Abstract
Monoamine uptake inhibitors are common treatments for depression; however, the therapeutic efficacy of these drugs varies widely. Two factors that are commonly linked to clinical outcome are age and serotonin transporter (SERT) genotype. Mouse models provide powerful tools to study consequences of age and genotype on antidepressant-like efficacy, however, to date systematic studies of this nature are lacking. Here we used the tail suspension test (TST), a preclinical assay for antidepressant efficacy, to gain insight into age- and SERT genotype-dependency of immobility time in the TST under control conditions (saline injection) and in response to the tricyclic antidepressant, desipramine (DMI). Immobility after saline injection in juvenile, adolescent, adult, mature adult, and middle-aged mice (postnatal day 21, 28, 90, 210, and 300 respectively) significantly increased with age; however, the rate of increase was slower for SERT null (−/−) mice than for wild type (+/+) or heterozygote (+/−) mice. DMI reduced immobility across ages and SERT genotypes. Middle-aged, but not adult, SERT−/− mice were significantly more sensitive to desipramine than age matched SERT+/+ or SERT+/− mice. DMI was less potent in middle-aged SERT+/+ and SERT+/− mice than in adult SERT+/+ or SERT+/− mice. Regardless of age, DMI's maximal effects were greater in SERT−/− mice than in SERT+/+ or SERT+/− mice. These results show that immobility time in the TST varies as a function of age and SERT genotype, underscoring the utility of the TST as a potential model to examine age- and genotype-dependent influences on antidepressant response.
Keywords: tail suspension test, serotonin transporter, desipramine, juvenile, adolescent, antidepressant, middle-age, efficacy, tricyclic, basal immobility
INTRODUCTION
Depression is a debilitating affective disorder for which most patients are not effectively treated. The most commonly prescribed antidepressants are monoamine uptake inhibitors, which act to increase extracellular levels of serotonin (5-HT), norepinephrine (NE) and/or dopamine (DA) by blocking the high-affinity transporters for these neurotransmitters, the 5-HT, NE and DA transporters (SERT [Slc6a4], NET [Slc6a2] and DAT [Slc6a3]) respectively. While monoamine uptake inhibitors are moderately effective in reducing depressive symptoms, they leave the majority of patients without full recovery (Sinyor et al., 2010; Kirsch et al., 2008). Additionally, these drugs can be less effective in certain subpopulations. For example, SERT blockers can be less effective in individuals carrying low expressing variants of SERT (Serritti et al., 2007) and there is some evidence that tricyclics may be less effective in treating geriatric patients (Kok et al., 2012). To date there is a paucity of preclinical studies investigating the efficacy of antidepressant drugs across age and as a function of SERT genotype. Such studies may help to provide mechanistic insight into variability in response to antidepressants among individuals. Here we investigate age- and SERT-genotype dependency of activity in the tail suspension test (TST), a common preclinical screen used to assess the antidepressant-like effects of clinically approved and experimental drugs.
The TST has been used to investigate antidepressant-like effects of drugs in adult mice, and shows predictive validity for drugs with antidepressant-like properties (for review see [Cryan et al., 2005]). Previous studies have, however, often ignored the specific age of the adult mouse. To date, there are no published studies that directly compare activity in the TST across different stages of adulthood. This fact is surprising as aged mice show less hippocampal neurogenesis, argued to be necessary for antidepressants’ therapeutic effect, than young adults (Cowen et al., 2008; Couillard-Despres et al., 2009). Experiments here test the hypothesis that the antidepressant-like activity of the tricyclic, desipramine (DMI), will be reduced in middle-aged mice compared with young adult mice. DMI is a norepinephrine transporter (NET) blocker. We selected DMI for these studies as it is a commonly used reference antidepressant and has become a standard for evaluating variation in antidepressant-like response among rodent strains and age groups (Lucki et al., 2000; Reed et al., 2007; 2008; Mitchell et al., 2013).
Studies using the TST to examine behavior of mice that lack SERT (SERT−/−) or have reduced SERT expression (SERT+/−) have been of great interest due to clinical observations linking SERT genotype to antidepressant response. Results support the idea that the TST can be used to reveal SERT genotype-dependent differences in response to antidepressants in adults (Holmes et al., 2002; for review see [Fox et al., 2007]), but studies examining age- and dose-dependency of sensitivity to antidepressant drugs among SERT genotypes are lacking. Here we test the hypotheses that basal immobility time in the TST will vary as a function of age and SERT genotype and that the antidepressant-like response to DMI in young and middle-aged adults will be SERT genotype-dependent. Our results show that age, desipramine dose and SERT genotype are all critical factors to consider when interpreting TST data.
MATERIALS and METHODS
Animals
Male SERT wild-type (SERT+/+), heterozygote (SERT+/−) or homozygote knockout (SERT−/−) mice (backcrossed to C57BL/6J for >10 generations) were used for all experiments. Only males were used, because previously we found no sex related differences in the effects of age on basal activity in the tail suspension test (TST) (Mitchell et al., 2013). Mice were obtained from our in-house breeding colony. Original breeding pairs were provided by Dr. Dennis Murphy (National Institute of Mental Health), and were generated as previously described (Bengel et al., 1998). In mice, P60 is generally considered young adult, P90 – P180 mature adult, P300 – P450 middle aged adult and P540 and older, senescent (Spear, 2000; Harrison, 2011; Allen and Cavanaugh, 2014). The first set of experiments, designed to assess immobility time as a function of age under vehicle conditions (saline injection), were conducted when mice were either post-partum day 21 (P21, juvenile, n = 10 SERT+/+, 10 SERT+/−, 10 SERT−/−), P28 (adolescent, n = 10 SERT+/+, 9 SERT+/−, 10 SERT−/−), P90 (adult, n = 11 SERT+/+, 11 SERT+/−, 11 SERT−/−), P210 (mature adult [comprising mice aged P180-P240], n = 10 SERT+/+, 10 SERT+/−, 10 SERT−/−), and P300 (middle-aged [comprising mice aged P240-P360], n = 7 SERT+/+, 10 SERT+/−, 10 SERT−/−). To verify no behavioral differences in basal immobility existed within the P300 group, we calculated Pearson's correlation coefficients for postnatal age and immobility in SERT+/+, SERT+/−, and SERT−/− mice. The correlation coefficients ranged from −0.07 to 0.28, and none of them reached statistical significance (P = 0.54, 0.85, and 0.74 for SERT+/+, SERT+/−, and SERT−/− respectively). Thus, the age range used to define P300 was deemed appropriate for this study.
The second set of experiments assessed if age-dependent variation in basal immobility would alter the response to the norepinephrine transporter (NET) blocker, desipramine (DMI) in the TST. Experiments were carried out in mice aged P90 (adult, n = 41 SERT+/+, 36 SERT+/−, 46 SERT−/−) and P300 (middle-aged [comprising mice aged P240-P360], n = 40 SERT+/+, 45 SERT+/−, 51 SERT−/−).
Animals were housed in a temperature-controlled (24°C) vivarium maintained on a 12/12-hr light/dark cycle (lights on at 7:00 am) in plastic cages (29cm × 18cm × 13 cm) containing rodent bedding (Sani-chips, Harlan Teklad, Madison, WI, USA) with free access to food (Rodent sterilizable diet, Harlan Teklad, Madison, WI, USA) and water. After weaning on P28, mice were housed in groups of five with same-sex peers. TST experiments were conducted prior to weaning in mice aged P21 and P28. Because the influence of weaning time on behavior is an important factor to consider, we used the same weaning protocol described in the only other published study investigating P21 and P28 mice in the TST (Mitchell et al., 2013). All procedures were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council 1996), and with the approval of the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio.
Tail suspension test
The tail suspension test (TST) was conducted as originally described by Steru et al. (1985) (for review see [Castagne et al., 2011]). All animals were behaviorally naïve before testing. Mice were moved from the colony room to the testing room and allowed a 1-2 hour acclimation period. All experiments were conducted between 8:00 am and 5:00 pm. The majority of experiments were conducted between 12:00 noon and 5:00 pm. Immobility of animals tested between 08:00 am - 12:00 noon did not differ systematically from animals tested in the afternoon. In the first set of experiments all mice received saline vehicle intraperitoneally (ip) one hour prior to testing, followed thirty minutes later by another ip injection of saline vehicle (control conditions). In the second set of experiments, mice received saline vehicle (ip) one hour prior to testing, followed thirty minutes later by ip injection of either saline or desipramine (DMI). This drug administration protocol was selected to be consistent with our previously published procedure (Baganz et al., 2008; Horton et al., 2013). In addition, ip administration of antidepressant 30 minutes prior to test is the standard (Steru et al., 1985; Holmes et al., 2002; Nipoll et al., 2003). This injection schedule produces dose-related effects of desipramine in the TST that can be compared directly with previously reported results. Immediately before testing, the distal portion of the tail was fastened to a flat aluminum (2 × 0.3 × 10 cm) bar using adhesive tape placed at a 90° angle to the longitudinal axis of the mouse tail, with 3-4 cm between the base of the mouse tail and the aluminum bar. A hole opposite the taped end of the bar was used to secure the bar to a hook on the ceiling of a visually isolated white box (40 × 40 × 40 cm). Each mouse was suspended by its tail for 6 minutes (min), allowing the ventral surface and the front and hind limbs to be recorded using a digital video camera facing the testing box. Total immobility time was measured (in seconds (s)) during the 6 min time period. Immobility was defined as the absence of initiated movements, and included passive swaying of the body. A mouse was excluded from the study if it climbed and held its tail or the aluminum bar for a period of 3 s or longer. Immobility was scored twice from the videos by observers who were blind to the treatment. Each mouse was tested once only and all treatments were randomly assigned.
Drugs
DMI hydrochloride [Sigma-Aldrich (St. Louis, MO, USA)] was dissolved in physiological saline and injected ip at doses expressed as salt weight per kilogram body weight. The injection volume was 10 ml/kg.
Data analysis
Statistical analyses were performed using Prism 6.0 (GraphPad, San Diego, CA, USA) and NCSS 2007 (Kaysville, UT, USA). TST and body weight data were analyzed using a two-factor ANOVA (age, genotype) followed by Tukey's and Dunnett's multiple comparisons tests. Additionally, P300 SERT−/− TST data were analyzed separately to include 1 mg/kg dose of desipramine using a one-factor ANOVA followed by Dunnett's multiple comparisons test. Figures 1 and 2 show data from two independent experiments. Values for table 1 were derived from data shown in figure 2.c,d. TST data were expressed in seconds (Figure 1.a, 2.a,b), as percent control (Figure 2.c,d, Table 1). All data are expressed as mean ± standard error of the mean (SEM). P < 0.05 was considered statistically significant.
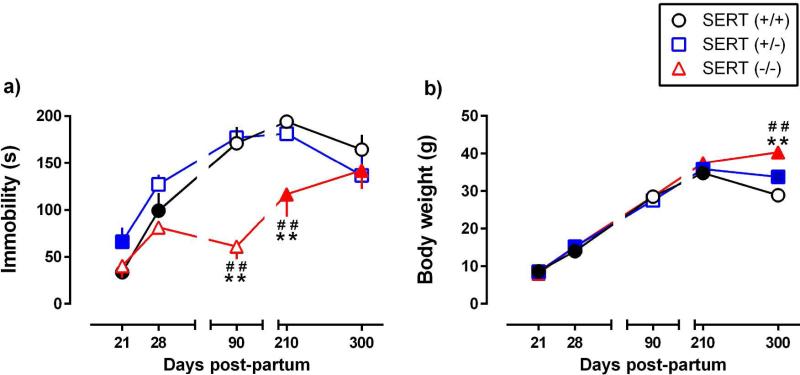
Figure 1.
Basal immobility time in the TST and body weight as a function of age. a) Immobility time in the TST after vehicle treatment in SERT mice aged P21, P28, P90, P210, or P300. Each mouse was tested only once. b) Body weights of mice used to generate data in figure 1.a. Data are expressed as mean ± SEM. Filled symbols indicate significant difference from P90 within each genotype; Dunnett's post hoc multiple comparisons test after a two-factor ANOVA. ** P < 0.01 SERT−/− vs SERT+/+ mice; # # P < 0.01 SERT−/− vs SERT+/− mice, within age group; Tukey's post hoc multiple comparisons test after a two-factor ANOVA. n = 7-12 per group. Where the error bars are not visible they are smaller than the symbol.
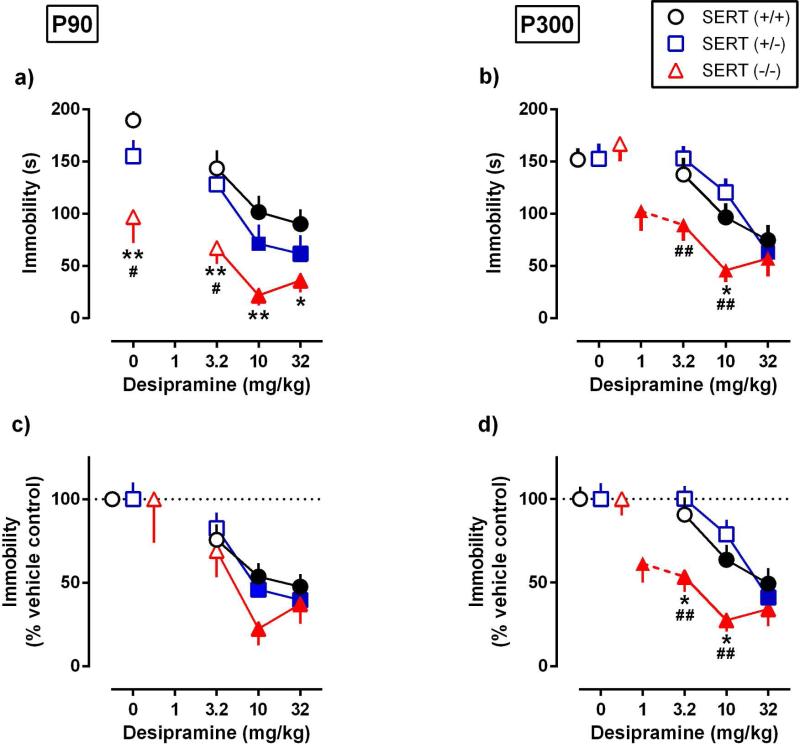
Figure 2.
Effects of acutely administered desipramine (DMI) on immobility time in the TST among SERT genotypes aged P90 and P300. a) P90 mice and b) P300 mice showed dose-dependent DMI-induced reductions in immobility time (s). c) Data from figure 2.a expressed as percent change from vehicle control. d) Data from figure 2.b expressed as percent change from vehicle control. Data are mean ± SEM; for each genotype; filled symbols indicate significant difference from vehicle control; Dunnett's post hoc multiple comparisons test after a two-factor ANOVA (excluding 1 mg/kg dose in figure 2.b,d). Data for SERT−/− mice in figure 2.b,d were further analyzed by one-factor ANOVA, followed by Dunnett's post hoc multiple comparisons test so as to include 1 mg/kg (represented with dashed line). * P < 0.05, ** P < 0.01 SERT−/− vs SERT+/+ mice; # P < 0.05, # # P < 0.01 SERT−/− vs SERT+/− mice; Tukey's post hoc multiple comparisons test; n = 8-12 per group. Where a symbol is not visible it is superimposed with that of another genotype.
Table 1.
Potency and maximal effect of DMI to induce antidepressant-like activity as a function of age and SERT genotype.
| Age | SERT | ED50 (mg/kg) | Maximal effect (% control) |
|---|---|---|---|
| P90 | +/+ | 11.0 | 47.6 ± 7.6 |
| +/− | 10.9 | 39.6 ± 11.6 | |
| −/− | 4.7 | 22.4 ± 9.8^ | |
| P300 | +/+ | 22.7 | 49.3 ± 9.6 |
| +/− | 29.2# | 41.2 ± 10.3 | |
| −/− | 2.8* | 27.5 ± 6.8^ |
Potency (ED50) and maximal effect of acutely administered desipramine to decrease immobility time in the TST among SERT genotypes aged P90 and P300. Data are mean ± SEM; Values calculated from data shown in figure 2.c,d
P < 0.01, different from +/+ and +/− within age
P < 0.01, different from P90 within genotype; F ratio test to compare intercepts
P < 0.05, different from +/+ (data collapsed across age groups); Tukey's post hoc multiple comparisons test; n = 8-12 per group.
To calculate the doses needed to reduce immobility by 50% (ED50), TST data were expressed as percent from vehicle control, and the linear portion of the dose-response curves were analyzed by log-linear regression of data from individual subjects, with the following equation: effect = slope X log(dose) + intercept, using methods detailed elsewhere (Koek et al., 2009). F ratio tests in GraphPad Prism were used to compare dose-response curves with respect to their slope and intercepts. For example, a non-significant F ratio for slopes and a significant F ratio for intercepts show that dose-response curves are parallel but occupy different positions on the dose axis. Maximal effects were defined as the greatest observed reduction in immobility. Data were analyzed using a two-factor ANOVA (age, genotype) followed by Tukey's multiple comparisons test.
RESULTS
Immobility time in the TST varies with age and SERT genotype
Immobility time under baseline conditions in the TST is known to vary as a function of both age (Mitchell et al., 2013) and mouse strain (Holmes et al., 2002). Here we investigated the effect of SERT genotype and age on immobility time in the TST.
Basal immobility time (s) was measured in SERT+/+, SERT+/− and SERT−/− mice aged P21, P28, P90, P210, and P300. Significant effects of age [F(4,133) = 28.28, P < 0.0001] and genotype [F(2,133) = 16.08, P < 0.0001] were revealed, with a significant interaction between age and genotype [F(8,133) = 3.74, P < 0.001]. Baseline immobility time increased with age in all SERT genotypes, however the rate at which immobility time increased with age was slower in SERT−/− mice (Figure 1.a). Among genotype comparisons showed significantly lower immobility times in SERT−/− mice than in SERT+/+ and SERT+/− mice at P90 and P210 (P < 0.001). Within genotype comparisons showed that P21 and P28 were significantly different from P90 SERT+/+ mice (P < 0.0001; P = 0.003). In SERT+/− mice, P21, but not P28, were significantly different from P90 (P < 0.01; P = 0.073). In SERT−/− mice, P210 and P300, but not P21 or P28, were significantly different from P90 (P = 0.03; P = 0.001).
Differences in body weight did not account for lower immobility times in SERT−/−mice aged P90 and P210. As expected, body weight increased with age [F(4,133) = 506.0, P < 0.0001]. Body weight also differed as a function of genotype [F(2,133) = 12.43, P < 0.0001], with a significant interaction between age and genotype [F(8,133) = 6.39, P < 0.0001]; however, post-hoc analyses showed no statistically significant body weight differences among SERT genotypes until P300 (P < 0.001) (Figure 1.b), when time spent immobile in the TST did not differ significantly among genotypes (Figure 1.a).
Ability of DMI to reduce immobility time is age- and SERT genotype-dependent
Stage of adulthood appears to be an important factor in determining immobility time in the TST under vehicle (saline injection) conditions for SERT−/− mice (Figure 1.a). This raises the possibility that stage of adulthood also influences response to drugs in the TST and in a manner that may be SERT genotype-dependent. We investigated this possibility using desipramine (DMI), a norepinephrine transporter (NET) blocker, as a reference antidepressant and measured its ability to reduce immobility time in all SERT genotypes aged either P90 (adult) or P300 (middle-aged). Note that DMI produces sedative effects at high doses (Tucker and File, 1986). For this reason, 32 mg/kg is generally the highest concentration used when evaluating antidepressant-like response with the TST (Cryan et al., 2005) and was the highest dose used in these studies.
In P90 mice, DMI reduced immobility time [main effect treatment: F(3,111) = 20.33, P < 0.0001], with the greatest effect in SERT−/− mice [main effect genotype: F(2,111) = 25.05, P < 0.0001] (Figure 2.a). No significant interaction between treatment and genotype was found [F(6,111) = 0.41, P = 0.87). Among genotype comparisons showed that SERT−/− mice spent less time immobile after saline injection than did SERT+/+ or SERT+/− mice (consistent with results shown in Figure 1.a). SERT−/− mice also spent less time immobile than SERT+/+ or SERT+/− mice after 3.2 mg/kg DMI. Additionally, SERT−/− mice spent less time immobile than SERT+/+ mice after 10 and 32 mg/kg DMI. Within genotype comparisons showed 10 mg/kg to be the lowest effective dose to significantly reduce immobility time compared with saline-injected counterparts in all P90 SERT genotype mice. As well, 32 mg/kg DMI produced a significant reduction of immobility compared with gene matched controls in all SERT genotype mice.
In P300 mice, DMI also reduced immobility time [main effect treatment: F(3,114) = 24.33, P < 0.0001], in a SERT genotype-dependent manner [main effect genotype: F(2,114) = 5.61, P < 0.05] (Figure 2.b). In contrast to P90 mice, there was a significant treatment × genotype interaction [F(6,114) = 2.52, P = 0.025]. Among genotype comparisons showed that SERT−/− mice spent less time immobile after 3.2 and 10 mg/kg DMI than did SERT+/− mice. SERT−/− mice also spent less time immobile after 10 mg/kg DMI than did SERT+/+ mice. Within genotype comparisons showed 10 mg/kg DMI to be the lowest dose to significantly reduce immobility time in SERT+/+ and SERT+/− genotypes. In SERT−/− mice 3.2 mg/kg DMI also significantly reduced immobility time. In an effort to identify a dose of DMI that is ineffective in reducing immobility time in SERT−/− mice we tested these mice with a dose of 1 mg/kg DMI. However, this dose also significantly reduced immobility time in SERT−/− mice (immobility time 166.8s ± 16.54s compared to 102.5s ± 19.08s following saline vs DMI 1 mg/kg respectively (40% reduction in immobility), P < 0.05 with Dunnett's post-test).
To account for variation in immobility time under control (saline-injection) conditions among the SERT genotypes, data were analyzed as a percent of immobility time in control mice (Figures 2.c and 2d). When analyzed as a percent of vehicle control, DMI reduced immobility time in P90 mice [main effect treatment: F(3,111) = 14.95, P > 0.0001]; however, this effect was no longer genotype dependent (Figure 2.c). This finding is consistent with lower immobility time (s) in SERT−/− mice under vehicle conditions driving the significantly lower immobility time following DMI in SERT−/− mice compared with SERT+/+ and SERT+/− mice (compare Figures 2.a and 2.c).
As expected, given that immobility time under saline-injected control conditions does not differ among SERT genotypes aged P300, in these mice DMI reduced immobility time [main effect: F(3,114) = 24.07, P < 0.0001], in a SERT genotype-dependent manner [main effect genotype: F(2,114) = 9.38, P < 0.001] (Figure 2.d). Once again in P300 mice, there was a significant treatment × genotype interaction [F(6,114) = 2.27, P = 0.0418] (compare Figures 2.b and 2.d).
Doses of DMI needed to decrease immobility by 50% (ED50) and maximal effects are summarized as a function of age and genotype in Table 1. P90 dose-response data, after 3.2 mg/kg and 10 mg/kg DMI administration, and P300 dose-response data, after 3.2 mg/kg, 10 mg/kg and 32 mg/kg DMI administration, were analyzed by log-linear regression. All six dose-response curves did not deviate from linearity (replicates test, P = 0.6), had a common slope (F(5,140) = 1.0, P = 0.4), but not a common intercept (F(5,146) = 10.8, P < 0.0001). Comparisons of intercepts were used to analyze age- and genotype-dependent potency differences. Not surprisingly, no significant effect of genotype on potency was found in P90 mice (F(2,56) = 1.8, P = 0.18). In P300 mice, potency significantly varied among genotypes (F(2,90) = 27.37, P = 0.0001). DMI was significantly less potent in SERT+/+ and SERT+/− mice than in SERT−/− mice (F(1,58) = 35.29, P < 0.0001; F(1,61) = 56.99, P < 0.0001). Within genotype comparisons showed significant potency differences for DMI at P90 and P300 in SERT+/− mice (F(1,48) = 5.7, P = 0.02), but not in SERT+/+ and SERT−/− mice (F(1,47) = 3.5, P = 0.07; F(1,51) = 1.2, 5.7 0.28). These data show the occurrence of age-dependent changes in sensitivity of mice to DMI within the adult period.
The maximal effect of DMI to reduce immobility increased in a SERT genotype-dependent manner [F(2,55) = 3.2, P = 0.049], and did not show significant effects of age [F(1,55) = 0.1, P = 0.7], nor a significant SERT genotype and age interaction [F(2,55) = 0.2, P = 0.9]. Among genotype comparisons found that the maximal effect of DMI was significantly greater in SERT−/− mice compared with SERT+/+ mice (P = 0.04). Within genotype comparisons showed no significant differences in maximal effects between P90 and P300. Genotype, but not age, influenced the maximal effect of DMI on immobility time in the TST.
DISCUSSION
Immobility time in the TST, under control conditions increases with age; however, the rate of increase is slower in SERT−/− mice compared with SERT+/+ and SERT+/− mice (Figure 1.a). The divergence in basal immobility time between SERT−/− mice and their wild-type and heterozygote counterparts was evident only during certain stages of adulthood. In addition, stage of adulthood, and SERT genotype, were important determinants for the amount of time spent immobile following administration of the NET blocker DMI. Dose-response analysis showed that P300 SERT−/− mice (i.e. middle-aged), but not P90 adult SERT−/− mice, were significantly more sensitive to DMI than age matched SERT+/+ or SERT+/− mice (Figures 2.a,b). Increasing age during adulthood decreased the potency (ED50) of DMI to reduce immobility time in SERT+/+ and SERT+/− mice and increased potency in SERT−/− mice (Table 1). Regardless of adult age, maximal effects of DMI on immobility time in the TST were greater in SERT−/− mice compared with SERT+/+ or SERT+/− mice (Table 1).
In rodents, P64 and older is considered adult (Bylund & Reed, 2007; Spear, 2000), and behavior is thought to remain stable through adulthood (Harris, 2011). Consistent with this notion, we found that immobility time in the TST under vehicle conditions is similar during the adult period (ranging P90 through P300) in C57BL/6 SERT+/+ and SERT+/− mice. In contrast, immobility time increased approximately 2-fold between P90 and P300 in SERT−/− mice. Body weight is unlikely to account for differences in immobility time among genotypes, since at ages where SERT−/− mice were less immobile than their SERT+/+ and SERT+/− counterparts (i.e. P90 and P210), body weight did not differ among genotypes. Consistent with previous reports (Murphy & Lesch, 2008), SERT−/− mice weighed significantly more than SERT+/+ and SERT+/−mice at P300, however, at this age immobility time did not differ among genotypes. Our findings suggest that behavior, at least in the TST, may vary markedly across adulthood for certain genotypes.
This study is the first aimed specifically at investigating SERT genotype-dependent variability in basal immobility time in the TST across a wide range of ages, including ages younger (i.e., juvenile) and older (i.e., middle-aged) than those previously reported. Holmes and co-workers (2002) found no consistent difference in basal immobility time among SERT genotypes bred on a C57BL/6 background. This study used two cohorts of mice. One group was five months or older, and the other P84-112, but the precise age of the animals at the time of testing was not indicated. Our data suggest that one parsimonious explanation for the inconsistent phenotype described by Holmes and colleagues was the age of the mice at the time of testing. Supporting this idea, and consistent with our findings, the only occasion where SERT−/−mice spent less time immobile than SERT+/+ and SERT+/− mice was in the P84-112 cohort (Holmes et al., 2002). Interestingly, in mice bred on a 129S6 background, SERT−/− mice had lower immobility times compared to SERT+/+ and SERT+/− mice across a range of adult ages (likely spanning P84 to P210, based on information provided in these publications) (Holmes et al., 2002; Lira et al., 2003), suggesting that the 129S6 background may be resilient to age-dependent shifts in basal immobility time throughout adulthood. This finding is perhaps not surprising given elegant studies by Lucki and colleagues, showing that responsiveness to the antidepressant, citalopram in the TST is highly dependent on genetic background of the mouse (Crowley et al., 2005).
In addition to age-dependent differences in basal immobility time among SERT genotypes, immobility time following antidepressants can also vary in a SERT genotype-dependent manner. For example, using adult C57BL/6 mice Holmes and colleagues found that DMI (20 mg/kg), decreased immobility in all SERT genotypes but with the largest effect observed in SERT−/− mice (Holmes et al., 2002). We extend these findings to determine the dose-response relationship for the anti-immobility effect of DMI among SERT genotypes aged P90 or P300. We found the anti-immobility effects of DMI to be greater in P300 SERT−/− mice compared with SERT+/+ or SERT+/−mice (Figure 2.b); however, no genotype-dependent effect was found in P90 mice. Age-dependent increases in sensitivity to DMI in SERT−/− mice could be driven by greater expression or function of NET compared with SERT+/+ and SERT+/− mice. To date, there is no evidence that genetic inactivation of SERT influences NET expression in adult mice, at least in CA3 region of hippocampus (Montañez et al., 2003). We cannot rule out the possibility that NET expression and/or function may be increased in other brain regions and/or at different stages of adulthood. Montañez and colleagues did not report the age of mice used in their studies of NET expression, so it is difficult to make conclusions relevant to the present findings. Future studies of NET expression and function throughout adulthood will be informative.
Of potential clinical significance are changes in potency of DMI to produce antidepressant-like effects during adulthood. In SERT+/+ and SERT+/− mice aged P300, ED50 values increased 2.1- and 2.7-fold, respectively, relative to mice aged P90. In contrast, the ED50 value decreased 1.7-fold in SERT−/− mice aged P300, relative to those aged P90. Within genotype, Emax values did not differ between P90 and P300. However, Emax was greater in SERT−/− mice compared to SERT+/+ mice, and though not significant, Emax values for SERT+/− mice tended to be intermediate between those for SERT+/+ and SERT−/− mice. These data suggest that stage of adulthood as well as SERT genotype could influence the ability of antidepressants to produce therapeutic effects in humans.
It is worth noting some potential caveats to these studies. Though the TST is a reliable screening test for antidepressants that has good predictive validity because it detects the majority of clinically-used antidepressants (for review, see Cryan et al., 2005), the TST also has limitations. For example, drug effects can vary in a mouse strain-dependent manner (Crowley et al., 2005). Likewise strain-dependent differences in basal immobility have been reported (Holmes et al., 2002). Further, antidepressants are active in the TST when administered acutely, whereas chronic treatment is usually required for clinical recovery in humans. Thus, the TST as commonly used appears to be unable to discriminate potentially rapid-acting from currently available antidepressants. Despite its limitations, the TST is generally considered a useful test to assess potential antidepressant activity.
Conceivably, differences in basal immobility in the TST could be related to differences in locomotor activity. However, the genotype- and age-dependent differences in basal immobility reported here do not appear to co-vary with previously reported genotype- and age-dependent differences in locomotion. For example, SERT−/− mice (C57BL/6 background), show no difference in locomotor activity compared with SERT+/+ mice (Bengel et al., 1998). In contrast, basal immobility in the TST was lower in P90 and P210 SERT−/− mice than in SERT+/+ mice (Figure 1.a). Concerning age-related effects, adolescent mice show less basal immobility in the TST than adults (present study) but also less locomotor activity in a novel environment than adult mice (Koek et al., 2011; Koek, 2013). It is also worth noting that DMI is known to exert antidepressant-like effects without increasing general activity in adult mice (e.g. Lew et al., 1971), therefore it is unlikely that antidepressant-like effects of DMI reported here were related to drug-induced increases in locomotor activity.
Few clinical studies have examined variation in antidepressant response during adulthood. One study reported subtle differences in response to combination psychotherapy and tricyclic medication between patients of mean age 38.5 years and those aged 67.9 years (Reynolds et al., 1996). These differences are difficult to interpret given that the older patients were treated with nortriptyline and the younger adults with imipramine; nor were patients’ genotypes known. Our data suggest further clinical investigation of SERT genotype and drug interaction may be warranted.
Clinical evaluation of age related variation in drug potency is challenging. Physicians often prescribe lower drug doses as patients age through mid-life and enter the geriatric period (65 years and up) to minimize side effects and drug-drug interactions (Dolder et al., 2010). That said, the refractory period for onset of antidepressant therapeutic effects in older adults is greater than younger adults (Kok et al., 2012). A greater refractory period may suggest lower antidepressant potency. Here we found that the potency of DMI to produce antidepressant-like effects decreases with age in SERT+/+ and SERT+/− mice. Future investigation may help determine which therapies are most effective across different stages of adulthood.
The current study shows distinct age-sensitive behavioral phenotypes in the TST, which are most prominent in SERT−/− mice. These data offer a possible explanation for discrepancies in the literature regarding behavioral responses of “adult” SERT−/− mice. These data also underscore the importance of dose-response analysis to capture shifts in potency and maximal effect of drugs among genotypes and ages. The present study encourages closer attention to age as a factor when studying “adult” mice as well as to the study of response to antidepressant drugs. Future studies of this nature may help to uncover age- and genotype-dependent influences on therapeutic response to antidepressant drugs.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the technical assistance of Ms. Melissa Vitela. Supported by NIH grants MH064489 (LCD), MH093320 (LCD & WK), MH106978 (LCD).
Footnotes
Conflict of interest statement: The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions: NCM performed experiments; NCM, WK & LCD designed experiments, analyzed data and wrote manuscript.
REFERENCES
- Allen EN, Cavanaugh JE. Loss of motor coordination in an aging mouse model. Behav. Brain Res. 2014;267:119–125. doi: 10.1016/j.bbr.2014.03.032. [DOI] [PubMed] [Google Scholar]
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2008;105:18976–18981. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mosser R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstacy”) in serotonin transporter-deficient mice. Mol. Pharmacol. 53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Altamirano AV, Jones DJ, Sanchez TA, Gould GG, Pardon MC, Morilak DA, Frazer A. Regulation of the norepinephrine tranporter by chonic administration of antidepressants. Biol. Psychiatry. 2004;55:313–316. doi: 10.1016/s0006-3223(03)00676-0. [DOI] [PubMed] [Google Scholar]
- Bourin M, Colombel MC, Redrobes JP, Nizard J, Hascoet M, Baker GB. Evaluation of efficacies of different classes of antidepressants in the forced swimming test in mice at different ages. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1998;22:343–51. doi: 10.1016/s0278-5846(98)00009-8. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Reed AL. Childhood and adolescent depression: why do children and adults respond differently to antidepressant drugs? Neurochem. Int. 2007;51:246–253. doi: 10.1016/j.neuint.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Harir AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am. J. Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braitherwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castagne V, Moser P, Roux S, Porsolt RD. Curr. Protoc. Neurosci. Wiley Online Library; 2011. Rodent models of depression: forced swim and tail suspension behavioral despair test in rats and mice. Chapter 8 unit 8.10A. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Wuertinger C, Kandasamy M, Caioni M, Stadler K, Aigner R, Bogdahn U, Aigner L. Ageing abolishes the effects of fluoxetine on neurogenesis. Mol. Psychiatry. 2009;14:856–864. doi: 10.1038/mp.2008.147. [DOI] [PubMed] [Google Scholar]
- Cowen DS, Takase LF, Fornal CA, Jacobs BL. Age-dependent decline in hippocampal neurogenesis is not altered by chronic treatment with fluoxetine. Brain Res. 2008;1228:14–19. doi: 10.1016/j.brainres.2008.06.059. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacol. (Berl.) 2005;183:257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout V. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Dolder C, Nelson M, Stump A. Pharmacological and clinical profile of newer antidepressants: implications for the treatment of elderly patients. Drugs Aging. 2010;27:625–640. doi: 10.2165/11537140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Fox MA, Andrews AM, Wendland JR, Lesch KP, Holmes A, Murphy DL. A pharmacological analysis of mice with a target disruption of the serotonin transporter. Psychopharmacol. (Berl) 195:147–166. doi: 10.1007/s00213-007-0910-0. [DOI] [PubMed] [Google Scholar]
- Haesich B, Bönisch H. Depression and antidepressants: insight from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol. Ther. 2011;129:352–368. doi: 10.1016/j.pharmthera.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Harrison HE. Life span as a biomarker. 2011 Available from: http://research.jax.org/faculty/harrison/ger1vLifespan1.html. [6 January 2015]
- Hetrick SE, Merry SN, McKenzie J, Sindahl P, Proctor M. The Cochrane Collaboration. John Wiley & Sons, Ltd.; 2009. Selective serotonin reuptake inhibitors (SSRIs) for depressive disorders in children and adolescents (Review). pp. 1–91. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuorpsychopharmacol. 2002;27:914–923. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- Horton RE, Apple DM, Owens WA, Baganz NL, Cano S, Mitchell NC, Vitela M, Gould GG, Koek W, Daws LC. Decynium-22 enhances SSRI-induced antidepressant-like effects in mice: uncovering novel targets to treat depression. J. Neurosci. 2013;33:10534–10543. doi: 10.1523/JNEUROSCI.5687-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keers R, Uher R, Huezo-Diaz P, Smith R, Jaffee S, Rietschel M, Henigsberg N, Kozel D, Mors O, Maier W, Zobel A, Hauser J, Souery D, Placentino A, Larsen ER, Dmitrzak-Weglarz M, Gupta B, Hoda F, Craig I, McGuffin P, Farmer AE, Aitchson KJ. Interaction between serotonin transporter gene variants and life events predicts response to antidepressant in the GENDEP project. Pharmacogenomics J. 2011;11:138–145. doi: 10.1038/tpj.2010.14. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: A meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;358:260–268. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W. Effect of repeated exposure to morphine in adolescent and adult male C57BL/6J mice: age-dependent differences in locomotor stimulation, sensitization, and body weight loss. Psychopharmacol. 2013;231:1517–1529. doi: 10.1007/s00213-013-3298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A, France CP. Behavioral effects of gamma-hydroxybutyrate, its precursor gamma-butyrolactone, and GABAB Receptor agonists: Time course and differential antagonism by the GABAB receptor antagonist 3-aminopropyl(diethoxymethyl)phosphinic acid (CGP35348). J. Pharmacol. Exp. Ther. 2009;330:876–883. doi: 10.1124/jpet.109.151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, France CP, Javors MA. Morphine-induced motor stimulation, motor incoordination, and hypothermia in adolescent and adult mice. Psychopharmacol. 2011;219:1027–1037. doi: 10.1007/s00213-011-2432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok RM, Nolen WA, Heeren TJ. Efficacy of treatment in older depressed patients: a systematic review and meta-analysis of double-blind randomized controlled trials with antidepressants. J. Affect Disord. 2012;141:103–115. doi: 10.1016/j.jad.2012.02.036. [DOI] [PubMed] [Google Scholar]
- Lew C, Iversen SD, Iversen LL. Effects of imipramine, desipramine and monoamine oxidase inhibitors on the metabolism and psychomotor stimulant actions of d-amphetamine in mice. Eur. J. Pharmacol. 1971;14:351–359. doi: 10.1016/0014-2999(71)90189-0. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, Kung HF, Hofer MA, Hen R, Gingrich JA. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol. Psychiatry. 54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Mason SS, Baker KB, Davis KW, Pogorelov VM, Malbari MM, Ritter R, Wray SP, Gergardt B, Lanthorn LH, Savelieva KV. Differential sensitivity to SSRI and tricyclic antidepressants in juvenile and adult mice of three strains. Eur. J. Pharmacol. 2009;602:306–315. doi: 10.1016/j.ejphar.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Mitchell NC, Gould GG, Smolik CM, Koek W, Daws LC. Anitdepressant-like drug effects in juvenile and adolescent mice in the tail suspension test: Relationshp with hippocampal serotonin and norepinephrine transporter expression and function. Front. Pharmacol. 2013;4:131. doi: 10.3389/fphar.2013.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanez S, Owens WA, Gould GG, Murphy DL, Daws LC. Exagerated effect pf fluvoxamine in heterozygote serotonin transporter knockout mice. J. Neurochem. 2003;86:210–209. doi: 10.1046/j.1471-4159.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insight into human neurobiology. Nat. Rev. Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Perona MT, Waters S, Hall FS, Sora I, Lesch KP, Murphy DL, Caron M, Uhl GR. Animal Models of depression in dopamine, serotonin, and norepinephrine transporter knockout mice: prominent effects of dopamine transporter deletions. Behav. Pharmacol. 2008;19:566–574. doi: 10.1097/FBP.0b013e32830cd80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen IT, Bates JE, Goodnight JA, Dodge KA, Landford JE, Pettit GS, Latendresse SJ, Dick DM. Interaction between serotonin transporter polymorphism (5-HTTLPR) and stressful life events in adolescents’ trajectories of anxious/depressed symptoms. Dev. Psychol. 2012;48:1463–1475. doi: 10.1037/a0027471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LA, Happe HK, Petty F, Bylund DB. Juvenile rats in the forced swim test model the human response to antidepressant treatment for pediatric depression. Psychopharmacol. (Berl.) 2008;197:433–441. doi: 10.1007/s00213-007-1052-0. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Frank E, Kupfer DJ, Thase ME, Perel JM, Mazumdar S, Houck PR. Treatment outcome in recurrent major depression: a post hoc comparison of elderly (“young old”) and midlife patients. Am. J. Psychiatry. 1996;153:1288–1292. doi: 10.1176/ajp.153.10.1288. [DOI] [PubMed] [Google Scholar]
- Ripoll N, David DJ, Dailly E, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the tail suspension test. Behav. Brain Res. 2003;143:193–200. doi: 10.1016/s0166-4328(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Salum GA, Bortoluzzi A, Silveira PP, Bosa VL, Schuch I, Goldani M, Blaya C, Leistner-Segal S, Manfro GG. Is puberty a trigger for 5HTTLPR polymorphism association with depressive symptoms? J. Psychiatry Res. 2012;46:831–833. doi: 10.1016/j.jpsychires.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Serritti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- Sinyor M, Schaffer A, Levitt A. The sequence treatment alternatives to relieve depression (STAR*D) trail: a review. Can. J. Psychiatry. 2010;55:126–135. doi: 10.1177/070674371005500303. [DOI] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res. Health. 2000;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacol. (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Tucker JC, File EF. The effects of tricyclic and ‘atypical’ antidepressants on spontaneous locomotor activity in rodents. Neurosci. and Biobehav. Rev. 1986;10:115–121. doi: 10.1016/0149-7634(86)90022-9. [DOI] [PubMed] [Google Scholar]