The lack of randomization is another limitation of the study. We would also have achieved better results for external validation if we had included an isolated group of LGMD patients like LGMD type 1a.
In conclusion, the results of this study indicate that HVPGS was more effective than moderate intense exercise therapy, in increasing the strength and the contraction capacity of the deltoideus and quadriceps femoris muscles, and improving periodic performance and independence in ADL. As no definitive treatments currently exist for LGMD patients, these results provide important information on the role of exercise therapy and electrical stimulation for clinicians working in rehabilitation. There is a further need for randomized controlled trials that will investigate and compare the effects of electrical stimulation and exercise therapy applications in different types of NMD patients (adult and children).
Acknowledgments
The authors would like to thank Assist. Prof. İlkim Çıtak Karakaya, PT, PhD for her valuable help in translating the manuscript and criticizing the draft.
Footnotes
Disclosure.
References
- 1.Guglieri M, Magri F, D’Angelo MG, Prelle A, Morandi L, Rodolico C, et al. Clinical, molecular, and protein correlations in a large sample of genetically diagnosed Italian limb girdle muscular dystrophy patients. Hum Mutat. 2008;29:258–266. doi: 10.1002/humu.20642. [DOI] [PubMed] [Google Scholar]
- 2.Stübgen JP. Limb girdle muscular dystrophy: an interval study of weakness and functional impairment. J Clin Neuromuscul Dis. 2008;9:333–340. doi: 10.1097/CND.0b013e318163c5ba. [DOI] [PubMed] [Google Scholar]
- 3.Bushby K. Diagnosis and management of the limb girdle muscular dystrophies. Pract Neurol. 2009;9:314–323. doi: 10.1136/jnnp.2009.193938. [DOI] [PubMed] [Google Scholar]
- 4.Krivickas LS. Exercise in neuromuscular disease. J Clin Neuromuscul Dis. 2003;5:29–39. doi: 10.1097/00131402-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Cup EH, Pieterse AJ, Ten Broek-Pastoor JM, Munneke M, van Engelen BG, Hendricks HT, et al. Exercise therapy and other types of physical therapy for patients with neuromuscular diseases: a systematic review. Arch Phys Med Rehabil. 2007;88:1452–1464. doi: 10.1016/j.apmr.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Fowler WM., Jr Role of physical activity and exercise training in neuromuscular diseases. Am J Phys Med Rehabil. 2002;81:S187–S195. doi: 10.1097/01.PHM.0000029726.80774.83. [DOI] [PubMed] [Google Scholar]
- 7.Kilmer DD. Response to aerobic exercise training in humans with neuromuscular disease. Am J Phys Med Rehabil. 2002;81:S148–S150. doi: 10.1097/00002060-200211001-00015. [DOI] [PubMed] [Google Scholar]
- 8.McDonald CM. Physical activity, health impairments, and disability in neuromuscular disease. Am J Phys Med Rehabil. 2002;81:S108–S120. doi: 10.1097/00002060-200211001-00012. [DOI] [PubMed] [Google Scholar]
- 9.Spector SA, Lemmer JT, Koffman BM, Fleisher TA, Feuerstein IM, Hurley BF, et al. Safety and efficacy of strength training in patients with sporadic inclusion body myositis. Muscle Nerve. 1997;20:1242–1248. doi: 10.1002/(sici)1097-4598(199710)20:10<1242::aid-mus6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 10.Aitkens SG, McCrory MA, Kilmer DD, Bernauer EM. Moderate resistance exercise program: its effect in slowly progressive neuromuscular disease. Arch Phys Med Rehabil. 1993;74:711–715. doi: 10.1016/0003-9993(93)90031-5. [DOI] [PubMed] [Google Scholar]
- 11.Milner-Brown HS, Miller RG. Muscle strengthening through high-resistance weight training in patients with neuromuscular disorders. Arch Phys Med Rehabil. 1988;69:14–19. [PubMed] [Google Scholar]
- 12.Lohi EL, Lindberg C, Andersen O. Physical training effects in myasthenia gravis. Arch Phys Med Rehabil. 1993;74:1178–1180. [PubMed] [Google Scholar]
- 13.Scott OM, Vrbová G, Hyde SA, Dubowitz V. Responses of muscles of patients with Duchenne muscular dystrophy to chronic electrical stimulation. J Neurol Neurosurg Psychiatry. 1986;49:1427–1434. doi: 10.1136/jnnp.49.12.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott OM, Hyde SA, Vrbová G, Dubowitz V. Therapeutic possibilities of chronic low frequency electrical stimulation in children with Duchenne muscular dystrophy. J Neurol Sci. 1990;95:171–182. doi: 10.1016/0022-510x(90)90240-n. [DOI] [PubMed] [Google Scholar]
- 15.Zupan A. Long-term electrical stimulation of muscles in children with Duchenne and Becker muscular dystrophy. Muscle Nerve. 1992;15:362–367. doi: 10.1002/mus.880150316. [DOI] [PubMed] [Google Scholar]
- 16.Zupan A, Gregoric M, Valencic V, Vandot S. Effects of electrical stimulation on muscles of children with Duchenne and Becker muscular dystrophy. Neuropediatrics. 1993;24:189–192. doi: 10.1055/s-2008-1071537. [DOI] [PubMed] [Google Scholar]
- 17.Newton R. High-voltage pulsed current: theoretical bases and clinical applications. In: Nelson RM, Currier DP, editors. Clinical electrotherapy. 2nd ed. East Norwalk (CT): Appleton & Lange; 1991. pp. 201–220. [Google Scholar]
- 18.Balogun JA, Onilari OO, Akeju OA, Marzouk DK. High voltage electrical stimulation in the augmentation of muscle strength: effects of pulse frequency. Arch Phys Med Rehabil. 1993;74:910–916. [PubMed] [Google Scholar]
- 19.Morris L, Newton RA. Use of high voltage pulsed galvanic stimulation for patients with levator ani syndrome. Phys Ther. 1987;67:1522–1525. doi: 10.1093/ptj/67.10.1522. [DOI] [PubMed] [Google Scholar]
- 20.Jensen MP, Abresch RT, Carter GT, McDonald CM. Chronic pain in persons with neuromuscular disease. Arch Phys Med Rehabil. 2005;86:1155–1163. doi: 10.1016/j.apmr.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 21.Lue YJ, Chen SS. Strength and functional performance of patients with limb-girdle muscular dystrophy. Kaohsiung J Med Sci. 2000;16:83–90. [PubMed] [Google Scholar]
- 22.Mendell JR, Florence J. Manual muscle testing. Muscle Nerve. 1990;13:S16–S20. doi: 10.1002/mus.880131307. [DOI] [PubMed] [Google Scholar]
- 23.Merlini L, Mazzone ES, Solari A, Morandi L. Reliability of hand-held dynamometry in spinal muscular atrophy. Muscle Nerve. 2002;26:64–70. doi: 10.1002/mus.10166. [DOI] [PubMed] [Google Scholar]
- 24.Allsop KG, Ziter FA. Loss of strength and functional decline in Duchenne's dystrophy. Arch Neurol. 1981;38:406–411. doi: 10.1001/archneur.1981.00510070040004. [DOI] [PubMed] [Google Scholar]
- 25.Rossier P, Wade DT. Validity and reliability comparison of 4 mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil. 2001;82:9–13. doi: 10.1053/apmr.2001.9396. [DOI] [PubMed] [Google Scholar]
- 26.Carter GT. Rehabilitation Management in Neuromuscular Disease. J Neurol Rehabil. 1997;11:69–80. [Google Scholar]
- 27.Kilmer DD, McCrory MA, Wright NC, Rosko RA, Kim HR, Aitkens SG. Hand-held dynamometry reliability in persons with neuropathic weakness. Arch Phys Med Rehabil. 1997;78:1364–1368. doi: 10.1016/s0003-9993(97)90311-7. [DOI] [PubMed] [Google Scholar]
- 28.Bumin G, Ergun A, Uyanık M, Kayıhan H. Sağ ve Sol Hemiplejik Hastalarda Duyu, Algı ve Fonksiyonel Durumun Karşılaştırılması. Fırat University Journal of Health Sciences. 2007;21:221–224. [Google Scholar]
- 29.Dirks ML, Hansen D, Van Assche A, Dendale P, Van Loon LJ. Neuromuscular electrical stimulation prevents muscle wasting in critically ill comatose patients. Clin Sci (Lond) 2015;128:357–365. doi: 10.1042/CS20140447. [DOI] [PubMed] [Google Scholar]
- 30.Citak-Karakaya I, Akbayrak T, Demirtürk F, Ekici G, Bakar Y. Short and long-term results of connective tissue manipulation and combined ultrasound therapy in patients with fibromyalgia. J Manipulative Physiol Ther. 2006;29:524–528. doi: 10.1016/j.jmpt.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Bourguignon GJ, Bourguignon LY. Electric stimulation of protein and DNA synthesis in human fibroblasts. FASEB J. 1987;1:398–402. doi: 10.1096/fasebj.1.5.3678699. [DOI] [PubMed] [Google Scholar]
- 32.Stübgen JP, Lahouter A. Limb girdle muscular dystrophy: weakness and disease duration as predictors of functional impairment. Muscle Nerve. 1994;17:873–880. doi: 10.1002/mus.880170806. [DOI] [PubMed] [Google Scholar]


