Abstract
Anterior gradient 2 (AGR2) promotes cancer growth, metastasis and resistance to therapy via unknown mechanisms. We investigated the effects of extracellular AGR2 signaling through the orphan GPI-linked receptor C4.4A in pancreatic ductal adenocarcinoma (PDAC). Proliferation, migration and invasion and apoptosis were measured using colorimetric, Boyden chamber, and fluorescence-activated cell sorting analyses. We developed blocking monoclonal antibodies against AGR2 and C4.4A and tested their effects, along with siRNAs, on cancer cell functions and on orthotopic tumors in nude mice. Extracellular AGR2 stimulated proliferation, migration, invasion and chemoresistance of PDAC cell lines. AGR2 interacted with C4.4A in cell lysates and mixtures of recombinant proteins. Knockdown of C4.4A reduced migration and resistance to gemcitabine. PDAC tissues, but not adjacent healthy pancreatic tissues, expressed high levels of AGR2 and C4.4A. AGR2 signaling through C4.4A required laminins 1 or 5 and integrin β1. Administration of antibodies against AGR2 and C4.4A reduced growth and metastasis and caused regression of aggressive xenograft tumors leading to increased survival of mice. These data support a model in which AGR2 binds and signals via C4.4A in an autocrine loop and promotes the growth of pancreas tumors in mice. Blocking monoclonal antibodies against AGR2 and C4.4A may have therapeutic potential against PDAC.
Keywords: Anterior Gradient 2, C4.4A, bioluminescence, pancreatic adenocarcinoma, AGR2/C4.4A blocking antibodies
Introduction
Anterior Gradient 2 (AGR2) is expressed in a wide variety of tumors formed in different tissues with diverse patterns of genetic alterations including pancreatic ductal adenocarcinoma (PDAC) (1) and cancers of the breast (2,3), prostate (4), lung (5), and colorectum (6). AGR2 supports aggressive growth and metastasis of a variety of cancer cells (7-9). Hence, understanding the function of AGR2 may serve as useful therapeutic target. However, little is known of the mechanisms through which AGR2 functions.
AGR2 [also called hAG-2 (2) or Gob-4 (10)] is the human orthologue of the Xenopus laevis, XAG-2. XAG-2 is secreted and takes part in ectodermal patterning of the frog embryo and in amphibian limb regeneration by interacting with the receptor Prod-1 of Ly6 superfamily (11-13). However, there is no human homologue of Prod-1. It is unknown whether AGR2 functions through a receptor on the cell surface or functions within cells in humans. The tissue distribution of AGR2 in healthy adult humans indicates that it is restricted to organs possessing mucin producing cells. A mouse genetic deletion model of AGR2 showed alterations in mucin synthesis (14). Other studies have supported the concept that AGR2 possesses sequence similarity to the protein disulfide isomerase (PDI) family (15-18). A member of the PDI protein family may catalyze formation, reduction and isomerization of disulfide bonds, thereby stabilizing intermediate conformations during protein maturation in the ER (17). However, a role of AGR2 in protein synthesis in normal cells does not resemble its actions in amphibians and also does not well explain its observed roles in cancer.
Previously it was reported that AGR2 could bind to dystroglycan-1 (DAG-1) and C4.4A based on yeast two-hybrid results (3). However, no evidence was provided to support the interactions of these molecules in cells or the biological function of these interactions. In the current study, we identified C4.4A (LYPD3) as the functional cell surface receptor for extracellular AGR2. Similar to Prod-1, C4.4A belongs to the Ly6 family of receptors including CD59 (13) and uPAR (19) and was associated with increased metastasis of PDAC (20,21) melanoma (22) and non-small cell lung cancer (23). AGR2 protein levels are correlated with poor prognosis in breast cancer (23) and colorectal cancer (24,25) Furthermore, like other glycosylphosphatidylinositol (GPI)-linked plasma membrane receptors, C4.4A lacks intracellular domains to mediate its downstream signaling. To date, the signaling mechanisms engaged by C4.4A have not been identified.
To support the idea that an AGR2/C4.4A autocrine loop may be a therapeutic target against cancer, we developed novel mouse monoclonal blocking antibodies against both AGR2 and C4.4A. In vivo treatment with these antibodies significantly reduced PDAC tumor weight and metastasis and prolonged survival. These results suggest that further investigation of AGR2/C4.4A as a potential target for cancer therapy is warranted.
Materials & Methods
Cell lines
NIH 3T3, BxPC3, SU86.86, MiaPaCa-2, AsPC-1 and Capan-2 cells were obtained from ATCC (Manassas, VA). Cell line identities were verified using DNA fingerprinting (Powerplex16 system, Promega). Cells were routinely cultured in DMEM containing 10% FBS and were maintained at 37°C in a humidified atmosphere of 5% CO2.
Antibodies and recombinant proteins
Antibodies were purchased for AGR2 (mouse polyclonal), DAG-1, CD59 (Cat# ab56703, ab105504, ab9182, AbCam, Cambridge, MA) C4.4A, uPAR (Cat# AF5428, MAB807, R&D Systems, Minneapolis, MN), laminin 1, 5, ITG β1, α6, and β4 (sc-74417, sc-20145, sc-9936, sc-10730, Santa Cruz Biotechnology, Dallas, TX), p-ERK (Cat # 9160, Cell Signaling, Danvers, MA), β-actin (Cat#A2066, Sigma, St. Louis, MO) and control IgG (Cat# OB010701; Southern Biotech, Birmingham, Alabama). Human and mouse AGR2 proteins have 96% homology (26), therefore human recombinant (rAGR2) (ab64013, AbCam, Cambridge, MA) was used for all studies. Recombinant C4.4A was purchased (5428-C4-050, R&D Systems, Minneapolis, MN).
Transient transfection of siRNA
The following pre-designed and pre-validated siRNAs were purchased from Qiagen (Los Angeles, CA): siControl(Cat#1027281), siAGR2(Cat#SI04274522), siC4.4A(Cat#SI00105700,707,714,721), siUPAR(Cat#SI03033289), siCD59(Cat#SI03052616), laminin1(α1)(Cat#SI02779511), laminin 5(β3)(Cat#SI02664116), integrin α6(Cat#SI02654078), integrin β1(Cat#SI00300573), integrin β2(Cat#SI03648848), and integrin β4(Cat#SI02664109). Cells were transfected with siRNAs (5 or 10nM) with HiPerFect transfection reagent (Cat#301705; Qiagen, Los Angeles, CA) and lysates were prepared after 72 hrs.
IP Studies
Commercial AGR2 Ab (Abcam) (2μg) was used to immunoprecipitate AGR2 from SU86.86 lysate (100μg) and western blot was conducted. The same membrane was then probed for each antibody individually (suppl. figs) and with pooled antibodies. In addition, rAGR2 and rC4.4A were suspended together (2μg each) in lysis buffer and IP was conducted. IgG (mouse) served as control. Western blot imaging and processing was done with an Odyssey imaging system (LI-COR Biosciences, Lincoln, NE).
Cell growth, migration, invasion and apoptosis assays
Wild type and siRNA transfected PDAC cells were grown with rAGR2 (0–500 nM) in the presence or absence of Abs (polyclonal commercial or newly developed mAbs) (1μM). The medium was refreshed daily. Cell numbers were estimated after 48hrs by MTS assay as described previously (27). Proliferation is shown as percent of viable cells over the appropriate control. In order to make the scales similar for easy comparisons, as well to avoid the differences in basal values for each cell line, the data are presented as the percent of viable cells compared to the appropriate controls. Migration and invasion assays were conducted at 24hrs, as described previously (27). Apoptosis assays were conducted 72hrs after adding Gemcitabine (Gem) to Gem-sensitive BxPC-3 cells (1μM) and Gem-resistant AsPC-1 cells (5μM) (28), as described previously (1). Because siRNA transfection itself was observed to increase basal apoptosis, cells transfected with siRNAs were treated with a lower concentration of Gem (BxPC3-0.5μM; AsPC-1-1μM).
Immunohistochemical (IHC) Staining
IHC was performed on tissue microarray (TMA) slides with mAbs (1:1000) and developed using the Vectastain Universal kit (Vector Laboratories, Burlingame, CA), as described previously (1). Results were blindly evaluated by pathologist (HW) and expression levels were categorized as positive or negative (cytoplasmic staining of >10% or <10% of the tumor cells, respectively) and staining intensity as strong, moderate, or no staining. Apoptotic cells were detected in paraffin sections by fluorescence labeled TUNEL staining (Promega, Cat # G3250). Activation of the MapK pathway was evaluated by analysis of the levels of p-ERK (Cell Signaling, Cat # 9160; 1:1000) and the proliferative index of the cancer cells was measured by Ki-67 staining (Thermo Scientific, Cat # RM-9106-S0; 1:200).
Blocking Monoclonal Antibody development
Mouse monoclonal antibodies (mAbs) against AGR2 and C4.4A were developed in the Monoclonal Antibody Core of UT MDACC using unconjugated antigenic peptides. Antigenic peptide sequence for AGR2 falls between 25-125 amino acids, and for C4.4A between 240-340 amino acids. Several hybridoma colonies (2400 for each Ab) were screened for target recognition by ELISA using KLH-conjugated peptides. Selected hybridoma colonies were then cloned and screened for ELISA again to select those with the highest affinity. Selected clones were sub-cloned and purified using protein A columns. Validation of antibody specificity, blocking ability, and purity was conducted by western blotting against recombinant and cell lysates proteins; functional screening by apoptosis assay; binding screening by ELISA assay; and analysis of the purity of selected Abs by SDS-PAGE. In vitro validation experiments with the selected antibodies included inhibition of cancer cell migration and invasion and ability to increase in Gem induced apoptosis. Top candidate antibodies with high affinity and functional blocking ability, one each against AGR2 and C4.4A, were purified and further used to conduct in vivo experiments. Final selected clones were 28B for AGR2 and 1A for C4.4A. Antibodies were sub-typed as IgG1 for AGR2 and IgG2b for C4.4A. Purified antibodies for in vivo experiments were produced in the monoclonal antibody core.
In Vivo Studies
In vivo experiments were conducted with athymic nude mice (B6.Cg-Foxn1nu/J – female – age 9 weeks) (NCI, Bethesda, MD) according to the UT MDACC regulatory standards and IUCAC committee approval. Orthotopic tumors were developed with luciferase-labeled cells (0.25×106). IgG (Cat# OB010701; Southern Biotech, Birmingham, Alabama) served as a control Ab.
Model 1 (AsPc-1-Aggressive Cell Model)
Two weeks after the injection of the aggressive AsPC-1 cells, when the tumors weighed less than 0.5g (as surgically confirmed from a parallel untreated group), mice (n=6) were treated with control or mAbs (5mg each of AGR2/C4.4A mAb in combination/kg/body weight/twice a week/i.p) and with or without Gem (100mg/kg body weight/once a week/i.p) until all of the control mice had died (7 weeks). Tumor weight and metastasis to liver and lung were compared between control and treated groups ex vivo at the end of the experiment.
Model 2 (CaPan-2-Stromal Model)
Two weeks after the injection of stroma forming Capan-2 cells, mice (n=7) were treated with 15mg AGR2 or C4.4A Ab /kg/body weight/twice a week/i.p. Treatment was stopped after 15 weeks (13 weeks of treatment) and tumor size in surviving animals was monitored by bioluminescence until 63 weeks.
Model 3 (CaPan-2-Regression Study)
Four weeks after the injection of Capan-2 cells, when tumors weighed more than 1g (as surgically confirmed from a parallel untreated group), mice (n=5) were treated with AGR2 or C4.4A Abs 15mg/kg/body weight/twice a week/i.p or with both Abs in combination (7.5mg each). Treatment was stopped after 12 weeks. Bioluminescence was monitored on surviving animals until 18 weeks. Tumor growth and metastasis were measured weekly with the IVIS live animal bioluminescence imaging system after injecting luciferin substrate (Xenogen, Alameda, CA). The number of mice surviving was recorded each week and shown as the percent of the original group size.
Statistical analysis
All in vitro experiments were conducted in triplicate and carried out on three or more separate occasions. Data presented are means of the three or more independent experiments ± SEM. In vivo experiments were conducted with groups of 7-10 mice. Statistically significant differences were determined by ANOVA analysis (Newman-Keuls Multiple Comparison Test) and were defined as a p value of <0.05.
Results
Extracellular AGR2 Stimulates PDAC Aggressiveness and Chemoresistance in vitro
We previously reported that AGR2 is highly expressed and secreted by PDAC cells and contributes to chemoresistance (1). In the current study, we assessed whether extracellular AGR2 (rAGR2) could mimic the effects of AGR2 expression. Because PDAC cell lines are heterogeneous, we used multiple cell models - BxPc-3 (epithelial phenotype, sensitive to Gem), AsPC-1 and MiaPaCa-2 cells (mesenchymal phenotype, highly resistant to Gem) (28). Results from the AsPC-1 cells are shown throughout the manuscript; similar results obtained with BxPC-3 and MiaPaCa-2 cells are provided in the supplementary figures.
In AsPC-1 cells, treatment with rAGR2 increased proliferation (3-fold), migration (10-fold) and invasion (3-fold) in a concentration-dependent manner (Fig.1A-C). Similar effects were observed with BxPC-3 and MiaPaCa-2 cell lines (Supplementary Fig.S1A–S1C). To determine the effects of rAGR2 on cancer cell resistance to therapeutic agents, we treated PDAC cells with Gem in the presence and absence of rAGR2. Although AsPC-1 cells are highly resistant, a significant 3-fold increase in apoptosis was induced at a concentration of 5μM Gem (Fig.1D). Simultaneous treatment with rAGR2 reduced the effect of Gem to nearly the control level (>50% reduction), demonstrating a strong survival effect. AGR2 treatment had even larger effects with BxPC3 cells, which are more Gem sensitive (Supplementary Fig.S1D). Thus, extracellular recombinant AGR2 recapitulated the effects on PDAC cells previously observed with AGR2 expression (1).
Figure 1. Extracellular AGR2 stimulates PDAC cell aggressiveness.
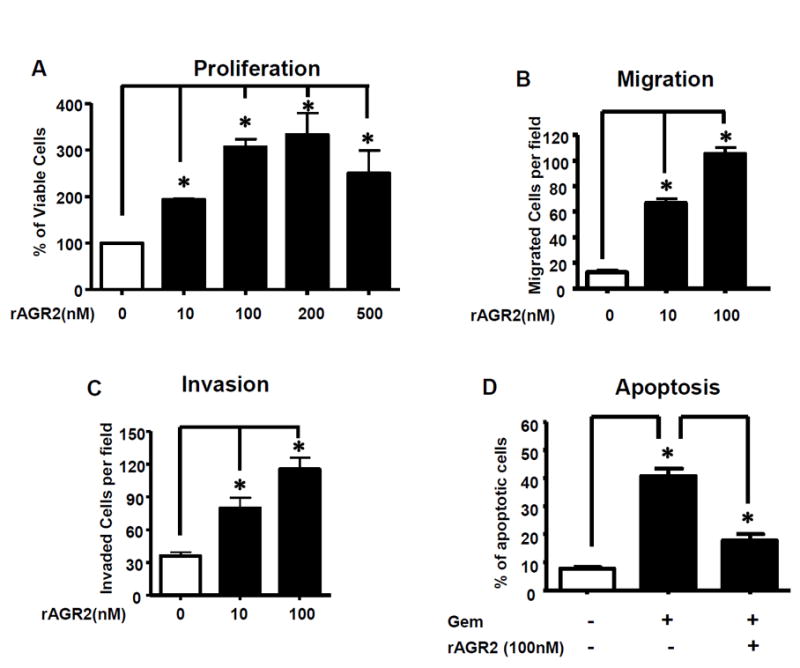
Extracellular addition of rAGR2 (0–500 nM) to AsPC-1 cells led to a dose-dependent increase in (A) cell proliferation, (B) migration and (C) invasion. (D) Gem addition resulted in increased apoptosis. Proliferation is shown as percent of viable cells over the control. However, extracellular AGR2 significantly reduced the level of Gem-induced apoptosis. Data shown are mean±SEM for 3 experiments (*p<0.05).
C4.4A is the Functional Receptor for AGR2
Candidate receptors for AGR2 were selected from the literature and examined for importance in AGR2 functions. Ly6 receptor family members - uPAR, C4.4A, and CD59 co-immunoprecipitated with AGR2 (Fig.2A), while DAG-1 did not co-immunoprecipitate. (See Supplementary Figs.S2A–S2C for individual immunoprecipitations). To determine the functional importance of each receptor, they were silenced using siRNAs and significant silencing was confirmed (Fig.2B). (See Figs.S2D–S2F for full blots). Only silencing of C4.4A significantly reduced basal and nearly completely abolished rAGR2-stimulated cell proliferation, migration and invasion in AsPC-1 cells (Fig.3A–C) and BxPC-3 cells (Supplementary Fig.S3A–S3C). On the other hand, silencing of CD59 and uPAR significantly increased AsPC-1 cell migration.
Figure 2. C4.4A and other LY6 family receptors interact with AGR2.
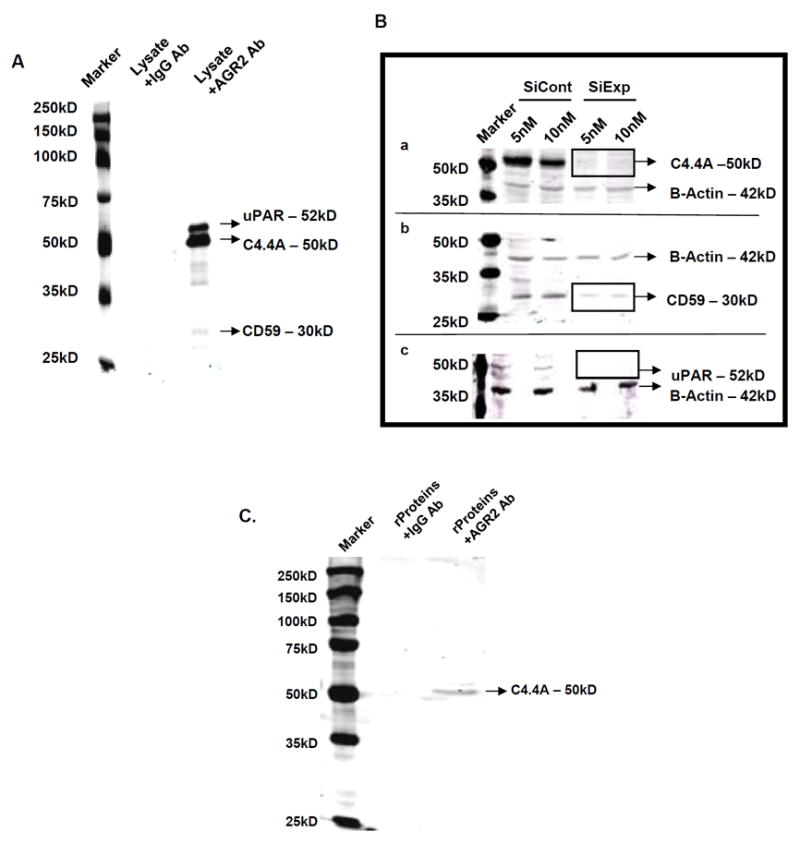
(A) Several candidate receptors (uPAR, C4.4A, and CD59) co-immunoprecipitated with AGR2, while DAG-1 did not. (B) Silencing of C4.4A, CD59, and uPAR was accomplished using siRNAs at two different concentrations. Significant silencing was shown by western blotting with respective antibodies and the same membranes were blotted for β-actin, which served as loading control. Both concentrations of siRNA showed significant silencing. Micrographs shown are representation of three independent experiments. (C) Purified recombinant AGR2 and C4.4A were also co-immunoprecipitated from their suspension, supporting a direct interaction of AGR2 and C4.4A.
Figure 3. C4.4A is required for rAGR2-mediated functions.
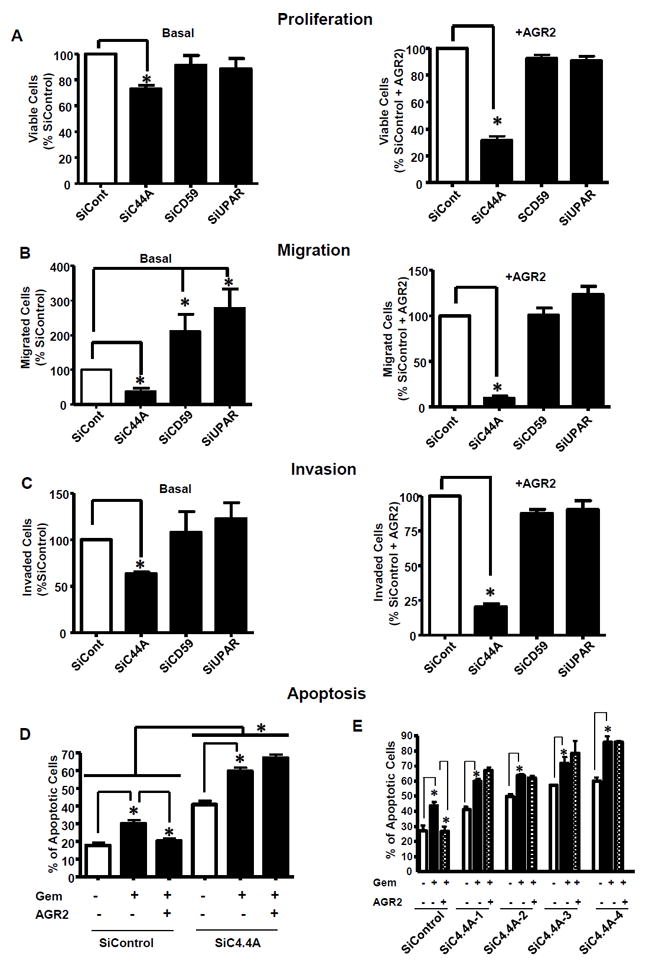
AsPC-1 cells were transfected with siRNAs to silence C4.4A, CD59 or uPAR then treated without (basal, left column) and with AGR2 (100nM, right column) daily. Only C4.4A silencing reduced both basal and rAGR2-stimulated (A) proliferation, (B) migration and (C) invasion. Proliferation is shown as percent of viable cells over the SiControl (basal) and SiControl+AGR2 (treated). (D) In SiControl cells, Gem stimulated apoptosis and this effect was ameliorated by AGR2. Silencing of C4.4A itself induced apoptosis; improved Gem-mediated apoptosis and abolished the survival effects of AGR2 showing significant increase in apoptosis. Data shown are mean±SEM for 3 experiments (*p<0.05). (E) To determine the effects of specific C4.4A siRNAs, four siRNAs (SiC4.4A 1–4) were used for apoptosis studies and had comparable results.
C4.4A silencing also blocked AGR2-mediated chemoresistance to Gem (Fig.3D and Supplementary Fig.S3D). Silencing of C4.4A alone, and in combination with Gem, resulted in significantly increased rates of apoptosis (2 fold), which was a greater increase than that observed with Gem treatment of control cells (Fig.3D). Importantly, the ability of AGR2 treatment to protect cells from Gem was abolished after C4.4A silencing. To control for off-target effects, we examined four siRNA sequences for C4.4A, each of which showed comparable results (Fig.3E). These data support the idea that the effects of extracellular AGR2 are mediated by interaction with C4.4A.
To determine whether AGR2 and C4.4A interact directly or only by association in a complex, rAGR2 and rC4.4A were combined in the absence of other proteins and co-immunoprecipitation was conducted. Direct interaction between rAGR2 and rC4.4A was indicated by the presence of an obvious band in this assay (Fig.2C). We also examined nine PDAC cell lines for C4.4A mRNA and protein expression and observed that it was present in all lines (See Supplementary Figs. S2G-I for full-length gels).
C4.4A Requires Integrin β1 and Laminins 1 and 5 for Activity
In light of previously identified signaling complexes of uPAR, a member of this receptor family (29), we investigated surface receptors, including integrins and extracellular matrix components that might be involved in C4.4A signaling. C4.4A was reported to bind laminins 1 and 5 although the functional consequences were unknown (30). Hence, candidate integrins and laminins 1 and 5 were silenced and AGR2-mediated Gem-resistance effects were assessed. Silencing of laminin 1, laminin 5 or integrin β1 completely abolished the protective effects of AGR2, while silencing of integrin β2, β4, or α6 had no effect (Fig.4A). Similarly, commercial blocking antibodies to laminins 1, laminins 5 and integrin β1 also abolished AGR2 mediated stimulation of proliferation and chemoprotective effects (Fig 4B,C). Unfortunately, our efforts to identify the participating integrin α subunits did not provide definitive answers. Similar results for the BxPC-3 cell line are shown in Supplementary Figs.S4A&B. Taken together these data suggest that laminins 1 and 5 and integrin β1 are involved in the AGR2/C4.4A receptor complex.
Figure 4. Effects of AGR2 are mediated by C4.4A interacting with integrin β1 and laminin 1 or laminin 5.
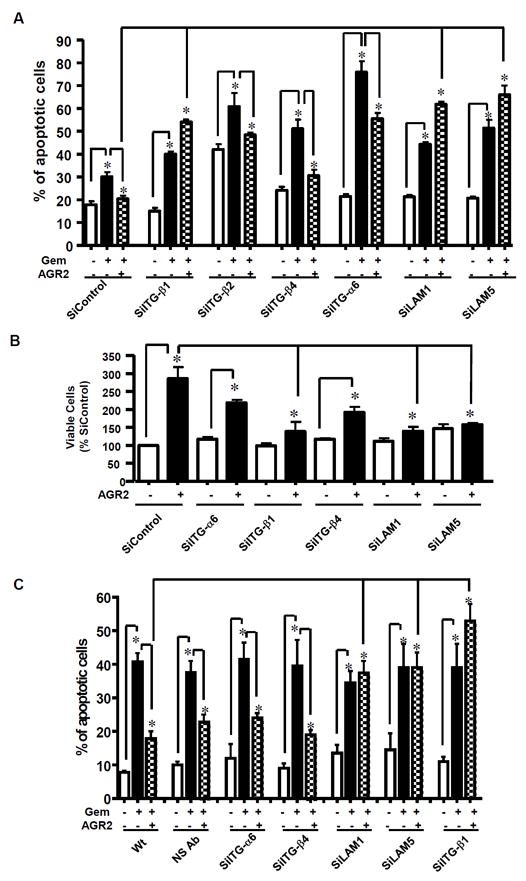
(A) In SiControl AsPC-1 cells, Gem stimulated apoptosis and addition of rAGR2 inhibited this effect. AsPC-1 cells were also transfected with siRNAs against ITG-β1, ITG-β2, ITG-β4, ITG-α6, laminin 1 and laminin 5. Only silencing of laminins 1 & 5 and integrin β1 increased Gem stimulated apoptosis and abolished the survival effects of AGR2. (B) Silencing of laminins 1 and 5 and integrin β1 by siRNA significantly abolished AGR2-mediated proliferation of AsPC-1 cells. (C) Commercially available blocking antibodies to ITG-β1, ITG-β2, ITG-β4, ITG-α6, laminin 1 and laminins 5 showed similar results as the siRNA treatments with only Abs to laminins 1 & 5 and integrin β1 blocking AGR2 mediated survival effects. Data shown are mean ± SEM for 3 experiments (*p<0.05)
Developed AGR2 and C4.4A monoclonal antibodies are highly specific and block the binding of AGR2 to C4.4A
To further understand the roles of AGR2 and C4.4A in cancer we wished to block their interactions using antibodies. Unfortunately, commercially available antibodies, while recognizing AGR2 (18kD) and C4.4A (50kD) (Fig.5A), did not block AGR2 induced cell migration (Fig.5B) or Gem resistance (Fig.5C). Therefore, we developed novel AGR2 and C4.4A mAbs which recognized their respective antigens and blocked their interactions (Fig 5A). The novel mABs were more specific than the commercial antibodies, as indicated by the lack of non-specific bands in western blots of pancreatic cancer cell lysates. We also observed that the novel mABs blocked AGR2 stimulation of cell migration and resistance to Gem while the commercially available antibodies were without effects (Fig 5B and 5C). Lead antibody binding and functional screening and purity testing has been added in Supplementary Figure 5 (A-G). Validation of antibody specificity, blocking ability, and purity was conducted by western blotting against recombinant and cell lysates proteins; functional screening by apoptosis assay; binding screening by ELISA assay; and analysis of the purity of selected Abs by SDS-PAGE.
Figure 5. Monoclonal antibodies with high specificity were developed that block AGR2/C4.4A binding and biological effects.
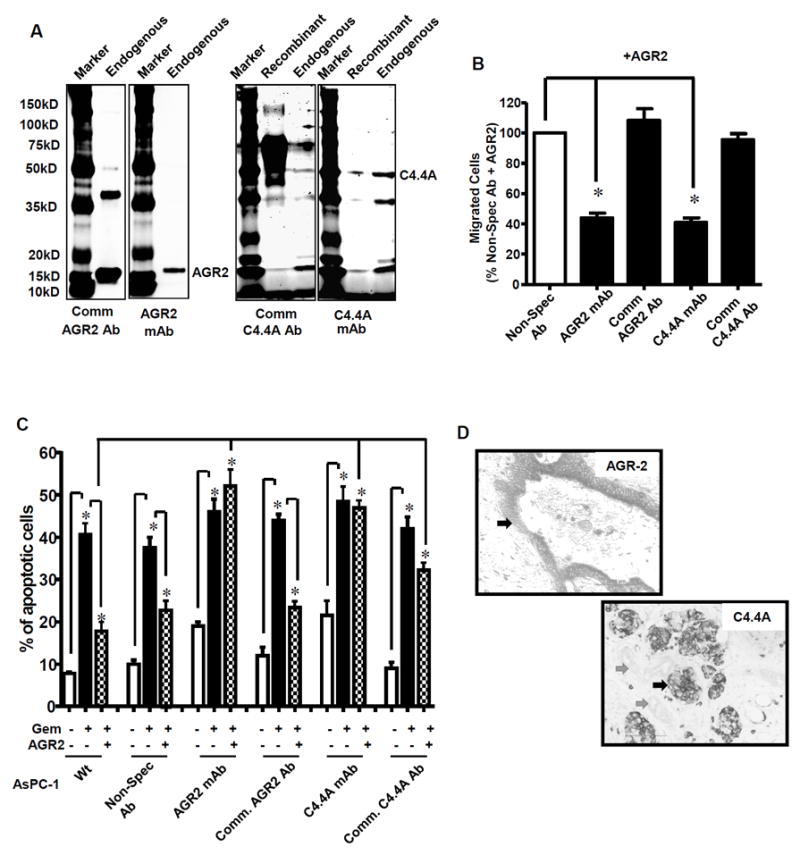
(A) From the endogenous PDAC cell lysate (SU86.86), AGR2 (18 kD) and C4.4A (50 kD) were identified by their respective newly developed mAbs. Extra bands identified with the endogenous protein were also observed with the recombinant protein and likely represent cleavage products. Commercially available antibodies also recognized these molecules. However, the commercially available Abs showed several non-specific bands. (B) Blocking mAbs reduced AGR2-stimulated PDAC cell migration and (C) blocked the survival effects of AGR2, whereas commercial Abs did not. (D) Immunohistochemical analysis on TMA using mAbs developed showed strong labeling of PDAC (indicated by arrow), but normal pancreas was not labeled.
AGR2/C4.4A are widely expressed in pancreatic cancer
The expression patterns of AGR2 and C4.4A were also assessed in patient tissues (TMA- Tissue Micro Array) using the mAbs developed (Fig.5D). Both antibodies showed strong labeling of PDAC, but normal pancreas was not labeled. For AGR2, 105 of 140 (75%) were positive with respective staining of 46% (high), 29% (moderate) and 25% (no staining). High levels of AGR2 expression was associated with higher frequency of lymph node metastasis in overall patient population and in stage II patients (p<0.05). There was also weak correlation between the AGR2 expression and differentiation. For C4.4A, 67 of 74 (91%) were positive with respective staining of 52% (high), 39% (moderate), and 9% (no staining). These data confirm that AGR2 and C4.4A are both highly expressed in advanced PDAC. Both molecules tend to be expressed together as the correlation between the expression of AGR2 and C4.4A in PDAC patients was significant (p<0.0001, correlation coefficient 0.74 (Spearman r).
Inhibition of the AGR2/C4.4A autocrine loop provides potential therapeutic benefits
To evaluate the potential therapeutic benefits of inhibiting the AGR2/C4.4A autocrine loop, we tested the effects of treatments with the blocking mAbs in pre-clinical models. In the aggressive cell model (Model 1) (Fig.6A), we used AsPC-1, a highly tumorigenic, metastatic and Gem-resistant cell line. We tested the effects of the combination of both mAbs with and without Gem. Mice were injected orthotopically with luciferase expressing AsPC-1 cells and tumors were allowed to form for 2 weeks prior to the start of treatments. After 4 weeks of treatment (6 weeks total), all mice in the control Ab group had died and the other mice were sacrificed to compare tumor weights and metastasis. At that time, 30% of the mice treated with the control Ab in combination with Gem, 100% of the mice with the combination of AGR2 and C4.4A mAbs and 80% of the mice with the combination of mAbs and Gem remained alive. Compared with control Ab, combined mAbs treatment reduced tumor weight by 33% (p<0.03) and incidence of metastasis by 66% (p<0.05) (Fig.7A&B). Combining Gem with the mABs did not have a significant advantage, as this combination resulted in a reduction in tumor weight by 40% (p<0.003) and incidence of metastasis by 50% (p<0.05). As no substantial benefits were obtained in combination with Gem treatment in Model 1, Gem treatment was not considered in Models 2 and 3. Tumor volume data for group mice and individual mice are included in the supplementary material (Fig S6A). Tumor volume was estimated weekly by bioluminescence imaging. Combo IgG with and without Gem showed reduction in tumor volume as compared to Control IgG with and without Gem. We also noticed that treatment with the mAbs did not reduce the animal’s body weight as compared to control Ab treated mice, suggesting a lack of systemic toxicity associated with blocking this pathway.
Figure 6. In Vivo treatment with AGR2/C4.4A antibodies reduced tumor volume and improved survival.
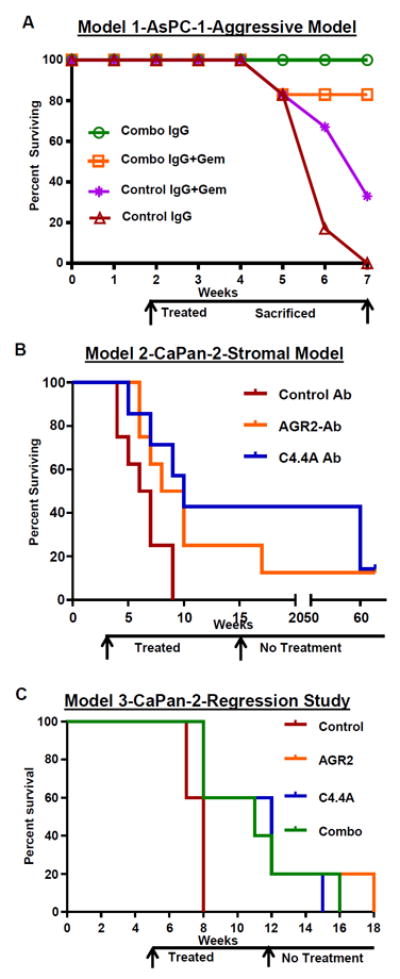
Tumor growth and metastasis was measured weekly with the IVIS live animal bioluminescence imaging system after injecting luciferin substrate (Xenogen, Alameda, CA). The number of mice which survived till end of the experiment was noted (Percent Surviving). A representative image of mice showing reduction/regression in tumor volume is also shown for each group. (A) Model 1-AsPc-1-Aggressive Model - Two weeks after the injection of the aggressive AsPC-1 cells, when the tumors weighed less than 0.5g (as surgically confirmed from a parallel untreated group), mice (n=6) were treated with control or mAbs (5mg each of AGR2/C4.4A mAb in combination/kg/body weight/twice a week/i.p) and with or without Gem (100mg/kg body weight/once a week/i.p) till 7 weeks. All mice treated with combined mAbs survived for at least 6 weeks, while all control mice perished within 6 weeks. (B) Model 2-CaPan2-Stromal Model - Two weeks after the injection of stromal forming Capan-2 cells, mice (n=7) were treated with 15mg AGR2 or C4.4A Ab /kg/body weight/twice a week/i.p. Treatment was stopped after 15 weeks (13 weeks of treatment) and tumor size in surviving animals was monitored by bioluminescence until 63 weeks. Treatment with either mAbs individually showed 24wks improvement in survival as compared to control mice which died by nine weeks. Forty-eight weeks past no-treatment, mice showed no tumor re-occurrence. (C) Model 3-CaPan-2-Regression Studies - Four weeks after the injection of Capan-2 cells, when tumors weighed more than 1g (as surgically confirmed from a parallel untreated group), mice (n=5) were treated with AGR2 or C4.4A Abs 15mg/kg/body weight/twice a week/i.p or with both Abs in combination (7.5mg each). Treatment was stopped after 12 weeks. Bioluminescence was monitored on surviving animals until 18 weeks. Treatment of mice possessing tumors larger than than 1g with mAbs improved their survival by 14wks compared to control mice which died by 4wks of treatment.
Figure 7. In Vivo treatment with AGR2/C4.4A antibodies reduced tumor weight and metastasis, increased apoptosis, reduced Erk activity, and showed reduced proliferation index.
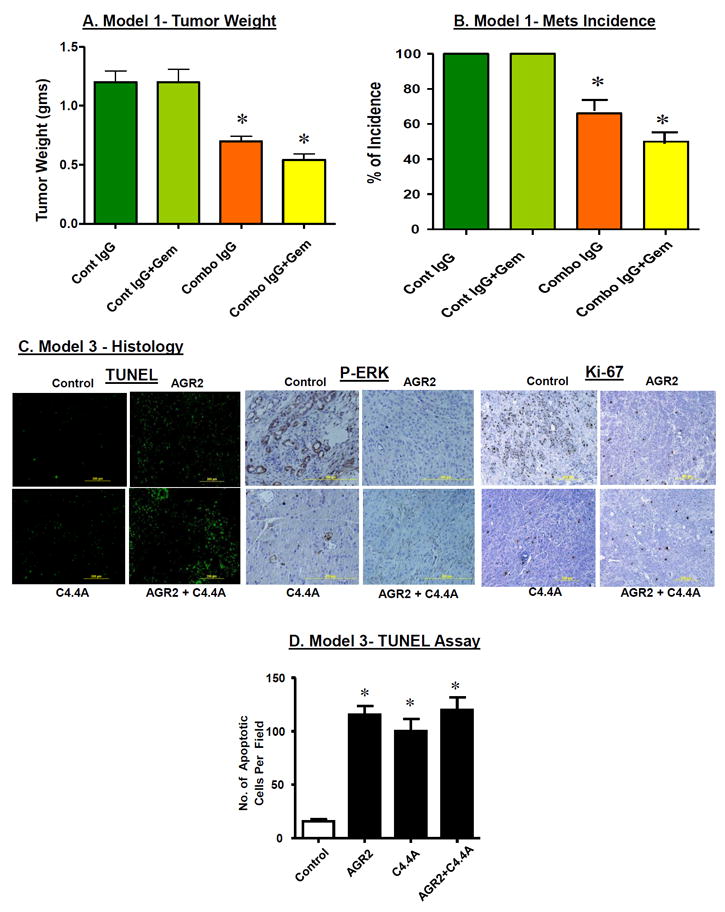
In Model 1, tumor weight and metastasis to liver and lung were compared between control and treated groups ex vivo at the end of the experiment. Mice treated with the mABs showed greatly reduced tumor growth (A) and mets incidence (B). Gemcitabine had no significant effect either alone or in combination with the mAbs. Histological examination of tumors developed in Model 3 was conducted. (C) TUNEL staining of paraffin sections showed increased apoptotic cells in antibody treated groups either alone or in combination compared to control. Staining for p-ERK and Ki-67 showed increased activity in cancer cells of control mice, while antibody treated mice showed no activity in the cancer cells. (D) Quantitation indicated a significant increase in the number of apoptotic cells per field in sections from tumors of mice treated with mABs. (*=p<0.05)
In the stromal model (Model 2) (Fig 6B), we used Capan-2, a Gem resistant, dense stroma forming but not metastasizing cell line. Mice in the control Ab group all died within 9 weeks (7 weeks of treatment). At that time, 43% of mice treated with the AGR2 Ab and 57% of mice treated with the C4.4A Ab were surviving. Treatments were discontinued after 15 weeks (13 weeks of treatment) and the animals were allowed to survive until they died or were severely morbid. Median survival (the point at which 50% of the animals survived) was 6wks for the control Ab, 9wks for the AGR2 Ab and 10wks for the C4.4A Ab) (p<0.05). After week 63, one mouse was surviving in each of the AGR2 Ab and C4.4A Ab groups. After sacrifice these animals were examined and no evidence of tumor was observed. Tumor volume data for group mice and individual mice are included in the supplementary material (Fig S6B). Mean tumor volume changes indicated that mice treated with either mAB showed slower growth. Both AGR2 and C4.4A Ab treatments reduced the tumor volume by 50% compared to control Ab (p<0.05). Some mice (1/7 of AGR2 mAb treated; 3/7 of C4.4A Ab treated) showed complete tumor regression as indicated by bioluminescence imaging and confirmed by surgical examination. In several mice, tumors were observed to disappear.
Regression studies (Model 3) were conducted on mice beginning 5 weeks after cancer cell implantation when tumors were more than 1g size (Fig 6C). In this study, all mice in the control Ab group died 3 weeks after initiation of treatment (8 weeks total). At that time, 60% of each mAB treated groups survived. Treatment with mABs was discontinued after 12 weeks and the mice were allowed to survive until they died or were severely morbid. Median survival times were 8 wks for control Ab treated animals, 12 weeks for AGR2 or C4.4A treated animals and 11wks for animals treated with the combination of AGR2 and C4.4A mABs (p<0.05). Tumor volume data for group mice and individual mice are included in the supplementary material (Fig S6C). Reduction in tumor volume for each treatment group is shown as measured by bioluminescence imaging. Control group mice showed an increase in tumor volume, while, AGR2/C4.4A/combo Ab treated mice showed regression in tumor volume. One of the five mice treated with AGR2 mAb showed complete regression of its tumor. Analysis of the residual tumor in surviving mice indicated a high level of apoptotic cells in mAB treated groups (Fig.7C&D). Analysis of p-ERK levels indicated that activity of this pathway was completely abolished in antibody treated groups (Fig 7C). Analysis of the proliferation indicator, Ki-67 showed no staining on mAbs treated groups (Fig.7C). Similar results were also observed in models 1&2.
DISCUSSION
AGR2 is associated with poor outcomes in several tumor types (26) but the mechanisms remain unknown. AGR2 has been reported to be involved in protein maturation and folding (14-16,31), to regulate cathepsins (32) and to modulate MUC-1 levels (14,33). However these roles of AGR2 do not explain its ability to act as an oncogene (34), or its ability to increase the aggressiveness of several types of cancer. It is therefore likely that this protein has multiple intracellular and extracellular functions. Potentially its physiologic and pathologic roles differ. In our current study, extracellular addition of rAGR2 stimulated the proliferation, migration, invasion, and chemoresistance of PDAC cells. These actions required the presence of cell surface receptors. Thus, based on these data, it seems likely that the important role of AGR2 in cancer is mechanistically similar to its roles in amphibians, where it is a secreted signaling molecule that interacts with a specific receptor.
In amphibians, AGR2 promotes limb growth by interacting with Prod1 (12,13), a GPI-linked receptor related to the Ly6 family of receptors in humans (19,35). The Lys6 family includes uPAR, C4.4A, and CD59 (19,13). Our current study indicated that Lys family receptors (uPAR, C4.4A, and CD59) were co-immunoprecipitated with AGR2, likely because of the structural homologies between these receptors (19). Nevertheless, only blocking the interaction of AGR2 with C4.4A by silencing or blocking antibodies reduced endogenous (basal) and extracellular rAGR2-stimulated PDAC cell functions. Though it was reported that in a yeast-two-hybrid system Dystoglycan-1 bound to AGR2, our co-immunoprecipitation study could not verify this interaction. Surprisingly, we observed that silencing of other two receptors, CD59 and uPAR, slightly increased the migration of PDAC cells. This observation was unexpected, as a previous report suggested that silencing uPAR inhibited PDAC cell migration (36). It is unclear what accounts for this difference, but it may be due to the studies being conducted in different cell lines. Nevertheless, the data showed here support a model in which AGR2 and C4.4A participate in an autocrine loop that activates survival mechanisms.
In our previous gene profiling studies, C4.4A was found to be highly expressed in pancreatic cancer but not in normal or chronic pancreatitis tissue (37). C4.4A is an orphan receptor described previously as a regulator of cancer cells metastasis (21,38). We have now identified for the first time C4.4A as a functional cell surface receptor for AGR2. Silencing or antibody mediated blocking of C4.4A eliminated the effects of extracellular AGR2, thus supporting AGR2 as the ligand for C4.4A. However, the mechanism of action of C4.4A had not previously been investigated. Hence, we further examined the signaling complex molecules that interact with C4.4A in order to identify a few specific molecules.
Like other GPI-linked receptors, C4.4A does not have an intracellular domain to mediate downstream signaling mechanisms. On the basis of homologies between C4.4A and uPAR, another member of the Ly6 family, these interactions likely include extracellular matrix proteins and specific integrin receptors. A recent study suggested that C4.4A promotes migration by associating with α6β4 (39). C4.4A was also previously reported to bind laminins 1 and 5, although functional studies were not conducted (30). Laminins 1 and 5 are thought to interact primarily with integrin α3β1 (40,41). Integrin α3β1 is expressed by pancreatic ductal cells (42). We observed that silencing of laminin 1 or 5 or integrin β1 abolished the effects of AGR2 treatments, thus suggesting their involvement in AGR2-mediated C4.4A receptor complex. Further studies will be required to fully understand the signaling mechanisms involved in the actions of C4.4A.
To examine the potential therapeutic benefits of blocking the AGR2/C4.4A autocrine loop, we developed blocking mAbs against the ligand (AGR2) and the receptor (C4.4A). Both Abs blocked basal and AGR2-mediated functions. Pre-clinical studies using the blocking mAbs in three different types of preclinical models resulted in significant reductions in tumor weight and metastasis and improved survival. We found that treatment with the mAbs let to better therapeutic benefits than treatment with Gem, the clinical standard of care for PDAC. Partial or complete regression of tumors was observed in several mice after mAbs treatment individually or in combination and even several weeks post treatment no tumor re-occurrence was observed.
In summary, this study indicated that AGR2 has extracellular functions to increase the aggressiveness of cancer cells and that C4.4A is the functional receptor of AGR2. The signaling complex of C4.4A likely includes laminins 1 and 5 and β1 integrin. Blocking mAbs developed against AGR2 and/or C4.4A significantly reduced tumor growth and metastasis; and led to tumor regression resulting in remarkably improved survival in preclinical mouse models. Thus, this study has provided valuable new insights into a poorly understood but clinically significant pathway and supports the further investigation of the AGR2/C4.4A autocrine loop as a potential target for cancer therapy.
Supplementary Material
Acknowledgments
Financial Support: This research was supported by funds from the Lockton Endowment (to C.D. Logsdon), by Cancer Center Support Core grant CA16672, Pancreatic Specialized Programs of Research Excellence (SPORE) grant P20 CA101936 (to The University of Texas MD Anderson Cancer Center), University Cancer Foundation (to V.Ramachandran) and GS Hogan Gastrointestinal Research funds (to C.D. Logsdon and V. Ramachandran). This research was also supported by funds from the Sheikh Ahmed Center for Pancreatic Cancer Research at The University of Texas M. D. Anderson Cancer Center (to V. Ramachandran). We also acknowledge the MDACC Monoclonal Antibody Core Facility for their help in developing the monoclonal antibodies and the core is funded by NCI#CA 16672.
Footnotes
Disclosures: None
References
- 1.Ramachandran V, Arumugam T, Wang H, Logsdon CD. Anterior gradient 2 is expressed and secreted during the development of pancreatic cancer and promotes cancer cell survival. Cancer Res. 2008;68:7811–7818. doi: 10.1158/0008-5472.CAN-08-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson DA, Weigel RJ. hAG-2, the human homologue of the Xenopus laevis cement gland gene XAG-2, is coexpressed with estrogen receptor in breast cancer cell lines. Biochem Biophys Res Commun. 1998;251:111–116. doi: 10.1006/bbrc.1998.9440. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher GC, Patel S, Tyson K, Adam PJ, Schenker M, Loader JA, et al. hAG-2 and hAG-3, human homologues of genes involved in differentiation, are associated with oestrogen receptor-positive breast tumours and interact with metastasis gene C4.4a and dystroglycan. Br J Cancer. 2003;88:579–85. doi: 10.1038/sj.bjc.6600740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang JS, Gong A, Cheville JC, Smith DI, Young CY. AGR2, an androgen-inducible secretory protein overexpressed in prostate cancer. Genes Chromosomes Cancer. 2005;43:249–259. doi: 10.1002/gcc.20188. [DOI] [PubMed] [Google Scholar]
- 5.Zhu H, Lam DC, Han KC, Tin VP, Suen WS, Wang E, et al. High resolution analysis of genomic aberrations by metaphase and array comparative genomic hybridization identifies candidate tumour genes in lung cancer cell lines. Cancer Lett. 2007;245:303–314. doi: 10.1016/j.canlet.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, et al. Global gene expression profiling of circulating tumor cells. Cancer Res. 2005;65:4993–4997. doi: 10.1158/0008-5472.CAN-04-4330. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Rudland PS, Sibson DR, Platt-Higgins A, Barraclough R. Human homologue of cement gland protein, a novel metastasis inducer associated with breast carcinomas. Cancer Res. 2005;65:3796–3805. doi: 10.1158/0008-5472.CAN-04-3823. [DOI] [PubMed] [Google Scholar]
- 8.Innes HE, Liu D, Barraclough R, et al. Significance of the metastasis-inducing protein AGR2 for outcome in hormonally treated breast cancer patients. Br J Cancer. 2006;94:1057–1065. doi: 10.1038/sj.bjc.6603065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Innes HE, Liu D, Barraclough R, Davies MP, O’Neill PA, Platt-Higgins A, et al. The metastasis-associated anterior gradient 2 protein is correlated with poor survival of breast cancer patients. Am J Pathol. 2009;175:1848–1857. doi: 10.2353/ajpath.2009.090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komiya T, Tanigawa Y, Hirohashi S. Cloning of the gene gob-4, which is expressed in intestinal goblet cells in mice. Biochim Biophys Acta. 1999;1444:434–438. doi: 10.1016/s0167-4781(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 11.Aberger F, Weidinger G, Grunz H, Richter K. Anterior specification of embryonic ectoderm: the role of the Xenopus cement gland-specific gene XAG-2. Mech Dev. 1998;72:115–30. doi: 10.1016/s0925-4773(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Gates PB, Brockes JP. Positional identity of adult stem cells in salamander limb regeneration. C R Biol. 2007;330:485–490. doi: 10.1016/j.crvi.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 13.da Silva SM, Gates PB, Brockes JP. The new ortholog of CD59 is implicated in proximodistal identity during amphibian limb regeneration. Dev Cell. 2002;3:547–555. doi: 10.1016/s1534-5807(02)00288-5. [DOI] [PubMed] [Google Scholar]
- 14.Park SW, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci U S A. 2009;106:6950–6955. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao F, Edwards R, Dizon D, Afrasiabi K, Mastroianni JR, Geyfman M, et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2-/- mice. Dev Biol. 2010;338:270–279. doi: 10.1016/j.ydbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persson S, Rosenquist M, Knoblach B, Khosravi-Far R, Sommarin M, Michalak M. Diversity of the protein disulfide isomerase family: identification of breast tumor induced Hag2 and Hag3 as novel members of the protein family. Mol Phylogenet Evol. 2005;36:734–740. doi: 10.1016/j.ympev.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Dong A, Lowe AW. AGR2 gene function requires a unique endoplasmic reticulum localization motif. J Biol Chem. 2012;287:4773–82. doi: 10.1074/jbc.M111.301531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galat A. The three-fingered protein domain of the human genome. Cell Mol Life Sci. 2008;65:3481–3493. doi: 10.1007/s00018-008-8473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Würfel J, Seiter S, Stassar M, Claas A, Kläs R, Rösel M, et al. Cloning of the human homologue of the metastasis-associated rat C4.4A. Gene. 2001;262:35–41. doi: 10.1016/s0378-1119(00)00515-1. [DOI] [PubMed] [Google Scholar]
- 21.Rösel M, Claas C, Seiter S, Herlevsen M, Zöller M. Cloning and functional characterization of a new phosphatidyl-inositol anchored molecule of a metastasizing rat pancreatic tumor. Oncogene. 1998;17:1989–2002. doi: 10.1038/sj.onc.1202079. [DOI] [PubMed] [Google Scholar]
- 22.Seiter S, Stassar M, Rappl G, Reinhold U, Tilgen W, Zöller M. Upregulation of C4.4A expression during progression of melanoma. J Invest Dermatol. 2001;116:344–347. doi: 10.1046/j.1523-1747.2001.01230.x. [DOI] [PubMed] [Google Scholar]
- 23.Hansen LV, Skov BG, Ploug M, Pappout H. Tumour cell expression of C4.4A, a structural homologue of the urokinase receptor, correlates with poor prognosis in non-small cell lung cancer. Lung Cancer. 2007;58:260–266. doi: 10.1016/j.lungcan.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Paret C, Hildebrand D, Weitz J, Kopp-Schneider A, Kuhn A, Beer A, Hautmann R, Zöller M. C4.4A as a candidate marker in the diagnosis of colorectal cancer. Br J Cancer. 2007;97:1146–1156. doi: 10.1038/sj.bjc.6604012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konishi K, Yamamoto H, Mimori K, Takemasa I, Mizushima T, Ikeda M, et al. Expression of C4.4A at the invasive front is a novel prognostic marker for disease recurrence of colorectal cancer. Cancer Sci. 2010;101:2269–2277. doi: 10.1111/j.1349-7006.2010.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brychtova V, Vojtesek B, Hrstka R. Anterior gradient 2: a novel player in tumor cell biology. Cancer Lett. 2011;304:1–7. doi: 10.1016/j.canlet.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran V, Arumugam T, Hwang RF, Greenson JK, Simeone DM, Logsdon CD. Adrenomedullin is expressed in pancreatic cancer and stimulates cell proliferation and invasion in an autocrine manner via the adrenomedullin receptor, ADMR. Cancer Res. 2007;67:2666–2675. doi: 10.1158/0008-5472.CAN-06-3362. [DOI] [PubMed] [Google Scholar]
- 28.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23–36. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
- 30.Paret C, Bourouba M, Beer A, Miyazaki K, Schnölzer M, Fiedler S, et al. Ly6 family member C4.4A binds laminins 1 and 5, associates with galectin-3 and supports cell migration. Int J Cancer. 2005;115:724–733. doi: 10.1002/ijc.20977. [DOI] [PubMed] [Google Scholar]
- 31.Higa A, Mulot A, Delom F, Bouchecareilh M, Nguyên DT, Boismenu D, et al. Role of the pro-oncogenic Protein Disulfide Isomerase (PDI)-family member Anterior Gradient 2 (AGR2) in the control of endoplasmic reticulum homeostasis. J Biol Chem. 2011;286:44855–68. doi: 10.1074/jbc.M111.275529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumartin L, Whiteman HJ, Weeks ME, Hariharan D, Dmitrovic B, Iacobuzio-Donahue CA, et al. AGR2 is a novel surface antigen that promotes the dissemination of pancreatic cancer cells through regulation of cathepsins B and D. Cancer Res. 2011;71:7091–7102. doi: 10.1158/0008-5472.CAN-11-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norris AM, Gore A, Balboni A, Young A, Longnecker DS, Korc M. AGR2 is a SMAD4-suppressible gene that modulates MUC1 levels and promotes the initiation and progression of pancreatic intraepithelial neoplasia. Oncogene. 2013;32(33):3867–76. doi: 10.1038/onc.2012.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Hao Y, Lowe AW. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res. 2008;68:492–497. doi: 10.1158/0008-5472.CAN-07-2930. [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee S, Mayor S. The GPI-anchor and protein sorting. Cell Mol Life Sci. 2001;58:1969–1987. doi: 10.1007/PL00000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue A, Xue M, Jackson C, Smith RC. Suppression of urokinase plasminogen activator receptor inhibits proliferation and migration of pancreatic adenocarcinoma cells via regulation of ERK/p38 signaling. Int J Biochem Cell Biol. 2009;41:1731–8. doi: 10.1016/j.biocel.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Logsdon CD, Simeone DM, Binkley C, Arumugam T, Greenson JK, Giordano TJ, et al. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 38.Jacobsen B, Ploug M. The urokinase receptor and its structural homologue C4.4A in human cancer: expression, prognosis and pharmacological inhibition. Curr Med Chem. 2008;5:2559–2573. doi: 10.2174/092986708785909012. [DOI] [PubMed] [Google Scholar]
- 39.Ngora H, Galli UM, Miyazaki K, Zoller M. Membrane-Bound and Exosomal Metastasis-Associated C4.4A Promotes Migration by Associating with the α(6)β(4) Integrin and MT1-MMP. Neoplasia. 2012;14:95–107. doi: 10.1593/neo.111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldfinger LE, Hopkinson SB, deHart GW, Collawn S, Couchman JR, Jones JC. The alpha3 laminin subunit, alpha6beta4 and alpha3beta1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J Cell Sci. 1999;112:2615–2629. doi: 10.1242/jcs.112.16.2615. [DOI] [PubMed] [Google Scholar]
- 41.Symington BE, Carter WG. Modulation of epidermal differentiation by epiligrin and integrin alpha 3 beta 1. J Cell Sci. 1995;108:831–838. doi: 10.1242/jcs.108.2.831. [DOI] [PubMed] [Google Scholar]
- 42.Jiang FX, Naselli G, Harrison LC. Distinct distribution of laminin and its integrin receptors in the pancreas. J Histochem Cytochem. 2002;50:1625–1632. doi: 10.1177/002215540205001206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


